Abstract
The lower urinary tract has two main functions, storage and periodic expulsion of urine, that are regulated by a complex neural control system in the brain and lumbosacral spinal cord. This neural system coordinates the activity of two functional units in the lower urinary tract: (1) a reservoir (the urinary bladder) and (2) an outlet (consisting of bladder neck, urethra and striated muscles of the external urethra sphincter). During urine storage the outlet is closed and the bladder is quiescent to maintain a low intravesical pressure. During micturition the outlet relaxes and the bladder contracts to promote efficient release of urine. This reciprocal relationship between bladder and outlet is generated by reflex circuits some of which are under voluntary control. Experimental studies in animals indicate that the micturition reflex is mediated by a spinobulbospinal pathway passing through a coordination center (the pontine micturition center) located in the rostral brainstem. This reflex pathway is in turn modulated by higher centers in the cerebral cortex that are involved in the voluntary control of micturition. Spinal cord injury at cervical or thoracic levels disrupts voluntary control of voiding as well as the normal reflex pathways that coordinate bladder and sphincter function. Following spinal cord injury the bladder is initially areflexic but then becomes hyperreflexic due to the emergence of a spinal micturition reflex pathway. However the bladder does not empty efficiently because coordination between the bladder and urethral outlet is lost. Studies in animals indicate that dysfunction of the lower urinary tract after spinal cord injury is dependent in part on plasticity of bladder afferent pathways as well as reorganization of synaptic connections in the spinal cord. Reflex plasticity is associated with changes in the properties of ion channels and electrical excitability of afferent neurons and appears to be mediated in part by neurotrophic factors released in the spinal cord and/or the peripheral target organs.
Keywords: Micturition, Urinary bladder, Nerve growth factor, Urethra sphincter, Neurogenic detrusor overactivity, Detrusor-sphincter-dyssynergia, Afferent nerves, Synaptic remodeling, Neuropeptides, Urothelium
Introduction
The functions of the lower urinary tract to store and periodically release urine are dependent upon neural circuits located in the brain, spinal cord and peripheral ganglia (Fowler et al., 2008). This dependence on central nervous control distinguishes the lower urinary tract from many other visceral structures (e.g., the gastrointestinal tract and cardiovascular system) that maintain a certain level of function even after elimination of extrinsic neural input.
The dependence of lower urinary tract functions on complex central neural networks renders these functions susceptible to a variety of neurological disorders including spinal cord injury (de Groat et al., 1993; Fowler et al., 2008). Spinal cord injury rostral to the lumbosacral level eliminates the voluntary and supraspinal control of voiding, leading initially to an areflexic bladder and complete urinary retention, and then a slow development of automatic micturition and neurogenic detrusor overactivity (NDO) that is mediated by spinal reflex pathways (de Groat and Yoshimura, 2006). However, voiding is commonly inefficient owing to simultaneous contractions of the bladder and urethral sphincter (detrusor-sphincter-dyssynergia, DSD).
The recovery of reflex bladder activity after spinal cord injury is dependent on the reorganization of reflex pathways in the spinal cord as well as alterations in the properties of bladder afferent neurons (de Groat and Yoshimura, 2006, 2009). This review will summarize the morphological and electrophysiological changes in bladder reflex pathways after spinal cord injury and the molecular mechanisms that might underlie these changes.
Anatomy and innervation of the lower urinary tract
Bladder and urethra
The storage and periodic elimination of urine are dependent upon the activity of two functional units in the lower urinary tract: (1) a reservoir (the urinary bladder) and (2) an outlet consisting of the bladder neck, urethra and striated muscles of the external urethral sphincter (Birder et al., 2009; Fowler et al., 2008). These structures are in turn regulated by three sets of peripheral nerves: sacral parasympathetic (pelvic nerves), thoracolumbar sympathetic (hypogastric nerves and sympathetic chain) and somatic nerves (pudendal nerves) that contain afferent and efferent axons (Birder et al., 2009; de Groat and Yoshimura, 2009).
Efferent nerves
The sacral parasympathetic outflow provides the major excitatory input to the urinary bladder. Parasympathetic preganglionic neurons (PGN) located in the intermediolateral region of the sacral spinal cord send axons via the pelvic nerves to ganglion cells in the pelvic plexus and in the wall of the bladder (Birder et al., 2009). Parasympathetic postganglionic nerves in turn excite bladder smooth muscle via the release of cholinergic (acetylcholine) and non-adrenergic, non-cholinergic transmitters (adenosine triphosphate, ATP), acting on muscarinic cholinoceptors and on P2X1 purinoceptors, respectively (Birder et al., 2009).
Sympathetic pathways to the lower urinary tract, that originate in the thoracolumbar spinal cord elicit various effects, including: 1) inhibition of the detrusor muscle via β-adrenoceptors and 2) excitation of the bladder base and urethra via α1-adrenoceptors (Birder et al., 2009). The efferent innervation of the urethral striated muscles originates from neurons in a circumscribed region of the lateral ventral horn that is termed Onuf’s nucleus (Thor and de Groat, 2010). Sphincter motor neurons send their axons into the pudendal nerve and excite sphincter muscles via activation of nicotinic receptors.
Afferent nerves
Afferent axons innervating the urinary tract are present in the three sets of nerves (de Groat and Yoshimura, 2009). The most important afferents for initiating micturition are those passing in the pelvic nerve to the sacral spinal cord. These afferents are small myelinated (Aδ) and unmyelinated (C) fibers, which convey information from receptors in the bladder wall to second-order neurons in the spinal cord (Fig. 1). Aδ bladder afferents in the cat respond in a graded manner to passive distension as well as to active contraction of the bladder. In contrast, unmyelinated C-fiber bladder afferents in the cat are insensitive to mechanical stimuli and commonly do not respond to even high levels of intravesical pressure (de Groat and Yoshimura, 2009; Habler et al., 1990). However, activity in these “silent” C-fiber afferents is unmasked by chemical irritation of the bladder mucosa. These findings indicate that C-fiber afferents in the cat have specialized functions, such as the signaling of inflammatory or noxious events in the lower urinary tract. In the rat, A-fiber and C-fiber bladder afferents cannot be distinguished on the basis of stimulus modality; both types of afferents consist of mechanosensitive and chemosensitive populations (de Groat and Yoshimura, 2009). The properties of A-fiber and C-fiber bladder afferent nerves in humans are unknown.
Fig. 1.
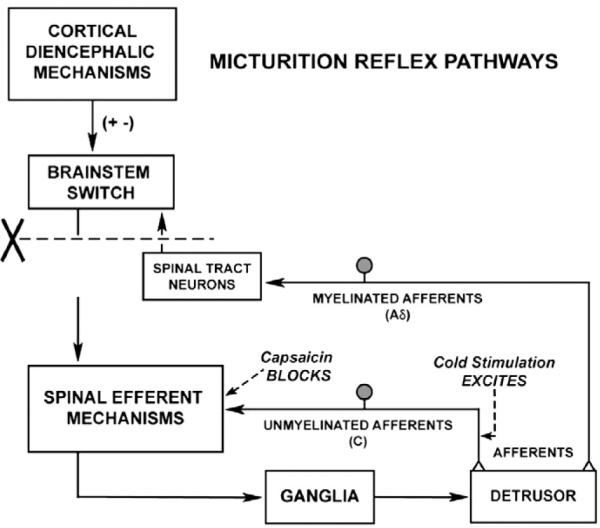
Diagram showing the organization of the parasympathetic excitatory reflex pathway to the detrusor muscle. Scheme is based on electrophysiologic studies in cats. In animals with an intact spinal cord, micturition is initiated by a supraspinal reflex pathway passing through the pontine micturition center in the brain stem that functions as a micturition switching circuit. The pathway is triggered by myelinated afferents (A δ fibers), which are connected to the tension receptors in the bladder wall. Injury to the spinal cord above the sacral segments (X) interrupts the connections between the brain and spinal autonomic centers and initially blocks micturition. However, over a period of several weeks following cord injury, a spinal reflex mechanism emerges, which is triggered by unmyelinated vesical afferents (C-fibers); the A-fiber afferent inputs are ineffective. The C-fiber reflex pathway is usually weak or undetectable in animals with an intact nervous system. Stimulation of the C-fiber bladder afferents by instillation of ice water into the bladder (cold stimulation) activates voiding responses in patients with spinal cord injury. Capsaicin (20–30 mg, subcutaneously) blocks the C-fiber reflex in chronic spinal cats, but does not block micturition reflexes in intact cats. Intravesical capsaicin also suppresses detrusor hyperreflexia and cold-evoked reflexes in patients with neurogenic bladder dysfunction.
C-fiber bladder afferent neurons synthesize and release various neuropeptides, including calcitonin-gene-related peptide (CGRP), vasoactive intestinal polypeptide (VIP), pituitary-adenylate cyclase-activating polypeptide (PACAP) and tachykinins (substance P and neurokinin A) (de Groat and Yoshimura, 2009) and express receptors for some of these peptides. The C-fiber afferent neurons also express receptors for other substances, including acetylcholine, ATP, prostaglandins and neurotrophic factors, that are released in the bladder by urothelial cells, efferent nerves and inflammatory cells. These substances modulate afferent nerve excitability and change the response of afferents to mechanical stimulation. Peptide-containing afferent axons project into the lumbosacral parasympathetic nucleus in the spinal cord. The C-fiber afferents also express transient receptor potential (TRP) receptors, such as TRPV1, and therefore are sensitive to the neurotoxins, capsaicin and resiniferatoxin (Fowler et al., 2008).
The properties of lumbosacral dorsal root ganglion cells innervating the bladder, urethra and external urethral sphincter in the rat and cat have been studied with patch clamp recording techniques (Yoshimura et al., 1996, 1998; Yoshimura and de Groat, 1997b). Based on responsiveness to capsaicin, approximately 70% of bladder afferent neurons in the rat are likely to be of the C-fiber type. These neurons have high threshold tetrodotoxin-resistant sodium channels (Yoshimura et al., 2003) and produce action potentials and phasic firing (one to two spikes) in response to prolonged depolarizing current pulses (Yoshimura and de Groat, 1999) (Fig. 2). A-fiber afferent neurons are resistant to capsaicin, have low threshold tetrodotoxin-sensitive sodium channels and produce tonic firing (multiple spikes) to depolarizing current pulses (Fig. 2). Approximately 90% of the bladder afferent neurons also are excited by ATP, which induces depolarization and firing through activation of P2X3 or P2X2/3 receptors (Dang et al., 2005).
Fig. 2.
Comparison of the electrophysiological characteristics of C-fiber (top traces) and A δ fiber (lower traces) bladder afferent neurons isolated from lumbar DRG of the rat. The left panels are voltage responses and action potentials evoked by 20 ms depolarizing current pulses asterisks with dashed lines indicate thresholds for spike activation (20 mV and 29 mV in the top and bottom traces, respectively). C-fiber neurons have higher thresholds and longer duration action potentials. The second panels show that C-fiber and A δ fiber afferent neurons have tetrodotoxin (TTX) resistant and sensitive action potentials, respectively. The third panel shows the firing patterns during long duration (600 ms) depolarizing currents pulses. C-fiber neurons fire phasically (1–2 spikes); whereas A δ fiber neurons fire tonically (12–15 spikes). The fourth panel shows that C-fiber neurons are capsaicin sensitive; whereas A δ fiber neurons are capsaicin insensitive.
Urothelium
The urothelium, which has been traditionally viewed as a passive barrier, also has specialized sensory and signaling properties that allow it to respond to chemical and mechanical stimuli and to engage in reciprocal chemical communication with neighboring nerves or myofibroblasts in the lamina propria (Birder et al., 2008; Birder, 2010). These properties include: (1) expression of receptors for acetylcholine, norepinephrine, tachykinins and agonists for TRP channels (TRPV1, TRPV4, TRPM8), (2) close physical association with afferent nerves and (3) ability to release chemical mediators, such as ATP, acetylcholine, nerve growth factor (NGF) and nitric oxide (Birder and de Groat, 2007; de Groat, 2004), that can regulate the activity of adjacent nerves (de Groat and Yoshimura, 2009) or myofibroblasts (Fry et al., 2009) and thereby influence reflex bladder contractions.
Anatomy of central nervous pathways controlling the lower urinary tract
The reflex circuitry controlling micturition consists of four basic components: primary afferent neurons, spinal efferent neurons, spinal interneurons and neurons in the brain that modulate spinal reflex pathways.
Afferent projections in the spinal cord
Afferent pathways from the bladder project into Lissauer’s tract at the apex of the dorsal horn and then send collaterals laterally and medially around the dorsal horn into laminae V–VII and X at the base of the dorsal horn (de Groat and Yoshimura, 2009; Morgan et al., 1981). The lateral pathway terminates in the region of the sacral parasympathetic nucleus.
Spinal efferent neurons
PGN innervating the bladder and urethra are located in the intermediolateral gray matter (laminae V–VII) in the lumbosacral segments of the spinal cord (Nadelhaft et al., 1980). Motor neurons supplying the external urethral sphincter are located in lamina IX in Onuf’s nucleus in the cat and in the dorsolateral motor nucleus in the rat (Thor and de Groat, 2010).
Parasympathetic PGN have dendrites projecting to four major areas: (1) the lateral and dorsolateral funiculus, (2) lamina I on the lateral edge of the dorsal horn, (3) the dorsal gray commissure and (4) the gray matter and lateral funiculus ventral to the sacral parasympathetic nucleus (Morgan et al., 1993). Motor neurons controlling the external urethral sphincter have a similar dendritic pattern (Thor and de Groat, 2010).
Spinal interneurons
Interneurons retrogradely labeled by injection of pseudorabies virus into the urinary bladder or urethra of the rat are located primarily in lamina I, the dorsal commissure and in regions dorsal and medial to the parasympathetic nucleus (Nadelhaft et al., 1992; Sugaya et al., 1997; Vizzard et al., 1995). Interneurons in these locations receive afferent input from the lower urinary tract (Birder and de Groat, 1993), make excitatory and inhibitory synaptic connections with the parasympathetic PGN (Araki and de Groat, 1996, 1997; de Groat et al., 1998; Miura et al., 2003) and also send projections to supraspinal centers involved in the control of micturition (Birder et al., 1999; Holstege and Mouton, 2003).
Supraspinal pathways
Pseudorabies virus tracing methods have identified many populations of neurons in the rat brain that are involved in the control of the bladder, urethra, and urethral sphincter. These areas include Barrington’s nucleus (the pontine micturition center, PMC), medullary raphé nuclei, locus coeruleus, periaqueductal gray (PAG), A5 noradrenergic cell group, hypothalamus and medial frontal cortex (Nadelhaft et al., 1992; Sugaya et al., 1997; Vizzard et al., 1995).
Descending projections from the brain to the lumbosacral spinal cord have been identified by injection of anterograde tracers into specific brain areas. Tracer injected into the paraventricular nucleus of the hypothalamus labeled axons in the sacral parasympathetic nucleus as well as axons within Onuf’s nucleus (Holstege and Mouton, 2003); while tracer injected into the PMC labeled axons primarily in the sacral parasympathetic nucleus, the lateral edge of the dorsal horn and the dorsal commissure. On the other hand, projections from neurons in the lateral pons in the cat, an area implicated in the control of the urethral sphincter, terminate rather selectively in the Onuf’s nucleus.
Organization of urine storage and voiding reflexes
Sympathetic storage reflex
Sympathetic urine storage reflexes are elicited by a sacrolumbar intersegmental spinal reflex pathway that is triggered by vesical afferent activity in the pelvic nerves (de Groat and Lalley, 1972). The reflex pathway is inhibited when bladder pressure is raised to the threshold for producing micturition. This inhibitory response is abolished by transection of the spinal cord at the lower thoracic level, indicating that it originates at a supraspinal site, possibly the PMC (de Groat and Lalley, 1972).
Urethral sphincter storage reflex
Motoneurons innervating the striated muscles of the urethral sphincter exhibit a tonic discharge that increases during bladder filling. This activity is mediated in part by low-level afferent input from the bladder. During micturition the firing of sphincter motoneurons is inhibited by descending input from the PMC (see review:Thor and de Groat, 2010).
Voiding reflexes
Studies in cats using brain-lesioning techniques revealed that neurons in the brainstem at the level of the PMC have an essential role in the control of the parasympathetic component of micturition (de Groat et al., 1993; Ruch and Tang, 1956). Bilateral lesions in this region of the pons or transections of the neuraxis at any point below this level abolish micturition; whereas electrical or chemical stimulation at this site suppresses urethral sphincter activity, triggers bladder contractions and releases urine (de Groat et al., 1993). These observations led to the concept of a spinobulbospinal micturition reflex pathway that is activated by Aδ bladder afferents and consists of ascending and descending limbs connected by a switching circuit in the PMC (Fig. 1).
Brain imaging studies in humans and animals have confirmed that the PAG and PMC as well as many areas in the forebrain are involved in the voluntary and reflex control of micturition (Athwal et al., 2001; Blok et al., 1998; Fowler, 2006; Fowler and Griffiths, 2010; Griffiths et al., 2007; Kavia et al., 2005; Tai et al., 2009). During bladder filling sensory input from the bladder is thought to be processed in the PAG and then transmitted to sites in the forebrain including the mid-cingulate gyrus and the frontal lobes. Voluntary micturition is accompanied by activation of the dorsolateral prefrontal cortex, anterior cingulate gyrus, as well as the PMC and PAG.
Plasticity after spinal cord injury
Spinal Cord injury (SCI) rostral to the lumbosacral level eliminates voluntary and supraspinal control of voiding, leading initially to an areflexic bladder and complete urinary retention followed by a slow development of automatic micturition, bladder hyperactivity mediated by spinal reflex pathways and emergence of neonatal perineal-to-bladder and bladder-to-bladder excitatory reflexes (de Groat and Yoshimura, 2006). However, voiding is commonly inefficient due to simultaneous contractions of the bladder and urethral sphincter (detrusor-sphincter-dyssynergia, DSD). Changes at various sites in the reflex pathways and in the target organs contribute to the LUT dysfunctions after SCI in humans and animals (de Groat and Yoshimura, 2006).
Changes in the bladder
Cystometrograms in animals and humans with suprasacral lesions exhibit intrinsic and reflex contractions (i.e., neurogenic detrusor overactivity, NDO) during bladder filling (de Groat and Yoshimura, 2006; Fowler et al., 2008; Kruse et al., 1993, 1994) that are not present in individuals with intact spinal cords. In addition maximal voiding pressure is increased, voiding efficiency is reduced and the bladder undergoes marked hypertrophy (Cheng and de Groat, 2004; Kruse et al., 1993, 1994). Recordings of contractile activity of in vitro whole bladders or bladder strips from SCI animals reveals large amplitude phasic contractions similar to those present in neonatal bladders or in adult bladders with chronic partial ligation of the urethral outlet (Ikeda et al., 2007; Ikeda and Kanai, 2008; Kanai et al., 2007; Ng et al., 2007; Sugaya and de Groat, 1994, 2000; Szell et al., 2003). Because this type of activity does not occur in bladders from animals with intact spinal cords, it has been proposed that the bladder changes induced by urethral outlet obstruction are adaptive responses to overdistention of the bladder and increased bladder work (Sugaya and de Groat, 2000). This is supported by the demonstration that the changes can be prevented by diverting urine into another pelvic organ and thereby eliminating bladder overdistension (Kanai et al., in press).
The epithelial layer of the bladder is also altered after SCI. In rats within two hours after spinal transection the continuity of the umbrella cell layer of the urothelium is disrupted and the transepithelial resistance (TER) is markedly reduced (Apodaca et al., 2003). By 24 h TER reaches a minimum and water and urea permeability are significantly increased. The changes are reversed within 2 weeks, but can be prevented by treatment with a ganglionic blocking agent indicating that they are mediated by activity in autonomic nerves. Long term changes include increased expression of gap junction proteins: connexin 26 in the urothelium and connexin 43 in lamina propria in the rat bladder (Ikeda et al., 2007). However connexin 45 in the detrusor smooth muscle is not changed after SCI.
These changes at the luminal surface of the bladder may contribute to the changes in bladder function after SCI. Optical imaging techniques revealed that phasic contractions in bladders from neonatal (Kanai et al., 2007) or SCI animals (McCarthy et al., 2009) originate from localized sites in the bladder dome and are driven by activity arising in the urothelium. Removal of the mucosa eliminates the phasic contractions in bladders from SCI rats (Ikeda and Kanai, 2008). In addition gap junction blockers suppress the phasic contractions (Ikeda et al., 2007). Based on these findings it was proposed that phasic activity in bladders after SCI is due to a signaling pathway that originates in the urothelium and then passes through gap junctions via a network of myofibroblasts in the lamina propria to smooth muscle and afferent nerves (Ikeda and Kanai, 2008).
Prejunctional facilitatory mechanisms mediated by muscarinic receptor activation at parasympathetic postganglionic nerve terminals in the rat bladder are also changed after SCI (Somogyi and de Groat, 1999). In spinal intact rats facilitation of transmitter release occurs at frequencies greater than 10 Hz and by activation of prejunctional M1 receptors; whereas in SCI rats facilitation occurs at lower frequencies (2 Hz) and by activation of M3 receptors (Somogyi et al., 2003). These changes would enhance the efferent excitatory pathway to the bladder after SCI.
Changes in bladder and sphincter reflexes
Electrophysiological studies in cats revealed that the recovery of bladder function after SCI is mediated by a change in the afferent limb of the micturition reflex pathway and remodeling of synaptic connections in the spinal cord. In chronic SCI cats unmyelinated C-fiber afferents rather than Aδ afferents initiate voiding (Fig. 1); and the spinal micturition reflex occurs with a short central delay (15 ms) in contrast to the long central delay (60 ms) of the spinobulbospinal micturition reflex in cats with an intact spinal cord (de Groat et al., 1981). These findings are supported by pharmacological studies showing that subcutaneous administration of capsaicin, a C-fiber neurotoxin, completely blocks reflex bladder contractions induced by bladder distention in chronic spinal cats; whereas capsaicin has no inhibitory effect on reflex bladder contractions in spinal intact cats (Cheng et al., 1999; de Groat et al., 1990). Thus, it is plausible that C-fiber bladder afferents which usually do not respond to bladder distention (i.e., silent C-fibers) (Habler et al., 1990) become mechanosensitive and initiate automatic micturition after spinal cord injury.
Chronic spinal injury in humans also causes the emergence of an unusual bladder reflex that is elicited by infusion of cold water into the bladder (the Bors Ice Water Test) (Geirsson et al., 1993, 1994). The response to cold water does not occur in normal adults but does occur in: (1) infants, (2) in patients with suprasacral cord lesions, (3) patients with multiple sclerosis and Parkinson’s disease, and (4) elderly patients with hyperactive bladders. Studies in animals indicate that cold temperature activates TRPM8 receptors and possibly other temperature sensitive receptors in bladder C-fiber afferents and/or urothelial cells (Birder et al., 2008; Fall et al., 1990; Stein et al., 2004). Intravesical administration of capsaicin to paraplegic patients blocks the cold-induced bladder reflexes, indicating that they are mediated by C-fiber afferents (Geirsson et al., 1995; Jiang et al., 2002; Mazieres et al., 1998). The presence of the cold reflex in infants, its disappearance with maturation of the nervous system, and its reemergence under conditions in which higher brain functions are disrupted suggests that it represents a primitive spinal involuntary voiding reflex activated by C-fiber afferents.
SCI in cats and rats also causes the re-emergence of a neonatal exteroceptive micturition reflex that is activated by tactile stimulation of cutaneous afferent axons passing though the pudendal nerve to the perineum (i.e., the perineal-to-bladder reflex) (de Groat, 2002). In neonatal animals, this reflex which is activated by the mother licking the perineal region of the young animal is essential for survival because isolation of the newborn from its mother leads to urinary retention. When the adult form of reflex voiding, triggered by bladder distension becomes functional several weeks after birth the neonatal perineal-to-bladder reflex is down regulated and eventually replaced by an inhibitory reflex (de Groat, 2002). However in adults the excitatory reflex re-emerges after SCI. A similar type of plasticity can be demonstrated with electrical stimulation of afferent axons in adult cats. Stimulation of pudendal nerve afferents evokes IPSPs in bladder PGN in cats with an intact spinal cord, but the same stimulation evokes short latency EPSPs in chronic SCI cats (de Groat and Ryall, 1969).
Changes in urethral sphincter reflexes after SCI causes DSD, reduced voiding efficiency and urinary retention. This is due in part to loss of descending inhibitory input from the brain to urethral sphincter motoneurons but also to changes in sphincter reflexes as a result of remodeling of spinal synapses (Beattie et al., 1993; Chang et al., 2007; Nout et al., 2006). In SCI rats DSD is associated with an increased tonic activity and reduced phasic activity of the sphincter electromyogram (Cheng and de Groat, 2004). The abnormal sphincter activity and voiding dysfunction are reduced by desensitization with capsaicin indicating that they are initiated by C-fiber bladder afferents. Cold stimulation of the rat bladder that activates capsaicin sensitive afferents also induces DSD (Cheng et al., 1997).
Morphological and chemical plasticity of bladder afferent neurons
Studies in humans have revealed an increased density of TRPV1-and P2X3-immunoreactivity as well as immunoreactivity to a panneuronal marker (PGP9.5) in suburothelial nerves and increased TRPV1-immunoreactivity in the basal layer of the urothelium in patients with neurogenic detrusor overactivity (NDO) (Apostolidis et al., 2005a; Brady et al., 2004a, 2004b). Treatment of NDO patients with intravesical capsaicin or another C-fiber neurotoxin, resiniferatoxin, produces symptomatic improvement in a subpopulation of these patients and reduces the density of TRPV1, P2X3 and PGP9.5 immunoreactive nerve fibers and urothelial TRPV1 immunoreactivity (Brady et al., 2004a, 2004b). Injections into the bladder wall of botulinum neurotoxin type A (BoNT/A), an agent that blocks the release of neurotransmitters from urothelial cells and from afferent and efferent nerves also reduces NDO (Apostolidis and Fowler, 2008; Popat et al., 2005; Schurch et al., 2007) and reduces the density of TRPV1- and P2X3-immunoreactive nerves (Apostolidis et al., 2005a, 2005b; Apostolidis and Fowler, 2008). These results suggest that an abnormality of the C-fiber afferent innervation contributes to NDO.
Changes in morphology, neuropeptide expression and function of C-fiber afferents have been detected in rats and cats after SCI. The changes include: (1) somal hypertrophy of bladder afferent neurons in the L6–S1 DRG (Kruse et al., 1995; Yoshimura and de Groat, 1997a; Yoshimura et al., 1998), (2) increase in expression of PACAP-IR in bladder DRG neurons and expansion of PACAP-IR afferent axons in the lumbosacral spinal cord (Zvarova et al., 2005), (3) expansion of VIP (Thor et al., 1986), CGRP, IB4 and substance P containing primary afferent fibers in the spinal cord (Weaver et al., 2006; Zinck and Downie, 2006, 2008; Zinck et al., 2007), (4) increase in Fos protein expression in the spinal cord in response to bladder distension (Vizzard, 2000a), (5) increase in nNOS-IR, galanin-IR, TrkA-IR and TrkB-IR in bladder DRG neurons and in the region of the lumbosacral parasympathetic nucleus (Vizzard, 2006). The somal hypertrophy of bladder afferent neurons is prevented by diversion of the urine into other pelvic organs which also prevents bladder hypertrophy (Kruse et al., 1995), suggesting that the morphological change is driven by factors activated by bladder overdistension. These observations raise the possibility that after SCI, C-fiber bladder afferents sprout and contribute to the synaptic remodeling of the spinal micturition reflex pathway.
Pudendal afferent projections in the region of the sacral dorsal horn and sacral autonomic nucleus are also increased in chronic SCI cats (Thor et al., 1986). This change is most obvious in lateral lamina I where the afferents exhibit a periodic distribution in the rostrocaudal axis in spinal intact animals but have a continuous distribution in chronic SCI animals.
Changes in the electrophysiological properties of bladder afferent neurons
The ionic mechanisms underlying the hyperexcitability of C-fiber bladder afferents were investigated using whole-cell patch clamp recording in bladder DRG neurons (Yoshimura and de Groat, 1997a). Dissociated bladder DRG neurons from chronic SCI rats are larger in size and have increased input capacitance. This is consistent with results from histologic studies showing that bladder afferent neurons in the L6–S1 DRG undergo somal hypertrophy in SCI rats (Kruse et al., 1995). The action potentials in bladder afferent neurons are also different after SCI in rats and cats. In contrast to neurons from spinal intact rats where the majority (approximately 70%) of bladder afferent neurons exhibit high threshold TTX-resistant action potentials (Yoshimura et al., 1996), in chronic SCI rats, 60% of bladder afferent neurons exhibit low threshold TTX-sensitive action potentials. In SCI cats bladder afferent neurons exhibit multiple action potentials (tonic firing) in response to long depolarizing current pulses (Fig. 3); whereas in cats and rats with an intact spinal cord the neurons usually respond with one or two action potentials (phasic firing) (de Groat and Yoshimura, 2010).
Fig. 3.
Effect of chronic spinal cord injury on the firing of a bladder sacral dorsal root ganglion neuron. Records on the left side which are from an afferent neuron from a cat with an intact spinal cord show action potentials (top trace) elicited by short (10 ms) and long (600 ms) duration depolarizing current pulses (bottom trace). This neuron is typical of small diameter bladder neurons which exhibit phasic firing. Records on right side show tonic firing during a long duration depolarizing current pulse in a small diameter bladder neuron from a spinal cord injured (SCI) cat.
The alteration of electrophysiological properties in bladder afferent neurons after SCI is also reflected in changes in Na+ current distribution (Yoshimura and de Groat, 1997a). Consistent with the increment in the proportion of neurons with TTX-sensitive spikes, the number of bladder afferent neurons which predominantly express TTX-sensitive Na+ currents (60–100% of total Na+ currents) is also increased. The density of TTX-sensitive Na+ currents in bladder afferent neurons significantly increased from 32.1 to 80.6 pA/pF, while TTX-resistant current density decreased from 60.5 to 17.9 pA/pF following SCI. These data indicate that SCI induces a switch in expression of Na+ channels from TTX-resistant type to TTX-sensitive type. Since TTX-sensitive Na+ currents have a lower threshold for activation than TTX-resistant currents, it is reasonable to assume that these changes in expression of Na+ channels in bladder afferent neurons after SCI contribute to a low threshold for spike activation in these neurons (Fig. 3) and the emergence of mechanosensitivity in silent C-fiber bladder afferent nerves.
Bladder afferent neurons with TTX-sensitive spikes in chronic SCI rats also do not exhibit membrane potential relaxation during low intensity depolarizing current pulses. Furthermore the voltage responses induced by current injections are not altered by application of 4-aminopyridine, a K+ channel blocker, as noted in cells from control animals (Yoshimura et al., 1996). Therefore it is likely that following SCI, A-type potassium channels are suppressed in parallel with an increased expression of TTX-sensitive Na+ currents, thereby increasing excitability of C-fiber bladder afferent neurons. Thus drugs that selectively modulate these types of ion channels might be useful in treating NDO.
Remodeling of synapses in the spinal cord
Plasticity in spinal circuitry after spinal cord injury has been studied during postnatal development using patch clamp recordings in identified parasympathetic PGN in spinal cord slice preparations obtained from 6 to 28 day old neonatal rats (Araki, 1994; Araki and de Groat, 1996, 1997; Miura et al., 2003). Excitatory synaptic connections between interneurons and PGN were analyzed by electrically stimulating neurons located within 100 μm of the PGN. Stimulation of interneurons located dorsal to the PGN elicited excitatory postsynaptic currents (EPSCs) consisting of fast and slow components that were blocked, respectively, by nonNMDA and NMDA glutamatergic receptor antagonists. Because dorsal interneurons are exclusively excitatory to PGN and receive excitatory inputs from presumptive afferent axons it is reasonable to think that these interneurons are part of a disynaptic parasympathetic reflex pathway that mediates spinal bladder reflexes. The effect of postnatal maturation and spinal cord transection on the efficiency of dorsal interneuron-to-PGN synaptic transmission was therefore evaluated to determine if changes in transmission could account for the alteration in the micturition reflex pathway in pathological conditions. In preparations removed from 1 and 2 week old rats the dorsal interneuron-evoked EPSCs are large and of constant amplitude (mean 40 pA). However, in preparations from 3 week old rats, an age when the spinobulbospinal micturition has emerged, the EPSCs are reduced in amplitude by 50% (20 pA). Quantal analysis of unitary EPSCs indicates that this reduction in amplitude is attributable to a decrease in the presynaptic release of glutamic acid (Araki and de Groat, 1997). Transection of the spinal cord between 1 and 2 weeks of age prevents the reduction in synaptic transmission that occurs at 3 weeks of age.
These observations led to a hypothesis linking synaptic remodeling after spinal cord injury to a reversal of the changes in spinal synapses that occur during postnatal maturation (Araki and de Groat, 1997). It was proposed that maturation of bulbospinal pathways to the PGN during the second to third postnatal week, at the same time when the mature bladder-to-bladder spinobulbospinal reflex is emerging, downregulates the interneuronal-PGN excitatory synapses, possibly by synapse elimination in response to competition for synaptic space between boutons of interneurons and descending axons. Transection of the spinal cord which causes degeneration of the descending axons blocks this synaptic reorganization and maintains the primitive neonatal reflex pathway. A similar degeneration of descending axons in adult animals after SCI could stimulate axonal sprouting in interneuronal pathways and cause the re-emergence of the neonatal bladder reflexes in chronic spinal animals.
Plasticity of neurotransmitter mechanisms in the spinal cord
Changes in the neurotransmitter mechanisms underlying the neural control of the lower urinary tract have been detected after spinal cord injury.
Neurokinins
Destruction of lumbosacral spinal neurons expressing neurokinin-1 (NK-1) receptors using a NK-1 ligand conjugated with saporin does not affect reflex voiding in rats with an intact spinal cord but reduces the bladder irritant effects of intravesical capsaicin (Seki et al., 2005) and reduces non-voiding contractions in SCI rats (Seki et al., 2004a). Similarly, intrathecal administration of a selective NK-1 receptor antagonist (L-733060) does not affect the micturition reflex in spinal intact rats but blocks the micturition reflex in SCI rats (Zhang et al., 2008). These data coupled with the increased expression of substance P in the region of the sacral parasympathetic nucleus in SCI rats (Zhang et al., 2008) indicate that activation of NK-1 receptors plays a role in micturition in paraplegic animals.
VIP and PACAP
The expression of VIP and PACAP in lumbosacral afferent pathways is increased after spinal cord injury (Thor et al., 1986; Zvarova et al., 2005). The pharmacological effect of VIP on bladder activity is also changed after SCI. Intrathecal administration of VIP which suppresses reflex bladder activity in cats with an intact spinal cord, enhances or unmasks reflex bladder activity in chronic SCI cats (de Groat et al., 1990).
PACAP-27 or PACAP-38 enhances reflex bladder activity when administered intrathecally (Ishizuka et al., 1995; Yoshiyama and de Groat, 2008); and PACAP-38 has excitatory effects on lumbosacral parasympathetic neurons in the rat (Miura et al., 2001). Intrathecal administration of PACAP6-38, an agent that blocks one type of PACAP receptor (PAC-1) suppresses bladder hyperreflexia in chronic SCT rats indicating a role for PACAP in NDO (Zvara et al., 2006).
GABA
Pharmacologic activation of spinal GABAA and GABAB receptors inhibits the C-fiber mediated nonvoiding bladder contractions and suppresses DSD in SCI rats (Miyazato et al., 2008a, 2008b). Reduced GABAergic inhibition could contribute to the development of NDO because mRNA levels of GAD67, an enzyme involved in GABA synthesis, are decreased in the spinal cord after SCI (Miyazato et al., 2008a). A possible therapy for NDO has emerged from an experimental study in which an HSV vector encoding the GAD gene was injected into the bladder of SCI rats to increase GAD expression in bladder afferent nerves. This treatment reduces NDO and DSD (Miyazato et al., 2009, 2010); an effect similar to that elicited in chronic SCI rats by desensitization of C-fiber bladder afferents with systemic capsaicin administration (Cheng et al., 1995; Cheng and de Groat, 2004).
Role of neurotrophic factors
Neurotrophic factors including nerve growth factor (NGF) have been implicated as chemical mediators of pathology-induced changes in C-fiber afferent nerve excitability and reflex bladder activity (Vizzard, 2000c; Yoshimura, 1999) (Fig. 4). After SCI in rats the levels of NGF increase in the bladder (Vizzard, 2000c, 2006), in the lumbosacral spinal cord and in the DRG (Seki et al., 2003). In the bladder NGF may originate from multiple sources including smooth muscle and urothelial cells. The stimulus for increased levels of NGF may be overdistension of the bladder due to DSD (Fig. 4) and decreased voiding efficiency; because NGF levels also increase in the bladder of rats after partial obstruction of the urethral outlet (Steers and Tuttle, 2006).
Fig. 4.

Diagram showing hypothetical mechanisms inducing lower urinary tract dysfunction following spinal cord injury (SCI). Injury to the spinal cord (1) causes detrusor-sphincter dyssynergia (DSD) (2), leading to functional urethral obstruction, reduced voiding efficiency, urinary retention and bladder hypertrophy (3), resulting in increased levels of NGF in the bladder wall (4). NGF is taken up by afferent nerves and transported to the dorsal root ganglion cells (5) where it changes gene expression leading to increased cell size, modulation of ion channels including a decrease in K+ channel function and increased neuronal excitability (6). The levels of NGF also increase in the spinal cord after SCI. Hyperexcitability of bladder afferent pathways causes or enhances neurogenic detrusor overactivity and DSD. Intrathecal application of NGF antibodies reduces NGF levels in DRGs and suppresses neurogenic detrusor overactivity and DSD.
Intravesical administration of NGF in rats acutely increases reflex bladder activity (Chuang et al., 2001) and chronic administration of NGF into the spinal cord or into the bladder wall of rats induces bladder hyperactivity and increases the firing frequency of dissociated bladder afferent neurons (Lamb et al., 2004; Seki et al., 2003; Yoshimura, 1999; Yoshimura et al., 2006; Zvara and Vizzard, 2007). NGF might act by multiple mechanisms because it is known that it upregulates PACAP and TRPV1 expression in DRG neurons (Steers and Tuttle, 2006; Vizzard, 2000b, 2006) and downregulates A-type K+ channel currents (Yoshimura et al., 2006).
Endogenous NGF seems to contribute to the lower urinary tract dysfunction after SCI because intrathecal application of NGF antibodies, which neutralize NGF in the spinal cord, suppresses NDO and DSD in SCI rats (Seki et al., 2002, 2004b). This treatment with NGF antibodies produces effects similar to the effect of desensitizing C-fiber afferents with capsaicin or resiniferatoxin (Cheng et al., 1993). Intrathecal administration of NGF antibodies also blocks autonomic dysreflexia induced by bladder or distal bowel distension in SCI rats (Weaver et al., 2006).
In humans with NDO or idiopathic detrusor overactivity (IDO), increased levels of NGF have been detected in bladder tissue (Giannantoni et al., 2006) and in the urine (Liu et al., 2009). After treatment with BoNT/A injections into the bladder wall, patients that exhibited reduced symptoms also had reduced tissue and urinary NGF levels (Giannantoni et al., 2006; Liu et al., 2009). Thus, it has been proposed (Liu et al., 2009) that NGF may be a sensitive biomarker for the diagnosis NDO and IDO and may be a useful tool for evaluating the therapeutic effect of BoNT/A injections. NGF and its receptors in the bladder and/or the spinal cord are potential targets for new therapies to reduce voiding dysfunction after SCI.
Influence of sacral neuromodulation on reflex plasticity after spinal cord injury
Tonic electrical stimulation of a sacral spinal nerve root with chronically implanted electrodes (termed sacral neuromodulation, SNM) has been used to treat IDO symptoms but is less effective in treating NDO and DSD after spinal cord injury. However a recent study (Sievert et al., 2010) in which bilateral SNM was initiated early (0.8–4.5 months) during the recovery from complete thoracic spinal cord injury prevented the development of NDO and urinary incontinence and reduced the rate of urinary tract infections. It was speculated that early SNM might prevent the plasticity in bladder C-fiber afferents that contributes to NDO.
Conclusions
Anatomical, neurochemical and electrophysiological studies have provided insights into the effects of SCI on the reflex circuitry controlling micturition. Changes in afferent neurons and remodeling of synaptic connections in the spinal cord induce the formation of new reflex mechanisms as well as re-emergence of neonatal reflexes that are down-regulated during postnatal development. This plasticity is due in part to a change in the function of peripheral target organs that increases the expression of neurotrophic factors and in turn induces plasticity in peripheral and spinal neural pathways.
Acknowledgments
The authors thank Mrs. Stephanie Daugherty for her help in preparing the manuscript. Supported by NIH grants to WCD (DK-049430 and DK-077783) and to NY (DK057267).
Abbreviations
- DSD
detrusor-sphincter-dyssynergia
- NDO
neurogenic detrusor overactivity
- IDO
idiopathic detrusor overactivity
- EPSP
excitatory post synaptic potential
- IPSP
inhibitory postsynaptic potential
- PGN
preganglionic neuron
- PMC
pontine micturition center
- SNM
sacral neuromodulation
- GABA
gamma-aminobutyric acid
References
- Apodaca G, Kiss S, Ruiz W, Meyers S, Zeidel M, Birder L. Disruption of bladder epithelium barrier function after spinal cord injury. Am. J. Physiol. Renal Physiol. 2003;284:F966–F976. doi: 10.1152/ajprenal.00359.2002. [DOI] [PubMed] [Google Scholar]
- Apostolidis A, Fowler CJ. The use of botulinum neurotoxin type A (BoNTA) in urology. J. Neural Transm. 2008;115:593–605. doi: 10.1007/s00702-007-0862-x. [DOI] [PubMed] [Google Scholar]
- Apostolidis A, Brady CM, Yiangou Y, Davis J, Fowler CJ, Anand P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology. 2005a;65:400–405. doi: 10.1016/j.urology.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, Dasgupta P, Fowler CJ, Anand P. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J. Urol. 2005b;174:977–982. doi: 10.1097/01.ju.0000169481.42259.54. [DOI] [PubMed] [Google Scholar]
- Araki I. Inhibitory postsynaptic currents and the effects of GABA on visually identified sacral parasympathetic preganglionic neurons in neonatal rats. J. Neurophysiol. 1994;72:2903–2910. doi: 10.1152/jn.1994.72.6.2903. [DOI] [PubMed] [Google Scholar]
- Araki I, de Groat WC. Unitary excitatory synaptic currents in preganglionic neurons mediated by two distinct groups of interneurons in neonatal rat sacral parasympathetic nucleus. J. Neurophysiol. 1996;76:215–226. doi: 10.1152/jn.1996.76.1.215. [DOI] [PubMed] [Google Scholar]
- Araki I, de Groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. J. Neurosci. 1997;17:8402–8407. doi: 10.1523/JNEUROSCI.17-21-08402.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athwal BS, Berkley KJ, Hussain I, Brennan A, Craggs M, Sakakibara R, Frackowiak RS, Fowler CJ. Brain responses to changes in bladder volume and urge to void in healthy men. Brain. 2001;124:369–377. doi: 10.1093/brain/124.2.369. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Leedy MG, Bresnahan JC. Evidence for alterations of synaptic inputs to sacral spinal reflex circuits after spinal cord transection in the cat. Exp. Neurol. 1993;123:35–50. doi: 10.1006/exnr.1993.1138. [DOI] [PubMed] [Google Scholar]
- Birder LA. Urothelial signaling. Auton. Neurosci. 2010;153:33–40. doi: 10.1016/j.autneu.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, de Groat WC. Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am. J. Physiol. 1993;265:R326–R333. doi: 10.1152/ajpregu.1993.265.2.R326. [DOI] [PubMed] [Google Scholar]
- Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat. Clin. Pract. Urol. 2007;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Roppolo JR, Erickson VL, de Groat WC. Increased c-fos expression in spinal lumbosacral projection neurons and preganglionic neurons after irritation of the lower urinary tract in the rat. Brain Res. 1999;834:55–65. doi: 10.1016/s0006-8993(99)01546-2. [DOI] [PubMed] [Google Scholar]
- Birder LA, de Groat WC, Apodaca G. Physiology of the urothelium. In: Shick E, Corcos J, editors. Textbook of the Neurogenic Bladder. Taylor and Francis; London, UK: 2008. pp. 19–39. [Google Scholar]
- Birder L, Drake M, de Groat WC, Fowler C, Mayer E, Morrison J, Paton J, Griffiths D, Mills I, Thor K. Neural control. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Health Publication Ltd; Paris: 2009. pp. 169–253. [Google Scholar]
- Blok BF, Sturms LM, Holstege G. Brain activation during micturition in women. Brain. 1998;121:2033–2042. doi: 10.1093/brain/121.11.2033. [DOI] [PubMed] [Google Scholar]
- Brady CM, Apostolidis A, Yiangou Y, Baecker PA, Ford AP, Freeman A, Jacques TS, Fowler CJ, Anand P. P2X3-immunoreactive nerve fibres inneurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur. Urol. 2004a;46:247–253. doi: 10.1016/j.eururo.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Brady CM, Apostolidis AN, Harper M, Yiangou Y, Beckett A, Jacques TS, Freeman A, Scaravilli F, Fowler CJ, Anand P. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int. 2004b;93:770–776. doi: 10.1111/j.1464-410X.2003.04722.x. [DOI] [PubMed] [Google Scholar]
- Chang HY, Cheng CL, Chen JJ, de Groat WC. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am. J. Physiol. Renal Physiol. 2007;292:F1044–F1053. doi: 10.1152/ajprenal.00175.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp. Neurol. 2004;187:445–454. doi: 10.1016/j.expneurol.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Cheng C-L, Ma C-P, de Groat WC. Effects of capsaicin on micturition and associated reflexes in the rat. Am. J. Physiol. 1993;265:R132–R138. doi: 10.1152/ajpregu.1993.265.1.R132. [DOI] [PubMed] [Google Scholar]
- Cheng CL, Ma CP, de Groat WC. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 1995;678:40–48. doi: 10.1016/0006-8993(95)00212-9. [DOI] [PubMed] [Google Scholar]
- Cheng CL, Chai CY, de Groat WC. Detrusor-sphincter dyssynergia induced by cold stimulation of the urinary bladder of rats. Am. J. Physiol. 1997;272:R1271–R1282. doi: 10.1152/ajpregu.1997.272.4.R1271. [DOI] [PubMed] [Google Scholar]
- Cheng CL, Liu JC, Chang SY, Ma CP, de Groat WC. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am. J. Physiol. 1999;277:R786–R794. doi: 10.1152/ajpregu.1999.277.3.R786. [DOI] [PubMed] [Google Scholar]
- Chuang YC, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J. Urol. 2001;165:975–979. [PubMed] [Google Scholar]
- Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J. Neurosci. 2005;25:3973–3984. doi: 10.1523/JNEUROSCI.5239-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC. Plasticity of bladder reflex pathways during postnatal development. Physiol. Behav. 2002;77:689–692. doi: 10.1016/s0031-9384(02)00919-8. [DOI] [PubMed] [Google Scholar]
- de Groat WC. The urothelium in overactive bladder: passive bystander or active participant? Urology. 2004;64:7–11. doi: 10.1016/j.urology.2004.08.063. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Lalley PM. Reflex firing in the lumbar sympathetic outflow to activation of vesical afferent fibres. J. Physiol. 1972;226:289–309. doi: 10.1113/jphysiol.1972.sp009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J. Physiol. 1969;200:87–108. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog. Brain Res. 2006;152:59–84. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb. Exp. Pharmacol. 2009;194:91–138. doi: 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Changes in afferent activity after spinal cord injury. Neurourol. Urodyn. 2010;29:63–76. doi: 10.1002/nau.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J. Auton. Nerv. Syst. 1981;3:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, Steers W, Roppolo JR. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J. Auton. Nerv. Syst. 1990;30:S71–S77. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The Autonomic Nervous System. Harwood Academic Publishers; London: 1993. pp. 227–289. [Google Scholar]
- de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR. Developmental and injury induced plasticity in the micturition reflex pathway. Behav. Brain Res. 1998;92:127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- Fall M, Lindstrom S, Mazieres L. A bladder-to-bladder cooling reflex in the cat. J. Physiol. 1990;427:281–300. doi: 10.1113/jphysiol.1990.sp018172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ. Integrated control of lower urinary tract—clinical perspective. Br. J. Pharmacol. 2006;147:S14–S24. doi: 10.1038/sj.bjp.0706629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths DJ. A decade of functional brain imaging applied to bladder control. Neurourol. Urodyn. 2010;29:49–55. doi: 10.1002/nau.20740. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat. Rev. Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CH, Kanai AH, Roosen A, Takeda M, Wood DN. Cell biology. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Health Publication Ltd; Paris: 2009. pp. 116–166. [Google Scholar]
- Geirsson G, Fall M, Lindstrom S. The ice-water test—a simple and valuable supplement to routine cystometry. Br. J. Urol. 1993;71:681–685. doi: 10.1111/j.1464-410x.1993.tb16065.x. [DOI] [PubMed] [Google Scholar]
- Geirsson G, Lindstrom S, Fall M, Gladh G, Hermansson G, Hjalmas K. Positive bladder cooling test in neurologically normal young children. J. Urol. 1994;151:446–448. doi: 10.1016/s0022-5347(17)34984-4. [DOI] [PubMed] [Google Scholar]
- Geirsson G, Fall M, Sullivan L. Clinical and urodynamic effects of intravesical capsaicin treatment in patients with chronic traumatic spinal detrusor hyperreflexia. J. Urol. 1995;154:1825–1829. [PubMed] [Google Scholar]
- Giannantoni A, Di Stasi SM, Nardicchi V, Zucchi A, Macchioni L, Bini V, Goracci G, Porena M. Botulinum-A toxin injections into the detrusor muscle decrease nerve growth factor bladder tissue levels in patients with neurogenic detrusor overactivity. J. Urol. 2006;175:2341–2344. doi: 10.1016/S0022-5347(06)00258-8. [DOI] [PubMed] [Google Scholar]
- Griffiths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the bladder in normal and urge-incontinent women. Neuroimage. 2007;37:1–7. doi: 10.1016/j.neuroimage.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J. Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G, Mouton LJ. Central nervous system control of micturition. Int. Rev. Neurobiol. 2003;56:123–145. doi: 10.1016/s0074-7742(03)56004-4. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Kanai A. Urotheliogenic modulation of intrinsic activity in spinal cord-transected rat bladders: role of mucosal muscarinic receptors. Am. J. Physiol. Renal Physiol. 2008;295:F454–F461. doi: 10.1152/ajprenal.90315.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Fry C, Hayashi F, Stolz D, Griffiths D, Kanai A. Role of gap junctions in spontaneous activity of the rat bladder. Am. J. Physiol. Renal Physiol. 2007;293:F1018–F1025. doi: 10.1152/ajprenal.00183.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka O, Alm P, Larsson B, Mattiasson A, Andersson KE. Facilitatory effect of pituitary adenylate cyclase activating polypeptide on micturition in normal, conscious rats. Neuroscience. 1995;66:1009–1014. doi: 10.1016/0306-4522(95)00038-k. [DOI] [PubMed] [Google Scholar]
- Jiang CH, Mazieres L, Lindstrom S. Cold- and menthol-sensitive C afferents of cat urinary bladder. J. Physiol. 2002;543:211–220. doi: 10.1113/jphysiol.2002.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder L, Griffiths D, de Groat W, Fry C. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am. J. Physiol. Renal Physiol. 2007;292:F1065–F1072. doi: 10.1152/ajprenal.00229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai A, Zabbarova I, Ikeda Y, Yoshimura N, Birder L, Hanna-Mitchell A, de Groat WC. Sophisticated models and methods for studying neurogenic bladder dysfunction. Neurourol. Urodyn. 2011;30:658–667. doi: 10.1002/nau.21120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavia RB, Dasgupta R, Fowler CJ. Functional imaging and the central control of the bladder. J. Comp. Neurol. 2005;493:27–32. doi: 10.1002/cne.20753. [DOI] [PubMed] [Google Scholar]
- Kruse MN, Belton AL, de Groat WC. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am. J. Physiol. 1993;264:R1157–R1163. doi: 10.1152/ajpregu.1993.264.6.R1157. [DOI] [PubMed] [Google Scholar]
- Kruse MN, Bennett B, de Groat WC. Effect of urinary diversion on the recovery of micturition reflexes after spinal cord injury in the rat. J. Urol. 1994;151:1088–1091. doi: 10.1016/s0022-5347(17)35189-3. [DOI] [PubMed] [Google Scholar]
- Kruse MN, Bray LA, de Groat WC. Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J. Auton. Nerv. Syst. 1995;54:215–224. doi: 10.1016/0165-1838(95)00011-l. [DOI] [PubMed] [Google Scholar]
- Lamb K, Gebhart GF, Bielefeldt K. Increased nerve growth factor expression triggers bladder overactivity. J. Pain. 2004;5:150–156. doi: 10.1016/j.jpain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. Eur. Urol. 2009;56:700–707. doi: 10.1016/j.eururo.2008.04.037. [DOI] [PubMed] [Google Scholar]
- Mazieres L, Jiang C, Lindstrom S. The C fibre reflex of the cat urinary bladder. J. Physiol. 1998;513:531–541. doi: 10.1111/j.1469-7793.1998.531bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy CJ, Zabbarova IV, Brumovsky PR, Roppolo JR, Gebhart GF, Kanai AJ. Spontaneous contractions evoke afferent nerve firing in mouse bladders with detrusor overactivity. J. Urol. 2009;181:1459–1466. doi: 10.1016/j.juro.2008.10.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura A, Kawatani M, de Groat WC. Effects of pituitary adenylate cyclase activating polypeptide on lumbosacral preganglionic neurons in the neonatal rat spinal cord. Brain Res. 2001;895:223–232. doi: 10.1016/s0006-8993(01)02112-6. [DOI] [PubMed] [Google Scholar]
- Miura A, Kawatani M, de Groat WC. Excitatory synaptic currents in lumbosacral parasympathetic preganglionic neurons evoked by stimulation of the dorsal commissure. J. Neurophysiol. 2003;89:382–389. doi: 10.1152/jn.00180.2002. [DOI] [PubMed] [Google Scholar]
- Miyazato M, Sasatomi K, Hiragata S, Sugaya K, Chancellor MB, de Groat WC, Yoshimura N. GABA receptor activation in the lumbosacral spinal cord decreases detrusor overactivity in spinal cord injured rats. J. Urol. 2008a;179:1178–1183. doi: 10.1016/j.juro.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazato M, Sasatomi K, Hiragata S, Sugaya K, Chancellor MB, de Groat WC, Yoshimura N. Suppression of detrusor-sphincter dysynergia by GABA-receptor activation in the lumbosacral spinal cord in spinal cord-injured rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008b;295:R336–R342. doi: 10.1152/ajpregu.90315.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazato M, Sugaya K, Goins WF, Wolfe D, Goss JR, Chancellor MB, de Groat WC, Glorioso JC, Yoshimura N. Herpes simplex virus vector-mediated gene delivery of glutamic acid decarboxylase reduces detrusor overactivity in spinal cord-injured rats. Gene Ther. 2009;16:660–668. doi: 10.1038/gt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazato M, Sugaya K, Saito S, Chancellor MB, Goins WF, Goss JR, de Groat WC, Glorioso JC, Yoshimura N. Suppression of detrusor-sphincter dyssynergia by herpes simplex virus vector mediated gene delivery of glutamic acid decarboxylase in spinal cord injured rats. J. Urol. 2010;184:1204–1210. doi: 10.1016/j.juro.2010.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C, Nadelhaft I, de Groat WC. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J. Comp. Neurol. 1981;201:415–440. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- Morgan CW, de Groat WC, Felkins LA, Zhang SJ. Intracellular injection of neurobiotin or horseradish peroxidase reveals separate types of preganglionic neurons in the sacral parasympathetic nucleus of the cat. J. Comp. Neurol. 1993;331:161–182. doi: 10.1002/cne.903310203. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, de Groat WC, Morgan C. Location and morphology of parasympathetic preganglionic neurons in the sacral spinal cord of the cat revealed by retrograde axonal transport of horseradish peroxidase. J. Comp. Neurol. 1980;193:265–281. doi: 10.1002/cne.901930118. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL, Card JP, Miselis RR. Central nervous system neurons labelled following the injection of pseudorabies virus into the rat urinary bladder. Neurosci. Lett. 1992;143:271–274. doi: 10.1016/0304-3940(92)90281-b. [DOI] [PubMed] [Google Scholar]
- Ng YK, de Groat WC, Wu HY. Smooth muscle and neural mechanisms contributing to the downregulation of neonatal rat spontaneous bladder contractions during postnatal development. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R2100–R2112. doi: 10.1152/ajpregu.00779.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nout YS, Leedy GM, Beattie MS, Bresnahan JC. Alterations in eliminative and sexual reflexes after spinal cord injury: defecatory function and development of spasticity in pelvic floor musculature. Prog. Brain Res. 2006;152:359–372. doi: 10.1016/S0079-6123(05)52024-7. [DOI] [PubMed] [Google Scholar]
- Popat R, Apostolidis A, Kalsi V, Gonzales G, Fowler CJ, Dasgupta P. A comparison between the response of patients with idiopathic detrusor overactivity and neurogenic detrusor overactivity to the first intradetrusor injection of botulinum-A toxin. J. Urol. 2005;174:984–989. doi: 10.1097/01.ju.0000169480.43557.31. [DOI] [PubMed] [Google Scholar]
- Ruch TC, Tang PC. Localization of brain stem and diencephalic areas controlling the micturation reflex. J. Comp. Neurol. 1956;106:213–245. doi: 10.1002/cne.901060108. [DOI] [PubMed] [Google Scholar]
- Schurch B, Denys P, Kozma CM, Reese PR, Slaton T, Barron RL. Botulinum toxin A improves the quality of life of patients with neurogenic urinary incontinence. Eur. Urol. 2007;52:850–858. doi: 10.1016/j.eururo.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J. Urol. 2002;168:2269–2274. doi: 10.1016/S0022-5347(05)64369-8. [DOI] [PubMed] [Google Scholar]
- Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor M, de Groat W, Yoshimura N. Detrusor overactivity induced by increased levels of nerve growth factor in bladder afferent pathways in rats. Neurourol. Urodyn. 2003;22:375–377. [Google Scholar]
- Seki S, Erickson KE, Seki M, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Targeting spinal neurokinin I receptor-expressing neurons for the treatment of neurogenic detrusor overactiving in spinal cord injury. J. Urol. 2004a;171:142–143. [Google Scholar]
- Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J. Urol. 2004b;171:478–482. doi: 10.1097/01.ju.0000088340.26588.74. [DOI] [PubMed] [Google Scholar]
- Seki S, Erickson KA, Seki M, Nishizawa O, Igawa Y, Ogawa T, de Groat WC, Chancellor MB, Yoshimura N. Elimination of rat spinal neurons expressing neurokinin 1 receptors reduces bladder overactivity and spinal c-fos expression induced by bladder irritation. Am. J. Physiol. Renal Physiol. 2005;288:F466–F473. doi: 10.1152/ajprenal.00274.2004. [DOI] [PubMed] [Google Scholar]
- Sievert KD, Amend B, Gakis G, Toomey P, Badke A, Kaps HP, Stenzl A. Early sacral neuromodulation prevents urinary incontinence after complete spinal cord injury. Ann. Neurol. 2010;67:74–84. doi: 10.1002/ana.21814. [DOI] [PubMed] [Google Scholar]
- Somogyi GT, de Groat WC. Function, signal transduction mechanisms and plasticity of presynaptic muscarinic receptors in the urinary bladder. Life Sci. 1999;64:411–418. doi: 10.1016/s0024-3205(98)00580-3. [DOI] [PubMed] [Google Scholar]
- Somogyi GT, Zernova GV, Yoshiyama M, Rocha JN, Smith CP, de Groat WC. Change in muscarinic modulation of transmitter release in the rat urinary bladder after spinal cord injury. Neurochem. Int. 2003;43:73–77. doi: 10.1016/s0197-0186(02)00193-6. [DOI] [PubMed] [Google Scholar]
- Steers WD, Tuttle JB. Mechanisms of disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat. Clin. Pract. Urol. 2006;3:101–110. doi: 10.1038/ncpuro0408. [DOI] [PubMed] [Google Scholar]
- Stein RJ, Santos S, Nagatomi J, Hayashi Y, Minnery BS, Xavier M, Patel AS, Nelson JB, Futrell WJ, Yoshimura N, Chancellor MB, De Miguel F. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J. Urol. 2004;172:1175–1178. doi: 10.1097/01.ju.0000134880.55119.cf. [DOI] [PubMed] [Google Scholar]
- Sugaya K, de Groat WC. Micturition reflexes in the in vitro neonatal rat brain stem-spinal cord-bladder preparation. Am. J. Physiol. 1994;266:R658–R667. doi: 10.1152/ajpregu.1994.266.3.R658. [DOI] [PubMed] [Google Scholar]
- Sugaya K, de Groat WC. Influence of temperature on activity of the isolated whole bladder preparation of neonatal and adult rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R238–R246. doi: 10.1152/ajpregu.2000.278.1.R238. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Roppolo JR, Yoshimura N, Card JP, de Groat WC. The central neural pathways involved in micturition in the neonatal rat as revealed by the injection of pseudorabies virus into the urinary bladder. Neurosci. Lett. 1997;223:197–200. doi: 10.1016/s0304-3940(97)13433-4. [DOI] [PubMed] [Google Scholar]
- Szell EA, Somogyi GT, de Groat WC, Szigeti GP. Developmental changes in spontaneous smooth muscle activity in the neonatal rat urinary bladder. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R809–R816. doi: 10.1152/ajpregu.00641.2002. [DOI] [PubMed] [Google Scholar]
- Tai C, Wang J, Jin T, Wang P, Kim SG, Roppolo JR, de Groat WC. Brain switch for reflex micturition control detected by FMRI in rats. J. Neurophysiol. 2009;102:2719–2730. doi: 10.1152/jn.00700.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor KB, de Groat WC. Neural control of the female urethral and anal rhabdosphincters and pelvic floor muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R416–R438. doi: 10.1152/ajpregu.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor K, Kawatani M, de Groat WC. In: Plasticity in the reflex pathways to the lower urinary tract of the cat during postnatal development and following spinal cord injury. Goldberger ME, Gorio A, Murray M, editors. Liviana Press; Padova: 1986. pp. 65–80. [Google Scholar]
- Vizzard MA. Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000a;279:R295–R305. doi: 10.1152/ajpregu.2000.279.1.R295. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J. Comp. Neurol. 2000b;420:335–348. [PubMed] [Google Scholar]
- Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp. Neurol. 2000c;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury. Prog. Brain Res. 2006;152:97–115. doi: 10.1016/S0079-6123(05)52007-7. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Erickson VL, Card JP, Roppolo JR, de Groat WC. Transneuronal labeling of neurons in the adult rat brainstem and spinal cord after injection of pseudorabies virus into the urethra. J. Comp. Neurol. 1995;355:629–640. doi: 10.1002/cne.903550411. [DOI] [PubMed] [Google Scholar]
- Weaver LC, Marsh DR, Gris D, Brown A, Dekaban GA. Autonomic dysreflexia after spinal cord injury: central mechanisms and strategies for prevention. Prog. Brain Res. 2006;152:245–263. doi: 10.1016/S0079-6123(05)52016-8. [DOI] [PubMed] [Google Scholar]
- Yoshimura N. Bladder afferent pathway and spinal cord injury: possible mechanisms inducing hyperreflexia of the urinary bladder. Prog. Neurobiol. 1999;57:583–606. doi: 10.1016/s0301-0082(98)00070-7. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Plasticity of Na+ channels in afferent neurones innervating rat urinary bladder following spinal cord injury. J. Physiol. 1997a;503:269–276. doi: 10.1111/j.1469-7793.1997.269bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Plasticity of Na+ channels in afferent neurones innervating rat urinary bladder following spinal cord injury. J. Physiol. 1997b;503:269–276. doi: 10.1111/j.1469-7793.1997.269bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J. Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, White G, Weight FF, de Groat WC. Different types of Na+ and A-type K+ currents in dorsal root ganglion neurones innervating the rat urinary bladder. J. Physiol. 1996;494:1–16. doi: 10.1113/jphysiol.1996.sp021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, Erdman SL, Snider MW, de Groat WC. Effects of spinal cord injury on neurofilament immunoreactivity and capsaicin sensitivity in rat dorsal root ganglion neurons innervating the urinary bladder. Neuroscience. 1998;83:633–643. doi: 10.1016/s0306-4522(97)00376-x. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Seki S, Erickson KA, Erickson VL, Chancellor MB, de Groat WC. Histological and electrical properties of rat dorsal root ganglion neurons innervating the lower urinary tract. J. Neurosci. 2003;23:4355–4361. doi: 10.1523/JNEUROSCI.23-10-04355.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J. Neurosci. 2006;26:10847–10855. doi: 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama M, de Groat WC. The role of vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide in the neural pathways controlling the lower urinary tract. J. Mol. Neurosci. 2008;36:227–240. doi: 10.1007/s12031-008-9090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Douglas KL, Jin H, Eldaif BM, Nassar R, Fraser MO, Dolber PC. Sprouting of substance P-expressing primary afferent central terminals and spinal micturition reflex NK1 receptor dependence after spinal cord injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R2084–R2096. doi: 10.1152/ajpregu.90653.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinck ND, Downie JW. Plasticity in the injured spinal cord: can we use it to advantage to reestablish effective bladder voiding and continence? Prog. Brain Res. 2006;152:147–162. doi: 10.1016/S0079-6123(05)52010-7. [DOI] [PubMed] [Google Scholar]
- Zinck ND, Downie JW. IB4 afferent sprouting contributes to bladder dysfunction in spinal rats. Exp. Neurol. 2008;213:293–302. doi: 10.1016/j.expneurol.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Zinck ND, Rafuse VF, Downie JW. Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Exp. Neurol. 2007;204:777–790. doi: 10.1016/j.expneurol.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol. 2007;7:9. doi: 10.1186/1472-6793-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvara P, Braas KM, May V, Vizzard MA. A role for pituitary adenylate cyclase activating polypeptide (PACAP) in detrusor hyperreflexia after spinal cord injury (SCI) Ann. N.Y. Acad. Sci. 2006;1070:622–628. doi: 10.1196/annals.1317.092. [DOI] [PubMed] [Google Scholar]
- Zvarova K, Dunleavy JD, Vizzard MA. Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp. Neurol. 2005;192:46–59. doi: 10.1016/j.expneurol.2004.10.017. [DOI] [PubMed] [Google Scholar]




