Abstract
Platelets and erythrocytes are major components of wound provisional scaffolding. In this study, we hypothesized that the concentration of platelets and erythrocytes would significantly affect fibroblast-mediated contraction of three-dimensional scaffolds or the release of cytokines from the scaffold. To test this hypothesis, human anterior cruciate ligament fibroblasts were cultured in one of four scaffolds: a collagen matrix, a collagen-fibrin matrix containing the same concentration of platelets as whole blood, a collagen-fibrin matrix containing a high platelet concentration, and a collagen–fibrin matrix containing a high platelet concentration and red blood cells. Cytokine release from the four groups of gels and gel contraction were measured over a 10-day period. The results of these assays supported greater cytokine release, fibroblast proliferation, and gel contraction in scaffolds with higher platelet concentration. In contrast, the addition of erythrocytes did not significantly stimulate or suppress scaffold contraction or growth factor release from the provisional scaffolds. We concluded that while platelet concentration can significantly impact cytokine release and scaffold retraction in a provisional scaffold, the inclusion of erythrocytes does not have a significant effect on these same behaviors. Therefore, while platelets may be an important regulator of repair processes after injury, it is less likely that erythrocytes have a similar function.
Enhancing healing of ligaments using growth factors has been an area of great interest and research. While the majority of studies have focused on the use of a single growth factor to stimulate healing,1,2 the natural wound healing process is an orchestration of multiple growth factors released by platelets and other cells over time.3 To try to reproduce this in the in vitro and in vivo environment, prior investigators have looked at sustained release carriers1,4 and viral vectors5 for release of these cytokines over days or weeks, as well as examining applications of multiple growth factors.6,7 These studies have shown some additive effects of applied combinations of growth factors on the wound healing of ligaments; however, even with advanced application techniques, the myriad possible combinations of growth factors, timing of release, and concentration of release make optimization of these systems a complex and daunting task.
An alternative method recently used to stimulate healing of the anterior cruciate ligament (ACL) is the application of a substitute provisional scaffolding material containing platelets, erythrocytes, and plasma proteins to the injured tissue.8 Each of these elements is likely to have a significant role in wound healing. When platelets are activated by the exposed collagen of a ligament injury, they begin to aggregate and release multiple growth factors including platelet-derived growth factor (PDGF-AA, PDGF-AB, PDGF-BB),3 members of the transforming growth factor-β family (TGF-β1,3,9,10 TGF-β2), and vascular endothelial growth factor (VEGF).10 Growth factor release typically occurs immediately upon platelet activation and is sustained at much lower levels for the life-span of the platelet—up to 9–10 days in humans. As seen in vivo, these levels of cytokines released locally by these platelet concentrates can result in up-regulation of type I collagen production11 and changes in collagen organization.12 The use of platelets in plasma rather than purified growth factor concentrates provides the added benefit of additional extracellular matrix (ECM) proteins, which also stimulate cellular adhesion and collagen synthesis, particularly in the presence of collagen fibrils.13 In addition, the fact that autologous platelets can be easily obtained and used avoids costs associated with recombinant forms of the individual cytokines and the complications of the disease transmission and immune reactions associated with the use of nonautologous platelets and proteins.
Two of the key cytokines released by platelets and associated specifically with wound contraction are TGF-β1 and the PDGF isoforms. TGF-β1 has been shown to stimulate the differentiation of fibroblasts into myofibroblasts and to also stimulate wound contraction of both collagen and fibrin matrices, likely through driving the resident fibroblasts to a myofibroblast phenotype.14 PDGF-AB has also been shown to stimulate contraction of a collagen matrix by fibroblasts, likely via an integrin-mediated pathway.15 While provisional scaffold contraction during early wound healing is required for initial wound closure as well as the mechanical function of ligamentous tissues, contracture-induced scarring is potentially motion limiting and could result in joint stiffness or arthrofibrosis. Thus, defining and controlling the rate of production of cytokines such as TGF-β1 and PDGF-AB is important in designing provisional substitute scaffolds for use within intra-articular wound sites.
Erythrocytes are also an integral part of the provisional scaffolding in most wounds. Recent reports have presented conflicting data on the ability of erythrocytes to stimulate cellular behaviors important in wound healing, including fibroblast proliferation, ECM contraction, and binding of cytokines within the wound site.16–20 However, prior studies have been limited to in vitro studies using two-dimensional culture techniques or three-dimensional gels21 devoid of many of the plasma proteins critical to the complex ECM of a provisional scaffold. This study is the first study to our knowledge to measure the effects of these cell types in a scaffold with an ECM composition more similar to the provisional scaffolds found in wound sites.
Erythrocytes have been reported to have anabolic effects of inducing fibrosis in the vitreous of the eye,16 as well as stimulating fibroblast secretion of fibronectin18 and matrix metalloproteinase-2.17 Erythrocytes also have been reported to accelerate fibroblast-mediated contraction of collagen gels.18 However, the presence of erythrocytes has also been shown to inhibit proliferation and stimulate apoptosis of fibroblasts in vitro,19 as well as bind inflammatory mediators,20 processes that may be considered more catabolic in the wound site.
However, no prior studies have examined the effect of red blood cells on a model of provisional scaffold, that contained the key elements of platelets and plasma proteins, including fibrin. However, given the results previously reported in other in vitro conditions, we hypothesized that the inclusion of red blood cells in a provisional scaffold model would result in significant changes in scaffold contraction, cellular proliferation, and cytokine release from a provisional scaffold model containing the cellular and ECM components found in vivo. In this study, we tested the hypothesis that platelet and erythrocyte concentration in the provisional scaffold would significantly affect three functional outcomes of the provisional scaffold, namely cytokine release, cumulative scaffold contraction, and cell proliferation.
MATERIALS AND METHODS
Experimental design
Soluble type I collagen was used as the basis of the provisional scaffold models used in this paper. Four types of provisional scaffold were studied: (1) a collagen matrix (COL), (2) a collagen–fibrin matrix containing the same concentration of platelets as whole blood (CF), (3) a collagen–fibrin matrix containing a high platelet concentration (PFC), and (4) a collagen–fibrin matrix containing both red blood cells and the high platelet concentration (R-PFC).
To prepare the provisional scaffolds, a neutralized solution of type I collagen was mixed in a 1 : 1 ratio with one of four cellular fractions. Each fraction was seeded with 6×105 human ACL fibroblasts per milliliter. For the collagen matrix (COL) group, the cells were suspended in phosphate-buffered saline (PBS). For the collagen–fibrin matrix (CF) group, the cells were suspended in a plasma fraction prepared by centrifugation of human blood, which results in retention of some platelets, but very few erythrocytes (details below). For the platelet–collagen–fibrin matrix (PFC), the cells were suspended in a platelet concentrate made using the HARVEST® Smart-PReP®2 System (Harvest Technologies, Plymouth, MA) with a second centrifugation step to remove the majority of the erythrocytes (RBCs). For the group containing erythrocytes (R-PFC), the s tandard protocol for the HARVEST° Smart-PReP®2 System was used.
The provisional scaffold gels were cultured for 10 days and media was collected for measurement of cytokine release at 12 hours, 24 hours, 3 days, 5 days, and 7 days. Scaffolds were measured for the contraction at 1, 5, and 10 days of culture. At those same time points, four samples of each provisional scaffold type were sacrificed for MTT analysis.
As the presence of erythrocytes in the R-PFC group was found to make MTT analysis in this group inaccurate, a second study was performed where ACL cell-seeded scaffolds, containing either platelets, red blood cells, or an aliquot of saline as a control group, were cultured and proliferation within the scaffolds measured using radiolabeling with tritiated thymidine. Histology was also performed on these scaffolds.
ACL cell explant culture
Human ACLs were obtained from patients undergoing ACL reconstruction after IRB approval had been obtained for this use. Synovium was carefully removed from the ligaments and the ACL fascicles cut into small explants 1–2 mm on each side. Explants were cultured in Dulbecco's modified Eagle's medium (DMEM) with 2% Antibiotic–Antimycotic solution (Cellgro, Mediatech Inc., Herndon, VA) and 10% Fetal Bovine Serum (HyClone Inc., South Logan, UT, Cat. #16777-006). When the explants showed sufficient outgrowth, the cells were trypsinized and replated into 75 cm2 flasks and cultured in the same media. At the time of the experiment, first passage human ACL outgrowth cells were trypsinized, counted, and resuspended in one of the four cellular solutions above at 6×105 cells/mL.
Preparation of the collagen hydrogel component
Soluble type I hydrogels were created by neutralizing and warming acid-solubilized type I collagen, which had a concentration of 11.5 mg/mL. The preparation of the collagen has been detailed previously.8 At the time of the experimental start, aliquots of the collagen solution were neutralized and kept on ice until mixing with the cellular fractions noted below.
Preparation of the cellular fractions
To prepare the fibrin, fibrin—platelet, and RBC–fibrin–platelet components of the scaffolds, blood was drawn from each of five hematologically normal volunteers meeting all criteria of the American Association of Blood Banks (Food and Drug Administration, Center for Biologics Evaluation and Research). The blood from each volunteer was drawn into five 60 cm3 syringes each containing 6 cm3 of acid-citrate dextrose. For each donor, the five syringes were pooled in a 300 mL transfer pack at the Center for Blood Research (Boston, MA). For each donor (n=5), all three types of cellular fractions (fibrin, platelet–fibrin, and RBC–platelet–fibrin) were prepared. For the fibrin group (CF), 45 mL from each donor was centrifuged for 6 minutes at 200 g (Beckman GS-6 Centrifuge, Fullerton, CA). The Rernatant was aspirated and collected as the fibrin fraction. For the RBC–platelet–fibrin fraction (R-PFC), the HARVEST® Smart-PReP®2 System was used. A platelet concentrate was produced by the method recommended by the manufacturer. Fifty-four milliliter of whole blood was anticoagulated using 6 mL acid-citrate dextrose and transferred to the blood chamber of the device, and 2 mL ACD was placed in the plasma chamber of the disposable blood processor. Following the separation of plasma from some of the red blood cells by centrifugation for 4 minutes, the platelets, plasma, white blood cells, and remaining red blood cells were decanted into a second chamber and the process continued for 9 minutes at a force to pellet the platelet concentrate. According to the manufacturer's directions, spacers were used, which allowed removal of a limited volume of platelet poor plasma (PPP), leaving 10 mL for the platelet concentrate. The platelet concentrate (PC) was then resuspended in the remaining PPP. This group was maintained as the R-PFC group. To make the PFC group (without erythrocytes), 30 cm3 of the PFC solution from each patient was centrifuged in the Smart-PReP®2 system for an additional 2 minutes. The Rernatant was then aspirated and kept as the RBC-reduced PFC (PFC group).
Provisional scaffold culture
For each experimental group, 12 cell-seeded provisional scaffolds were created in 2 mL centrifuge tubes. Additional ACL cell-free groups were also cultured as controls. Gels were weighed and placed in a 37 °C incubator for 20 minutes before transfer to sterile 12-well plates. One milliliter of DMEM with 2% Antibiotic–Antimycotic solution (Cellgro, Mediatech Inc.) was added to each gel. Samples were cultured in a 37 °C humidified incubator. For donor #1, we did not have enough of each provisional scaffold type to produce the required number of scaffolds for the enzyme-linked immunosorbent assay (ELISA), and thus, the results are only reported for n=4 donors for that assay.
Cytokine release using ELISA assay
Interval release of PDGF-AB, TGF-β1, and VEGF were measured at 12 hours, 24 hours, 3 days, 5 days, and 7 days after provisional scaffold formation. At each time point, media was aspirated from around each sample and replaced with 1 mL of fresh media. Media samples were stored at −80 °C until all samples were collected. Concentrations of human PDGF-AB, TGF-β1, and VEGF were measured using the Quantikine colorimetric sandwich ELISA kits (R&D Systems, Minneapolis, MN). Assays were performed in duplicate on media samples as described in the instructions of the manufacturer and experimental values compared with a standard curve produced with each kit. The color change of the final reaction was measured at a wavelength of 450 nm for the optical density and the standard curve concentrations vs. absorbencies was linear using a four-parameter logistic fit curve. Because of the media sampling technique described above, growth factor concentrations reported in the results section reflect the growth factor release in the time period since the prior media change.
Collagen gel contraction assay
Fibroblast-mediated collagen gel contraction was assessed. The degree of contraction of collagen gels was determined by measuring the area of each gel over time in culture. At 1, 5, and 10 days of culture, the length and width at the gel midpoint was measured directly and recorded.
Effect on cell proliferation: MTT assay
Cell proliferation was measured using a MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay using four scaffolds for each group (n=4) at 1, 5, and 10 days. The MTT was prepared at a concentration of 1 mg/mL in serum-free DMEM from a sterile stock MTT solution (Sigma-Aldrich, St. Louis, MO, Cat. #M5655-500MG) (5 mg/mL PBS). After the media was removed from each well, a sterile spatula was used to transfer each gel to individual wells of a fresh sterile 12-well plate. MTT solution (1.2 mL of 1 mg/mL) was added to each well. Each gel was fully immersed in the MTT solution and incubated for 3 hours (37 °C, 5% CO2). Subsequently, the excess MTT solution was removed and 1 mL of sterile 1×PBS (EMD Chemicals, Gibbstown, NJ, Cat. #B10241-34) was added to each well, placed on a horizontal agitator (Fisher Scientific Clinical Rotator, Pittsburgh, PA) and left to rinse at room temperature for 30 minutes. Rinses were repeated until absorbencies of the wash were < 0.100. All PBS was then removed and each gel transferred with a sterile spatula into a sterile 3.0 mL centrifuge tube. The gels were then placed in 1 mL of a detergent containing 20% aqueous sodium dodecyl sulfate/formamide (1 : 1 volume ratio) and incubated for 5 hours in a 37 °C water bath. Finally, the tubes were centrifuged for 5 minutes at 365 g, and aliquots of the Rernatant from each tube (200 μL) were transferred onto a sterile 96-well plate. The absorbencies were measured at 562 nm. Control groups of gels without cells for each plasma fraction were also prepared and evaluated.
We were unable to assess the effect of the red cell concentration on cell proliferation as the control readings for the cell-free gels were higher than the absorbance reader tolerance, likely due to the high number of red blood cells in the R-PFC preparation; therefore, this group was not included in the results analysis for the MTT assay. To therefore complete testing of our hypotheses, an additional set of experiments was run. Primary outgrowth porcine ACL cells were obtained from six porcine ACLs and seeded in collagen gels containing either porcine platelets, porcine red blood cells, or saline. These scaffolds were cultured in an identical fashion and a radiolabeling assay with tritiated thymidine used to assess cellular proliferation in each group (n=5 per group at each time point).
Statistical analysis
Repeated measures analysis of variance (ANOVA) was used to compare the growth factor release from the four types of provisional scaffolds over time, with values of p < 0.05 considered statistically significant. Two-factor ANOVA for time and group was used to determine the effect of provisional scaffold type on cell proliferation and gel contraction in the cell-seeded gels, with values of p < 0.05 considered statistically significant. Bonferroni–Dunn post hoc testing was used to determine the significance of observed differences between groups in a pairwise analysis.
RESULTS
Platelet and erythrocyte count in the experimental groups
Platelet counts for the whole blood and cellular fractions for each patient are detailed in Table 1. The COL group had no platelets in the scaffolds. The CF fraction had platelets in the cellular component, which averaged 1.6× that of whole blood; thus, the hydrogels in the CF group had a platelet concentration 0.8× that of whole blood when the cellular component was mixed 1 : 1 with the collagen solution.
Table 1.
Platelet counts for each of the blood component preparations
| Platelet concentration (×103(μL) (times baseline) |
||||
|---|---|---|---|---|
| Patient # | Whole blood (baseline) | CF | PFC | R-PFC |
| 1 | 316 | 434 (1.37) | 1287 (4.07) | 919 (2.91) |
| 2 | 204 | 286 (1.40) | 1000 (4.90) | 860 (4.22) |
| 3 | 194 | 249 (1.28) | 794 (4.09) | 715 (3.68) |
| 4 | 318 | 573 (1.80) | 1385 (4.36) | 1215 (3.82) |
| 5 | 246 | 479 (1.95) | 1131 (4.60) | 1057 (4.30) |
| Average | 256 | 404 (1.58) | 1119 (4.38) | 953 (3.73) |
CF, collagen–fibrin; PFC, platelet–fibrin–collagen; R-PFC, RBC–platelet–fibrin–collagen.
The R-PFC and PFC groups had similar levels of platelets (Table 1; Bonferroni–Dunn, post hoc testing, p > 0.10). In both groups, a significant enrichment of the platelet fraction was seen, averaging 3.7× in the R-PFC fraction and 4.4× in the PFC fraction, leading to gels in each group with 1.85× and 2.2× the platelet count in whole blood in the R-PFC and PFC groups, respectively. In the additional set of scaffolds to evaluate cell proliferation, the platelet count was 1,110×103/μL in the platelet solution (enrichment of 3.0× over blood levels), a level similar to that in the PFC group.
The erythrocyte count in the whole blood samples averaged 4.1×106 cells/μL, with an average hematocrit (HCT) of 36.9%. The erythrocyte count in the provisional scaffolding in the COL group was 0, in the CF and PFC groups was 0.044×106 cells/μL (HCT < 0.1% for all scaffolds). For the PFC group, the erythroctye count was 0.12×106 cells/μL (HCT < 2% for all scaffolds) and in the R-PFC group, the erythrocyte count averaged 1.5×106 cells/μL (HCT > 12% for all donors). In the additional set of scaffolds to evaluate cell proliferation, the erythrocyte count in the whole blood samples was 7.36×106 cells/μL with a HCT of 40.8%, levels similar to those in the R-PFC group.
The R-PFC group had total white cell counts over twice as high as the whole blood, a difference that was significant (Table 2; two-factor ANOVA; Bonferroni–Dunn post hoc testing p < 0.007); however, the PFC erythrocyte samples had a similar level to whole blood (p > 0.27). The FC group had a white blood cell concentration of < 20% that in whole blood, a significant reduction (p < 0.007). Granulocyte count was also significantly affected by processing method (two-factor ANOVA, p < 0.001), with post hoc testing showing significant decreases in the FC and PFC erythrocyte groups (p < 0.007), but no reduction in the R-PFC group when compared with whole blood (p > 0.77).
Table 2.
Differential cell counts in total cells per microliter
| WBC and granulocyte concentration (×103 cell/μL_) (times baseline) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Whole blood baseline |
CF |
R-PFC |
PFC |
|||||
| Patient # | WBC | GRN | WBC | GRN | WBC | GRN | WBC | GRN |
| 1 | 3.5 | 2.0 | 1.4 (0.4) | 0.1 (0.05) | 7.7 (2.2) | 0.7 (0.35) | 7.6 (2.2) | 0.3 (0.15) |
| 2 | 3.9 | 2.6 | 1.0 (0.25) | 0 (0) | 7.6 (2.0) | 1.0 (0.4) | 5.4 (1.4) | 0.2 (0.07) |
| 3 | 6.7 | 4.8 | 0.5 (0.07) | 0 (0) | 13.6 (2.0) | 5.6 (1.2) | 4.9 (0.7) | 0.6 (0.13) |
| 4 | 5.5 | 3.3 | 1.1 (0.2) | 0.1 (0.03) | 16.0 (2.9) | 4.3 (1.3) | 8.0 (1.45) | 1.3 (0.4) |
| 5 | 5.0 | 3.3 | 0.5 (0.1) | 0 (0) | 11.0 (2.2) | 3.3 (1.0) | 6.1 (1.22) | 0.7 (0.21) |
| Average | 4.92 | 3.20 | 0.90 (0.18) | 0.04 (0.01) | 11.2 (2.3) | 2.98 (0.9) | 6.40 (1.3) | 0.62 (0.2) |
CF, collagen–fibrin; R-PFC, RBC–platelet–fibrin–collagen; PFC, platelet–fibrin–collagen.
The summary of the cellular elements in the four provisional scaffold models studied here is illustrated schematically in Table 3.
Table 3.
Comparison of blood cell components in the provisional scaffold types
| Provisional Scaffold Group | Collagen | Fibrin | Platelets | RBCs |
|---|---|---|---|---|
| Collagen (COL) | + | − | − | − |
| Collagen–Fibrin (CF) | + | + | + | − |
| Platelet–fibrin–collagen (PFC) | + | + | ++ | − |
| RBC–platelet–fibrin–collagen (R-PFC) | + | + | ++ | + |
−, minimal presence in provisional scaffold; +, concentration in provisional scaffold similar to that in whole blood; ++, concentration in provisional scaffold approximately twice that in whole blood.
Growth factor release
Release of PDGF-AB from the scaffolds was highest in the first 24 hours after scaffold formation for the CF, PFC, and R-PFC groups (Figure 1). No PDGF-AB release was detected from the COL group, which did not contain platelets. Total release over the first 12-hour period ranged from 48±19 ng/mL (mean±standard deviation [SD]) in the CF group to 160±13 ng/mL in the R-PFC group and 206±52 ng/mL in the PFC group. Over time, the levels eluted by all scaffolds decreased, and by day 7, the release from the CF group was < 1 ng/mL while the release from the R-PFC group averaged 2.8±2.5 ng/mL and the PFC group averaged 2.7±0.8 ng/mL. Differences over time between groups were significant, with the R-PFC and PFC groups both having greater release than the CF groups; however, there was no significant difference in PDGF-AB release between the R-PFC and PFC groups (repeated measures ANOVA p < 0.009; Bonferroni–Dunn post hoc testing p < 0.007 for both compare the R-PFC and PFC groups on PDGF-AB release [p > 0.99]).
Figure 1.
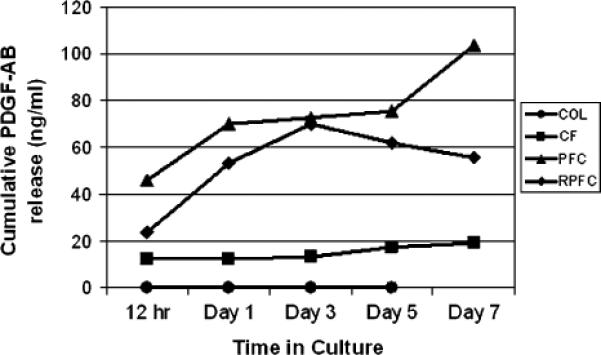
Cumulative release of platelet-derived growth factor-AB (PDGF-AB) over the 7 days of culture. Value markers are the mean with error bars representing the standard deviation for each group. N=5 for all groups and time points with the exception of the day 7 control group where the scaffolds had disintegrated, precluding an accurate measure of PDGF-AB at that time point for that group at that time point.
TGF-β1 release was also time dependent, with the greatest release seen in the first 3 days. In the first 12 hours after scaffold formation, the cumulative release from the scaffolds was 118±100 ng/mL from the CF group, 293±90 ng/mL from the R-PFC group, and 332±128 ng/mL from the PFC group (all values mean±SD). After 3 days, the levels dropped down to zero for all of the scaffolds in the CF group. Elution over the 48-hour period between 5 and 7 days ranged from 7 to 55 ng/mL in the R-PFC scaffolds and from 0 to 76 ng/mL in the PFC scaffolds. There was no significant effect of erythrocyte concentration on TGF-β1 release (repeated measures ANOVA p > 0.20).
VEGF release was also highest in the first 3 days, with values at 12 hours of 20±17 pg/mL for FC group, 646±91 pg/mL for R-PFC, and 477±87 pg/mL for the PFC group (all values are mean±SD). The R-PFC group had the highest release of VEGF (repeated measures ANOVA p < 0.002; BFD p < 0.007 for PC vs. R-PFC and p < 0.001 for PC vs. CF groups).
When all groups were analyzed together, there was a strong positive correlation between platelet count in the plasma preparations and TGF-β1 and PDGF-AB release (Figure 2). For TGF-β1 and PDGF-AB, this was strongest at the 12-hour time point (r2=0.80 and 0.90, respectively) and at the 24-hour time point (r2=0.77 and 0.83, respectively). The strongest correlation between platelet count and VEGF release was seen at 12 hours (r2=0.43), and was similar to the correlation observed between VEGF release and granulocyte count within the scaffolds (r2=0.44).
Figure 2.
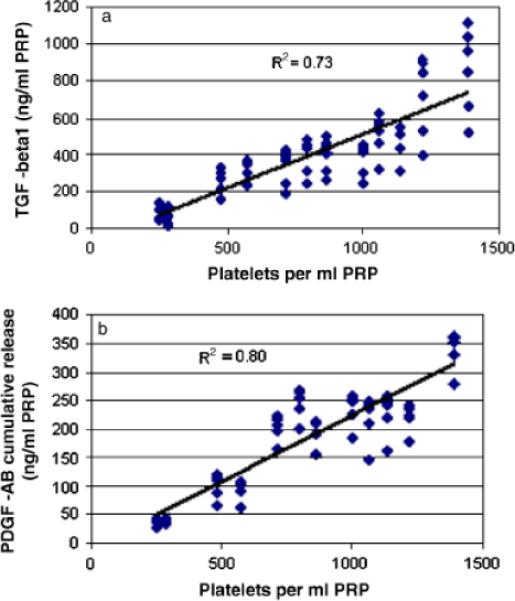
(A) Cumulative transforming growth factor-β1 (TGF-β1) release as a function of platelet concentration in the cellular fractions for the 7-day culture period for all specimens. (B) Cumulative platelet-derived growth factor-AB (PDGF-AB) release as a function of platelet concentration in the plasma preparations for the 7-day culture period for all specimens. For both figures, note the strong direct correlation between cytokine release and platelet concentration (r2=0.73 for TGF-β1 and r2=0.80 for PDGF-AB).
Scaffold contraction as a function of provisional scaffold type
In the fibroblast-free control gels, the greatest amount of scaffold contraction occurred in the first 24 hours after scaffold formation in the cell-free group (Figure 3). By that time, the COL scaffolds had contracted 18±6% and no further contraction was seen in this group. The CF group contracted 25±8% in the first 24 hours, increasing to 37±15% by 10 days. The R-PFC group had contracted 52±16% by 24 hours and an additional 10% by 10 days, and similarly, the PFC group contracted 58±11% by 24 hours, contracting only an additional 8% by the 10-day point.
Figure 3.
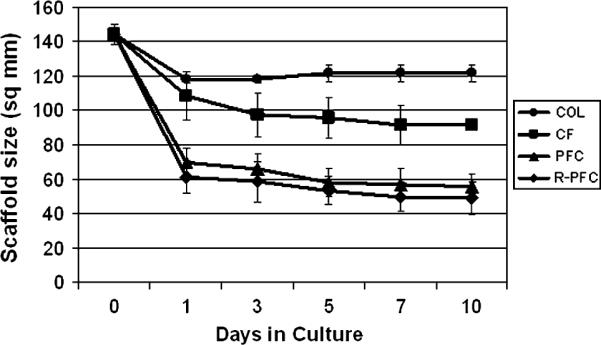
Scaffold size as a function of time in culture for the cell-free scaffolds. The scaffolds containing higher concentration of platelets (PFC and R-PFC groups; p < 0.0001) had significantly greater contraction than those with lower concentrations of platelets (COL and CF groups). The addition of erythrocytes did not have a significant effect on scaffold contraction (PFC vs. R-PFC, p > 0.8). COL, collagen; CF, Collagen–fibrin; PFC, platelet–fibrin–collagen; R-PFC, RBC–platelet–fibrin–collagen.
In the cell-seeded gels, similar contraction results were observed. The majority of the contraction in the R-PFC and PFC groups occurred in the first 24 hours. By 10 days, the CF group had contracted 34±3%, the R-PFC group 52±16%, and the PFC group had contracted 53±19%. In this group, by 10 days, the COL group had started to disintegrate and contraction could not be measured. In addition, scaffolds that were seeded with cells had lower rates of contraction than the cell-free scaffolds in all groups (p < 0.0001).
There was no difference noted between R-PFC and PFC groups (BFD, p > 0.84) for scaffold contraction. However, scaffolds that contained platelets had significantly greater contraction than scaffolds made with CF plasma or COL groups (two-factor ANOVA, p < 0.001 for time and p < 0.0001 for PRP processing group, Bonferroni–Dunn post hoc testing, p < 0.0005 for all comparisons). These results were found in the both the cell-seeded and cell-free gels.
Cell proliferation
The incorporation of CF or PFC cellular fractions in the collagen hydrogel both stimulated cell proliferation in the scaffolds. In the PFC group, there was a 40% increase in cell number over the 10-day period, while in the CF group, there was a 17% increase in the same time period and in the COL scaffolds, the cell number stayed essentially static. This resulted in almost twice as many cells in the PFC scaffolds as in the COL scaffolds at the 10-day time point 0.32±0.04 vs. 0.17±0.03; mean±SD; one-factor ANOVA, p < 0.009). No significant difference was seen between the CF and PFC groups (Bonferroni–Dunn post hoc testing, p > 0.40).
As the cell-free gels in the R-PFC group had a large amount of uptake of the MTT dye, this was thought to be an invalid assay for measuring proliferation within this group. Therefore, a second assay using tritiated thymidine was used to determine the effect of red blood cells on cell proliferation. The results of this assay showed that fibroblast proliferation was completely Rpressed in the presence of erythrocytes in the scaffold at 4 days in culture, with cell-seeded values not higher than the cell-free control values (7,215±761 vs. 11,564±826 c.p.m.; mean±SEM). This was in stark contrast to the platelet and saline groups, which showed significantly higher rates of tritiated thymidine uptake when compared with the erythrocyte group at 4 days (24,682±1,839 c.p.m. for platelet and 23,887±4,279 c.p.m. for saline; mean±SEM; p < 0.0001 for both comparisons). At 14 days, the proliferation rate in the erythrocyte group still remained below the background level (7,268±1,627 c.p.m.), and the proliferation rate in the platelet group had decreased to only 1,080±106 higher than control, while the saline group continued to have high cell proliferation (20,307±1,396 c.p.m. higher than control). These findings were corroborated by the histology of scaffolds at each of these time points, histology scaffolds containing saline (Figure 4A and D), platelets (B and E), and erythrocytes (C and F). At 3 days, the cells in the scaffolds containing saline were round; however, after 2 weeks in culture, the fibroblasts had started to elongate (D). ACL fibroblasts within the scaffolds containing platelets had elongated cytoplasmic extensions even at 3 days, although these were not organized in any preferred direction at this time point (B). By 3 weeks, the fibroblasts in the platelet scaffold had maintained their elongated phenotype (E). In contrast, the scaffolds containing erythrocytes at 3 days had ACL fibroblasts with minimal cell spreading visualized (C) and after 2 weeks in culture, only a few, rounded, ACL fibroblasts were seen in the erythrocyte-rich scaffold (F).
Figure 4.
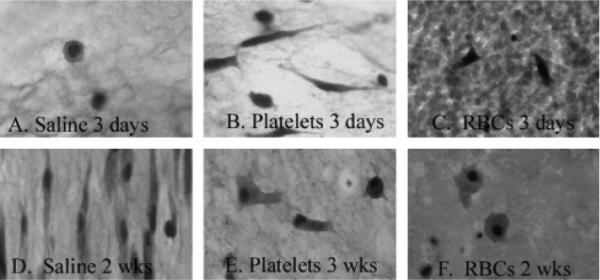
Histology scaffolds containing saline (A and D), platelets (B and E), and erythrocytes (C and F). At 3 days, the cells in the scaffolds containing saline were round; however, after 2 weeks in culture, the fibroblasts had started to elongate (D). Anterior cruciate ligament (ACL) fibroblasts within the scaffolds containing platelets had elongated cytoplasmic extensions even at 3 days (B), a finding maintained at 3 weeks (E). In contrast, the scaffolds containing erythrocytes at 3 days had ACL fibroblasts with minimal cell spreading visualized (C) and after 2 weeks in culture, only a few, rounded, ACL fibroblasts were seen in the erythrocyte-rich scaffold (F).
DISCUSSION
In this study, we tested the hypothesis that platelet and erythrocyte concentration in the provisional scaffold would significantly affect two functional outcomes of the provisional scaffold, namely cytokine release and scaffold contraction. The results showed that platelet concentration had a positive correlation with the release of TGF-β1 and PDGF-AB from the provisional scaffold, as well as on the contraction of the provisional scaffold. The presence of erythrocytes, however, did not result in significant changes in either of the measured parameters in this study. However, the presence of erythrocytes in the scaffold did result in a Rpression of fibroblast proliferation within the collagen-based provisional scaffolds.
Growth factors such as TGF-β1 and PDGF-AB are thought to play an important role in the regulation of ECM deposition and fibrosis in a wide range of tissues, including ligaments.3 TGF-β1 is found in human plasma in both the latent and activated forms, with mean latent levels of approximately 35–40 ng/mL22,23 and activated forms reported to range from 1.23 to 5.3 ng/mL.24–26 In synovial fluid, the TGF-β1 level in osteoarthritic joints is 3.8 ng/mL.27 Increases of two to three times the normal plasma value of active TGF-β1 have been shown to cause renal fibrosis.28 Therefore, changes in activated TGF-β1 concentration as small as 1 ng/mL can have clinically important biologic effects. Thus, in this paper, the fact that collagen can stimulate platelet release of TGF-β1 in the range of 300 ng/mL over the first 12 hours with sustained release of 15 ng/mL over 48 hours at 10 days after platelet activation suggests that the sustained release of TGF-β1 from collagen–PRP gels is in a clinically significant range. The positive correlation between platelet count and TGF-β1 release is consistent with that previously reported for calcium-stimulated PRP activation.10
PDGF-AB has a normal plasma level of approximately 41 ng/mL.23 In this paper, the PDGF-AB release from the collagen–PRP gels within the first 12 hours was between 150 and 400 ng/mL, and even at 10 days remained elevated at 1.9 ng/mL over 48 hours. Thus, the initial release of PDGF-AB into the wound milieu is at Rra-physiologic levels, but quickly drops off to levels well below that in the circulating plasma. The correlation observed in this paper between platelet concentration and PDGF-AB release is consistent with previous reports.10
The major contribution of VEGF to the wound healing process is currently thought to be in stimulating angiogenesis; however, the roles of this complex molecule in wound healing are still being defined29 with roles postulated in ECM production,30 cell recruitment,31 and cellular proliferation.32 VEGF is found in concentrations of 97–266 pg/mL in normal control serum,33 and is elevated in the serum of patients with rheumatoid arthritis (590 pg/mL) and osteoarthritis (287 pg/mL).33 Thus, the levels of initial release in this study of 250–1,000 pg/mL are in a range approaching that of differences between normal and diseased serum and may be clinically relevant. The positive correlation between granulocyte count and VEGF release is consistent with the fact that VEGF is stored in granulocytes specifically.34 VEGF is also known to be secreted by fibroblasts, a finding consistent with increased release of VEGF from ACL cell-seeded gels seen in this study. Of note, the R-PFC group had total white cell counts over twice as high as the whole blood, a difference that was significant (Table 1; two-factor ANOVA; Bonferroni–Dunn post hoc testing p < 0.007); however, the PFC samples had a similar level to whole blood (p > 0.27). It is possible that these differences in white blood cell concentration may be at least partially responsible for the differences in VEGF release from the groups of provisional scaffolds with and without erythrocytes.
The amount of scaffold contraction seen in these experiments is lower than that in previous reports.12 Possible explanations for this phenomenon is that the collagen-based scaffolds used here had a greater collagen concentration in the lattices (3.5 mg/mL here vs. 1.95 mg/mL in previous studies12), which resulted in stiffer collagen scaffolds. Other studies where a higher collagen density has been used have reported rates of collagen contraction similar to those reported here (60% at 24 hours35 vs. 50% reported here for the platelet-containing scaffolds). This higher collagen concentration has proved extremely useful in constructing scaffolds for applications where minimal scaffold contraction is desirable, particularly for filling wound sites in vivo. The stiffer collagen matrices may require greater forces to contract, which may explain in part the lower rates of contraction reported here.
In addition, in this study for scaffolds where no platelets were incorporated, there was far less contraction of the scaffolds—only 14%. As all other parameters for the scaffolds in this study were similar, this suggests that the presence of platelets significantly increases the rate of collagen-fibrin scaffold contraction, a finding consistent with that reported for human lung fibroblasts.21 The fact that the scaffolds containing ACL cells actually contracted less than the scaffolds that did not contain cells suggests that the early contraction is more likely to be related to the contractile force generated by the platelet microfilament system36 than a fibroblast-mediated response.
The higher concentration of platelets in the PFC group resulted in increased cell proliferation of ACL cells within the scaffolds over scaffolds made with a lower platelet concentration (CF) and also resulted in increased cell contraction of the scaffolds. Increased cell proliferation may be useful in situations where few cells are available in situ to repopulate a wound scaffold. In addition, the association between increased growth factor release from the gels and increased cell proliferation is also consistent with prior studies documenting the mitogenic effects of PDGF-AB on ligament cells.14,37 However, the higher contraction of the scaffolds may be detrimental, as in these applications, premature loss of filling volume due to contraction may result in incomplete wound healing.
The suppression of cellular proliferation seen in the scaffolds containing erythrocytes is consistent with prior reports of erythrocytes inhibiting proliferation and stimulating apoptosis of fibroblasts in vitro.19 However, it is more difficult to reconcile these results with prior reports of erythrocytes inducing fibrosis,16 which is typically a fibroblast-mediated process. The histologic sections of erythrocyte populated scaffolds would suggest that the presence of erythrocytes inhibits fibroblast attachment and spreading to the collagenous matrices; however, more work is certainly needed to evaluate this observation even as a hypothesis.
In our prior studies, we have not had difficulties with ACL fibroblasts proliferating, producing collagen, or contracting collagen–PRP scaffolds made in our laboratory,5,38 and indeed, in this study, we did not see any problems with cell proliferation in all scaffolds that did not contain high numbers of erythrocytes, which suggests the cells were viable. Additional studies using this same formulation in vivo have found it to be effective in vivo in stimulating both return of mechanical function of a healing wound8,39 and stimulating histologic healing of a nonunion site within a ligament as well.40 Thus, it is relatively unlikely that the lack of proliferation in the erythrocyte containing scaffolds was a result of the other components of this scaffold.
In conclusion, the level of platelets in the provisional scaffold significantly affects the release of growth factors, as well as cellular behaviors in the scaffolds and scaffold contraction. Although the levels of cytokine release measured in this study are within the range of physiologic levels, future work is needed to determine if the amounts of release measured here will have a significant effect on in vivo wound healing.
ACKNOWLEDGMENTS
This study was Rported by CIMIT through DoD funding under cooperative agreement no. DAMD17-02-2-0006 and NIH grant R01AR054099. Salary Rport was provided by NIH grant K02 AR049346 (MMM). Rport was also provided by the Center for Blood Research, Boston, MA.
REFERENCES
- 1.Spindler KP, Dawson JM, Stahlman GC, Davidson JM, Nanney LB. Collagen expression and biomechanical response to human recombinant transforming growth factor beta (rhTGF-beta2) in the healing rabbit MCL. J Orthop Res. 2002;20:318–24. doi: 10.1016/S0736-0266(01)00107-3. [DOI] [PubMed] [Google Scholar]
- 2.Batten ML, Hansen JC, Dahners LE. Influence of dosage and timing of application of platelet-derived growth factor on early healing of the rat medial collateral ligament. J Orthop Res. 1996;14:736–41. doi: 10.1002/jor.1100140509. [DOI] [PubMed] [Google Scholar]
- 3.Lee J, Harwood FL, Akeson WH, Amiel D. Growth factor expression in healing rabbit medial collateral and anterior cruciate ligaments. Iowa Orthop J. 1998;18:19–25. [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi D, Kurosaka M, Yoshiya S, Mizuno K. Effect of basic fibroblast growth factor on the healing of defects in the canine anterior cruciate ligament [see comments] Knee Surg Sports Traumatol Arthrosc. 1997;5:189–94. doi: 10.1007/s001670050049. [DOI] [PubMed] [Google Scholar]
- 5.Pascher A, Steinert AF, Palmer GD, Betz O, Gouze JN, Gouze E, Pilapil C, Ghivizzani SC, Evans CH, Murray MM. Enhanced repair of the anterior cruciate ligament by in situ gene transfer: evaluation in an in vitro model. Mol Ther. 2004;10:327–36. doi: 10.1016/j.ymthe.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Spindler KP, Murray MM, Detwiler KB, Tarter JT, Dawson JM, Nanney LB, Davidson JM. The biomechanical response to doses of TGF-beta 2 in the healing rabbit medial collateral ligament. J Orthop Res. 2003;21:245–9. doi: 10.1016/S0736-0266(02)00145-6. [DOI] [PubMed] [Google Scholar]
- 7.Letson AK, Dahners LE. The effect of combinations of growth factors on ligament healing. Clin Orthop. 1994;308:207–12. [PubMed] [Google Scholar]
- 8.Murray MM, Spindler KP, Devin C, Snyder BS, Muller J, Takahashi M, Ballard P, Nanney LB, Zurakowski D. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820–30. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 9.Frechette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84:434–9. doi: 10.1177/154405910508400507. [DOI] [PubMed] [Google Scholar]
- 10.Anitua E, Andia I, Sanchez M, Azofra J, del Mar Zalduendo M, de la Fuente M, Nurden P, Nurden AT. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res. 2005;23:281–6. doi: 10.1016/j.orthres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Kawase T, Okuda K, Wolff LF, Yoshie H. Platelet-rich plasma-derived fibrin clot formation stimulates collagen synthesis in periodontal ligament and osteoblastic cells in vitro. J Periodontol. 2003;74:858–64. doi: 10.1902/jop.2003.74.6.858. [DOI] [PubMed] [Google Scholar]
- 12.MacNeil RL, D'Errico J, Strayhorn C, Pickrum H, Somerman MJ. Agents with periodontal regenerative potential regulate cell-mediated collagen lattice contraction in vitro. J Dent Res. 1996;75:903–11. doi: 10.1177/00220345960750030701. [DOI] [PubMed] [Google Scholar]
- 13.Kawase T, Okuda K, Saito Y, Yoshie H. In vitro evidence that the biological effects of platelet-rich plasma on periodontal ligament cells is not mediated solely by constituent transforming-growth factor-beta or platelet-derived growth factor. J Periodontol. 2005;76:760–7. doi: 10.1902/jop.2005.76.5.760. [DOI] [PubMed] [Google Scholar]
- 14.Murray MM, Rice K, Wright RJ, Spector M. The effect of selected growth factors on human anterior cruciate ligament cell interactions with a three-dimensional collagen-GAG scaffold. J Orthop Res. 2003;21:238–44. doi: 10.1016/S0736-0266(02)00142-0. [DOI] [PubMed] [Google Scholar]
- 15.Grundstrom G, Mosher DF, Sakai T, Rubin K. Integrin alphavbeta3 mediates platelet-derived growth factor-BB-stimulated collagen gel contraction in cells expressing signaling deficient integrin alpha2beta1. Exp Cell Res. 2003;291:463–73. doi: 10.1016/j.yexcr.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Katakami C, Raymond LA, Lipman MJ, Appel A, Kao WW. Change in the synthesis of glycosaminoglycans by fibrotic vitreous induced by erythrocytes. Biochim Biophys Acta. 1986;880:40–5. doi: 10.1016/0304-4165(86)90117-0. [DOI] [PubMed] [Google Scholar]
- 17.Fredriksson K, Liu XD, Lundahl J, Klominek J, Rennard SI, Skold CM. Red blood cells increase secretion of matrix metalloproteinases from human lung fibroblasts in vitro. Am J Physiol Lung Cell Mol Physiol. 2006;290:L326–33. doi: 10.1152/ajplung.00057.2005. [DOI] [PubMed] [Google Scholar]
- 18.Fredriksson K, Lundahl J, Fernvik E, Liu XD, Rennard SI, Skold CM. Red blood cells stimulate fibroblast-mediated contraction of three dimensional collagen gels in co-culture. Inflamm Res. 2002;51:245–51. doi: 10.1007/pl00000300. [DOI] [PubMed] [Google Scholar]
- 19.Fredriksson K, Stridh H, Lundahl J, Rennard SI, Skold CM. Red blood cells inhibit proliferation and stimulate apoptosis in human lung fibroblasts in vitro. Scand J Immunol. 2004;59:559–65. doi: 10.1111/j.1365-3083.2004.01433.x. [DOI] [PubMed] [Google Scholar]
- 20.Neote K, Darbonne W, Ogez J, Horuk R, Schall TJ. Identification of a promiscuous inflammatory peptide receptor on the surface of red blood cells. J Biol Chem. 1993;268:12247–9. [PubMed] [Google Scholar]
- 21.Zagai U, Fredriksson K, Rennard SI, Lundahl J, Skold CM. Platelets stimulate fibroblast-mediated contraction of collagen gels. Respir Res. 2003;4:13. doi: 10.1186/1465-9921-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114:1502–8. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 23.Okuda K, Kawase T, Momose M, Murata M, Saito Y, Suzuki H, Wolff LF, Yoshie H. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol. 2003;74:849–57. doi: 10.1902/jop.2003.74.6.849. [DOI] [PubMed] [Google Scholar]
- 24.Wang XL, Liu SX, Wilcken DE. Circulating transforming growth factor beta 1 and coronary artery disease. Cardiovasc Res. 1997;34:404–10. doi: 10.1016/s0008-6363(97)00033-3. [DOI] [PubMed] [Google Scholar]
- 25.Clausen T, Djurovic S, Reseland JE, Berg K, Drevon CA, Henriksen T. Altered plasma concentrations of leptin, transforming growth factor-beta(1) and plasminogen activator inhibitor type 2 at 18 weeks of gestation in women destined to develop pre-eclampsia. Circulating markers of disturbed placentation? Placenta. 2002;23:380–5. doi: 10.1053/plac.2002.0828. [DOI] [PubMed] [Google Scholar]
- 26.Dziadzio M, Smith RE, Abraham DJ, Black CM, Denton CP. Circulating levels of active transforming growth factor beta1 are reduced in diffuse cutaneous systemic sclerosis and correlate inversely with the modified Rodnan skin score. Rheumatology (Oxford) 2005;44:1518–24. doi: 10.1093/rheumatology/kei088. [DOI] [PubMed] [Google Scholar]
- 27.Fava R, Olsen N, Keski-Oja J, Moses H, Pincus T. Active and latent forms of transforming growth factor beta activity in synovial effusions. J Exp Med. 1989;169:291–6. doi: 10.1084/jem.169.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanderson N, Factor V, Nagy P, Kopp J, Kondaiah P, Wakefield L, Roberts AB, Sporn MB, Thorgeirsson SS. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci USA. 1995;92:2572–6. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu C, Chang J. Clinical implications of growth factors in flexor tendon wound healing. J Hand Surg [Am] 2004;29:551–63. doi: 10.1016/j.jhsa.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Le AD, Zhang Q, Wu Y, Messadi DV, Akhondzadeh A, Nguyen AL, Aghaloo TL, Kelly AP, Bertolami CN. Elevated vascular endothelial growth factor in keloids: relevance to tissue fibrosis. Cells Tissues Organs. 2004;176:87–94. doi: 10.1159/000075030. [DOI] [PubMed] [Google Scholar]
- 31.Ohtani K, Egashira K, Hiasa K, Zhao Q, Kitamoto S, Ishibashi M, Usui M, Inouye S, Yonemitsu Y, Sueishi K, Sata M, Shibuya M, Sunagawa K. Blockade of vascular endothelial growth factor Rpresses experimental restenosis after intraluminal injury by inhibiting recruitment of monocyte lineage cells. Circulation. 2004;110:2444–52. doi: 10.1161/01.CIR.0000145123.85083.66. [DOI] [PubMed] [Google Scholar]
- 32.Herve MA, Buteau-Lozano H, Mourah S, Calvo SF, Perrot-Applanat M. VEGF189 stimulates endothelial cells proliferation and migration in vitro and up-regulates the expression of Flk-1/KDR mRNA. Exp Cell Res. 2005;309:24–31. doi: 10.1016/j.yexcr.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Lee SS, Joo YS, Kim WU, Min DJ, Min JK, Park SH, Cho CS, Kim HY. Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2001;19:321–4. [PubMed] [Google Scholar]
- 34.Nielsen HJ, Werther K, Mynster T, Brunner N. Soluble vascular endothelial growth factor in various blood transfusion components. Transfusion. 1999;39:1078–83. doi: 10.1046/j.1537-2995.1999.39101078.x. [DOI] [PubMed] [Google Scholar]
- 35.Garrett Q, Khaw PT, Blalock TD, Schultz GS, Grotendorst GR, Daniels JT. Involvement of CTGF in TGF-beta1-stimulation of myofibroblast differentiation and collagen matrix contraction in the presence of mechanical stress. Invest Ophthalmol Vis Sci. 2004;45:1109–16. doi: 10.1167/iovs.03-0660. [DOI] [PubMed] [Google Scholar]
- 36.Jen CJ, McIntire LV. The structural properties and contractile force of a clot. Cell Motil. 1982;2:445–55. doi: 10.1002/cm.970020504. [DOI] [PubMed] [Google Scholar]
- 37.Matsuda N, Lin WL, Kumar NM, Cho MI, Genco RJ. Mitogenic, chemotactic, and synthetic responses of rat periodontal ligament fibroblastic cells to polypeptide growth factors in vitro. J Periodontol. 1992;63:515–25. doi: 10.1902/jop.1992.63.6.515. [DOI] [PubMed] [Google Scholar]
- 38.Murray MM, Forsythe B, Chen F, Lee SJ, Yoo JJ, Atala A, Steinert A. The effect of thrombin on ACL fibroblast interactions with collagen hydrogels. J Orthop Res. 2006;24:508–15. doi: 10.1002/jor.20054. [DOI] [PubMed] [Google Scholar]
- 39.Murray MM, Spindler KP, Abreu E, Muller JA, Nedder A, Kelly M, Frino J, Zurakowski D, Valenza M, Snyder BD, Connolly SA. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 40.Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen–platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–17. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]


