Abstract
Human cells, cell cultures, and organ cultures have been extremely useful for studying the events that occur when gonococci and meningococci encounter human mucosal surfaces. The specificity and selectivity of these events for human cells are striking and correlate with the adaptation of these pathogens for survival on human mucous membranes. To colonize these sites, meningococci and gonococci have developed mechanisms to damage local host defenses such as the mucociliary blanket, to attach to epithelial cells, and to invade these cells. Attachment to epithelial cells mediated by pili, and to some types of cells mediated by PIIs, serves to anchor the organism close to sources of nutrition and allows multiplication. Intracellular invasion, possibly initiated by the major porin protein, may provide additional nutritional support and protection from host defenses. Mucosal invasion may also result in access of gonococci and meningococci to the bloodstream, leading to dissemination.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton F. E., Pasieka A. E., Collins F., Wallace R., Diena B. B. Inhibition of attachment of Neisseria gonorrhoeae to tissue cells by goat milk antigonococcal immunoglobulin G. Can J Microbiol. 1977 Aug;23(8):975–980. doi: 10.1139/m77-145. [DOI] [PubMed] [Google Scholar]
- Bessen D., Gotschlich E. C. Chemical characterization of binding properties of opacity-associated protein II from Neisseria gonorrhoeae. Infect Immun. 1987 Jan;55(1):141–147. doi: 10.1128/iai.55.1.141-147.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen D., Gotschlich E. C. Interactions of gonococci with HeLa cells: attachment, detachment, replication, penetration, and the role of protein II. Infect Immun. 1986 Oct;54(1):154–160. doi: 10.1128/iai.54.1.154-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur B. R., Johnson W. M., Johnson K. G., Diena B. B. In vitro interaction of Neisseria gonorrhoeae type 1 and type 4 with tissue culture cells. Infect Immun. 1977 Feb;15(2):560–567. doi: 10.1128/iai.15.2.560-567.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Pearce W. A. Pili as a mediator of the attachment of gonococci to human erythrocytes. Infect Immun. 1976 May;13(5):1483–1489. doi: 10.1128/iai.13.5.1483-1489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Jeffery C., Dever C. A. Electron microscope studies of attachment to human fallopian tube mucosa by a gonococcal IgA1 protease deficient mutant and wild type parent. Scan Electron Microsc. 1984;(Pt 4):1925–1930. [PubMed] [Google Scholar]
- Cooper M. D., McGee Z. A., Mulks M. H., Koomey J. M., Hindman T. L. Attachment to and invasion of human fallopian tube mucosa by an IgA1 protease-deficient mutant of Neisseria gonorrhoeae and its wild-type parent. J Infect Dis. 1984 Nov;150(5):737–744. doi: 10.1093/infdis/150.5.737. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., McGraw P. A., Melly M. A. Localization of gonococcal lipopolysaccharide and its relationship to toxic damage in human fallopian tube mucosa. Infect Immun. 1986 Feb;51(2):425–430. doi: 10.1128/iai.51.2.425-430.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper D. L., Donegan E. A., James J. F., Sweet R. L., Brooks G. F. In vitro modeling of acute salpingitis caused by Neisseria gonorrhoeae. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):996–1002. doi: 10.1016/0002-9378(80)91095-9. [DOI] [PubMed] [Google Scholar]
- Evans B. A. Ultrastructural study of cervical gonorrhea. J Infect Dis. 1977 Aug;136(2):248–255. doi: 10.1093/infdis/136.2.248. [DOI] [PubMed] [Google Scholar]
- Farley M. M., Stephens D. S., Mulks M. H., Cooper M. D., Bricker J. V., Mirra S. S., Wright A. Pathogenesis of IgA1 protease-producing and -nonproducing Haemophilus influenzae in human nasopharyngeal organ cultures. J Infect Dis. 1986 Nov;154(5):752–759. doi: 10.1093/infdis/154.5.752. [DOI] [PubMed] [Google Scholar]
- Garcia-Kutzbach A., Beachey E. H., Chandler R. W., Townes A. S., Masi A. T. Identification of Neisseria gonorrhoeae in synovial membrane by electron microscopy. J Infect Dis. 1974 Aug;130(2):183–186. doi: 10.1093/infdis/130.2.183. [DOI] [PubMed] [Google Scholar]
- Goldman W. E., Klapper D. G., Baseman J. B. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect Immun. 1982 May;36(2):782–794. doi: 10.1128/iai.36.2.782-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J. J., Floyd K., Philipps M. E., Frasch C. E. Morphological differences in Neisseria meningitidis pili. Infect Immun. 1988 Sep;56(9):2356–2362. doi: 10.1128/iai.56.9.2356-2362.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C. R., Johnson A. P., Taylor-Robinson D., Melly M. A., McGee Z. A. Host species-specific damage to oviduct mucosa by Neisseria gonorrhoeae lipopolysaccharide. Infect Immun. 1981 Dec;34(3):1056–1058. doi: 10.1128/iai.34.3.1056-1058.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C. R., Melly M. A., Hellerqvist C. G., Coniglio J. G., McGee Z. A. Toxic activity of purified lipopolysaccharide of Neisseria gonorrhoeae for human fallopian tube mucosa. J Infect Dis. 1981 Mar;143(3):432–439. doi: 10.1093/infdis/143.3.432. [DOI] [PubMed] [Google Scholar]
- Gregg C. R., Melly M. A., McGee Z. A. Gonococcal lipopolysaccharide: a toxin for human fallopian tube mucosa. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):981–984. doi: 10.1016/0002-9378(80)91092-3. [DOI] [PubMed] [Google Scholar]
- Gubish E. R., Jr, Chen K. C., Buchanan T. M. Attachment of gonococcal pili to lectin-resistant clones of Chinese hamster ovary cells. Infect Immun. 1982 Jul;37(1):189–194. doi: 10.1128/iai.37.1.189-194.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubish E. R., Jr, Mace M. L., Jr, Steiner S. M., Williams R. P. Assessment of attachment of Neisseria gonorrhoeae to HeLa cells by double radiolabeling. Infect Immun. 1979 Sep;25(3):1043–1050. doi: 10.1128/iai.25.3.1043-1050.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckels J. E., Virji M., Zak K., Fletcher J. N. Immunobiology of gonococcal outer membrane protein I. Antonie Van Leeuwenhoek. 1987;53(6):461–464. doi: 10.1007/BF00415503. [DOI] [PubMed] [Google Scholar]
- James J. F., Lammel C. J., Draper D. L., Brown D. A., Sweet R. L., Brooks G. F. Gonococcal attachment to eukaryotic cells. Sex Transm Dis. 1983 Oct-Dec;10(4):173–179. doi: 10.1097/00007435-198311000-00002. [DOI] [PubMed] [Google Scholar]
- Johnson A. P., Clark J. B., Osborn M. F., Taylor-Robinson D. A comparison of the association of Neisseria gonorrhoeae with human and guinea-pig genital mucosa maintained in organ culture. Br J Exp Pathol. 1980 Oct;61(5):521–527. [PMC free article] [PubMed] [Google Scholar]
- Johnson A. P., Taylor-Robinson D., McGee Z. A. Species specificity of attachment and damage to oviduct mucosa by Neisseria gonorrhoeae. Infect Immun. 1977 Dec;18(3):833–839. doi: 10.1128/iai.18.3.833-839.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Lynch E. C., Blake M. S., Gotschlich E. C., Mauro A. Studies of Porins: Spontaneously Transferred from Whole Cells and Reconstituted from Purified Proteins of Neisseria gonorrhoeae and Neisseria meningitidis. Biophys J. 1984 Jan;45(1):104–107. doi: 10.1016/S0006-3495(84)84127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson K. E., Davies J., Grundström T., Kihlström E., Normark S. Surface charge and hydrophobicity of Salmonella, E. coli, Gonococci in relation to their tendency to associate with animal cells. Scand J Infect Dis Suppl. 1980;Suppl 24:135–140. [PubMed] [Google Scholar]
- McGee Z. A., Gorby G. L., Wyrick P. B., Hodinka R., Hoffman L. H. Parasite-directed endocytosis. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S311–S316. doi: 10.1093/cid/10.supplement_2.s311. [DOI] [PubMed] [Google Scholar]
- McGee Z. A., Johnson A. P., Taylor-Robinson D. Human fallopian tubes in organ culture: preparation, maintenance, and quantitation of damage by pathogenic microorganisms. Infect Immun. 1976 Feb;13(2):608–618. doi: 10.1128/iai.13.2.608-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Johnson A. P., Taylor-Robinson D. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J Infect Dis. 1981 Mar;143(3):413–422. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- Melly M. A., Gregg C. R., McGee Z. A. Studies of toxicity of Neisseria gonorrhoeae for human fallopian tube mucosa. J Infect Dis. 1981 Mar;143(3):423–431. doi: 10.1093/infdis/143.3.423. [DOI] [PubMed] [Google Scholar]
- Melly M. A., McGee Z. A., Rosenthal R. S. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J Infect Dis. 1984 Mar;149(3):378–386. doi: 10.1093/infdis/149.3.378. [DOI] [PubMed] [Google Scholar]
- Mårdh P. A., Baldetorp B., Håkansson C. H., Fritz H., Weström L. Studies of ciliated epithelia of the human genital tract. 3: Mucociliary wave activity in organ cultures of human Fallopian tubes challenged with Neisseria gonorrhoeae and gonococcal endotoxin. Br J Vener Dis. 1979 Aug;55(4):256–264. doi: 10.1136/sti.55.4.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota F., Pontefract R., Ashton F. E., Diena B. B. Studies on gonococcal infection. II. Attachment and fate of gonococci in tissue-culture cells. Can J Microbiol. 1975 Nov;21(11):1698–1704. doi: 10.1139/m75-249. [DOI] [PubMed] [Google Scholar]
- Pearce W. A., Buchanan T. M. Attachment role of gonococcal pili. Optimum conditions and quantitation of adherence of isolated pili to human cells in vitro. J Clin Invest. 1978 Apr;61(4):931–943. doi: 10.1172/JCI109018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. P., Sadoff J. C. Induced engulfment of Neisseria gonorrhoeae by tissue culture cells. Infect Immun. 1988 Sep;56(9):2512–2514. doi: 10.1128/iai.56.9.2512-2514.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal L., Danielsson D. Induction of DNA synthesis in lymphocytes in vitro by various bacteria, with special reference to Neisseria gonorrhoeae, in patients with uro-arthritis (Reiter's disease). Scand J Rheumatol. 1978;7(2):101–108. doi: 10.3109/03009747809098845. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Fernandez R., Wang L., Teng N. N., Schoolnik G. K. Antibodies to peptides corresponding to a conserved sequence of gonococcal pilins block bacterial adhesion. Proc Natl Acad Sci U S A. 1985 Feb;82(3):915–919. doi: 10.1073/pnas.82.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Fernandez R., Tai J. Y., Rothbard J., Gotschlich E. C. Gonococcal pili. Primary structure and receptor binding domain. J Exp Med. 1984 May 1;159(5):1351–1370. doi: 10.1084/jem.159.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. H., Falkow S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect Immun. 1988 Jun;56(6):1625–1632. doi: 10.1128/iai.56.6.1625-1632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. H., Hayes F., Brooks G. F., Falkow S. Development of a tissue culture model for gonococcal invasion. Antonie Van Leeuwenhoek. 1987;53(6):485–491. doi: 10.1007/BF00415507. [DOI] [PubMed] [Google Scholar]
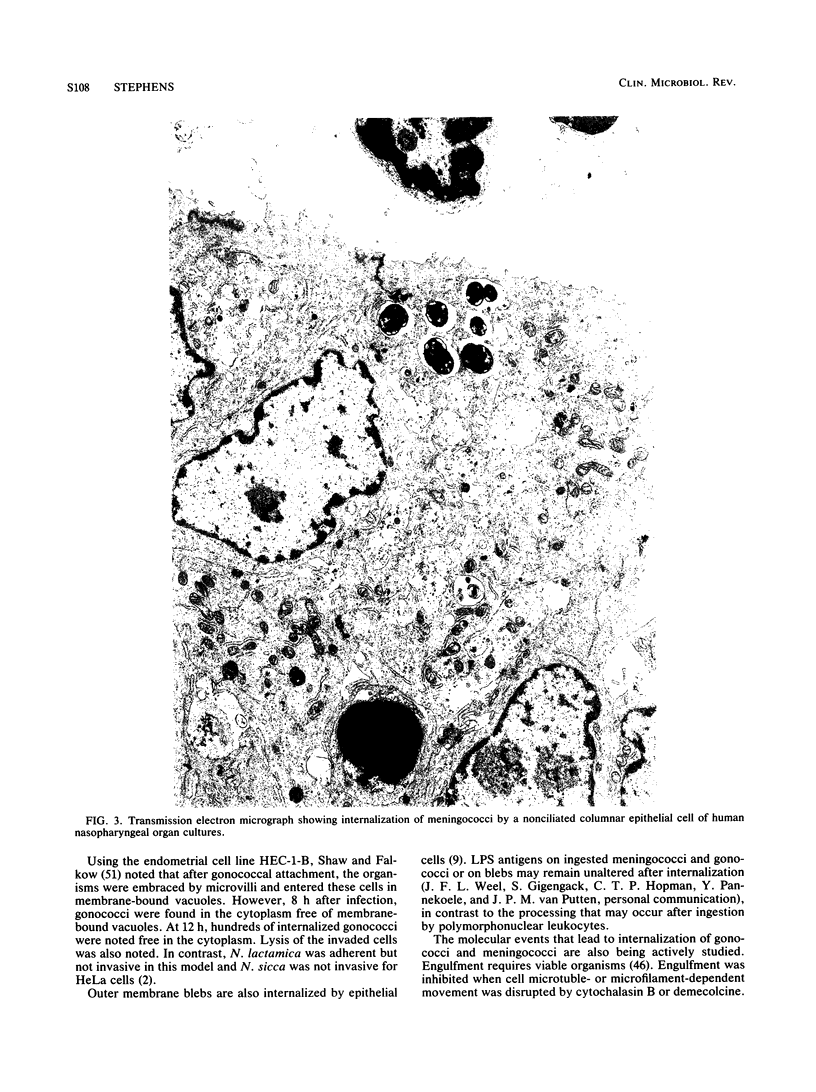
- Stephens D. S., Hoffman L. H., McGee Z. A. Interaction of Neisseria meningitidis with human nasopharyngeal mucosa: attachment and entry into columnar epithelial cells. J Infect Dis. 1983 Sep;148(3):369–376. doi: 10.1093/infdis/148.3.369. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., McGee Z. A. Attachment of Neisseria meningitidis to human mucosal surfaces: influence of pili and type of receptor cell. J Infect Dis. 1981 Apr;143(4):525–532. doi: 10.1093/infdis/143.4.525. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., McGee Z. A., Cooper M. D. Cytopathic effects of the pathogenic Neisseria. Studies using human fallopian tube organ cultures and human nasopharyngeal organ cultures. Antonie Van Leeuwenhoek. 1987;53(6):575–584. doi: 10.1007/BF00415519. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., Whitney A. M., Melly M. A., Hoffman L. H., Farley M. M., Frasch C. E. Analysis of damage to human ciliated nasopharyngeal epithelium by Neisseria meningitidis. Infect Immun. 1986 Feb;51(2):579–585. doi: 10.1128/iai.51.2.579-585.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., Whitney A. M., Schoolnik G. K., Zollinger W. D. Common epitopes of pilin of Neisseria meningitidis. J Infect Dis. 1988 Aug;158(2):332–342. doi: 10.1093/infdis/158.2.332. [DOI] [PubMed] [Google Scholar]
- Sugasawara R. J., Cannon J. G., Black W. J., Nachamkin I., Sweet R. L., Brooks G. F. Inhibition of Neisseria gonorrhoeae attachment to HeLa cells with monoclonal antibody directed against a protein II. Infect Immun. 1983 Dec;42(3):980–985. doi: 10.1128/iai.42.3.980-985.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Robbins K., Barrera O., Corwin D., Boslego J., Ciak J., Blake M., Koomey J. M. Gonococcal pilin variants in experimental gonorrhea. J Exp Med. 1987 May 1;165(5):1344–1357. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Surface components affecting interactions between Neisserai gonorrhoeae and eucaryotic cells. J Infect Dis. 1977 Aug;136 (Suppl):S138–S143. doi: 10.1093/infdis/136.supplement.s138. [DOI] [PubMed] [Google Scholar]
- Tramont E. C. Specificity of inhibition of epithelial cell adhesion of Neisseria gonorrhoeae. Infect Immun. 1976 Aug;14(2):593–595. doi: 10.1128/iai.14.2.593-595.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trust T. J., Gillespie R. M., Bhatti A. R., White L. A. Differences in the adhesive properties of Neisseria meningitidis for human buccal epithelial cells and erythrocytes. Infect Immun. 1983 Jul;41(1):106–113. doi: 10.1128/iai.41.1.106-113.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Everson J. S. Comparative virulence of opacity variants of Neisseria gonorrhoeae strain P9. Infect Immun. 1981 Mar;31(3):965–970. doi: 10.1128/iai.31.3.965-970.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Everson J. S., Lambden P. R. Effect of anti-pilus antisera on virulence of variants of Neisseria gonorrhoeae for cultured epithelial cells. J Gen Microbiol. 1982 May;128(5):1095–1100. doi: 10.1099/00221287-128-5-1095. [DOI] [PubMed] [Google Scholar]
- Virji M., Heckels J. E. The role of common and type-specific pilus antigenic domains in adhesion and virulence of gonococci for human epithelial cells. J Gen Microbiol. 1984 May;130(5):1089–1095. doi: 10.1099/00221287-130-5-1089. [DOI] [PubMed] [Google Scholar]
- Waitkins S. A., Flynn J. Intracellular growth and type variation of Neisseria gonorrhoeae in tissue cell-cultures. J Med Microbiol. 1973 Aug;6(3):399–403. doi: 10.1099/00222615-6-3-399. [DOI] [PubMed] [Google Scholar]