Abstract
Adverse early care is associated with attention regulatory problems, but not all so exposed develop attention problems. In a sample of 612 youth (girls=432, M=11.82 yrs, SD=1.5) adopted from institutions (e.g., orphanages) in 25 countries, we examined whether the Val66Met polymorphism of the BDNF gene moderates attention problems associated with the duration of institutional care. Parent-reported attention problem symptoms were collected using the MacArthur Health and Behavior Questionnaire. DNA was genotyped for the BDNF Val66Met (rs6265) SNP. Among youth from SE Asia, the predominant genotype was Val/Met, while among youth from Russia/Europe and Caribbean/South America the predominant genotype was Val/Val. For analysis, youth were grouped as carrying Val/Val or AnyMet alleles. Being female, being from SE Asia, and being younger when adopted were associated with fewer attention regulatory problem symptoms. Youth carrying at least one copy of the Met allele were more sensitive to the duration of deprivation, yielding an interaction that followed a differential susceptibility pattern. Thus, youth with Val/Met or Met/Met genotypes exhibited fewer symptoms than Val/Val genotypes when adoption was very early and more symptoms when adoption occurred later in development. Similar patterns were observed when SE Asian youth and youth from other parts of the world were analyzed separately.
Introduction
Attention regulation is highly sensitive to early life conditions. Increases in attention regulatory problems are observed for infants born prematurely and young children who experience neglect and multiple changes in primary caregivers (Hildyard & Wolfe, 2002). Consistent with these findings, children adopted from institutions (e.g. orphanages) are at high risk of attention regulatory problems that are often severe enough to be classified as attention-deficit/hyperactivity disorder (ADHD) (e.g., Gunnar & van Dulmen, 2007). Attention regulatory problems in post-institutionalized children appear to be influenced by deprivation in care, in addition to heritable factors and family background (Roy, Rutter, & Pickles, 2000). Indeed, it is argued that attention regulatory deficits and hyperactivity problems among children reared in institutions constitutes part of a deprivation-specific syndrome (Kreppner, O’Connor, & Rutter, 2001; Stevens, et al., 2008). Duration of exposure to early deprivation increases risk of attention problems, with evidence that children placed in a supportive family by six to seven months of age may show few effects of institutional rearing, while those placed later and especially beyond two years of age show attention problems that do not resolve with time (Gunnar & van Dulmen, 2007). Prevalence estimates of clinically significant attention problems in post-institutionalized children vary from 20 to 40%, depending on the strictness of criteria and the duration and severity of early deprivation. This is much higher than the worldwide estimate for ADHD of 5%–6% of children (Polanczyk, de Lima, Horta, Biedereman, & Rohde, 2007). Nonetheless, it is clear that not all children exposed to prolonged early institutional care develop significant attention regulatory problems.
It has been argued that ADHD due to deprivation has a different etiology and developmental mechanisms than ADHD more generally (Stevens et al., 2008); however, because ADHD behaves as a multifactorial disorder in which different combinations of genetic and environmental factors contribute to risk (Poelmans, Pauls, Buitelaar, & Franke, 2011), deprivation-induced attention regulatory problems may simply reflect a larger environmental contribution than observed when these problems arise among children with more advantageous early life histories. Nonetheless, genetic variations among children might help explain variations in the vulnerability of attention regulatory functions to early deprivation.
Most researchers expect that the genetic model for attention regulatory problems will be one in which multiple genetic factors of small to moderate effect sizes contribute (Faraone et al., 2005). Furthermore, because complex behaviors result from interplay between genetic and environmental factors, gene-by-environment interactions are expected (Wermter et al., 2010). Nonetheless, despite high heritability estimates, the search for genes associated with ADHD risk has been elusive, whether one adopts a candidate gene or genome-wide association approach (Branaschewski, Becker, Scherag, Franke, & Coghill, 2010; Faraone et al., 2005). Gene-environment interaction (GEI) approaches have had some success. As with main effect candidate gene analyses, most GEI studies of attention regulatory problems have focused on genes regulating the activity of dopamine. The Mannheim Study of Risk Children reported that the dopamine transporter (DAT1) haplotype comprising the 6-repeat and 10-repeat alleles resulted in increased risk of ADHD among youth growing up under conditions of high but not low psychosocial adversity with a moderate genetic effect size among the high adversity group (Laucht, Skowronek, Becker, Schmidt, Esser, & Schulze, 2007). Similarly, studying post-institutionalized children to index early deprivation, again the DAT1 but not the dopamine receptor D4 (DRD4) polymorphism was associated with increased ADHD symptoms with more prolonged early deprivation (Stevens, Kumsta, Kreppner, Brookes, Rutter, & Sonuga-Barke, 2009).
In candidate gene studies of ADHD risk there has been only slight attention to genes involved in brain growth and repair (see for review, Branaschewski et al., 2010). However, given the sensitivity of attention systems to early adverse experience, an exploration of the role of such genes as moderators of early adversity effects is warranted. Here we focus on the gene coding for brain derived neurotrophic factor (BDNF). BDNF’s neurotrophic actions are essential for brain development (Bartkowska, Turlejski, & Djavadian, 2010) and activity-dependent transcription of the BDNF gene is critical in neural plasticity (Kuczewski, Porcher, & Gaiarsa, 2010). Because of its role in cell differentiation, cell survival, neurotransmission and synaptic plasticity, BDNF has been the focus of a number of studies examining early adverse care, brain development and behavioral outcomes. Over a wide variety of animal models of early life adversity, results indicate that altered outcomes are associated with changes in BDNF gene transcription and protein expression which typically are decreased in a graded fashion with the level of adverse early care (see for review, Roth & Sweatt, 2011).
Stress is believed to play a role in mediating the impact of early adversity on BDNF activity. In adult and juvenile mammals, acute stressors tend to facilitate BDNF expression, while chronic stressors exert the opposite effects (see review, Calabrese, Molteni, Racagni, & Riva, 2009). During development BDNF activity responds differentially to early adverse care depending on brain region. Thus, for example, timing and intensity appear to affect how and whether early maternal separation affects the development of hippocampal BDNF activity (Lippmann, Bress, Nemeroff, Poltsky, & Monteggia, 2007; Roceri et al., 2004; Roceri, Hendriks, Racagni, Ellenbroek, & Riva, 2002). In the prefrontal cortex, duration of adverse care appears to matter more than timing, with longer durations being associated with larger reductions in BDNF activity (see review, Calabrese et al., 2009). Glucocorticoids, via transcriptional activity of the glucocorticoid receptor (GR), acutely increased tropomyosin-related kinase (Trk) receptor signaling including activity of the Trk receptor with high affinity binding for BDNF. The impact of chronic stress in down regulation of GR is believed to be one mechanism in shifting stress-BDNF effects from increases in response to acute stress to decreases in response to chronic stress (Numakawa, Yokomaku, Richards, Hori, Adachi, & Kunugi, 2010). Epigenetic changes in the BDNF gene are also suspected, which has been demonstrated for rodents using several early adverse care paradigms (see review, Roth & Sweatt, 2011). Notably, repeated bouts of adverse care lasting throughout early development produce hyper-methylation of the BDNF gene in the rat prefrontal cortex, but not in the hippocampus which last through adolescence and into adulthood (Roth, Lubin, Funk, & Sweatt, 2009). The effect was specific to exon IV, which plays a critical role in GABAergic transmission and synaptic plasticity in the prefrontal cortex (Sakata et al., 2009).
In addition to epigenetic changes in the BDNF gene induced by chronic stress, a single-nucleotide polymorphism (SNP) in the human gene has been examined as source of greater or lesser resilience to adverse life conditions. This guanine-to-adenine SNP at nucleotide 196 (rs6265) results in a substitution of methionine (met) for valine (val) at codon 66 (i.e., Val66Met). This appears to be a functional polymorphism that affects the 5′ proregion of the protein and reduces activity-dependent secretion of BDNF (Egan, et al., 2003). Most of the work on the role of Val66Met BDNF genotype in moderating early adversity has focused on risk for depression (Aguilera et al., 2009; Kaufman et al., 2006) or endophenotypes of depression (Gatt et al., 2009; Hayden et al., 2010). However, in addition to depression, there is some evidence of GEI effects on impulsivity and self-regulatory behavior (Gatt et al., 2009; Nederhof et al., 2010). For example, Gatt and colleagues (2009) studied adults with varying (zero to five plus) stress indicators (abuse, neglect, exposure to violence) prior to age 18 years. Those who were Met carriers and who had more than two indicators exhibited decreases in hippocampal, lateral prefrontal cortex, amygdala and associated medial prefrontal cortex gray matter. These effects were associated with increased depressive symptoms, working memory impairments and decreased sustained attention. Using a large sample of adolescents in the Tracking Adolescents’ Individual Lives Survey (TRAILS), researchers found that carrying one or two copies of the Met allele was associated with poorer effortful control (e.g., attention regulation and inhibitory control) scores as a function of childhood adversity (Nederhof et al., 2010). However, gene-gene interactions were also noted in that study such that an anomalous enhancement of effortful control was noted with childhood adversity for those adolescents who also carried one of two short repeat versions of the serotonin transporter gene.
In addition to this complexity, in both the Gatt and colleagues (2009) and Hayden and colleagues (2010) studies, the opposite pattern of BDNF Val66Met findings were noted for individuals with no or few childhood adversities. That is, in these cases those with one or more Met versions of the gene functioned better than those with Val/Val genotypes under no or low early life adversity conditions. Such a pattern is consistent with the argument that genetic polymorphisms that are common in the population, like the BDNF Val66Met, may function as plasticity or differential susceptibility genes, increasing the child’s sensitivity to variations in the environment which can mean better than average functioning under supportive conditions, and worse than average under adverse or chronically stressful conditions (Belsky, Bakermans-Kranenburg, van Ijzendoorn, M., 2007; Belsky & Pluess, 2009; Belsky, Jonassaint, Pluess, Stanton, Brummet, & Williams, 2009)
The following study examined internationally adopted children who had lived for some period of time prior to adoption in institutional care. We examined parent-reported attention and impulsivity problems when the children were 8 to 13 years of age. We chose to focus on attention problems rather than depression because at this age, attention regulatory problems are far more prevalent than problems with depression in post-institutionalized samples, (e.g., Colvert, et al., 2008; Rutter, Kreppner, & O’Connor, 2001). Institutional rearing for infants and young children is a chronic stressor. It is associated with a decrease in the amplitude of the diurnal cortisol rhythm, consistent with chronic or prolonged stress (Carlson & Earls, 1997), with a slowing of linear growth, a reflection of allostatic load in young children (Johnson, Bruce, Tarullo, & Gunnar, 2011), and marked delays in cognitive and social development (see for review, Gunnar, 2001). While adoption produces a marked rebound in physical, cognitive and social development, problems with attention regulation, among other deficits, often continue (Pollak, et al., 2010). These finding are consistent with evidence of altered frontal-striatal development and development of monoaminergic fiber tracks in the frontal cortex following early life stress as noted in animal models (e.g., Bock, Gruss, Becker, & Braun, 2005; Braun, Lange, Metzger, & Poegoel, 2000). We predicted that attention problems would increase with the duration of early institutional care and thus with older ages at adoption. We further predicted that these effects would be greater for those children carrying the Met version of the Val66Met polymorphism. Given questions about genes that confer differential susceptibility to the environment, we also entertained the possibility that the Met version of the gene might be associated with fewer attention problem symptoms when children were adopted quite earlier but with more attention problem symptoms when they were adopted later and thus spent longer periods in institutional care.
The sample consisted of children from many regions of the world, including Asia. This created challenge in analysis. Population genetic studies of the Val66Met polymorphism reveal striking variation in frequency of the Met allele (Val/Met and Met/Met combined), ranging from near zero in sub-Saharan Africa and some indigenous groups in the Americas, to approximately 20% in European populations to roughly 44% in Asia (Petryshen et al., 2010). In addition, children of Asian descent are sometimes advanced on attention regulatory task performance, particularly tasks involving inhibitory control (e.g., Oh & Lewis, 2008). Thus, we needed to control for potential confounding of ethnic differences in both attention and impulsivity scores and in gene allele frequency. We did this by including SE Asian as a factor in our analyses and analyzing interactions that addressed whether the Met allele bore functionally different relations to attention regulatory functioning in SE Asia where the allele predominates relative to other regions of the world where it is less frequent.
Methods and Materials
Participants
The participants were 612 youth (M=11.82 yrs, Sd=1.48 yrs). The majority (66%) had spent their entire (M=93%, Sd=13%) preadoption lives in institutional care. All but 3% were adopted by 72 months of age (M=18.63, Sd=18.35). They came from 25 different countries, including Russia, Eastern Europe and India (108 boys, 158 girls), South America/Caribbean (36 males, 41 girls), Africa (4 males, 3 girls) and SE Asia (32 males, 230 girls). The majority resided in homes with parents who had completed a 4 year college degree or more (78.7%) and who earned 85 thousand dollars or more in the preceding year (63.4%).
Procedures
The sample was recruited from a registry of families of internationally-adopted children who were interested in being contacted about research. The registry was formed by contacting all of the families who had adopted internationally into the state of Minnesota between 1990 and 1998 in a study designed in conjunction with the state’s department of human resources (see Hellerstedt, Madsen, Gunnar, Grotevant, Lee, & Johnson, 2008) and then subsequently continuing to contact all families adopting internationally through the major agencies in the state. Families joining the registry reflected approximately 60–75% of all families adopting from countries using institutions to care for wards of the state over the period reflected in this manuscript.
The present analysis constituted part one of a two-part study, where part one involved the collection of genetic material and parent-reported behavior problems and part two involved collecting behavioral and neuroimaging data on a subset of the sample based on their genotype and eligibility for magnetic resonance imaging. The youth were included in part one if they would be 12–13 years of age during the funding period and were adopted from institutional care. Parents were contacted by phone and those agreeing to participate were mailed consent forms, questionnaires and a gene collection kit. These materials were returned through the mail. Of the children who met criteria, 82% agreed to participate and 70% returned the completed questionnaire and gene sampling material.
The study was reviewed and approved by the university’s institutional review board. During the phone recruitment the goals of the study were described along with a detailed description of the procedures used to maintain the family’s anonymity. Parents were asked to answer questions that probed their understanding of the procedures. The gene collection kit and questionnaire packet that was mailed to the homes included a detailed letter describing the study along with two copies of the consent form, one for the family to keep. A child assent letter and form were also included. Both had to be signed prior to analysis. Participants were identified by participant number only.
Measures
Demographics and Background
Parents provided information about family income, parent education, family composition, and child’s adoption history (birth country, age at adoption, and time in institutional care). Age at adoption and time in institutional care were positively skewed and thus were log10 transformed. These two variables were almost perfectly correlated, r=.95, n=612, p<.0001. In subsequent analyses, because institutionalized children may also be deprived prior to institutionalization, age at adoption was used to index duration of deprivation.
DNA Collection, Extraction and Analysis
Saliva samples (~4 ml total) were collected and DNA extracted using the Oragene system (DNA Genotek). A TaqMan 5′ exonuclease assay (ABI) was used to genotype DNA samples at the BDNF Val66Met (rs6265) SNP. Assays were performed on a 7900HT apparatus (ABI) in real-time PCR mode using standardized cycling parameters for ABI Assays-on-Demand. Fluorescence intensities were also collected in Allelic Discrimination mode after thermal cycling. Visual inspection of the amplification curves and endpoint ratios for each allele of rs6265 led to determination of the genotype. All samples were required to give clear and concordant results in real time and endpoint analyses that were in agreement and all samples that did not were re-run and/or re-extracted until they provided clear genotype calls. No template controls and a panel of samples with known genotypes at rs6265 representing both homozygote and heterozygous genotypes were run in parallel with experimental samples.
Preliminary analyses indicated that the distribution of alleles in each of the three major racial/geographic groups were in Hardy-Weinberg equilibrium. As expected, very few (<10%) of the sample were Met/Met genotypes. We therefore grouped Met/Mets with Val/Mets and analyzed Val/Val genotypes compared to Any Met genotypes. Table 1, however, shows also as expected, that for the youth from SE Asia, the Val/Met genotype was the most common genotype, while this was not the case for youth from other regions of the world. Indeed, using 2 (region) by 3 (genotype) analyses, we noted that youth adopted from SE Asia differed significantly in genotype distribution from those adopted from Russia and Indo-European countries, χ(2)=117.3, p<.001, as well as from those adopted from Central and South American countries, χ(2)=78.5, p<.001; however youth adopted from the latter two regions did not differ, χ(2)=3.37, p=.18. There were too few African youth to examine in these analyses. For some analyses (see below), we controlled for being from SE Asia and we also re-examined our results for SE Asian versus youth adopted from other regions of the world. We considered excluding the 1% of the sample adopted from Africa from these analyses because they only exhibited the Val/Val genotype, but decided against it, as with so few participants (n=7), it would be unlikely to affect the results either way.
Table 1.
Frequency counts of Val66Met genotype x region of origin
| Val/Val | Val/Met | Met/Met | Totals | |
|---|---|---|---|---|
| SE Asian | 69 | 140 | 53 | 262 |
| European | 190 | 70 | 6 | 266 |
| So. American | 63 | 13 | 1 | 77 |
| African | 7 | 0 | 0 | 7 |
| Totals | 329 | 223 | 60 | 612 |
ADHD symptoms
Parents completed the mental health symptomatology section of the MacArthur Health and Behavior Questionnaire (HBQ; Essex, Boyce, Goldstein, Armstrong, Kraemer, & Kupfer, 2002). Both parents completed the questionnaire for 73% of the youth. Only one parent completed the form in the remaining cases. The HBQ was derived from the Ontario Child Health Study measure designed to map onto DSM symptom criteria (Boyle, Offord, Racine, Szatmari, & Sanford, 1993). The HBQ has strong psychometric properties and has been used to assess child mental health across multiple ages from 4.5 years into adolescence (Ablow, Measelle, Kraemer, Harrington, Luby, & Smider, 1999; Shirtcliff & Essex, 2008). The HBQ is administered in questionnaire format, and assesses symptoms on a 0 (“never or not true”) to 2 (“often or very true”) scale. We analyzed the Attention-Deficit and Hyperactivity Disorder (ADHD) symptoms scale, as well as its two subscales, inattention and impulsivity. When both parents completed the scale, the inter-parent correlation was .81, p<.001. Responses were averaged so that each child had one score for the ADHD scale and for each of its two subscales. Reliability for the ADHD scale in our sample was α=.96. We also identified youth who met or exceeded the clinical cut-off for ADHD symptoms (≥ 1.2) (Lemery-Chalfant, Schreiber, Schmidt, Van Hulle, & Essex, 2007). The ADHD symptoms scale and its subscales were log10 transformed to improve normality of distribution.
Analysis Plan
The hypothesis that the BDNF genotype would interact with duration of deprivation to predict symptoms of attention regulatory problems was tested using hierarchical linear regression with the HBQ ADHD scale as the dependent measure. Control measures (child sex and whether the child was born in SE Asia or not) were entered in step one. In step two, age at adoption was entered as an index of duration of deprivation. Genotype (Val/Val versus AnyMet) was entered in step three. In step four the centered interaction of age at adoption and genotype was entered. Significant interactions were plotted and tested using procedures described by Aiken and West (1991). To determine whether a similar pattern of findings would be noted for the two subscales, this analysis was repeated twice, once with impulsivity and once with inattention as the dependent measure.
Several analyses were computed to examine whether genotype bore different associations with ADHD symptoms as a function of birth region. First, two additional steps were added to the regression model. Step five examined the interaction of genotype and whether or not the child was born in SE Asia in predicting ADHD symptoms. Step six examined the three way interaction between age at adoption, genotype and SE Asian or not. Finally, we split the file by SE Asian birth status and recomputed the regression analysis, removing SE Asian as a control variable, and examining the effects of sex, genotype, age at adoption and the centered interaction of genotype and age at adoption.
Results
Table 2 presents the correlations among all of the variables used in the regression analyses. Table 3 presents the results of the regression predicting ADHD symptoms. As shown, being a girl and being born in SE Asia were associated with fewer ADHD symptoms. Having controlled for the variance associated with these factors, age at adoption was still positively correlated with ADHD symptoms. For descriptive purposes, we also examined the percentage of children meeting clinical cut-off as a function of age at adoption (before 12 months of age, between 12 and 24 months, or over 24 months of age). The results were 9%, 16% and 28%, respectively, χ2=23.56, df=2, p<.001.
Table 2.
Correlations Among Variables in the Regression Equation (N=588)
| 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|
| 1. Sexa | .33*** | .15*** | −.06 | −.34*** | −.31*** | −.33** |
| 2. SE Asianb | -- | .48*** | −.10** | −.33*** | −.33*** | −.29*** |
| 3. Genotypec | -- | −.08* | −.16*** | −.16*** | −.14** | |
| 4. Age Adoptd | -- | .29*** | .27*** | .26*** | ||
| 5. ADHD symptomsd | -- | .95*** | .94*** | |||
| 6. Inattention.d | -- | .77*** | ||||
| 7. Impulsivity.d | -- |
p < .05,
p < .01,
p < .001 Pearson product-moment correlations
scored boys=1, girls=2
Scored as other areas of the world=1, SE Asia=2.
Scored Val/Val=1, any Met allele=2.
log10 transformed variables
Table 3.
Summary of Hierarchical Regression Predicting ADHD Symptoms (N=588)
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| B | SE(B) | β | B | SE(B) | β | B | SE(B) | β | B | SE(B) | β | |
| SE Asia | −.059 | .010 | −.231*** | −.054 | .010 | −.212*** | −.056 | .011 | −.218*** | −.055 | .011 | −.213** |
| Sex | −.076 | .011 | −.273*** | −.070 | .011 | −.251*** | −.07 | .011 | −.251*** | −.072 | .011 | −.258*** |
| Adoption Age | .086 | .013 | .242*** | .086 | .013 | .242*** | −.005 | .038 | −.014 | |||
| Genotype | .003 | .010 | .013 | .004 | .010 | .015 | ||||||
| Adopt Age X Genotype | .024 | .009 | .272*** | |||||||||
| Multivariate F for model | F (2, 611) = 61.94*** | F (3, 611) = 59.27*** | F (4, 611) = 44.41*** | F (5, 611) = 37.11*** | ||||||||
| Total R2 | .17 | .226 | .226 | .23 | ||||||||
| Δ R2 | .057*** | .00 | .008* | |||||||||
=p<.05,
=p<.001
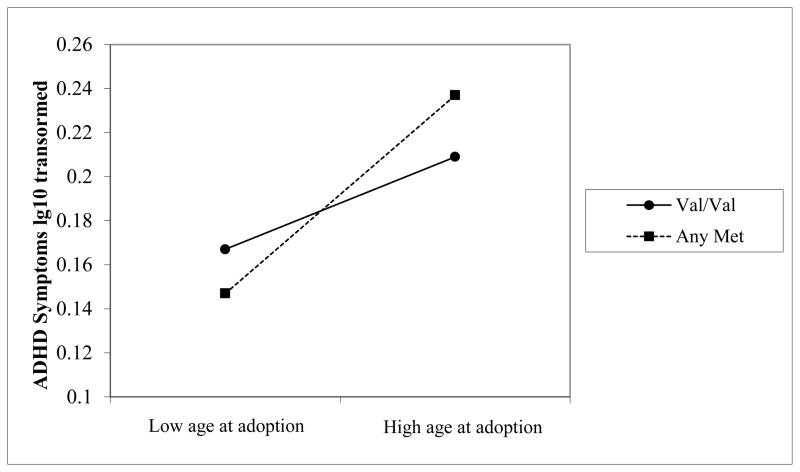
Genotype had no main effect on ADHD symptoms. However, there was a significant interaction between age at adoption and genotype. As shown in Figure 1, age at adoption was more closely associated with ADHD symptoms for youth with at least one Met allele than for youth with the Val/Val genotype. Notably, at younger ages at adoption, youth with at least one Met allele had fewer ADHD symptoms than those with the Val/Val genotype, while the reverse was the case at later ages of adoption. The analysis was repeated for the two subscales. All factors that were significant for the combined ADHD scale were significant when inattention and impulsivity were analyzed separately. For inattention, the full model explained 17% of the variance and the β for the interaction of age at adoption and genotype was .39, p<.01. For impulsivity, the full model explained 16% of the variance and the interaction of age at adoption and genotype was β =.32, p<.01.
Figure 1.
Interaction of the Val66Met polymorphism (Val/Val vs Any Met allele) and duration of institutional care in predicting ADHD symptoms. Results of the hierarchical regression analysis controlling for sex and whether the child was from SE Asia or not are plotted using procedures described by Aiken and West (1991).
The regression predicting ADHD symptoms was recomputed adding the centered interaction of genotype and SE Asian birth and the centered three way interaction of age at adoption, genotype and SE Asian birth. Neither of these equations was significant, p’s > .10. We then split the data set by whether the child was born in SE Asia or not and recomputed the regression analysis. For Non-SE Asians the interaction of age at adoption and genotype yielded a β =.31, p<.05; while for SE Asians this interaction yielded a β =.57, p<.05. Thus the Met allele appeared to be similarly related to ADHD symptoms as a function of duration of deprivation in both children from SE Asia and those from other areas of the world.
Discussion
As expected, attention regulatory problems increased with the duration of deprivation. ADHD scores were lower for children adopted within the first year of life and increased as adoption age got older. This was true even though in the regression equation, variance associated sex (boys exhibited more ADHD symptoms) and birth region (SE Asian children displayed fewer ADHD symptoms) had already been removed. Importantly, however, the Val66Met genotype moderated the association between duration of deprivation and ADHD scores. Individuals with at least one Met Allele exhibited a more marked positive association of age at adoption and ADHD symptoms than did individuals with no Met alleles (i.e., Val/Val genotype). This pattern of findings held for the ADHD symptoms scale as well as for its two subscales.
The pattern of this interaction was not consistent with a diathesis-stress model in which at low duration of adversity little or no impacts of the polymorphism would be noted, while with increasing duration of adversity those with Met alleles would exhibit increasing symptoms of regulatory problems. Instead, the pattern conformed to a differential susceptibility model in which the Met allele serves as a plasticity gene, supporting the development of attention regulatory competence at low durations of adversity and suppressing it at high durations of adversity. While the pattern is clearly a differential susceptibility pattern, it is challenging to identify a biologically-plausible explanation for why carrying one or more Met alleles might be advantageous for attention regulation under conditions of brief early adversity. Another way to state this is why carrying one or more Met alleles might be more encouraging of attention regulatory development than having two Val alleles for children who spend the majority of infancy in the context of a supportive, high resourced, adoptive home.
As noted earlier, activity-dependent transcription of the BDNF gene is critical for neuroplasticity and the Met allele is associated with reduced availability of BDNF (Bartkowska et al., 2010). Biologically-plausible models thus would involve reduced sculpting of attention regulatory circuitry in response to stimulation, particularly when the stimulation needed to sculpt developing attention regulatory circuits is meager. In that vein, one possibility is that for children adopted earlier, carrying the Met allele resulted in less sculpting of the attention circuits while the children were in institutional care, permitting the longer period in supportive care to have a bigger influence on their developing attentional systems. Viewed this way, the Met allele did not increase sensitivity to the rearing context, but rather reduced it. For children adopted later, those carrying the Met allele had lived in the institutional setting long enough to be influenced by it, and were less able than children with the Val/Val genotype to benefit from the enriching context of the adoptive home once they arrive there. According to this interpretation, had we been able to follow the children longitudinally we would have seen that children with the Val/Val genotype would have become more rapidly impaired in attention regulation than those with Met alleles with time spent in institutional care, but would have rebounded more rapidly and fully following adoption.
The present results add to a growing body of literature suggesting differential susceptibility effects of the Val66Met BDNF polymorphism. Hayden et al. (2010) found that Met allele carriers of the BDNF polymorphism functioned better than those with the Val/Val genotype at low levels of adversity and worse at high levels with respect to negative emotionality. Gatt and colleagues (2009) found very similar genotype by early life stress interactions for hippocampal volume and working memory accuracy. Suzuki and colleagues (2011) likewise noted that adults with one or more Met alleles were differentially susceptible to variations in childhood maternal care with regards to harm avoidance and self-directedness aspects of personality. Studying institutionally-reared children randomly assigned to care as usual compared to study-designed foster care, Drury and colleagues (2011) found that indiscriminately friendly behavior, a problem correlated with attention regulatory difficulties (e.g., Bruce, Tarullo, & Gunnar, 2009), was elevated for children with one or more Met alleles if they were in the care-as-usual group, but was exhibited less than for Val/Val genotypes if they had been randomly assigned to leave the institution and enter foster care. Notably in all of these studies, as in the present one, no main effects of the BDNF polymorphism were noted. Indeed, in a differential susceptibility pattern, the genotype would not likely exhibit a main effect as the susceptible version of the gene would be associated with better functioning than the non-susceptible version under some conditions and worse under others.
The present results also add to growing evidence that genes are involved in the variations in outcomes for children adopted from conditions of adversity. As noted, with regards to attention, both the Mannheim Study of Risk Children (Laucht et al., 2007) and the English and Romanian Adoption Study (Stevens, et al., 2009) found that the DAT1 genotype was associated with increased attention regulatory problems particularly among those who experienced more severely adverse or more prolonged exposure to adversity during childhood. Notably neither of these studies yielded evidence of a differential susceptibility pattern, although other studies that included the DAT1 genotype have (e.g., Pluess & Belsky, 2010).
The results also confirmed previous evidence that carrying at least one BDNF Met allele is common among SE Asians (Petryshen et al., 2000). If carrying one or more Met alleles impaired attention regulation, we would have expected the youth from SE Asia to have had more attention problems than youth from other regions. In contrast, and consistent with other findings (Oh & Lewis, 2008), they had fewer attention regulatory problem symptoms. However, we were concerned that there might be functional differences in the Met allele with regards to moderating the impact of early adversity in SE Asians, perhaps because other polymorphisms in the BDNF gene might have emerged to counter the impact of the Met allele. We tried several methods of determining whether the association between the Met allele, attention, and duration of deprivation differed among SE Asian adoptees versus youth adopted from other regions. In all cases we found no evidence to suggest differential effects. First, we found no significant interaction between genotype and SE Asian birth in predicting ADHD symptoms. Nor was the three way interaction of genotype, SE Asian birth, and age at adoption significant. When we split the children into two groups and repeated the regression analysis, we found significant interactions of the same pattern between genotype and age at adoption among both the SE Asian children and those from other regions of the world. Thus, despite the marked difference in frequency, the present results suggest that with regards to attention regulatory problems and early institutional care, the Val66Met polymorphism functions similarly in individuals from populations where carrying a Met allele is less frequent than the Val/Val genotype and in populations where it is the more frequent genotype.
While there are a number of strengths to the present analyses, including the large sample size, there are also limitations. First, we are dealing with parent report which can be biased. Second, while we know the children were adopted from institutions overseas, we do not have objective measures of the quality of those institutions. There was likely a great range of care represented in the sample that, along with duration of exposure, contributed to the effects observed. Our lack of information would have added noise to the analysis, making it more rather than less difficult to obtain significant effects. Third, it is very likely that some, but not all, of the children were born prematurely or at low birth weight and those from Russia/Eastern Europe were likely exposed to some level of alcohol prenatally (Johnson, 2000). These are also factors that impair attention regulatory competence and are unaccounted for in our analysis. Again, however, this should have added noise to the analysis, reducing rather than increasing our ability to detect GEI effects on attention regulatory problems. Finally, we were working with a sample of volunteers and thus one must always wonder what segment of the population agreed to participate. In the case of the present study, we do have some idea of sample bias because the registry grew out of an epidemiological study during which all of the families who adopted internationally through agencies in our state were identified. We know from analyses of the characteristics of who did and did not respond that we do have a small bias to better educated parents, even among a population with generally highly educated and higher income people who have the resources to adopt internationally (Hellerstedt et al., 2008). We also know that parents adopting children from orphanage/institutional care were more likely to respond (70%) than those who adopted children from foster care overseas (50%). Since in the present study we only attempted to recruit those families with children adopted from institutions, the bias in the registry likely work in our favor to increase the representativeness of our sample.
Even with these limitations, the results indicate that genetic variations may help explain some of the variation in outcomes for children adopted from institutions and, perhaps, other contexts of adverse early care. The fact that the effects were consistent with a differential susceptibility model indicates the complexity we are likely to find as we incorporate research on gene-behavior relations into studies of early life stress and deprivation. Certainly, findings such as those in the present study strongly argue against ever using common genetic variations as selection factors in adoption. For genes with variations common in the population, all the variants are likely to be advantageous under some conditions and disadvantageous under others.
However, the results also indicated that institutional care has significant main effects on the development of attention regulatory skills. For both genotypes, being older at adoption was associated with poorer attention regulation. When we examined the percentage of children meeting or exceeding the clinical cut-off by age at adoption, 9% of those adopted by 12 months, 16% of those by 24 months and 28% of those adopted between 2 and 6 years appeared clinically impaired according to parent report. The expected frequency in the population is around 5–6%, thus institutional rearing is associated with marked increases in the risk of clinically-significant attention regulatory problems (Kreppner, et al., 2001). While the BDNF Val66Met and likely other polymorphisms may moderate the effect of duration of institutional care, clearly time spent in deprived institutional rearing conditions early in life is a highly significant factor in these children’s attention regulatory difficulties.
Acknowledgments
The authors wish to thank the families of the Minnesota International Adoption Project and Bonny Donzella for their help with the project. The research was supported by an NIMH Center grant MH079513 for the Center for Brain, Gene and Behavioral Research Across Development, B.J. Casey (Director), Megan Gunnar and Kathleen Thomas Co-PIs of project 2.
Contributor Information
Megan R. Gunnar, University of Minnesota
A. Wenner Jennifer, University of Minnesota.
Kathleen M. Thomas, University of Minnesota
Charles E. Glatt, Weill Medical College of Cornell University
Morgan C. McKenna, Weill Medical College of Cornell University
Andrew G. Clark, Cornell University
References
- Ablow JC, Measelle JR, Kraemer HC, Harrington R, Luby J, Smider N. The MacArthur three-city outcome study: Evaluating multi-informant measures of young children’s symptomatology. Journal of American Academy of Child and Adolescent Psychiatry. 1999;38:1580–1590. doi: 10.1097/00004583-199912000-00020. [DOI] [PubMed] [Google Scholar]
- Aguilera MB, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, van Os J, Ibanez MI, Ruiperez MA, Ortet G, Fananas L. Early adversity and 5-HTT/BDNF genes: New evidence of gene-environment interactions on depressive symptoms in a general population. Psychological Medicine. 2009;39:1425–1432. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park; London, Sage: 1991. [Google Scholar]
- Bartkowska KK, Turlejski K, Djavadian RL. Neurotrophins and their receptors in early development of the mammalian nervous system. Acta Neurobiologiae Experimentalis (Wars) 2010;70:454–467. doi: 10.55782/ane-2010-1816. [DOI] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg M, van Ijzendoorn M. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:305–309. [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummet B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis-stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bock J, Gruss M, Becker S, Braun K. Experience-induced changes of dendritic spine densities in the prefrontal and sensory cortex: Correlations with developmental time windows. Cerebral Cortex. 2005;15:802–808. doi: 10.1093/cercor/bhh181. [DOI] [PubMed] [Google Scholar]
- Boyle MH, Offord DR, Racine Y, Szatmari P, Sanford M. Evaluation of the revised Ontario Health Study Scales. Journal of Child Psychology & Psychiatry. 1993;34:189–213. doi: 10.1111/j.1469-7610.1993.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Branaschewski T, Becker KK, Scherag S, Franke B, Coghill D. Molecular genetics of attention deficit/hyperactivity disoder: An overview. European Child and Adolescent Psychiatry. 2010;19:237–257. doi: 10.1007/s00787-010-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K, Lange E, Metzger M, Poegoel G. Maternal separation followed by early social deprivation afffects the development of monoaminergic fiber systems in the medial prefrontal cortex of Octodon degus. Neuroscience. 2000;95:309–318. doi: 10.1016/s0306-4522(99)00420-0. [DOI] [PubMed] [Google Scholar]
- Bruce J, Tarullo AR, Gunnar MR. Disinhibited social behavior among internationally adopted children. Development & Psychopathology. 2009;21:151–171. doi: 10.1017/S0954579409000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Racagni G, Riva MA. Neuronal plasticity: A link between stress and mood disorders. Psychoneuoendocrinology. 2009;34(suppl1):S208–216. doi: 10.1016/j.psyneuen.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Colvert E, Rutter M, Beckett C, Castle J, Groothues C, Hawkins A, Kreppner J, O’Connor TG, Stevens S, Sonuga-Barke EJS. Emotional difficulties in early adolescence following severe early deprivation: Findings from the English and Romanian Adoptees study. Development & Psychopathology. 2008;20:547–567. doi: 10.1017/S0954579408000278. [DOI] [PubMed] [Google Scholar]
- Drury SS, Gleason MM, Theall KP, Smyke AT, Nelson CA, Fox NA, Zeanah CH. Genetic sensitivity to the caregiving context: The influence of 5httlpr and BDNF val66met on indiscriminate social behavior. Physiology & Behavior. 2011 doi: 10.1016/j.physbeh.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Boyce T, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer D. The confluence of mental, physical, social and academic difficulties in middle childhood. II: Developing the MacArthur Health and Behavior Questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, Williams LM. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Molecular Psychiatry. 2009;14:681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Effects of early deprivation: Findings from orphanage-reared infants and children. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge, MA: MIT Press; 2001. pp. 617–629. [Google Scholar]
- Gunnar MR, van Dulmen M. Behavior problems in post-institutionalized internationally-adopted children. Development & Psychopathology. 2007;19:129–148. doi: 10.1017/S0954579407070071. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Dougherty LR, Olino TM, Dyson MW, Durbin CE, Sheikh HI, Singh SM. The role of brain-derived neurotrophic factor genotype, parental depression, and relationship discord in predicting early-emerging negative emotionality. Psychological Science. 2010;21:1678–1685. doi: 10.1177/0956797610385357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstedt WL, Madsen NJ, Gunnar MR, Grotevant HD, Lee RM, Johnson DE. The international adoption project: Population-based surveillance of Minnesota parents who adopted children internationally. Maternal and Child Health Journal. 2008;12:162–171. doi: 10.1007/s10995-007-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildyard KL, Wolfe DA. Child neglect: Developmental issues and outcomes. Child Abuse & Neglect. 2002;26:679–695. doi: 10.1016/s0145-2134(02)00341-1. [DOI] [PubMed] [Google Scholar]
- Johnson DE. Medical and developmental sequale of early childhood institutionalization in Eastern European adoptees. Minnesota Symposium on Child Psychology. 2000;31:113–162. [Google Scholar]
- Johnson AE, Bruce J, Tarullo AR, Gunnar MR. Growth delay as an index of allostatic load in young children: Predictions to disinhibited social approach and diurnal cortisol activity. Development & Psychopathology. 2011;23:859–871. doi: 10.1017/S0954579411000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor-5HTTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kreppner JA, O’Connor TG, Rutter M. Can inattention/overactivity be an institutional deprivation syndrome? Journal of Abnormal Child Psychology. 2001;29:513–528. doi: 10.1023/a:1012229209190. [DOI] [PubMed] [Google Scholar]
- Kuczewski N, Porcher C, Gaiarsa JL. Activity-dependent dendritic secretion of brain derived neurotrophic factor modulates synaptic plasticity. European Journal of Neuroscience. 2010;32:1239–1244. doi: 10.1111/j.1460-9568.2010.07378.x. [DOI] [PubMed] [Google Scholar]
- Laucht M, Skowronek MH, Becker K, Schmidt MH, Esser G, Schulze TG. Interacting effects of the dopamine transporter gene and psychosocial adversity on attention-deficit/hyperactivity disorder symptoms among 15-year-olds from a high-risk community sample. Archives of General Psychiatry. 2007;64:585–590. doi: 10.1001/archpsyc.64.5.585. [DOI] [PubMed] [Google Scholar]
- Lemery-Chalfant K, Schreiber JE, Schmidt NL, Van Hulle CA, Essex MJ, Goldsmith HH. Assessing internalizing, externalizing, and attention problems in young children: Validation of the MacArthur HBQ. Journal American Academy of Child and Adolescent Psychiatry. 2007;46:1315–1323. doi: 10.1097/chi.0b013e3180f616c6. [DOI] [PubMed] [Google Scholar]
- Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. European Journal of Neuroscience. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- Nederhof E, Bouma EM, Riese H, Laceulle OM, Ormel J, Oldehinkel AJ. Evidence for plasticity genotypes in a gene-gene environment interaction: the TRAILS study. Genes, Brain and Behavior. 2010;9:968–973. doi: 10.1111/j.1601-183X.2010.00637.x. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Yokomaku D, Richards M, Hori H, Adachi N, Kunugi H. Functional interactions between steroid hormones and neurotrophin BDNF. World Journal of Biological Chemistry. 2010;1:133–143. doi: 10.4331/wjbc.v1.i5.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Lewis C. Korean preschoolers’ advanced inhibitory control and its relation to other executive skills and mental state understanding. Child Development. 2008;79:80–99. doi: 10.1111/j.1467-8624.2007.01112.x. [DOI] [PubMed] [Google Scholar]
- Petryshen TL, Sabeti PC, Aldinger KA, Fry B, Fan JB, Schaffner SF, Waggoner SG, Tahl AR, Sklar P. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Molecular Psychiatry. 2010;15:810–815. doi: 10.1038/mp.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M, Belsky J. Differential susceptibility to parenting and quality child care. Developmental Psychology. 2010;46:379–390. doi: 10.1037/a0015203. [DOI] [PubMed] [Google Scholar]
- Poelmans G, Pauls DL, Buitelaar JK, Franke B. Integrated genome-wide association study findings: Identification of a neurodevelopmental network for attention deficit hyperacticity disorder. American Journal of Psychiatry. 2011;168:365–377. doi: 10.1176/appi.ajp.2010.10070948. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. American Journal of Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, Frenn KA, Loman MM, Gunnar MR. Neurodevelopmental effects of early deprivation in post-institutionalized children. Child Development. 2010;81:224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roceri M, Cirulli F, Pessina C, Peretto P, Racagni G, Riva MA. Postnatal repeated maternal deprivation produces age dependent changes in brain-derive neurotrophic factor expression in selected rat brain regions. Biological Psychiatry. 2004;55:708–714. doi: 10.1016/j.biopsych.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Molecular Psychiatry. 2002;7:609–616. doi: 10.1038/sj.mp.4001036. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biological Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sweatt JD. Epigenetic marking of the BDNF gene by early-life adverse experience. Hormones and Behavior. 2011;59:315–320. doi: 10.1016/j.yhbeh.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P, Rutter M, Pickels A. Institutional care: Risk from family background or pattern of rearing? Journal of Child Psychology and Psychiatry. 2000;41:139–149. [PubMed] [Google Scholar]
- Rutter ML, Kreppner JM, O’Connor TG. Specificity and heterogeneity in children’s responses to profound institutional privation. British Journal of Psychiatry. 2001;179:97–103. doi: 10.1192/bjp.179.2.97. [DOI] [PubMed] [Google Scholar]
- Sakata K, Woo NH, Martinowich K, Greene JS, Schloesser RJ, Shen L, Lu B. Critical role of promoter IV-driven BDNF transcription in GABAergic tranmission and synaptic platicity in the prefrontal cortex. Proceedings of the National Academy of Science. 2009;106:5942–5947. doi: 10.1073/pnas.0811431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens S, Kumsta R, Kreppner J, Brookes K, Rutter M, Sonuga-Barke EJS. Dopamine transporter gene polymorphism moderates the effects of severe deprivation on ADHD symptoms: Developmental continuities in gene environment inter-play. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics. 2009;150B:753–761. doi: 10.1002/ajmg.b.31010. [DOI] [PubMed] [Google Scholar]
- Stevens SE, Sonuga-Barke EJ, Kreppner JM, Beckett C, Castle J, Colvert E, Groothues C, Hawkins A, Rutter M. Inattention/overactivity following early severe institutional deprivation: presentation and associations in early adolescence. Journal of Abnormal Child Psychology. 2008;36:385–398. doi: 10.1007/s10802-007-9185-5. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Matsumoto Y, Shibuya N, Sadahiro R, Kamata M, Goto K, Otani K. The brain-derived neurotrophic factor Val66Met polymorphism modulates the effects of parental rearing on personality traits in healthy subjects. Genes, Brain and Behavior. 2011;10:385–391. doi: 10.1111/j.1601-183X.2010.00673.x. [DOI] [PubMed] [Google Scholar]
- Wermter AK, Laucht M, Schimmelmann BG, Banaschweski T, Sonuga-Barke EJ, Rietschel M, Becker K. From nature versus nurture, via nature and nurture, to gene x environment interaction in mental disorders. European Child and Adolescent Psychiatry. 2010;19:199–210. doi: 10.1007/s00787-009-0082-z. [DOI] [PubMed] [Google Scholar]