Abstract
Objective
To summarize recommended updates to the 2001 Stages of Reproductive Aging Workshop (STRAW) criteria. The 2011 STRAW+10 workshop reviewed advances in understanding of the critical changes in hypothalamic-pituitary-ovarian function that occur before and after the final menstrual period (FMP).
Methods
Scientists from five countries and multiple disciplines evaluated data from cohort studies of midlife women and in the context of chronic illness and endocrine disorders, on change in menstrual, endocrine and ovarian markers of reproductive aging including anti-mullerian hormone (AMH), inhibin B, follicle-stimulating hormone (FSH), and antral follicle count (AFC). Modifications were adopted by consensus.
Results
STRAW+10 simplified bleeding criteria for the early and late menopausal transition, recommended modifications to criteria for the late reproductive stage (Stage − 3) and the early post-menopause stage (Stage +1), provided information on the duration of the late transition (Stage −1) and early post-menopause (Stage +1) and recommended application regardless of women’s age, ethnicity, body size or lifestyle characteristics.
Conclusion
STRAW+10 provides a more comprehensive basis for assessing reproductive aging in research and clinical contexts. Application of the STRAW+10 staging system should improve comparability of studies of midlife women and facilitate clinical decision-making. Nonetheless, important knowledge gaps persist and seven research priorities are identified.
Keywords: reproductive aging; ovarian aging; menopause; follicle-stimulating hormone; anti-mullerian hormone; antral follicle count, inhibin B
The 2001 Stages of Reproductive Aging Workshop (STRAW) proposed nomenclature and a staging system for ovarian aging including menstrual and qualitative hormonal criteria to define each stage.1-4 The STRAW criteria are widely considered the gold standard for characterizing reproductive aging through menopause, just as the Marshall-Tanner Stages characterize pubertal maturation5. Research conducted over the past ten years has advanced knowledge of the critical changes in hypothalamic-pituitary and ovarian function that occur before and after the final menstrual period. These advances were the topic of a follow-up workshop STRAW+10: Addressing the Unfinished Agenda of Staging Reproductive Aging (STRAW+10). STRAW+10, held in Washington DC on September 20 and 21, 2011, reviewed these scientific advances and updated the STRAW model. The sponsors were the National Institutes of Health National Institute of Aging (NIA) and Office of Research on Women’s Health (ORWH), the North American Menopause Society (NAMS), the American Society for Reproductive Medicine (ASRM), the International Menopause Society (IMS) and The Endocrine Society. The STRAW+10 workshop achieved the following aims:
To re-evaluate criteria for the onset of late reproductive life and the early menopause given new population-based data relating to follicle-stimulating hormone (FSH), antral follicle count (AFC), anti-mullerian hormone (AMH) and inhibin-B;
To re-evaluate criteria for staging the postmenopause given new population based data on FSH and estradiol trajectories following the final menstrual period (FMP);
To re-evaluate exclusion of women based on body size, lifestyle characteristics and health status; and,
4. To identify remaining gaps in scientific knowledge and research priorities.
Background and Significance
The menopausal transition marks a period of physiologic changes as women approach reproductive senescence. Evidence supports the clinical importance of the transition for many women as a period of temporal changes in health and quality of life (i.e., vasomotor symptoms, sleep disturbance, depression) and longer term changes in several health outcomes (i.e., urogenital symptoms, bone, lipids)6-15, that may influence women’s quality of life and likelihood of healthy aging. As a standardized staging system for reproductive aging, STRAW made a substantial contribution to women’s health research by providing consistent classification of menopausal status for studies of midlife women. Importantly, STRAW facilitated research aimed at distinguishing health effects of ovarian versus somatic aging. STRAW criteria also serve as a clinical tool for women and their health care providers to guide assessment of fertility, contraceptive needs and healthcare decision-making.16, 17
Building upon prior consensus meetings of the World Health Organization and the Council of Affiliated Menopause Societies18, STRAW re-evaluated nomenclature, proposed a standardized staging system and recommended criteria for defining onset of each stage. STRAW participants evaluated potential criteria including menstrual cycles, endocrinologic parameters including FSH, estradiol, AMH, and Inhibin-B, symptoms, fertility, and ovarian imaging including AFC. Of the candidate biomarkers considered in 2001, only FSH was consistently measurable in a clinical setting. Data were insufficient to define quantitative criteria for FSH or to clarify the precise timing of change in FSH trajectories. Information on AFC and on the relationship between AMH, inhibin B and the timing of ovarian aging was limited. Symptoms were acknowledged to be subjective and not universally experienced. STRAW therefore restricted staging recommendations to menstrual cycle bleeding criteria and qualitative FSH criteria.
Seven Stages of STRAW
STRAW divided the adult female life into 3 broad phases: reproductive, menopausal transition, and postmenopause. These 3 phases included a total of 7 stages centered around the final menstrual period (FMP, Stage 0).1-4 The reproductive phase was divided into Stage −5, −4 and −3 corresponding to early, peak and late, respectively. The menopausal transition phase consisted of Stage −2 (early) and Stage −1 (late), and the postmenopause phase contained Stage +1 (early) and +2 (late). Stage −3 was characterized by regular menstrual cycles and increasing levels of FSH. Stage −2 was characterized by variability in menstrual cycle length and increased levels of FSH. Stage −1 was characterized by onset of skipped cycles or amenorrhea of at least 60 days and continued elevation of FSH.
The ReSTAGE Collaboration subsequently conducted empirical analyses to assess the validity and reliability of STRAW’s menstrual cycle criteria in four cohort studies – the TREMIN study, the Melbourne Women’s Midlife Health Project (MWMHP), the Seattle Midlife Women’s Health Study (SMWHS) and the Study of Women’s Health Across the Nation (SWAN). Findings supported STRAW’s recommendations, provided more precise specification of menstrual criteria for early and late transition and recommended a quantitative cutpoint for FSH levels characteristic of the late transition19-23.
Generalizability
A limitation of the original STRAW was its recommendation, based on the available evidence, that the criteria only be applied to healthy women. STRAW explicitly recommended against applying the criteria to 7 subgroups of women1-4, including smokers (19% of US women aged 45-6424), women with a body mass index (BMI) >30 kg/m2 (38% of US women25) and women who had undergone hysterectomy (35% of US women26). Women engaged in heavy aerobic exercise and women with chronic menstrual cycle irregularities, uterine abnormalities or ovarian abnormalities were also excluded. Another limitation of STRAW was lack of insight regarding staging in diverse populations. In 2001 few data were available from studies of multi-ethnic or diverse socio-economic populations. Recent data from multi-ethnic cohorts now permit assessment of generalizability17, 22, 27-34, although data from low resource countries remain quite limited.35, 36
STRAW had a sustained influence on research in the field, prompting assessment of trajectories of change in endocrine levels and biomarkers of ovarian senescence as well as evaluation of how these trajectories vary by body size, smoking, ethnicity and other factors27-34, 37-53. Ten years later, understanding of ovarian aging and its endocrine and clinical correlates has advanced considerably, providing a more nuanced understanding of the critical junctures that occur during reproductive aging before and after the final menstrual period (FMP). The role of AMH and inhibin B as markers of fertility decline and ovarian aging is more clearly understood as are the relationships among patterns of decline in AMH, inhibin B, AFC and primordial follicle counts27, 41, 42, 44-46, 49, 51, 54-59. The goal of STRAW+10 was to review significant advances in the field and develop recommendations for updating the original STRAW criteria.
Methods
STRAW+10 involved a 2-day, in-person meeting hosted at the 2011 Annual Meeting of NAMS. On the first day, international experts gave oral presentations reviewing recent data bearing on the goals, as part of a public symposium, followed by comments and discussion from the audience. The first two sessions focused on data from prospective cohort studies of midlife women, clinical findings related to trajectories of change in menstrual, endocrine and ovarian markers of reproductive aging, and data relevant to how these trajectories vary by ethnicity, body size, and smoking status. A particular focus was on patterns of change in AMH, inhibin B, FSH, estradiol and AFC and their inter-relationships. A third session focused on emerging evidence related to staging reproductive aging in the context of cancer treatment, chronic illness including cancer and HIV-AIDS, and endocrine disorders including polycystic ovarian syndrome (PCOS) and primary ovarian insufficiency (POI, otherwise known as premature ovarian failure). At the end of day one, a panel reviewed and participants discussed modifications that had been proposed by symposium speakers. STRAW+10 explicitly considered feasibility of applying criteria in low resource countries.
Subsequently, 41 invited scientists convened to develop consensus and propose modifications to the STRAW model. These participants had clinical and/or research experience in female reproductive aging and included scientists from several key research groups in the United States, Canada, Australia, the Netherlands and South Africa, representatives from the NIH funded cohort studies of midlife women that have biological samples60 including SWAN, the Michigan Bone Health and Metabolism Study (MBHMS), SMWHS, Biodemographic Models of Reproductive Aging (BIMORA), and the Penn Ovarian Aging Study (POAS) as well as the Australian MWMHP, as well as junior investigators who submitted qualifying posters.
Three breakout groups were formed based on scientific expertise and interest. Group 1 reviewed criteria for STRAW Stages −4 to −2. Group 2 reviewed criteria for STRAW Stages −1 to +2. Each of these two groups was subdivided into two subgroups and assigned a rapporteur. Each subgroup proposed modifications to the STRAW paradigm separately, considering criteria for the relevant stages in healthy women and the weight of evidence concerning the appropriateness of applying these criteria to smokers and women regardless of body size. Each subgroup of Group 1 and of Group 2 then reviewed the recommendations of their paired subgroup and discussed points of disagreement until consensus was reached. Group 3 discussed staging in the context of endocrine disorders and chronic illness and proposed modifications. This group then integrated with one of the Group 1 or Group 2 subgroups.
On the second day, the 41 scientists convened to review and discuss proposed modifications. First, Group 1 and Group 2 reviewed the other group’s recommendations proposed on the previous day. In this way, all groups reviewed all stages under consideration (Stages −4 to +2) Then, the group at-large met to discuss each proposal and final recommendations were adopted by consensus. Preliminary recommendations of the STRAW+10 Workshop were presented at the NAMS annual meeting on September 22 with comments and requests for clarification considered by the STRAW+10 program committee.
Results
STRAW+10 retained the criteria for an ideal staging system employed by the 2001 Workshop. Thus a staging system should:
Rely on objective data;
Use widely available, reliable, noninvasive and inexpensive tests;
Allow for prospective classification of women; and
Permit unambiguous classification of women into a unique stage.
In addition, it was concluded that the modified staging system should:
5. Retain widely accepted nomenclature
6. Consider menstrual cycle criteria to be paramount given continuing lack of international standardization of biomarker assays as well as their cost and/or invasiveness, particularly in the context of resource poor countries
7. Consider biomarkers only as supportive criteria given the lack of standardization. Supportive criteria are to be used only as necessary and should not be interpreted as required for diagnosis.
8. Use criteria that are independent of age, symptoms and pathology. As no universal menopausal syndrome has been established across ethnic groups61, two key symptoms are incorporated only as descriptive additional information that may be supportive62.
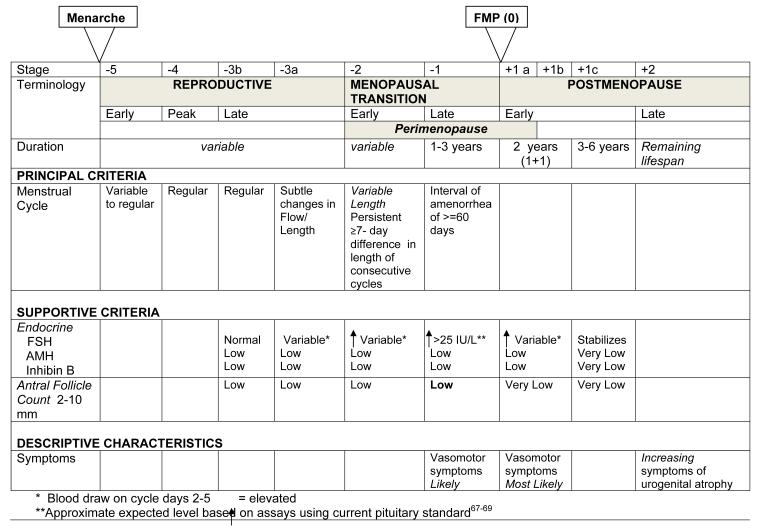
The revised STRAW+10 Staging System is presented in Figure 1. STRAW+10 recommended acceptance of the ReSTAGE Collaboration’s more precise and simplified specification of the menstrual cycle criteria for the early and late menopausal transition and concurred with ReSTAGE recommendations that quantification of the FSH criteria in STAGE −1 is possible given improved standardization of this assay. In addition, STRAW+10 recommended modifications to criteria for the late reproductive stage (Stage −3) as well as the early post-menopause stage (Stage +1) and provided information on the duration of the late transition (Stage −1) and early post-menopause (Stage +1) stages. Although additional biomarkers, especially AMH and AFC, have considerable promise, lack of standardized assays and data from non-infertility populations remain important limitations to their incorporation into a staging model and their utility as a clinical tool for staging reproductive aging. Nonetheless, the revised STRAW+10 Staging System includes qualitative criteria for these biomarkers during late reproductive life when relative changes in these parameters have important consequences for fertility potential.
Figure 1.
The STRAW+10 Staging System for Reproductive Aging in Women
The definition and rationale for key revisions to the staging criteria include: LATE REPRODUCTIVE STAGE (Stage −3). The late reproductive stage marks the time when fecundability begins to decline and during which a woman may begin to notice changes in her menstrual cycles. Given that critical endocrine parameters begin to change prior to overt changes in menstrual cyclicity and that these endocrine changes are important to fertility assessments, STRAW+10 recommended that the late reproductive stage be subdivided into two substages (−3b and −3a). In Stage −3b, menstrual cycles remain regular without change in length and FSH, in the context of a simultaneously measured estradiol level, remains normal ; however, AMH and antral follicle counts are low. Most51, 63 but not all64 studies suggest inhibin B is also low. In Stage −3a, subtle changes in menstrual cycle characteristics, specifically shorter cycles65, 66, begin. Early follicular phase (cycle days 2-5) FSH increases above normal, with the other three markers of ovarian aging being low.
EARLY MENOPAUSAL TRANSITION (Stage-2)
The early menopausal transition is marked by increased variability in menstrual cycle length, defined as a persistent difference of ≤7 days in the length of consecutive cycles. Persistence is defined as recurrence within 10 cycles of the first variable length cycle. Cycles in the early menopausal transition are also characterized by elevated, but variable, early follicular phase FSH levels and low AMH levels and AFC.
LATE MENOPAUSAL TRANSITION (Stage −1)
The late menopausal transition is marked by the occurrence of amenorrhea of 60 days or longer. Menstrual cycles in the late menopausal transition are characterized by variability in cycle length, extreme fluctuations in hormonal levels, and frequent anovulation. In this stage, FSH levels are sometimes elevated into the menopausal range and sometimes within the range characteristic of the earlier reproductive years, particularly in association with high estradiol levels. AMH falls to undetectable levels. Development of international standards and availability of substantive population based data now permit definition of quantitative FSH criteria, with levels > 25 IU/L characteristic of being in late transition, based on current international pituitary standards.67-69 Empirical analyses should be undertaken to confirm this recommendation, and researchers and clinicians should carefully evaluate the appropriate FSH value depending on the assay they use. Based on studies of menstrual calendars and on changes in FSH and estradiol, this stage is estimated to last on average 1-3 years. Symptoms, most notably vasomotor symptoms, are likely to occur during this stage.
EARLY POSTMENOPAUSE (Stage +1a, +1b, +1c)
New data on the trajectories of change in mean levels of FSH and estradiol22, 31, 33, 39, 40, 50, 52, 53 indicate that FSH continues to increase and estradiol continues to decrease until approximately two years after the FMP, after which levels of each of these hormones stabilize. Thus, STRAW +10 recommended that the Early Post-Menopause be subdivided into 3 substages (+1a, +1b and +1c).
Stages +1a and +1b each last one year and end at the time point at which FSH and estradiol levels stabilize. Stage +1a marks the end of the 12 month period of amenorrhea required to define that the FMP has occurred. It corresponds to the end of the “perimenopause”, a term still in common usage that means the time around the menopause and begins at Stage −2 and ends 12 months after the FMP. Stage +1b includes the remainder of the period of rapid changes in mean FSH and estradiol levels. Based on studies of hormonal changes, Stage +1a and +1b together are estimated to last on average 2 years. Symptoms, most notably vasomotor symptoms, are most likely to occur during this stage.
Stage +1c represents the period of stabilization of high FSH levels and low estradiol values that is estimated to last 3 to 6 years, thus the entire early post-menopause lasts approximately 5-8 years. Further specification of this stage will require additional studies of trajectories of change in FSH and estradiol from the FMP through the late post-menopause.
LATE POSTMENOPAUSE (Stage +2)
Stage +2 represents the period in which further changes in reproductive endocrine function are more limited and processes of somatic aging become of paramount concern. Symptoms of vaginal dryness and urogenital atrophy become increasingly prevalent at this time. However, mean FSH levels fall again many years after menopause in the very elderly70; future studies are needed to determine if an additional stage near the end of life is warranted.
INCLUSIVENESS OF THE STRAW+10 CRITERIA
Evidence now supports the applicability of the STRAW+10 recommendations for the majority of women. Epidemiologic and clinical studies have documented that the process of reproductive aging, although influenced by demographic factors, lifestyle and BMI, follows a robust and predictable pattern.22, 27, 28, 33, 34, 40, 71 While smoking and BMI influence hormonal levels and the timing of transition, these factors do not alter the trajectory of change in bleeding patterns or hormonal levels with reproductive aging. Thus the STRAW+10 staging system is applicable to women regardless of age, demographic, BMI or lifestyle characteristics.
The STRAW+10 model does not use age as a criteria for determining reproductive staging. However, women meeting the criteria for Primary Ovarian Insufficiency/Premature Ovarian Failure (POI/POA; age < 40 with 4 months of amenorrhea and 2 serum FSH levels [at least a month apart] in the menopausal range) may not easily fit into this model. The course of reproductive aging in women with POI/POA may be considerably more variable than that of normal reproductive aging. Not only are there several potential etiologies but a substantial proportion of women have spontaneous resumption of menstrual function even once the diagnosis has been confirmed.72. Additional research is needed to better document the process of ovarian aging in these women and whether the course of ovarian aging differs by etiology of POI. Studies of reproductive aging in POI are considered to be a research priority.
HYSTERECTOMY AND ENDOMETRIAL ABLATION
Women who have undergone hysterectomy or endometrial ablation cannot be staged by menstrual bleeding criteria73. Reproductive stage in these women can only be assessed by the supportive criteria of biomarkers of ovarian aging. It is recommended that clinicians and researchers wait at least 3 months post surgery to assess endocrine status given emerging evidence that pelvic surgeries may transiently raise FSH levels74-77. Further research on the nature and duration of alterations in biomarkers of ovarian aging secondary to pelvic surgery is warranted. In most cases, staging will be limited to classification of whether such women are premenopausal or postmenopausal. A single sample for measurement of FSH and estradiol may be ambiguous or misleading, and a repeated measurement is often required.
POLYCYSTIC OVARIAN SYNDROME (PCOS)
Women with PCOS frequently experience oligomenorrhea that is not attributable to ovarian aging. Thus, the current menstrual cycle criteria used to stage reproductive aging are not applicable to this population. Understanding of the changes occurring prior to menopause in this group of women is limited. Some data suggest that women with PCOS may experience a later age at menopause56, 78, however, the experience of reproductive aging in PCOS is not well understood. Similarly, menstrual cycle criteria are not applicable in women with hypothalamic amenorrhea. Studies of reproductive aging in these subgroups of women are considered to be a research priority.
WOMEN WITH CHRONIC ILLNESS AND UNDERGOING CHEMOTHERAPY
Several important subgroups remain difficult to stage yet deserve attention in any staging system.57, 79-82 Depending on age at treatment and cancer treatment type, a significant proportion of women who undergo cancer treatment, particularly with alkylating agents, may experience transient increases in FSH and decreases in AMH and AFC with return of bleeding even after 12 months or more of amenorrhea.57, 81-83 In these patients, resumption of menstrual cycles may not indicate return of normal menstrual function. Women undergoing treatment with tamoxifen pose an additional problem as FSH and estradiol levels may be altered by this treatment which can also cause abnormal bleeding through direct effects on the endometrium. Women with chronic illnesses such HIV/AIDS also pose a problem in staging of reproductive aging due to lack of reliability of bleeding patterns and hormonal markers.79, 80 Staging in these women will require assessment with menstrual cycle criteria, the supportive criteria using relevant biomarkers, and age, to better characterize their ovarian function. Large prospective cohort studies are needed to better characterize the trajectories of ovarian aging in these populations.
CONCLUSION AND RESEARCH PRIORITIES
STRAW+10 revised and extended the STRAW recommendations to include additional criteria for defining specific stages of reproductive life. The revised staging system provides a more comprehensive basis for classification and assessment from the late reproductive stage through the menopausal transition and into the postmenopause. Its application should improve comparability of studies of midlife women by establishing clear criteria for ascertaining women’s reproductive stage. The STRAW+10 recommendations are expected to improve guidance for classifying the ovarian status of midlife women in the research setting while advancing efforts to translate this new science for clinicians and women.
Although scientific understanding of ovarian aging has advanced considerably in the last decade, important gaps in scientific knowledge persist. The workshop participants identified seven research priorities.
Lack of standardized assays for key biomarkers remains an important limitation in efforts to stage reproductive aging and to translate research findings to cost-effective clinical tools. Given the importance of AMH in relation to fertility and its relative stability across the menstrual cycle, development of an international standard for assessment of AMH is of paramount importance.
Empirical analysis across multiple cohorts is needed to specify precise menstrual cycle criteria for Stages −3b and −3a.
Studies are needed to characterize the hormonal changes of the postmenopause from Stage +1 to +2 as data across these stages are limited; several cohort studies are well positioned to provide this information.
Given that the large cohort studies of midlife women were initiated before the STRAW staging system was developed, these cohorts should be supported to apply the STRAW+10 staging criteria in order to reanalyze key findings on the clinical changes that occur across the menopausal transition.
Improved characterization of the pattern, timing, and level of reproductive biomarkers across nations is necessary, especially to provide data on the experience of women from low resource countries.
Research is needed to better understand the process of reproductive aging and appropriate staging criteria for women with PCOS and POI, and when removal of a single ovary and/or hysterectomy has occurred.
Research is needed to better evaluate staging in women with chronic illness such as HIV and those undergoing cancer treatment.
ACKNOWLEDGEMENTS
The STRAW+10 workshop had grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA) (AG039961) and the NIH Office of Research on Women’s Health (ORWH) as well as from the North American Menopause Society (NAMS), the American Society for Reproductive Medicine (ASRM), the International Menopause Society (IMS) and the Endocrine Society. This paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, ORWH or the NIH.
Footnotes
This article is being simultaneously published in the journals Climacteric, Fertility and Sterility, the Journal of Clinical Endocrinology and Metabolism, and Menopause
DISCLOSURES MG, RR, SS declare no conflict of interest. SDH has grant support from NIA and NICHD and receives travel support from the NAMS. JH has grant support from NIA and receives travel support from the Endocrine Society. RL is past president of ASRM. PM receives grant support from the NIMH, NIA, NIAID and NIDA, is a member of the Board of Trustees for NAMS and has previously consulted for Noven Pharmaceuticals, received lecture fees from the Royal Ottawa Foundation for Mental Health, the Mayo Clinic, Baycrest, and Northwestern University and received travel support from the Society for Women’s Health Research, the International Menopause Society, Pfizer, the Australasian Pacific Menopause Society, VCU Institute for Women’s Health. PS received travel support from The Endocrine Society. TdeV declares no direct conflict of interest as regards the submitted paper but has in the past received consultancy fees from Adcock Ingram and Pfizer, speaker’s fees from Servier and travel assistance to meetings from Amgen ,Pfizer and Bayer.
Contributor Information
Siobán D. Harlow, Department of Epidemiology, University of Michigan.
Margery Gass, The North American Menopause Society.
Janet E. Hall, The Endocrine Society; Department of Medicine, Harvard Medical School.
Roger Lobo, Department of Obstetrics and Gynecology, Columbia University.
Pauline Maki, Department of Psychiatry and Psychology, University of Illinois.
Robert W. Rebar, American Society for Reproductive Medicine.
Sherry Sherman, National Institute of Aging.
Patrick M. Sluss, Department of Pathology, Harvard Medical School.
Tobie J. de Villiers, International Menopause Society.
References
- 1.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Climacteric. 2001;4(4):267–72. [PubMed] [Google Scholar]
- 2.Soules MR, Sherman S, Parrott E, et al. Stages of Reproductive Aging Workshop (STRAW) J Womens Health Gend Based Med. 2001;10(9):843–8. doi: 10.1089/152460901753285732. [DOI] [PubMed] [Google Scholar]
- 3.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Fertil Steril. 2001;76(5):874–8. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 4.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Park City, Utah, July, 2001. Menopause. 2001;8(6):402–7. doi: 10.1097/00042192-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bromberger JT, Schott LL, Kravitz HM, et al. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women’s Health Across the Nation (SWAN) Arch Gen Psychiatry. 2010;67(6):598–607. doi: 10.1001/archgenpsychiatry.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351–8. doi: 10.1016/s0029-7844(00)00930-3. [DOI] [PubMed] [Google Scholar]
- 8.Freeman EW, Grisso JA, Berlin J, Sammel M, Garcia-Espana B, Hollander L. Symptom reports from a cohort of African American and white women in the late reproductive years. Menopause. 2001;8(1):33–42. doi: 10.1097/00042192-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Hollander LE, Freeman EW, Sammel MD, Berlin JA, Grisso JA, Battistini M. Sleep quality, estradiol levels, and behavioral factors in late reproductive age women. Obstet Gynecol. 2001;98(3):391–7. doi: 10.1016/s0029-7844(01)01485-5. [DOI] [PubMed] [Google Scholar]
- 10.Lee CG, Carr MC, Murdoch SJ, et al. Adipokines, inflammation, and visceral adiposity across the menopausal transition: a prospective study. J Clin Endocrinol Metab. 2009;94(4):1104–10. doi: 10.1210/jc.2008-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maki PM, Freeman EW, Greendale GA, et al. Summary of the National Institute on Aging-sponsored conference on depressive symptoms and cognitive complaints in the menopausal transition. Menopause. 2010;17(4):815–22. doi: 10.1097/gme.0b013e3181d763d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54(25):2366–73. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neer RM. Bone loss across the menopausal transition. Ann N Y Acad Sci. 2010;1192:66–71. doi: 10.1111/j.1749-6632.2009.05233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sowers MR, Jannausch M, McConnell D, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91(4):1261–7. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 15.Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Sleep. 2010;33(4):539–49. doi: 10.1093/sleep/33.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastian LA, Smith CM, Nanda K. Is this woman perimenopausal? JAMA. 2003;289(7):895–902. doi: 10.1001/jama.289.7.895. [DOI] [PubMed] [Google Scholar]
- 17.Santoro N, Brockwell S, Johnston J, et al. Helping midlife women predict the onset of the final menses: SWAN, the Study of Women’s Health Across the Nation. Menopause. 2007;14(3 Pt 1):415–24. doi: 10.1097/gme.0b013e31802cc289. [DOI] [PubMed] [Google Scholar]
- 18.Utian WH. Menopause-related definitions. International Congress Series. 2004;1266:133–8. [Google Scholar]
- 19.Harlow SD, Cain K, Crawford S, et al. Evaluation of four proposed bleeding criteria for the onset of late menopausal transition. J Clin Endocrinol Metab. 2006;91(9):3432–8. doi: 10.1210/jc.2005-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harlow SD, Crawford S, Dennerstein L, Burger HG, Mitchell ES, Sowers MF. Recommendations from a multi-study evaluation of proposed criteria for staging reproductive aging. Climacteric. 2007;10(2):112–9. doi: 10.1080/13697130701258838. [DOI] [PubMed] [Google Scholar]
- 21.Harlow SD, Mitchell ES, Crawford S, Nan B, Little R, Taffe J. The ReSTAGE Collaboration: defining optimal bleeding criteria for onset of early menopausal transition. Fertil Steril. 2008;89(1):129–40. doi: 10.1016/j.fertnstert.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randolph JF, Jr., Zheng H, Sowers MR, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab. 2011;96(3):746–54. doi: 10.1210/jc.2010-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taffe JR, Cain KC, Mitchell ES, Woods NF, Crawford SL, Harlow SD. “Persistence” improves the 60-day amenorrhea marker of entry to late-stage menopausal transition for women aged 40 to 44 years. Menopause. 17(1):191–3. doi: 10.1097/gme.0b013e3181b5540e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vital signs: current cigarette smoking among adults aged >or=18 years --- United States. MMWR Morb Mortal Wkly Rep. 2009;59(35):1135–40. [PubMed] [Google Scholar]
- 25.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 26.Merrill RM, Layman AB, Oderda G, Asche C. Risk estimates of hysterectomy and selected conditions commonly treated with hysterectomy. Ann Epidemiol. 2008;18(3):253–60. doi: 10.1016/j.annepidem.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF., 3rd Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007;87(1):101–6. doi: 10.1016/j.fertnstert.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 28.Gracia CR, Freeman EW, Sammel MD, Lin H, Nelson DB. The relationship between obesity and race on inhibin B during the menopause transition. Menopause. 2005;12(5):559–66. doi: 10.1097/01.gme.0000172268.24949.94. [DOI] [PubMed] [Google Scholar]
- 29.Huddleston HG, Cedars MI, Sohn SH, Giudice LC, Fujimoto VY. Racial and ethnic disparities in reproductive endocrinology and infertility. Am J Obstet Gynecol. 2010;202(5):413–9. doi: 10.1016/j.ajog.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Manson JM, Sammel MD, Freeman EW, Grisso JA. Racial differences in sex hormone levels in women approaching the transition to menopause. Fertil Steril. 2001;75(2):297–304. doi: 10.1016/s0015-0282(00)01723-4. [DOI] [PubMed] [Google Scholar]
- 31.Randolph JF, Jr., Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab. 2004;89(4):1555–61. doi: 10.1210/jc.2003-031183. [DOI] [PubMed] [Google Scholar]
- 32.Randolph JF, Jr., Sowers M, Gold EB, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88(4):1516–22. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- 33.Sammel MD, Freeman EW, Liu Z, Lin H, Guo W. Factors that influence entry into stages of the menopausal transition. Menopause. 2009;16(6):1218–27. doi: 10.1097/gme.0b013e3181a8f62b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su HI, Sammel MD, Freeman EW, Lin H, DeBlasis T, Gracia CR. Body size affects measures of ovarian reserve in late reproductive age women. Menopause. 2008;15(5):857–61. doi: 10.1097/gme.0b013e318165981e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sievert LL, Begum K, Sharmeen T, Chowdhury O, Muttukrishna S, Bentley G. Patterns of occurrence and concordance between subjective and objective hot flashes among Muslim and Hindu women in Sylhet, Bangladesh. Am J Hum Biol. 2008;20(5):598–604. doi: 10.1002/ajhb.20785. [DOI] [PubMed] [Google Scholar]
- 36.Sievert LL, Saliba M, Reher D, et al. The medical management of menopause: a four-country comparison care in urban areas. Maturitas. 2008;59(1):7–21. doi: 10.1016/j.maturitas.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Battistini M, Freeman EW, Grisso JA, Sammel M, Hollander L, Garcia-Espana B. Pilot study of racial differences and longitudinal changes in inhibin B in the late reproductive years. Fertil Steril. 2002;77(1):193–5. doi: 10.1016/s0015-0282(01)02946-6. [DOI] [PubMed] [Google Scholar]
- 38.Burger HG, Hale GE, Dennerstein L, Robertson DM. Cycle and hormone changes during perimenopause: the key role of ovarian function. Menopause. 2008;15(4 Pt 1):603–12. doi: 10.1097/gme.0b013e318174ea4d. [DOI] [PubMed] [Google Scholar]
- 39.Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum Reprod Update. 2007;13(6):559–65. doi: 10.1093/humupd/dmm020. [DOI] [PubMed] [Google Scholar]
- 40.Freeman EW, Sammel MD, Lin H, Gracia CR. Obesity and reproductive hormone levels in the transition to menopause. Menopause. 2010;17(4):718–26. doi: 10.1097/gme.0b013e3181cec85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab. 2007;92(8):3060–7. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 42.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–5. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Hansen KR, Morris JL, Thyer AC, Soules MR. Reproductive aging and variability in the ovarian antral follicle count: application in the clinical setting. Fertil Steril. 2003;80(3):577–83. doi: 10.1016/s0015-0282(03)00741-6. [DOI] [PubMed] [Google Scholar]
- 44.Klein NA, Houmard BS, Hansen KR, et al. Age-related analysis of inhibin A, inhibin B, and activin a relative to the intercycle monotropic follicle-stimulating hormone rise in normal ovulatory women. J Clin Endocrinol Metab. 2004;89(6):2977–81. doi: 10.1210/jc.2003-031515. [DOI] [PubMed] [Google Scholar]
- 45.Knauff EA, Eijkemans MJ, Lambalk CB, et al. Anti-Mullerian hormone, inhibin B, and antral follicle count in young women with ovarian failure. J Clin Endocrinol Metab. 2009;94(3):786–92. doi: 10.1210/jc.2008-1818. [DOI] [PubMed] [Google Scholar]
- 46.La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16(2):113–30. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 47.O’Connor KA, Ferrell R, Brindle E, et al. Progesterone and ovulation across stages of the transition to menopause. Menopause. 2009;16(6):1178–87. doi: 10.1097/gme.0b013e3181aa192d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Connor KA, Ferrell RJ, Brindle E, et al. Total and unopposed estrogen exposure across stages of the transition to menopause. Cancer Epidemiol Biomarkers Prev. 2009;18(3):828–36. doi: 10.1158/1055-9965.EPI-08-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robertson DM. Anti-Mullerian hormone as a marker of ovarian reserve: an update. Womens Health (Lond Engl) 2008;4(2):137–41. doi: 10.2217/17455057.4.2.137. [DOI] [PubMed] [Google Scholar]
- 50.Robertson DM, Hale GE, Jolley D, Fraser IS, Hughes CL, Burger HG. Interrelationships between ovarian and pituitary hormones in ovulatory menstrual cycles across reproductive age. J Clin Endocrinol Metab. 2009;94(1):138–44. doi: 10.1210/jc.2008-1684. [DOI] [PubMed] [Google Scholar]
- 51.Sowers MR, Eyvazzadeh AD, McConnell D, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93(9):3478–83. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sowers MR, Zheng H, McConnell D, Nan B, Harlow S, Randolph JF., Jr. Follicle stimulating hormone and its rate of change in defining menopause transition stages. J Clin Endocrinol Metab. 2008;93(10):3958–64. doi: 10.1210/jc.2008-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sowers MR, Zheng H, McConnell D, Nan B, Harlow SD, Randolph JF., Jr. Estradiol rates of change in relation to the final menstrual period in a population-based cohort of women. J Clin Endocrinol Metab. 2008;93(10):3847–52. doi: 10.1210/jc.2008-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Broekmans FJ, Faddy MJ, Scheffer G, te Velde ER. Antral follicle counts are related to age at natural fertility loss and age at menopause. Menopause. 2004;11(6 Pt 1):607–14. doi: 10.1097/01.gme.0000123643.76105.27. [DOI] [PubMed] [Google Scholar]
- 55.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30(5):465–93. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 56.Mulders AG, Laven JS, Eijkemans MJ, de Jong FH, Themmen AP, Fauser BC. Changes in anti-Mullerian hormone serum concentrations over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertility. Hum Reprod. 2004;19(9):2036–42. doi: 10.1093/humrep/deh373. [DOI] [PubMed] [Google Scholar]
- 57.Su HI, Sammel MD, Green J, et al. Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer. 2010;116(3):592–9. doi: 10.1002/cncr.24746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Disseldorp J, Faddy MJ, Themmen AP, et al. Relationship of serum antimullerian hormone concentration to age at menopause. J Clin Endocrinol Metab. 2008;93(6):2129–34. doi: 10.1210/jc.2007-2093. [DOI] [PubMed] [Google Scholar]
- 59.van Rooij IA, Broekmans FJ, Scheffer GJ, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83(4):979–87. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 60.Ferrell RJ, Sowers M. Longitudinal, epidemiologic studies of female reproductive aging. Ann N Y Acad Sci. 2010;1204:188–97. doi: 10.1111/j.1749-6632.2010.05525.x. [DOI] [PubMed] [Google Scholar]
- 61.Avis NE, Brockwell S, Randolph JF, Jr., et al. Longitudinal changes in sexual functioning as women transition through menopause: results from the Study of Women’s Health Across the Nation. Menopause. 2009;16(3):442–52. doi: 10.1097/gme.0b013e3181948dd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Avis NE, Zhao X, Johannes CB, Ory M, Brockwell S, Greendale GA. Correlates of sexual function among multi-ethnic middle-aged women: results from the Study of Women’s Health Across the Nation (SWAN) Menopause. 2005;12(4):385–98. doi: 10.1097/01.GME.0000151656.92317.A9. [DOI] [PubMed] [Google Scholar]
- 63.Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab. 1999;84(1):105–11. doi: 10.1210/jcem.84.1.5381. [DOI] [PubMed] [Google Scholar]
- 64.Robertson DM, Hale GE, Fraser IS, Hughes CL, Burger HG. A proposed classification system for menstrual cycles in the menopause transition based on changes in serum hormone profiles. Menopause. 2008;15(6):1139–44. doi: 10.1097/gme.0b013e3181735687. [DOI] [PubMed] [Google Scholar]
- 65.Johannes CB, Crawford SL, Longcope C, McKinlay SM. Bleeding patterns and changes in the perimenopause: a longitudinal characterization of menstrual cycles. Clinical Consultations in Obstetrics and Gyncecology. 1996;8:9–20. [Google Scholar]
- 66.Mitchell ES, Woods NF, Mariella A. Three stages of the menopausal transition from the Seattle Midlife Women’s Health Study: toward a more precise definition. Menopause. 2000;7(5):334–49. doi: 10.1097/00042192-200007050-00008. [DOI] [PubMed] [Google Scholar]
- 67.Roche Diagnostics . Package Insert: Elecsys and Cobas e analyzers. V 13. Roche Diagnostics; Indianapolis, IN: 2006-11. FSH, Follicle Stimulating Hormone. [Google Scholar]
- 68.Abbott Laboratories FSH. Package Insert: Architect Analyzers; 6C24, 34-4680/R1. 2007-10.
- 69.Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab Med. 2006;44(7):883–7. doi: 10.1515/CCLM.2006.160. [DOI] [PubMed] [Google Scholar]
- 70.Hall JE. Neuroendocrine physiology of the early and late menopause. Endocrinol Metab Clin North Am. 2004;33(4):637–59. doi: 10.1016/j.ecl.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 71.Schmitz KH, Lin H, Sammel MD, et al. Association of physical activity with reproductive hormones: the Penn Ovarian Aging Study. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2042–7. doi: 10.1158/1055-9965.EPI-07-0061. [DOI] [PubMed] [Google Scholar]
- 72.Bidet M, Bachelot A, Bissauge E, et al. Resumption of Ovarian Function and Pregnancies in 358 Patients with Premature Ovarian Failure. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2011-1038. [DOI] [PubMed] [Google Scholar]
- 73.Johnson BD, Merz CN, Braunstein GD, et al. Determination of menopausal status in women: the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. J Womens Health (Larchmt) 2004;13(8):872–87. doi: 10.1089/jwh.2004.13.872. [DOI] [PubMed] [Google Scholar]
- 74.Gelbaya TA, Nardo LG, Fitzgerald CT, Horne G, Brison DR, Lieberman BA. Ovarian response to gonadotropins after laparoscopic salpingectomy or the division of fallopian tubes for hydrosalpinges. Fertil Steril. 2006;85(5):1464–8. doi: 10.1016/j.fertnstert.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 75.Hehenkamp WJ, Volkers NA, Broekmans FJ, et al. Loss of ovarian reserve after uterine artery embolization: a randomized comparison with hysterectomy. Hum Reprod. 2007;22(7):1996–2005. doi: 10.1093/humrep/dem105. [DOI] [PubMed] [Google Scholar]
- 76.Qu X, Cheng Z, Yang W, Xu L, Dai H, Hu L. Controlled clinical trial assessing the effect of laparoscopic uterine arterial occlusion on ovarian reserve. J Minim Invasive Gynecol. 2010;17(1):47–52. doi: 10.1016/j.jmig.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 77.Sezik M, Ozkaya O, Demir F, Sezik HT, Kaya H. Total salpingectomy during abdominal hysterectomy: effects on ovarian reserve and ovarian stromal blood flow. J Obstet Gynaecol Res. 2007;33(6):863–9. doi: 10.1111/j.1447-0756.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 78.Dahlgren E, Johansson S, Lindstedt G, et al. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: a long-term follow-up focusing on natural history and circulating hormones. Fertil Steril. 1992;57(3):505–13. doi: 10.1016/s0015-0282(16)54892-4. [DOI] [PubMed] [Google Scholar]
- 79.Santoro N, Arnsten JH, Buono D, Howard AA, Schoenbaum EE. Impact of street drug use, HIV infection, and highly active antiretroviral therapy on reproductive hormones in middle-aged women. J Womens Health (Larchmt) 2005;14(10):898–905. doi: 10.1089/jwh.2005.14.898. [DOI] [PubMed] [Google Scholar]
- 80.Santoro N, Lo Y, Moskaleva G, et al. Factors affecting reproductive hormones in HIV-infected, substance-using middle-aged women. Menopause. 2007;14(5):859–65. doi: 10.1097/GME.0b013e31802f7369. [DOI] [PubMed] [Google Scholar]
- 81.Su HI, Alton J, Sherman L, Stankiewicz C, Ratcliffe S, DeMichele A. Relationships between clinical and biochemical predictors of chemotherapy related amenorrhea in premenopausal adjuvant breast cancer patients. American Society of Clinical Oncology Annual Meeting Proceedings.p. 11011. [Google Scholar]
- 82.Su HI, Sammel MD, Velders L, et al. Association of cyclophosphamide drug-metabolizing enzyme polymorphisms and chemotherapy-related ovarian failure in breast cancer survivors. Fertil Steril. 2009;94(2):645–54. doi: 10.1016/j.fertnstert.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sukumvanich P, Case LD, Van Zee K, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: a prospective study. Cancer. 2010;116(13):3102–11. doi: 10.1002/cncr.25106. [DOI] [PubMed] [Google Scholar]