Abstract
Background
Renal transplant recipients regularly fail to take their prescribed immunosuppressive medications, frequently leading to adverse outcomes.
Methods
Medication vials incorporating electronic monitor circuits in their caps compiled prospective data files on the azathioprine dosing patterns of 180 adult renal transplant recipients monitored up to 4 years. These patients were followed for a mean of 8.7 years posttransplantation.
Results
Patients were divided into three groups by the medication doses missed during the first 6 months posttransplant. These initial dosing patterns remained remarkably consistent up to 4 years. Patients (n=47) missing the most doses (≥5%) experienced earlier and more frequent acute rejection episodes (P=0.025). This group also demonstrated significantly longer interdose intervals (P=0.005), with more frequent (P<0.001) and longer (P<0.001) “drug holidays.” A patient subgroup with early declining medication adherence (n=23) experienced dramatically poorer outcomes, with significantly increased acute rejection (P<0.001), chronic rejection (P=0.034), graft loss before death (P<0.001), and death (P=0.04). In all tertiles there was a trend toward missing more medication over time.
Conclusions
Excellent posttransplant medication adherence is critical to improved outcomes. Individual dosing patterns are established early after hospital discharge and remain remarkably consistent, despite gradual erosion in adherence over time. The later consequences of medication nonadherence, especially early declines in adherence, include increased frequencies of rejection, graft loss, and death.
Keywords: Transplantation, Medication, Adherence, Compliance, Rejection
Successful renal transplantation has dramatically altered the outcome of chronic kidney disease. Over the past few decades, several generations of newer immunosuppressive agents have propelled the 1-year patient and graft survival rates to greater than 90% (1-3). But to fully benefit from these pharmaceutical advances, first, patients must actually take their prescribed medications. The advent of this remarkably effective immunosuppressive drug therapy has highlighted the issue of medication nonadherence and its consequences (4-7). Previously, transplant recipients who overtly discontinued their medications usually were easily identified, whereas those patients with less dramatic lapses in their prescribed drug use more frequently went undetected. Today, physicians involved in transplant patient care suspect the role of even subtle nonadherence in initiating adverse events and impacting longer-term graft survival (4, 8, 9).
Prospective information about the frequency and magnitude of posttransplant medication nonadherence is limited (9-12). Most studies of nonadherence after transplantation have been retrospective, with nonadherence suspected or confirmed only in recipients with adverse outcomes (e.g., rejection or graft loss). Consequently, the definitions of nonadherence have largely been qualitative and variable.
Employing electronic medication monitors (MEMS), we obtained prospective, dynamic, and quantitative data on medication adherence in renal transplant recipients. Our initial report of this cohort (with daily monitored azathioprine [Aza] dosing) demonstrated that nonadherence appeared early in the posttransplant period and was pervasive (9). More than 50% of patients missed one or more days of Aza during the first 90 days after hospital discharge. Early adverse consequences, including acute transplant rejection and later loss, were proportional to the timing and extent of early nonadherence (9). We report here the results of multi-year electronic monitoring and later clinical follow-up of that prospective patient cohort.
MATERIALS AND METHODS
Patients
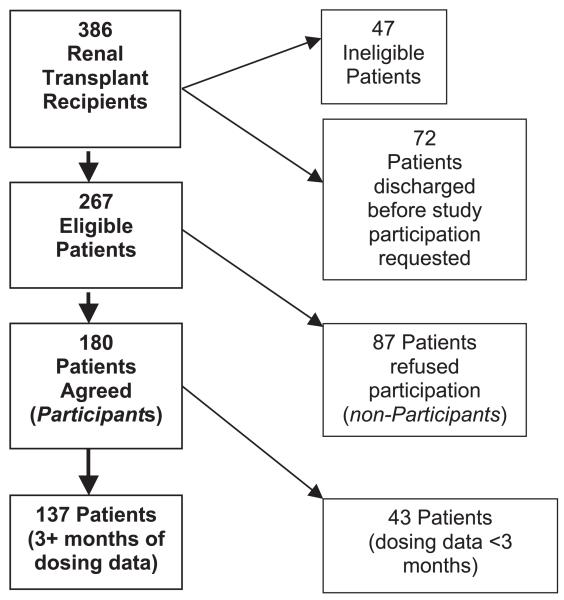
Study design and recruitment were described in the initial report (9). Briefly, between March 1993 and October 1995, 386 consecutive patients received kidney-only transplants at the University of Minnesota and were discharged with functioning renal allografts. Study-eligible patients received Aza tablets as part of their immunosuppressive regimen and were responsible for their own medication administration. Of the 386 patients, 47 were ineligible because of: language barriers; use of Aza suspension; or care setting (e.g., nursing home), and 72 patients were discharged before they could be invited to participate. Of the 267 remaining patients, 180 (67.4%) agreed to enroll, whereas 87 declined study participation (nonparticipants – Fig. 1). Excluded from the analyses were 43 enrolled patients with less than 3 months of evaluable monitor data. These patients included 21 who withdrew, 18 with missing data, two grafts lost, and two deaths occurring within the first 90 days. The remaining 137 patients are included in all analyses.
FIGURE 1.
The final distribution of all study-eligible subjects.
Electronic Medication Monitors
All study participants received their Aza tablets in a medication vial with an electronic monitor sealed in the vial cap (Medication Event Management System, MEMS Track-Cap [Aprex Corp., Union City, CA]). These monitors generate a date and time record each time the cap is removed. By study design, 4 years of continuous medication monitoring was planned and participants were informed that their Aza use was being electronically monitored. By using techniques previously described (9), we compiled a cumulative record of daily cap openings for each study patient, beginning at initial hospital discharge after transplant surgery. Each cap removal was presumed to represent the patient ingesting one dose of Aza. All MEMS records were maintained separate from clinical records, so medication adherence information would not influence clinical decisions regarding medication dosing, transplant kidney biopsy, or hospitalization. This study was initially approved by the University of Minnesota Institutional Review Board and continues to be reviewed and approved annually.
Adherence Definition
Aza was usually prescribed as a single daily dose. Dosage changes were documented by careful review of each patient’s medical record. From hospital discharge after transplantation through study termination (due to dropout, patient death, or study completion), each day was assigned to three mutually exclusive categories; adherent, nonadherent, or missing. Missing data days are those when a patient was hospitalized, the monitor cap malfunctioned, or the cap was physically lost. Because no reliable estimation of adherence was possible for missing data days, they were excluded from calculations. All the remaining days were evaluable as adherent or nonadherent. Days were deemed nonadherent, if on that day there were no cap openings, between 3:00 a.m. that morning and 2:59 a.m. the next day. This interval was chosen to minimize confusion when patients took their Aza after midnight. A adherent day was one when there was at least the prescribed cap opening during that 24-hr interval, and extra openings were ignored. On days when Aza was prescribed not to be taken, no cap opening was defined as an adherent day.
Time after hospital discharge was divided into 30-day increments (months). Each month had to contain a minimum of five evaluable days of cap data to be considered for analysis. Adherence rates were calculated as the percent adherent days per evaluable days during that interval.
The interdose interval was defined as the time from the date and hour of the first dose to the date and hour of the second dose, as long as the entire interval was evaluable. Drug holidays were defined as interdose intervals of 48 hr or longer.
Outcomes
All 267 eligible patients were followed for the end points of acute rejection, allograft survival, and patient survival. Patient follow-up is through March 1, 2006. Outcome events occurring during the first 90 days after initial posttransplant hospital discharge are defined as early and those after 90 days are designated as late. Acute and chronic rejection was diagnosed in kidney biopsy or nephrectomy specimens by the Banff criteria (13). When a tissue diagnosis was not available, the clinical diagnosis of acute rejection was based on an otherwise unexplained elevation of creatinine, coupled with appropriate physical signs (including fever, hypertension, or oliguria) resulting in the clinical decision to treat rejection.
Time to graft loss was based on the date of retransplantation or resumption of regular dialysis treatments. Death also defined graft loss, regardless of renal function at death.
Statistical Methods
Demographic factors and outcomes were compared using chi-square or Fisher’s exact test; continuous demographic variables were compared with two-sample t tests. Adherence-related measures were skewed and so groups were compared using the Kruskal-Wallis rank analysis of variance, and Wilcoxon two-sample tests. Event percents were compared using chi-square, event rates were calculated and compared using Poisson regression. Logistic regression was used to compare odds of outcomes between adherence subgroups adjusting for age, sex, race, diabetes, kidney source, and early rejection. Individual regressions for adherence trend were performed by fitting a mixed-effects model with a random slope and intercept for each participant.
Statistical significance was assumed at P<0.05, but exact p values are presented. All analyses were performed using SAS release 9.1 (14) and graphics were made using R (15).
RESULTS
Participants and Nonparticipants
Study participants did not differ from eligible nonparticipants statistically significantly by age, race, sex, donor source, diabetic status (9), or length of follow-up. The occurrence of early and late acute rejection, and chronic rejection, was similar among participants and nonparticipants. Nonparticipants had increased mortality (P=0.045) and a higher late death rate (P=0.027), but actual death-censored graft loss rates were nearly identical to participants (P=0.827). Since the initial report (9), death rates doubled for both participants and nonparticipants but the ratio did not change. The additional 5 years of follow-up lowered standard errors, explaining the current statistical significance.
Natural History of Medication Adherence During the First 4 Years After Transplantation
Electronic monitoring of each participant ended after 4 years, by study design. Median length of the MEMS record was 3.8 years (range: 0.1–5.1 years). One hundred thirty-four participants provided adequate medication monitor data during the first 90-day interval after hospital discharge. Three additional patients, missing those early data for technical reasons, subsequently completed more than 12 months of monitored dosing, and are also included (Fig. 1). One hundred eleven participants had medication record data through month 12 after discharge, whereas 91 patients had at least 2 years of data and 69 completed 4 years of monitored dosing. Collectively, the monitor records captured 125,487 presumed Aza doses over 139,797 evaluable days, comprising 383 patient-years, with an overall average medication adherence rate of 90%.
Dose Monitor Data
By using their first 6-month monitor records, we divided the 137 participants into tertiles by initial mean adherence: those missing less than 1.5% of their Aza doses (group A; n=45); those missing 1.5% to less than 5% (group B; n=45); and those missing more than or equal to 5% (group C; n=47). The groups were demographically similar; however, the middle and poorest adherence groups had larger proportions of younger (P=0.047) and non-white participants (P=0.012).
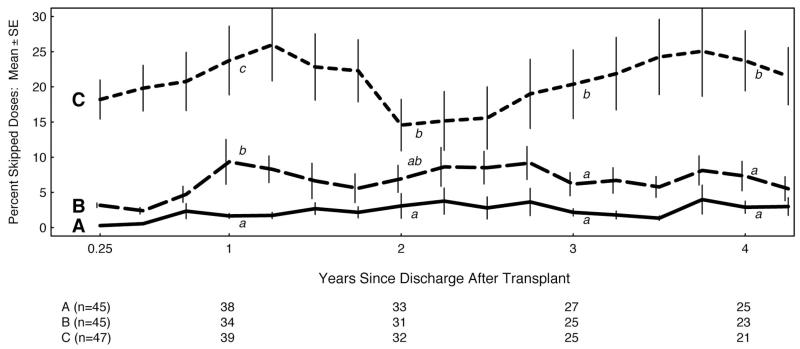
The groups showed considerable variation over time in the mean percentage of missed doses for each tertile at 90-day intervals, but maintained their relative ordering throughout the study (Fig. 2). In tertile C, the rate of missed doses remained statistically the highest, although all tertiles experienced a subtle increase in the mean number of missed doses.
FIGURE 2.
The mean percent (±SE) of skipped azathioprine doses quarterly for each initial adherence tertile. The three tertiles, labeled A, B, and C, were statistically compared at 1, 2, 3, and 4 years after transplant. For each annual comparison, curves with different lower case letters (a, b, or c) are statistically significantly different (P<.05), while those sharing a letter are not significantly different. The number of participants remaining in each tertile annually is shown in the table immediately below the graph.
By individual regression analyses, more than 70% of all participants had an increasing trend in skipped doses. Also comparing each participant’s rate of missed doses during their first 6-month record to their rate during their final 6-month record, 54% skipped more doses in their final 6-month record.
During the first 6 months, interdose intervals for the best and middle tertiles were close to the prescribed interval of 24 hr, whereas tertile C intervals (30.4±2.8 hr) were significantly longer (P=0.005). Mean interdose intervals in all tertiles increased by the end of the cap record. During the first 6 months, drug holidays were more frequent and longer in tertile C than in A or B, as expected because the tertiles were defined by adherence. However, during their final 6 months, each group exhibited longer and more frequent drug holidays (Table 1).
TABLE 1.
Initial-adherence tertile comparisons
| Skipped doses in months 1–6: (initial tertiles) |
||||
|---|---|---|---|---|
| A <1.5% (n=45) | B 1.5%–5% (n=45) | C ≥5% (n=47) | P | |
| Missed doses (%) | ||||
| Months 1–6 | 0 (0–1.3) a | 2.4 (1.6–5) b | 12 (4–84) c | <0.001 |
| Months 19–24 | 0.6 (0–42) a | 2.8 (0–53) b | 10 (0–99) c | <0.001 |
| Months 43–48 | *0.9 (0–27) a | *3.9 (0–41) b | 16(0–97)c | <0.001 |
| No. holidays ≥2 d | ||||
| Months 1–6 | 0(0–2)a | 1 (0-4)b | 4(0-37)c | <0.001 |
| Months 19–24 | 1 (0–25)A | *2 (0–25) b | 4(0–33)b | <0.001 |
| Months 43–48 | *1(0–5)a | *2 (0–28) b | 8(0–42)b | <0.001 |
| Maximum holiday (d) | ||||
| Months 1–6 | 0(0-3)a | 2(0–5)b | 4(0–34)c | <0.001 |
| Months 19–24 | *2 (0–10) a | 2(0–32)b | 4(0–175)c | <0.001 |
| Months 43–48 | 2(0-41)a | *2 (0–7) b | 3(0–119)b | 0.008 |
Values are medians (minimum–maximum). Sample sizes are given in Figure 2.
Between-group comparisons are within rows, with the overall P value given in the table. Significant differences between groups are indicated by lower case letters (a, b, or c): those with different letters are significantly different (P<0.05), whereas those sharing the same letter are not.
Significant within-group changes from the first 6 mo are indicated with an asterisk (*).
We previously identified a patient subgroup whose monthly dose counts declined during the first 3 months after discharge (9). These 23 patients skipped their medication on at least two more days in the second month compared with the first month after hospital discharge (“drop-2”). Their demographic characteristics differed from the larger group of “steady” adherers only by race. Membership in this drop-2 subgroup was the strongest adherence-based predictor of clinical outcome. Throughout the longer follow-up reported here, the drop-2 subgroup continued to have significantly higher rates of rejection, graft loss, and death (Table 2).
TABLE 2.
Outcomes by steady vs. declining (“drop-2”) adherence groups of 137 patients with adequate monitor data
| Outcomes | Declining (n=23) |
Steady (n=114) |
P |
|---|---|---|---|
| Rejection | |||
| Late acute rejectionsa | 8 | 24 | 0.156 |
| Late acute rejection rate | 9.6 (±2.5) | 2.7 (±0.5) | <0.001 |
| Chronic rejection | 8 | 23 | 0.127 |
| Chronic rejection rate | 5.1 (±1.8) | 2.1 (±0.4) | 0.034 |
| Graft loss | |||
| Graft loss before death | 11 | 21 | 0.002 |
| Graft loss before death rate | 7.0 (±2.1) | 2.0 (±0.4) | <0.001 |
| Death | |||
| Late death | 11 | 30 | 0.040 |
| Late death rate | 4.9 (±1.5) | 2.6 (±0.5) | 0.066 |
| Late death with graft function | 6 | 20 | 0.341 |
| Any event | |||
| No events before death | 11 | 77 | 0.072 |
| No events including death | 6 | 61 | 0.016 |
Late events occurred 90 d or more after discharge from hospital at transplantation, and are reported two ways: by number and as an event rate (± standard error) per 100 patient-years.
Declining adherence patients provided 20 of 47 (43%) subjects in tertile C. Although the group was demographically indistinguishable from the rest of tertile C, they had earlier and higher rates of acute rejection (P=0.008) and graft loss before death (P=0.009). On average, they also missed twice as many doses during both the first and last 6-month records and took twice as many drug holidays (data not shown).
Associations Between Nonadherence and Clinical Events
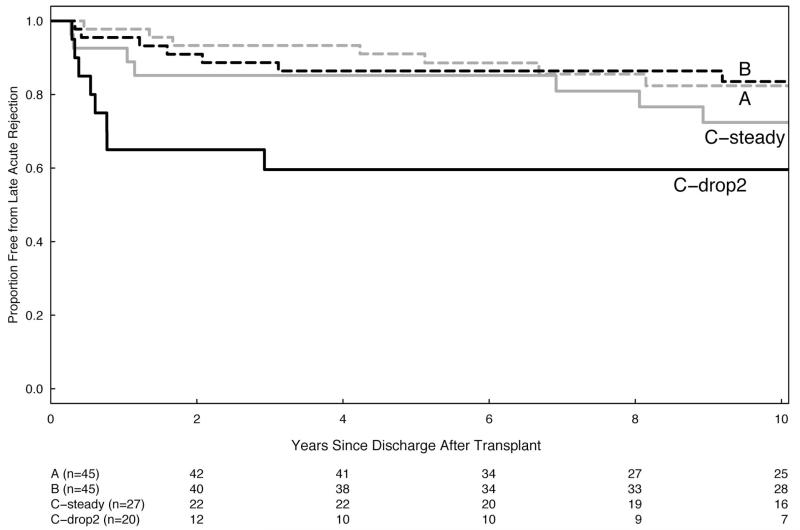
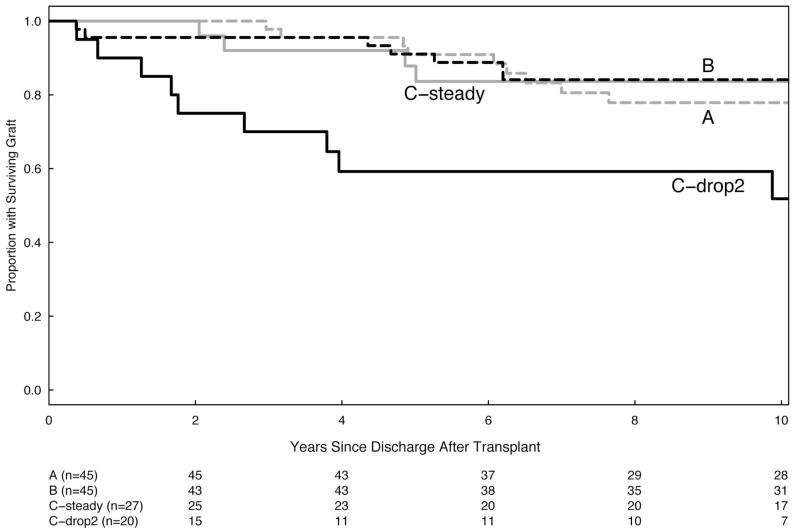
The patients with acute rejection, graft loss before death, or the experience of any adverse clinical outcome all increased with increasing nonadherence (Table 3). Initial adherence tertiles were associated with the rate of acute rejection episodes (P=0.025), but not with rates of chronic rejection, graft loss, or death, even after adjusting for age, sex, race, diabetes, kidney source, and early rejection. But time to the event was also important, so that during the first 5 years after transplant, rates for acute rejection and graft loss before death in tertiles A and B are significantly better than tertile C. However, after 5 years, the rates in all three groups are similar (Table 3; Fig. 3).
TABLE 3.
Outcomes by initial-adherence tertiles of 137 patients with adequate monitor data
| Skipped dosses in months 1–6: (initial tertile) |
||||
|---|---|---|---|---|
| Late outcomesa | A <1.5% (n=45) | B 1.5%–5% (n=45) | C >5% (n=47) | Overall P |
| Rejection | ||||
| Late acute rejection | 8 | 9 | 15 | 0.224 |
| Late acute rejection rate | 2.2 (±.7) a | 2.5 (±.8) a | 6.4 (±1.3) b | 0.025 |
| Late acute rejection rate (≤5 yr) | 1.9 (±1) a | 3.9 (±1.4) a | 10.8 (±2) b | 0.001 |
| Late acute rejection rate (>5 yr) | 2.4 (±1) | 1.3 (±.7) | 1.6 (±1) | 0.436 |
| Graft loss | ||||
| Late graft loss before death | 9 | 9 | 14 | 0.438 |
| Late graft loss before death rate | 2.2 (±0.7) | 2.1 (±0.7) | 3.7 (±1) | 0.357 |
| Late graft loss before death rate (≤5 yr) | 1.9 (±1) a | 2.0 (±1) a | 5.7 (±2) b | 0.036 |
| Late graft loss before death rate (>5 yr) | 2.4 (±1) | 2.1 (±1) | 1.6 (±1) | 0.811 |
| Any event | ||||
| No event before death | 32 | 28 | 28 | 0.484 |
| No event including death | 24 | 23 | 20 | 0.549 |
Between-group comparisons are within rows, with the overall P value given in the table. Significant differences between groups are indicated by lower case letters (a, b, or c): those with different letters are significantly different (P<0.05), whereas those sharing a letter are not significantly different.
Late events occurred 90 d or more after discharge from hospital at transplantation, and are reported and compared in two ways: number of affected patients; event rate (± standard error) per 100 patient-years during all followup before and after the 5th year post-transplant.
FIGURE 3.
(A and B) Kaplan-Meier plots of event-free survival beginning 90 days after posttransplant hospital discharge. Patients are grouped by their initial adherence tertiles. Tertile C (worst adherence) is further divided to separate the steady and the declining adherence patients. The number of participants remaining in each tertile is shown in the table immediately below the graph. (A) Late rejection-free graft survival is statistically different between groups (P=0.018, Wilcoxon test). (B) Late death-censored graft survival is also significantly different (P=0.003, Wilcoxon test).
Based on the adherence rate over their entire MEMS record, there were 32 participants with an overall missed dose rate less than 1.5% (the limit defined for initial-tertile A), and they experienced the fewest adverse outcomes (P=0.049). However, for the 46 patients with overall missed dose rates less than 5% (the limit for initial-tertile B), and 59 participants with overall missed dose rates more than 5%, adverse outcomes were not significantly associated with these groupings (data not shown).
Through March 2006, the average participant follow-up was 8.7 years (maximum 13 years). Thirty-two participants experienced a total of 44 late acute rejections (Table 2; Fig. 3), but 27 of these first acute rejections occurred after monitoring was discontinued. Only 8 of 32 grafts lost before death and none of the 41 deaths occurred during the affected participants’ monitored record. Further, 17 rejections, 23 graft losses before death, and 35 deaths all occurred more than a year after monitoring ended.
DISCUSSION
This prospective cohort study of renal transplant recipients presents a unique opportunity to examine the natural history and dynamics of medication adherence. These newer data extend our earlier description of Aza adherence after renal transplantation (9). More than half of the 137 patients completed 48 months of monitoring. These records confirm generally good medication adherence in our transplant population.
Newly identified in this report are three distinct trends in medication-taking behavior. The first pattern was the persistent difference between the initial tertiles, which were defined by their adherence rates during the first 6 months. These tertile groupings maintained their order and remained statistically distinct throughout 4 years of follow-up. The two better tertiles also showed an overall smaller mean rate decline than tertile C. The overall benefit of this consistent medication adherence was also clear. Those patients missing less than 1.5% of their Aza doses experienced the fewest acute rejections and had the best late allograft outcomes.
The second pattern was the early decline in medication adherence, detectable shortly after hospital discharge. We initially reported (9) significantly higher rates of acute rejection and graft loss in the subgroup that skipped their medication on at least two more days (“drop-2”) during the second versus the first posttransplant month. This pattern of early declining adherence remained the single strongest predictor of clinical outcomes. Patients with this pattern represent nearly half of the subjects in the poorest adherence tertile and experienced significantly higher rejection rates and death-censored graft losses than the remainder of tertile C. Remarkably, after the earlier occurrence of adverse events, this group then experienced striking clinical stability over the ensuing years (Fig. 3). Although speculative, this survival may reflect an emerging selection bias for patients with relatively high degree of “accommodation” to their graft.
The third pattern, confirming predictions in other studies (17-19), was the erosion of average adherence in each monitored tertile up to 4 years posttransplantation. While maintaining their overall rank order, the better two tertiles showed smaller but persistent reductions in mean adherence rates. Patient adherence in tertile C declined markedly, exhibiting longer interdose intervals and twice as frequent medication holidays (Table 1), resulting in substantially lower drug exposure over time.
Our results fit with the view that early nonadherence with immunosuppression is a critical factor increasing the risk of earlier adverse events. Initially acute rejection is more frequent in patients missing as few as 10 Aza doses during the first 6 months of study (9). Tertile C, with the worst early adherence, had significantly more acute rejections and death-censured graft losses during the first 5 years posttransplant. However, later, adverse events appeared even in initially adherent patient groups, leading to convergent event-free survival curves and similar rates of acute rejection and graft loss after 5 years.
Earlier cross-sectional studies (8, 10, 11) similarly found later events were associated with early medication adherence behaviors. Using electronic medication monitors, increased acute rejection rates and decreased event-free survival were demonstrated in minimally (“subclinical”) nonadherent cardiac transplant patients (10). Revisiting the same patient cohort after an additional 5 years of follow-up, these investigators also observed a convergence of the event-free survival curves because adverse events progressively occurred in their more adherent patient group (8). A similar study from Belgium (11) noted that renal transplant patients who, by interview, self-identified as nonadherent also experienced more frequent and earlier acute rejections. Once again on later follow-up, even the more adherent patients in this study demonstrated decreasing rejection-free survival.
Overall, it may be that patients who are at highest risk for acute rejection are “unmasked” by early nonadherence, whereas later declining adherence for any reason provokes late acute rejection in some initially adherent but “at-risk” patients. Instead of examining averaged values for rejection and graft loss in large patient groups, this dynamic monitoring permits grouping of patients by their individual behavior and then examines outcomes as a function of that quantitative medication adherence. Graft survival curves, and their half-life calculations (1), may simply represent a summation of curves similar to those in Figure 3(B). Hypothetically then, medication nonadherence may occupy a central role in explaining why recently reviewed graft survival slopes seem only modestly affected by era or drug regimen (20, 21).
There are several weaknesses in our study. First, although cited as the most sensitive measure of medication adherence (22, 23), the MEMS technology is indirect, only documenting the actual cap opening but not the ingestion of a prescribed medication dose. Second, in this study 17 of the 32 first late acute rejections occurred over 1 year after active medication monitoring ended, so it is not possible to define the role contemporaneous medication adherence may have played. With the gradual declines, we noted in adherence during the monitoring period, it is possible that later reductions in drug exposure might permit rejection in susceptible patients. It is also possible that after completing active dose monitoring, patients may become less consistent in their medication taking. Finally, we only monitored a single drug and the reliability of Aza adherence because a surrogate for other medication taking is unproven.
In conclusion, this prospective study documents the natural history and consequences of Aza adherence after renal transplantation. With up to 4 years of electronic monitoring, we identify broad patterns of medication nonadherence. There is an early segregation with roughly two thirds of patients missing remarkably few medication doses, whereas the remaining patients miss considerably more than 5% of their Aza doses. Within this latter group, we find a patient subgroup exhibiting a sharp, early decline in medication adherence and experiencing adverse outcomes. With later monitored followup, a slower but pervasive pattern of adherence erosion is also documented. Additionally, with longer follow-up, more patients experience adverse events including late acute rejection, graft loss, and death. The observation that critical nonadherence emerges early posttransplantation, accurately predicting later behavior and outcomes, should permit focused interventions on discrete patient subpopulations before adverse outcomes develop.
ACKNOWLEDGMENTS
The authors acknowledge the consistent support and assistance of Drs. Arthur Matas, Michael Mauer, and William Robiner. They also thank Linda Kruse, study coordinator, and Lois McHugh of the Transplant Database. Also the contributions of Qian An, Ying Fan, Dongfeng Qi, Melissa Skeans, Jia Xu, and Yanni Zhu in Biostatistics are gratefully appreciated.
This work was supported entirely by a grant from the National Institutes of Health; NIDDK, DK-013083.
Footnotes
The authors declare no conflict of interests.
REFERENCES
- 1.Hariharan S, Johnson CP, Bresnahan BA, et al. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Gaston RS, Gourishankar S, et al. Long-term deterioration of kidney allograft function. Am J Transplant. 2005;5:1405. doi: 10.1111/j.1600-6143.2005.00853.x. [DOI] [PubMed] [Google Scholar]
- 3.Vincenti F. A decade of progress in kidney transplantation. Transplantation. 2004;77:S52. doi: 10.1097/01.tp.0000126928.15055.dc. [DOI] [PubMed] [Google Scholar]
- 4.Butler JA, Roderick P, Mullee M, et al. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: A systematic review. Transplantation. 2004;77:769. doi: 10.1097/01.tp.0000110408.83054.88. [DOI] [PubMed] [Google Scholar]
- 5.Nevins TE, Matas AJ. Medication noncompliance: Another iceberg’s tip. Transplantation. 2004;77:776. doi: 10.1097/01.tp.0000110409.71847.6f. [DOI] [PubMed] [Google Scholar]
- 6.Denhaerynck K, Dobbels F, Cleemput I, et al. Prevalence, consequences, and determinants of nonadherence in adult renal transplant patients: A literature review. Transpl Int. 2005;18:1121. doi: 10.1111/j.1432-2277.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 7.Dew MA, DiMartini AF, De Vito Dabbs A, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83:858. doi: 10.1097/01.tp.0000258599.65257.a6. [DOI] [PubMed] [Google Scholar]
- 8.Dobbels F, De Geest S, van Cleemput J, et al. Effect of late medication non-compliance on outcome after heart transplantation: A 5-year follow-up. J Heart Lung Transplant. 2004;23:1245. doi: 10.1016/j.healun.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Nevins TE, Kruse L, Skeans MA, et al. The natural history of azathioprine compliance after renal transplantation. Kidney Int. 2001;60:1565. doi: 10.1046/j.1523-1755.2001.00961.x. [DOI] [PubMed] [Google Scholar]
- 10.De Geest S, Abraham I, Moons P, et al. Late acute rejection and subclinical noncompliance with cyclosporine therapy in heart transplant recipients. J Heart Lung Transplant. 1998;17:854. [PubMed] [Google Scholar]
- 11.Vlaminck H, Maes B, Evers G, et al. Prospective study on late consequences of subclinical non-compliance with immunosuppressive therapy in renal transplant patients. Am J Transplant. 2004;4:1509. doi: 10.1111/j.1600-6143.2004.00537.x. [DOI] [PubMed] [Google Scholar]
- 12.Denhaerynck K, Steiger J, Bock A, et al. Prevalence and risk factors of non-adherence with immunosuppressive medication in kidney transplant patients. Am J Transplant. 2007;7:108. doi: 10.1111/j.1600-6143.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 13.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 14.SAS version 9.1, copyright 2003. SAS Institute; Cary NC: [Google Scholar]
- 15. [Accessed 26 March 2008];R: A language and environment for statistical computing, R Foundation for Statistical Computing. Available at: http://www.r-project.org/
- 16.Vrijens B, Goetghebeur E. Electronic monitoring of variation in drug intakes can reduce bias and improve precision in pharmacokinetic/pharmacodynamic population studies. Stat Med. 2004;23:531. doi: 10.1002/sim.1619. [DOI] [PubMed] [Google Scholar]
- 17.Laederach-Hofmann K, Bunzel B. Noncompliance in organ transplant recipients: A literature review. Gen Hosp Psychiatry. 2000;22:412. doi: 10.1016/s0163-8343(00)00098-0. [DOI] [PubMed] [Google Scholar]
- 18.Dew MA, Roth LH, Thompson ME, et al. Medical compliance and its predictors in the first year after heart transplantation. J Heart Lung Transplant. 1996;15:631. [PubMed] [Google Scholar]
- 19.Loghman-Adham M. Medication noncompliance in patients with chronic disease: Issues in dialysis and renal transplantation. Am J Manag Care. 2003;9:155. [PubMed] [Google Scholar]
- 20.Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4:1289. doi: 10.1111/j.1600-6143.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 21.Hwang AH, Cho YW, Cicciarelli J, et al. Risk factors for short- and long-term survival of primary cadaveric renal allografts in pediatric recipients: A UNOS analysis. Transplantation. 2005;80:466. doi: 10.1097/01.tp.0000168090.19875.b0. [DOI] [PubMed] [Google Scholar]
- 22.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 23.Farley J, Hines S, Musk A, et al. Assessment of adherence to antiviral therapy in HIV-infected children using the medication event monitoring system, pharmacy refill, provider assessment, caregiver self-report, and appointment keeping. J Acquir Immune Defic Syndr. 2003;33:211. doi: 10.1097/00126334-200306010-00016. [DOI] [PubMed] [Google Scholar]