Abstract
A pair of novel dipicolylamine ligands bearing isothiocyanate groups were used as conjugation reagents to prepare multivalent molecules with anionic recognition capability. The isothiocyanates were reacted with two classes of dendritic scaffolds bearing primary amines, squaraine rotaxanes and PAMAM dendrimers, and the products were converted into water soluble zinc(II) coordination complexes. The multivalent squaraine rotaxanes exhibit high fluorescence quantum yields in water and are very well suited for biological imaging applications.
Keywords: zinc dipicolylamine, multivalent, squaraine rotaxane, fluorescence, dendrimer
There is a need for molecular probes that can selectively target phosphorylated biomolecules in various types of biomedical samples. Supramolecular chemists have explored a wide array of organic and inorganic structures.1 For operation in aqueous solution, metal cation coordination complexes are especially effective because they have the ability to form strong coordination bonds with the phosphate oxygens. One of the most prominent family of synthetic phosphate receptors uses zinc(II)-dipicolylamine (Zn(II)-DPA) coordination complexes as the molecular recognition units.2 We have contributed to this topic by demonstrating that Zn(II)-DPA complexes can selectively target membrane surfaces that are rich in anionic phospholipids.2c This discovery has high biomedical relevance since several cell systems associated with disease, such as bacterial cells and apoptotic mammalian cells, have anionic surfaces. We have developed a number of fluorescent Zn(II)-DPA probes and used them to image bacterial infection and cell death in vitro and in vivo.3
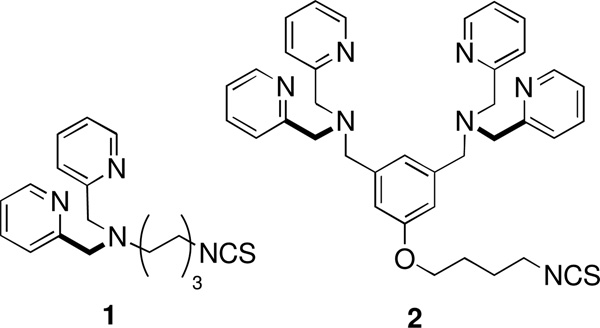
To date, our probe designs have incorporated one or two Zn(II)-DPA recognition units, and we have observed a general trend that the divalent systems exhibit higher and more selective affinity for anionic membranes over near-neutral membranes.3 These results have motivated us to investigate higher order multivalent systems. To access these compounds, we needed to develop convenient synthetic methods for attaching multiple copies of dipicolylamine (DPA) or bis(dipicolylamine) (BDPA) ligands to a molecular scaffold. There are several literature reports of nanoparticles that are coated with Zn(II)-DPA complexes,4,5 but we wanted to prepare smaller structures using organic scaffolds bearing primary amines.6 Thus, a logical conjugation choice was reaction of the primary amines with isothiocyanates to form the corresponding thioureas.7 This reaction typically proceeds under convenient conditions with good yields and does not require a catalyst or coupling agent. Additionally, isothiocyanates are easily prepared and can be stored for extended periods of time. These reasons lead us to synthesize the DPA isothiocyanate 1 and BDPA isothiocyanate 2 (Chart 1) and react them with dendritic structures having multiple branches terminating in primary amines
Chart 1.
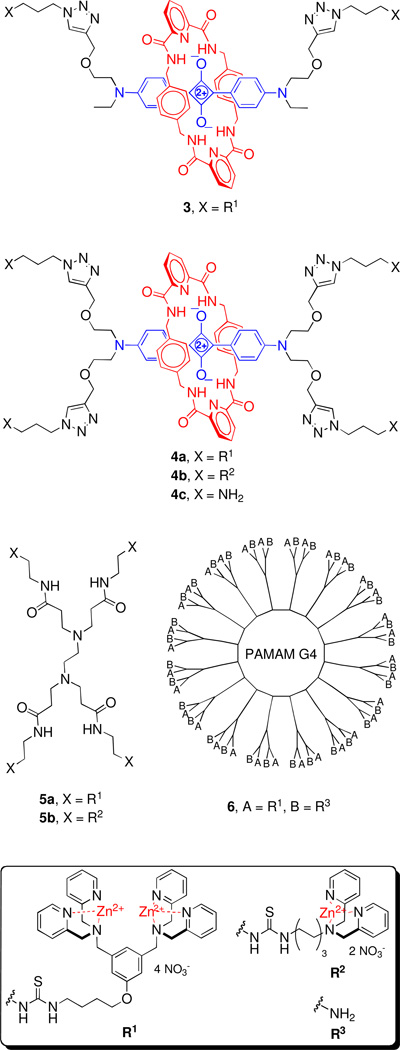
We chose to modify two classes of dendritic scaffolds, squaraine rotaxanes and PAMAM dendrimers. Squaraine rotaxanes, which were developed in our laboratory,8 are interlocked molecules each comprised of a highly fluorescent squaraine dye encapsulated inside a tetralactam macrocycle. The steric protection provided by the surrounding macrocycle inhibits decomposition of the squaraine by nucleophilic attack.9 Squaraine rotaxanes emit strong and narrow fluorescence in the deep red/near-infrared region, and their high stabilities makes them very well suited for animal imaging studies as tracer dyes or as targeted probes for molecular imaging.10 We chose to make multivalent versions, 3, 4a, 4b for eventual study as fluorescence imaging probes. In addition, isothiocyanate reagents 1 and 2 were used them to convert PAMAM dendrimers into multivalent recognition molecules.11 Specifically, we modified commercially available G0 and G4 PAMAM dendrimers and prepared the multivalent Zn(II)-DPA complexes 5a, 5b, 6 (Chart 2).
Chart 2.
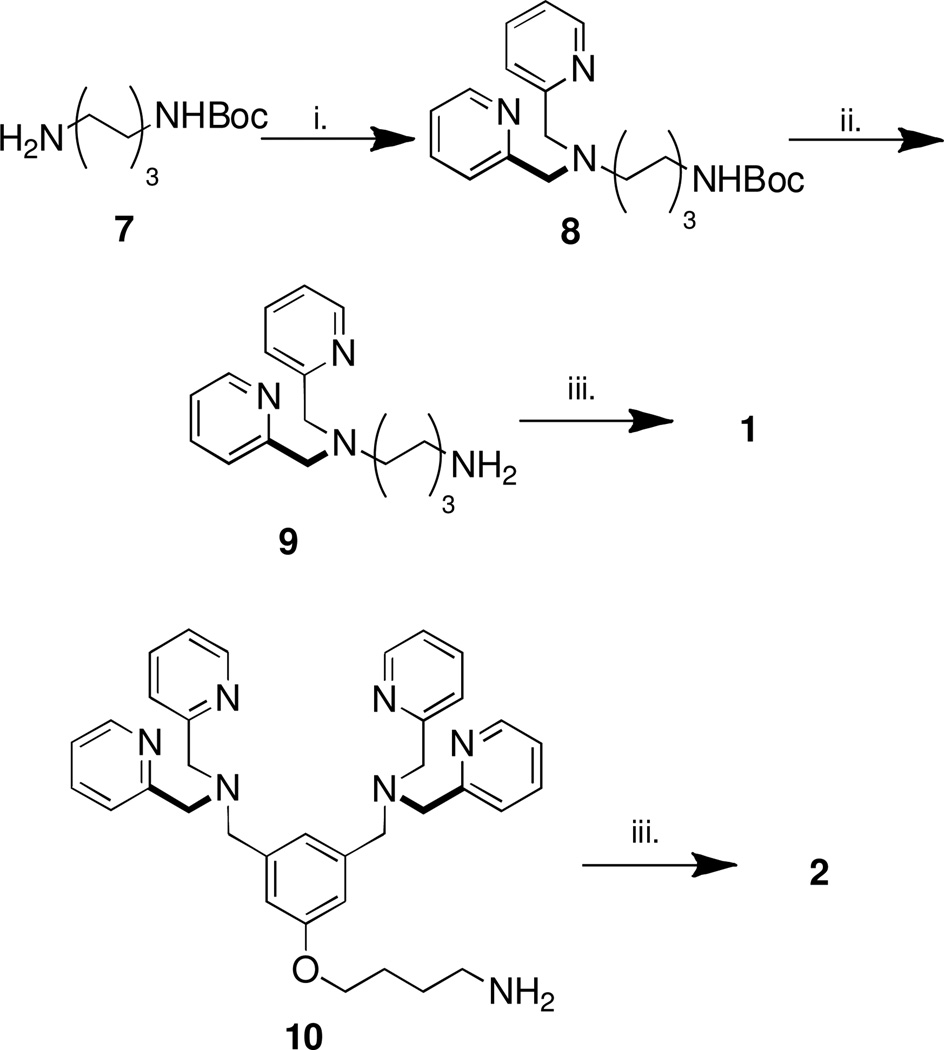
A number of methods exist for preparing isothiocyanates. Primary amines can be reacted with carbon disulfide (CS2) in ammonium hydroxide solution and the resulting ammonium dithiocarbonate salts are treated with lead nitrate or tosyl chloride to give the corresponding isothiocyanates.12 Another method involves the thermal fragmentation of 1,4,2-oxathiazoles.13 In our hands, reaction of primary amines with CS2 and subsequent treatment with hydrogen peroxide proved to be a convenient method.14 Preparation of isothiocyanate (1) required the synthesis of precursor DPA-hexylamine, 9. Thus, monoprotected 1,6-bisaminohexane was refluxed in acetonitrile with 2-(chloromethyl)pyridine in the presence of Na2CO3 to furnish the N-protected DPA derivative 8 in good yield which was quantitatively deprotected using TFA in CH2Cl2 to give 9. Treatment of 9 with CS2 followed by H2O2 afforded the desired isothiocyanate 1 in moderate yield. The isothiocyanate 2 was prepared in similar fashion (Scheme 1) starting with the known BDPA-butylamine 10.15
Scheme 1.
i. 2-chloromethyl pyridine, Na2CO3, MeCN, reflux, 16 h, 80%; ii. CH2Cl2, TFA, rt, 16h, quant.; iii. CS2, NEt3, THF, 0°C, 30 min. followed by H2O2, THF, 0°C, 40% (30% for 10).
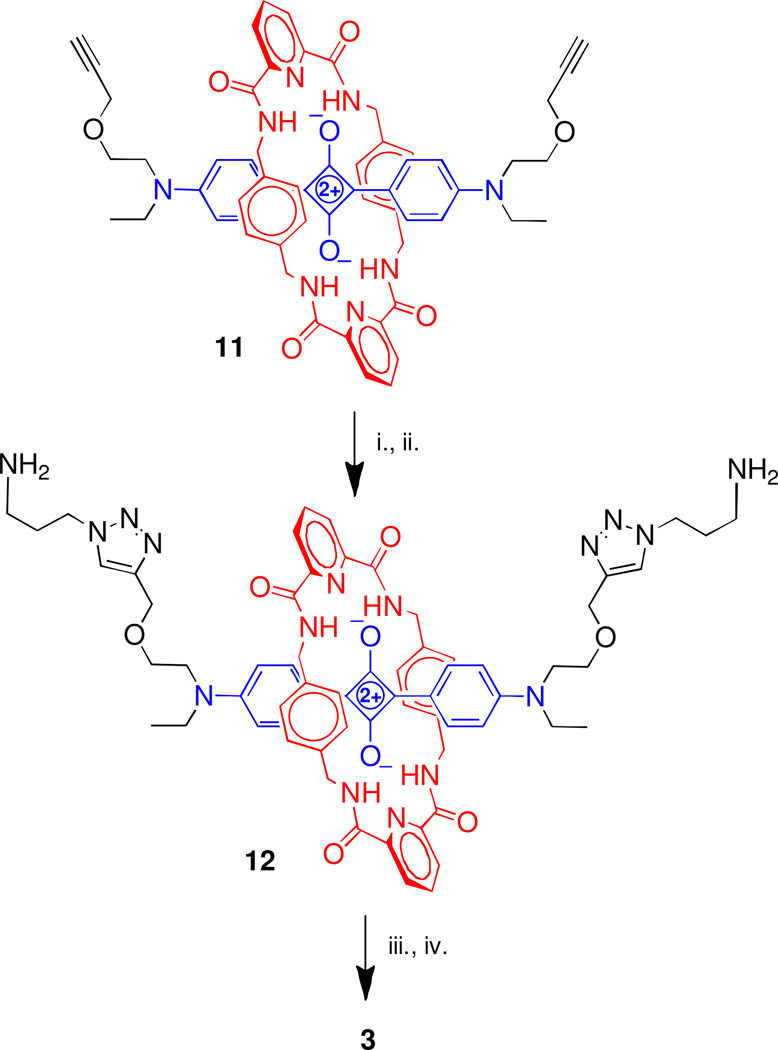
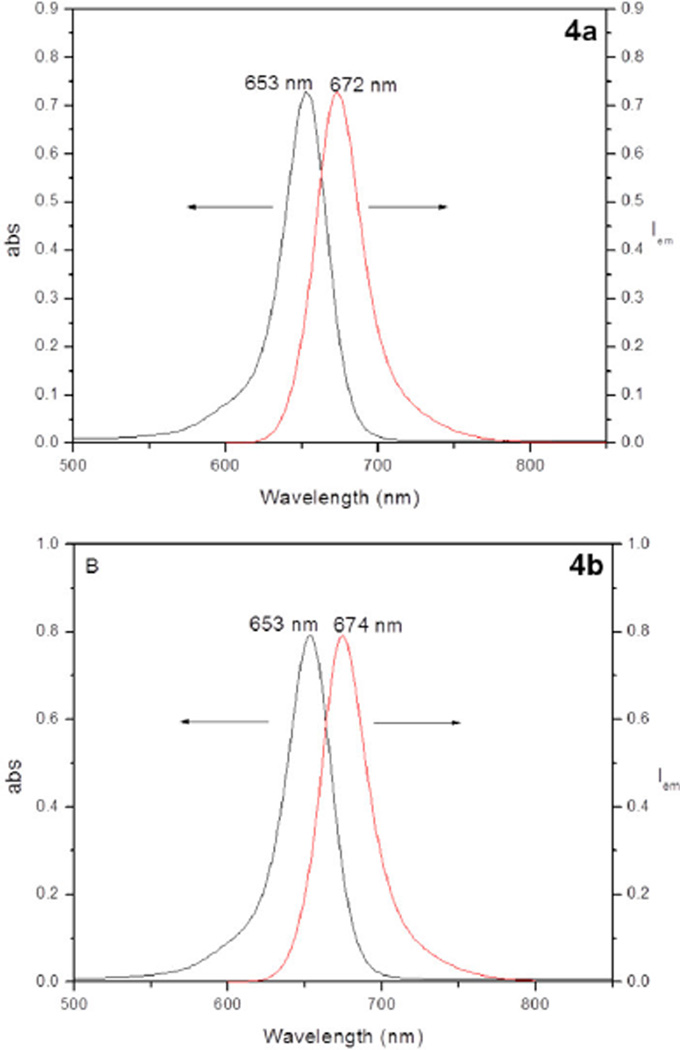
With isothiocyanates 1 and 2 in hand, we prepared squaraine rotaxanes 3, 4a, and 4b bearing multiple copies of DPA or BDPA ligands. The bis(amine) 12 was prepared by conducting a copper-catalyzed cycloaddition reaction using the known bisalkyne 11 and N-tert-butoxycarbonyl-3-azidopropylamine and then removing the Boc protecting groups using TFA in CH2Cl2.16 Reaction of 12 and 2 in the presence of NEt3 in DMF, followed by complexation with Zn(NO3)2 in methanol gave the targeted squaraine rotaxane 3. Squaraine rotaxanes 4a and 4b were prepared in a similar fashion by treating the known tetra(amine) 4c (Chart 2) with 1 or 2 followed by complexation with Zn(NO3)2 (Scheme 2).17 Squaraine rotaxanes 3, 4a, and 4b exhibited high water solubility and excellent solution phase stability even when exposed to light. The spectral plots in Figure 1 show the characteristic narrow intense absorption and emission bands, with no evidence for intermolecular aggregation. The photophysical properties listed in Table 1 include the impressive high quantum yields in water of 0.25–0.30. Taken together, the chemical and photophysical properties of squaraine rotaxanes 3, 4a, and 4b indicate that they are well suited for both in vitro and in vivo biological imaging applications.18
Scheme 2.
i. N3(CH2)3NHBoc, CuSO4, sodium ascorbate, CHCl3, H2O, rt, 16h, 90%; ii. TFA, CH2Cl2, 0°C to rt, 16 h, quant.; iii. 2, DMF, rt, 16 h, 40%; iv. Zn(NO3)2, MeOH, 16 h, quant.
Figure 1.
Absorption and emission spectra for squaraine rotaxanes 4a (upper panel) and 4b (lower panel).
Table 1.
Photophysical parameters for multivalent squaraine rotaxanes in water.
| Compound | λabs(nm) | λem(nm) | log ε | Φf |
|---|---|---|---|---|
| 3 | 653 | 672 | 5.16 | 0.30 |
| 4a | 653 | 672 | 5.16 | 0.30 |
| 4b | 653 | 674 | 5.20 | 0.25 |
We also modified commercially available PAMAM G0 (4 primary amines) and PAMAM G4 (64 primary amines) dendrimers. Reaction of PAMAM G0 with 1 or 2 in DMF overnight furnished the corresponding thioureas in good yields (78% and 75% respectively) and complexation of these products with Zn(NO3)2 in methanol afforded 5a and 5b as water soluble compounds. The reaction of PAMAM G4 with 2 was much slower, and 1H NMR of the product 6 indicated that only ~50% of the peripheral amines had reacted after stirring in DMF for one week. This sluggish reactivity is attributed to steric crowding at the surface of the dendrimer. Thus, there appears to be a steric limit to the number of Zn(II)-BDPA units that can be appended to the surface of high generation polyamine dendrimers. This finding is not unexpected, and is not a major drawback since partially coated high generation dendrimers are known to exhibit powerful multivalent molecular recognition effects.19,20 Indeed, preliminary membrane recognition experiments that added the above multivalent Zn(II)-DPA compounds to dispersions of anionic liposomes composed of 1:1 phosphatidylcholine/phosphatidylserine induced crosslinking and precipitation of the liposomes, with the higher order multivalent systems producing the most liposome precipitation. Conversely, no liposome precipitation was observed in control experiments using liposomes composed of entirely zwitterionic phosphatidylcholine. In due course we will describe the impressive ability of these multivalent Zn(II)-DPA complexes to target the anionic membrane surfaces of apoptotic mammalian cells and bacteria.
In summary, we prepared novel dipicolylamine isothiocyanates 1 and 2 and showed that they are convenient conjugation reagents for attaching Zn(II)-DPA molecular recognition units to dendritic scaffolds terminating in primary amines. The multivalent squaraine rotaxanes 3, 4a, and 4b exhibit high fluorescence quantum yields in water and are very well suited for biological imaging applications. We anticipate that compounds 1 and 2 will be broadly useful for conjugation to a wide range of amine-containing molecules, including peptides and proteins.
Supplementary Material
Acknowledgements
We are grateful for funding support from NIH and the Walther Cancer Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Data: Synthetic procedures and spectral data can be found in the online version of this article at http://dx.doi.org/10.1016/.
References and notes
- 1.For reviews of phosphate recognition see: Kubik S, Reyheller C, Stuwe S. J. Incl. Phenom. Macro. 2005;52:137–187. Hirsch AKH, Fischer FR, Diederich F. Angew. Chem. Int. Ed. Eng. 2007;46:338–352. doi: 10.1002/anie.200603420. Blondeau P, Segura M, Perez-Fernandez R, de Mendoza J. Chem. Soc. Rev. 2007;36:198–210. doi: 10.1039/b603089k. Kim SK, Lee DH, Hong J-I, Yoon J. Accounts Chem. Res. 2009;42:23–31. doi: 10.1021/ar800003f. Bazziacalupi C, Bencini A, Lippolis V. Chem. Soc. Rev. 2010;39:3709–3728. doi: 10.1039/b926161n. Hargrove AE, Nieto S, Zhang T, Sessler JL, Anslyn EV. Chem. Rev. 2011;111:6603–6782. doi: 10.1021/cr100242s.
- 2.For reviews on Zn(II)-DPA complexes as phosphate receptors see: Sakamoto T, Ojida A, Hamachi I. Chem. Comm. 2009;2:141. doi: 10.1039/b812374h. Kruppa M, König B. Chem. Rev. 2006;106:3520. doi: 10.1021/cr010206y. O’Neil EJ, Smith BD. Coord. Chem. Rev. 2006;250:3068. Kim SK, Lee DH, Hong J, Yoon J. Acc. Chem. Res. 2009;42:23. doi: 10.1021/ar800003f. Zhou Y, Xu Z, Yoon J. Chem. Soc. Rev. 2011;40:2222. doi: 10.1039/c0cs00169d. Ngo HT, Liu X, Jolliffe KA. Chem. Soc. Rev. 2012;41:4928. doi: 10.1039/c2cs35087d.
- 3.(a) Smith BA, Akers WJ, Leevy WM, Lampkins AJ, Xiao S, Wolter W, Suckow MA, Achilefu S, Smith BD. J. Am. Chem. Soc. 2010;132:67–69. doi: 10.1021/ja908467y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Smith BA, Gammon ST, Xiao S, Wang W, Chapman S, Ryan McDermott R, Suckow MA, Johnson JR, Piwnica-Worms D, Gokel GW, Smith BD, Leevy WM. Mol. Pharmaceutics. 2011;8:583–590. doi: 10.1021/mp100395u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lee J-J, White AG, Baumes JM, Smith BD. Chem. Commun. 2010;46:1068–1068. doi: 10.1039/b924350j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Smith BA, Xiao S, Wolter W, Wheeler J, Suckow MA, Smith BD. Apoptosis. 2011;16:722–731. doi: 10.1007/s10495-011-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) White AG, Gray BD, Pak KY, Smith BD. Biorg. Med. Chem. Lett. 2012;22:2833–2836. doi: 10.1016/j.bmcl.2012.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Yamaguchi S, Yoshimura I, Kohira T, Tamaru S, Hamachi I. J. Am. Chem. Soc. 2005;127:11835–11841. doi: 10.1021/ja052838y. [DOI] [PubMed] [Google Scholar]; (b) Zhang S, Wang J, Han L, Li C, Wang W, Yuan Z. Sensors and Actuators B. 2010;147:687–690. [Google Scholar]; (c) Oh DJ, Kim KM, Ahn KH. Chem. Asian J. 2011;6:2034–2039. doi: 10.1002/asia.201100149. [DOI] [PubMed] [Google Scholar]; (d) Moro AJ, Schmidt J, Doussineau T, Lapresta-Fernandez A, Wegener J, Mohr GJ. Chem. Commun. 2011;47:6066–6068. doi: 10.1039/c1cc10419e. [DOI] [PubMed] [Google Scholar]; (e) Liu DJ, Credo GM, Su X, Wu K, Lim HC, Elibol OH, Bashir R, Varma M. Chem. Commun. 2011;47:8310–8312. doi: 10.1039/c1cc12073e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Liu G, Choi KY, Bhirde A, Swierczewska M, Yin J, Lee SW, Park JH, Hong J-I, Xie J, Niu G, Kiesewetter DO, Lee S, Chen X. Angew. Chem. Int. Ed. Eng. 2012;51:445–449. doi: 10.1002/anie.201105565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leevy WM, Lambert TN, Johnson JR, Morris J, Smith BD. Chem. Commun. 2008:2331–2333. doi: 10.1039/b803590c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For other ways of converting amines into DPA ligands, see: DiVittorio KM, Johnson JR, Johansson E, Reynolds AJ, Jolliffe KA, Smith BD. Org. Biomol. Chem. 2006;4:1966–1976. doi: 10.1039/b514748d. Carolan JV, Butler SJ, Jolliffe KA. J. Org. Chem. 2009;74:2992–2996. doi: 10.1021/jo802555u.
- 7.Hermanson GT. Bioconjugate Techniques. 2nd Ed. New York: Academic Press; 2008. p. 170. [Google Scholar]
- 8.Gassensmith JJ, Baumes JM, Smith BD. Chem. Commun. 2009:6329–6338. doi: 10.1039/b911064j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arunkumar E, Forbes CC, Noll BC, Smith BD. J. Am. Chem. Soc. 2005;127 doi: 10.1021/ja042404n. 3288-332891. [DOI] [PubMed] [Google Scholar]
- 10.(a) Johnson JR, Fu N, Arunkumar E, Leevy WM, Gammon ST, Piwnica-Worms D, Smith BD. Angew. Chem. Int. Ed. 2007;46:5528–5531. doi: 10.1002/anie.200701491. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Smith BA, Xie B-W, van Beek ER, Ivo Que I, Blankevoort V, Xiao S, Cole EL, Hoehn M, Kaijzel EL, Löwik CWGM, Smith BD. ACS Chemical Neuroscience. 2012;3:530–537. doi: 10.1021/cn3000197. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cole EL, Arunkumar E, Xiao S, Smith BA, Smith BD. Org. Biomol. Chem. 2012;10:5769–5773. doi: 10.1039/c2ob06783h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.For examples of other multivalent systems based on the PAMAM scaffold see: Yordanov AT, Kobayashi H, English SJ, Rejinders K, Milenic D, Krishna MC, Mitchell JB, Brechbiel MW. J. Mater. Chem. 2003;13:1523–1525. Roy R, Baek M-G. Method. Enzymol. 2003;362:240–249. doi: 10.1016/S0076-6879(03)01017-6. Howell BA, Fan D. Proc. R. Soc. A. 2010;466:1515–1526. Myung JH, Gajja KA, Saric J, Eddington DT, Hong S. Angew. Chem. Int. Ed. 2011;50:1–5. doi: 10.1002/anie.201105508.
- 12.Wong R, Dolma SJ. J. Org. Chem. 2007;72:3969–3971. doi: 10.1021/jo070246n. [DOI] [PubMed] [Google Scholar]
- 13.(a) Kim NJ, Jung KS, Lee HJ, Son JS. Tetrahedron Lett. 1997;38:1597–1598. [Google Scholar]; (b) O’Reilly RJ, Radom L. Org. Lett. 2009;11:1325–1328. doi: 10.1021/ol900109b. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Tajima H, Ohtani T. J. Org. Chem. 1997;62:4539–4540. doi: 10.1021/jo970100w. [DOI] [PubMed] [Google Scholar]
- 15.Lakshmi C, Hanshaw RG, Smith BD. Tetrahedron. 2004;60:11307–11315. [Google Scholar]
- 16.Gassensmith JJ, Arunkumar E, Lorna Barr L, Baumes JM, Noll BC, Smith BD. J. Am. Chem. Soc. 2007;129:15054–15060. doi: 10.1021/ja075567v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao S, Fu N, Peckham K, Smith BD. Org. Lett. 2010;12:140–143. doi: 10.1021/ol902546m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.For recent reviews of fluorescent sensors for biological applications see: Lavis LD, Raines RT. ACS Chem. Biol. 2008;3:142–155. doi: 10.1021/cb700248m. Panigrahi M, Dash S, Patel S, Mishra BK. Tetrahedron. 2012;68:781–805.
- 19.Incomplete derivatization of terminal amines in high generation PAMAM dendrimers is precedented; see reference 11a.
- 20.For an example of enhanced multivalent recognition using partially coated dendrimers, see reference 11d.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.