Abstract
To explore immunologic risk factors for death within 90 days of highly active antiretroviral therapy (HAART) initiation, CD4+ and CD8+ T cell subsets were measured by flow cytometry and characterized by logistic regression in 149 Zambian children between 9 months and 10 years of age enrolled in a prospective, observational study of the impact of HAART on measles immunity. Of 21 children who died during follow-up, 17 (81%) had known dates of death and 16 (76%) died within 90 days of initiating HAART. Young age and low weight-for-age z-scores were associated with increased risks of mortality within 90 days of starting HAART, whereas CD4+ T cell percentage was not associated with mortality. After adjusting for these factors, each 10% increase in CD8+ effector T cells increased the odds of overall mortality [OR=1.43 (95% CI: 1.08, 1.90)] and was marginally associated with early mortality [OR=1.29 (95% CI: 0.97, 1.72)]. Conversely, each 10% increase in CD4+ central memory T cells decreased the odds of overall [OR=0.06 (95% CI: 0.01, 0.59)] and early mortality [OR=0.09 (95% CI: 0.01, 0.97)]. Logistic regression prediction models demonstrated areas under the receiver-operator characteristic curves of ≥85% for early and overall mortality, with bootstrapped sensitivities of 82–85% upon validation, supporting the predictive accuracy of the models. CD4+ and CD8+ T cell subsets may be more accurate predictors of early mortality than CD4+ T cell percentages and could be used to identify children who would benefit from more frequent clinical monitoring after initiating HAART.
Introduction
The scale-up of highly active antiretroviral therapy (HAART) in sub-Saharan Africa has resulted in substantial reductions in mortality due to human immunodeficiency virus (HIV).1 However, mortality rates of children during the first few months of HAART remain comparable to rates prior to HAART eligibility and approximately 10–20% of children die within the first few months of starting HAART.2–7 In Kenyan children, 90% of deaths occurred within 90 days of starting HAART, and 77% of deaths in children in the Democratic Republic of the Congo were within 60 days of starting HAART.6,8 Similarly, a recent meta-analysis estimated that 17% of HIV-infected adults initiating HAART in sub-Saharan Africa died within 12 months.9
Several studies assessed clinical and demographic risk factors for mortality in HIV-infected children beginning HAART and consistently identified low CD4+ T cell percentage or count, low weight-for-age z-score, young age, and increased severity of coinfections to be associated with mortality after treatment initiation.2,4,5,8,10 Only one assessed the relationship with plasma HIV viral load and found higher HIV viral load was associated with an increased risk of mortality.4 T cell activation markers, particularly HLA-DR and CD38, have been associated with HIV disease progression,11–13 but beyond CD4+ T cell levels, few reports link specific T cell subsets with the risk of mortality.14 In addition to depleting CD4+ T cells, HIV infection induces a chronic state of immune activation that impairs emigration of naive T cells from the thymus,15 increases T cell activation and turnover,16,17 and results in increased levels of effector cells as well as decreased proliferative and functional capacity.18,19 The objective of this study was to explore whether levels of CD4+ and CD8+ T cell subsets were associated with an increased risk of mortality in HIV-infected Zambian children beginning HAART.
Materials and Methods
Study design and population
We conducted a prospective, observational study from January 2009 to February 2012 of HIV-infected children 9 months to 10 years of age initiating HAART at two public clinics in Lusaka, Zambia to assess the impact of HAART on general immune reconstitution and measles virus-specific immunity. Enrollment occurred during a routine clinic visit 2 weeks after the initial evaluation for HAART eligibility at the referral clinic. Children initiating HAART with a history of prior measles vaccination were eligible for enrollment. Follow-up study visits occurred every 3 months in concert with routine clinical care. During the study visit, a questionnaire was administered and a 3–5 ml peripheral blood sample was collected. Clinical information obtained during the study visit included height, weight, date of birth, and recent and current illnesses. Recent illness was defined as presentation to a clinic or hospital with an illness within 4 weeks prior to the study visit. Current illness was defined as illness on the day of the study visit. Underweight, stunting, and wasting were determined using the World Health Organization standards of less than 2 standard deviations below weight-for-age (WAZ), height-for-age (HAZ), and weight-for-height (WHZ) z-scores from a standard reference population, respectively.20 The date of death was ascertained by phone contact or home visit after a child failed to return for follow-up. When the date of death was unknown but confirmed by a parent or guardian, death was assumed to have occurred midway between the previous study visit and the next scheduled 3-month follow-up visit.
Laboratory assays
Blood samples arrived in the laboratory within 6 h of collection and were aliquotted for hemoglobin and immunophenotyping. Hemoglobin was measured on a Sysmex Kx-21 automated hematology analyzer (Sysmex Corporation, Kobe, Japan) and reported in grams per deciliter (g/dl). Immunophenotyping was performed to differentiate T cell subsets. Briefly, 50 μl of heparinized whole blood was mixed with monoclonal antibodies against cell surface markers (described below), red blood cells were lysed with FACSLyse (BD Biosciences, Franklin Lakes, NJ), and cells were washed with phosphate-buffered saline (PBS) and resuspended in 1% paraformaldehyde.
To differentiate T cell subsets, cells were stained with monoclonal antibodies against CD3-peridinin chlorophyll-A protein (PerCP), CD4 or CD8 conjugated to allophycocyanin (APC), CD45RA conjugated to fluorescein isothiocyanate (FITC), and CCR7-phycoerythrin (PE). Total T cells were defined by the expression of the pan-T cell marker CD3. To distinguish functional T cell subsets, CD4+ and CD8+ T cells were defined as naive (CCR7+CD45RA+), effector memory (CCR7−CD45RA−), effector (CCR7−CD45RA+), and central memory (CCR7+CD45RA−).21,22 Activated phenotypes were detected using HLA-DR-FITC and CD38-PE,23 and interleukin 7 receptor α (IL-7Rα) was used to detect CD8+ T cells with the potential to develop into long-lived memory cells.24,25 All monoclonal antibodies were obtained from BD Biosciences. Flow cytometry data were acquired with CellQuest Pro software (BD Biosciences) on a BD FACSCalibur flow cytometer and immunophenotypes were analyzed using FlowJo v7.5 (Treestar, Ashland, OR). Lymphocytes were gated based on side and forward scatter, and gating of CD4+ or CD8+ T cells and respective subsets was determined with the use of isotype controls.
Data analysis
Clinical and laboratory data collected at enrollment, corresponding to the day of HAART initiation, were used to assess potential risk factors for mortality. Characteristics of children were compared using Mann–Whitney U-tests for continuous outcomes and χ2 tests for binary outcomes. Age, z-scores, hemoglobin, and cell populations were centered by subtracting the mean value. Age was scaled by 3-month intervals and cell populations were scaled by 10%. p-values less than 0.05 were considered statistically significant.
As dates of death were unavailable for some children, mortality was assessed as a binary outcome. Logistic regression was used to explore immunologic risk factors for overall mortality and mortality within 90 days of HAART initiation after adjusting for age, CD4+ T cell percentage, and WAZ as well as to predict the probability of mortality after HAART initiation. Given the potential collinearity between T cell subsets, relationships between subsets not achieving statistically significant univariable associations with mortality were assessed using correlations. Cellular subsets demonstrating statistically significant correlations with other subsets were selected for multivariable models. Model fit was assessed using Hosmer–Lemeshow goodness-of-fit tests, grouping observations into deciles.26
After identification of immunologic risk factors for mortality, concordance (C)-statistic/area under the receiver-operator characteristic curves (AUC) were used to assess the predictive accuracy of the logistic regression models for mortality within 90 days of HAART initiation and overall mortality. For each model, a cut-off for the predicted probability of mortality, also known as a criterion of positivity, was selected to categorize children into high and low mortality risk groups. The discriminatory abilities of these criteria were measured using sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The criteria of positivity maximizing sensitivity and the proportion of correctly classified individuals were selected to increase the probability of properly differentiating children with mortality outcomes. Discrimination measures were validated by bootstrapping with 100 replicates.26 An additional measure of accuracy, optimism, which estimates the degree of model overfitting, was calculated as the mean difference between the AUC from the model of the observed data and the AUCs from the models of the bootstrapped data.27 Statistical analyses were performed using Stata/IC version 10.1 (College Park, TX).
Results
Characteristics of the study population
A total of 169 HIV-infected children were enrolled at the time of HAART initiation in a study of the impact of HAART on immune reconstitution and measles immunity. Children were excluded from this analysis if the percents of CD4+ and CD8+ T cells were missing (n=6), date of birth was missing (n=1), baseline data were unavailable (n=3), or if children were enrolled after November 29, 2011, which was within 3 months of the study end date of February 28, 2012, as mortality outcomes from these children were not ascertained (n=11). Consequently, 149 HIV-infected children initiating HAART were eligible for analysis and contributed 127.3 person-years (py). Children were observed for a median of 274 days (range: 1–988). One hundred and twenty-eight children (86%) were alive at the conclusion of the study and 21 children (14%) died during follow-up. Of those who died, 16 (76%) died within 90 days of HAART initiation, four of whom had unknown dates of death but contact tracing revealed these children died before the first follow-up study visit 3 months after beginning HAART. Five children (24%) died more than 90 days after starting HAART. The overall mortality rate was 4.17 per 100 py (95% CI: 2.72, 6.40) and a mortality rate of 7.95 per 100 py (95% CI: 4.87, 13.0) was observed within 90 days of starting HAART, which was 4.8 times higher (95% CI: 1.7, 16.7) than that observed after 90 days of HAART [1.66 per 100 py (95% CI: 0.69, 3.99)].
The median age of children who died was younger than survivors at the time of HAART initiation (Table 1). The median WAZ score of children who died less than 90 days after initiating HAART was −3.50 (IQR: −4.34, −2.60), significantly lower than the WAZ score of −2.33 (IQR: −3.15, −1.31) for survivors (p<0.001) (Table 1). Moreover, 94% of children who died within 90 days of HAART initiation were underweight compared to 57% of survivors and 40% of children who died after 90 days of HAART (p=0.01 by χ2 test). The WHZ scores of children who died within 90 days of HAART initiation also were significantly lower than those of survivors. In contrast, the median HAZ scores were less than zero for all groups but did not differ significantly between groups (Table 1).
Table 1.
Characteristics of HIV-Infected Children Who Survived or Died Within 90 Days or During Follow-up After Starting Highly Active Antiretroviral Therapy
| Alive during follow-up N=128 | Death within 90 days N=16 | Death during follow-up N=21 | p-value* (alive vs. death <90 days, overall) | ||||
|---|---|---|---|---|---|---|---|
| Female | 61 (48%) | 8 (50%) | 10 (48%) | 0.86, 1.00 | |||
| Age, months | 29.1 (19.9, 54.3) | 20.4 (14.9, 29.4) | 19.1 (14.4, 27.4) | 0.02, <0.01 | |||
| Weight-for-age z-score | −2.33 (−3.15, −1.31) | −3.50 (−4.34, −2.60) | −3.28 (−4.00, −2.16) | <0.01, 0.02 | |||
| Underweight | 73 (57%) | 15 (94%) | 17 (81%) | <0.01, 0.04 | |||
| Height-for-age z-score | −2.94 (−4.16, −1.79) | N=124 | −3.34 (−4.66, −2.19) | N=13 | −3.31 (−4.66, −2.19) | N=18 | 0.55, 0.54 |
| Stunting | 86 (69%) | N=124 | 10 (77%) | N=13 | 14 (78%) | 0.57, 0.46 | |
| Weight-for-height z-score | −0.73 (−1.85, 0.33) | N=101 | −2.19 (−3.04, −0.73) | N=13 | −1.46 (−3.04, −0.39) | 0.03, 0.14 | |
| Wasting | 21 (21%) | N=101 | 7 (54%) | N=13 | 8 (44%) | <0.01, 0.03 | |
| Recent illness | 97 (76%) | 16 (100%) | 20 (95%) | 0.03, 0.04 | |||
| Current illness | 38 (30%) | 8 (50%) | 11 (52%) | 0.10, 0.04 | |||
| Hemoglobin, g/dl | 9.8 (8.8, 10.9) | N=109 | 9.3 (9.1, 10.2) | N=12 | 9.3 (9.0, 10.2) | 0.34, 0.37 |
p-values determined using Mann–Whitney U-tests and χ2 tests.
Dichotomous characteristics are expressed as N (%) and continuous characteristics as median (interquartile range).
Hemoglobin concentrations and the proportion of mothers receiving perinatal antiretroviral therapy did not differ between children who died and survivors. All children who died within 90 days of beginning HAART had a recent illness and half were ill on the day of HAART initiation; in contrast, 76% and 30% of survivors were recently and currently ill (p=0.08 and p=0.11, respectively). Nineteen (90%) of the 21 children who died had more than one symptom of illness. Signs and symptoms consisted of cough (n=18), diarrhea (n=18), fever (n=16), sore throat (n=8), vomiting (n=8), ear discharge (n=5), and rash (n=5). Children were receiving cotrimoxazole prophylaxis.
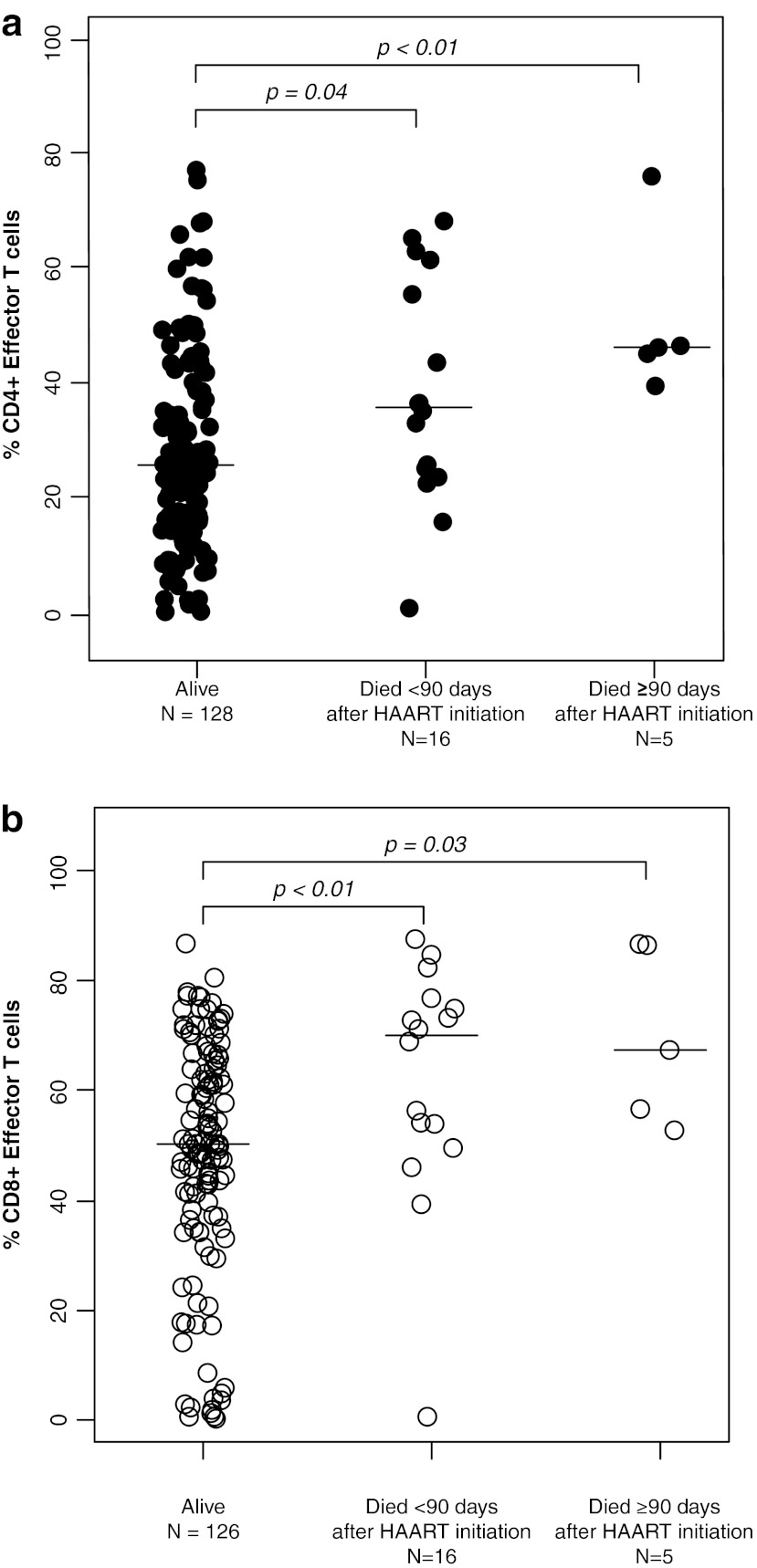
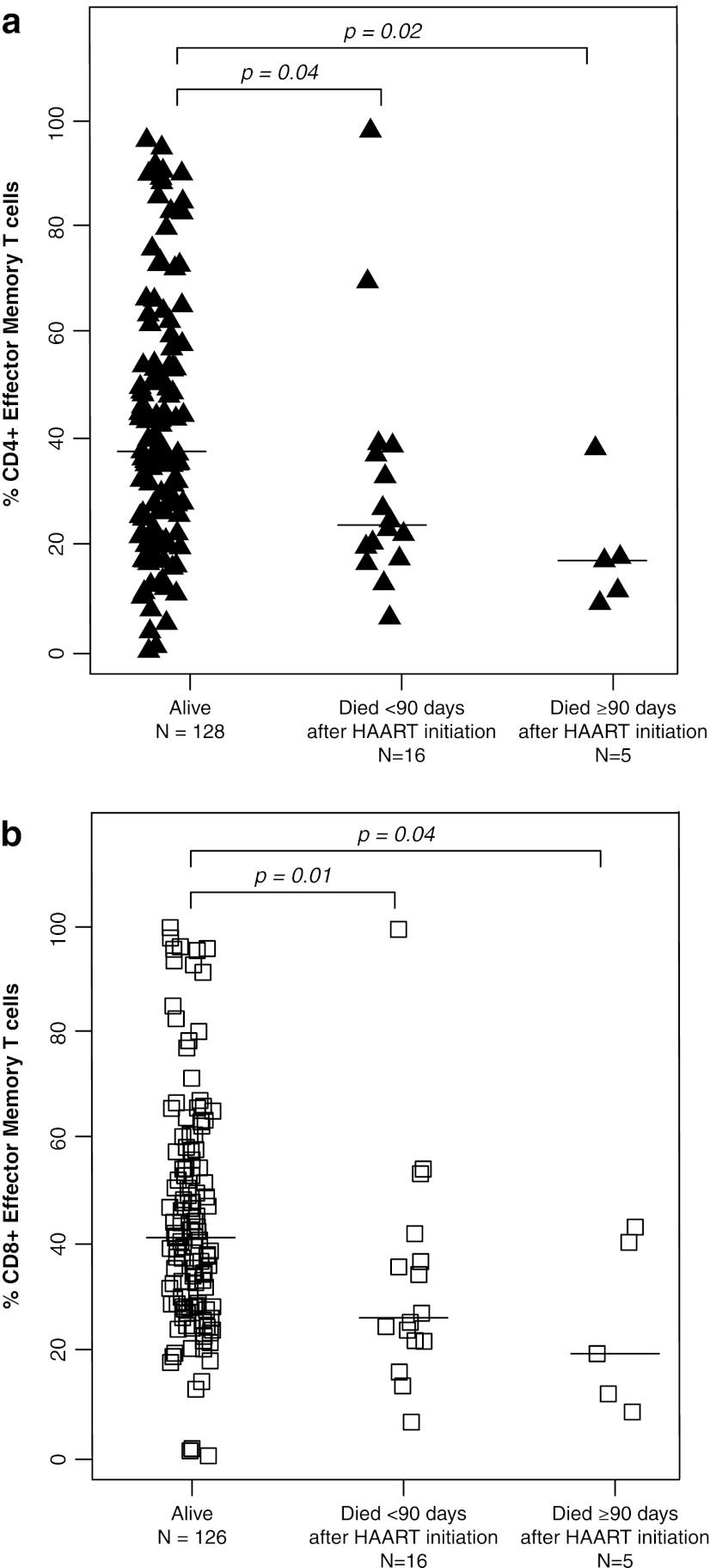
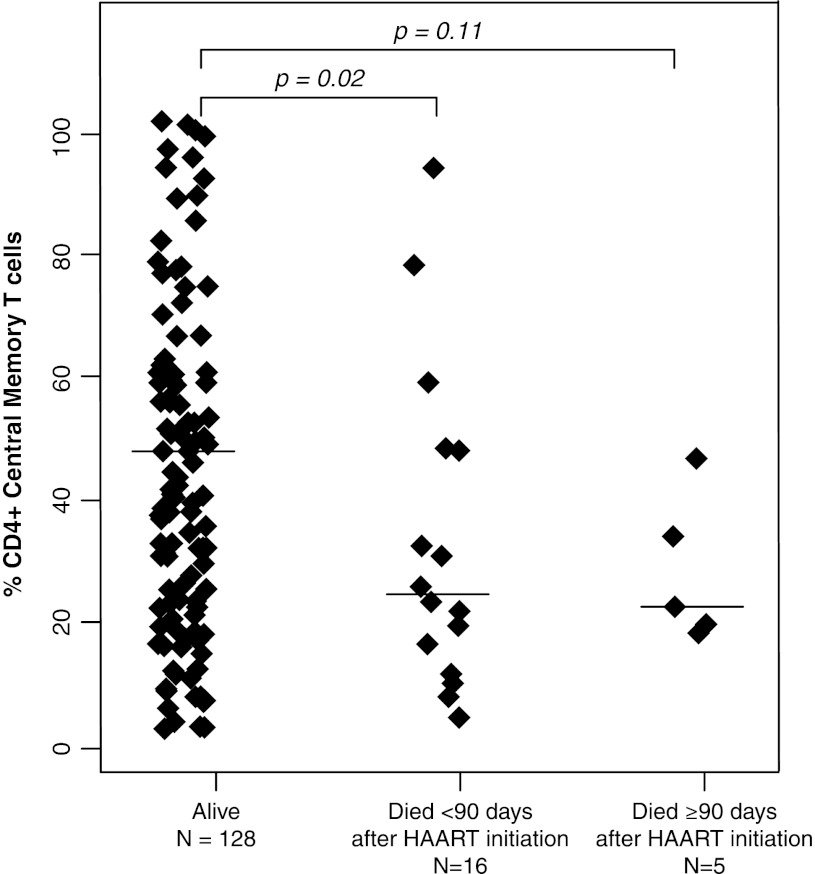
The percentages of CD4+ T cells did not differ between children who died and those who survived with medians of 11.3% (IQR: 7.5%, 16.8%) and 12.3% (IQR: 6.7%, 15.7%), respectively (p=0.72 and 0.76 by Mann–Whitney U-test) (Table 2). Activated T cells, defined by the expression of CD38 and HLA-DR, also did not differ between survivors and children who died. In contrast, CD4+ effector and CD8+ effector T cell percentages were ≥13.9% higher among those who died (all p-values ≤0.04) (Fig. 1), whereas effector memory T cell percentages for both CD4+ and CD8+ T cell populations were ≥10.1% lower among children who died (all p-values ≤0.04) (Fig. 2). CD4+ and CD8+ central memory T cells were 2-fold higher in survivors compared to children who died within 90 days of HAART initiation (p=0.02 and 0.08) (Fig. 3).
Table 2.
T Cell Subset Percentages Among HIV-Infected Zambian Children Who Survived or Died Within 90 Days or During Follow-up After Starting Highly Active Antiretroviral Therapy
| Alive during follow-up N=128 | Death within 90 days N=16 | Death during follow-up N=21 | p-value* (alive vs. died <90 days, overall) | ||
|---|---|---|---|---|---|
| % CD4+ T cells | 11.3 (7.5, 16.8) | 11.6 (6.6, 15.8) | 12.3 (6.7, 15.7) | 0.72, 0.76 | |
| % Naive | 19.6 (7.3, 35.8) | 28.6 (14.4, 37.5) | 28.9 (14.1, 38.4) | 0.41, 0.33 | |
| % Effector | 25.6 (15.7, 38.6) | 35.7 (24.3, 58.5) | 39.5 (25.7, 55.5) | 0.04, <0.01 | |
| % Effector memory | 38.3 (25.8, 54.3) | 24.4 (19.1, 38.5) | 22.7 (17.7, 37.7) | 0.04, <0.01 | |
| % Central memory | 4.8 (2.4, 7.6) | 2.4 (1.3, 4.8) | 2.3 (1.8, 4.7) | 0.02, <0.01 | |
| % Activated | 11.4 (6.8, 16.3) | N=123 | 9.7 (7.4, 11.1) | 10.4 (7.8, 11.7) | 0.39, 0.56 |
| % CD8+ T cells | 43.6 (38.0, 52.4) | N=126 | 46.3 (32.9, 51.8) | 48.1 (38.3, 53.6) | 0.90, 0.56 |
| % Naive | 3.2 (1.2, 7.1) | N=126 | 3.3 (1.9, 6.2) | 3.4 (1.9, 6.5) | 0.72, 0.60 |
| % Effector | 50.2 (37.0, 64.0) | N=126 | 70.0 (51.7, 75.8) | 68.9 (53.9, 76.8) | <0.01, <0.01 |
| % Effector memory | 41.2 (28.6, 57.4) | N=126 | 26.0 (21.7, 39.3) | 25.2 (19.3, 40.3) | 0.01, <0.01 |
| % Central memory | 0.08 (0.03, 0.19) | N=126 | 0.03 (0.00, 0.11) | 0.04 (0.02, 0.09) | 0.08, 0.06 |
| % Activated | 34.4 (20.8, 45.4) | N=126 | 30.1 (24.2, 47.9) | 30.7 (25.1, 47.4) | 0.94, 0.89 |
| % IL-7Rα | 10.4 (4.4, 16.8) | N=125 | 10.9 (5.2, 15.7) | 9.7 (6.2, 14.9) | 0.75, 0.80 |
p-values determined using Mann–Whitney U-tests.
Subsets of CD4+ or CD8+ T cells are presented as median (interquartile range) and defined as naive: CCR7+CD45RA+, effector memory: CCR7−CD45RA−, effector: CCR7−CD45RA+, central memory: CCR7+CD45RA−, and activated: HLA-DR+CD38+.
FIG. 1.
(a) CD4+ effector T cells and (b) CD8+ effector T cells among HIV-infected Zambian children who survived and died less than or greater than 90 days after beginning highly active antiretroviral therapy.
FIG. 2.
(a) CD4+ effector memory T cells and (b) CD8+ effector memory T cells among HIV-infected Zambian children who survived and died less than or greater than 90 days after beginning highly active antiretroviral therapy.
FIG. 3.
CD4+ central memory T cell subsets among HIV-infected Zambian children who survived and died less than or greater than 90 days after beginning highly active antiretroviral therapy.
Associations between subsets were assessed using correlations to reduce the potential for including collinear subsets in multivariable models. Percentages of CD4+ T cells were marginally correlated with CD4+ effector memory T cell percentages (ρ=−0.16, p=0.05) and CD4+ effector T cell percentages (ρ=0.14, p=0.09) (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid). CD8+ effector T cells were strongly correlated with CD4+ effector and CD4+ effector memory T cell percentages (ρ=0.56 and −0.64, both p<0.001) as well as CD8+ effector memory and CD8+ central memory T cell percentages (ρ=−0.79 and −0.34, p<0.001), but not with total CD4+ or CD4+ central memory T cell percentages. Therefore, total CD4+, CD8+ effector, and CD4+ central memory T cell percentages were included in the multivariable models.
Risk of overall mortality after initiating HAART
Risk factors for overall mortality and mortality within 90 days of beginning HAART were assessed separately. In univariable analysis, each 3-month increase in age and 1-unit increase in WAZ score were associated with 12% (95% CI: 2, 20) and 32% (95% CI: 4, 53) decreased risks of mortality, respectively, while total CD4+ T cell percentage was not associated with the risk of overall mortality [univariable OR: 0.82 (95% CI: 0.40, 1.65)] (Table 3). Increases in the percentages of CD4+ effector and CD8+ effector T cells were associated with a 1.47-fold (95% CI: 1.14, 1.90) and 1.59-fold (95% CI: 1.17, 2.16) increased odds of overall mortality, while increased percentages of CD4+ effector memory and central memory T cells decreased the risk of overall mortality (Table 3). After adjusting for age, WAZ score, and CD4+ T cell percentage, 10% increases in CD8+ effector and CD4+ central memory T cells remained independently associated with overall mortality, producing 1.43-fold (95% CI: 1.08, 1.90) and 0.06-fold (95% CI: 0.01, 0.59) changes in the odds of mortality after HAART initiation (Table 3).
Table 3.
Clinical and Immunological Risk Factors for Overall Mortality and Mortality Within 90 Days of Initiating Highly Active Antiretroviral Therapy
| |
Death during follow-up |
Death within 90 days |
||||
|---|---|---|---|---|---|---|
| Univariable OR (95% CI) | N | Multivariable OR (95% CI) N=147 | Univariable OR (95% CI) | N | Multivariable OR (95% CI) N=147 | |
| 3-month increase in age | 0.88 (0.80, 0.98) | 0.86 (0.77, 0.98) | 0.89 (0.79, 0.99) | 0.87 (0.76, 1.00) | ||
| Weight-for-age z-score | 0.68 (0.47, 0.96) | 0.69 (0.48, 1.00) | 0.53 (0.35, 0.82) | 0.54 (0.34, 0.86) | ||
| Height-for-age z-score | 0.91 (0.71, 1.17) | 142 | 0.91 (0.68, 1.22) | 142 | ||
| Weight for-height z-score | 0.86 (0.66, 1.10) | 119 | 0.76 (0.56, 1.01) | 119 | ||
| Hemoglobin (g/dl) | 0.91 (0.71, 1.17) | 126 | 0.91 (0.69, 1.22) | 126 | ||
| Recent illness | 6.39 (0.82, 49.6) | —* | ||||
| Current illness | 2.61 (1.02, 6.65) | 2.24 (0.79, 6.39) | ||||
| 10% increase in | ||||||
| % CD4+ T cells | 0.82 (0.40, 1.65) | 0.69 (0.22, 1.46) | 0.84 (0.38, 1.86) | 0.59 (0.21, 1.67) | ||
| % Naive | 1.08 (0.85, 1.37) | 1.07 (0.81, 1.40) | ||||
| % Effector | 1.47 (1.14, 1.90) | 1.31 (0.99, 1.73) | ||||
| % Effector memory | 0.73 (0.56, 0.95) | 0.82 (0.63, 1.06) | ||||
| % Central memory | 0.10 (0.02, 0.64) | 0.06 (0.01, 0.59) | 0.13 (0.02, 0.99) | 0.09 (0.01, 0.97) | ||
| % Activated | 0.82 (0.46, 1.46) | 144 | 0.80 (0.40, 1.57) | 144 | ||
| % CD38+ | 1.10 (0.81, 1.51) | 144 | 0.80 (0.40, 1.57) | 144 | ||
| % HLA-DR+ | 0.86 (0.54, 1.38) | 144 | 1.00 (0.73, 1.36) | 144 | ||
| % CD8+ T cells | 1.00 (0.68, 1.44) | 147 | 0.84 (0.57, 1.25) | 147 | ||
| % Naive | 0.86 (0.41, 1.80) | 147 | 0.80 (0.32, 1.98) | 147 | ||
| % Effector | 1.59 (1.17, 2.16) | 147 | 1.43 (1.08, 1.90) | 1.43 (1.04, 1.96) | 147 | 1.29 (0.97, 1.72) |
| % Effector memory | 0.69 (0.52, 0.92) | 147 | 0.76 (0.57, 1.02) | 147 | ||
| % Central memory | 0.09 (<0.01, 5103) | 147 | 0.19 (<0.01, 1328) | 147 | ||
| % Activated | 1.01 (0.77, 1.32) | 147 | 1.01 (0.75, 1.37) | 147 | ||
| % CD38+ | 1.22 (0.84, 1.76) | 147 | 1.12 (0.79, 1.60) | 147 | ||
| % HLA-DR+ | 0.96 (0.75, 1.24) | 147 | 0.97 (0.73, 1.29) | 147 | ||
| % IL-7Rα | 1.03 (0.76, 1.40) | 146 | 1.07 (0.77, 1.48) | 146 | ||
All children who died within 90 days of HAART initiation were recently ill.
N=149 children unless otherwise noted; OR=odds ratio; g/dl=grams per deciliter.
Risk of mortality within 90 days of initiating HAART
Similar associations were observed between T cell subsets and mortality within 90 days of HAART initiation. Increased age and WAZ score were associated with decreased risks of mortality within 90 days of HAART initiation in univariable analyses, whereas CD4+ T cell percentage was not associated with mortality (Table 3). Ten percent increases in CD4+ effector and CD8+ effector T cells were associated with 1.31-fold (95% CI: 0.99, 1.73) and 1.43-fold (95% CI: 1.04, 1.96) increased odds of death within 90 days of beginning HAART (Table 3). The point estimates for both CD4+ memory populations were consistent with those observed with overall mortality, although only that for central memory cells was statistically significant. Adjusting for age, WAZ score, and CD4+ T cell percentage attenuated the association between early mortality and CD8+ effector T cell percentage [odds ratio (OR): 1.29 (95% CI: 0.97, 1.72)], while an increased percentage of CD4+ central memory T cells remained negatively associated with the risk of death [OR: 0.09 (95% CI: 0.01, 0.97)] (Table 3).
Discriminatory characteristics of models for mortality
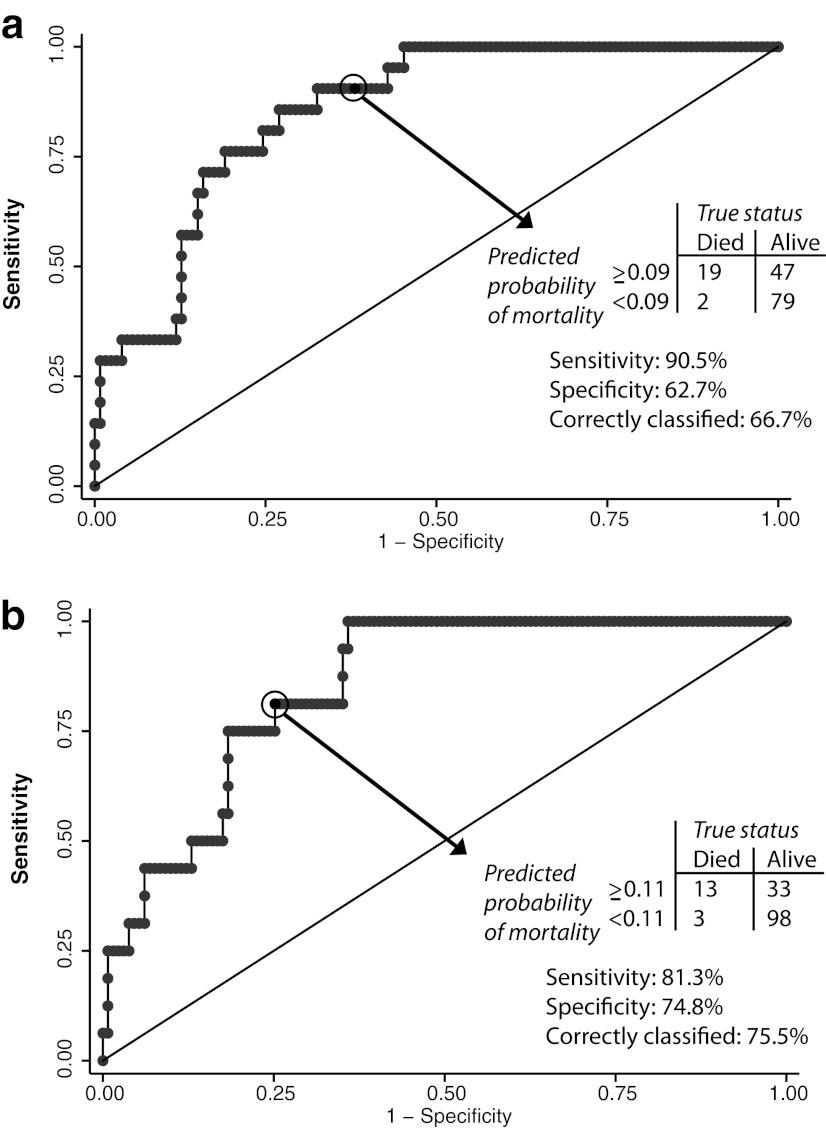
We assessed the ability of the multivariable models to distinguish children who died from those who survived. For the models of overall mortality and mortality within 90 days of HAART initiation, inclusion of both CD8+ effector T cells and CD4+ central memory T cells after adjusting for age, WAZ score, and CD4+ T cell percentage produced the highest bootstrapped AUC values of 85.4% and 85.3%, respectively. The optimism, or estimate of model overfitting, was 4.5% for the model of mortality within 90 days of HAART initiation and 3.8% for the overall mortality model, producing corrected AUC values of 80.8% and 81.6%.
Each point on the receiver-operator characteristic curves represents the predicted probability of mortality from a logistic regression model (similar to a continuous result from a screening or diagnostic test) and choosing a cut-off among these probabilities makes it possible to categorize children into groups of high and low mortality risk. The cut-off or “criterion of positivity” was selected from these probabilities to calculate measures of model discrimination that maximized sensitivity and the proportion of correctly classified children. A criterion of positivity of 0.09 (i.e., the probability of mortality) for the model of overall mortality properly classified mortality outcomes for 66.7% of children and resulted in a sensitivity of 90.5% (Fig. 4a). Moreover, a predicted probability of overall mortality of less than 9% corresponded to a negative predictive value of 97.5% (79 of 81 children alive with a predicted probability of less than 9%). Thus, a majority of children at low risk of mortality outcomes were properly classified using this model. For mortality within 90 days of HAART initiation, a criterion of positivity of 0.11 captured the highest number of children who had post-HAART mortality outcomes with a sensitivity of 81.3%, while simultaneously misclassifying the fewest number of children (Fig. 4b).
FIG. 4.
Receiver-operator characteristic (ROC) curves for the models of (a) overall mortality and (b) mortality within 90 days of beginning highly active antiretroviral therapy. Note: Models for overall mortality and mortality within 90 days of highly active antiretroviral therapy (HAART) initiation included covariates for CD4+ central memory T cell percentage, CD8+ effector T cell percentage, total CD4+ T cell percentage, weight-for-age z-score, and 3-month increases in age, as outlined in the multivariable logistic regression models of Table 3.
Discussion
Mortality was high within the first 3 months of starting HAART in this cohort of HIV-infected children receiving care in urban Zambia. Previously identified risk factors for mortality among children initiating HAART, including younger age and undernutrition, were associated with mortality; however, anemia and immune suppression, as measured by CD4+ T cell percentage, were not associated with mortality. This may have been due in part to the younger age of this cohort in comparison with other studies.5 Importantly, children with a higher percentage of CD8+ effector T cells and those with a lower percentage of CD4+ central memory T cells were at increased risk of mortality. These T cell subsets may better reflect immunologic dysfunction than total CD4+ T cell percentage.
Effective immune responses are critical for protection against opportunistic infections and other HIV-related illnesses, and require virologic suppression and immune reconstitution with HAART. Consistent with previous reports, approximately 75% of the mortality observed in this cohort occurred within 90 days of HAART initiation.6,8 The overall and early mortality rates of 4.17 and 7.95 deaths per 100 py observed in this cohort were lower than rates observed in other studies of children receiving HAART in sub-Saharan Africa.3–6,8,28 A mortality rate of 17.4 deaths per 100 py was observed within the first 90 days of HAART in a large study of Zambian children in urban Lusaka.5 Within 4 months of beginning HAART, Kenyan children died at the strikingly high rate of 46 deaths per 100 py.6 These early deaths may reflect late clinical presentation, advanced immunosuppression, and severe opportunistic infections, as well as the inability of HAART to rapidly reverse immunologic damage caused by HIV infection.
Among healthy individuals, naive T cells are activated following antigen exposure and differentiate into memory and effector cells. Effector T cells induce cytolysis of infected cells or activate other lymphocytes and immune effector cells, whereas memory T cells maintain the capacity to respond rapidly to previously encountered antigens. We found no differences in the percentages of CD8+ naive T cells between children who died and survived. However, children who died had 20% higher CD8+ effector T cell percentages and half the CD4+ central memory T cell percentages of survivors, suggesting altered differentiation pathways in children who died. Elevated levels of HIV-specific and other pathogen-specific effector T cells have been observed in persons with chronic HIV infection,29–32 and may reflect immune exhaustion, a state characterized by the loss of cytokine production, cytotoxicity, and proliferation after continual antigen stimulation.33,34 Conversely, central memory T cells are frequently lower in HIV-infected individuals regardless of treatment status but are necessary for long-term protective immune responses.19,35 This combination of immunophenotypes suggests children who died were unable to mount effective immune responses to coinfecting pathogens.
We attempted to identify thresholds of effector and memory T cell percentages that could aid in differentiating mortality risk but found these had poor discriminatory ability. Additionally, this approach neglected to account for confounding by age, nutritional status, and disease status. Substantial maturational changes occur in the composition of T cell subsets in younger children, making summary measures potentially misleading among children spanning a broad age range. Therefore, models were developed to adjust for these factors as well as allow for the contribution of multiple cellular subsets to mortality risk. After adjusting for age, WAZ score, and CD4+ T cell percentage, associations between mortality and effector and central memory T cells remained. Importantly, total CD4+ T cell percentage at the time of starting HAART was not associated with mortality in this cohort. After compensating for model overfitting, the AUC values suggested these models provided good measures of discrimination within this cohort, although the ability of CD4+ central memory T cells and CD8+ effector T cells to predict mortality should be confirmed in larger studies as the small number of deaths limited statistical power.
The process of immune reconstitution following HAART can unmask underlying illnesses and result in paradoxical exacerbation of opportunistic illnesses such as tuberculosis and contribute to immune reconstitution inflammatory syndrome (IRIS).36,37 Associations between increased CD8+ effector T cell percentages at HAART initiation and both overall and early mortality may signal unrecognized, severe opportunistic infections or higher levels of HIV-specific effector cells corresponding to higher viral loads.29 IRIS may have contributed to some of the deaths observed in this cohort but causes of death were not ascertained. The lack of HIV viral load data was a limitation, although only one prior study found that a plasma HIV viral load of >750,000 copies/ml was associated with mortality in univariate analysis but not after adjustment for CD4+ T cell percentage, age, and WAZ score.4
The inclusion criteria limit the generalizability of study findings to children older than 9 months of age accessing medical care. By requiring evidence of prior measles vaccination on an Under-5 Immunization Card, this study may have excluded children with poorer access to care and potentially more advanced immunosuppression; however, medical care is free at the clinics where enrollment occurred.
The cellular subsets associated with early mortality after HAART initiation may provide insight into the mechanisms contributing to immunological dysfunction, impaired immune reconstitution, and preventable mortality. Further research linking changes in specific cellular subsets with clinical markers and specific disease conditions may aid in identifying HIV-infected children who would benefit from starting HAART earlier than current guidelines suggest or the need for more frequent clinical monitoring after starting HAART.
Supplementary Material
Acknowledgments
W.J.M., C.B.M., and M.M.M. conceived of the study, C.B.M. and M.M.M. led the collection of clinical data, K.R.L. performed statistical analyses, H.N. optimized and performed laboratory analyses, and K.R.L. and W.J.M. drafted the manuscript. All authors read and reviewed the manuscript. We thank the participants of this study and the clinicians and study staff who collected data and cared for the participating children. This study was funded by the U.S. National Institute of Allergy and Infectious Diseases (R01AI070018).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.WHO, UNAIDS, UNICEF: Global HIV/AIDS response. Epidemic update, health sector progress towards Universal Access. www.who.int/entity/hiv/pub/progress_report2011/en/ [Jan 16;2011 ]. www.who.int/entity/hiv/pub/progress_report2011/en/
- 2.Sutcliffe CG. Scott S. Mugala N, et al. Survival from 9 months of age among HIV-infected and uninfected Zambian children prior to the availability of antiretroviral therapy. Clin Infect Dis. 2008;47(6):837–844. doi: 10.1086/591203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutcliffe CG. van Dijk JH. Bolton C. Persaud D. Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8(8):477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 4.van Dijk JH. Sutcliffe CG. Munsanje B, et al. HIV-infected children in rural Zambia achieve good immunologic and virologic outcomes two years after initiating antiretroviral therapy. PLoS One. 2011;6(4):e19006. doi: 10.1371/journal.pone.0019006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolton-Moore C. Mubiana-Mbewe M. Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298(16):1888–1899. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 6.Wamalwa DC. Obimbo EM. Farquhar C, et al. Predictors of mortality in HIV-1 infected children on antiretroviral therapy in Kenya: A prospective cohort. BMC Pediatr. 2010;10:33. doi: 10.1186/1471-2431-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutcliffe CG. van Dijk JH. Munsanje B, et al. Risk factors for pre-treatment mortality among HIV-infected children in rural Zambia: A cohort study. PLoS One. 2011;6(12):e29294. doi: 10.1371/journal.pone.0029294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callens SF. Shabani N. Lusiama J, et al. Mortality and associated factors after initiation of pediatric antiretroviral treatment in the Democratic Republic of the Congo. Pediatr Infect Dis J. 2009;28(1):35–40. doi: 10.1097/INF.0b013e318184eeb9. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A. Nadkarni G. Yang WT, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): A systematic review and meta-analysis. PLoS One. 2011;6(12):e28691. doi: 10.1371/journal.pone.0028691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker AS. Mulenga V. Sinyinza F, et al. Determinants of survival without antiretroviral therapy after infancy in HIV-1-infected Zambian children in the CHAP Trial. J Acquir Immune Defic Syndr. 2006;42(5):637–645. doi: 10.1097/01.qai.0000226334.34717.dc. [DOI] [PubMed] [Google Scholar]
- 11.McCloskey TW. Kohn N. Lesser M. Bakshi S. Pahwa S. Immunophenotypic analysis of HIV-infected children: Alterations within the first year of life, changes with disease progression, and longitudinal analyses of lymphocyte subsets. Cytometry. 2001;46(3):157–165. doi: 10.1002/cyto.1100. [DOI] [PubMed] [Google Scholar]
- 12.Appay V. Sauce D. Immune activation and inflammation in HIV-1 infection: Causes and consequences. J Pathol. 2008;214(2):231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 13.Leng Q. Borkow G. Weisman Z, et al. Immune activation correlates better than HIV plasma viral load with CD4 T-cell decline during HIV infection. J Acquir Immune Defic Syndr. 2001;27(4):389–397. doi: 10.1097/00126334-200108010-00010. [DOI] [PubMed] [Google Scholar]
- 14.Hunt PW. Cao HL. Muzoora C, et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25(17):2123–2131. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douek DC. McFarland RD. Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396(6712):690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 16.Ensoli F. Fiorelli V. Alario C, et al. Decreased T cell apoptosis and T cell recovery during highly active antiretroviral therapy (HAART) Clin Immunol. 2000;97(1):9–20. doi: 10.1006/clim.2000.4915. [DOI] [PubMed] [Google Scholar]
- 17.Hazenberg MD. Stuart JW. Otto SA, et al. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: A longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95(1):249–255. [PubMed] [Google Scholar]
- 18.Douek DC. Brenchley JM. Betts MR, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417(6884):95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 19.Ladell K. Hellerstein MK. Cesar D, et al. Central memory CD8+ T cells appear to have a shorter lifespan and reduced abundance as a function of HIV disease progression. J Immunol. 2008;180(12):7907–7918. doi: 10.4049/jimmunol.180.12.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO Multicentre Growth Reference Study Group: WHO Child Growth Standards. Length/height-for-age, weight-for-age, weight-for-length, weight-for-height, body mass index-for-age: Methods, development. www.who.int/childgrowth/publications/en/ [May 19;2010 ]. www.who.int/childgrowth/publications/en/
- 21.Sallusto F. Lenig D. Forster R. Lipp M. Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 22.Michie CA. McLean A. Alcock C. Beverley PC. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360(6401):264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z. Cumberland WG. Hultin LE, et al. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16(2):83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 24.Kaech SM. Tan JT. Wherry EJ, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4(12):1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 25.van Leeuwen EM. de Bree GJ. Remmerswaal EB, et al. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood. 2005;106(6):2091–2098. doi: 10.1182/blood-2005-02-0449. [DOI] [PubMed] [Google Scholar]
- 26.Vittinghoff E. Glidden DV. Shiboski SC. McCulloch CE. Springer Science+Business Media, Inc.; New York: 2004. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. [Google Scholar]
- 27.Massie AB. Desai NM. Montgomery RA. Singer AL. Segev DL. Improving distribution efficiency of hard-to-place deceased donor kidneys: predicting probability of discard or delay. Am J Transplant. 2010;10:1613–1620. doi: 10.1111/j.1600-6143.2010.03163.x. [DOI] [PubMed] [Google Scholar]
- 28.Sutcliffe CG. van Dijk JH. Bolton-Moore C, et al. Differences in presentation, treatment initiation, and response among children infected with human immunodeficiency virus in urban and rural Zambia. Pediatr Infect Dis J. 2010;29(9):849–854. doi: 10.1097/INF.0b013e3181e753a8. [DOI] [PubMed] [Google Scholar]
- 29.Kalams SA. Goulder PJ. Shea AK, et al. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73(8):6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Resino S. Seoane E. Gutierrez MD. Leon JA. Munoz-Fernandez MA. CD4(+) T-cell immunodeficiency is more dependent on immune activation than viral load in HIV-infected children on highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;42(3):269–276. doi: 10.1097/01.qai.0000222287.90201.d7. [DOI] [PubMed] [Google Scholar]
- 31.Montesano C. Anselmi A. Palma P, et al. HIV replication leads to skewed maturation of CD8-positive T-cell responses in infected children. New Microbiol. 2010;33(4):303–309. [PubMed] [Google Scholar]
- 32.Tussey LG. Nair US. Bachinsky M, et al. Antigen burden is major determinant of human immunodeficiency virus-specific CD8+ T cell maturation state: Potential implications for therapeutic immunization. J Infect Dis. 2003;187(3):364–374. doi: 10.1086/367707. [DOI] [PubMed] [Google Scholar]
- 33.Wherry EJ. Blattman JN. Murali-Krishna K. van der Most R. Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Far M. Halwani R. Said E, et al. T-cell exhaustion in HIV infection. Curr HIV/AIDS Rep. 2008;5(1):13–19. doi: 10.1007/s11904-008-0003-7. [DOI] [PubMed] [Google Scholar]
- 35.Elrefaei M. McElroy MD. Preas CP, et al. Central memory CD4+ T cell responses in chronic HIV infection are not restored by antiretroviral therapy. J Immunol. 2004;173(3):2184–2189. doi: 10.4049/jimmunol.173.3.2184. [DOI] [PubMed] [Google Scholar]
- 36.Muller M. Wandel S. Colebunders R, et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: A systematic review and meta-analysis. Lancet Infect Dis. 2010;10(4):251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haddow LJ. Easterbrook PJ. Mosam A, et al. Defining immune reconstitution inflammatory syndrome: Evaluation of expert opinion versus 2 case definitions in a South African cohort. Clin Infect Dis. 2009;49(9):1424–1432. doi: 10.1086/630208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.