Abstract
A rapid increase in the number of HIV cases in the men who have sex with men (MSM) population has been observed in China; however, little information is available on the genetic characterization of HIV prevalent in this population. In this study, 95 HIV-1-seropositive drug-naive patients from the Beijing MSM population were enrolled. The genetic characterization and transmission of drug resistance of HIV-1 were examined based on full-length gag, pol, and partial env gene sequences. Three subtypes, including CRF01_AE (56.0%), B (30.8%), and CRF07_BC (12.6%), were identified. Close phylogenetic relationships were found among these strains with isolates from other populations in Beijing and MSM isolates from Hebei province, which suggested that the Beijing MSM population might act as a bridge for HIV transmission between MSM and other high-risk populations. Drug-resistant mutations were identified in 5.3% of sampled individuals. Our results provided detailed genetic data and would be helpful for understanding the transmitting pattern of HIV strains between MSM and other populations.
Since its initial identification in men who have sex with men (MSM) 30 years ago, HIV has continued to spread in this group in virtually all regions other than sub-Saharan Africa, especially in industrialized countries. A study carried out in industrialized countries revealed that HIV infections among MSM have increased by ∼3% per year from 2000 through 2005 even though the prevalence of HIV infections has generally decreased in other populations.1 Furthermore, in the United States, more than half (53%) of the new cases in 2006 were among MSM.2 In China, the percentages of newly reported HIV cases attributed to MSM have also increased dramatically in the past decade. In 2001, only 0.2% of new Chinese HIV cases were estimated to be infected through homosexual contacts, but the proportion increased to 12.2% in 2007 and 32.5% in 2009.3–6 In Shenyang City, the capital of Liaoning Province in northeast China, MSM transmission accounted for 39.6% of new HIV infections in 2009.7 Undoubtedly, MSM was also among the population most vulnerable to HIV infection in China,8 especially in certain metropolitan areas of China, where the estimated infectious rates reach 3.0–4.6%.6, 9,10
Beijing, the capital of China, is a metropolitan area with a large MSM population. In the past few years, the ratios of MSM carrying HIV in Beijing have increased quickly, from 3.1% in 200211 to 3.23% in 200512 and 4.80% in 2006,13 which is much higher than the corresponding rates for MSM populations in other cities in China (1.3–1.6%).14–16 Due to the severity of HIV in MSM in Beijing, several studies have been published in the past few years on the prevalence of HIV in this population; however, most of them concentrated on social behavior and HIV seroprevalence.8,11 Very limited information is available on the genetic characteristics of HIV-1 strains within the MSM group in Beijing. In this study, we performed the most comprehensive genetic characterization of HIV-1 strains prevalent in the MSM group in Beijing to date, based on analysis of the sequences of the HIV-1 full-length gag, pol, and partial env genes.
A total of 95 HIV-positive treatment-naive MSM were randomly recruited into this study at the local HIV/AIDS sentinel surveillance sites in Beijing Youan Hospital, Capital Medical University, with informed consent from 2007 to 2010. The subjects all resided in Beijing, and 74 of 95 subjects were natives of Beijing. The average age of the HIV-infected MSM was 33.2 years old (ranging from 23 to 57 years). All subjects except subject 2832 were of Han ethnicity. Peripheral blood was collected within 3 months of being confirmed as HIV positive. The CD4 T cell counts varied widely, ranging from 109 to 1,220 cells/μl. Of 95 patients 14 presented at least one typical symptom, including fever, oral thrush, nausea, debility, diarrhea, and depression.
Viral full length gag, pol, and partial env genes (C2V3 region) were amplified separately using reverse transcriptional nested PCR with RNA extracted from plasma as templates and sequenced as described previously.17 BLAST search (http://hiv-web.lanl.gov/content/index) was used to exclude any potential contaminations. All of the sequenced fragments were edited and assembled into contiguous sequences.18 Eighty full length gag genes (89.5%), 76 full length pol genes (79.2%), and 78 env C2V3 genes (82.1%) were successfully obtained. The inconsistency in obtaining different genes was probably due to the sequence variations at the primer binding sites or genetic diversity of HIV quasispecies in different gene regions. Four samples failed to amplify any portion of the three genes probably due to low viral load, transport conditions, or sequence variations at the primer binding sites. Analysis of sequences showed that all of the gene structures were normal with the correct open reading frames (ORFs).
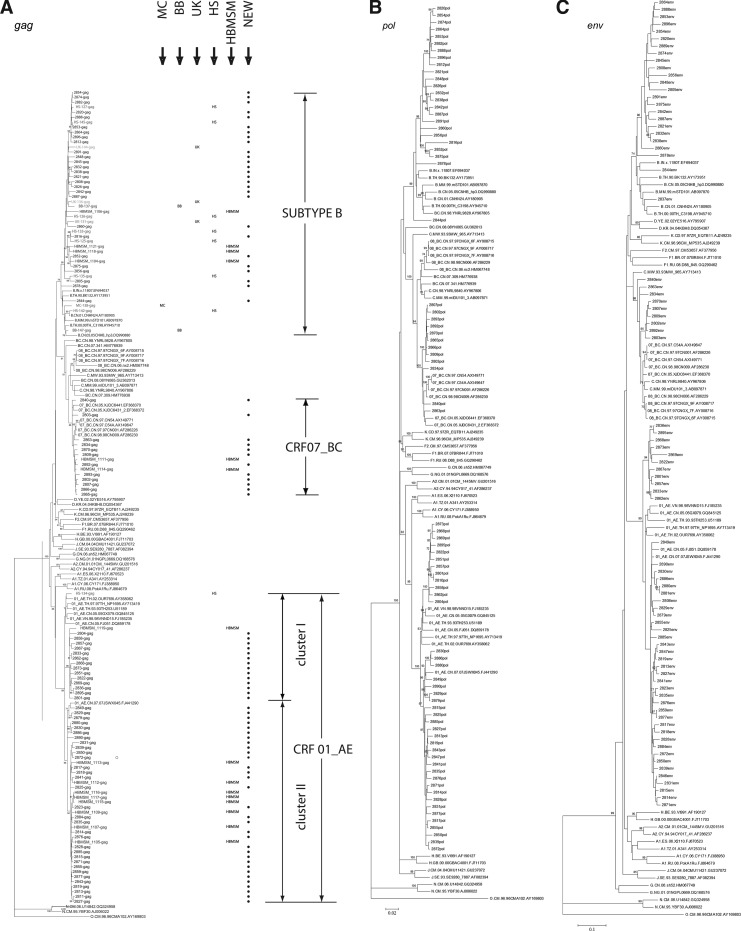
All assembled sequences were submitted to the NCBI viral genotyping tool (www.ncbi.nih.gov/projects/genotyping/formpage.cgi) to determine HIV genotypes, which were further confirmed by phylogenetic analysis using the reference sequences representing subtypes A–D, F–H, J, K, CRF01_AE, CRF07_BC, and CRF08_BC (www.hiv.lanl.gov) (Fig. 1). The results of three gene regions were combined together to finally determine the strain's subtype. Of the 91 positive samples 51 were CRF01_AE (56.0%), 28 strains were subtype B (30.8%), and 12 strains were CRF07_BC (12.6%). In previous studies in MSM in China, subtype B was found to be dominant.19,20 Different from those reports, CRF01_AE strains were found to surpass subtype B strains in Beijing MSM. This finding is also supported in another study that reported that subtype CRF01_AE had displaced subtype B as the dominant subtype in samples collected between 2008 and 2009 in Beijing MSM.21 In China, CRF01_AE was primarily associated with transmission through heterosexual activities, and the rapid increase of CRF01_AE strains in MSM suggests that there might be an overlap between these two populations.
FIG. 1.
Phylogenetic tree analysis. Maximum likelihood trees created with the full length gag gene (A), pol gene (B), and partial env gene (C) of HIV-1 sequences from men who have sex with men (MSM) in Beijing and a selection of reference sequences of subtype A–D, F–H, J, K, CRF01_AE, CRF07_BC, CRF08_BC, and group O (http://hiv-web.lanl.gov). The lengths of the gag, pol, and partial env sequences obtained in the study were 1503, 3056, and 574 base pairs, respectively, by using HXB2 as the reference genomic. Each reference sequence is labeled with the HIV-1 subtype, followed by country, sampling year, name, and accession number. Bootstrap probabilities greater than 70% are indicated at the corresponding nodes of the tree. The scale bar represents 5% genetic distance (0.05 substitutions per site). The gag tree also contains reference sequences from different Beijing HIV-positive populations. From left to right: MC, mother to child; BB, blood borne; UK, unknown; HS, heterosexual transmission; HBMSM, MSM samples from Shijizhuang city in Hubei; NEW, new MSM sequences collected in this study.
To investigate the relationship of strains identified in this study with those from other reports, further phylogenetic analysis was carried out. To do that, T_COFFEE software was used to align sequences and the BioEdit software package (version 7.0.0; T. Hall, North Carolina State University, Raleigh, NC) was used to check visually and edit the final alignments. Phylogenetic trees were generated using maximum likelihood methods in the MEGA4 software package. The reliability of topologies was estimated by performing bootstrap analysis with 1,000 replicates. In the phylogenetic tree, the samples were generally tightly clustered within their respective subtypes, with the exception of the CRF01_AE strains, which seemed to form two distinct clusters with strong bootstrap support. To estimate the diversity of the sequences in this study within their respective subtypes and clusters (marked in Fig. 1A) the genetic distances of different subtype and cluster sequences were analyzed using the Kimura two-parameter model based on the gag gene (Table 1). The largest mean genetic distance was found among subtype B (0.055±0.003), suggesting that subtype B may have been introduced into the population for a relatively longer period of time than other subtypes. Relatively smaller mean genetic distances were found in CRF01_AE (0.037±0.003) and CRF07_BC (0.025±0.002). One-way ANOVA analysis showed that genetic distances were significant different among subtypes by using SPSS17.0 software (p<0.01).
Table 1.
Genetic Distances Among Sequences Belonging to Different Subtypes or Clusters
| |
Genetic distances(mean±SD) |
|||||
|---|---|---|---|---|---|---|
| Gene | CRF01_AE | Subtype B | CRF07_BC | CRF01_AE cluster I | CRF01_AE cluster II | Subtype B cluster |
| gag | 0.037±0.003 | 0.055±0.003 | 0.025±0.002 | 0.029±0.002 | 0.025±0.002 | 0.049±0.003 |
| pol | 0.032±0.002 | 0.045±0.002 | 0.018±0.001 | 0.019±0.001 | 0.025±0.001 | 0.041±0.002 |
| env (C2V3) | 0.099±0.008 | 0.129±0.011 | 0.095±0.008 | 0.064±0.006 | 0.092±0.007 | — |
In a previous study carried out in HIV-positive MSM in Shijiazhuang City, the capital of Hebei province (approximately 250 km to the southwest), a close phylogenetic relationship was identified among HIV strains prevalent in MSM from a different area; however, no close relationship was observed among HIV strains from MSM and other local high-risk populations. In this study, we also checked whether a similar phenomenon could be observed. To check whether there is similarity between HIV strains identified in MSM from Beijing and other Chinese cities, 15 HIV gag sequences identified in the Shjiazhuang MSM population were added to the alignment.17 To check the relationship among HIV strains prevalent in Beijing MSM and those in other populations, 14 HIV gag sequences (accession numbers JQ900929–JQ900942) identified in Beijing individuals in 2005 who acquired HIV through other routes, including heterosexual transmission (HS), mother-to-child transmission (MC), unknown routes (UK), and blood-borne transmission (BB), were added to the alignment.
In the phylogenetic tree, subtype B strains from Beijing MSM, Beijing other populations, and MSM residing in Shijiazhuang grouped into a cluster supported by a high bootstrap value (95%) (Fig. 1), indicating that these different populations share a close epidemiological relationship. In previous reports, subtype B strains isolated in MSM in China were always most similar to subtype B strains prevalent in Europe and America but less similar to those in Thailand, which were popular in former paid blood donors. The same phenomenon was also observed in our study; however, one sequence was identified as being more similar to the strains in former paid blood donors, suggesting the possibility of HIV transmission between MSM and former paid blood donors in Beijing. For the CRF01_AE cluster, the MSM samples from Beijing and Shijiazhuang were intermixed, with all but one of the Shijiazhuang strains placed in the second cluster. Since only one CRF01_AE strain isolated from other local high-risk populations in Beijing was included in the alignment, it was difficult to infer the relationship basing on only CRF01_AE strains.
Anyway, the grouping of subtype B strains from different populations suggested that MSM residing in Beijing might have played an important role in HIV transmission between MSM and other populations in China.
Highly active antiretroviral therapy (HAART) has been widely employed in China since the start of the “Four Free and One Care” policy in 2003. With the wide use of antiretroviral drugs, HIV-1 drug resistance became severe in some areas of China.22 Accordingly, the transmission of drug-resistant strains was also reported in some areas in China. According to an investigation in Liaoning province among ART-naive AIDS patients, 4.4% of HIV-1-infected people had drug-resistant strains although they had never undertaken a course of ART treatment.23 In this study, all of strains were obtained from drug-naive individuals, which made it possible to investigate the prevalence of drug resistance in the drug-naive HIV-infected population. The 76 full-length pol genes obtained in the study were submitted to the online Calibrated Population Resistance tool (v5.0 beta)24 (http://hivdb.stanford.edu) to identify the presence of transmitted drug resistance mutations using the WHO 2009 list of mutations for surveillance of TDR. None of the 76 strains under investigation showed any major mutation that is known to be associated with drug resistance to any class of reverse transcriptase inhibitors (RTI). Four CRF01_AE sequences (5.3%) contained a major mutation (M46I) to protease inhibitor (PI). The prevalence of drug-resistant variants in HIV-1-infected drug-naive Beijing MSM (5.3%) was not as high as reported previously (15%), which is encouraging because it indicates that patients with failing therapy are not a major source of new infections. However, the discrepancies among different studies highlight the need for an analysis based on a larger population as well as observing how the properties in this population change over an extended period as this would provide more insight into the prevalence and significance of HIV drug resistance in drug-naive MSM in Beijing.
The most recent estimate of the MSM population in China is ∼17.82 million.25 Due to social discrimination and cultural stigma associated with homosexual behavior in China, 31.5% of HIV-positive MSM are estimated to engage in sex with both men and women within and out of marriage.20 This behavior facilitates the transmission of HIV from high-risk populations to the general population, and may contribute to the growing number of HIV-positive women infected through unprotected sex. The distinct characteristics of HIV cases within the MSM population in China mean that it is important to understand how this can impact the spread of the virus. Our study of the genetic background of HIV in Beijing MSM will contribute toward a better understanding of the distribution and evolution of HIV-1 in MSM in China and provide clues to determining effective programs for intervening to change the existing behavior.
Sequence data: The gene sequences were deposited in GenBank with accession numbers JQ900844–JQ901097.
Acknowledgments
This work was supported by the National Key S&T Special Projects on Major Infectious Diseases (grant no.2008ZX10001-004, 2012ZX10001-002) and the National Natural Science Foundation of China (no. 81072348). We also want to show our thanks to Dr. Feng Gao in Duke Human Vaccine Institute, Duke University Medical Center for providing the technical support in reverse transcription and nest-PCR. Lin Li and Na Han contributed equally to this work.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sullivan PS. Hamouda O. Delpech V, et al. Reemergence of the HIV epidemic among men who have sex with men in North America, Western Europe, and Australia, 1996–2005. Ann Epidemiol. 2009;19(6):423–431. doi: 10.1016/j.annepidem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention: HIV and AIDS among gay and bisexual men. Fast Fact Sheet. Atlanta, GA: Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 3.China Ministry of Health, UNAIDS, WHO: 2005 Update on the HIV/AIDS epidemic and response in China. Beijing: National Center for AIDS/STD Prevention and Control; 2006. [Google Scholar]
- 4.Beijing CCMoH. China Ministry of Health, UNAIDS (WHO) WHO: 2009 estimates for the HIV/AIDS epidemic in China. www.unaids.org.cn/download/2009%20China%20Estimation%20Report-En.pdf. 2010. www.unaids.org.cn/download/2009%20China%20Estimation%20Report-En.pdf
- 5.Beijing CMoH, UNAIDS China Office. China Ministry of Health, UN Theme Group on HIV/AIDS in China: A joint assessment of HIV/AIDS prevention, treatment and care in China. http://data.unaids.org/UNA-docs/china_joint_assessment_2003_en.pdf. 2003. http://data.unaids.org/UNA-docs/china_joint_assessment_2003_en.pdf
- 6.China Ministry of Health, UN Theme Group on HIV/AIDS in China. Beijing: State Council AIDS Working Committee Office UTGoAiC; 2007. A joint assessment of HIV/AIDS prevention, treatment and care in China. [Google Scholar]
- 7.Zhang M. Chu Z. Wang H. Xu J. Lu C. Shang H. A rapidly increasing incidence of HIV and syphilis among men who have sex with men in a major city of China. AIDS Res Hum Retroviruses. 2011;27(11):1139–1140. doi: 10.1089/aid.2010.0356. [DOI] [PubMed] [Google Scholar]
- 8.Zhang BC. Chu QS. MSM and HIV/AIDS in China. Cell Res. 2005;15(11–12):858–864. doi: 10.1038/sj.cr.7290359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi KH. Lui H. Guo Y. Han L. Mandel JS. Lack of HIV testing and awareness of HIV infection among men who have sex with men, Beijing, China. AIDS Educ Prev. 2006;18(1):33–43. doi: 10.1521/aeap.2006.18.1.33. [DOI] [PubMed] [Google Scholar]
- 10.UNAIDS/WHO. AIDS epidemic update: December 2006. www.unaids.org/ 2007. www.unaids.org/
- 11.Choi KH. Liu H. Guo Y. Han L. Mandel JS. Rutherford GW. Emerging HIV-1 epidemic in China in men who have sex with men. Lancet. 2003;361(9375):2125–2126. doi: 10.1016/S0140-6736(03)13690-2. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XY. Li XX. Zhang XX, et al. Study of HIV infection co-infection with STDs and HCV and related changes in immunological indicators and viral loads among men who have sex with men in Beijing. Chin J AIDS STD. 2006;12:294–296. [Google Scholar]
- 13.Li SW. Zhang XY. Li XX, et al. Detection of recent HIV-1 infections among men who have sex with men in Beijing during 2005–2006. Chin Med J (Engl) 2008;121(12):1105–1108. [PubMed] [Google Scholar]
- 14.Cai WD FT. Tan JG. Chen L. Shi XD. Chen PL. Jiang LZ. Tao XY. A survey of the characteristics and STD/HIV infection of homosexuality in Shenzhen. Modern Prevent Med. 2005;32:328–330. [Google Scholar]
- 15.Choi KH. Ning Z. Gregorich SE. Pan QC. The influence of social and sexual networks in the spread of HIV and syphilis among men who have sex with men in Shanghai, China. J Acquir Immune Defic Syndr. 2007;45(1):77–84. doi: 10.1097/QAI.0b013e3180415dd7. [DOI] [PubMed] [Google Scholar]
- 16.Gu Y QP. Xu L. Luo M. Wang XL. Gu J. Zhao LL. Lu YH. Zhou BS. Survey of knowledge, attitude, behavior and practices related to STI/HIV among male homosexuality in Shenyang. Chin J Publ Health. 2004;20:573–574. [Google Scholar]
- 17.Li L. Lu X. Li H, et al. High genetic diversity of HIV-1 was found in men who have sex with men in Shijiazhuang, China. Infect Genet Evol. 2011;11(6):1487–1492. doi: 10.1016/j.meegid.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Li L. Liang S. Chen L, et al. Genetic characterization of 13 subtype CRF01_AE near full-length genomes in Guangxi, China. AIDS Res Hum Retroviruses. 2010;26(6):699–704. doi: 10.1089/aid.2010.0026. [DOI] [PubMed] [Google Scholar]
- 19.Yao J ZF. He ZP. Zhao HX. Li XW. Feng X. Xu KY. Subtype and sequence analysis of the C2-V3 region of env gene among HIV-1 infected homosexual men in Beijing. Chin J STD/AIDS Prev Cont. 2002;8:131–133. [Google Scholar]
- 20.Zhang D. Bi P. Lv F. Zhang J. Hiller JE. Changes in HIV prevalence and sexual behavior among men who have sex with men in a northern Chinese city: 2002–2006. J Infect. 2007;55(5):456–463. doi: 10.1016/j.jinf.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Wang W. Xu J. Jiang S, et al. The dynamic face of HIV-1 subtypes among men who have sex with men in Beijing, China. Curr HIV Res. 2011;9(2):136–139. doi: 10.2174/157016211795569096. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z. Liang Y. Xin T. Investigation on curative effect and drug resistance of HIV/AIDS patients receiving HAART. J Med Forum. 2005;26:1–3. [Google Scholar]
- 23.Han XX. Zhang M. Dai D, et al. Background study of HIV-1 drug resistant mutations in treatment-naive patients in Liaoning province. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006;28(5):632–636. [PubMed] [Google Scholar]
- 24.Bennett DE. Camacho RJ. Otelea D, et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One. 2009;4(3):e4724. doi: 10.1371/journal.pone.0004724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang C. Yang R. Xia X, et al. High prevalence of HIV-1 and hepatitis C virus coinfection among injection drug users in the southeastern region of Yunnan, China. J Acquir Immune Defic Syndr. 2002;29(2):191–196. doi: 10.1097/00042560-200202010-00014. [DOI] [PubMed] [Google Scholar]