Abstract
Introduction. Soft tissue sarcomas (STSs) represent 1 percent of all adult malignancies and sarcomas only rarely spread to the regional lymph nodes. Case Presentation. We present a case of a woman with a dermatofibrosarcoma protuberans and a sarcoma not therwise specified of the lower extremity. The patient had no distant metastasis during follow-up, but did develop a regional lymph nodemetastasis (RLNM) in the groin. We reviewed the literature about RLNM in STSs. Discussion. Reviewing the literature we see that within specific histological types RLNM occurs as often as distant metastasis. Furthermore RLNM occurs in over 10% for specific histological types and in 24% of all patients with a soft tissue sarcoma of the lower extremity. Except for radical lymphadenectomy with a 5-year survival rate of 46% there is no appropriate treatment. Conclusion. The risk for a RLNM in certain histological types and anatomical locations might transcend the risk for a distant lung metastasis.
1. Introduction
Soft tissue sarcomas (STSs) represent 1 percent of all adult malignancies and up to 6 percent of all childhood cancers [1–4]. In 2010, there were 10.520 cases of soft tissue sarcomas in the United States and 3920 patients died from this disease [5]. For dermatofibrosarcoma protuberans (DFSP) the annual incidence in the United States between 1973 and 2002 was 4.2 per 1.000.000 [6]. In the Netherlands the annual incidence of STS was 34 per 1.000.000 for men and 28 per 1.000.000 for women in 1997 [3].
The pattern of metastatic spread is usually haematogenous; lymphatic spread is very rare [7, 8]. We present a case of a patient with a inguinal lymph node metastasis 4 years after resection of a STS. A review of the literature will be described.
2. Case Presentation
A 68-year-old Caucasian woman with hypertension and diabetes type 2 was treated in 2001 because of a mass of unknown histological origin of the right upper leg. She underwent a local resection of the tumor and pathological examination showed a DFSP. It was found to be a DFSP in histological research due to proliferation of atypical fusiform mesenchymal cells, with multinuclear giant cells which were sometimes arranged in rosettes (Figure 1). Moreover, the immunoprofile showed mainly positivity with vimentin (++), desmin (+), and CD-68 (+) which would mostly fit the diagnosis of a DFSP, a malignant fibrous histiocytoma was in the differential diagnosis. Due to tight surgical margins, a re-resection found place with margins of more than 2 centimetres.
Figure 1.
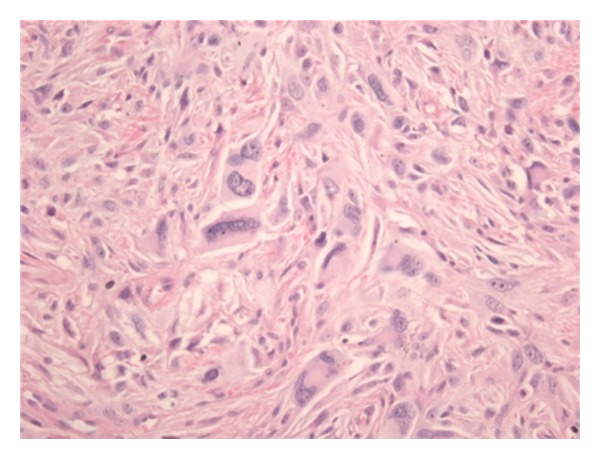
Microscopic view (400x) of the tumor of the right upper leg. The histopathological pattern, immunoprofile, and localization of this subcutaneous tumor favor the diagnosis of DFSP.
During followup a new mass was discovered at the site of the scar 6 years later. Local surgical excision was performed and pathological examination showed a high grade sarcoma not otherwise specified (NOS), grade III. Histological research showed proliferation of a more cellular tumor process with more and bizarre mitotic figures (Figure 2). The tumor cells showed, compared to the primary tumor in 2001, less differentiation. The diagnosis of a NOS sarcoma was also based on the immunoprofile which was only positive for vimentin and the previously positive markers were considered negative (CD-68, desmin). After surgical excision the tumor was treated with radiotherapy. Ultrasound of the right groin showed no regional lymph node metastasis (RLNM) and there were no abnormalities seen at the chest X-ray.
Figure 2.
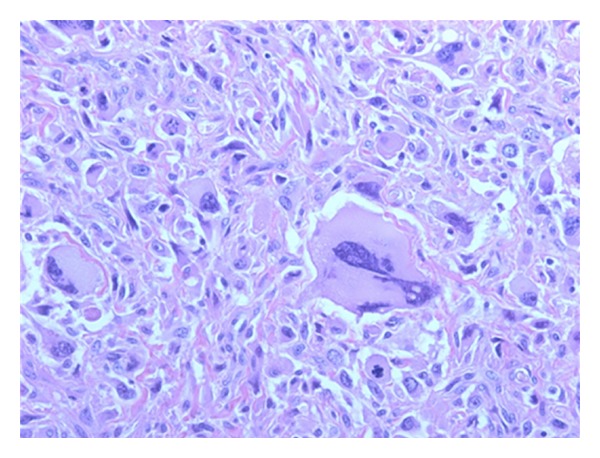
Microscopic view (400x) of the second tumor of the right upper leg. Based on the morphology, clinical history, and immunoprofile it was diagnosed to be a localization of a high grade sarcoma NOS.
During further follow-up a groin mass was found 4 years later. CT examination revealed a large lymph node (Figure 3) without any other evidence of distant disease. An ultrasound-guided biopsy was performed and showed a metastases of the sarcoma. A superficial regional lymph node dissection was performed which showed one positive lymph node out of nine dissected and Cloquet's node negative. Pathological-anatomical study showed a metastasis of a high grade sarcoma NOS (Figure 4). Until now, further followup with chest X-ray and CT scan showed no abnormalities.
Figure 3.
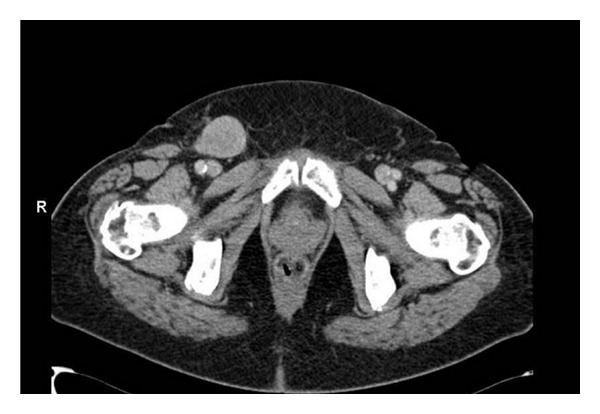
A 68-year-old Caucasian woman with a high grade sarcoma NOS presenting with a RLNM.
Figure 4.
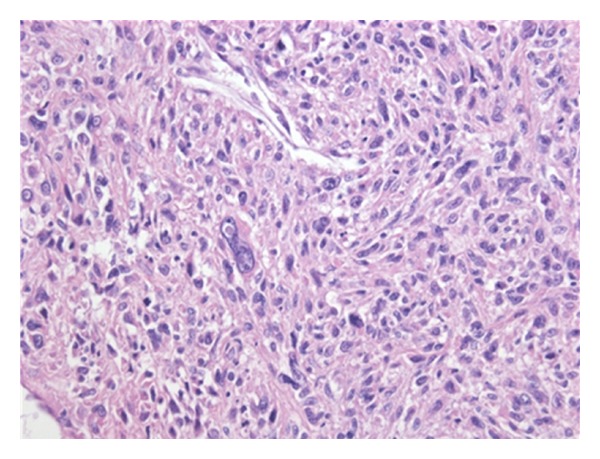
Microscopic view (400x) of the tumor located in a lymph node, diagnosed to be a localization of a high grade sarcoma NOS. It developed as a result of the differentiation of the primary tumor 10 years ago. This was concluded based on the morphology, clinical history, and immunoprofile (vimentin positive) of this tumor.
3. Discussion
3.1. Metastatic Pattern
In STS, distant metastases are relatively common, occurring in approximately 10% of patients. In patients with a sarcoma of the extremity, metastases will develop in up to 25% of patients [13, 14]. In contrary only 1% of patients with DFSP have distant metastases [6, 15]. Distant metastases occur especially in patients having had large, high grade tumors that are deep located to or nearby the fascia. Up to 83 percent of the distant metastases in STS occur in the lung [16, 17].
3.2. Lymph Node Metastases
Unlike distant metastases, regional lymph node metastases (RLNMs) in soft tissue sarcoma are rare. STS progress to regional lymph node metastases in 1.75%–5.9% [9–12]. Furthermore lymphatic spread is more frequently associated with certain histologic types, such as rhabdomyosarcoma, epitheloid sarcoma, clear cell sarcoma, or vascular sarcoma. Regional lymph node involvement is seen in over 10% of all patients [9–12]. Dermatofibrosarcoma protuberans and NOS-type sarcomas rarely spread to the regional lymph nodes (2%) (Table 1). Moreover RLNMs occur more often in patients having sarcomas of the lower extremities. The incidence of a RLNM in patients with a sarcoma of the lower extremity is reported up to 24% (Table 2) [9–12].
Table 1.
Four studies on the number of patients with a STS who develop RLNM.
| Type of tumor | Number of patients | Number of RLNMs | Percentage |
|---|---|---|---|
| Synovial sarcoma | |||
| Mazeron and Suit [9] | 15 | 0 | 0% |
| Review Mazeron and Suit [9] | 851 | 117 | 14% |
| Fong et al. [10] | 145 | 2 | 1% |
| Daigeler et al. [11] | 111 | 4 | 4% |
| Behranwala et al. [12] | 171 | 7 | 4% |
|
| |||
| Total | 1293 | 130 | 10% |
|
| |||
| Fibrosarcoma | |||
| Mazeron and Suit [9] | 45 | 0 | 0% |
| Review Mazeron and Suit [9] | 215 | 54 | 25% |
| Fong et al. [10] | 162 | 0 | 0% |
| Daigeler et al. [11] | 45 | 1 | 2% |
| Behranwala et al. [12] | 132 | 1 | 1% |
|
| |||
| Total | 599 | 56 | 9% |
|
| |||
| Malignant fibrohistiocytoma | |||
| Mazeron and Suit [9] | 48 | 1 | 2% |
| Review Mazeron and Suit [9] | 823 | 84 | 10% |
| Fong et al. [10] | 316 | 8 | 3% |
| Behranwala et al. [12] | 235 | 3 | 1% |
|
| |||
| Total | 1422 | 96 | 7% |
|
| |||
| Neurofibrosarcoma | |||
| Mazeron and Suit [9] | 20 | 1 | 5% |
| Review Mazeron and Suit [9] | 476 | 3 | 1% |
| Fong et al. [10] | 96 | 2 | 2% |
| Daigeler et al. [11] | 94 | 3 | 3% |
| Behranwala et al. [12] | 95 | 4 | 4% |
|
| |||
| Total | 781 | 13 | 2% |
|
| |||
| Liposarcoma | |||
| Mazeron and Suit [9] | 55 | 2 | 4% |
| Review Mazeron and Suit [9] | 504 | 16 | 3% |
| Fong et al. [10] | 403 | 3 | 1% |
| Daigeler et al. [11] | 333 | 1 | 0% |
| Behranwala et al. [12] | 340 | 3 | 1% |
|
| |||
| Total | 1635 | 25 | 2% |
|
| |||
| Rhabdomyosarcoma | |||
| Mazeron and Suit [9] | 15 | 5 | 33% |
| Review Mazeron and Suit [9] | 1354 | 201 | 15% |
| Fong et al. [10] | 123 | 13 | 11% |
| Daigeler et al. [11] | 50 | 3 | 6% |
| Behranwala et al. [12] | 54 | 12 | 22% |
|
| |||
| Total | 1596 | 234 | 15% |
|
| |||
| Leiomyosarcoma | |||
| Mazeron and Suit [9] | 30 | 1 | 3% |
| Review Mazeron and Suit [9] | 524 | 21 | 4% |
| Fong et al. [10] | 328 | 9 | 3% |
| Daigeler et al. [11] | 167 | 1 | 1% |
| Behranwala et al. [12] | 483 | 13 | 3% |
|
| |||
| Total | 1532 | 45 | 3% |
|
| |||
| Vascular sarcoma | |||
| Mazeron and Suit [9] | 14 | 2 | 14% |
| Fong et al. [10] | 37 | 5 | 14% |
| Daigeler et al. [11] | 38 | 3 | 8% |
| Behranwala et al. [12] | 46 | 5 | 11% |
|
| |||
| Total | 135 | 15 | 11% |
|
| |||
| Epithelioid sarcoma | |||
| Mazeron and Suit [9] | 7 | 5 | 71% |
| Review Mazeron and Suit [9] | 70 | 14 | 20% |
| Fong et al. [10] | 12 | 2 | 17% |
| Daigeler et al. [11] | 28 | 6 | 21% |
| Behranwala et al. [12] | 27 | 5 | 19% |
|
| |||
| Total | 144 | 32 | 22% |
|
| |||
| Clear cell | |||
| Review Mazeron and Suit [9] | 40 | 11 | 28% |
| Daigeler et al. [11] | 14 | 3 | 21% |
| Behranwala et al. [12] | 25 | 1 | 4% |
|
| |||
| Total | 79 | 15 | 19% |
|
| |||
| NOS | |||
| Mazeron and Suit [9] | 42 | 2 | 5% |
| Fong et al. [10] | 27 | 0 | 0% |
| Daigeler et al. [11] | 268 | 2 | 1% |
| Behranwala et al. [12] | 10 | 1 | 10% |
|
| |||
| Total | 347 | 5 | 1% |
|
| |||
| Dermatofibrosarcoma Protuberans | |||
| Daigeler et al. [11] | 48 | 1 | 2% |
| Behranwala et al. [12] | 43 | 1 | 2% |
|
| |||
| Total | 91 | 2 | 2% |
Table 2.
Three studies on the number of patients with a STS who developed RLNM in various anatomical locations.
| Anatomical location | Number of patients |
Number of RLNMs |
Percentage |
|---|---|---|---|
| Head and Neck | |||
| Mazeron and Suit [9] | 20 | 3 | 15% |
| Fong et al. [10] | 45 | 5 | 11% |
| Behranwala et al. [12] | 75 | 6 | 8% |
|
| |||
| Total | 140 | 14 | 10% |
|
| |||
| Upper extremity | |||
| Mazeron and Suit [9] | 42 | 6 | 14% |
| Fong et al. [10] | 47 | 7 | 15% |
| Behranwala et al. [12] | 70 | 7 | 10% |
|
| |||
| Total | 159 | 20 | 13% |
|
| |||
| Lower extremity | |||
| Mazeron and Suit [9] | 122 | 6 | 5% |
| Fong et al. [10] | 46 | 19 | 41% |
| Behranwala et al. [12] | 73 | 33 | 45% |
|
| |||
| Total | 241 | 58 | 24% |
|
| |||
| Trunk | |||
| Mazeron and Suit [9] | 102 | 4 | 4% |
| Fong et al. [10] | 500 | 5 | 1% |
| Behranwala et al. [12] | 73 | 11 | 15% |
|
| |||
| Total | 675 | 20 | 3% |
|
| |||
| Abdominal and Thoracic Viscera | |||
| Mazeron and Suit [9] | 23 | 0 | 0% |
| Fong et al. [10] | 43 | 3 | 7% |
| Behranwala et al. [12] | 73 | 11 | 15% |
|
| |||
| Total | 139 | 14 | 10% |
There is no research done in survival rates for patients with DFSP or NOS-type sarcomas and RLNMs. The median overall survival of patients with RLNM was 12.7 months, ranging from 0 to 40.7 months [9–12]. The 1-, 5-, and 10-year overall survival rates are 81.5%, 33.3%, and 20%. After the diagnosis of a regional lymph node the 1- and 5-year survival rates dropped to 55.5% and 12.8% [9–12]. The average time to develop RLNM is 27 months ranging from 1 month to 16 years after primary surgical resection [9–12]. For DFSP in general the 5-year survival rate was up to 99% [6, 15].
3.3. Therapy
To our knowledge there is no evidence of the single use of radiotherapy or chemotherapy in the treatment of STS with RLNM, nor is there any evidence for the specific treatment of primary NOS-type STS. For DFSP radiation therapy is only used in extremely large and recurrent tumors, and there is currently only a little role for chemotherapy [18].
In general for the treatment of a primary STS, radiation therapy combined with surgery is recommended only for intermediate and high grade malignancies [19, 20]. For low grade malignancies reexcision alone is favored over radiotherapy, and radiation therapy is not recommended with negative margins after surgery [19, 20]. The optimal timing of adjuvant radiation therapy in primary STS is not clear. In a prospective study randomizing for pre- or postoperative radiotherapy (RT) among 190 patients, there was a higher rate of acute wound complications with preoperative RT. Moreover there was a higher amount of patients with late complications, such as edema or fibrosis, for postoperative RT. Nevertheless there was no difference in survival rate between the two types of radiotherapy [21].
In general chemotherapy as part of the treatment of a primary STS in adults has not demonstrated an overall survival advantage [22, 23], nor is adjuvant chemotherapy considered to be a standard practice, in some studies even showing a worse 5-year survival rate [24, 25]. Furthermore, neo-adjuvant chemotherapy failed to show any benefit, awaiting the results of larger randomized trials [26, 27]. In contrast, hyperthermia has proven its efficacy in combination with neoadjuvant chemotherapy. In high-risk patients (>5 cm tumor size, grade 2 or 3, and/or deep to the fascia) it increases the benefit of chemotherapy alone [28–30].
The role of a sentinel lymph node biopsy(SLNB) for staging of patients with primary STS is unknown. In many cases, despite radical lymphadenectomy, patients with positive lymph node found with a SLNB procedure developed distant metastases; also a serious number of patients with positive lymph nodes remained disease-free. A multicenter trial would be necessary to determine the efficacy of SLNB [31–36].
At last we know that radical lymphadenectomy in case of RLNM remains the appropriate treatment so far [9]. Patients not treated with a radical lymphadenectomy had a shorter median survival than patients without a radical lymphadenectomy. Patients without appropriate treatment had a 5-year survival rate of 0% (median survival of 4.3 months) versus 46% (median survival of 16.3 months) after a radical excision of the regional lymph nodes [9].
4. Conclusion
To the best of our knowledge our case was the first description of a case of a DFSP followed by a high grade sarcoma NOS presenting with a RLNM. If we review the literature, there was a minor a priori chance of a RLNM in both types of STS. For DFSP the risk for a regional lymph node metastasis is as big as the risk for a distant metastasis; this is unknown for NOS-type soft tissue sarcoma. Nevertheless the standard of care in the Netherlands does not include a regular analysis of the regular nodes, but does include an X-ray for lung metastasis.
Furthermore, we should urge caution in specific histological types and also in certain anatomical locations as the risk for an RLNM in rhabdomyosarcoma, epitheloid sarcoma, clear cell sarcoma, or a vascular sarcoma and also in STS of the lower extremities might transcend the risk for a distant lung metastasis.
According to the literature the best practice for a RLNM is radical surgery. There is yet no evidence on the therapy of RNLM with radiotherapy or chemotherapy but in primary tumors radiotherapy is proven effective.
Consent
Informed consent was obtained from the patient for publication of this case report and any accompanying images.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- 1.Coebergh JWW, van Dijck JAAM, Janssen-Heijnen MLG, Visser O. Childhood Cancer in the Netherlands, 1989–1997. Utrecht, The Netherlands: Dutch Association of Cancer Registries; 2000. [Google Scholar]
- 2.Kaatsch P. Epidemiology of childhood cancer. Cancer Treatment Reviews. 2010;36(4):277–285. doi: 10.1016/j.ctrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Kankercentra VVI. Incidence of Cancer in the Netherlands 1997. Ninth Report of the Netherlands Cancer Registry; 1997. [Google Scholar]
- 4.Nijhuis PHA, Schaapveld M, Otter R, Molenaar WM, Van Der Graaf WTA, Hoekstra HJ. Epidemiological aspects of soft tissue sarcomas (STS)-consequences for the design of clinical STS trials. European Journal of Cancer. 1999;35(12):1705–1710. doi: 10.1016/s0959-8049(99)00152-5. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer Journal for Clinicians. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 6.Criscione VD, Weinstock MA. Descriptive epidemiology of dermatofibrosarcoma protuberans in the United States, 1973 to 2002. Journal of the American Academy of Dermatology. 2007;56(6):968–973. doi: 10.1016/j.jaad.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Huth JF, Eilber FR. Patterns of metastatic spread following resection of extremity soft-tissue sarcomas and strategies for treatment. Seminars in Surgical Oncology. 1988;4(1):20–26. doi: 10.1002/ssu.2980040106. [DOI] [PubMed] [Google Scholar]
- 8.Trovik CS, Bauer HCF, Alvegård TA, et al. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. European Journal of Cancer. 2000;36(6):710–716. doi: 10.1016/s0959-8049(99)00287-7. [DOI] [PubMed] [Google Scholar]
- 9.Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60(8):1800–1808. doi: 10.1002/1097-0142(19871015)60:8<1800::aid-cncr2820600822>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults: analysis of data from a prospective database of 1772 sarcoma patients. Annals of Surgery. 1993;217(1):72–78. doi: 10.1097/00000658-199301000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daigeler A, Kuhnen C, Moritz R, et al. Lymph node metastases in soft tissue sarcomas-a single center analysis of 1,597 patients. Langenbeck’s Archives of Surgery. 2009;394(2):321–329. doi: 10.1007/s00423-008-0371-x. [DOI] [PubMed] [Google Scholar]
- 12.Behranwala KA, A’Hern R, Omar AM, Thomas JM. Prognosis of lymph node metastasis in soft tissue sarcoma. Annals of Surgical Oncology. 2004;11(7):714–719. doi: 10.1245/ASO.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of disease and postmetastasis survival. Annals of Surgery. 1999;229(5):602–612. doi: 10.1097/00000658-199905000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisters PWT, Leung DHY, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. Journal of Clinical Oncology. 1996;14(5):1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 15.Bowne WB, Antonescu CR, Leung DHY, et al. Dermatofibrosarcoma protuberans: a clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000;88(12):2711–2720. [PubMed] [Google Scholar]
- 16.Al-Refaie WB, Andtbacka RHI, Ensor J, et al. Lymphadenectomy for isolated lymph node metastasis from extremity soft-tissue sarcomas. Cancer. 2008;112(8):1821–1826. doi: 10.1002/cncr.23363. [DOI] [PubMed] [Google Scholar]
- 17.Christie-Large M, James SLJ, Tiessen L, Davies AM, Grimer RJ. Imaging strategy for detecting lung metastases at presentation in patients with soft tissue sarcomas. European Journal of Cancer. 2008;44(13):1841–1845. doi: 10.1016/j.ejca.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Bogucki B, Neuhaus I, Hurst EA. Dermatofibrosarcoma protuberans: a review of the literature. doi: 10.1111/j.1524-4725.2011.02292.x. Dermatologic Surgery. In press. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities. Prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Annals of Surgery. 1982;196(3):305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheplan LJ, Juliano JJ. Use of radiation therapy for patients with soft-tissue and bone sarcomas. Cleveland Clinic Journal of Medicine. 2010;77(supplement 1):S27–S29. doi: 10.3949/ccjm.77.s1.06. [DOI] [PubMed] [Google Scholar]
- 21.O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. The Lancet. 2002;359(9325):2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 22.Bramwell VH, Anderson D, Charette ML. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database of Systematic Reviews. 2003;(3) doi: 10.1002/14651858.CD003293.CD003293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wesolowski R, Budd GT. Use of chemotherapy for patients with bone and soft-tissue sarcomas. Cleveland Clinic Journal of Medicine. 2010;77(supplement 1):S23–S26. doi: 10.3949/ccjm.77.s1.05. [DOI] [PubMed] [Google Scholar]
- 24.Casali PG, Picci P. Adjuvant chemotherapy for soft tissue sarcoma. Current Opinion in Oncology. 2005;17(4):361–365. doi: 10.1097/01.cco.0000166652.15546.4f. [DOI] [PubMed] [Google Scholar]
- 25.Sarcoma Meta-Analysis Collaboration. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. The Lancet. 1997;350(9092):1647–1654. [PubMed] [Google Scholar]
- 26.Gortzak E, Azzarelli A, Buesa J, et al. A randomised phase II study on neo-adjuvant chemotherapy for 'high-risk' adult soft-tissue sarcoma. European Journal of Cancer. 2001;37(9):1096–1103. doi: 10.1016/s0959-8049(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 27.Grobmyer SR, Maki RG, Demetri GD, et al. Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Annals of Oncology. 2004;15(11):1667–1672. doi: 10.1093/annonc/mdh431. [DOI] [PubMed] [Google Scholar]
- 28.Issels RD, Abdel-Rahman S, Wendtner CM, et al. Neoadjuvant chemotherapy combined with regional hyperthermia (RHT) for locally advanced primary or recurrent high-risk adult soft-tissue sarcomas (STS) of adults: long-term results of a phase II study. European Journal of Cancer. 2001;37(13):1599–1608. doi: 10.1016/s0959-8049(01)00183-6. [DOI] [PubMed] [Google Scholar]
- 29.Issels RD, Lindner LH, Verweij J, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. The Lancet Oncology. 2010;11(6):561–570. doi: 10.1016/S1470-2045(10)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wendtner CM, Abdel-Rahman S, Krych M, et al. Response to neoadjuvant chemotherapy combined with regional hyperthermia predicts long-term survival for adult patients with retroperitoneal and visceral high-risk soft tissue sarcomas. Journal of Clinical Oncology. 2002;20(14):3156–3164. doi: 10.1200/JCO.2002.07.146. [DOI] [PubMed] [Google Scholar]
- 31.Andreou D, Tunn PU. Sentinel node biopsy in soft tissue sarcome. Recent Results in Cancer Research. 2009;179:25–36. doi: 10.1007/978-3-540-77960-5_3. [DOI] [PubMed] [Google Scholar]
- 32.Baratti D, Pennacchioli E, Casali PG, et al. Epithelioid sarcoma: prognostic factors and survival in a series of patients treated at a single institution. Annals of Surgical Oncology. 2007;14(12):3542–3551. doi: 10.1245/s10434-007-9628-9. [DOI] [PubMed] [Google Scholar]
- 33.Blazer DG, III, Sabel MS, Sondak VK. Is there a role for sentinel lymph node biopsy in the management of sarcoma? Surgical Oncology. 2003;12(3):201–206. doi: 10.1016/s0960-7404(03)00030-6. [DOI] [PubMed] [Google Scholar]
- 34.Maduekwe UN, Hornicek FJ, Springfield DS, et al. Role of sentinel lymph node biopsy in the staging of synovial, epithelioid, and clear cell sarcomas. Annals of Surgical Oncology. 2009;16(5):1356–1363. doi: 10.1245/s10434-009-0393-9. [DOI] [PubMed] [Google Scholar]
- 35.Tunn PU, Andreou D, Illing H, Fleige B, Dresel S, Schlag PM. Sentinel node biopsy in synovial sarcoma. European Journal of Surgical Oncology. 2008;34(6):704–707. doi: 10.1016/j.ejso.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 36.van Akkooi ACJ, Verhoef C, van Geel AN, Kliffen M, Eggermont AMM, de Wilt JHW. Sentinel node biopsy for clear cell sarcoma. European Journal of Surgical Oncology. 2006;32(9):996–999. doi: 10.1016/j.ejso.2006.03.044. [DOI] [PubMed] [Google Scholar]


