Abstract
Human immunodeficiency virus type 2 (HIV-2) is less transmissible and less pathogenic compared to HIV-1 and, when matched for CD4+ T cell count, the plasma viral load in HIV-2-infected individuals is approximately one log lower than in HIV-1-infected individuals. The explanation for these observations is elusive, but differences in virus controlling immunity generated in the two infections may be contributing factors. In the present study, we investigated neutralization by immunoglobulin A (IgA), in parallel with IgG, purified from plasma of HIV-1, HIV-2, and HIV-1/HIV-2 dually (HIV-D) infected individuals. Neutralization was analyzed against HIV-1 and HIV-2 isolates using a plaque reduction assay. In HIV-2 infection, intratype-specific neutralization by IgA was frequently detected, although at a lesser magnitude then the corresponding IgG neutralizing titers. In contrast, neutralization by IgA could rarely be demonstrated in HIV-1 infection despite similar plasma IgA levels in both infections. In addition, IgA and IgG of HIV-D plasma neutralized the HIV-2 isolate more potently than the HIV-1 isolate, suggesting that the difference between neutralizing activity of plasma IgA and IgG depends on the virus itself. Taken together, these findings suggest that both IgA and IgG add to the potent intratype neutralizing activity detected in HIV-2 plasma, which may contribute to virus control in HIV-2 infection.
Introduction
In an effort to understand the correlates of protective immunity to human immunodeficiency virus type 1 (HIV-1) infection, marked attention has been given to the small group of HIV-1 long-term nonprogressors (LTNP) and elite controllers that either do not develop disease or do so with slower pace.1,2 However, less attention has been given to individuals infected with HIV-2, an infection that is compatible with a normal lifespan in most infected individuals and, if disease develops at all, the progression rate is lower than in HIV-1 infection.3 Also vertical and heterosexual transmission of HIV-2 occurs less frequently than with HIV-1.4–6 Moreover, a recent study found that HIV-1 disease progression was inhibited by concomitant HIV-2 infection.7 Although the underlying mechanisms behind differences between HIV-1 and HIV-2 infections remain to be clarified, viral replication and the envelope glycoprotein complex (Env) characteristics could be important. Indeed, similar levels of proviral load but approximately one log lower plasma virus load in HIV-2 infection compared to HIV-1 infection could indicate more effective control of viral replication in HIV-2 infection.8–11 In addition, the HIV-2 Env seems to have a more open configuration characterized by multiple broadly cross-reactive epitopes and reduced CD4 dependence, which are uncommon characteristics of HIV-1.12,13 Moreover, as shown in our earlier work, HIV-2-infected individuals display plasma with potent neutralizing activity (NAc) against HIV-2 isolates (intratype neutralization), whereas NAc of HIV-1 plasma against HIV-1 isolates is of lower magnitude.14
The magnitude and frequency of virus-specific serum immunoglobulin A (IgA) has been studied and found to be low in chronic HIV-1 infections in HIV-1-infected chimpanzees as well as in SIV-infected rhesus macaques.15–18 The knowledge on HIV-2-specific serum IgA is limited, and to our knowledge, only one study reports on neutralization by IgA in HIV-2 infection.19
HIV-1 is mainly a mucosal pathogen targeting CD4+CCR5+ memory T cells abundantly present in gut-associated lymphoid tissue (GALT).20 The predominant antibody isotype in GALT is IgA. Interestingly, in humans, including HIV-1-infected cases, it has been shown that part of the mucosal IgA is also secreted directly into the blood.21–23 Furthermore, it is known that a large proportion of the circulating antibody secreting cells (ASC) express IgA and most of them are derived from mucosal immune reactions.16,24,25 IgA may also display antiinflammatory effects mediated by FcαRI binding.26,27 Instead, effector functions beyond binding to antigen and classical neutralization, i.e., complement activation and induction of phagocytosis, have been reported to be a minor feature of IgA.28,29
In the present study, we examined, side by side, the neutralizing activity of IgA and IgG in plasma of HIV-1, HIV-2, and HIV-1/HIV-2 dually (HIV-D) infected individuals. Our results show that intratype neutralization by plasma IgA is frequently detected in HIV-2 but not in HIV-1 infection. Furthermore, this study suggests that the magnitude of HIV-2 plasma IgA, as well as IgG, intratype neutralizing activity exceeds the magnitude of HIV-1 plasma IgA and IgG neutralization.
Materials and Methods
Study population
Women attending the Agiubef sexual health and family planning clinic for urogenital problems in Bissau, Guinea-Bissau from November 2006 to January 2011 were invited to participate in the study. After informed consent, HIV test counseling was given. Subsequently, a blood sample was drawn and general and gynecological examinations were performed. At the clinics, all women were offered free treatment for the diagnosis of other sexually transmitted diseases and free condoms. In this screening, including 1,287 individuals, women with HIV-1 (n=47), HIV-2 (n=21), and HIV-D (n=22) infections were identified and after one more blood sampling were referred to HIV treatment and support centers. For the current study, 10 HIV-1, 10 HIV-2, and 8 HIV-D-infected women were selected on the basis of CD4+ T cell count availability (Table 1). The study participants were further characterized by assessment of age, plasma viral load, and plasma total IgG and IgA levels (Table 1). None of the study participants received antiretroviral treatment at the time of blood sampling. HIV-negative controls (n=16) were selected on the basis of plasma sample availability.
Table 1.
Reciprocal Titers of IgG and IgA Neutralizing Activity in Individual Plasma Against HIV-1 and HIV-2 Isolates
| HIV-1 positive (p=10) | HIV-2 positive (p=10) | HIV-D positive (n=8) | p valuea | |
|---|---|---|---|---|
| Age: median (IQR) | 26.5 (22.0–28.0) | 32.0 (29.2–39.2) | 34.0 (24.0–41.0) | 0.113 |
| Viral load, RNA copies/ml | ||||
| <282 | 20% | 80% | 25% | |
| 282–10,000 | 60% | 25% | ||
| >10,000 | 20% | (data missing for 2) | 25% (data missing for 2) | 0.006 |
| CD4+ T cell count/μl: | ||||
| median (IQR) | 472 (333–633) | 493 (184–839) | 352 (259–788) | 0.891 |
| CD4+ T cell count/μl | ||||
| >499 | 40% | 50% | 38% | |
| 200–499 | 50% | 10% | 50% | |
| <200 | 10% | 40% | 12% | 0.294 |
| Total IgG, mg/ml: | ||||
| median (IQR) | 22.3 (16.4–31.2) | 14.9 (7.4–19.4) | 22.3 (12.7–30.2) | 0.05 |
| Total IgA, mg/ml: | ||||
| median (IQR) | 1.0 (0.8–1.3) | 1.0 (0.5–1.2) | 0.8 (0.6–1.1) | 0.454 |
p-values calculated using Kruskal–Wallis, comparing means over the columns or Fisher's exact test when appropriate.
IQR, interquartile range.
Blood sampling, HIV-1, HIV-2 status, and CD4+ T cell determination
Venous blood samples were drawn and collected in vacutainer tubes (BD Biosciences, San Jose, CA) with EDTA as anticoagulant. Plasma was then kept frozen at −80°C until use in neutralization assays, viral load determinations, and other laboratory analyses. Serological testing for HIV antibodies was done using the Behring Enzygnost HIV-1/HIV-2 ELISA (Behring, Marburg, Germany). Confirmation and differential HIV-1/HIV-2 diagnoses were performed using Capillus HIV-1/HIV-2 (Cambridge Biotech Limited, Galway, Ireland) and Immunocomb II HIV-1 and HIV-2 BiSpot RST (Origenics, Yavne, Israel). CD4+ T cell counts were determined by flow cytometry using the CyFlow instrument (Partec GmbH, Münster, Germany) and the CD4 % antibody kit (CyTecs GmbH, Görlitz, Germany) according to the manufacturer's instructions.
HIV plasma viral load
Viral RNA was extracted and purified from 200 μl blood plasma samples using the RNeasy Lipid Tissue Mini Kit (Qiagen, Stockholm, Sweden) according to the manufacturer's instructions. RNA was eluted in 30 μl RNase-free water, treated with DNase I (Fermentas, St Leon-Rot, Germany), and reverse transcribed using random primers and the Maxima Reverse Transcriptase (Fermentas) according to the manufacturer's instructions. HIV-1 viral load and HIV-2 viral load were determined using an in-house TaqMan qPCR with primers and probes as described.30,31 Briefly, HIV-1 RNA was quantified using primers LTR HIV-1 U (5′-GCCTCAATAA AGCTTGCCTTGA-3′), LTR HIV-1 L (5′-GGCGCCACTGCTA GAGATTTT-3′), and probe Pr LTR 1 (5′-FAM-AAGTAGTG TGTGCCCGTCTGTTRTKTGACT-TAMRA-3′). HIV-2 RNA was quantified using primers 1108 F3 (5′-GCGCGAGAAAC TCCGTCTTG-3′), 1234 L140 (5′-TCCAACAGGCTCTCTGC TAATCC-3′), and probe S65GAG2 (5′-FAM-TAGGTTACGG CCCGGCGGAAAGA-TAMRA-3′). All primers were synthesized by Invitrogen (Stockholm, Sweden) and probes by Applied Biosystems. qPCR was done using the AB StepOnePlus System (Applied Biosystems, Stockholm, Sweden) and AmpliTaq Fast DNA polymerase (Applied Biosystems) according to the manufacturer's instructions. Polymerase chain reaction (PCR) conditions were as follows: 95°C for 10 min, 40 cycles of 95°C for 20 s, and 60°C for 45 s. Stocks of HIV-1 strain IIIB and HIV-2 strain NIH-Z, which had been quantified by electron microscopy (Advanced Biotechnology Incorporated, Columbia MD), were used as standards. Known virus copy numbers were spiked into HIV-negative human plasma and extracted as described above. Both HIV-1 and HIV-2 assays had a limit of quantification of five RNA copies/qPCR reaction resulting in a cut-off value for quantification of 281 RNA copies/ml plasma. Both assays had a linear range between 106 and 5 RNA copies/reaction. Standards and samples were treated under the same conditions.
Assessment of IgG and IgA levels
IgG levels in plasma were measured by an in-house enzyme-linked immunosorbent assay (ELISA).13,32 Briefly, plates were coated overnight with AffiniPure goat antihuman IgG (20 μg/ml) (Jackson Immunotech, Marseille, France). Alkaline phosphatase-conjugated antihuman IgG (diluted to 1:5,000) (Jackson Immunotech, Marseille, France) was used as a detection antibody and human IgG (R&D Systems, Minneapolis, MN) was used as a standard. IgA levels were also measured by an in-house ELISA (the protocol was kindly provided by Dr. Carol Ann Fraser from Imperial College London, London, UK). Briefly, plates were coated overnight with goat antihuman IgA (2 μg/ml) (Jackson Immunotech). Alkaline phosphatase-conjugated AffiniPure goat antihuman IgA (diluted to 1:2,500) (Jackson Immunotech) was used as a detection antibody and human serum IgA (Jackson Immunotech, Marseille, France) was used as a standard. The ELISA was repeated in triplicate for each sample.
Plasma IgG purification
IgG was purified from plasma using protein G Sepharose HP prepacked into spin columns (GE Healthcare Life Sciences, Buckinghamshire, UK). Briefly, plasma samples were first inactivated at 56°C for 30 min and then clarified by centrifugation at 4,000×g for 20 min. Then 200 μl plasma and 400 μl binding buffer (20 mM Na phosphate pH 7.0) (GE Healthcare Life Sciences) were added to the equilibrated protein G Sepharose HP and the mixture was incubated at room temperature for 4 min on a tube rotator. Thereafter unbound fractions were removed by centrifugation at 100×g for 30 s and kept in the refrigerator. The columns were then washed two times with 600 μl of binding buffer. Neutralization buffer (30 μl 1 M Tris–HCl, pH 9.0) (GE Healthcare Life Sciences) was added to the bottom of fresh collection tubes and bound IgG was eluted with elution buffer (400 μl, 0.1 M glycine–HCl, pH 2.7) (GE Healthcare Life Sciences) and centrifuged at 70×g for 30 s into the neutralization buffer. Elution was repeated with another 400 μl of elution buffer. IgG purification was repeated with unbound fractions in the refrigerator. The eluted IgG and non-IgG fractions were concentrated using Amicon Ultra-15 centrifugal filter units with a 30,000-Da cut-off (Merck Millipore, Billerica, MA). Purified IgGs were kept at −80°C until use. Non-IgG fractions were kept at 4°C until purification of IgA on the same day. Residual IgA in the IgG fraction was assessed by IgA ELISA, as described above, and found not to exceed 0.1 μg/ml.
IgA purification from non-IgG plasma fraction
IgA was purified from non-IgG fractions of plasma using a peptide M/Agarose gel slurry (InvivoGen, Toulouse, France). Briefly, 400 μl of peptide M/Agarose gel slurry (equals to 200 μl of resin) was loaded into a Pierce microcentrifuge spin column (Thermo Fisher Scientific, Hampton, NH); then IgG-depleted plasma was loaded onto the column and incubated with rocking at room temperature for 30 min. After incubation, the column was washed and the flow through fractions were collected. The bound antibodies were then eluted with IgG elution buffer (6×200 μl, Thermo Scientific, Rockford, IL) and neutralized immediately with neutralization buffer (6×20 μl 1 M Tris–HCl, pH 9.0) (GE Healthcare Life Sciences). The column was subsequently regenerated by washing with regeneration buffer [2×300 μl phosphate-buffered saline (PBS) with 20% ethanol] and used for another round of purification of the flow through to increase the yield. Finally, the eluted fractions from the two consecutive purification processes were combined, buffer exchanged with PBS, and concentrated using Amicon Ultra-15 centrifugal filter units with a 30,000-Da cut-off (Merck Millipore). Purified IgAs were kept at 4°C until use. Residual IgG in the IgA fraction was assessed by IgG ELISA, as described above, and found not to exceed 0.1 μg/ml.
Primary HIV-1 and HIV-2 isolates
For the analysis of NAc of HIV-positive plasma IgG and IgA, one HIV-1 and one HIV-2 isolate were used. HIV-1 C Br (92BR025) originated from Brazil and was isolated within the framework of the WHO Network for HIV Isolation and Characterization.33 The HIV-2 A isolate GB/1812 (original name 1812) originated from Guinea Bissau.34,35 Both viruses were isolated from peripheral blood mononuclear cells (PBMCs)35,36 and were found to use CCR5 but not CXCR4 for cell entry,34,36 which was the rationale for the use of GHOST(3)-CCR5 cells as targets in the neutralization assays. Based on our previous experience, the viruses were chosen such as to ensure neutralization sensitivity with samples collected in Guinea-Bissau.14
Neutralization assay using GHOST(3)-CCR5 cells
NAc of plasma Ig (IgG or IgA) from HIV-1-infected and/or HIV-2-infected individuals was determined in a plaque reduction assay using the GHOST(3)-CCR5 cells as targets and plaque reduction, assessed by expression of green fluorescent protein, as readout.32 In brief, GHOST(3)-CCR5 cells were maintained in Dulbecco's modified Eagle's medium (DMEM; GIBCO) (Life Technologies, Paisley, UK) complemented with 7.5% fetal calf serum (FCS) and 10,000 IU/ml penicillin-streptomycin (Invitrogen, Carlsbad, CA) in 25-cm2 culture flasks and split twice a week at a ratio of 1:15–20. Briefly, virus stocks and plasma Ig were mixed and diluted in DMEM infection medium, i.e., complete DMEM medium supplemented with 2 μg/ml polybrene (Sigma Aldrich, St. Louis, MO), such that the final virus concentration gave 10–40 fluorescent plaque-forming units (PFU) per well and the final Ig concentrations corresponded to plasma Ig levels (starting at an 1:20 plasma dilution). Prior to infection, the medium in the well was replaced with 50 μl fresh medium. The virus and Ig mixtures were incubated at 37°C for 1 h and subsequently titrated in triplicate (150 μl/well). The day after infection, plates were washed once and fresh medium was added (200 μl). As positive neutralization controls IgG and IgA from one of the plasma with high neutralizing activity were included throughout the experiments; positive virus controls consisted of wells with cells and virus without Ig; negative controls consisted of wells with virus mixed with a pool of plasma Ig from six HIV-uninfected women from the same cohort; and cell controls consisted of cells only. Three days after infection, green fluorescent expressing plaques were enumerated under the fluorescent microscope. Percentage neutralization was expressed as % plaque reduction in the virus cultures containing Ig relative to virus without sample Ig and calculated by the following formula37: Plaque reduction (%)=[1 – (PFU with sample Ig/PFU without sample Ig)]×100. The cut-off for neutralization in this assay was based on intraassay variation, determined in three consecutive assays run on the same day. Since the standard deviation (SD) of intraassay variation was repeatedly found to be less than 10%, a 30% reduction in fluorescent PFU, corresponding to >3 SD, was chosen as the cut-off.37 Reduction of PFU by negative control plasma Ig, tested individually from six HIV-uninfected subjects, was repeatedly below 30% (data not shown).
Magnitude of plasma IgG and IgA neutralizing activity
For analysis of the magnitude of plasma Ig NAc, plasma Ig was titrated against one HIV-1 and one HIV-2 isolate. The magnitude of NAc in an individual plasma Ig was determined by its neutralization score defined as log-transformed titers.38 Log-transformed titers were calculated by dividing the highest dilution giving neutralization by 100 before applying a log-base 3 transformation and then adding 1 [Y=log 3 (dilution/100)+1]. All titers below 1:40 were given a value of 33 for the sake of calculation of a neutralization score.
HIV-specific neutralizing activity in relation to total plasma IgG and IgA
For determination of proportions of IgG and IgA with specific, i.e., neutralizing activity, the reciprocal intratype neutralizing titers of plasma IgG and IgA were related to total plasma IgG and IgA levels. Reciprocal intratype neutralizing titers of plasma IgG and IgA were divided by the amount of IgG and IgA, respectively, present in 150 μl, corresponding to the volume assessed in the neutralization assay setup (see above).
Statistical methods
For comparisons between unrelated categorical variables, Fisher's exact test was used. Differences between independent subgroups of numerical variables were assessed by Mann–Whitney U test or Kruskal Wallis test as appropriate. The magnitudes of IgG and IgA derived from HIV-D plasma directed against HIV-1 and HIV-2 isolates were compared using the Wilcoxon signed rank test. Spearman's rank test was used to assess correlations. Analyses were performed using SPSS and GraphPad Prism 5 version 5.2.
Ethical considerations
The study was approved by the Ethics Committee at the Ministry of Health in Guinea-Bissau and the Ethical Committee at Lund University, Sweden. Study participants were counseled and provided informed verbal consent. Participants were offered free medical examination with free essential medications. Study participants who tested positive for HIV were referred to HIV treatment and support centers.
Results
Neutralization of HIV-1 and HIV-2 by plasma IgG and IgA
To study neutralizing activity mediated by plasma IgG and IgA in HIV-1 and HIV-2 infections, neutralization of one HIV-1 and one HIV-2 isolate was assessed in a plaque reduction assay. Purified IgG and IgA were tested in dilution steps starting from the concentration corresponding to a 1:20 plasma dilution. IgG from HIV-1-infected individuals showed a moderate level neutralization of the HIV-1 isolate (intratype neutralization), similar to our previous experience with HIV-1 plasma, median reciprocal titer 50 (Table 2). Neutralization of the HIV-2 isolate with HIV-1 IgG (intertype neutralization) was of a similar level, median reciprocal titer 60, with the exception of IgG of two plasma (Table 2). In contrast, intratype neutralization mediated by HIV-2 plasma IgG was found to be stronger, median reciprocal titer 163,840 (Table 2).The strong intratype NAc of HIV-2 IgG contrasted with the lack of demonstrable intertype neutralization. Plasma IgA neutralization, whether intratype or intertype, was absent in 9/10 HIV-1-infected individuals. In HIV-2 infection, plasma IgA with intratype neutralization was instead found more frequently, 7/10 cases (p<0.05). Quantitative comparison of the magnitude of neutralization confirmed that intratype neutralization with plasma IgG as well as IgA differed significantly between HIV-1 and HIV-2 infections (Fig. 1A and B) (p<0.001 and p<0.01, respectively). Likewise, HIV-2 IgG intratype neutralization was of significantly higher magnitude than intertype neutralization (p<0.001), whereas HIV-1 IgG intertype and intratype neutralization were at a similarly low level. Moreover, the magnitude of IgG-mediated intratype neutralization was found to be higher than the corresponding IgA neutralization in HIV-2 infection (p<0.001). In addition, we noted that the magnitudes of HIV-2 intratype neutralizing IgG and IgA were correlated (p<0.05, r=0.72). In summary, these findings suggest that intratype-specific neutralization by plasma IgA is frequently detected in HIV-2, but not in HIV-1 infection. Moreover, the magnitudes of intratype neutralization mediated by both IgG and IgA of HIV-2 plasma exceeded that of HIV-1 IgG and IgA, respectively.
Table 2.
Characteristics of Study Participants
| |
|
Neutralizing activity of IgG |
Neutralizing activity of IgA |
||
|---|---|---|---|---|---|
| Plasma | Code | HIV-1 C Br | HIV-2 A GB/1812 | HIV-1 C Br | HIV-2 A GB/1812 |
| HIV-1 | 1215 | 80 | <20 | <20 | <20 |
| 1218 | 20 | 640 | <20 | <20 | |
| 1239 | 80 | <20 | <20 | <20 | |
| 1242 | 160 | 80 | <20 | <20 | |
| 1246 | 20 | 40 | <20 | <20 | |
| 1264 | <20 | 80 | <20 | <20 | |
| 1381 | 20 | 40 | <20 | <20 | |
| 1388 | 80 | 40 | <20 | 80 | |
| 1426 | 20 | 40,960 | <20 | <20 | |
| 1411 | 640 | 5,120 | 20 | <20 | |
| HIV-2 | 150 | <20 | 327,680 | <20 | 160 |
| 313 | <20 | 81,920 | <20 | 80 | |
| 323 | <20 | 163,840 | <20 | 160 | |
| 512 | <20 | 1,310,720 | <20 | 1,280 | |
| 646 | <20 | 1,310,720 | <20 | 5,120 | |
| 914 | <20 | 163,840 | <20 | <20 | |
| 1071 | 20 | 40,960 | <20 | <20 | |
| 1075 | <20 | 81,920 | <20 | <20 | |
| 1277 | <20 | 163,840 | <20 | 2,560 | |
| 1397 | <20 | 327,680 | <20 | 80 | |
| HIV-D | 404 | 160 | 327,680 | <20 | 640 |
| 413 | 20 | 327,680 | 20 | 640 | |
| 423 | 80 | 5,242,880 | <20 | 320 | |
| 427 | 160 | 163,840 | <20 | 40 | |
| 636 | 320 | 163,840 | <20 | 80 | |
| 637 | 160 | 655,360 | <20 | 320 | |
| 1438 | <20 | 655,360 | <20 | 640 | |
| 1458 | 20 | 163,840 | <20 | 160 | |
Given reciprocal titers correspond to 1/dilution of plasma giving IC30 in the plaque reduction neutralization assay applied and boxes are color coded as follows white, <20; light gray, 20–640; medium gray, 1,280–81,920; dark gray, 163,840–5,242,880.
FIG. 1.
Magnitude of intratype and intertype neutralization of HIV-1, HIV-2, and HIV-D plasma IgG and IgA. Median magnitude scores of intratype and intertype neutralization of HIV-1 (n=10), HIV-2 (n=10), and HIV-1/HIV-2 dually (HIV-D) (n=8) plasma IgG (A) and IgA (B) against HIV-1 and HIV-2 isolates. The diameters of circles correspond to the magnitude of neutralization of plasma IgG and IgA. Titer-based magnitudes of 0, 0.4, 0.5, 1.1, 2.1, 7.8, and 8.6 correspond to reciprocal titers of <40, 50, 60, 120, 335, 176,000, and 423,000, respectively. *p<0.05, **p<0.01, ***p<0.001.
Neutralization by IgG and IgA derived from HIV-D plasma
For the purpose of analyzing HIV-1-directed and HIV-2-directed neutralizing antibody responses generated within the same host we tested IgG and IgA purified from plasma of HIV-D individuals. Results showed that HIV-D IgG neutralized both the HIV-1 and HIV-2 isolates (Table 2). Furthermore, the magnitude of HIV-2-directed IgG neutralization was found to be higher than that of HIV-1-directed IgG (p<0.001) (Fig. 1A). In fact, the magnitude of HIV-1 neutralization was similar to that seen with plasma IgG from HIV-1 singly infected individuals. HIV-D IgA did not neutralize the HIV-1 isolate, with the exception of IgA from one plasma, similar to HIV-1 IgA, but neutralized the HIV-2 isolate as frequently and potently as IgA from HIV-2 singly infected individuals (Table 2 and Fig. 1). Taken together, this indicates that the type of virus plays a role in eliciting the frequency and strength of neutralization by both plasma IgG and IgA.
IgG and IgA intratype neutralizing activity in relation to total plasma IgG and IgA
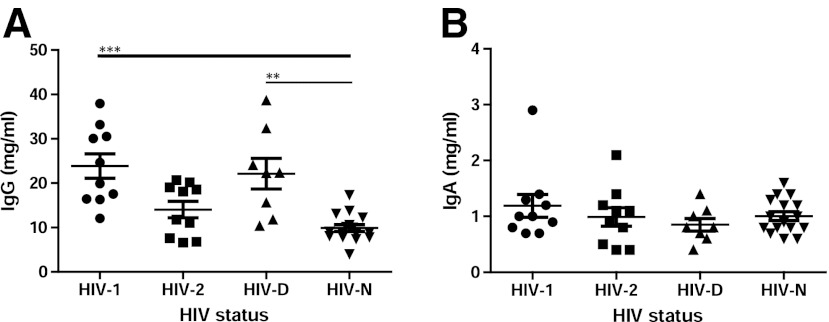
For the analysis of proportions of IgG and IgA with specific activity, i.e., neutralizing capacity, in plasma of HIV-1-infected and HIV-2-infected individuals the total plasma levels of IgG and IgA were determined. It was noted that plasma IgG levels varied depending on the type of HIV infection. Plasma of HIV-1-infected and HIV-D-infected individuals displayed significantly higher levels of plasma IgG (p<0.0001 and <0.01, respectively) compared to plasma of HIV-uninfected individuals, while IgG levels in HIV-2 plasma, although somewhat elevated, were not significantly different from HIV-negative individuals (Fig. 2A). Thus, the elevated IgG levels in HIV-1-infected individuals may be the sign of hypergammaglobulinemia and reflect chronic immune activation, not as prevalent in HIV-2 infection. Unlike IgG, plasma IgA levels were similar in the four groups examined (Fig. 2B).
FIG. 2.
Plasma IgG and IgA levels. The total amounts of IgG (A) and IgA (B) in plasma of HIV-1 (n=10), HIV-2 (n=10), HIV-1/HIV-2 dually (HIV-D) (n=8) infected, and HIV-negative (HIV-N) (n=16) individuals were quantified by ELISA. **p<0.01, ***p<0.001.
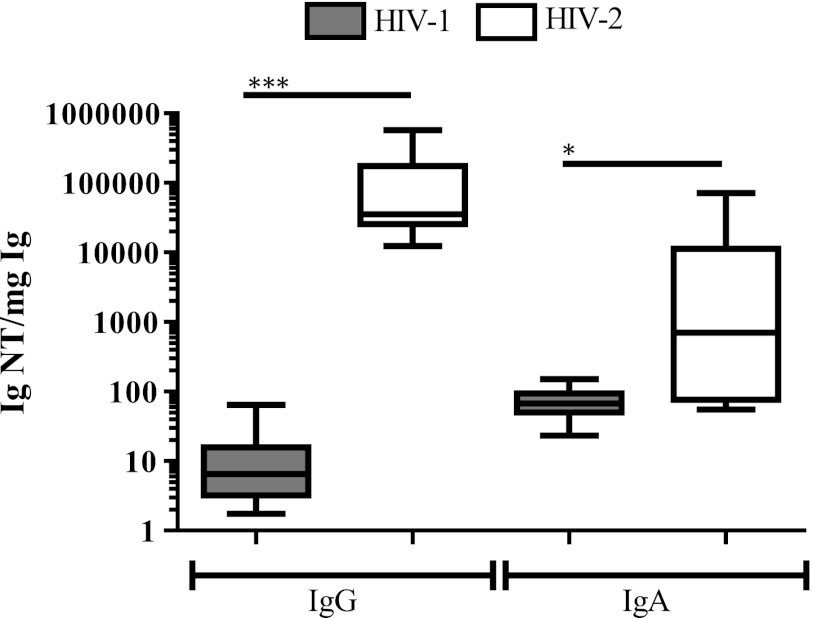
Next, analysis of the proportion of IgG and IgA with intratype neutralizing capacity in plasma of HIV-1-infected and HIV-2-infected individuals was assessed by the division of reciprocal neutralization titers with total plasma IgG and IgA levels expressed in milligrams. Results showed that HIV-2 intratype neutralizing titer per mg IgG was elevated, in median 5,400-fold, as compared to the corresponding HIV-1 IgG titer, p<0.001 (Fig. 3). The proportion of HIV-2 IgA with intratype neutralizing capacity was also found to be increased as compared to the corresponding HIV-1 IgA; however, the median difference was more moderate, around 10-fold. Thus, despite lower or similar total Ig levels detected in plasma of HIV-2-infected individuals, as compared with plasma of HIV-1-infected individuals, the proportion of antibodies with neutralizing capacity appeared higher in HIV-2 infection. Moreover, the HIV-1 and HIV-2 neutralizing IgG and IgA titers in plasma of dually infected individuals displayed similar proportions in relation to total IgG and IgA as corresponding intratype reactivates in the singly infected individuals (data not shown). Since variation in plasma IgG levels could reflect disease progression, we went on to analyze NAc in relation to clinical parameters. An inverse correlation between CD4 count and magnitude of intratype IgG neutralization was noted in infection with HIV-1 (p<0.05, r=–0.70) but not with HIV-2. Moreover, since all plasma of the HIV-2-infected individuals displayed viral load levels below the level of quantification (<281 RNA copies/ml) (Table 1), no correlations were noted between magnitudes of HIV-2 IgG or IgA intratype neutralization and viral load. Thus, although the power of the statistical analyses was limited by the low numbers of participants, these results suggest that the magnitude of HIV-1 intratype IgG neutralization is elevated with severity of the disease, while the magnitude of HIV-2 IgG and IgA intratype neutralization does not appear to be.
FIG. 3.
Intratype functionality of plasma IgG and IgA in HIV-1 and HIV-2 infections. Immunoglobulin functionality was calculated by dividing the highest reciprocal neutralizing titer of immunoglobulin by the corresponding plasma immunoglobulin level (mg) present in the assay. IgG and IgA titers where neutralization was not detected at 1:20 dilution were given a reciprocal titer of 10. Boxes illustrate interquartile range with median indicated and whiskers minimum and maximum. *p<0.05, ***p<0.001.
Discussion
In the present work, we compared side-by-side plasma IgA- and IgG-mediated neutralization in HIV-1, HIV-2, and HIV-D infections. We show that plasma IgA-mediated intratype neutralization is more frequent in HIV-2 infection than in HIV-1 infection. Moreover, the magnitudes of both IgG and IgA intratype neutralization in HIV-2 plasma exceeded the magnitude of neutralization by HIV-1 IgG and IgA.
In agreement with recent publications, on different cohorts, by our group and others,14,39,40 the current study provides evidence that HIV-2-directed neutralizing antibody responses in HIV-2 infection are of high magnitude. Moreover, we reveal that both IgG and IgA contribute to this phenomenon, since the magnitude of intratype neutralization by HIV-2 IgG and IgA was higher than that of HIV-1 IgG and IgA, respectively. Furthermore, in HIV-2 infection we found a correlation between intratype neutralizing IgG and IgA titers. This correlation could not be found in HIV-1 infection since only one out of 10 plasma samples displayed intratype neutralizing IgA. The paucity of plasma IgA neutralization in HIV-1 infection is in agreement with another study.41 In contrast, we noted that plasma IgA from seven out of 10 HIV-2-infected individuals displayed intratype neutralizing capacity. In support of this finding, the only previous study that pursued the analysis of HIV-2 neutralizing IgA in HIV-2-infected individuals detected this with similar frequency.19
There may be several explanations for the differences in magnitude of IgG and IgA intratype neutralization between HIV-1 and HIV-2 infections. Differences seem to be dependent on the viruses themselves, since we observed, in a manner similar to our previous study on plasma neutralizing activity,14 that HIV-D individuals harbored IgG and IgA that neutralized HIV-1 and HIV-2 at magnitudes comparable to the corresponding intratype neutralization by IgG and IgA from singly infected individuals. Moreover, the common phenotypes of HIV-1 and HIV-2 seem to differ in neutralization sensitivity. Therefore, isolates to be used in the present study were selected based on our previous experience.14 HIV-1 C Br was in our earlier study found to be the most sensitive HIV-1 isolate in neutralization assays using HIV-1 plasma from Guinea-Bissau. We also avoided use of the most neutralization-sensitive HIV-2 isolate, thereby bringing the virus sensitivities closer to each other. Interestingly, we noted that intertype neutralization by plasma IgG was significantly stronger in HIV-1 than in HIV-2 infection. Also in this case, the overall higher neutralization sensitivity of HIV-2 may provide an explanation.14,39,40 The difference in frequency of neutralization-sensitive virus variants in HIV-1 and HIV-2 infections is further revealed by previous studies of ours and others14,39,40 and goes along with studies showing that escape from neutralizing antibodies is rare in HIV-2 infection while it is common in HIV-1 infection.13,35,42
Interestingly, our findings also revealed that the magnitude of IgG, as well IgA intratype neutralization in relation to the level of plasma IgG and IgA was higher in HIV-2 than in HIV-1 infection. Thus, despite the fact that HIV-1 plasma contained elevated levels of IgG the virus-specific effector function was not increased, but rather appears to be diluted. It is well known that progressive HIV-1 infection is characterized by chronic immune activation, including aberrant cytokine secretion and nonspecific activation of T and B cells.43,44 Chronic B cell immune activation is also characterized by the manifestation of hypergammaglobulinemia in parallel with disease progression.45 In line with this, the present study showed that IgG levels in plasma of HIV-1-infected individuals, either singly or dually infected, were elevated as compared with HIV-negative individuals, while HIV-2 plasma IgG was not. This is in spite of the unexpected observation that four out of 10 HIV-2-infected individuals had a CD4+ T cell count <200/μl, while this low CD4 count was recorded for only one out of the 10 HIV-1-positive participants. This finding might be explained by the fact that the participants in the current study had attended a sexual health and family planning clinic for urogenital problems, some of which could be AIDS defining. Nevertheless, we also noted that none of the HIV-2-infected individuals, where plasma viral load could be assessed, displayed plasma viral RNA above 281 copies/ml. This observation together with observations of others suggests that the low degree of immune activation present in HIV-2 infection is associated with the relatively low level of plasma viral load.46,47 Thus, it is even more intriguing that both IgG and IgA of HIV-2-infected individuals display potent intratype neutralizing activity in the absence of strong antigenic stimulation. However, the magnitude of intratype IgG neutralization appeared elevated with the severity of HIV-1 disease, here detected as decreasing CD4+ T cell counts, while this was not the case in HIV-2 infection.
It is known that HIV-1 preferentially infects CD4+CCR5+ memory T cells in GALT, and thereby affects the structure of germinal centers in this tissue. Since GALT hosts ASC that almost exclusively secrete IgA, defects in the ability to generate high-affinity HIV-1-specific IgA may be the result.48,49 Although the extent of GALT involvement and lymphocyte depletion in HIV-2 infection has not been explored yet, the frequent HIV-2-specific plasma IgA responses detected indicate better preservation of germinal centers in the GALT of HIV-2-infected individuals. In contrast, aberrant chronic immune activation and augmented replication of HIV-1 may lead to destruction of IgA-secreting cells in GALT, depriving the HIV-1-infected host of anti-HIV-1 IgA. The presence of powerful IgA-mediated neutralization in the presence of intact gut mucosa in HIV-1-infected LTNP also supports this hypothesis.50,51
Monomeric serum IgA has been described as an antibody isotype with antiinflammatory properties27,52,53 inasmuch as it lacks complement-activating capacity and suppresses chemotaxis, phagocytosis, as well as cytokine release.29,54–56 We speculate that blunting of inflammatory processes via anti-HIV-2 plasma IgA may contribute to the lower immune activation in HIV-2 infection.
Taken together, HIV-2 displays features that allow potent intratype neutralization by both IgG and IgA, characteristics that are distinct from those of HIV-1. We believe that continued studies on the interaction between HIV-2 and neutralizing antibodies may reveal important knowledge on the makeup of potent humoral immune responses.
Acknowledgments
The authors are indebted to expert technical assistance provided by the staff of the National Laboratory of Public Health (LNSP), Guinea-Bissau. The work was supported by grants provided from the Swedish International Development Agency/Department for Research Cooperation (Sida/SAREC), the Swedish Research Council, the European Commission Sixth and Seventh Framework Programmes, FP6 grant agreement 037611 (EUROPRISE), and FP7 grant agreement 201433 (NGIN). Grants were also provided by the Royal Physiographic Society in Lund, Sweden, and the Crafoord Foundation, Lund, Sweden.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cao Y. Qin L. Zhang L, et al. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332(4):201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 2.Bello G. Velasco-de-Castro CA. Bongertz V, et al. Immune activation and antibody responses in non-progressing elite controller individuals infected with HIV-1. J Med Virol. 2009;81(10):1681–1690. doi: 10.1002/jmv.21565. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Steele E. Awasana AA. Corrah T, et al. Is HIV-2-induced AIDS different from HIV-1-associated AIDS? Data from a West African clinic. AIDS. 2007;21(3):317–324. doi: 10.1097/QAD.0b013e328011d7ab. [DOI] [PubMed] [Google Scholar]
- 4.van der Loeff MF. Larke N. Kaye S, et al. Undetectable plasma viral load predicts normal survival in HIV-2-infected people in a West African village. Retrovirology. 2010;7:46. doi: 10.1186/1742-4690-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanki PJ. Travers KU. S MB, et al. Slower heterosexual spread of HIV-2 than HIV-1. Lancet. 1994;343(8903):943–946. doi: 10.1016/s0140-6736(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 6.Adjorlolo-Johnson G. De Cock KM. Ekpini E, et al. Prospective comparison of mother-to-child transmission of HIV-1 and HIV-2 in Abidjan, Ivory Coast. JAMA. 1994;272(6):462–466. [PubMed] [Google Scholar]
- 7.Esbjornsson J. Mansson F. Kvist A, et al. Natural inhibition of HIV-1 disease progression by contemporaneous HIV-2 infection. N Engl J Med. 2012;367:224–232. doi: 10.1056/NEJMoa1113244. [DOI] [PubMed] [Google Scholar]
- 8.Simon F. Matheron S. Tamalet C, et al. Cellular and plasma viral load in patients infected with HIV-2. AIDS. 1993;7(11):1411–1417. doi: 10.1097/00002030-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Andersson S. Norrgren H. da Silva Z, et al. Plasma viral load in HIV-1 and HIV-2 singly and dually infected individuals in Guinea-Bissau, West Africa: Significantly lower plasma virus set point in HIV-2 infection than in HIV-1 infection. Arch Intern Med. 2000;160(21):3286–3293. doi: 10.1001/archinte.160.21.3286. [DOI] [PubMed] [Google Scholar]
- 10.Ariyoshi K. Berry N. Wilkins A, et al. A community-based study of human immunodeficiency virus type 2 provirus load in rural village in West Africa. J Infect Dis. 1996;173(1):245–248. doi: 10.1093/infdis/173.1.245. [DOI] [PubMed] [Google Scholar]
- 11.MacNeil A. Sarr AD. Sankale JL, et al. Direct evidence of lower viral replication rates in vivo in human immunodeficiency virus type 2 (HIV-2) infection than in HIV-1 infection. J Virol. 2007;81(10):5325–5330. doi: 10.1128/JVI.02625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endres MJ. Clapham PR. Marsh M, et al. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87(4):745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y. Brandin E. Vincic E, et al. Evolution of human immunodeficiency virus type 2 coreceptor usage, autologous neutralization, envelope sequence and glycosylation. J Gen Virol. 2005;86(12):3385–3396. doi: 10.1099/vir.0.81259-0. [DOI] [PubMed] [Google Scholar]
- 14.Ozkaya Sahin G. Holmgren B. da Silva Z, et al. Potent intratype neutralizing activity distinguishes human immunodeficiency virus type 2 (HIV-2) from HIV-1. J Virol. 2012;86(2):961–971. doi: 10.1128/JVI.06315-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozlowski PA. Chen D. Eldridge JH. Jackson S. Contrasting IgA and IgG neutralization capacities and responses to HIV type 1 gp120 V3 loop in HIV-infected individuals. AIDS Res Hum Retroviruses. 1994;10(7):813–822. doi: 10.1089/aid.1994.10.813. [DOI] [PubMed] [Google Scholar]
- 16.Mestecky J. Jackson S. Moldoveanu Z, et al. Paucity of antigen-specific IgA responses in sera and external secretions of HIV-type 1-infected individuals. AIDS Res Hum Retroviruses. 2004;20(9):972–988. doi: 10.1089/aid.2004.20.972. [DOI] [PubMed] [Google Scholar]
- 17.Israel ZR. Marx PA. Nonclassical mucosal antibodies predominate in genital secretions of HIV-1 infected chimpanzees. J Med Primatol. 1995;24(2):53–60. doi: 10.1111/j.1600-0684.1995.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 18.Schafer F. Kewenig S. Stolte N, et al. Lack of simian immunodeficiency virus (SIV) specific IgA response in the intestine of SIV infected rhesus macaques. Gut. 2002;50(5):608–614. doi: 10.1136/gut.50.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lizeng Q. Skott P. Sourial S, et al. Serum immunoglobulin A (IgA)-mediated immunity in human immunodeficiency virus type 2 (HIV-2) infection. Virology. 2003;308(2):225–232. doi: 10.1016/s0042-6822(02)00088-0. [DOI] [PubMed] [Google Scholar]
- 20.Brenchley JM. Schacker TW. Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200(6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woof JM. Kerr MA. The function of immunoglobulin A in immunity. J Pathol. 2006;208(2):270–282. doi: 10.1002/path.1877. [DOI] [PubMed] [Google Scholar]
- 22.Macpherson AJ. Geuking MB. Slack E, et al. The habitat, double life, citizenship, and forgetfulness of IgA. Immunol Rev. 2012;245(1):132–146. doi: 10.1111/j.1600-065X.2011.01072.x. [DOI] [PubMed] [Google Scholar]
- 23.Quesnel A. Moja P. Lucht F, et al. Is there IgA of gut mucosal origin in the serum of HIV1 infected patients? Gut. 1994;35(6):803–808. doi: 10.1136/gut.35.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mei HE. Yoshida T. Sime W, et al. Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood. 2009;113(11):2461–2469. doi: 10.1182/blood-2008-04-153544. [DOI] [PubMed] [Google Scholar]
- 25.van Vlasselaer P. Punnonen J. de Vries JE. Transforming growth factor-beta directs IgA switching in human B cells. J Immunol. 1992;148(7):2062–2067. [PubMed] [Google Scholar]
- 26.Boehm MK. Woof JM. Kerr MA. Perkins SJ. The Fab and Fc fragments of IgA1 exhibit a different arrangement from that in IgG: A study by X-ray and neutron solution scattering and homology modelling. J Mol Biol. 1999;286(5):1421–1447. doi: 10.1006/jmbi.1998.2556. [DOI] [PubMed] [Google Scholar]
- 27.Kerr MA. The structure and function of human IgA. Biochem J. 1990;271(2):285–296. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai H. Chen A. Wyatt RJ. Rifai A. Lack of complement activation by human IgA immune complexes. Clin Exp Immunol. 1988;73(3):479–483. [PMC free article] [PubMed] [Google Scholar]
- 29.Wilton JM. Suppression by IgA of IgG-mediated phagocytosis by human polymorphonuclear leucocytes. Clin Exp Immunol. 1978;34(3):423–428. [PMC free article] [PubMed] [Google Scholar]
- 30.Gueudin M. Damond F. Braun J, et al. Differences in proviral DNA load between HIV-1- and HIV-2-infected patients. AIDS. 2008;22(2):211–215. doi: 10.1097/QAD.0b013e3282f42429. [DOI] [PubMed] [Google Scholar]
- 31.Damond F. Gueudin M. Pueyo S, et al. Plasma RNA viral load in human immunodeficiency virus type 2 subtype A and subtype B infections. J Clin Microbiol. 2002;40(10):3654–3659. doi: 10.1128/JCM.40.10.3654-3659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozkaya Sahin G. Bowles EJ. Parker J, et al. Generation of neutralizing antibodies and divergence of SIVmac239 in cynomolgus macaques following short-term early antiretroviral therapy. PLoS Pathog. 2010;6(9):e1001084. doi: 10.1371/journal.ppat.1001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.HIV type 1 variation in World Health Organization-sponsored vaccine evaluation sites: Genetic screening, sequence analysis, and preliminary biological characterization of selected viral strains. WHO Network for HIV Isolation and Characterization. AIDS Res Hum Retroviruses. 1994;10(11):1327–1343. doi: 10.1089/aid.1994.10.1327. [DOI] [PubMed] [Google Scholar]
- 34.Morner A. Bjorndal A. Albert J, et al. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J Virol. 1999;73(3):2343–2349. doi: 10.1128/jvi.73.3.2343-2349.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albert J. Bottiger B. Biberfeld G. Fenyo EM. Replicative and cytopathic characteristics of HIV-2 and severity of infection. Lancet. 1989;1(8642):852–853. doi: 10.1016/s0140-6736(89)92321-0. [DOI] [PubMed] [Google Scholar]
- 36.Bjorndal A. Deng H. Jansson M, et al. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71(10):7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lauren A. Thorstensson R. Fenyo EM. Comparative studies on mucosal and intravenous transmission of simian immunodeficiency virus (SIVsm): The kinetics of evolution to neutralization resistance are related to progression rate of disease. J Gen Virol. 2006;87(3):595–606. doi: 10.1099/vir.0.81409-0. [DOI] [PubMed] [Google Scholar]
- 38.Simek MD. Rida W. Priddy FH, et al. Human immunodeficiency virus type 1 elite neutralizers: Individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83(14):7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Silva TI. Aasa-Chapman M. Cotten M, et al. Potent autologous and heterologous neutralizing antibody responses occur in HIV-2 infection across a broad range of infection outcomes. J Virol. 2012;86(2):930–946. doi: 10.1128/JVI.06126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong R. Li H. Bibollet-Ruche F, et al. Broad and potent neutralizing antibody responses elicited in natural HIV-2 infection. J Virol. 2012;86(2):947–960. doi: 10.1128/JVI.06155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burrer R. Salmon-Ceron D. Richert S, et al. Immunoglobulin G (IgG) and IgA, but also nonantibody factors, account for in vitro neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by serum and plasma of HIV-infected patients. J Virol. 2001;75(11):5421–5424. doi: 10.1128/JVI.75.11.5421-5424.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bjorling E. Scarlatti G. von Gegerfelt A, et al. Autologous neutralizing antibodies prevail in HIV-2 but not in HIV-1 infection. Virology. 1993;193(1):528–530. doi: 10.1006/viro.1993.1160. [DOI] [PubMed] [Google Scholar]
- 43.Haas A. Zimmermann K. Oxenius A. Antigen-dependent and -independent mechanisms of T and B cell hyperactivation during chronic HIV-1 infection. J Virol. 2011;85(23):12102–12113. doi: 10.1128/JVI.05607-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moir S. Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9(4):235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lane HC. Masur H. Edgar LC, et al. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309(8):453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 46.Leligdowicz A. Feldmann J. Jaye A, et al. Direct relationship between virus load and systemic immune activation in HIV-2 infection. J Infect Dis. 2010;201(1):114–122. doi: 10.1086/648733. [DOI] [PubMed] [Google Scholar]
- 47.Chollet-Martin S. Simon F. Matheron S, et al. Comparison of plasma cytokine levels in African patients with HIV-1 and HIV-2 infection. AIDS. 1994;8(7):879–884. doi: 10.1097/00002030-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Ferre AL. Hunt PW. Critchfield JW, et al. Mucosal immune responses to HIV-1 in elite controllers: A potential correlate of immune control. Blood. 2009;113(17):3978–3989. doi: 10.1182/blood-2008-10-182709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levesque MC. Moody MA. Hwang KK, et al. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 2009;6(7):e1000107. doi: 10.1371/journal.pmed.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ciccone EJ. Greenwald JH. Lee PI, et al. CD4+ T cells, including Th17 and cycling subsets, are intact in the gut mucosa of HIV-1-infected long-term nonprogressors. J Virol. 2011;85(12):5880–5888. doi: 10.1128/JVI.02643-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Planque S. Salas M. Mitsuda Y, et al. Neutralization of genetically diverse HIV-1 strains by IgA antibodies to the gp120-CD4-binding site from long-term survivors of HIV infection. AIDS. 2010;24(6):875–884. doi: 10.1097/QAD.0b013e3283376e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pasquier B. Launay P. Kanamaru Y, et al. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: Dual role of FcRgamma ITAM. Immunity. 2005;22(1):31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 53.Monteiro RC. The role of IgA and IgA Fc receptors as anti-inflammatory agents. J Clin Immunol. 2010;30(Suppl 1):S61–64. doi: 10.1007/s10875-010-9397-2. [DOI] [PubMed] [Google Scholar]
- 54.Van Epps DE. Williams RC:, Jr Suppression of leukocyte chemotaxis by human IgA myeloma components. J Exp Med. 1976;144(5):1227–1242. doi: 10.1084/jem.144.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolf HM. Fischer MB. Puhringer H, et al. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes. Blood. 1994;83(5):1278–1288. [PubMed] [Google Scholar]
- 56.Wolf HM. Hauber I. Gulle H, et al. Anti-inflammatory properties of human serum IgA: Induction of IL-1 receptor antagonist and Fc alpha R (CD89)-mediated down-regulation of tumour necrosis factor-alpha (TNF-alpha) and IL-6 in human monocytes. Clin Exp Immunol. 1996;105(3):537–543. doi: 10.1046/j.1365-2249.1996.d01-793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]