Abstract
The relationship between exogenous contraceptive hormones and permissiveness of the female genital tract to human immunodeficiency virus type 1 (HIV-1) is the subject of renewed debate. To better characterize the effect of depot medroxyprogesterone acetate (DMPA) on HIV-1 cellular targets and epithelial integrity in the vagina, we compared leukocyte populations, markers of activation and proliferation, and the density of intercellular junctional proteins in the vaginal epithelium of women during the follicular and luteal phases of the menstrual cycle and approximately 12 weeks after receiving a DMPA injection. This prospective cohort study involved 15 healthy women. Vaginal biopsies were obtained in the follicular and luteal phases of the menstrual cycle, and approximately 12 weeks following a 150-mg intramuscular injection of DMPA. Leukocyte populations, activation phenotype, and epithelial tight junction and adherens proteins were evaluated by immunohistochemistry. After receiving DMPA, the numbers of CD45, CD3, CD8, CD68, HLA-DR, and CCR5 bearing immune cells were significantly (p<0.05) increased in vaginal tissues, compared to the follicular and/or luteal phases of untreated cycles. There were no significant differences in immune cell populations between the follicular and luteal phases of the control cycle. There were also no statistically significant differences in epithelial thickness and density of epithelial tight junction and adherens proteins among the follicular, luteal, and post-DMPA treatment sampling points. In this pilot study, vaginal immune cell populations were significantly altered by exogenous progesterone, resulting in increased numbers of T cells, macrophages, and HLA-DR- and CCR5-positive cells.
Introduction
Depot medroxyprogesterone acetate (DMPA, Depo-Provera) is one of the most widely used contraceptives in the world1 and the only injectable contraceptive currently available in the United States.2 It is a highly effective, long-acting, reversible contraceptive with very few absolute contraindications for use.3 However, concern has been raised as to the effect of DMPA on the female genital tract, specifically regarding a potential facilitating effect on mucosal human immunodeficiency virus type 1 (HIV-1) infection.4–7 This issue has been rekindled by a recent study showing increased acquisition of HIV-1 in women from serodiscordant couples using DMPA.8 In response to this growing debate, the World Health Organization convened an international technical consultation to determine if changes to the medical eligibility criteria (MEC) for contraceptive use should be made. A technical statement upholding the MEC category 1 for DMPA was released, but, due to the inconsistent nature of existing data, clarified that women using progestogen-only injectable contraception should be strongly advised to also use condoms and other measures to prevent HIV-1 acquisition.9
Therefore, elucidating the interaction between exogenous hormones and mucosal susceptibility to HIV-1 is critical, as the main route of HIV-1 infection in women is via heterosexual intercourse.10 Several large epidemiologic studies in Africa and Thailand have found that women using DMPA were significantly more likely to acquire HIV-1 as compared to women not using hormonal contraception, even after controlling for certain confounding variables such as frequency of intercourse, age, and number of sexual partners.8,11–14 As is the case with most observational studies, however, not all methodological considerations, e.g., consistent condom use or other important, unknown confounders, could be properly controlled.15
Theories as to how exogenous progesterone might increase genital tissue susceptibility to HIV-1 infection center on epithelial thinning and alteration of local mucosal immunity. A decrease in the number of epithelial cell layers or the density of intercellular junction proteins potentially enhances exposure of cervicovaginal mucosal target cells to HIV-1. In nonhuman primate models of HIV infection, progesterone and DMPA administration cause a dramatic atrophy of the vaginal epithelium, and DMPA (at 30 mg) is routinely used to enhance the efficiency of vaginal infection by simian immunodeficiency virus (SIV) or a chimeric variant of HIV (SHIV).16–21 However, data regarding the effect of exogenous progestins on epithelial thickness in the human vagina are mixed, ranging from no change to either increased or decreased thickness,5,22–24 in short-term (3–6 months)5,22 and long-term (2–3 year)23 DMPA users.
DMPA could also affect mucosal susceptibility to HIV-1 infection through alterations of the cervicovaginal mucosal immune response. Although the cervix and vagina are likely the initial site of entry of HIV-1 in women, the effects of exogenous estrogen and progesterone on the local immune environment of the lower genital tract have not been clearly elucidated,24–29 with most data focusing on biological mechanisms within the endometrium.30
The objective of this study was to compare immune cell populations and markers of epithelial integrity in women during the normal menstrual cycle and 3 months after receiving a single injection of DMPA, to determine if exogenous and endogenous progesterone alters the cellular immunoinflammatory status of the vaginal mucosa. To this end, we examined vaginal leukocytes bearing CD45, CD3, CD4, CD8, CD1a, and CD68 antigens, markers of activation and proliferation such as HLA-DR, CCR5, and Ki-67, and the epithelial junctional proteins zona occludin 1 (ZO-1) and E-cadherin. Because the dual need for contraception and protection from HIV-1 exists for so many women, especially in high-risk areas such as sub-Saharan Africa, the possible biological mechanisms underpinning a relationship between effective contraceptives such as DMPA and enhanced HIV-1 susceptibility need to be urgently explored.
Materials and Methods
Clinical study
This study was approved by the Institutional Review Board of Eastern Virginia Medical School. Details of the clinical study have been previously described.5 In brief, 20 women were screened for the study. After verifying the absence of exclusionary factors, such as sexually transmitted infections (STIs), women were examined in the luteal phase of the menstrual cycle (days 22–26), the follicular phase of the menstrual cycle (days 8–12), and finally 12 weeks (range 81–96 days) after receiving an injection of 150 mg of DMPA intramuscularly. Phase of the menstrual cycle was verified by menstrual calendar and serum estradiol and progesterone measurements. At each visit, two vaginal biopsies, colposcopy, swabs of cervicovaginal secretions, and serum samples for estradiol, progesterone, and MPA were obtained. All women enrolled in this study were otherwise not at risk of pregnancy due to female tubal sterilization or had a male sexual partner with a vasectomy and were not using any exogenous hormones during the study.5
Tissue analysis
Paraffin-embedded tissue blocks of the vaginal biopsies were cut into 5-μm sections. Immunohistochemistry (IHC) staining of tissue sections was performed using the ABC method [avidin:biotinylated enzyme complex from Vector labs (Burlingame, CA)]. Briefly, the slides were deparaffinized, dehydrated, and rehydrated followed by antigen retrieval in citrate buffer (pH 6.2, DAKO) at high temperature. Nonspecific binding was blocked using specific protein block for 30 min at room temperature. After washing with phosphate-buffered saline (PBS), the slides were incubated overnight with primary antibody at 4°C. Slides were then washed with PBS and subjected to biotinylated secondary antibody followed by ABC reagent. The antigen was localized by incubation with AEC chromogen–substrate (skyTek Labs, Mississauga, Ontario, Canada) and finally mounted with Accergyl mounting media (Accurate Chemicals, NY) with a cover slip. Positive stained cells were either counted under the microscope (Nikon E-800) or analyzed using Image J software (NIH, Bethesda, MD), as described below.
The main endpoint of this secondary analysis was the number of cell types associated with mucosal immunity and inflammatory responses and the density of epithelial junctional proteins (ZO-1 and E-cadherin) in the vaginal tissue biopsies. Cell phenotype was identified using specific unconjugated monoclonal antibodies against CD1a, CD3, CD4, CD8, CD45, CD68, CCL5, HLA-DR, and Ki67 markers and cell density was expressed per square millimeter of tissue (cells/mm2). The following mouse monoclonal antibodies were used: CD45 (X16/99 clone, Leica Microsystems), CD3 (LN10 clone, Leica Microsystems), and HLA-DR (TAL 1B5 clone, Santa Cruz). The following monoclonal mouse antihuman antibodies were used: CD4 (4B12 clone, DAKO), CD8 (1A5 clone, Leica Microsystems), CD68 (KP1 clone, ZYMED), CD1a (O10 clone, Gene Tex), and Ki-67 (MIB-1 clone, DAKO). The following rabbit monoclonal antibodies were used: E-cadherin (EP700Y clone, Abcam) and ZO-1 (Polyclonal, Invitrogen). Finally, for CCR5 a monoclonal antihuman antibody, 45523 clone, from R&D Systems was used.
All cell types were counted in the epithelial barrier, except CD4 and CCR5, which were counted in the lamina propria. For epithelial E-Cadherin and ZO-1, the immunolabeling of the protein was analyzed semiquantitatively, using Image J software (NIH, Bethesda MD). In brief, five to six areas were randomly selected using a Nikon E800 microscope from each section and these images were captured using a CCD camera (Spot Camera, Diagnostic Instruments, MI). The integrated optical density (IOD) in each area was calculated for the positive staining color. The IOD of the negative control (no primary antibody) was subtracted from the IOD values for each tissue and the mean value was calculated for the areas of each tissue sample.
Epithelial thickness was previously reported,5 but the samples were reanalyzed in this study in association with intercellular protein expression. Briefly, epithelial thickness was measured using cross-line reticules (Klarmann Rulings Inc., Litchfield, NH), fitted to the objective of a Nikon Eclipse 600 microscope. The epithelium was measured from the basal lamina to the apical surface in micrometers in 10 random areas per tissue sample and the mean was reported. The mean number of cell layers in the same 10 selected areas of the tissue sample was also reported.
Statistical analysis
Descriptive statistics were obtained by calculating the median, interquartile range, means, and standard deviations for each variable. Since each woman served as her own control, paired tests were used. Paired comparisons were done across the three phases: (1) luteal phase versus follicular phase, (2) luteal phase versus post-DMPA administration, and (3) follicular phase versus post-DMPA administration. For the immune cell and epithelial proteins, the data were not normally distributed and therefore statistical analyses were performed using Wilcoxon signed rank tests. For epithelial thickness and number of epithelial cell layers, the data were normally distributed and therefore a paired t test was used. The test results were interpreted with p-values of 0.05 as statistically significant. Distribution of the data was evaluated by examination of normal quantile plots and with the Shapiro Wilk test. Statistical analyses were performed by use of the SAS statistical analysis software package (SAS for Windows, Version 9.3; SAS Institute, Cary, NC).
Results
Of the 20 women enrolled in the study, results were originally reported on 16 women who completed all study visits.5 Of these 16 women, 15 had archived paraffin tissue sections suitable for analysis in this study. Women were on average 35.9 (±3.1) years-old, weighed 158.5 (±25.0) pounds, and had an average menstrual cycle length of 29.0 (±1.4) days. All 15 women had previously been pregnant with an average gravidity of 2.7 (±1.1).
We found no significant differences between numbers of vaginal immune cells, activation markers, epithelial junction proteins, number of cell layers, or epithelial thickness in tissues from the luteal and follicular phases of the control menstrual cycle (all p>0.05).
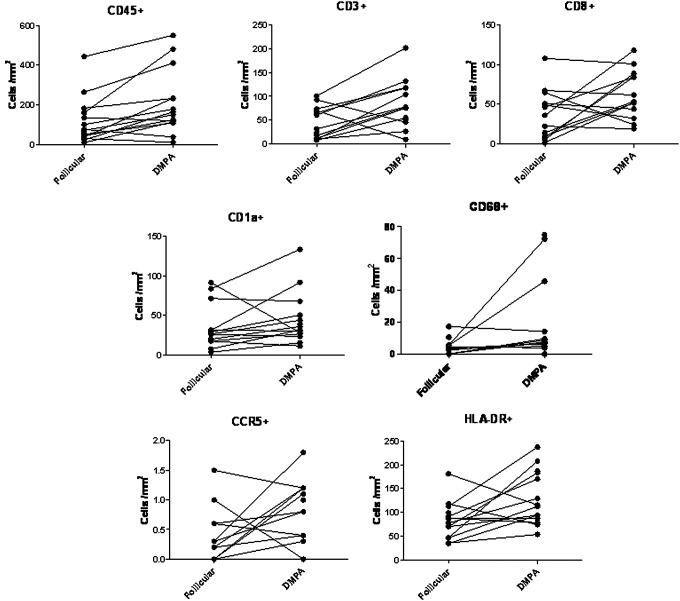
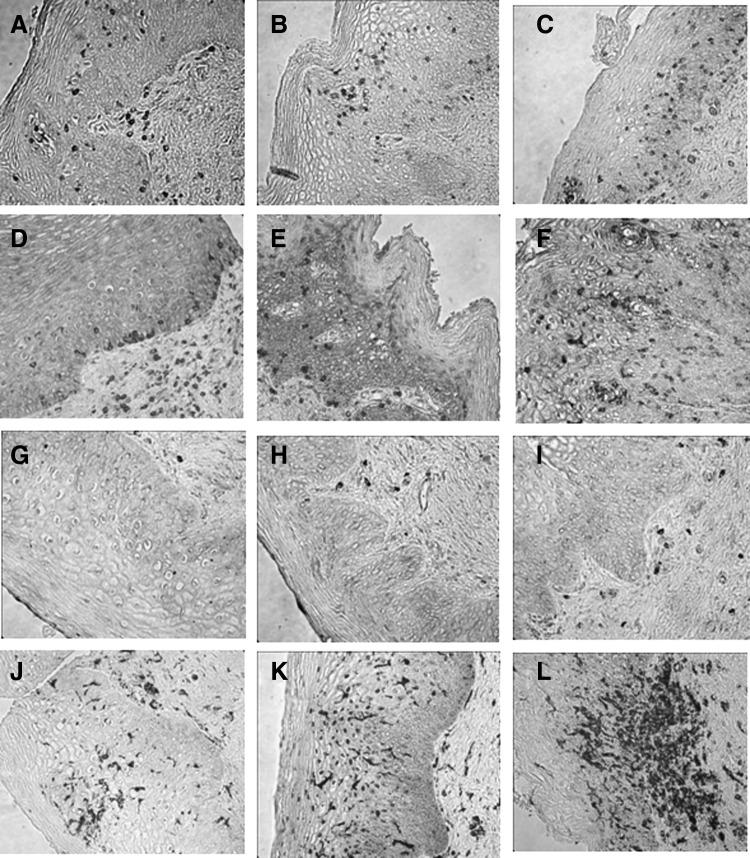
Table 1 shows the differences in the median numbers of immune cell populations between the follicular and luteal phases and after treatment with DMPA. There were significant increases in the median numbers of CD45, CD3, CD8, CD68, CCR5+, and HLA-DR+ immune cells after DMPA treatment compared to the follicular and/or luteal phases of the control cycle. Immune cells displayed a random distribution in the epithelial tissue, with no observed clustering or differential localization across any of the phases of the menstrual cycle or after DMPA treatment. Figure 1 (dot plots) shows increased number of immune cells in tissues obtained after DMPA exposure when compared to the follicular phase. It also demonstrates interindividual variability in the response to DMPA, with some subjects showing a greater change in immune cells than others. Compared with luteal phase samples, vaginal tissues exposed to DMPA showed an increased density of CD3+, CD8+, and CCR5+ immune cells. They also showed an increase in HLA-DR+ cells, which is statistically significant when compared with tissues taken during the follicular phase. Figure 2 shows a composite picture of these cells in the vaginal mucosa during follicular, luteal, and DMPA phases.
Table 1.
Vaginal Leukocytes and Activation Markers in the Follicular and Luteal Phases of a Control (No Treatment) Cycle Versus 12 Weeks Post-Depot Medroxyprogesterone Acetate Treatment
| |
Median cell count (cells/mm2) (25th, 75th percentile, quantile)a |
Wilcoxon signed rank p value |
|||
|---|---|---|---|---|---|
| Phenotype of immune cells in the vaginal epithelium | Follicular phase | Luteal phase | DMPA treatment | Follicular vs. DMPA | Luteal vs. DMPA |
| CD45 | 70.6 (37.5, 159.6) | 73.5 (31.7, 120.3) | 156.9 (112.4, 277.3) | 0.03 | 0.22 |
| CD3 | 40.0 (23.8, 60.0) | 45.9 (11.3, 70.7) | 76.5 (47.8, 117.9) | 0.08 | 0.04 |
| CD4b | 0 (0, 0) | 0 (max 11.6) | 0 (max 9.3) | NA | NA |
| CD8 | 28.1 (18.4, 44.9) | 29.1 (9.3, 54.4) | 52.6 (40.9, 85.6) | 0.15 | 0.01 |
| CD1a | 26.4 (16.7, 60.9) | 27.6 (17.8, 41.7) | 33.9 (26.0, 54.9) | 0.13 | >0.99 |
| CD68 | 1.5 (0, 5.1) | 3.3 (0, 5.4) | 7.2 (3.9, 22.0) | 0.01 | 0.07 |
| CCR5b | 0.2 (0, 0.5) | 0.2 (0, 0.6) | 0.8 (0, 1.2) | 0.09 | 0.05 |
| HLA-DR | 97.4(81.0, 120.0) | 82.4 (46.5, 102.6) | 113.9 (85.1, 184.3) | 0.02 | 0.35 |
Data are not normally distributed and are thus expressed as median (25th percentile, 75th percentile).
CD4 and CCR5-positive cells counted in lamina propria.
Bold numbers indicate statistically significant differences.
DMPA, depot medroxyprogesterone acetate.
FIG. 1.
Individual changes in vaginal immune cell populations comparing follicular phase and after depot medroxyprogesterone acetate (DMPA) treatment. Total cell density (cells/mm2) for each subject was plotted for the selected immune cell markers. In spite of substantial variability in individual responses, the majority of the samples showed increased numbers of immune cells and activation markers. In some cases, the increase was several fold in magnitude. All cells were quantified in the epithelium, except for CCR5+ and CD4+ cells, which were detected only in the lamina propria.
FIG. 2.
Vaginal immune cells in follicular, luteal, and post-DMPA treatment. Immunohistochemistry staining of paraffin-embedded tissue blocks of vaginal biopsies taken during the follicular phase (A, D, G, J), luteal phase (B, E, H, K), and 3 months after DMPA injection (C, F, I, L). Vaginal tissues exposed to DMPA showed significantly increased density of CD3+ (A, B, C), CD8+ (D, E, F), and CCR5+ (G, H, I) cells compared to the luteal phase, and greater expression of HLA-DR+ (J, K, L) cells compared to the follicular phase. Cell phenotypes were identified using specific monoclonal antibodies against each marker, and cell density was expressed per square millimeter of tissue (cells/mm2).
Notably, we identified very few CD4+ cells in only three vaginal tissue samples (from a total of 43 evaluable samples for CD4). These cells were exclusively confined to the lamina propria in each tissue sample. CD4+ cell counts were 11.6 cells/mm2 (for one subject in the follicular phase) and 9.3 and 6.3 cells/mm2 (for two subjects after DMPA treatment). Of note, in the three tissue samples where CD4+ cells were seen, there was histological evidence of inflammation, with increased numbers of leukocytes.
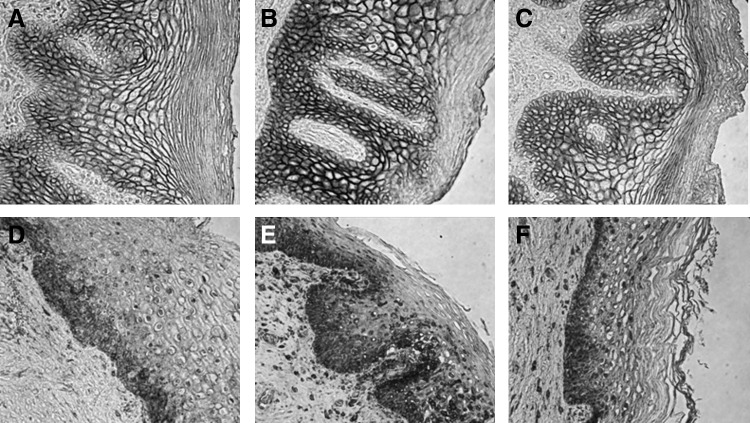
In terms of measures of epithelial integrity, there was a trend, although not statistically significant, toward decreased epithelial thickness and number of cell layers in the luteal phase and after DMPA administration when compared to the follicular phase (Table 2). In fact, there was an increase in Ki-67+ epithelial cells, a marker of cell proliferation, when comparing DMPA treatment to either the follicular or luteal phase baselines (Table 2). There were no differences in the density of epithelial tight junction and adherens proteins (E-cadherin and ZO-1) among any of the groups (Table 2 and Fig. 3).
Table 2.
Effect of Depot Medroxyprogesterone Acetate on Markers of Epithelial Integrity
| |
Mean epithelial thickness (in micrometers) or mean number of epithelial cell layers (standard deviation) or median cell count (cells/mm2) (25th, 75th percentile, quantile) |
Paired t test p value |
|||
|---|---|---|---|---|---|
| Epithelial characteristics | Follicular phase | Luteal phase | DMPA treatment | Follicular vs. DMPA | Luteal vs. DMPA |
| Epithelial thickness | 307.8±96.8 | 269.6±80.1 | 272.3±94.6 | 0.33 | 0.94 |
| Number of cell layers | 31.2±8.1 | 28.2±7.2 | 28.9±6.5 | 0.42 | 0.79 |
| Number of Ki-67+ epithelial cells | 35.7 (19.3, 64.3) | 17.1 (9.0, 68.4) | 72.6 (43.2, 126.9) | 0.004 | 0.005 |
| |
Median integrated optical density (IOD) per stained field (25th, 75th percentile, quantile)a |
Wilcoxon signed rank p value |
|||
|---|---|---|---|---|---|
| Epithelial adhesion proteins | Follicular phase | Luteal phase | DMPA treatment | Follicular vs. DMPA | Luteal vs. DMPA |
| E-cadherin | 3.5×106 (4.3×105, 4.7×106) | 1.6×106 (6.9×105, 8.1×106) | 1.6×106 (9.5×105, 3.2×106) | 0.64 | 0.42 |
| ZO-1 | 1.1×105 (2.9×104, 9.3×105) | 1.5×105 (3.5×104, 6.5×105) | 1.5×105 (3.7×104, 3.3×105) | 0.38 | 0.22 |
Data are not normally distributed and are thus expressed as median (25th percentile, 75th percentile).
Bold numbers indicate statistically significant differences.
FIG. 3.
DMPA treatment does not alter epithelial junction protein density. Immunolabeling for the epithelial tight junction protein E-cadherin (A–C) and adherens junction protein ZO-1 (D–F) in vaginal tissue biopsies obtained during the follicular (A, D) and luteal phases (B, E), and following administration of DMPA (C, F). No statistically significant difference in density was observed. Protein expression was calculated using the integrated optical density (IOD) in each area based on positive staining color.
Discussion
Exposure to a single dose of DMPA induced changes in the immune cell populations of the human vaginal mucosa, as compared with the follicular and luteal phases of the normal menstrual cycle. These changes consisted of increased numbers of leukocytes (CD45+ cells) and immune cells bearing CD3, CD8, CD68, CCR5, and HLA-DR. Since CD4+ cells were so scarce in the vaginal tissue biopsies, we could not detect statistically significant differences in this cell subset among any of the sampling times. Contrary to the alterations observed in mucosal immune cell populations, we did not see a statistically significant change in epithelial thickness, number of cell layers, or intercellular junctional proteins.
Changes in the mucosal immune system may contribute to the increased risk of acquiring HIV-1 infection reported in certain epidemiological studies of women using DMPA and other progestin-only injectable contraceptives.8,11–14 An expansion in CCR5 expressing cells is one of the key changes in the vaginal mucosa that could potentially enhance susceptibility to HIV-1 infection. We found a significant increase in cells bearing this marker in vaginal tissues after DMPA treatment. In addition to being a receptor for the chemokine CCL5 (RANTES), CCR5 is the main coreceptor for mucosal HIV-1 infection in humans, and is thought to be the predominant target for viral entry in the mucosal tissue of the lower female reproductive tract.31–33 Human cervical mucosal CD4+ T cells express CCR5, often in conjunction with other secondary receptors for HIV-1 entry.34,35 In addition, within the lower reproductive tract of both humans and macaques, CCR5 is expressed on mucosal dendritic cells and macrophages, also primary HIV cell targets. While most groups have found that CCR5 is not expressed on intraepithelial CD1a+ Langerhans cells, there is some evidence that the receptor may be present in these cells also.36–38 Our findings are in accordance with results from an in vitro model showing that progesterone treatment of peripheral blood mononuclear cells (PBMCs) caused a 5- to 10-fold up-regulation of CCR5 in CD14+ monocytes/macrophages.39 Furthermore, women in various progesterone-dominant states have been found to have increased expression of cervical and vaginal lymphocytes expressing CCR5.39–41 Interestingly, they have also been shown to have increased susceptibility to acquire HIV-1.42–45
CCR5 is known to be expressed by activated lymphocytes.46 Another marker of lymphocyte activation is the histocompatibility antigen HLA-DR. HLA-DR+ T cells are present in the early phases of HIV-1 infection47–49 and are thought to account for the majority of the cell population responsible for dissemination of HIV-1 from the mucosal portal to draining lymph nodes and distant sites.50 Animal models show that HLA-DR+-activated T cells and macrophages are productively infected during the early stage of SIV/HIV infection and constitute one of the main targets for the virus.51,52
In our study, DMPA increased CD3+ T cells and HLA-DR+ cells. Our findings are consistent with a large longitudinal study that found that white blood cells (WBCs) and polymorphonuclear monocytes (PMNs) were increased in the cervicovaginal fluid lavage (CVL) of women using hormonal contraception.53 CD3+ cells are widely reported to be the predominant lymphocyte population of the vagina.54–57 Although not as numerous in the cervix and vagina as in the upper reproductive tract, vaginal CD3+ T cell populations are not known to be affected by hormonal fluctuations of the menstrual cycle.54,56 The two main subsets of CD3+ T cells are CD4+ and CD8+ cells56,57; however, CD8+ T cells can outnumber CD4+ T cells in the vaginal epithelium by as much as 8:1.58,59 CD4+ T cells are a key target for cervicovaginal mucosal HIV-1 infection.32 Other CD4-bearing cells in the lower female genital tract are dendritic cells (DCs) and macrophages.37 In vivo and ex vivo data indicate that intraepithelial and submucosal DCs and CD4+ T lymphocytes and macrophages are the first cells targeted by HIV-1.32,50,60–63 We detected few vaginal tissue biopsies containing CD4+ cells, and the observed cells were confined exclusively to the lamina propria. Of note, in the three tissue samples in which CD4+ cells were detected, subclinical inflammation was noted. This is in agreement with previous reports describing limited numbers and distribution of CD4+ cells in the vaginal epithelium, especially in the absence of infections or other inflammatory conditions.28,34,36,59,64 In this study, the presence of STIs or other symptomatic inflammatory vaginal infections such as bacterial vaginosis or trichomoniasis was exclusionary. We have found similar low numbers and confined localization of CD4+ cells to the lamina propria in the mucosa of fresh, noninflamed vaginal tissue obtained from patients undergoing anterior and posterior surgical repairs (data not shown). Furthermore, parallel positive controls using lymph node tissue displayed robust labeling of CD4+ cells, indicating our findings were not due to technical issues in detection (data not shown).
The presence of CD4+ cells in a small percentage of biopsies does not rule out their importance in cervicovaginal HIV-1 acquisition, given the low incidence of HIV transmission, the ability of HIV to penetrate intact epithelium, and the increase in CD4+ cell numbers at mucosal sites of inflammation.65,66 Furthermore, the average increased susceptibility to HIV-1 reported in observational studies of DMPA users has an approximate mean adjusted HR of 1.50 (1.07–2.09).6,14,67,68 Therefore, an increase in the number of HIV cell targets, even if present only in a small percentage of the users, may justify the relatively small increased risk for acquiring the infection seen in the population of DMPA users. In our study, although not consistently across all markers, certain subjects showed marked increases of T cells, macrophages, or activated immune cells (HLA-DR+ and CCR5+) (Fig. 2).
We also found significant increases in CD8+ cells in vaginal tissues after DMPA treatment, in agreement with a previous study of long-term DMPA users.24 CD8+ T cells can exert cytolytic or suppressive functions. Cytolytic activity has been demonstrated in the cervix and vagina throughout the menstrual cycle.69 However, vaginal CD8+ T cells largely lack TIA1, a marker indicative of cytolytic function in lymphocytes.26 In addition to being more prevalent than CD4+, CD8+ T cells in the cervicovaginal tissue have a higher expression of activation markers and an increased effector memory phenotype.70 A sequestered population of CD8+ tissue-resident memory T (TRM) cells appears to be common at skin and mucosal surfaces, including that of the vaginal, and can be recalled by antigen-specific and antigen-nonspecific stimuli.71,72 Although the presence of CD8+ T cells in the vaginal epithelium appears to be protective against various pathogens,73 CD8+ T cells coexpressing the transcription factor Foxp3 can also exert suppressive functions through a regulatory T cell phenotype (Treg).74 Progesterone and its derivative medroxyprogesterone have been associated with immunosuppression of the reproductive tract, inhibiting antibody production, cell-mediated immune responses, and innate immunity.7 Innate antimicrobial levels decrease during the luteal phase of the menstrual cycle, and exogenous progesterone inhibits human beta defensin-2 production in vaginal epithelial cells.75,76 MPA also decreases TLR9-induced interferon (IFN)-α production by human and mouse dendritic cells, thereby attenuating potential antiviral responses.77 It is possible that DMPA suppresses mucosal antiviral mechanisms via CD8+ T cells. Treatment with DMPA stimulated the circulating CD8+ Treg response in macaques.78 Since we only measured the density of CD8+ cells in this study, and could not perform functional assays or determine cofactor expression, we are unable to clarify the immune function of these cells in the vaginal epithelium.
DMPA has been associated with thinning of the vaginal epithelium.22 We observed a trend toward decreased epithelial thickness in the luteal phase and DMPA biopsies as compared to those from the follicular phase. This trend, however, was not statistically significant as analyzed by ANOVA with paired t tests for comparison of each of the three sampling intervals. The trend in the data suggests that, on average, women lose cell layers and thus epithelial thickness, when comparing the follicular phase with the luteal phase or DMPA use. However, individual variability is present. For example, 7 of 15 women increased the mean number of vaginal epithelial cell layers, when comparing the follicular phase to after DMPA treatment or from the follicular to the luteal phase. In the previous analysis of these tissues, epithelial thickness and number of cell layers were measured by two different methods and it was noted that there was up to a 34% interobserver variability in measurements.5 The issue of changes in epithelial thickness after DMPA administration has been investigated by several groups, and it is likely that the inconsistent results reported reflect differences in the method of measurement, statistical analyses, observer bias, timing of tissue sampling, pharmacokinetics, and interindividual variability in the biological response to DMPA. In addition, DMPA administration in humans does not appear to lead to the consistent and marked epithelial atrophy observed in monkey models, although a better dose–response study should be carried out in monkeys to determine a dose of DMPA that leads to pharmacokinetic equivalence with humans.
To investigate the issue of epithelial integrity further, we measured the density of the intercellular adhesion proteins, E-cadherin and ZO-1, which have both been localized in the human vagina and cervix,79 hypothesizing that a decrease in these proteins could lead to a more permeable epithelium. It has been previously shown that vaginal estrogen alters the concentration of vaginal epithelial tight junctions,80 but no data exist on the effect of DMPA on epithelial tight junction proteins. We found that a single administration of DMPA did not significantly alter the density of E-cadherin or ZO-1 in the vaginal epithelium. It is known that intercellular adhesion proteins are less concentrated in the apical portions of the vaginal epithelium, as these epithelial cells lose cell-to-cell adhesions and are eventually sloughed into the lumen.79 We also found that epithelial cell proliferation, as determined by the Ki-67 marker, was significantly increased after DMPA administration, as compared to the luteal and follicular phases. However, the increased proliferation was not reflected in any morphological changes in the number of cell layers or epithelial cell thickness. Women in this study were biopsied at 12 weeks post-DMPA administration, which is the standard length of DMPA treatment. Our findings of no statistical differences in epithelial thickness and number of cell layers, with a statistically significant increase in the Ki-67 marker, could also suggest that at the 12-week time point the vaginal tissue may be in the initial process of reverting back to its normal cyclic state, thereby increasing proliferation, thickness, and cellular junction integrity. It would be ideal to examine epithelial integrity at shorter intervals post-DMPA administration or after prolonged DMPA use. Proliferation of vaginal epithelium after long-term DMPA use has been previously reported by one group.81 Taken as a whole, our data do not suggest that DMPA causes significant epithelial thinning or compromises epithelial integrity at the 12 week sampling interval. It is unknown if a relatively small reduction in the thickness of the epithelial barrier may facilitate access of virions to mucosal-activated HIV-1 target cells
The data presented in this report represent a pilot study conducted on archived tissue. Since HIV-1 infection was not an endpoint, our data cannot provide a conclusive explanation as to how DMPA use may increase a woman's susceptibility to HIV-1 acquisition. These data, however, do demonstrate a significant effect of exogenous MPA on the number of immune cell populations within the vagina. Importantly, they show changes in immune cell populations known to be involved in the early phases of HIV-1 infection, following exposure to a single injection of DMPA. Lacking functional endpoints and dual marker labeling, we cannot determine if these changes are associated with a mucosal proinflammatory or immunosuppressive state. However, either through increased numbers of activated target cells or reduced antiviral responses, DMPA may be associated with increased cervicovaginal mucosal susceptibility to HIV-1 infection.
These findings are even more critical in the context of recent HIV-1 prevention trials. The majority of these trials require enrolled participants to be on effective contraception; for example, in the CAPRISA 004 trial, 80% of participants used injectable progestin-only contraception, primarily DMPA.82 Of note, it is not clear if our findings are applicable to other exogenous progestins, widely used as contraceptives. Because millions of women need dual protection from unintended pregnancy and HIV-1, it is essential to characterize the impact of the exogenous hormones on mucosal susceptibility to HIV-1 and the effect of exogenous hormones on microbicide efficacy. A prospective study of approximately 70 women who select combined oral contraceptives or DMPA is planned to further elucidate the effect of exogenous hormones on mucosal susceptibility to HIV-1.
Acknowledgments
This work was supported by intramural CONRAD funds from the U.S. Agency for International Development (Grant GPO-A-00-08-00005-00). The views expressed by the authors do not necessarily reflect those of the funding agency or CONRAD.
Neelima Chandra, PhD, performed the immunohistochemistry (IHC) analyses of all samples. Andrea Ries Thurman, MD, analyzed the IHC data and wrote the manuscript. Sharon Anderson, PhD, analyzed the IHC data and edited the manuscript. Tina Duong Cunningham, PhD, performed the primary statistical analyses of the data and edited the manuscript. Nazita Yousefieh, PhD, performed quality control analyses of the IHC experiments. Christine Mauck, MD, MPH, designed the clinical study and edited the manuscript. Gustavo F. Doncel, MD, PhD, designed the clinical study, analyzed the IHC data, and edited the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.UNAIDS. World AIDS Day Report. 2011.
- 2.Kaunitz AM. Long-acting injectable contraception with depot medroxyprogesterone acetate. Am J Obstet Gynecol. 1994;170(5 Pt 2):1543–1549. [PubMed] [Google Scholar]
- 3.WHO. Medical Eligibility Criteria for Contraceptive Use 2008 Update. 2008.
- 4.Daly CC. Helling-Giese GE. Mati JK. Hunter DJ. Contraceptive methods and the transmission of HIV: Implications for family planning. Genitourin Med. 1994;70(2):110–117. doi: 10.1136/sti.70.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauck CK. Callahan MM. Baker J, et al. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999;60(1):15–24. doi: 10.1016/s0010-7824(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 6.Baeten JM. Lavreys L. Overbaugh J. The influence of hormonal contraceptive use on HIV-1 transmission and disease progression. Clin Infect Dis. 2007;45(3):360–369. doi: 10.1086/519432. [DOI] [PubMed] [Google Scholar]
- 7.Hel Z. Stringer E. Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev. 2010;31(1):79–97. doi: 10.1210/er.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heffron R. Donnell D. Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: A prospective cohort study. Lancet Infect Dis. 2012;12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Hormonal Contraception and HIV: Technical Statement. 2012. [PubMed]
- 10.UNAIDS: 09 AIDS epidemic update. http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf. 2009. http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdfhttp://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf
- 11.Ungchusak K. Rehle T. Thammapornpilap P, et al. Determinants of HIV infection among female commercial sex workers in northeastern Thailand: Results from a longitudinal study. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12(5):500–507. doi: 10.1097/00042560-199608150-00010. [DOI] [PubMed] [Google Scholar]
- 12.Martin HL., Jr Nyange PM. Richardson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178(4):1053–1059. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]
- 13.Baeten JM. Benki S. Chohan V, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS. 2007;21(13):1771–1777. doi: 10.1097/QAD.0b013e328270388a. [DOI] [PubMed] [Google Scholar]
- 14.Morrison CS. Chen PL. Kwok C, et al. Hormonal contraception and HIV acquisition: Reanalysis using marginal structural modeling. AIDS. 2010;24(11):1778–1781. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shelton JD. HIV/AIDS. ARVs as HIV prevention: A tough road to wide impact. Science. 2011;334(6063):1645–1646. doi: 10.1126/science.1212353. [DOI] [PubMed] [Google Scholar]
- 16.Marx PA. Spira AI. Gettie A, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2(10):1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 17.Smith SM. Baskin GB. Marx PA. Estrogen protects against vaginal transmission of simian immunodeficiency virus. J Infect Dis. 2000;182(3):708–715. doi: 10.1086/315776. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y. Tian B. Agy MB. Saifuddin M. Tsai CC. Macaca fascicularis are highly susceptible to an RT-SHIV following intravaginal inoculation: A new model for microbicide evaluation. J Med Primatol. 2009;38(Suppl 1):39–46. doi: 10.1111/j.1600-0684.2009.00374.x. [DOI] [PubMed] [Google Scholar]
- 19.Pal R. Nuttall J. Galmin L. Weiss D. Chung HK. Romano J. Characterization of vaginal transmission of a simian human immunodeficiency virus (SHIV) encoding the reverse transcriptase gene from HIV-1 in Chinese rhesus macaques. Virology. 2009;386(1):102–108. doi: 10.1016/j.virol.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Salle B. Brochard P. Bourry O, et al. Infection of macaques after vaginal exposure to cell associated simian immunodeficiency virus. J Infect Dis. 2010;202:337–344. doi: 10.1086/653619. [DOI] [PubMed] [Google Scholar]
- 21.Veazey RS. Shattock RJ. Pope M, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9(3):343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 22.Miller L. Patton DL. Meier A. Thwin SS. Hooton TM. Eschenbach DA. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol. 2000;96(3):431–439. doi: 10.1016/s0029-7844(00)00906-6. [DOI] [PubMed] [Google Scholar]
- 23.Bahamondes L. Trevisan M. Andrade L, et al. The effect upon the human vaginal histology of the long-term use of the injectable contraceptive Depo-Provera. Contraception. 2000;62(1):23–27. doi: 10.1016/s0010-7824(00)00132-3. [DOI] [PubMed] [Google Scholar]
- 24.Ildgruben AK. Sjoberg IM. Hammarstrom ML. Influence of hormonal contraceptives on the immune cells and thickness of human vaginal epithelium. Obstet Gynecol. 2003;102(3):571–582. doi: 10.1016/s0029-7844(03)00618-5. [DOI] [PubMed] [Google Scholar]
- 25.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 26.Pudney J. Quayle AJ. Anderson DJ. Immunological microenvironments in the human vagina and cervix: Mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73(6):1253–1263. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 27.Hladik F. Sakchalathorn P. Ballweber L, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26(2):257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patton DL. Thwin SS. Meier A, et al. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am J Obstet Gynecol. 2000;183(4):967–973. doi: 10.1067/mob.2000.108857. [DOI] [PubMed] [Google Scholar]
- 29.Wieser F. Hosmann J. Tschugguel W, et al. Progesterone increases the number of Langerhans cells in human vaginal epithelium. Fertil Steril. 2001;75(6):1234–1235. doi: 10.1016/s0015-0282(01)01796-4. [DOI] [PubMed] [Google Scholar]
- 30.Wira CR. Fahey JV. Ghosh M, et al. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol. 2011;63(6):544–565. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choe H. Farzan M. Sun Y, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85(7):1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 32.Hladik F. Sakchalathorn P. Ballweber L, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26(2):257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng H. Liu R. Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381(6584):661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 34.Prakash M. Kapembwa MS. Gotch F. Patterson S. Higher levels of activation markers and chemokine receptors on T lymphocytes in the cervix than peripheral blood of normal healthy women. J Reprod Immunol. 2001;52(1–2):101–111. doi: 10.1016/s0165-0378(01)00114-0. [DOI] [PubMed] [Google Scholar]
- 35.McKinnon LR. Nyanga B. Chege D, et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011;187(11):6032–6042. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 36.Poonia B. Wang X. Veazey RS. Distribution of simian immunodeficiency virus target cells in vaginal tissues of normal rhesus macaques: Implications for virus transmission. J Reprod Immunol. 2006;72(1–2):74–84. doi: 10.1016/j.jri.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Hirbod T. Kaldensjo T. Broliden K. In situ distribution of HIV-binding CCR5 and C-type lectin receptors in the human endocervical mucosa. PLoS One. 2011;6(9):e25551. doi: 10.1371/journal.pone.0025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen R. Richter HE. Smith PD. Early HIV-1 target cells in human vaginal and ectocervical mucosa. Am J Reprod Immunol. 2011;65(3):261–267. doi: 10.1111/j.1600-0897.2010.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patterson BK. Landay A. Andersson J, et al. Repertoire of chemokine receptor expression in the female genital tract: Implications for human immunodeficiency virus transmission. Am J Pathol. 1998;153(2):481–490. doi: 10.1016/S0002-9440(10)65591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prakash M. Kapembwa MS. Gotch F. Patterson S. Oral contraceptive use induces upregulation of the CCR5 chemokine receptor on CD4(+) T cells in the cervical epithelium of healthy women. J Reprod Immunol. 2002;54(1–2):117–131. doi: 10.1016/s0165-0378(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 41.Sheffield JS. Wendel GD., Jr McIntire DD. Norgard MV. The effect of progesterone levels and pregnancy on HIV-1 coreceptor expression. Reprod Sci. 2009;16(1):20–31. doi: 10.1177/1933719108325510. [DOI] [PubMed] [Google Scholar]
- 42.Leroy V. Van de Perre P. Lepage P, et al. Seroincidence of HIV-1 infection in African women of reproductive age: A prospective cohort study in Kigali, Rwanda, 1988–1992. AIDS. 1994;8(7):983–986. doi: 10.1097/00002030-199407000-00017. [DOI] [PubMed] [Google Scholar]
- 43.Gray RH. Li X. Kigozi G, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: A prospective study. Lancet. 2005;366(9492):1182–1188. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 44.Aaby P. Ariyoshi K. Buckner M, et al. Age of wife as a major determinant of male-to-female transmission of HIV-2 infection: A community study from rural West Africa. AIDS. 1996;10(13):1585–1590. doi: 10.1097/00002030-199611000-00019. [DOI] [PubMed] [Google Scholar]
- 45.Mugo NR. Heffron R. Donnell D, et al. Increased risk of HIV-1 transmission in pregnancy: A prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25(15):1887–1895. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaspan HB. Liebenberg L. Hanekom W, et al. Immune activation in the female genital tract during HIV infection predicts mucosal CD4 depletion and HIV shedding. J Infect Dis. 2011;204(10):1550–1556. doi: 10.1093/infdis/jir591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z. Schuler T. Zupancic M, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286(5443):1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed SM. Al-Doujaily H. Johnson MA, et al. Immunity in the female lower genital tract and the impact of HIV infection. Scand J Immunol. 2001;54(1–2):225–238. doi: 10.1046/j.1365-3083.2001.00927.x. [DOI] [PubMed] [Google Scholar]
- 49.Miller CJ. Shattock RJ. Target cells in vaginal HIV transmission. Microbes Infect. 2003;5(1):59–67. doi: 10.1016/s1286-4579(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 50.Hu J. Gardner MB. Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74(13):6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veazey RS. Tham IC. Mansfield KG, et al. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: Highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74(1):57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore AC. Bixler SL. Lewis MG. Verthelyi D. Mattapallil JJ. Mucosal and peripheral Lin- HLA-DR+ CD11c/123- CD13+ CD14- mononuclear cells are preferentially infected during acute simian immunodeficiency virus infection. J Virol. 2012;86(2):1069–1078. doi: 10.1128/JVI.06372-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghanem KG. Shah N. Klein RS, et al. Influence of sex hormones, HIV status, and concomitant sexually transmitted infection on cervicovaginal inflammation. J Infect Dis. 2005;191(3):358–366. doi: 10.1086/427190. [DOI] [PubMed] [Google Scholar]
- 54.Givan AL. White HD. Stern JE, et al. Flow cytometric analysis of leukocytes in the human female reproductive tract: Comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38(5):350–359. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 55.White HD. Yeaman GR. Givan AL. Wira CR. Mucosal immunity in the human female reproductive tract: Cytotoxic T lymphocyte function in the cervix and vagina of premenopausal and postmenopausal women. Am J Reprod Immunol. 1997;37(1):30–38. doi: 10.1111/j.1600-0897.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 56.Yeaman GR. White HD. Howell A. Prabhala R. Wira CR. The mucosal immune system in the human female reproductive tract: potential insights into the heterosexual transmission of HIV. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S57–62. [PubMed] [Google Scholar]
- 57.Saba E. Grivel JC. Vanpouille C, et al. HIV-1 sexual transmission: Early events of HIV-1 infection of human cervico-vaginal tissue in an optimized ex vivo model. Mucosal Immunol. 2010;3:280–290. doi: 10.1038/mi.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edwards JN. Morris HB. Langerhans' cells and lymphocyte subsets in the female genital tract. Br J Obstet Gynaecol. 1985;92(9):974–982. doi: 10.1111/j.1471-0528.1985.tb03080.x. [DOI] [PubMed] [Google Scholar]
- 59.Ma Z. Lu FX. Torten M. Miller CJ. The number and distribution of immune cells in the cervicovaginal mucosa remain constant throughout the menstrual cycle of rhesus macaques. Clin Immunol. 2001;100(2):240–249. doi: 10.1006/clim.2001.5058. [DOI] [PubMed] [Google Scholar]
- 60.Spira AI. Marx PA. Patterson BK, et al. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183(1):215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang ZQ. Schuler T. Zupancic M, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286(5443):1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 62.Collins KB. Patterson BK. Naus GJ. Landers DV. Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med. 2000;6(4):475–479. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- 63.Gupta P. Collins KB. Ratner D, et al. Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol. 2002;76(19):9868–9876. doi: 10.1128/JVI.76.19.9868-9876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pudney J. Quayle AJ. Anderson DJ. Immunological microenvironments in the human vagina and cervix: Mediators of cellular immunity are concentrated in the cervical transformation zone 1. Biol Reprod. 2005;73(6):1253–1263. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 65.Wawer MJ. Gray RH. Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 66.Hladik F. Hope TJ. HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep 2009. 2009;6(1):20–28. doi: 10.1007/s11904-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 67.Morrison CS. Richardson BA. Mmiro F, et al. Hormonal contraception and the risk of HIV acquisition. AIDS. 2007;21(1):85–95. doi: 10.1097/QAD.0b013e3280117c8b. [DOI] [PubMed] [Google Scholar]
- 68.Heffron R. Donnell D. Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: A prospective cohort study. Lancet Infect Dis. 2012;12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.White HD. Crassi KM. Givan AL, et al. CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J Immunol. 1997;158(6):3017–3027. [PubMed] [Google Scholar]
- 70.Saba E. Grivel JC. Vanpouille C, et al. HIV-1 sexual transmission: Early events of HIV-1 infection of human cervico-vaginal tissue in an optimized ex vivo model. Mucosal Immunol. 2010;3(3):280–290. doi: 10.1038/mi.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gebhardt T. Whitney PG. Zaid A, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477(7363):216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 72.Mackay LK. Stock AT. Ma JZ, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci USA. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Musey L. Hu Y. Eckert L. Christensen M. Karchmer T. McElrath MJ. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J Exp Med 20. 1997;185(2):293–303. doi: 10.1084/jem.185.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang J. Kelly KA. Phenotype and function of regulatory T cells in the genital tract. Curr Trends Immunol. 2011;12:89–94. [PMC free article] [PubMed] [Google Scholar]
- 75.Keller MJ. Guzman E. Hazrati E, et al. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS. 2007;21(4):467–476. doi: 10.1097/QAD.0b013e328013d9b5. [DOI] [PubMed] [Google Scholar]
- 76.Han JH. Kim MS. Lee MY, et al. Modulation of human beta-defensin-2 expression by 17beta-estradiol and progesterone in vaginal epithelial cells. Cytokine. 2010;49(2):209–214. doi: 10.1016/j.cyto.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 77.Hughes GC. Thomas S. Li C. Kaja MK. Clark EA. Cutting edge: Progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. J Immunol. 2008;180(4):2029–2033. doi: 10.4049/jimmunol.180.4.2029. [DOI] [PubMed] [Google Scholar]
- 78.Genesca M. McChesney MB. Miller CJ. Depo-provera treatment does not abrogate protection from intravenous SIV challenge in female macaques immunized with an attenuated AIDS virus. PLoS One. 2010;5(3):e9814. doi: 10.1371/journal.pone.0009814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blaskewicz CD. Pudney J. Anderson DJ. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol Reprod. 2011;85(1):97–104. doi: 10.1095/biolreprod.110.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gorodeski GI. Estrogen decrease in tight junctional resistance involves matrix-metalloproteinase-7-mediated remodeling of occludin. Endocrinology. 2007;148(1):218–231. doi: 10.1210/en.2006-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ildgruben AK. Sjoberg IM. Hammarstrom ML. Influence of hormonal contraceptives on the immune cells and thickness of human vaginal epithelium. Obstet Gynecol. 2003;102(3):571–582. doi: 10.1016/s0029-7844(03)00618-5. [DOI] [PubMed] [Google Scholar]
- 82.Karim Q. Karim SS. Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]