Abstract
On the basis of studies in experimental animals demonstrating that AdVEGF121, an E1−E3− serotype 5 adenovirus coding the 121 isoform of vascular endothelial growth factor (VEGF), could mediate the generation of new blood vessels and reverse coronary ischemia, a clinical study of direct myocardial administration of AdVEGF121 was initiated in patients with late-stage, diffuse coronary artery disease. This study provides long-term (median, 11.8 years) follow-up on these patients. From 1997 to 1999, AdVEGF121 was administered by direct myocardial injection to an area of reversible ischemia in 31 patients with severe coronary disease, either as an adjunct to conventional coronary artery bypass grafting (group A) or as minimally invasive sole (MIS) therapy, using a minithoracotomy (group B). There was no control group; the study participants served as the control subjects. The 5- and 10-year survival was 10 of 15 (67%) and 6 of 15 (40%) for the group A patients, and 11 of 16 (69%) and 5 of 16 (31%) for group B sole therapy patients, respectively. In comparison, maximal medical therapy in comparable groups in the literature have a 3- to 5-year survival rate of 52 to 59%. For the survivors, the angina score for group A was 3.4±0.5 at time 0 and 1.9±1.0 at last follow-up, and for group B it was 3.4±0.6 and 2.0±1.1, respectively. The incidences of malignancy and retinopathy were no greater than that expected for the age-matched general population. We conclude that adenovirus-mediated VEGF direct myocardial administration to patients with severe coronary artery disease is safe, and future larger trials are warranted to assess efficacy.
Rosengart and colleagues report long-term safety data (median, 11.8 years) from a clinical study involving 31 research subjects with late-stage, diffuse coronary artery disease. Research subjects who received direct myocardial administration of an adenovirus encoding the 121 isoform of vascular endothelial growth factor (Ad-VEGF121) did not have an increased incidence of malignancy and retinopathy compared with the age-matched general population.
Introduction
Therapeutic cardiac angiogenesis is a strategy of administering biologics to induce the growth of coronary artery collateral vessels to enhance blood flow to the chronically ischemic myocardium (Schumacher et al., 1998; Symes et al., 1999; Rosengart et al., 1999c; Simons et al., 2000; Freedman and Isner, 2002; Carmeliet, 2003). Most human trials of cardiac angiogenesis have used direct myocardial transfer of adenovirus or nonviral vectors encoding a mediator that will induce angiogenesis, such as one of the isoforms of vascular endothelial growth factor (VEGF) or fibroblast growth factors (Laham et al., 1999; Rosengart et al., 1999a,b; Symes et al., 1999; Vale et al., 2000; Losordo et al., 2002; Kastrup et al., 2005; Stewart et al., 2006; Mitsos et al., 2012). Nearly all of these trials had a short-term follow-up, typically less than 1 year (Rosengart et al., 1999a,b; Simons et al., 2000; Sarkar et al., 2001; Hedman et al., 2003; Yang et al., 2009).
In 1997 we initiated cardiac angiogenesis trials using direct administration to the wall of the left ventricle of a serotype 5, E1−E3− adenoviral gene transfer vector encoding the VEGF121 isoform (AdVEGF121) (Rosengart et al., 1999a,b). The study population all had severe, diffuse coronary artery disease, with a Canadian Cardiovascular Society angina score of III to IV (Rosengart et al., 1999b). A total of 31 patients were studied in 2 groups; group A (n=15) as an adjunct to coronary artery bypass grafting (CABG) and group B (n=16) as sole therapy using a minithoracotomy to gain access to the left ventricle (Rosengart et al., 1999a,b).
Our study was designed as phase 1/2 evaluations of short-term (6 months) evaluation of safety and efficacy. The focus of the present study is to assess what has happened over a median of nearly 12 years to the 31 patients enrolled in our study. Together with the 8-year follow-up of cardiac angiogenesis by Hedman and colleagues (2009), these data support the concept that direct myocardial adenovirus-mediated VEGF gene therapy for diffuse coronary artery disease is safe and that future, randomized placebo-controlled trials are warranted.
Materials and Methods
Patient selection and study design
Individuals were selected on the basis of their having refractory coronary artery disease and reversible left ventricular ischemia assessed by an independent cardiologist using rest and stress 99mTc-sestamibi studies, as well as angiographic findings of severe or diffuse disease and/or target coronaries that were deemed as not bypassable (Rosengart et al., 1999a,b). Two different treatment protocols were used for patient subgroups A and B, respectively. In group A (adjunct-to-CABG group; n=15), AdVEGF121 was administered by direct intramyocardial injection to an ischemic area of the heart that could not be bypassed because of complete obstruction or diffuse disease in coronary targets during conventional, on-pump CABG surgery performed for standard clinical indications. For group B (minimally invasive sole therapy [MIS]; n=16), in patients for whom CABG could not be carried out because of a lack of suitable coronary targets, AdVEGF121 was administered by direct intramyocardial injection through a minithoracotomy. In both cases, ischemic myocardium was identified by 99mTc-sestamibi nuclear medicine studies and suitability of bypass was determined by the surgeon on the basis of angiographic data.
Study recruitment included both men and women with ages ranging from 18 to 85 years with FEV1>1.2 liters, room air PO2>60 torr, PCO2<50 torr, hematocrit >30%, white blood cell count <10,000, blood urea nitrogen <40 U/liter, and creatinine <2.5 mg/dl. Exclusion criteria included individuals with life-threatening arrhythmias, an ejection fraction <25% for group A, or an ejection fraction <30% for group B (see below for group definitions). Other than the severity of ischemic heart disease, the study subjects were typical in their preoperative risk characteristics of other CABG populations reported in the literature (Rosengart et al., 1999a,b; Cheng et al., 2006).
The original studies were approved by the Weill-Cornell Institutional Review Board, and Biosafety Committee, the National Institutes of Health (NIH) DNA Recombinant Advisory Committee, and the Food and Drug Administration. The trial was registered through ClinicalTrials.gov (ID NCT01174095). After the conclusion of the original study, a follow-up longitudinal study was approved by both the Stony Brook University and Weill-Cornell Institutional Review Boards.
Vector
The AdVEGF121 vector has been described (Rosengart et al., 1999a,b; Lee et al., 2000). Briefly, AdVEGF121 (GenVec, Rockville, MD) is composed of an Ad5 serotype backbone, with deletions in the E1 and E3 regions. The expression cassette in the E1 region contains (right-to-left orientation) the cytomegalovirus early/immediate enhancer/promoter, an artificial splice sequence, the human VEGF121 cDNA, and the simian virus 40 (SV40) poly(A)/stop signal. The vector was produced and stored at −70°C as previously described (Patel et al., 1999; Lee et al., 2000). At the time of vector delivery, the vector was thawed, immediately diluted, drawn up into a 27-gauge needle (Becton Dickinson, Franklin Lakes, NJ), and placed into a sterile container for transport to the operating room.
For group A, the AdVEGF121 vector was administered directly to the myocardium as 10 subepicardial injections (100 μl/injection; 10 sites/patient; each site 1 to 1.5 cm apart) into a single coronary myocardial territory that demonstrated reversible ischemia. For group A, the CABG procedure was performed via a standard median sternotomy with vector administered during cardiopulmonary bypass after graft completion and rewarming. In group A, five dose groups were evaluated (n=3 patients per dose group), with total doses: 4×108, 4×108.5, 4×109, 4×109.5, and 4×1010 particle units (PU).
For group B, the vector was administered directly to the myocardium as 10 subepicardial injections (100 μl/injection; each site approximately 1 cm apart). The vector was injected through a 4- to 5-cm minithoracotomy with direct visualization of the region of reversible ischemia. For group B, total vector dose was 4×109 PU/patient.
Follow-up
To initiate this follow-up study, the original research records of all 31 patients were reviewed. The individuals were contacted to coordinate their informed (re-) consent and HIPAA authorization to conduct this longitudinal follow-up. The follow-up interviews and questionnaires were performed by a research coordinator, including an independent assessment of the severity of each patient's symptoms, using both the Canadian Cardiovascular Society and New York Heart Association classifications. In addition, a copy of the patients' medical records for the period since the index surgical procedure was reviewed to identify potential treatment-related complications as well as subsequent cardiac-related events. Deaths reported were verified using the online Social Security Death Index. Median follow-up time of survivors was 11.8 years; median survival time for the entire cohort (with two patients lost to follow-up) was 9.6 years.
Statistical analysis
Follow-up was obtained in 29 (94%) of these patients with a median follow-up of 11.8 years. Univariate statistical tests were performed as well as t tests, chi-square tests, and Kaplan–Meier survival curves, to compare the patient baseline characteristics and follow-up outcomes. All statistical analyses were performed with SPSS software version 18.0 (IBM, Somers, NY).
Results
As previously reported, in the first 6-month follow-up period, there were two perioperative deaths (within 40 days of the operative procedure), one additional sudden death of unknown cause on postoperative day 145 in group A, and no deaths in group B (Rosengart et al., 1999b). There was no unexpected morbidity in either group during this initial 6-month evaluation period. One group A patient underwent percutaneous coronary intervention (PCI) during the initial follow-up period, and one group B patient underwent PCI and subsequent CABG during this interval.
Over the average of the nearly 12-year follow-up period, cardiac revascularization procedures were performed in a total of eight patients, including the two in the initial, previously reported follow-up period (Table 1). Six received implantable cardioverter defibrillators (ICDs; Table 1). Of the 31 individuals, 1 developed retinopathy. One group A patient and three group B patients developed malignancies (prostate cancer, colon cancer, squamous cell cancer of the head, and breast cancer, respectively). One group A patient had a small right atrial mass (<9 mm) noted on an echocardiogram 10 years after AdVEGF121 therapy. This did not change when reevaluated 1 year later. The subject had undergone PCI and ICD, but refused surgical intervention or biopsy. At the time of this study, 13 years after the AdVEGF121 therapy, this study subject is alive and doing well.
Table 1.
Adverse Eventsa
| Group A (n=12)b | Group B (n=15)b | Total (n=27)b | Incidence in general population (%)c | |
|---|---|---|---|---|
| Cancer | ||||
| Prostate | 1 | 0 | 1 | 8 |
| Colon | 0 | 1 | 1 | 0.4 |
| Squamous cell (head/neck) | 0 | 1 | 1 | NA |
| Breast | 0 | 1 | 1 | 3 |
| Total cancer | 1 | 3 | 4 | 17 |
| Retinopathy | 0 | 1 | 1 | 20d |
| Cardiac intervention | ||||
| PCI | 3 | 4e | 7 | |
| CABG | 0 | 1e | 1 | |
| ICD | 3 | 3 | 6 | |
| Radiofrequency ablation | 1 | 0 | 1 | |
| Heart transplant | 0 | 1 | 1 | |
| Stroke | 0 | 1 | 1 | |
| Otherf | 1 | 0 | 1 | |
CABG, coronary artery bypass grafting; ICD, implantable cardioverter-defibrillator; NA, not available; PCI, percutaneous coronary intervention.
Includes data in our original 6-month follow-up report (Rosengart et. al., 1999a).
Number of patients with complete follow-up data.
SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations), National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data posted 2012 (tabulated incidence corrected to 10-year follow-up interval and age group).
National Eye Institute Data and Statistics (www.nei.nih.gov/eyedata/pbd3.asp); tabulated on the basis of incidence of diabetes in cohort population and incidence of retinopathy for diabetics in database.
One individual underwent both PCI and CABG during initial follow-up.
Right atrial mass.
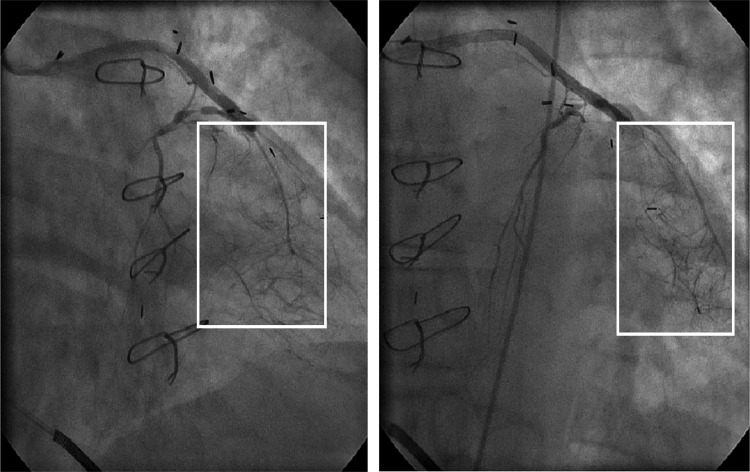
One group B patient underwent heart transplantation 9.5 years after vector administration. The explanted heart was obtained for postmortem examination, but no evidence of enhanced angiogenesis was evident in this specimen postfixation. In contrast, coronary angiograms of three patients (one in group A and two in group B) obtained at our institution 10 years after AdVEGF121 therapy had evidence of a “tumor blush,” suggesting collateral vessel formation in the treated territory (Fig. 1).
FIG. 1.
Example of an angiogram 10 years after direct myocardial administration of AdVEGF121 as sole therapy (group B). The vector was administered in the region outlined by the white box. The two views show an area of significant collateralization distal to the primary occlusion.
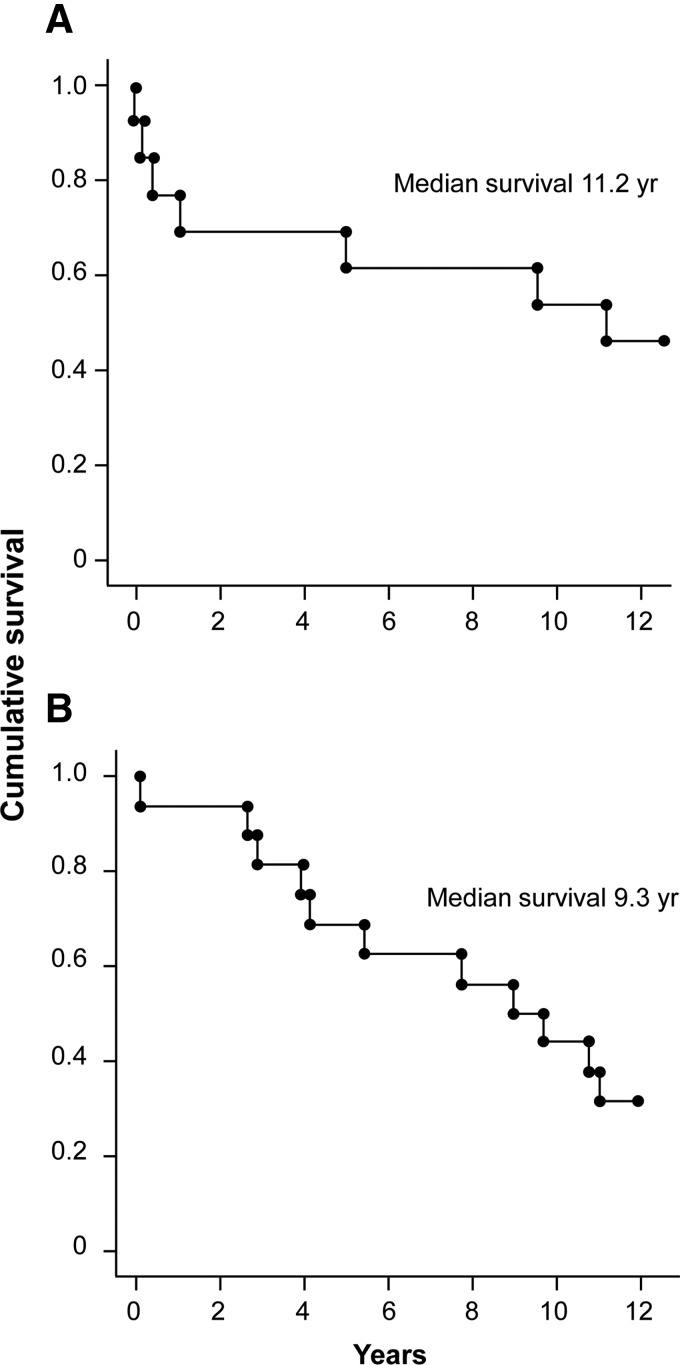
For group A, survival at 5 years was 10 of 15 (67%) and survival at 10 years was 6 of 15 (40%). Group B survival at 5 and 10 years was 11 of 16 (69%) and 5 of 16 (31%), respectively (Figs. 2 and 3). Of the total of 18 patients known to have died over the follow-up period, 2 had an unknown cause of death, 1 died from causes related to breast cancer, and the remainder (n=15) died of cardiac-related causes.
FIG. 2.
Kaplan–Meier 10-year survival data. (A) Group A adjunct to bypass. (B) Group B, sole therapy.
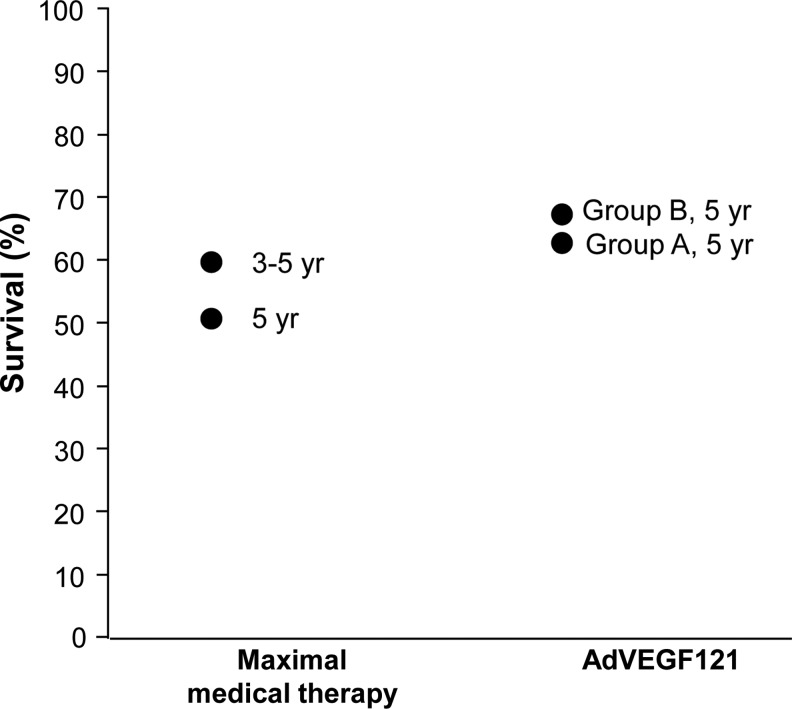
FIG. 3.
Five-year survival rates for groups A and B versus comparator groups with similar groups of diffuse coronary artery disease treated with maximal medical therapy from the literature.
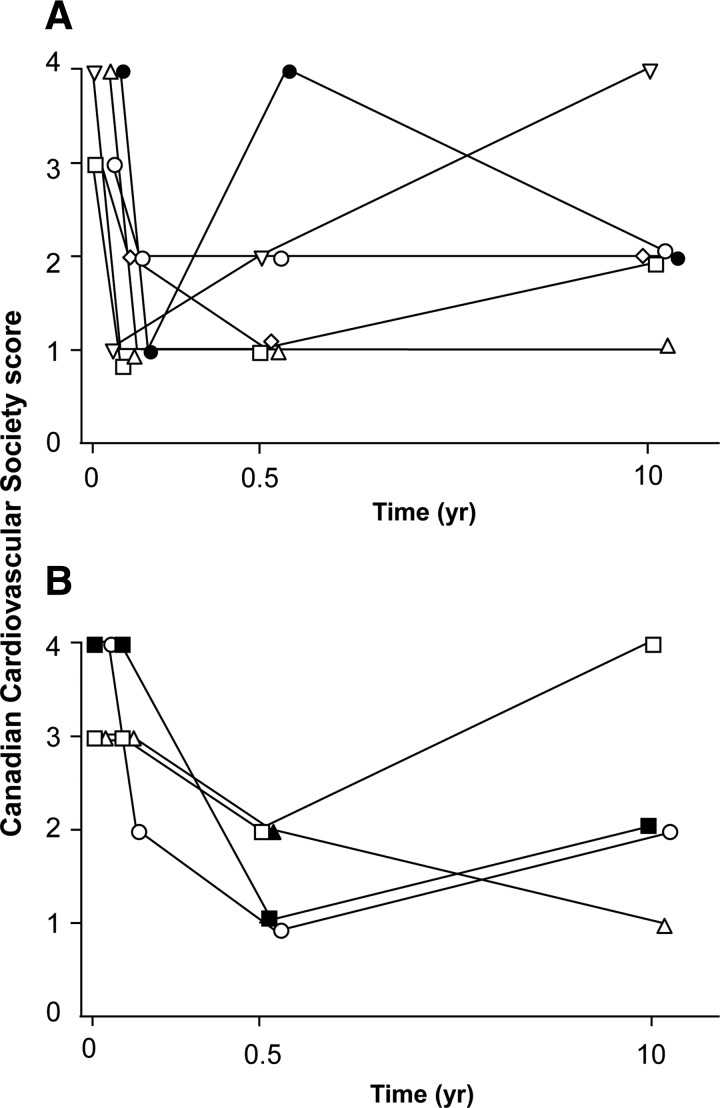
The patients still alive were more frequently male (10 males vs. 1 female were alive at follow-up vs. 11 males and 7 females that had died). The survivors had presented at a younger age (57±9 vs. 63±10 years) and had lower incidences of diabetes (36 vs. 61%) and hypertension (64 vs. 88%) at the time of initial surgery compared with nonsurvivors (Table 2). Of the 10 patients available for questioning, 5 of 6 living (83%) group A patients and 3 of 4 (75%) living group B patients (excluding the transplant patient) had a CCS angina class of I or II (Fig. 4). The baseline CCS angina class in all of these patients was either class III or IV.
Table 2.
Potential Risk Factors for Mortalitya
| Alive (n=11) | Dead (n=18) | p Value | |
|---|---|---|---|
| Age (yr) | 57±9 | 63±10 | 0.1 |
| Range | 42–69 | 43–83 | |
| Male:female | 10:1 | 11:7 | 0.1 |
| CCS Score (%) | 0.4 | ||
| CCS I/II | 0 | 0 | |
| CCS III | 5 (46%) | 12 (67%) | |
| CCS IV | 6 (55%) | 6 (33%) | |
| Cardiovascular (%) | |||
| CHF | 2 (18%) | 5 (28%) | 1.0 |
| Diabetes | 4 (36%) | 11 (61%) | 0.3 |
| Hypertension | 7 (64%) | 16 (88%) | 0.2 |
| Prior CABG | 7 (64%) | 9 (50%) | 0.7 |
| Prior PCI | 2 (27%) | 2 (17%) | 0.7 |
CABG, coronary artery bypass grafting; CCS, Canadian Cardiovascular Society classification; CHF, congestive heart failure; PCI, percutaneous coronary intervention.
Baseline demographics for all patients with known survival status (29 of 31).
FIG. 4.
Canadian Cardiovascular Society scores for surviving patients. (A) Group A. (B) Group B, excluding the one cardiac transplant patient.
Discussion
The current study represents the longest documented follow-up of a cohort of individuals having been administered a gene transfer vector in vivo. There is only one other report of long-term follow-up of in vivo gene transfer in the literature, describing the 8-year follow-up of a cohort of individuals receiving adenovirus encoding VEGF165 for the treatment of coronary artery disease (Hedman et al., 2009). Although not originally designed as a long-term follow-up study, the current report provides an encouraging safety profile after a median of 11.8 years after myocardial administration of an adenovirus encoding VEGF.
Despite early concerns that direct cardiac adenovirus-mediated angiogenic gene transfer might invoke deleterious acute local or systemic adverse responses, there were minimal elevations of markers of inflammation or myocardial injury after direct vector administration to the heart in this and other clinical studies (Rosengart et al., 1999c; Simons et al., 2000; Crystal et al., 2002; Hedman et al., 2003; Stewart et al., 2006; Gupta et al., 2009; Yang et al., 2009; Mitsos et al., 2012). Relevant to the VEGF transgene, although there were theoretical concerns of propensity for malignancy with VEGF-mediated cardiac gene therapy, no increase in malignancy was seen in the present study cohort compared with the general population, refuting concerns of tumor propagation supported by promiscuous neovascularization, or due to activation of latent oncogenes. The occurrence of four malignancies over nearly 12 years of follow-up (13% incidence) in our relatively elderly patient population is not different from the 17% rate expected for the general population at this age (Altekruse et al., 2012). Further, despite the fact that 52% of our study cohort were diabetics and that 40 to 50% of this population can be anticipated to develop diabetic retinopathy (National Eye Institute Data and Statistics; www.nei.nih.gov/eyedata/pbd3.asp), there was a low incidence (<5%) of retinopathy observed in our cohort. Together, these findings provide reassurance that concerns regarding promiscuous neovascularization may be unwarranted, consistent with our expectations that by using localized cardiac delivery of the angiogenic agent, there was minimal risk of systemic adverse neovascularization.
The survival over 5 and 10 years of individuals receiving AdVEGF121 in our cohort was as good as, if not better than, that seen in large cohorts of similar patients with coronary artery disease treated with medical therapy. In this regard, the 69% 5-year survival of our sole therapy group B patients compares favorably with the 52% 5-year survival of a similar-risk cohort of individuals randomized to maximal medical therapy in a study of transmyocardial revascularization (TMR) reported by Allen and colleagues (2004). Likewise, the 5-year survival of our sole therapy cohort exceeded the 59% 3- to 5-year survival of maximal medical therapy patients in a meta-analysis of randomized controlled TMR trials (Cheng et al., 2006). Survival in the current study also exceeded the 40% 5-year survival for patients undergoing TMR (Horvath et al., 2001). Survival in our patient population was not as great as in the 8-year follow-up of the Kuopio Angiogenesis Trial (KAT) involving the administration of VEGF via adenovirus or plasmid (Hedman et al., 2009). However, that trial used a patient population likely with a higher survival rate because of its exclusion of patients with diabetes or unstable angina and inclusion of individuals with less extensive coronary artery disease and only CCS class II to III angina.
Because our original study design did not include a placebo control, and given the many confounding variables to outcome that have ensued over the past 11.8 years, assessments of the efficacy of our angiogenic treatment strategy are difficult at best. Nevertheless, in addition to the survival results noted previously, it is interesting that a number of the sole therapy group B patients in this study improved from class III/IV to class I/II angina symptomatology, especially as these changes appear to have persisted beyond the typical 6-month “placebo effect” interval (Finniss et al., 2010). Likewise, although anecdotal and certainly not probative of persistent treatment-induced neovascularization, the angiographic evidence of “tumor blush” seen in three of our treated patients is of interest, and reminiscent of the autopsy-documented neovascularization pattern seen in a treated patient from the “REVASC” AdVEGF121 trial (Stewart et al., 2006).
The current study reports the longest known outcomes and survival results after human in vivo gene transfer from one of the earliest cohorts of patients to have undergone myocardial angiogenic gene therapy. Although interpretation of these data is limited by our lack of more formalized trial design over this prolonged interval, they nevertheless suggest the long-term safety of this intervention, even in patients with late-stage, diffuse critical coronary artery disease. Future placebo-controlled clinical trials that include a rigorous focus on long-term event-free survival rates and/or sustained reduction of symptoms and health-related quality of life appear warranted to substantiate these preliminary findings regarding safety, as well as to evaluate the differential treatment efficacy of gene therapies. In this regard, we are initiating a new, prospective placebo-controlled randomized trial of direct adenovirus-mediated angiogenic gene therapy in an attempt to definitively address this issue (Crystal et al., 2012).
Acknowledgments
Supported in part by a grant from the Lisa and James Cohen Foundation and supported, in part, by the Qatar Foundation and the Weill-Cornell Medical College in Qatar; and NIH R01HL085095 (TKR).
Author Disclosure Statement
No competing financial interests exist.
References
- Allen K.B. Dowling R.D. Angell W.W., et al. Transmyocardial revascularization: 5-year follow-up of a prospective, randomized multicenter trial. Ann. Thorac. Surg. 2004;77:1228–1234. doi: 10.1016/j.athoracsur.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Altekruse S.F. Kosary C.L. Krapcho M., et al. SEER Cancer Statistics Review, 1975–2007, National Cancer Institute. 2012. http://seer.cancer.gov/csr/1975_2007/ http://seer.cancer.gov/csr/1975_2007/
- Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Cheng D. Diegeler A. Allen K., et al. Transmyocardial laser revascularization: A meta-analysis and systematic review of controlled trials. Innovations. (Phila) 2006;1:295–313. doi: 10.1097/IMI.0b013e31802fe0a2. [DOI] [PubMed] [Google Scholar]
- Crystal R.G. Harvey B.G. Wisnivesky J.P., et al. Analysis of risk factors for local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of comorbid conditions. Hum. Gene Ther. 2002;13:65–100. doi: 10.1089/10430340152712647. [DOI] [PubMed] [Google Scholar]
- Crystal R.G. Kaminsky S.M. Hackett N.R., et al. Double-blinded, placebo-controlled, randomized gene therapy using surgery for vector delivery. Hum. Gene Ther. 2012;23:438–441. doi: 10.1089/hum.2012.062. [DOI] [PubMed] [Google Scholar]
- Finniss D.G. Kaptchuk T.J. Miller F., et al. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman S.B. Isner J.M. Therapeutic angiogenesis for coronary artery disease. Ann. Intern. Med. 2002;136:54–71. doi: 10.7326/0003-4819-136-1-200201010-00011. [DOI] [PubMed] [Google Scholar]
- Gupta R. Tongers J. Losordo D.W. Human studies of angiogenic gene therapy. Circ. Res. 2009;105:724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman M. Hartikainen J. Syvanne M., et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: Phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- Hedman M. Muona K. Hedman A., et al. Eight-year safety follow-up of coronary artery disease patients after local intracoronary VEGF gene transfer. Gene Ther. 2009;16:629–634. doi: 10.1038/gt.2009.4. [DOI] [PubMed] [Google Scholar]
- Horvath K.A. Aranki S.F. Cohn L.H., et al. Sustained angina relief 5 years after transmyocardial laser revascularization with a CO2 laser. Circulation. 2001;104:I81–I84. doi: 10.1161/hc37t1.094774. [DOI] [PubMed] [Google Scholar]
- Kastrup J. Jorgensen E. Ruck A., et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris: A randomized double-blind placebo-controlled study: The Euroinject One trial. J. Am. Coll. Cardiol. 2005;45:982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- Laham R.J. Sellke F.W. Edelman E.R., et al. Local perivascular delivery of basic fibroblast growth factor in patients undergoing coronary bypass surgery: Results of a phase I randomized, double-blind, placebo-controlled trial. Circulation. 1999;100:1865–1871. doi: 10.1161/01.cir.100.18.1865. [DOI] [PubMed] [Google Scholar]
- Lee L.Y. Patel S.R. Hackett N.R., et al. Focal angiogen therapy using intramyocardial delivery of an adenovirus vector coding for vascular endothelial growth factor 121. Ann. Thorac. Surg. 2000;69:14–23. doi: 10.1016/s0003-4975(99)01102-9. [DOI] [PubMed] [Google Scholar]
- Losordo D.W. Vale P.R. Hendel R.C., et al. Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation. 2002;105:2012–2018. doi: 10.1161/01.cir.0000015982.70785.b7. [DOI] [PubMed] [Google Scholar]
- Mitsos S. Katsanos K. Koletsis E., et al. Therapeutic angiogenesis for myocardial ischemia revisited: Basic biological concepts and focus on latest clinical trials. Angiogenesis. 2012;15:1–22. doi: 10.1007/s10456-011-9240-2. [DOI] [PubMed] [Google Scholar]
- Patel S.R. Lee L.Y. Mack C.A., et al. Safety of direct myocardial administration of an adenovirus vector encoding vascular endothelial growth factor 121. Hum. Gene Ther. 1999;10:1331–1348. doi: 10.1089/10430349950018012. [DOI] [PubMed] [Google Scholar]
- Rosengart T.K. Lee L.Y. Patel S.R., et al. Six-month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA. Ann. Surg. 1999a;230:466–470. doi: 10.1097/00000658-199910000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengart T.K. Lee L.Y. Patel S.R., et al. Angiogenesis gene therapy: Phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999b;100:468–474. doi: 10.1161/01.cir.100.5.468. [DOI] [PubMed] [Google Scholar]
- Rosengart T.K. Patel S.R. Crystal R.G. Therapeutic angiogenesis: Protein and gene therapy delivery strategies. J. Cardiovasc. Risk. 1999c;6:29–40. doi: 10.1177/204748739900600106. [DOI] [PubMed] [Google Scholar]
- Sarkar N. Ruck A. Kallner G., et al. Effects of intramyocardial injection of phVEGF-A165 as sole therapy in patients with refractory coronary artery disease—12-month follow-up: Angiogenic gene therapy. J. Intern. Med. 2001;250:373–381. doi: 10.1046/j.1365-2796.2001.00905.x. [DOI] [PubMed] [Google Scholar]
- Schumacher B. Pecher P. von Specht B.U., et al. Induction of neoangiogenesis in ischemic myocardium by human growth factors: First clinical results of a new treatment of coronary heart disease. Circulation. 1998;97:645–650. doi: 10.1161/01.cir.97.7.645. [DOI] [PubMed] [Google Scholar]
- Simons M. Bonow R.O. Chronos N.A., et al. Clinical trials in coronary angiogenesis: Issues, problems, consensus: An expert panel summary. Circulation. 2000;102:E73–E86. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- Stewart D.J. Hilton J.D. Arnold J.M., et al. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: A phase 2 randomized, controlled trial of AdVEGF121 (AdVEGF121) versus maximum medical treatment. Gene Ther. 2006;13:1503–1511. doi: 10.1038/sj.gt.3302802. [DOI] [PubMed] [Google Scholar]
- Symes J.F. Losordo D.W. Vale P.R., et al. Gene therapy with vascular endothelial growth factor for inoperable coronary artery disease. Ann. Thorac. Surg. 1999;68:830–836. doi: 10.1016/s0003-4975(99)00807-3. [DOI] [PubMed] [Google Scholar]
- Vale P.R. Losordo D.W. Milliken C.E., et al. Left ventricular electromechanical mapping to assess efficacy of phVEGF165 gene transfer for therapeutic angiogenesis in chronic myocardial ischemia. Circulation. 2000;102:965–974. doi: 10.1161/01.cir.102.9.965. [DOI] [PubMed] [Google Scholar]
- Yang Z.J. Zhang Y.R. Chen B., et al. Phase I clinical trial on intracoronary administration of Ad-hHGF treating severe coronary artery disease. Mol. Biol. Rep. 2009;36:1323–1329. doi: 10.1007/s11033-008-9315-3. [DOI] [PubMed] [Google Scholar]