Abstract
Viral infections cause an immunological disequilibrium that provokes CD8 T cell responses. These cells play critical roles in purging acute infections, limiting persistent infections, and conferring life-long protective immunity. At every stage of the response anti-viral CD8 T cells are sensitive to signals from cytokines. Initially cytokines operate as immunological warning signs that inform of the presence of an infection, and also influence the developmental choices of the responding cells. Later during the course of the response other sets of cytokines support the survival and maintenance of the differentiated anti-viral CD8 T cells. Although many cytokines promote virus-specific CD8 T cells, other cytokines can suppress their activities and thus favor viral persistence. In this review we discuss how select cytokines act to regulate anti-viral CD8 T cells throughout the response and influence the outcome of viral infections.
Keywords: Cytokines, T cells, Effector cells, Immunological memory
Introduction
A defining property of the immune system is its exquisite ability to discriminate between self and foreign antigens. This power enables the host to sense and respond to viral infections and usually results in rapid mobilization of immune defense mechanisms. The elaboration of anti-viral CD8 T cell responses following infection is a cornerstone of the adaptive immune response and they are vital for controlling acute infections, containing persistent infections, and conferring long-lived protection to viral re-exposures.
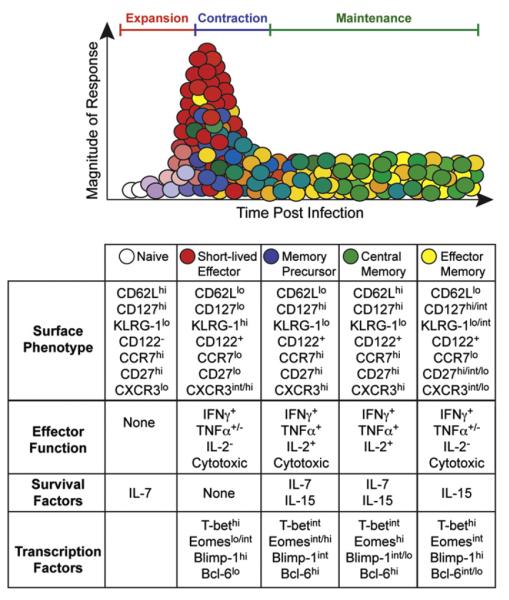
The progression of the anti-viral CD8 T cell response can be divided into distinct developmental stages defined by characteristic phenotypic and functional properties (Fig. 1) (Cox and Zajac, 2010; Lanzavecchia and Sallusto, 2005; Zhang and Bevan, 2011). Throughout the response the CD8 T cells receive instructions from the cytokine milieu, which changes in composition in reaction to viral infections (Cox et al., 2011). The cytokine network acts in conjunction with antigenic signals to amplify the clonal expansion of the naïve T cells that are triggered to respond, and cytokines also function as differentiation and survival factors which influence the developmental choices of the anti-viral CD8 T cells. This includes favoring the emergence of short-lived terminally differentiated effector cells, which are highly functional, and migrate from secondary lymphoid organs to other sites that may harbor the infection. Cytokine levels can also promote the emergence of memory precursor cells, which are competent effector cells but preferentially mature into long-lived memory T cells (Kaech and Wherry, 2007). Other cytokines act as party-poopers and suppress CD8 T cell responses, limiting their ability to cause immunopathology but possibly favoring viral persistence. During later stages of the response cytokines are also crucial for maintaining memory T cell populations, which form if the initial infection is cleared, and also influence inferior, exhausted, T cell responses which arise if the virus is not well controlled (Frebel et al., 2010; Wherry, 2011; Yi et al., 2010b). In addition, certain cytokines can stimulate effector and memory CD8 T cells independently of antigenic activation and thus allow these populations to mount some level of response to a broader range of pathogens (Berg and Forman, 2006).
Fig. 1.
The development of phenotypically and functionally distinct subsets of anti-viral CD8 T cells over time during acute viral infections. As the infection becomes established the small number of available naïve virus-specific CD8 T cells are activated and modify their expression of adhesion molecules, cytokine receptors, and transcription factors. These activated CD8 T cells undergo clonal expansion and differentiate into expanded populations of fully-fledged but short-lived effector T cells and this often overwhelming response operates to purge the virus infection. Distinct populations of effector CD8 T cells also develop which attain a memory precursor phenotype. As the infection is cleared the overall T cell response contracts and the short-lived effector cells are disposed by apoptosis, whereas the memory precursor cells are preferentially maintained and mature into the long-lived anti-viral memory T cell pool. Further phenotypic and functional diversity is observed within the memory T cell population and shifts in these attributes can occur over time.
Defining the cytokines that control CD8 T cell activities, and how they imprint the phenotypic and functional attributes of these cells, provides fundamental information about how this component of antiviral immunity is regulated. Understanding the stage dependent effects of specific cytokines on CD8 T cells, and how these signals integrate with other factors to dictate the outcome of the response, provides rationale for tactically manipulating cytokine levels to improve viral control and immunity. Cytokines are attractive therapeutic candidates as administering these molecules, neutralizing their activities, blocking or stimulating their receptors, or targeting their cellular sources, can alter their availability which in turn can modulate their biological actions. In this article we discuss the impact of select cytokines on the induction, differentiation, maintenance, and function of anti-viral CD8 T cells, and how this potentially determines the outcome of viral infections.
Cytokines as immunological warning signs
The detection of presented antigen is not sufficient to fully activate naïve CD8 T cells as they sense the infection for the first time. Instead, the response is restrained until the cells receive additional cell–cell contact dependent costimulatory signals, and inflammatory warning signs which are delivered in the form of specific cytokines induced by the infection (Cox et al., 2011; Curtsinger and Mescher, 2010). These activatory checkpoints prevent illegitimate triggering of naive CD8 T cells, but also permit swift and overwhelming responses to the immunological disequilibrium caused by infection.
Viral infections promote the production of various proinflammatory cytokines which can augment CD8 T cells. This includes type I interferons (IFN), IL (interleukin)-12 family members, IL-6 and related cytokines, as well as IL-1 family members including IL-18 and IL-33. The relative abundance and kinetics of expression of these factors depend upon the properties of the infecting pathogen, but they all impact the ensuing CD8 T cell response (Fig. 2).
Fig. 2.
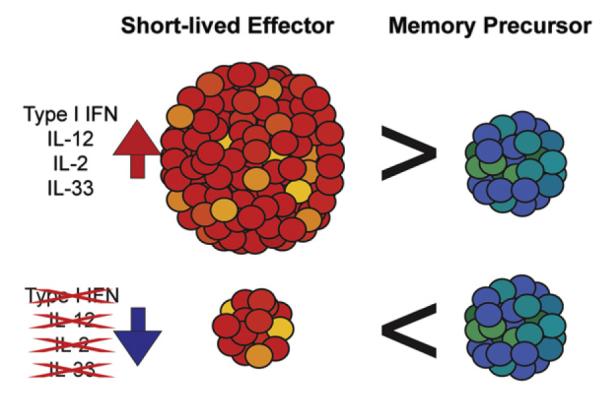
Cytokines influence the developmental choices of anti-viral CD8 T cells. Although CD8 T cell responses are triggered by the detection of viral antigens, other factors amplify and guide their development. The presence of several cytokines including type I interferons, IL-12, IL-33, and high levels of IL-2 have been shown to drive the differentiation of short-lived terminally differentiated effector CD8 T cells during the initial stages of the response. The magnitude of the effector response may be lower if these cytokine signals are ablated, but memory T cell development can still occur.
Type I interferons and IL-12
Type I IFNs (IFNα and IFNβ) form the first line of defense against viral infections. Their production is initiated as the infected cell senses the presence of virally encoded “non-self” nucleic acids via RIG-I-like, Toll-like, and NOD-like receptors (Aoshi et al., 2011). Type I IFNs are rapidly but transiently produced and can be synthesized by all cell types. Certain viruses, including influenza, hepatitis C virus (HCV), and West Nile virus, deploy immune evasion mechanisms to avoid inducing type I IFNs (Diamond, 2009; Garcia-Sastre, 2011; Lemon, 2010), and different viral infections induce distinct profiles of inflammatory cytokines. For example, vaccinia virus (VV) and vesicular stomatitis virus (VSV) induce less type I IFN than lymphocytic choriomeningitis virus (LCMV), however, they generate significant amounts of the inflammatory cytokine IL-12 (Keppler et al., 2012; Thompson et al., 2006), which is primarily produced by dendritic cells (DCs) and macrophages.
The biological effects of type I IFNs and IL-12 on CD8 T cell differentiation are overlapping as they both directly signal to the responding cells and act in conjunction with antigenic and costimulatory signals to promote the development and expansion of short-lived effector cells (Fig. 2) (Cui et al., 2009; Curtsinger et al., 1999, 2005; Pearce and Shen, 2007; Schmidt and Mescher, 1999, 2002; Valenzuela et al., 2002; Welsh et al., 2012). IL-12 signaling induces expression of T-box expressed in T cells (T-bet), a transcription factor which determines the differentiation state of the cell. High levels of T-bet galvanize the terminal differentiation of short-lived effector T cells, while lower levels of T-bet are associated with the development of memory precursor T cells which can go on to form the long-lived memory pool (Joshi et al., 2007; Takemoto et al., 2006). CD8 T cells lacking the IL-12 receptor (R), IFNAR, or both inflammatory cytokine receptors, are defective in the formation of short-lived cells following infection with LCMV, VSV, or the intracellular bacteria Listeria monocytogenes (LM) (Keppler et al., 2012; Thompson et al., 2006; Wiesel et al., 2012). These receptor deficient CD8 T cells express lower levels of T-bet and higher levels of the related T-box transcription factor Eomesodermin (Eomes) (Keppler et al., 2012; Wiesel et al., 2012). Although these transcription factors possess some overlapping functions, as both are sufficient to drive IFNγ (Intlekofer et al., 2008; Pearce et al., 2003), the expression of Eomes is preferentially associated with the formation of memory CD8 T cells (Banerjee et al., 2010; Intlekofer et al., 2005; Takemoto et al., 2006). Eomes may operate to recruit cells into the memory pool by upregulating expression of CD122, the β-chain of the IL-2 receptor, which is also required for IL-15 signaling (Intlekofer et al., 2005; Zhou et al., 2010). Increasing the ability of the T cells to receive IL-15 signals leads to upregulation of anti-apoptotic molecules and potentiates their transition into and maintenance within the memory pool. Thus, cooperation between cytokines shapes both short-term and long-lived anti-viral CD8 T cell development.
While the induction of type I IFN or IL-12 following infection boosts the expansion of highly cytolytic short-lived effector cells (Fig. 2), curtailing the inflammatory conditions surrounding CD8 T cells still permits the formation of long-lived memory populations that operate to confer protection against re-exposure to the infection (Badovinac et al., 2004; Joshi et al., 2007). Nevertheless, modulating the inflammatory milieu can be disastrous for viral control. Blockade of IFNα/β during the priming phase of LCMV Armstrong infection results in failure to clear the virus (Sandberg et al., 1994) and, similarly, IFNAR-deficient (−/−) mice poorly control the low virulence VV strain Lancy or LCMV Armstrong or WE (Muller et al., 1994; van den Broek et al., 1995; Zhou et al., 2012). Infection of IFNAR−/− mice with more persistent strains of LCMV, such as LCMV Docile or clone 13, results in a high titer life-long infection and exacerbated T cell exhaustion (Ou et al., 2001), as opposed to the slowly controlled persistent infection observed in wild type mice.
The amount of type I IFN induced by the virus also likely influences whether or not the infection persists. For example, Wang et al. (2012), found that less IFNα is produced in the early stages of infection with the chronic strain of LCMV, clone 13, than during infection with the acute LCMV Armstrong strain. This may be because of the enhanced tropism of LCMV clone 13 for plasmacytoid DCs and viral nucleoprotein inhibition of type I IFN production in these cells. Administering IFNα early following LCMV clone 13 infection promoted continued expansion of CD8 T cells and the control of the virus (Wang et al., 2012). Thus, virus-dependent differences in the induction of inflammatory warning signs impact control of the infection and the sustainability of the anti-viral CD8 T cells.
IL-1, IL-18, and IL-33
Members of the IL-1 family of cytokines include IL-1, IL-18, and IL-33, and each of these factors influence anti-viral CD8 T cells. Pro-IL-1 is cleaved by caspase 1 into active IL-1α and IL-1β in activated macrophages as well as other cell types (Sims and Smith, 2010). IL-1 signaling drives the production of the inflammatory cytokine IL-6 in numerous cell types, which can impact CD8 T cell differentiation, and IL-1 also augments the cytolytic capacity of effector CD8 T cells. Reductions in the overall numbers of CD8 T cells is observed in IL-1R−/− animals. Following infection of IL-1R−/− mice with LCMV, the virus-specific CD8 T cells that do develop have poor expression of granzymes and display markedly reduced cytotoxic effector functions. This loss of cytotoxic T lymphocyte (CTL) functions is consequential as IL-1R−/− mice failed to control LCMV infection (Joeckel et al., 2012).
The production of IL-18 is upregulated by activated macrophages and DCs. Classically, IL-18 is described as an IFNγ inducing factor (Okamura et al., 1995) and, as discussed later, this property allows IL-18, especially in conjunction with other inflammatory cytokines such as IL-12, to induce IFNγ production by effector and memory CD8 T cells without antigenic activation (Beadling and Slifka, 2005; Berg et al., 2003; Ingram et al., 2011; Raue et al., 2004). This cytokine, however, is not required for the generation of effector and memory CD8 T cells but it can have some influence on their functional properties and survival (Haring and Harty, 2009). Exposure to IL-18 can enhance the proliferation of CD8 T cells as well as limit activation-induced cell death, which is consistent with the induction of IL-18 associated genes during the contraction phase of the response (Haring and Harty, 2009; Iwai et al., 2008; Li et al., 2007). The expression of IL-18R has also been reported to correlate with the self-renewal potential of peripheral CD8 T cell populations, which is a key trait of memory T cells (Turtle et al., 2009). Effector and memory CD8 T cells express the IL-18R, which becomes downregulated if the responding cells succumb to exhaustion in chronically infected hosts (Haining et al., 2008; Luckey et al., 2006; Wherry et al., 2007). During chronic LCMV infection the expression of IL-18Rα appears to help preserve anti-viral CD8 T cells during the initial contraction phase of the response, but this effect is lost as the levels of the receptor decrease and exhaustion is established (Ingram et al., 2011).
As viruses begin to replicate the release of damage associate molecular patterns (DAMPs) from injured target cells also serve as immunological warning signs and operate as potent activators of cellular immune responses. Recent studies have highlighted the role of the IL-1 superfamily member IL-33 as an alarmin which launches anti-viral CD8 T cell responses. IL-33 is expressed as a nuclear precursor (Moussion et al., 2008) but lysis of cells following infection or necrotic cell death releases this factor (Cayrol and Girard, 2009; Haraldsen et al., 2009; Zhao and Hu, 2010), signaling the presence of cellular damage.
The cellular receptor for IL-33 is formed by the heterodimerization of ST2 (IL1RL1) and the IL-1 receptor accessory protein. ST2, the principle signaling chain of the receptor, is not detectable on naïve CD8 T cells but is upregulated in a T-bet dependent manner following activation (Bonilla et al., 2012). In vitro priming studies have demonstrated that IL-12 and IL-33 enhance the viability and effector differentiation of CD8 T cells and enforce expression of the transcription factors T-bet and B lymphocyte-induced maturation protein (Blimp)-1 (Prdm1), while repressing Eomes, lymphoid enhancer-binding factor (LEF)1 and T cell factor (TCF)-1 (Yang et al., 2011). This is consistent with the roles of these cytokines in driving terminally differentiated effector T cell development.
Infection of mice lacking IL-33 or ST2 with LCMV, murine gammaherpesvirus (MHV)-68, or VV results in drastically reduced anti-viral CD8 T cell responses, whereas the responses to replication-defective LCMV or attenuated VV are not affected, illustrating the importance of IL-33 during productive infections which cause tissue damage (Bonilla et al., 2012). Co-administration of IL-33 during vaccination robustly boosts expansion of CD8 T cells responses. In addition to bolstering the size of the response, IL-33 acts directly on the responding CD8 T cells to promote the acquisition of effector functions. ST2−/− CD8 T cells primed in wild-type animals lack the capacity to produce the effector cytokines TNFα and IL-2, and only ~5% of these cells attain the capacity to produce IFNγ. Degranulation and granzyme B expression are also severely compromised, and these cells also express reduced levels of the anti-apoptotic molecule Bcl-2, which likely contributes to the poor accumulation of these cells in vitro and in vivo (Bonilla et al., 2012). Thus, the inability to respond to IL-33 impedes effector CD8 T cell development following infection. ST2−/− CD8 T cells do however, express a surface phenotype more closely related to memory precursor cells, characterized by expression of lower levels of KLRG-1 and higher levels of CD127. This same pattern of compromised effector development but emergence of memory precursors is observed in Blimp-1 deficient mice infected with LCMV or influenza virus. Blimp-1 deficient memory cells also fail to develop into secondary effectors (Kallies et al., 2009) but vaccinated ST2−/− mice efficiently control subsequent LCMV infection (Bonilla et al., 2012), indicating that IL-33 is critical for primary effector responses, but not for memory T cell formation or the elaboration of recall responses by these cells.
Effector CD8 T cells are amplified by IL-33, as well as IFNα/β and IL-12 signals, but the timing of these different signals is critical for optimally promoting these responses. Signaling through TLR3 by administration of the synthetic dsRNA analog, polyinosinic:polycytidylic acid (poly(I:C)), induces the expression of IFNα/β, particularly in plasmacytoid DCs, and acts as an adjuvant during vaccination. Injection of poly(I:C) 3 days before immunization, however, impeded CD8 expansion and differentiation, and reduced their ability to respond to IL-33 (Ngoi et al., 2012). Thus, although these cytokines which signify the presence of an infection can act synergistically, temporal coordination is required to ensure the full amplification of the subsequent T cell response.
gp130 family members
The gp130 receptor is used by several cytokines including IL-6 and IL-27, which both impact CD8 T cell responses (Taga and Kishimoto, 1997). IL-6 is produced by monocytes and endothelial cells as well as by lymphocytes, keratinocytes, and a variety of other cells types. IL-6 affects multiple aspects of the immune response (Taga and Kishimoto, 1997), and has both direct and indirect influences on CD8 T cell development. IL-6 plays a role in control of several viral infections, including Herpes simplex virus (HSV)-1, ectromelia, VV, Friend Leukemia Virus (FLV), and the chronic strain of LCMV, clone 13 (Harker et al., 2011; Kopf et al., 1994; Murphy et al., 2008; O’Gorman et al., 2010; Strestik et al., 2001). Mice deficient in IL-6 are severely impaired in their capacity to control HSV-1 (Murphy et al., 2008) and the poxvirus ectromelia (O’Gorman et al., 2010), and infected animals rapidly succumbed to infection. Infection with LCMV clone 13 induces two waves of IL-6 production, one within the first few days of infection, and a second peak approximately 25 days post infection, which is primarily produced by follicular dendritic cells (Harker et al., 2011). This second wave of IL-6 is critical for control of viremia, as neutralization of IL-6 during this period by antibody or receptor blockade abrogates viral control in wild type mice (Harker et al., 2011).
IL-6 both directly and indirectly effects anti-viral CD8 T cell responses. IL-6 signaling to CD4 T cells induces expression of IL-21, which is critical for sustaining anti-viral CTL responses during persistent infections. Moreover, induction of IL-21 may divert CD4 T cells into the T follicular helper (Tfh) lineage, which help B cell responses. Accordingly, IL-6 deficient mice demonstrate reduced levels of virus-neutralizing antibodies compared to wild-type controls during VV, LCMV, or FLV infections (Harker et al., 2011; O’Gorman et al., 2010; Strestik et al., 2001). In the case of chronic LCMV infection, the resulting higher viral loads likely further exacerbate the development of CD8 T cell exhaustion.
In addition to indirect effects on anti-viral CD8 T cells, IL-6 also directly signals to CD8 T cells via a receptor comprised of the IL-6Rα chain and gp130. In naïve CD8 T cells this results in phosphorylation of signal transducer and activator of transcription (STAT)1 and STAT3, and supports cell survival (Teague et al., 1997). Interestingly, IL-6 signaling alone is not sufficient to protect activated or effector memory phenotype cells from cell death in vitro (Teague et al., 2000). Nevertheless, when IL-6 is combined with other cytokines, such as the homeostatic cytokines IL-7 and IL-15, it elevates the expression of granzyme B and increases cytolytic capacity (Gagnon et al., 2008). IL-6 also synergizes with IL-7 and IL-15 to augment T cell proliferation (Gagnon et al., 2008). Thus, IL-6 can enhance the survival or homeostatic expansion of memory cells when combined with other signals. In accordance with this finding, Castellino and Germain (2007) have found that CD8 T cells expressing both IL-6Rα and IL-7Rα, but not either receptor alone, preferentially survive and form the memory pool after vaccination. This protective effect of IL-6 may be due to inhibition of activation-induced cell death.
Like IL-6, the gp130 family member IL-27 can also steer CD8 T cell development. CD8 T cells activated in vitro in the presence of IL-27 demonstrate enhanced proliferation, increased T-bet and Eomes expression, greater IFNγ production, and more potent cytolytic properties (Morishima et al., 2005; Schneider et al., 2011). In addition to promoting CD8 T cell effector functions, IL-27 may indirectly support CD8 T cell responses by driving the differentiation of Th1 phenotype CD4 T cells (Hibbert et al., 2003; Owaki et al., 2005; Pflanz et al., 2002) and facilitating the expression of IL-21 by CD4 T cells (Pot et al., 2009), similar to IL-6. IL-27 signaling to CD4 T cells enhances expression of T-bet (Hibbert et al., 2003; Owaki et al., 2005), potentiating the Th1 lineage, and also induces c-maf (Pot et al., 2009), a transcription factor which activates IL-21 production to facilitate generation of Tfh CD4 T cells. IL-27 signaling also enhances the expression of Blimp-1 (Sun et al., 2011), a transcription factor that, similar to T-bet, acts in a rheostatic manner to progressively drive effector functions and the terminal differentiation of anti-viral CD8 T cells (Kallies et al., 2009; Rutishauser et al., 2009; Shin et al., 2009). During influenza infection, IL-27 signals coordinate with CD4 T cell help to enhance Blimp-1 expression in the responding CD8 T cells, promoting the production of the immunoregulatory cytokine IL-10 (Sun et al., 2011). This immunosuppressive cytokine dampens lung inflammation and prevents lethal immunopathology, and neutralization of IL-10 signaling results in more rapid mortality of influenza virus infected mice (Sun et al., 2009).
Guidance and support by common-γ chain cytokines
As a viral infection begins to take hold certain members of the common-γ chain family of cytokines guide the continuation of the anti-viral CD8 T cell response and influence the magnitude, differentiation state, and longevity of the participating T cells. These cytokine instructions are delivered at critical times, as encouraging an overwhelming immune response at early stages limits the release of progeny virus, restricts dissemination, and facilitates the eradication of the infection. Although these are desirable outcomes, a fine balance exists between eliminating infected cells and causing tissue damage which can further compromise the health of the infected individual. By contrast, a temporally slower or unsustainable response can in essence be outcompeted by the pathogen, delaying the possible clearance of the infection, permitting spread to immunoprivileged sites, and encouraging establishment of viral persistence. In addition, the failure to clear the infection can result in prolonged antigenic stimulation, causing varying degrees of T cell exhaustion and corrupting bona fide memory T cell formation. At later stages other sets of common-γ chain family members regulate the survival of memory anti-viral CD8 T cells, and thus impact long-term immunity to infections.
IL-2
A key cytokine which regulates T cell fate decisions and functions is IL-2. This cytokine has been reported to be expressed by DCs (Granucci et al., 2001), mast cells (Hershko et al., 2011), and natural killer T (NKT) cells (Jiang et al., 2005), although activated T cells are the main source of this cytokine. The production of IL-2 can have profound effects on T cell differentiation, but the role of this cytokine in immunity to infection extends beyond T cells and include supporting the activation and effector properties of NK cells (Gismondi et al., 2004), which can aid with infection control, regulating the proliferation of activated B cells (Mingari et al., 1984; Nakanishi et al., 1992), restricting Tfh development (Ballesteros-Tato et al., 2012), and augmenting regulatory CD4 T cell (Treg) levels (Setoguchi et al., 2005).
IL-2 signals through a receptor comprised of three independent subunits, which are differentially expressed and inducible (Bachmann and Oxenius, 2007; Malek, 2008). CD25 (IL-2Rα) serves as the high affinity receptor chain and is upregulated on several cell types, including T cells, upon activation. CD122 (IL-2Rβ) is a signaling chain shared by both the IL-2 and IL-15 receptors. The third chain of the IL-2 receptor is CD132, which is also incorporated into the receptors for several other “common-γ chain” cytokine family members including IL-4, IL-7, IL-9, IL-15, and IL-21. Upon heterodimerization the cytoplasmic domains of CD122 and CD132 contribute to signal transduction, resulting in activation of the JAK-STAT pathway via phosphorylation of STAT5, as well as activation of the phosphoinositiol 3-kinase and mitogen-activated protein kinase pathways (Liao et al., 2011).
As naïve CD8 T cells are initially activated a wave of IL-2 production occurs, but expression is short-lived (D’Souza and Lefrancois, 2004; Deeths et al., 1999); however, subsets of effector and memory T cells regain the ability to secrete this cytokine as the response ensues (Tham and Mescher, 2002; Tham et al., 2002). During the initial activation phase this transient release of IL-2 by both CD4 and CD8 T cells, as well as exposure to antigen and other proinflammatory cytokines, including IL-12, stimulates upregulation of CD25, which enhances the affinity of the IL-2 receptor by 100-fold (Bachmann and Oxenius, 2007; Cox et al., 2011; Malek, 2008), thereby increasing the sensitivity of the responding T cells to this cytokine. Although anti-viral CD8 T cells can expand independently of IL-2, this cytokine plays a potential role in fine-tuning overall magnitude of the response (Bachmann et al., 2007; Williams et al., 2006), and may be more critical for supporting responses in non-lymphoid tissues (D’Souza and Lefrancois, 2003; D’Souza et al., 2002). Nevertheless, IL-2 does function as a pivotal differentiation factor dictating qualitative aspects of CD8 T cell response.
The levels and kinetics of CD25 expression on responding CD8 T cells, as well as their exposure to IL-2, is not uniform. T cell populations which express higher levels of CD25, or that are exposed to higher levels of IL-2, preferentially attain a more terminally differentiated, short lived effector phenotype, signified by the expression of the transcription factors Blimp-1 and T-bet, and the effector molecules perforin, granzyme B, and IFNγ (Kalia et al., 2010; Pipkin et al., 2010). By contrast, CD8 T cells that express lower levels CD25 or encounter lower amounts of IL-2 express increased levels of the transcription factor Bcl-6, the central memory T cell marker CD62L, and IL-2, but lower levels of Blimp-1 and perforin (Kalia et al., 2010; Pipkin et al., 2010). These T cells have an increased ability to survive over time and give rise to the long-lived memory compartment. Furthermore, manipulation of CD8 T cell responses to reduce CD25 expression, either by genetic ablation or removal of CD4 T cells, results in reductions in the generation of the effector CD8 T cell pool (Obar et al., 2010; Williams et al., 2006).
In addition to the influence of IL-2 on the effector and memory fate decisions of CD8 T cells, it is also apparent that early exposure to IL-2 does not necessarily compromise memory T cell formation. Instead, IL-2 may be essential for programming the ability of these T cells to mount pronounced secondary immune responses. Analyses of experimental mice containing a mixture of CD25−/− and wild-type T cells, in combination with IL-2 treatments and adoptive transfer studies, have demonstrated that exposure to IL-2 during the priming phase allows memory T cell populations to form which are imprinted with the ability to proliferate vigorously to subsequent challenges (Bachmann et al., 2007; Williams et al., 2006). Strong IL-2 signals during priming are not always a prerequisite for the development of highly proliferative memory cells, however, as CD25−/− CD8 T cells primed with LM are still capable of mounting normal secondary responses (Obar et al., 2010), highlighting the importance of the pathogen-induced milieu in programming effector and memory CD8 T cell functions. Notably, CD8 T cells can express IL-2, and both cell intrinsic (autocrine) and extrinsic (paracrine) production of this cytokine has been shown to be sufficient for the generation of memory T cells with robust recall potential (Feau et al., 2011; Williams et al., 2006). Thus, the abundance, timing, and source of IL-2, as well as integration with other system-specific cellular and soluble factors, together with viral loads, all dictate precisely how IL-2 regulates CD8 T cell responses.
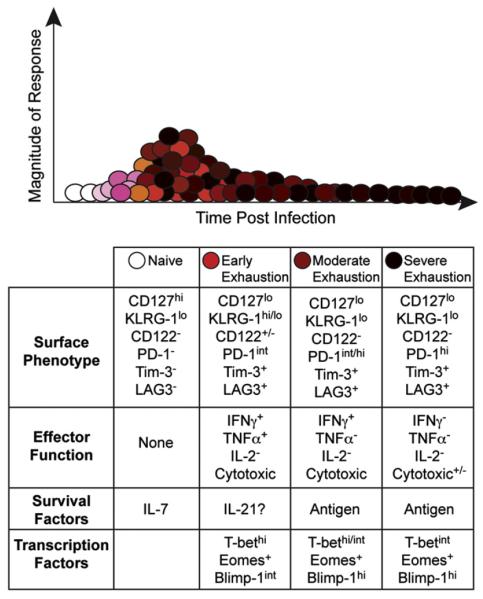
While the strength of IL-2 signaling plays a significant role in the modulation of the immune response, examining the consequences of IL-2 treatment during the expansion, contraction, and memory response have also revealed variances in the temporal requirement of IL-2 signaling. Early IL-2 treatment may be disadvantageous, causing decreased survival and function of the expanding cells (Blattman et al., 2003; Hamilton et al., 2010), consistent with the role of IL-2 in promoting short-lived, terminally differentiated, effector T cell development. Nevertheless, during certain chronic viral infections IL-2 signals are stringently required, as under these conditions CD25−/− T cells initially expand but subsequently decay (Bachmann et al., 2007). In addition, IL-2 therapy can bolster CD8 T cell responses during chronic infections, including LCMV and MHV-68 (Blattman et al., 2003; Hamilton et al., 2010; Molloy et al., 2009). Notably, the ability of virus-specific CD8 T cells to produce IL-2 is rapidly lost as they succumb to exhaustion (Fig. 3) (Fuller et al., 2004; Fuller and Zajac, 2003; Wherry et al., 2003) and therefore, extrinsic sources of IL-2, likely from CD4 T cells, may be most critical under these conditions.
Fig. 3.
The differentiation of anti-viral CD8 T cells is corrupted during chronic viral infections. Various factors including high viral loads, the composition of the cytokine milieu, and ineffective CD4 T cell help contribute to the induction of ineffective anti-viral CD8 T cell responses. Under these conditions the responding T cells usually attain a highly activated phenotype and express ensembles of inhibitory receptors as they progressively lose their ability to elaborate key anti-viral effector activities. Unlike their memory counterparts, these exhausted T cells usually require the continued presence of viral antigen for their maintenance and may succumb to deletion over time.
IL-21
Whereas IL-2 pushes effector CD8 T cell differentiation, the related common-γ chain family member, IL-21, helps sustain CD8 T cell responses and promotes memory T cell populations. This cytokine is primarily produced by CD4 T cells but has also been shown to be expressed by NKT cells. IL-21 regulates many aspects of the immune response that can indirectly impact CD8 T cell responses during the course of viral infections including NK cell effector activities, antibody responses, and the differentiation of CD4 T cell subsets–spanning promoting the development of Th17 cells, sustaining Tfh responses, and suppressing Tregs (Yi et al., 2010a). Additionally, as discussed below, IL-21 acts directly on CD8 T cells and can impact these responses during both acute and chronic viral infections.
During acute viral infections the effect of IL-21 on anti-viral CD8 T cells differs depending upon the pathogen. The responses to acute LCMV infection are relatively unperturbed by either IL-21 or IL-21R deficiency, although the ability of the virus-specific CD8 T cells to produce IL-2 may be lower, and their recall potential reduced, especially under competitive conditions (Elsaesser et al., 2009; Frohlich et al., 2009; Yi et al., 2010c). The magnitude of primary CD8 T cell responses and the size of the memory pool elicited by replication defective recombinant adenovirus (Ad) is lower in IL-21R−/− mice (Barker et al., 2010). A comparable, although less penetrant, trend has also been observed following recombinant VV infections (Barker et al., 2010; Novy et al., 2011). Following both rAd and rVV infections the secondary recall responses of IL-21R−/− CD8 T cells are curtailed. This has been in part attributed to overexpression of the apoptosis inducing molecule, TRAIL, caused by the absence of IL-21 signaling (Barker et al., 2010). Notably, certain populations of “helpless” CD8 T cells, which develop without CD4 T cell help show similar inability to mount vigorous recall responses which is also associated with increased TRAIL expression (Janssen et al., 2005).
IL-21 plays an especially significant role in sustaining anti-viral CD8 T cell responses during chronic infections (Elsaesser et al., 2009; Frohlich et al., 2009; Yi et al., 2009). During HIV infection IL-21 has been shown to be produced by both CD4 and CD8 T cells (Chevalier et al., 2011; Iannello et al., 2010; Williams et al., 2011; Yue et al., 2010). The levels of IL-21 producing CD4 T cells are greater in individuals with controlled infections than in subjects with progressive infections, and the fraction of IL-21+ CD4 T cells predicts the functional quality of the HIV-specific CD8 T cell responses. Exposure of HIV-specific CD8 T cells to IL-21 enhances their cytolytic and viral control abilities (Chevalier et al., 2011; Parmigiani et al., 2011; White et al., 2007). Moreover, therapeutic studies of simian immunodeficiency virus (SIV) infected rhesus (rh) macaques support the observation that IL-21 enhances CD8 T cell killing functions, although the regimen used did not impact overall T cell counts or viral levels (Pallikkuth et al., 2011).
The importance of IL-21 during chronic viral infections is also well-described following LCMV infections (Elsaesser et al., 2009; Frohlich et al., 2009; Yi et al., 2009). Mice deficient in IL-21 or IL-21R mount an initial CD8 T cell response to strains of LCMV which usually establish chronic infections, but the response subsequently collapses as the functional capacity of the anti-viral CD8 T cells deteriorates and these cells succumb to deletion. This is consequential as without IL-21 signaling viral titers remain high and the infection is never resolved. This contrasts with the pattern of LCMV clone 13 resolution in immunocompetent mice, which slowly bring the infection under control in many organs. Adoptive transfer and mixed bone marrow chimera studies have shown that the anti-viral CD8 T cells respond directly to IL-21 and that the loss of IL-21R on CD8 T cells results in severe T cell exhaustion (Elsaesser et al., 2009; Frohlich et al., 2009; Yi et al., 2009). Moreover, CD8 T cell responses can be rescued in CD4−/− mice by the administration of IL-21 early following LCMV clone 13 infection (Yi et al., 2009).
The role of IL-21 in regulating CD8 T cell responses may reflect the ability of this cytokine to restrict differentiation and promote the longevity of CD8 T cells. The overexpression of IL-21 results in massive accumulation of memory CD8 T cells (Allard et al., 2007), which have self-renewal properties and are less terminally differentiated than their effector counterparts. In addition, IL-21 in conjunction with IL-10 has been implicated in memory T cell formation through promoting STAT3 phosphorylation (Cui et al., 2012). Moreover, IL-21 induces the transcription factors TCF-1 and LEF1 (Hinrichs et al., 2008), both of which participate in the Wnt/β-catenin pathway and imprint memory T cell properties (Jeannet et al., 2010; Zhou et al., 2010). Perplexingly, IL-21 signaling also drives expression of Blimp-1 (Kwon et al., 2009), a transcriptional repressor which drives terminal differentiation of CD8 T cells, and is required for the development of exhaustion during viral persistence (Shin et al., 2009). In B cells and CD4 T cells, IL-21 signaling also promotes the expression of Bcl-6, a transcriptional repressor which is mutually antagonistic to Blimp-1. So IL-21 signaling in CD8 T cells may induce both transcription factors and their relative abundance, in conjunction with other factors, may determine the ultimate fate of the response.
IL-7 and IL-15
In addition to the roles of common-γ chain cytokines in determining the effector and memory preferences of anti-viral CD8 T cells during the initial stage of the response, another set of these cytokines operate to sustain the memory T cells which do form. CD127, the alpha chain of the IL-7 receptor, is expressed on naïve CD8 T cells but becomes downregulated upon activation (Fig. 1) (Bachmann et al., 2005; Huster et al., 2004; Kaech et al., 2003; Klonowski et al., 2006; Schluns et al., 2000). Although many of these activated CD127lo CD8 T cells become terminally differentiated and succumb to apoptosis, a subset re-express this receptor. These CD127hi cells, often referred to as memory precursor effector CD8 T cells, emerge early following infection and are preferentially recruited to the memory pool (Bachmann et al., 2005; Huster et al., 2004; Kaech et al., 2003). It has been hypothesized that these cells are better able to endure the contraction phase due to increased survival signals from IL-7. Nevertheless, this concept has been questioned as the forced overexpression of CD127 on effector cells does not prevent normal contraction of the response (Hand et al., 2007; Haring et al., 2008), and it is plausible that some CD127lo effector CD8 T cells later upregulate this receptor and mature into memory T cells.
The expression of CD127 is necessary to maintain memory CD8 T cells following the clearance of an infection (Bachmann et al., 2005; Huster et al., 2004; Kaech et al., 2003; Schluns et al., 2000) but, curiously, these cells survive in IL-7 deficient mice (Klonowski et al., 2006). This may reflect a role for thymic stromal lymphopoietin (TSLP), a closely related cytokine that also signals through CD127 and the TSLP receptor. TSLP can compensate for IL-7 deficiency in lymphopoiesis, as well as augment the survival and homeostatic proliferation of CD8 T cells (Chappaz et al., 2007). The contribution of TSLP to memory CD8 T cell formation is not fully understood and warrants further study.
In addition to IL-7 signals, memory CD8 T cells are dependent upon IL-15 signaling for their continued maintenance (Tan et al., 2002). In the absence of IL-15 memory T cell populations are severely reduced (Becker et al., 2002; Wu et al., 2002), although CD127lo effector T cell populations are also especially sensitive to IL-15 withdrawal (Mitchell et al., 2010; Sandau et al., 2010). Interestingly secondary memory CD8 T cells, which arise following rechallenge of immunized hosts, are also highly dependent on IL-15, as transfer of these cells into IL-15−/− mice results in their rapid decay which is associated with decreased expression of the anti-apoptotic protein Bcl-2 (Sandau et al., 2010). IL-15 can bind to the IL-15Rα chain, which is not necessary for signaling, but increases the half-life and bioavailability of this cytokine, and allows it to be presented in trans to cells expressing CD122 (IL-2Rβ) and the common-γ chain CD132. This transpresentation may maintain and bolster effector cell numbers in tissues. IL-15 presented in trans by pulmonary DCs is required to maintain effector cells in the lung following influenza infection, and either depletion of the DCs or blockade of IL-15 in the lung results in a dramatic increase in apoptosis of the antigen-specific CD8 T cells (McGill et al., 2010). Additionally, administration of IL-15-IL-15Rα complexes in tumor bearing mice revitalized the anti-tumor CTL responses, enhancing proliferation and cytotoxicity of the CTLs and reducing tumor burden (Epardaud et al., 2008; Stoklasek et al., 2006), further suggesting a role for IL-15 in sustaining specific CD8 T cell subsets in the tissues.
A recently described distinct population of memory cells are sessile and reside in specific tissues, particularly barrier tissues such as the skin and intestinal mucosa (Casey et al., 2012; Jiang et al., 2012; Mackay et al., 2012; Wakim et al., 2010). These tissue resident memory cells (TRM) provide superior site specific protection from certain infections, including herpesvirus (Jiang et al., 2012; Mackay et al., 2012) and SIV. CD8 T cells undergo antigen-independent, inflammation driven, recruitment to these sites, and are able to persist in the absence of antigenic recognition. TRM cells do not express detectable amounts of either CD127 or CD122 (Jiang et al., 2012), so should not be sensitive to IL-7 or IL-15, and thus the factors which maintain these local sentinels still need to be deciphered.
During persistent infections exhausted CD8 T cells downregulate both CD127 and CD122 (Fuller et al., 2005; Lang et al., 2005; Shin et al., 2007), and are therefore maintained independently of IL-7 and IL-15 (Fig. 3). Instead, these cells become antigen-addicted and require the presence of persistent viral antigen for their continued survival (Shin et al., 2007; Wherry et al., 2004). Paradoxically, the sustained antigenic activation, which occurs as a consequence of the failure to clear the infection, drives the functional exhaustion of anti-viral T cells that are maintained (Blattman et al., 2009; Mueller and Ahmed, 2009; Richter et al., 2012; Wherry et al., 2004). This antigen-addiction has been well described following LCMV clone 13 infection of mice, which is characterized by persistently high viral loads. Moreover, CD8 T cells responding to latent γ-herpesvirus infection (Obar et al., 2004) or cerebral mouse hepatitis virus (Zuo et al., 2009) can also persist in the absence of IL-15 signals.
Although exhausted CD8 T cells have unique maintenance requirements, and downregulate the IL-7 and IL-15 receptors, treatment with IL-7 during chronic LCMV infection can bolster anti-viral immunity (Nanjappa et al., 2011; Pellegrini et al., 2011). Administration of IL-7 to LCMV clone 13 infected mice increased the total numbers of CD4, CD8, and B cells, and expanded the virus-specific CD8 T cell population. This is surprising given that CD127 expression is low during the course of persistent LCMV infection, but may reflect expansion of a small pool of less exhausted cells, or may indicate that high levels of IL-7 can signal to cells with suboptimal expression of the cognate receptor. The IL-7 driven expansion of anti-viral CD8 T cells results in accelerated viral clearance, enhanced functionality of the CD8 T cells, and a reduction in the expression of inhibitory and activation markers, which are hallmarks of the exhausted state (Nanjappa et al., 2011; Pellegrini et al., 2011). The timing and duration of IL-7 administration is crucial as treatment of LCMV clone 13 infected mice during the early contraction phase (day 8 to day 15) is far less effective than treatment later (day 15 to day 25), potentially indicating some time dependent re-expression of CD127 on a subset of virus-specific CD8 T cells (Nanjappa et al., 2011). The most effective IL-7 treatment regimen is between days 8 and 30 following infection which causes durable expansion of anti-viral CD8 T cells. The CD8 T cells in IL-7 treated animals attained a resting phenotype and regained expression of CD127, likely perpetuating the responsiveness of the cells to therapy.
In accordance with the observations made in mouse models, IL-7 treatment in SIV infected macaques (Beq et al., 2006; Fry et al., 2003; Moniuszko et al., 2004; Nugeyre et al., 2003; Vassena et al., 2012) or HIV infected patients (Levy et al., 2009; Sereti et al., 2009) similarly bolsters T cell numbers, predominantly increasing naïve and central memory cells within the CD4 T cell compartment. This is in contrast to IL-15 therapy, which predominantly expands effector-phenotype cells (Mueller et al., 2005), and appears to be detrimental during SIV infection (Lugli et al., 2011; Mueller et al., 2008). Therefore, in the instance of SIV and potentially HIV infection, IL-7 and not IL-15 therapy may facilitate expansion of appropriate populations for viral control and rejuvenation of the immune system.
IL-7 treatment may act to resurrect T cell responses by down-regulating suppressor of cytokine signaling 3 (SOCS3). Mice genetically deficient in SOCS3 exhibit robust T cell responses to LCMV clone 13 and accelerated control of the infection, implicating this signaling inhibitor in the establishment of T cell exhaustion and persistent infection (Pellegrini et al., 2011). IL-7 treatment in vitro also renders CD4 and CD8 T cells refractory to Treg mediated suppression and decreases expression of transforming growth factor (TGF)-βRII, as well as reduces phosphorylation of the downstream signaling adaptors Smad2 and Smad3, indicating these IL-7 treated cells are also resistant to TGF-β mediated suppression (Pellegrini et al., 2009). Thus, the mechanism of immune rejuvenation by IL-7 treatment likely varies depending on the type of infection, however theses studies indicate an exciting new approach for restoring immune responses by cytokine therapy.
Cytokine-driven antigen-independent activation of CD8 T cells
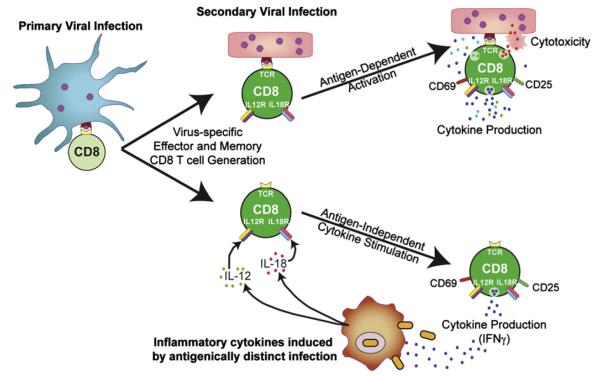
Antigen-specificity is a cardinal trait of adaptive immunity and allows the CD8 T cells to exquisitely target virus-infected cells while avoiding the possible mis-firing of potentially potent cytotoxic and cytokine-mediated anti-viral defense mechanisms. Nevertheless, effector and memory anti-viral CD8 T cells also express assortments of cytokine receptors which enable them to respond to shifts in the cytokine milieu and can circumvent some of their requirements for antigen-dependent activation (Berg and Forman, 2006). This cytokine-driven, but antigen-independent activation, allows anti-viral CD8 T cells to react to a broader range of pathogens, including bacterial pathogens, and mount some level of bystander response (Fig. 4).
Fig. 4.
Antigen-dependent and independent activation of effector and memory virus-specific CD8 T cells. Effector and memory CD8 T cells that develop in response to viral-infections are endowed with the ability to become rapidly activated and deploy effector functions if they re-encounter their cognate viral antigen. This allows virus-specific T cells to swiftly target virus-infected cells, and limit and control the infection. Effector and memory CD8 T cells can also become activated and produce effector cytokines, including IFNγ, if they are exposed to certain cytokine combinations, most notably IL-12 and IL-18. This sensitivity to cytokine activation enables the CD8 T cells to mount bystander responses, which may operate to help control the new infection and mobilize the host immune response.
These innate-like features of effector and memory CD8 T cells are most well characterized following exposure to IL-18 in combination with IL-12 or type I IFNs (Beadling and Slifka, 2005; Berg and Forman, 2006; Cousens et al., 1999; Ingram et al., 2011; Kambayashi et al., 2003; Pien et al., 2002; Sareneva et al., 1998). They have also been shown to occur following infections including LM and Burkholderia pseudomallei which provoke the production of inflammatory cytokines (Berg et al., 2003; Lertmemongkolchai et al., 2001). Recently the sensitivity of anti-viral effector and memory CD8 T cells to an expansive range of cytokine combinations has been profiled (Freeman et al., 2012). Notably, it has also been shown that IL-21 together with IL-18, and IL-12 in combination with IL-33, have synergistic effects on CD8 T cell activation, but many other cytokine pairings can also elicit responses.
Most commonly, the cytokine-driven activation of effector and memory CD8 T cells causes their upregulation of CD25 and the early activation marker CD69, induces the production of IFNγ, and stimulates proliferation. The induction of these innate-like responses likely allows existing memory CD8 T cell populations to confer some level of protection against other pathogens which may be encountered over time, providing an additional immunological safety net (Fig. 4) (Berg and Forman, 2006). In addition, this ability to sense shifts in the inflammatory milieu possibly pre-triggers the T cells in readiness to mount strong responses if they also re-encounter their cognate antigen.
Exhausted CD8 T cells which develop during chronic infections, including LCMV infection of mice and HIV infection of humans, are distinct from their effector and memory counterparts as their potential to elaborate effector functions is curtailed (Fig. 3) (Frebel et al., 2010; Wherry, 2011; Yi et al., 2010b). Moreover, unlike effector and memory CD8 T cells which express IL-18Rα, this receptor is downregulated on exhausted CD8 T cells (Haining et al., 2008; Ingram et al., 2011; Wherry et al., 2007). This loss of IL-18Rα expression, and the dampening of effector potential, correlates with an inability to produce IFNγ and upregulate CD25 in response to stimulation with cytokines, including IL-12, IL-18, and IL-21, which usually cause innate-like activation of anti-viral CD8 T cells (Ingram et al., 2011). This loss of sensitivity to antigen-independent cytokine activation mirrors the failure of exhausted CD8 T cells to deploy effector activities in response to antigenic-activation in the chronically infected host. The consequences of this global corruption not only include compromised viral control by the anti-viral CD8 T cells but also an inability to respond to bystander bacterial infections (Ingram et al., 2011). During HIV infection the integrity of the gut mucosal is reduced, permitting the translocation of intestinal bacteria which stimulate inflammatory cytokine production, including IL-12 and IL-18 (Brenchley et al., 2006; Jiang et al., 2009). Thus, the development of the exhausted state recalibrates the anti-viral CD8 T cells to the chronically infected environment, and it is plausible that they also adjust their responsiveness to the unique inflammatory conditions that evolve.
Cytokine suppression of CD8 T cells
Cytokine networks not only promote CD8 T cell responses following infection but can also function to limit their magnitude and quality. Since over exuberant responses can cause immunopathology, curbing this damage can be beneficial, although the suppression of the immune response can result in viral persistence. In this section we discuss how two cytokines, IL-10 and TGF-β, modulate virus-specific CD8 T cell responses.
IL-10
IL-10 signaling to T cells reduces their proliferation and effector activity, including the production of effector cytokines such as IFNγ. IL-10 can also indirectly inhibit T cell responses by decreasing the antigen presenting capacity of macrophages and DCs and reducing their expression of proinflammatory cytokines which amplify effector responses (Pestka et al., 2004; Wilson and Brooks, 2010). IL-10 deficient mice acutely infected with LCMV have greater numbers of virus-specific CD4 and CD8 T cells at the peak of the response (Brooks et al., 2010), highlighting the role of this cytokine in restricting immune responses. Elevated levels of IL-10 are detected during several chronic human viral infections including HIV, Epstein–Barr virus (EBV), hepatitis B virus (HBV), and HCV (Brockman et al., 2009; Clerici et al., 1994; Hyodo et al., 2004; Kaplan et al., 2008; Ohga et al., 2004). Abundant amounts of IL-10 also suppress cell-mediated immune responses during chronic LCMV infections (Brooks et al., 2006; Ejrnaes et al., 2006; Maris et al., 2007). Strikingly, IL-10 deficient mice can rapidly control chronic LCMV infection and blockade of IL-10R in immunocompetent mice facilitates viral clearance and improves cellular immune responses (Brooks et al., 2006; Ejrnaes et al., 2006; Maris et al., 2007). IL-10R blockade has also been shown to augment the responses to therapeutic DNA vaccination (Brooks et al., 2008), further demonstrating the inhibition of this suppressive cytokine as a possible therapeutic option for the treatment of established persistent infections. It has also been reported that IL-10−/− mice initially mount a superior response to LCMV clone 13 but that the infection subsequently breaks through and the CD8 T cells ultimately succumb to exhaustion (Maris et al., 2007). These different outcomes may reflect variables such as differences in microbial flora or in the virulence of the exact viral isolate used for the studies, and likely illustrate that the overall cellular immune response and full control of the infection is shaped by multiple factors.
The importance of IL-10 during persistent infections is also illustrated by human beta- and gamma-herpesviruses such as human cytomegalovirus (CMV) and EBV which encode IL-10 homologs (Moore et al., 1990; Nachtwey and Spencer, 2008; Slobedman et al., 2009). These virally encoded immune evasion molecules possess structural and function similarities to human IL-10, indicating that elevating the availability of this suppressive cytokine favors the infection. This is further supported by studies in rh macaques infected with wild type rhCMV or a virus in which the virally encoded IL-10 homolog is mutated. Animals infected with the mutant virus develop robust innate and adaptive responses to rhCMV, and sustain far greater numbers of virus-specific CD4 and CD8 T cells following immunization (Chang and Barry, 2010). Other viruses, such as HIV, do not encode a viral IL-10 homolog, but do induce high levels of cellular IL-10. HIV gp41 and gp120 can both drive production of IL-10 by the monocyte/macrophage population (Barcova et al., 1998).
TGF-β
TGF-β signaling is critical for down-modulating immune responses and preventing immunopathology. By comparison with acute LCMV infection, there is heightened TGF-β signaling during the early stages of chronic LCMV clone 13 infection, as measured by phosphorylation of the downstream signaling molecule Smad2 (Tinoco et al., 2009). Strikingly, infection of mice expressing a dominant negative receptor for TGF-β (dnTGFBRII) with LCMV clone 13 results in rapid clearance of this usually slowly contained infection and mitigates the development of CD8 T cell exhaustion, suggesting TGF-β plays an active role in suppressing the anti-viral T cell response and enhancing viral persistence (Garidou et al., 2012; Tinoco et al., 2009). CD8 T cells expressing dnTGFBRII expand more robustly than wild type cells, implicating direct TGF-β signaling regulating the response. Lack of TGF-β signaling does, however, alter the activation status of CD8 T cells even without infection (Garidou et al., 2012; Tinoco et al., 2009). Therefore, pre-activation of dnTGFBRII CD8 T cells in the murine model may account for their enhanced responsiveness. Accordingly, neutralization of TGF-β during persistent LCMV infection is not sufficient to induce viral control (Garidou et al., 2012), suggesting the intrinsic differences in dnTGFBRII cells may account for their ability to rapidly contain a usually chronic infection.
Similar to hijacking IL-10 signaling, some viruses also utilize the TGF-β immunosuppressive pathway to downregulate the immune response and promote their own persistence. The HBV encoded oncoprotein pX amplifies TGF-β signaling by stabilizing complexes containing the downstream transcription factor Smad4 and enhancing its nuclear translocation (Lee et al., 2001). The potential for viruses to harness these immunomodulatory pathways to facilitate their own persistence highlights the importance of studying the roles of cytokines in health and disease, as a pathway to devise better strategies to control infections and limit their pathology.
Acknowledgments
We wish to thank all members of the Zajac laboratory and also Laurie Harrington for advice and comments about this article. Certain findings discussed in this review were supported in part by grants R01 AI049360, R01 AI067933, and U01 AI082966 (to A.J.Z) and T32 AI007051 (to M.A.C and S.M.K) from the National Institutes of Health. We regret that due to space limitations, we were unable to cite all our colleagues who have contributed to our current knowledge of how cytokines impact anti-viral CD8 T cell responses.
References
- Allard EL, Hardy MP, Leignadier J, Marquis M, Rooney J, Lehoux D, Labrecque N. Overexpression of IL-21 promotes massive CD8 memory T cell accumulation. Eur. J. Immunol. 2007;37:3069–3077. doi: 10.1002/eji.200637017. [DOI] [PubMed] [Google Scholar]
- Aoshi T, Koyama S, Kobiyama K, Akira S, Ishii KJ. Innate and adaptive immune responses to viral infection and vaccination. Curr. Opin. Virol. 2011;1:226–232. doi: 10.1016/j.coviro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Oxenius A. Interleukin 2: from immunostimulation to immunoregulation and back again. EMBO Rep. 2007;8:1142–1148. doi: 10.1038/sj.embor.7401099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J. Immunol. 2005;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur. J. Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat. Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, Randall TD. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. Cutting edge: the transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 2010;185:4988–4992. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcova M, Kacani L, Speth C, Dierich MP. gp41 envelope protein of human immunodeficiency virus induces interleukin (IL)-10 in monocytes, but not in B, T, or NK cells, leading to reduced IL-2 and interferon-gamma production. J. Infect. Dis. 1998;177:905–913. doi: 10.1086/515230. [DOI] [PubMed] [Google Scholar]
- Barker BR, Gladstone MN, Gillard GO, Panas MW, Letvin NL. Critical role for IL-21 in both primary and memory anti-viral CD8+ T-cell responses. Eur. J. Immunol. 2010;40:3085–3096. doi: 10.1002/eji.200939939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadling C, Slifka MK. Differential regulation of virus-specific T-cell effector functions following activation by peptide or innate cytokines. Blood. 2005;105:1179–1186. doi: 10.1182/blood-2004-07-2833. [DOI] [PubMed] [Google Scholar]
- Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beq S, Nugeyre MT, Ho Tsong Fang R, Gautier D, Legrand R, Schmitt N, Estaquier J, Barre-Sinoussi F, Hurtrel B, Cheynier R, Israel N. IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy. J. Immunol. 2006;176:914–922. doi: 10.4049/jimmunol.176.2.914. [DOI] [PubMed] [Google Scholar]
- Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J. Exp. Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RE, Forman J. The role of CD8 T cells in innate immunity and in antigen non-specific protection. Curr. Opin. Immunol. 2006;18:338–343. doi: 10.1016/j.coi.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J. Virol. 2009;83:4386–4394. doi: 10.1128/JVI.02524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla WV, Frohlich A, Senn K, Kallert S, Fernandez M, Johnson S, Kreutzfeldt M, Hegazy AN, Schrick C, Fallon PG, Klemenz R, Nakae S, Adler H, Merkler D, Lohning M, Pinschewer DD. The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses. Science. 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, Porichis F, Le Gall S, Waring MT, Moss K, Jessen H, Pereyra F, Kavanagh DG, Walker BD, Kaufmann DE. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114:346–356. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J. Exp. Med. 2008;205:533–541. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Walsh KB, Elsaesser H, Oldstone MB. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proc. Nat. Acad. Sci. U.S.A. 2010;107:3018–3023. doi: 10.1073/pnas.0914500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, Vezys V, Masopust D. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F, Germain RN. Chemokine-guided CD4+ T cell help enhances generation of IL-6RalphahighIL-7Ralpha high prememory CD8+ T cells. J. Immunol. 2007;178:778–787. doi: 10.4049/jimmunol.178.2.778. [DOI] [PubMed] [Google Scholar]
- Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Nat. Acad. Sci. U.S.A. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WL, Barry PA. Attenuation of innate immunity by cytomegalovirus IL-10 establishes a long-term deficit of adaptive antiviral immunity. Proc. Nat. Acad. Sci. U.S.A. 2010;107:22647–22652. doi: 10.1073/pnas.1013794108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappaz S, Flueck L, Farr AG, Rolink AG, Finke D. Increased TSLP availability restores T- and B-cell compartments in adult IL-7 deficient mice. Blood. 2007;110:3862–3870. doi: 10.1182/blood-2007-02-074245. [DOI] [PubMed] [Google Scholar]
- Chevalier MF, Julg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, Kwon DS, Rychert J, Lian J, Muller MI, Cutler S, McAndrew E, Jessen H, Pereyra F, Rosenberg ES, Altfeld M, Walker BD, Streeck H. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J. Virol. 2011;85:733–741. doi: 10.1128/JVI.02030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M, Wynn TA, Berzofsky JA, Blatt SP, Hendrix CW, Sher A, Coffman RL, Shearer GM. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J. Clin. Invest. 1994;93:768–775. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens LP, Peterson R, Hsu S, Dorner A, Altman JD, Ahmed R, Biron CA. Two roads diverged: interferon alpha/beta- and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J. Exp. Med. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MA, Harrington LE, Zajac AJ. Cytokines and the inception of CD8 T cell responses. Trends Immunol. 2011;32:180–186. doi: 10.1016/j.it.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MA, Zajac AJ. Shaping successful and unsuccessful CD8 T cell responses following infection. J. Biomed. Biotechnol. 2010;2010:159152. doi: 10.1155/2010/159152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2012;35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J. Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- D’Souza WN, Lefrancois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J. Immunol. 2003;171:5727–5735. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- D’Souza WN, Lefrancois L. Frontline: an in-depth evaluation of the production of IL-2 by antigen-specific CD8 T cells in vivo. Eur. J. Immunol. 2004;34:2977–2985. doi: 10.1002/eji.200425485. [DOI] [PubMed] [Google Scholar]
- D’Souza WN, Schluns KS, Masopust D, Lefrancois L. Essential role for IL-2 in the regulation of antiviral extralymphoid CD8 T cell responses. J. Immunol. 2002;168:5566–5572. doi: 10.4049/jimmunol.168.11.5566. [DOI] [PubMed] [Google Scholar]
- Deeths MJ, Kedl RM, Mescher MF. CD8+ T cells become nonresponsive (anergic) following activation in the presence of costimulation. J. Immunol. 1999;163:102–110. [PubMed] [Google Scholar]
- Diamond MS. Mechanisms of evasion of the type I interferon antiviral response by flaviviruses. J. Interferon Cytokine Res. 2009;29:521–530. doi: 10.1089/jir.2009.0069. [DOI] [PubMed] [Google Scholar]
- Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, Hamerman JA, Goldrath AW, Turley SJ. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68:2972–2983. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- Feau S, Arens R, Togher S, Schoenberger SP. Autocrine IL-2 is required for secondary population expansion of CD8(+) memory T cells. Nat. Immunol. 2011;12:908–913. doi: 10.1038/ni.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frebel H, Richter K, Oxenius A. How chronic viral infections impact on antigen-specific T-cell responses. Eur. J. Immunol. 2010;40:654–663. doi: 10.1002/eji.200940102. [DOI] [PubMed] [Google Scholar]
- Freeman BE, Hammarlund E, Raue HP, Slifka MK. Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc. Nat. Acad. Sci. U.S.A. 2012;109:9971–9976. doi: 10.1073/pnas.1203543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- Fry TJ, Moniuszko M, Creekmore S, Donohue SJ, Douek DC, Giardina S, Hecht TT, Hill BJ, Komschlies K, Tomaszewski J, Franchini G, Mackall CL. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood. 2003;101:2294–2299. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]
- Fuller MJ, Hildeman DA, Sabbaj S, Gaddis DE, Tebo AE, Shang L, Goepfert PA, Zajac AJ. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J. Immunol. 2005;174:5926–5930. doi: 10.4049/jimmunol.174.10.5926. [DOI] [PubMed] [Google Scholar]
- Fuller MJ, Khanolkar A, Tebo AE, Zajac AJ. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J. Immunol. 2004;172:4204–4214. doi: 10.4049/jimmunol.172.7.4204. [DOI] [PubMed] [Google Scholar]
- Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 2003;170:477–486. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- Gagnon J, Ramanathan S, Leblanc C, Cloutier A, McDonald PP, Ilangumaran S. IL-6, in synergy with IL-7 or IL-15, stimulates TCR-independent prolifera tion and functional differentiation of CD8+ T lymphocytes. J. Immunol. 2008;180:7958–7968. doi: 10.4049/jimmunol.180.12.7958. [DOI] [PubMed] [Google Scholar]
- Garcia-Sastre A. Induction and evasion of type I interferon responses by influenza viruses. Virus Res. 2011;162:12–18. doi: 10.1016/j.virusres.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garidou L, Heydari S, Gossa S, McGavern DB. Therapeutic blockade of transforming growth factor beta fails to promote clearance of a persistent viral infection. J. Virol. 2012;86:7060–7071. doi: 10.1128/JVI.00164-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gismondi A, Cifaldi L, Mazza C, Giliani S, Parolini S, Morrone S, Jacobelli J, Bandiera E, Notarangelo L, Santoni A. Impaired natural and CD16-mediated NK cell cytotoxicity in patients with WAS and XLT: ability of IL-2 to correct NK cell functional defect. Blood. 2004;104:436–443. doi: 10.1182/blood-2003-07-2621. [DOI] [PubMed] [Google Scholar]
- Granucci F, Vizzardelli C, Pavelka N, Feau S, Persico M, Virzi E, Rescigno M, Moro G, Ricciardi-Castagnoli P. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat. Immunol. 2001;2:882–888. doi: 10.1038/ni0901-882. [DOI] [PubMed] [Google Scholar]
- Haining WN, Ebert BL, Subrmanian A, Wherry EJ, Eichbaum Q, Evans JW, Mak R, Rivoli S, Pretz J, Angelosanto J, Smutko JS, Walker BD, Kaech SM, Ahmed R, Nadler LM, Golub TR. Identification of an evolutionarily conserved transcriptional signature of CD8 memory differentiation that is shared by T and B cells. J. Immunol. 2008;181:1859–1868. doi: 10.4049/jimmunol.181.3.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SE, Schenkel JM, Akue AD, Jameson SC. IL-2 complex treatment can protect naive mice from bacterial and viral infection. J. Immunol. 2010;185:6584–6590. doi: 10.4049/jimmunol.1001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc. Nat. Acad Sci. U.S.A. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Kuchler AM. Interleukin-33—cytokine of dual function or novel alarmin? Trends Immunol. 2009;30:227–233. doi: 10.1016/j.it.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Haring JS, Harty JT. Interleukin-18-related genes are induced during the contraction phase but do not play major roles in regulating the dynamics or function of the T-cell response to Listeria monocytogenes infection. Infect. Immun. 2009;77:1894–1903. doi: 10.1128/IAI.01315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring JS, Jing X, Bollenbacher-Reilley J, Xue HH, Leonard WJ, Harty JT. Constitutive expression of IL-7 receptor alpha does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. J. Immunol. 2008;180:2855–2862. doi: 10.4049/jimmunol.180.5.2855. [DOI] [PubMed] [Google Scholar]
- Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334:825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko AY, Suzuki R, Charles N, Alvarez-Errico D, Sargent JL, Laurence A, Rivera J. Mast cell interleukin-2 production contributes to suppression of chronic allergic dermatitis. Immunity. 2011;35:562–571. doi: 10.1016/j.immuni.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J. Interferon Cytokine Res. 2003;23:513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Nat. Acad. Sci. U.S.A. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo N, Nakamura I, Imawari M. Hepatitis B core antigen stimulates interleukin-10 secretion by both T cells and monocytes from peripheral blood of patients with chronic hepatitis B virus infection. Clin. Exp. Immunol. 2004;135:462–466. doi: 10.1111/j.1365-2249.2003.02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannello A, Boulassel MR, Samarani S, Debbeche O, Tremblay C, Toma E, Routy JP, Ahmad A. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J. Immunol. 2010;184:114–126. doi: 10.4049/jimmunol.0901967. [DOI] [PubMed] [Google Scholar]
- Ingram JT, Yi JS, Zajac AJ. Exhausted CD8 T cells downregulate the IL-18 receptor and become unresponsive to inflammatory cytokines and bacterial co-infections. PLoS Pathog. 2011;7:e1002273. doi: 10.1371/journal.ppat.1002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Hemmi H, Mizenina O, Kuroda S, Suda K, Steinman RM. An IFN-gamma-IL-18 signaling loop accelerates memory CD8+ T cell proliferation. PLoS One. 2008;3:e2404. doi: 10.1371/journal.pone.0002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- Jeannet G, Boudousquie C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc. Nat. Acad. Sci. U.S.A. 2010;107:9777–9782. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Game DS, Davies D, Lombardi G, Lechler RI. Activated CD1d-restricted natural killer T cells secrete IL-2: innate help for CD4+ CD25+ regulatory T cells? Eur. J. Immunol. 2005;35:1193–1200. doi: 10.1002/eji.200425899. [DOI] [PubMed] [Google Scholar]
- Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, Landay A, Martin J, Sinclair E, Asher AI, Deeks SG, Douek DC, Brenchley JM. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J. Infect. Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joeckel LT, Wallich R, Metkar SS, Froelich CJ, Simon MM, Borner C. Interleukin-1R signaling is essential for induction of proapoptotic CD8 T cells, viral clearance, and pathology during lymphocytic choriomeningitis virus infection in mice. J. Virol. 2012;86:8713–8719. doi: 10.1128/JVI.00682-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin seven receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Kambayashi T, Assarsson E, Lukacher AE, Ljunggren HG, Jensen PE. Memory CD8+ T cells provide an early source of IFN-gamma. J. Immunol. 2003;170:2399–2408. doi: 10.4049/jimmunol.170.5.2399. [DOI] [PubMed] [Google Scholar]
- Kaplan DE, Ikeda F, Li Y, Nakamoto N, Ganesan S, Valiga ME, Nunes FA, Rajender Reddy K, Chang KM. Peripheral virus-specific T-cell interleukin-10 responses develop early in acute hepatitis C infection and become dominant in chronic hepatitis. J. Hepatol. 2008;48:903–913. doi: 10.1016/j.jhep.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler SJ, Rosenits K, Koegl T, Vucikuja S, Aichele P. Signal 3 cytokines as modulators of primary immune responses during infections: The interplay of type I IFN and IL-12 in CD8 T cell responses. PLoS One. 2012;7:e40865. doi: 10.1371/journal.pone.0040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonowski KD, Williams KJ, Marzo AL, Lefrancois L. Cutting edge: IL-7-independent regulation of IL-7 receptor alpha expression and memory CD8 T cell development. J. Immunol. 2006;177:4247–4251. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, Carotta S, Donovan CE, Goldman ML, Tailor P, Ozato K, Levy DE, Nutt SL, Calame K, Leonard WJ. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang KS, Recher M, Navarini AA, Harris NL, Lohning M, Junt T, Probst HC, Hengartner H, Zinkernagel RM. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur. J. Immunol. 2005;35:738–745. doi: 10.1002/eji.200425828. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr. Opin. Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Lee DK, Park SH, Yi Y, Choi SG, Lee C, Parks WT, Cho H, de Caestecker MP, Shaul Y, Roberts AB, Kim SJ. The hepatitis B virus encoded oncoprotein pX amplifies TGF-beta family signaling through direct interaction with Smad4: potential mechanism of hepatitis B virus-induced liver fibrosis. Genes Dev. 2001;15:455–466. doi: 10.1101/gad.856201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon SM. Induction and evasion of innate antiviral responses by hepatitis C virus. J. Biol. Chem. 2010;285:22741–22747. doi: 10.1074/jbc.R109.099556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J. Immunol. 2001;166:1097–1105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, Boue F, Molina JM, Rouzioux C, Avettand-Fenoel V, Croughs T, Beq S, Thiebaut R, Chene G, Morre M, Delfraissy JF. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J. Clin. Invest. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kashiwamura S, Ueda H, Sekiyama A, Okamura H. Protection of CD8+ T cells from activation-induced cell death by IL-18. J. Leukocyte Biol. 2007;82:142–151. doi: 10.1189/jlb.0706431. [DOI] [PubMed] [Google Scholar]
- Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr. Opin. Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey CJ, Bhattacharya D, Goldrath AW, Weissman IL, Benoist C, Mathis D. Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc. Nat. Acad. Sci. U.S.A. 2006;103:3304–3309. doi: 10.1073/pnas.0511137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli E, Mueller YM, Lewis MG, Villinger F, Katsikis PD, Roederer M. IL-15 delays suppression and fails to promote immune reconstitution in virally suppressed chronically SIV-infected macaques. Blood. 2011;118:2520–2529. doi: 10.1182/blood-2011-05-351155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Nat. Acad. Sci. U.S.A. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek TR. The biology of interleukin-2. Annu. Rev. Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- Maris CH, Chappell CP, Jacob J. Interleukin-10 plays an early role in generating virus-specific T cell anergy. BMC Immunol. 2007;8:8. doi: 10.1186/1471-2172-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill J, Van Rooijen N, Legge KL. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J. Exp. Med. 2010;207:521–534. doi: 10.1084/jem.20091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingari MC, Gerosa F, Carra G, Accolla RS, Moretta A, Zubler RH, Waldmann TA, Moretta L. Human interleukin-2 promotes proliferation of activated B cells via surface receptors similar to those of activated T cells. Nature. 1984;312:641–643. doi: 10.1038/312641a0. [DOI] [PubMed] [Google Scholar]
- Mitchell DM, Ravkov EV, Williams MA. Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells. J. Immunol. 2010;184:6719–6730. doi: 10.4049/jimmunol.0904089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy MJ, Zhang W, Usherwood EJ. Cutting edge: IL-2 immune complexes as a therapy for persistent virus infection. J. Immunol. 2009;182:4512–4515. doi: 10.4049/jimmunol.0804175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniuszko M, Fry T, Tsai WP, Morre M, Assouline B, Cortez P, Lewis MG, Cairns S, Mackall C, Franchini G. Recombinant interleukin-7 induces proliferation of naive macaque CD4+ and CD8+ T cells in vivo. J. Virol. 2004;78:9740–9749. doi: 10.1128/JVI.78.18.9740-9749.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein–Barr virus gene BCRFI. Science. 1990;248:1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J. Immunol. 2005;175:1686–1693. doi: 10.4049/jimmunol.175.3.1686. [DOI] [PubMed] [Google Scholar]
- Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Nat. Acad. Sci. U.S.A. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller YM, Do DH, Altork SR, Artlett CM, Gracely EJ, Katsetos CD, Legido A, Villinger F, Altman JD, Brown CR, Lewis MG, Katsikis PD. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J. Immunol. 2008;180:350–360. doi: 10.4049/jimmunol.180.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller YM, Petrovas C, Bojczuk PM, Dimitriou ID, Beer B, Silvera P, Villinger F, Cairns JS, Gracely EJ, Lewis MG, Katsikis PD. Interleukin-15 increases effector memory CD8+ t cells and NK Cells in simian immunodeficiency virus-infected macaques. J. Virol. 2005;79:4877–4885. doi: 10.1128/JVI.79.8.4877-4885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Murphy EA, Davis JM, Brown AS, Carmichael MD, Ghaffar A, Mayer EP. Effect of IL-6 deficiency on susceptibility to HSV-1 respiratory infection and intrinsic macrophage antiviral resistance. J. Interferon Cytokine Res. 2008;28:589–595. doi: 10.1089/jir.2007.0103. [DOI] [PubMed] [Google Scholar]
- Nachtwey J, Spencer JV. HCMV IL-10 suppresses cytokine expression in monocytes through inhibition of nuclear factor-kappaB. Viral Immunol. 2008;21:477–482. doi: 10.1089/vim.2008.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K, Hirose S, Yoshimoto T, Ishizashi H, Hiroishi K, Tanaka T, Kono T, Miyasaka M, Taniguchi T, Higashino K. Role and regulation of interleukin (IL)-2 receptor alpha and beta chains in IL-2-driven B-cell growth. Proc. Nat. Acad. Sci. U.S.A. 1992;89:3551–3555. doi: 10.1073/pnas.89.8.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjappa SG, Kim EH, Suresh M. Immunotherapeutic effects of IL-7 during a chronic viral infection in mice. Blood. 2011;117:5123–5132. doi: 10.1182/blood-2010-12-323154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngoi St SM, Rose MC, Menoret AM, Smith DE, Tovey MG, Adler AJ, Vella AT. Presensitizing with a Toll-like receptor 3 ligand impairs CD8 T-cell effector differentiation and IL-33 responsiveness. Proc. Nat. Acad. Sci. U.S.A. 2012;109:10486–10491. doi: 10.1073/pnas.1202607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novy P, Huang X, Leonard WJ, Yang Y. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J. Immunol. 2011;186:2729–2738. doi: 10.4049/jimmunol.1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugeyre MT, Monceaux V, Beq S, Cumont MC, Ho Tsong Fang R, Chene L, Morre M, Barre-Sinoussi F, Hurtrel B, Israel N. IL-7 stimulates T cell renewal without increasing viral replication in simian immunodeficiency virus-infected macaques. J. Immunol. 2003;171:4447–4453. doi: 10.4049/jimmunol.171.8.4447. [DOI] [PubMed] [Google Scholar]
- O’Gorman WE, Sampath P, Simonds EF, Sikorski R, O’Malley M, Krutzik PO, Chen H, Panchanathan V, Chaudhri G, Karupiah G, Lewis DB, Thorne SH, Nolan GP. Alternate mechanisms of initial pattern recognition drive differential immune responses to related poxviruses. Cell Host Microbe. 2010;8:174–185. doi: 10.1016/j.chom.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar JJ, Crist SG, Leung EK, Usherwood EJ. IL-15-independent proliferative renewal of memory CD8+ T cells in latent gammaherpesvirus infection. J. Immunol. 2004;173:2705–2714. doi: 10.4049/jimmunol.173.4.2705. [DOI] [PubMed] [Google Scholar]
- Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrancois L. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc. Nat. Acad. Sci. U.S.A. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohga S, Nomura A, Takada H, Tanaka T, Furuno K, Takahata Y, Kinukawa N, Fukushima N, Imai S, Hara T. Dominant expression of interleukin-10 and transforming growth factor-beta genes in activated T-cells of chronic active Epstein–Barr virus infection. J. Med. Virol. 2004;74:449–458. doi: 10.1002/jmv.20197. [DOI] [PubMed] [Google Scholar]
- Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- Ou R, Zhou S, Huang L, Moskophidis D. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 2001;75:8407–8423. doi: 10.1128/JVI.75.18.8407-8423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owaki T, Asakawa M, Morishima N, Hata K, Fukai F, Matsui M, Mizuguchi J, Yoshimoto T. A role for IL-27 in early regulation of Th1 differentiation. J. Immunol. 2005;175:2191–2200. doi: 10.4049/jimmunol.175.4.2191. [DOI] [PubMed] [Google Scholar]
- Pallikkuth S, Rogers K, Villinger F, Dosterii M, Vaccari M, Franchini G, Pahwa R, Pahwa S. Interleukin-21 administration to rhesus macaques chronically infected with simian immunodeficiency virus increases cytotoxic effector molecules in T cells and NK cells and enhances B cell function without increasing immune activation or viral replication. Vaccine. 2011;29:9229–9238. doi: 10.1016/j.vaccine.2011.09.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmigiani A, Pallin MF, Schmidtmayerova H, Lichtenheld MG, Pahwa S. Interleukin-21 and cellular activation concurrently induce potent cytotoxic function and promote antiviral activity in human CD8 T cells. Hum. Immunol. 2011;72:115–123. doi: 10.1016/j.humimm.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J. Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Calzascia T, Elford AR, Shahinian A, Lin AE, Dissanayake D, Dhanji S, Nguyen LT, Gronski MA, Morre M, Assouline B, Lahl K, Sparwasser T, Ohashi PS, Mak TW. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat. Med. 2009;15:528–536. doi: 10.1038/nm.1953. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M, Assouline B, Lahl K, Sparwasser T, Tedder TF, Paik JH, DePinho RA, Basta S, Ohashi PS, Mak TW. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu. Rev. Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- Pien GC, Nguyen KB, Malmgaard L, Satoskar AR, Biron CA. A unique mechanism for innate cytokine promotion of T cell responses to viral infections. J. Immunol. 2002;169:5827–5837. doi: 10.4049/jimmunol.169.10.5827. [DOI] [PubMed] [Google Scholar]
- Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, Kuchroo VK. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raue HP, Brien JD, Hammarlund E, Slifka MK. Activation of virus-specific CD8+ T cells by lipopolysaccharide-induced IL-12 and IL-18. J. Immunol. 2004;173:6873–6881. doi: 10.4049/jimmunol.173.11.6873. [DOI] [PubMed] [Google Scholar]
- Richter K, Brocker T, Oxenius A. Antigen amount dictates CD8 T-cell exhaustion during chronic viral infection irrespective of the type of antigen presenting cell. Eur. J. Immunol. 2012;42:2290–2304. doi: 10.1002/eji.201142275. [DOI] [PubMed] [Google Scholar]
- Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandau MM, Kohlmeier JE, Woodland DL, Jameson SC. IL-15 regulates both quantitative and qualitative features of the memory CD8 T cell pool. J. Immunol. 2010;184:35–44. doi: 10.4049/jimmunol.0803355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg K, Kemper P, Stalder A, Zhang J, Hobbs MV, Whitton JL, Campbell IL. Altered tissue distribution of viral replication and T cell spreading is pivotal in the protection against fatal lymphocytic choriomeningitis in mice after neutralization of IFN-alpha/beta. J. Immunol. 1994;153:220–231. [PubMed] [Google Scholar]
- Sareneva T, Matikainen S, Kurimoto M, Julkunen I. Influenza a virus-induced IFN-alpha/beta and IL-18 synergistically enhance IFN-gamma gene expression in human T cells. J. Immunol. 1998;160:6032–6038. [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Schmidt CS, Mescher MF. Adjuvant effect of IL-12: conversion of peptide antigen administration from tolerizing to immunizing for CD8+ T cells in vivo. J. Immunol. 1999;163:2561–2567. [PubMed] [Google Scholar]
- Schmidt CS, Mescher MF. Peptide antigen priming of naive, but not memory, CD8 T cells requires a third signal that can be provided by IL-12. J. Immunol. 2002;168:5521–5529. doi: 10.4049/jimmunol.168.11.5521. [DOI] [PubMed] [Google Scholar]
- Schneider R, Yaneva T, Beauseigle D, El-Khoury L, Arbour N. IL-27 increases the proliferation and effector functions of human naive CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur. J. Immunol. 2011;41:47–59. doi: 10.1002/eji.201040804. [DOI] [PubMed] [Google Scholar]
- Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, Battaglia CA, Landay AL, Pahwa S, Fischl MA, Asmuth DM, Tenorio AR, Altman JD, Fox L, Moir S, Malaspina A, Morre M, Buffet R, Silvestri G, Lederman MM. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113:6304–6314. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, Wherry EJ. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- Slobedman B, Barry PA, Spencer JV, Avdic S, Abendroth A. Virusencoded homologs of cellular interleukin-10 and their control of host immune function. J. Virol. 2009;83:9618–9629. doi: 10.1128/JVI.01098-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strestik BD, Olbrich AR, Hasenkrug KJ, Dittmer U. The role of IL-5, IL-6 and IL-10 in primary and vaccine-primed immune responses to infection with friend retrovirus (Murine leukaemia virus) J. Gen. Virol. 2001;82:1349–1354. doi: 10.1099/0022-1317-82-6-1349. [DOI] [PubMed] [Google Scholar]
- Sun J, Dodd H, Moser EK, Sharma R, Braciale TJ. CD4+ T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nat. Immunol. 2011;12:327–334. doi: 10.1038/ni.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J. Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teague TK, Marrack P, Kappler JW, Vella AT. IL-6 rescues resting mouse T cells from apoptosis. J. Immunol. 1997;158:5791–5796. [PubMed] [Google Scholar]
- Teague TK, Schaefer BC, Hildeman D, Bender J, Mitchell T, Kappler JW, Marrack P. Activation-induced inhibition of interleukin 6-mediated T cell survival and signal transducer and activator of transcription 1 signaling. J. Exp. Med. 2000;191:915–926. doi: 10.1084/jem.191.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham EL, Mescher MF. The poststimulation program of CD4 versus CD8 T cells (death versus activation-induced nonresponsiveness) J. Immunol. 2002;169:1822–1828. doi: 10.4049/jimmunol.169.4.1822. [DOI] [PubMed] [Google Scholar]
- Tham EL, Shrikant P, Mescher MF. Activation-induced nonresponsiveness: a Th-dependent regulatory checkpoint in the CTL response. J. Immunol. 2002;168:1190–1197. doi: 10.4049/jimmunol.168.3.1190. [DOI] [PubMed] [Google Scholar]
- Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J. Immunol. 2006;177:1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI. Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity. 2009;31:145–157. doi: 10.1016/j.immuni.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtle CJ, Swanson HM, Fujii N, Estey EH, Riddell SR. A distinct subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity. 2009;31:834–844. doi: 10.1016/j.immuni.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J. Immunol. 2002;169:6842–6849. doi: 10.4049/jimmunol.169.12.6842. [DOI] [PubMed] [Google Scholar]
- van den Broek MF, Muller U, Huang S, Aguet M, Zinkernagel RM. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassena L, Miao H, Cimbro R, Malnati MS, Cassina G, Proschan MA, Hirsch VM, Lafont BA, Morre M, Fauci AS, Lusso P. Treatment with IL-7 prevents the decline of circulating CD4+ T cells during the acute phase of SIV infection in rhesus macaques. PLoS Pathog. 2012;8:e1002636. doi: 10.1371/journal.ppat.1002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Nat. Acad. Sci. U.S.A. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Swiecki M, Cella M, Alber G, Schreiber RD, Gilfillan S, Colonna M. Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe. 2012;11:631–642. doi: 10.1016/j.chom.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RM, Bahl K, Marshall HD, Urban SL. Type 1 interferons and antiviral CD8 T-cell responses. PLoS Pathog. 2012;8:e1002352. doi: 10.1371/journal.ppat.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Nat. Acad. Sci. U.S.A. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- White L, Krishnan S, Strbo N, Liu H, Kolber MA, Lichtenheld MG, Pahwa RN, Pahwa S. Differential effects of IL-21 and IL-15 on perforin expression, lysosomal degranulation, and proliferation in CD8 T cells of patients with human immunodeficiency virus-1 (HIV) Blood. 2007;109:3873–3880. doi: 10.1182/blood-2006-09-045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel M, Crouse J, Bedenikovic G, Sutherland A, Joller N, Oxenius A. Type-I IFN drives the differentiation of short-lived effector CD8+ T cells in vivo. Eur. J. Immunol. 2012;42:320–329. doi: 10.1002/eji.201142091. [DOI] [PubMed] [Google Scholar]
- Williams LD, Bansal A, Sabbaj S, Heath SL, Song W, Tang J, Zajac AJ, Goepfert PA. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J. Virol. 2011;85:2316–2324. doi: 10.1128/JVI.01476-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Brooks DG. The role of IL-10 in regulating immunity to persistent viral infections. Curr. Top. Microbiol. Immunol. 2010;350:39–65. doi: 10.1007/82_2010_96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TS, Lee JM, Lai YG, Hsu JC, Tsai CY, Lee YH, Liao NS. Reduced expression of Bcl-2 in CD8+ T cells deficient in the IL-15 receptor alpha-chain. J. Immunol. 2002;168:705–712. doi: 10.4049/jimmunol.168.2.705. [DOI] [PubMed] [Google Scholar]
- Yang Q, Li G, Zhu Y, Liu L, Chen E, Turnquist H, Zhang X, Finn OJ, Chen X, Lu B. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+ T cells. Eur. J. Immunol. 2011;41:3351–3360. doi: 10.1002/eji.201141629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JS, Cox MA, Zajac AJ. Interleukin-21: a multifunctional regulator of immunity to infections. Microbes Infect. 2010a;12:1111–1119. doi: 10.1016/j.micinf.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010b;129:474–481. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JS, Ingram JT, Zajac AJ. IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J. Immunol. 2010c;185:4835–4845. doi: 10.4049/jimmunol.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue FY, Lo C, Sakhdari A, Lee EY, Kovacs CM, Benko E, Liu J, Song H, Jones RB, Sheth P, Chege D, Kaul R, Ostrowski MA. HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. J. Immunol. 2010;185:498–506. doi: 10.4049/jimmunol.0903915. [DOI] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Hu Z. The enigmatic processing and secretion of interleukin-33. Cell Mol. Immunol. 2010;7:260–262. doi: 10.1038/cmi.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Cerny AM, Fitzgerald KA, Kurt-Jones EA, Finberg RW. Role of IRF7 in T cell responses during an acute lymphocytic choriomeningitis virus infection. J. Virol. 2012 doi: 10.1128/JVI.00576-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Stohlman SA, Parra GI, Bergmann CC. IL-15 independent maintenance of virus-specific CD8(+) T cells in the CNS during chronic infection. J. Neuroimmunol. 2009;207:32–38. doi: 10.1016/j.jneuroim.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]