Abstract
Data on the effectiveness of second-line combination antiretroviral therapy (cART) are limited. We evaluated virologic outcomes of second cART in a multicenter cohort collaboration. The study population initiated first and second modern cART between 1996 and 2010. The second cART required a switch in at least the anchor agent of first cART. We evaluated time to virologic failure of second cART and factors associated with greater risk of failure using multivariable Cox proportional hazards models. Of 488 patients who switched to second-line cART, 67% were black and 32% were women. The median HIV-1 RNA at second cART initiation was 9,565 copies/ml [interquartile range (IQR); 123, 94,108]. The time to virologic failure of second cART was longer if HIV-1 RNA was undetectable at switch (p=0.001), although 12% and 17% of patients with undetectable and detectable HIV-1 RNA experienced virologic failure within 6 months of second cART initiation, respectively. A lower CD4 cell count at second cART initiation was associated with a greater risk of virologic failure. Failure rates decreased in more recent calendar years [adjusted relative hazard of 0.40 comparing 2008 to 2010 with 1996 to 1998 (95% confidence interval; 0.15, 1.00)]; however, type of anchor agent was not associated with failure. In conclusion, virologic failure of second cART was less likely if patients switched with undetectable HIV-1 RNA, although risk of early failure was similar. The effectiveness of second cART regimens improved over calendar time and was independent of the anchor agent in the regimen.
Introduction
The potent efficacy of currently recommended combination antiretroviral therapy (cART) regimens for ART-naive patients in suppressing HIV RNA levels has been clearly demonstrated in randomized clinical trials.1–4 Effective initial regimens with two nucleoside reverse transcriptase inhibitors (NRTIs) and an anchor agent are well established,5,6 and preferred agents have good toxicity profiles and most have a high barrier to resistance evolution.7–9 However, the durability of first cART is limited by factors as varied as preexisting resistance, toxicity, and/or intolerance, adherence, and virologic failure.10,11
In clinical care in North America over 20% and 50% of patients are no longer on their first cART by 6 and 24 months following initiation, respectively.12 Although first cART regimens have been simplified in recent calendar years, substantial heterogeneity and complexity remain in antiretroviral combinations provided for second cART.13 Moreover, little research exists on the management and outcomes of second cART, and adequately powered randomized comparative trials of second-line therapy, particularly in resource-rich settings, are not available.14,15
Achieving virologic suppression on second cART may be challenging as a switch to second-line therapy, whether motivated by virologic rebound, intolerability, or toxicity, is frequently complicated by difficulty with adherence and may involve more complex regimens.6 The long-term prognosis of individuals with virologic failure on second cART has not been well studied, but these individuals are likely to be at higher risk of HIV-related morbidity and mortality in part due to ongoing viral replication and further accumulation of HIV-1 resistance to antiretroviral agents.16 Therefore, in the present multicenter cohort collaboration we evaluated virologic outcomes of second cART in clinical care, including time to failure and patient demographic and clinical factors associated with greater risk of failure.
Materials and Methods
Study population
The study population included patients enrolled in one of three large U.S. clinical cohort studies, including the Vanderbilt-Meharry Center for AIDS Research (CFAR) Cohort, the Johns Hopkins HIV Clinical Cohort, and the UNC CFAR HIV Clinical Cohort, all described previously, which combined include over 15,000 HIV-infected individuals.17–19 In brief, these observational studies follow rigorous and standardized data collection methods, including electronic data capture from institutional records and periodic medical record reviews containing demographic, clinical, laboratory, and medication data. Institutional review board approval was obtained at each of the participating institutions.
For this study we included all patients who were receiving HIV care at one of the three clinical sites and who were at least 18 years old. Patients must have initiated a first cART followed by a second cART regimen. We excluded all patients who initiated first cART prior to June 1996 (i.e., prior to the introduction of cART) and patients without at least 8 weeks of follow-up on their second cART.
Measures
A first and second cART regimen was defined as two or more NRTIs with an anchor agent [i.e., a nonnucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI), a ritonavir-boosted PI (PI/r), or an integrase strand-transfer inhibitor (INSTI)]. To be considered as switching from first to second cART required a switch in the anchor agent (e.g., PI to NNRTI, NNRTI to PI, PI or NNRTI to INSTI), irrespective of changes in the NRTI backbone. Neither the addition of ritonavir to a PI nor a switch in any or all NRTIs alone was considered as a switch from first to second cART.
Because the sensitivity of HIV-1 RNA testing assays increased during the time frame of the study, an HIV-1 RNA level <400 copies/ml was considered successful virologic suppression. For first cART, virologic failure was defined as not reaching a decrease in an HIV-1 RNA level of at least 0.5 log10 copies/ml from cART initiation to 24 weeks or an HIV-1 RNA level of >400 copies/ml after 24 weeks. The definition of virologic failure for second cART was conditional on the HIV-1 RNA level at the start of the second cART regimen. In patients with an HIV-1 RNA level >400 copies/ml, virologic failure was defined as a failure to achieve a decrease in the HIV-1 RNA level of at least 0.5 log10 HIV-1 RNA by 24 weeks or an HIV-1 RNA level of >400 copies/ml after 24 weeks. For patients with an HIV-1 RNA of <400 copies/ml, virologic failure was defined as an HIV-1 RNA>400 copies/ml 8 weeks after switch.
Statistical analysis
Patients were followed from first cART initiation until the first of either their last reported HIV-1 RNA level while receiving second cART, or June 2010. Basic bivariable contrasts included performing the Pearson's Chi-square test for categorical variables and the Mann–Whitney rank sum test for continuous variables. We used Kaplan–Meier analyses and the log-rank test to assess time to virologic failure on second cART overall and stratified by patient demographic and clinical characteristics. Multivariable Cox proportional hazards regression models were fit to estimate relative hazards and 95% confidence intervals (95% CI) of time to second cART virologic failure. Analyses included assessing bivariable associations first and then fitting full and reduced models. We evaluated the proportional hazards assumption and all models were adjusted for clinical site. All tests for assessing statistical significance were two-sided with an α of 0.05, and analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC). Sensitivity analyses were conducted using HIV RNA limits of 200 and 50 copies/ml in those patients who were tested with a more sensitive plasma HIV RNA assay.
Results
The median age of the 488 patients meeting inclusion criteria was 37 years [interquartile range (IQR); 31, 44], and 32% were women, 67% black, and 25% white (Table 1). At first cART initiation the median CD4 cell count was 135 cells/mm3 (IQR; 27, 302) and the HIV-1 RNA level was 83,875 copies/ml (IQR; 22,218, 284,817). Almost one-half of patients initiated cART with an NNRTI (49%), while the remainder initiated cART with either a PI (34%) or PI/r (19%). No patient on INSTI-based initial therapy met the criteria for inclusion in this analysis.
Table 1.
Demographic and Clinical Characteristics of Patients at First Combination Antiretroviral Therapy Initiation, Among Persons Who Subsequently Initiated a Second Regimen and Met the Study Inclusion Criteria
| Characteristic | N (%) or median (IQR) |
|---|---|
| Total | 488 |
| Age, years | 37 (31–44) |
| Sex | |
| Male | 333 (68.2%) |
| Female | 155 (31.8%) |
| Race/ethnicity | |
| Black/African-American | 325 (66.6%) |
| White | 124 (25.4%) |
| Hispanic | 19 (3.9%) |
| Other | 20 (4.1%) |
| HIV risk group | |
| MSM | 140 (28.7%) |
| IDU | 118 (24.2%) |
| Heterosexual | 200 (41.0%) |
| Other/Unknown | 30 (6.1%) |
| Prior ADI | 165 (33.8%) |
| CD4 cell count, cells/mm3 | 135 (27–302) |
| HIV-1 RNA level, copies/ml | 83,875 (22,218–284,817) |
| cART anchor agent | |
| NNRTI | 237 (48.6%) |
| PI (unboosted) | 160 (33.8%) |
| PI/r | 91 (18.7%) |
IQR, interquartile range; MSM, men who have sex with men; IDU, intravenous drug use; ADI, AIDS-defining clinical condition; cART, combination antiretroviral therapy; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PI/r, ritonavir-boosted protease inhibitor.
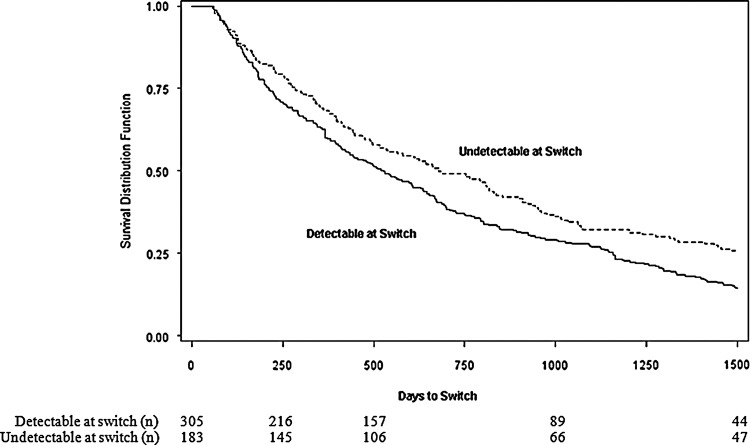
Over one-third of patients switched from their first to second cART regimen when their plasma HIV RNA was <400 c/ml (38%) and the overall median HIV-1 RNA level at start of second cART was 9,565 log10 copies/ml (IQR; 123, 94,108). The time from first to second cART initiation was on average just over 1.5 years, but depended on whether the patient had an HIV RNA<400 c/ml when they started second cART. Among patients with detectable HIV-1 RNA at second cART initiation, the median time from first to second cART initiation was 520 days (IQR; 205, 1158) compared to 670 days (IQR; 280, 1535) among patients with HIV-1 RNA<400 c/ml at switch (Fig. 1) (p=0.01). On average, the time between patients discontinuing their first cART and starting their second cART was 3 months (median 96 days: IQR; 0, 567).
FIG. 1.
Time from first to second combination antiretroviral therapy initiation stratified by HIV-1 RNA level at time of antiretroviral switch, multicohort collaboration 1996–2010. Note: undetectable defined as HIV-1 RNA<400 copies/ml, detectable defined as HIV-1 RNA≥400 copies/ml.
At second cART initiation the median CD4 cell count was 247 cells/mm3 (IQR; 85, 433) (Table 2), on average approximately 100 cells/mm3 higher than at first cART initiation. Over one-third of patients (37%) started their second cART with a CD4 cell count >350 cells/mm3. The most commonly used anchor agent of second cART was a PI (51%), with the vast majority ritonavir-boosted (∼90%). Most switches were either a modification from an NNRTI or PI to a PI (47%) or from a PI to an NNRTI (42%). Sixty percent of second-line treatment regimens in patients who switched with an HIV RNA>400 c/ml were PI based; use of INSTI-based regimens were uncommon (4.6%). As newer antiretroviral agents became available these were used in second cART, for example 10% (n=26/274) of patients switching between 2004 and 2010 were switched from either a PI or NNRTI to a raltegravir-based or etravirine-based regimen although most of these patients had HIV RNA<400 c/ml.
Table 2.
Clinical Characteristics of Patients at Start of Second Combination Antiretroviral Therapy Stratified by HIV-1 RNA Level at Time of Antiretroviral Switch, Multicohort Collaboration 1996–2010
| Characteristic | All | Undetectable | Detectable |
|---|---|---|---|
| N | 488 | 183 | 305 |
| CD4 cell count, cells/mm3 | 247 (85–433) | 433 (292–712) | 175 (44–308) |
| ≤50 | 87 (17.8%) | 6 (3.2%) | 81 (26.6%) |
| 51–200 | 113 (23.1%) | 25 (13.7%) | 88 (28.9%) |
| 201–350 | 109 (22.3%) | 33 (18.0%) | 76 (24.9%) |
| 351–500 | 84 (17.2%) | 44 (24.0%) | 40 (13.1%) |
| ≥501 | 95 (19.5%) | 75 (42.0%) | 20 (21.1%) |
| HIV-1 RNA level, copies/ml | 9,565 (123–94,108) | 50 (50–128) | 49,177 (11,094–160,653) |
| ≤400 | 183 (37.5%) | 183 (100%) | 0 (0.0%) |
| 401–1,000 | 13 (2.7%) | 13 (4.3%) | |
| 1,001–10,000 | 58 (11.9%) | 58 (19.0%) | |
| 10,001–100,000 | 131 (26.9%) | 131 (43.0%) | |
| ≥100,001 | 103 (21.1%) | 103 (33.8%) | |
| Second cART anchor agent | |||
| NNRTI in first cART | |||
| PI | 228 (46.7%) | 60 (32.8%) | 168 (55.1%) |
| INSTI | 8 (1.6%) | 4 (2.2%) | 4 (1.3%) |
| NNRTI (ETV) | 2 (0.4%) | 2 (1.1%) | 0 (0.0%) |
| PI in first cART | |||
| NNRTI | 207 (42.4%) | 93 (50.8%) | 114 (37.4%) |
| PI (DRV or TPV) | 25 (5.1%) | 10 (5.5%) | 15 (4.9%) |
| INSTI | 24 (4.9%) | 17 (9.3%) | 7 (2.3%) |
| Calendar year | |||
| 1996–2003 | 214 (43.9%) | 60 (32.8%) | 154 (50.5%) |
| 2004–2010 | 274 (56.2%) | 123 (65.2%) | 151 (49.5%) |
Undetectable and detectable defined as HIV-1 RNA<and ≥400 copies/ml, respectively; IQR, interquartile range; cART, combination antiretroviral therapy; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; INSTI, integrase strand-transfer inhibitor; ETV, etravirine; DRV, darunavir; TPV, tipranavir.
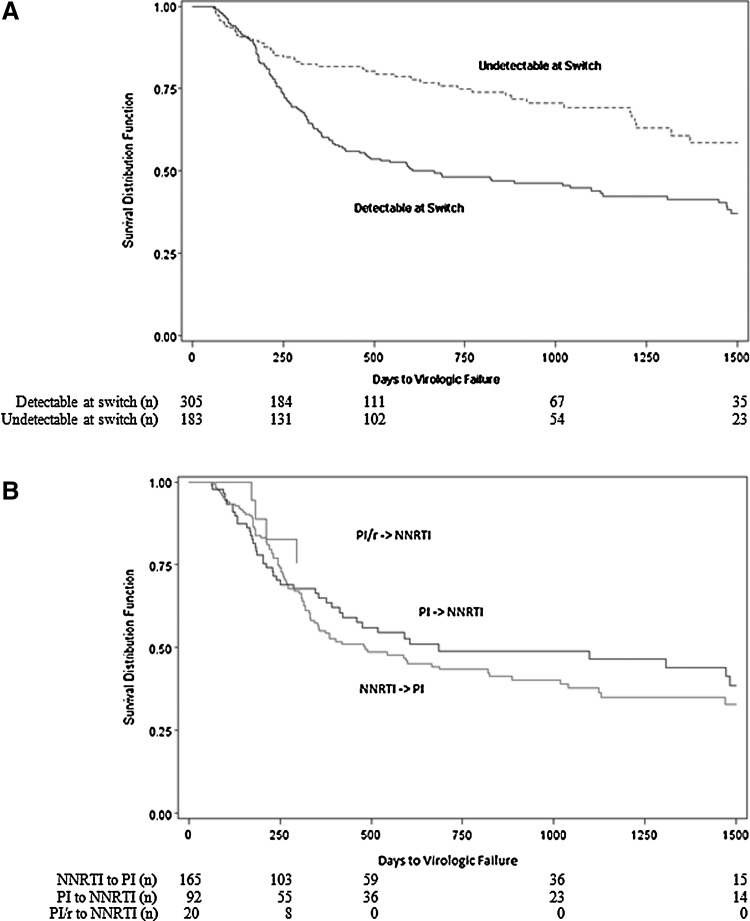
Time to virologic failure of second cART was significantly different based on the HIV-1 RNA level at switch. Among patients with a detectable HIV-1 RNA at second cART initiation the median time to virologic failure was 605 days (IQR; 240, 2250), in comparison to >1,500 days (IQR; 730, >1500) among patients with undetectable HIV-1 RNA (p=0.001) (Fig. 2A). However, a similar proportion of patients had virologic failure early after switching, with 12% and 17% failing virologically by 6 months after start of second cART among patients undetectable and detectable at switch, respectively. The time to virologic failure of second cART among patients who initiated second cART with detectable HIV-1 RNA was somewhat shorter (but not significantly) among persons whose second cART anchor agent was a PI in comparison to NNRTI, irrespective of whether the PI in the first cART regimen was ritonavir-boosted (Fig. 2B).
FIG. 2.
Time to virologic failure of second combination antiretroviral therapy, multicohort collaboration 1996–2010: (A) among all patients stratified by HIV-1 RNA level at initiation (n=488); (B) among patients with detectable HIV-1 RNA at initiation stratified by anchor agents received (n=277). Note: undetectable defined as HIV-1 RNA<400 copies/ml, detectable defined as HIV-1 RNA≥400 copies/ml. NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PI/r, ritonavir-boosted PI.
Using different cut-points for virologic suppression (<400, <200, and <50 copies/ml) did not significantly alter the time to virologic failure in patients who were below the limit at the time of switch (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid). We had data available on resistance testing for two of the three sites. At these two sites of 270 patients who were virologically detectable at switch 158 (59%) had an HIV genotype available after starting their first cART and prior to starting their second cART. Among those with a genotype 49% (77 of 158) subsequently experienced virologic failure, and 47% (52 of 112) of those without a genotype subsequently failed virologically (p=0.71).
In multivariable analyses of time to second cART virologic failure, both higher CD4 cell count and more recent calendar year at second cART initiation were independently associated with a lower risk of failure (Table 3). The risk of virologic failure declined consistently across calendar years, with the most notable reductions in 2006 and 2008. The relative hazard of virologic failure when second cART was initiated between 2008 and 2010, in comparison to starting between 1996 and 1998, was 0.40 (95% CI; 0.15, 1.00). Additional patient demographic and clinical characteristics considered were not associated with time to second cART virologic failure in multivariable analyses, including sex, age, race, HIV transmission risk group, history of AIDS-defining clinical condition, CD4 cell count at first cART initiation, HIV-1 RNA at second cART initiation, time between end of first cART and start of second cART, and anchor agent received.
Table 3.
Characteristics Associated with Virologic Failure on Second Combination Antiretroviral Therapy, Multicohort Collaboration 1996–2010
| Characteristica | Relative hazard (95% CI) |
|---|---|
| CD4 cell count at second cART start (per 100 cell/mm3 increase) | 0.82 (0.75, 0.89) |
| Calendar year of second cART start | |
| 1996–1998 | 1. |
| 1999–2001 | 0.79 (0.32, 1.81) |
| 2002–2004 | 0.88 (0.38 2.06) |
| 2005–2007 | 0.61 (0.25, 1.45) |
| 2008–2010 | 0.40 (0.15, 1.00) |
Estimates based on fitting a multivariable Cox proportional hazards model including all characteristics listed and clinical site.
95% CI, 95% confidence interval; cART, combination antiretroviral therapy.
Discussion
In this multicenter clinical cohort study of HIV patients in care who were observed to switch from first to second cART, the average time from therapy initiation to a second cART switch was approximately 1.5 years and over one-third had HIV RNA<400 c/ml at therapy switch. Approximately one-quarter of patients experienced virologic failure on their second cART by 1 and 2 years after starting their second cART depending on whether their HIV RNA was above 400 c/ml or suppressed at initiation, respectively. These results are comparable to recently reported studies in modestly treatment-experienced patients who received modern ritonavir-boosted PIs.20,21
The number of early second cART virologic failures observed among patients who had a suppressed HIV RNA at the time of switch from first to second cART regimens was surprising. These findings emphasize caution when switching cART in successfully treated patients. Optimistically we observed notable reductions in the time to virologic failure of second cART in more recent calendar years, with improvements evident through 2008 to 2010, the latest calendar years for which we had available data. Sample size restrictions limited our ability to assess the contribution that increasing use of newer ritonavir-boosted PIs, the NNRTI etravirine and the INSTI raltegravir had on study results. However, this finding is consistent with generally increasing HIV-1 RNA suppression rates in more recent calendar years among patients with varying exposure to cART in clinical care.22 We hypothesized that the time between the end of first-line therapy and the start of second-line therapy might be a marker of adherence to care, thereby influencing time to detectable HIV RNA on second-line therapy; however, in our multivariable analysis this gap in treatment was not a significant predictor of virologic failure.
The higher a patient's CD4 cell count at second cART initiation the greater their probability was of achieving and/or maintaining virologic suppression across time, even when HIV RNA level at switch was included in the model. This observation is consistent with prior work indicating that higher CD4 cell counts at cART initiation are associated with superior HIV-1 RNA outcomes,12 and clinical outcomes including morbidity and mortality.23–25 Among patients with detectable HIV-1 RNA at second cART initiation we observed a shorter time to virologic failure in patients whose second cART included a PI versus an NNRTI. The explanation for this finding is not obvious. Most of the switches from NNRTI to PI were among patients receiving older cART regimens, including ritonavir-unboosted PIs, which for the most part were less well tolerated and more complex to adhere to. While these patients met our definition of virologic failure, they may have had virologic rebound for reasons of tolerability and adherence, and therefore the simplicity of the NNRTI regimen or its better tolerability may have contributed to this finding.
The three large cohorts used for this study are highly representative of the HIV epidemic in the United States, with a substantial proportion of patients of black race, women, and a distribution of HIV risk factors. Although combined these three cohorts include over 15,000 HIV-infected patients, fewer individuals met our inclusion criteria than anticipated. In part this may be due to the high virologic suppression rates among patients initiating first cART,12,17 and in part to our inclusion criteria, specially the requirement that patients switch anchor agents.
Our results emphasize that the orderly treatment cascade from first NNRTI to second ritonavir-boosted PI-based therapy is only one of many scenarios in clinical practice. The majority of patients did not follow this particular sequence and reached approximately 50% only in more recent years. Randomized trials of early treatment failure have been difficult to conduct in the developed world setting in part due to the diversity of initial regimens, reasons for treatment failure and challenges with therapy, and visit adherence in this population. Our findings suggest that obtaining data on specific sequencing strategies will also be difficult using observational cohort data and will likely require very large collaborative cohorts, with any specific treatment sequence representing only a minority of patients.
Our study benefits from the use of data from three large clinical care sites with well-established HIV cohorts, comparable data collection methods, and extensive prior collaborative experience.26 Additionally, our results have direct relevance to clinical care given the representativeness of the cohorts. However, our study, as other observational analyses, may be subject to residual confounding, including confounding by indication and selection bias. For example, if certain patients were differentially prescribed an NNRTI versus a PI as first ART based on factors not collected in this study our results may be either underestimating or overestimating the time to second ART virologic failure. Another limitation we encountered was sample size, which hindered our ability to conduct subgroup analyses and evaluate additional secondary outcomes. Moreover, a number of factors that may have affected our results were not considered, including presence of antiretroviral resistance.
In summary, we present some of the first evidence of second cART virologic outcomes among HIV patients in clinical care. Our findings suggest that although therapeutic outcomes are improving, notable numbers of patients have virologic failure of their second cART in a relatively short period of time after switching therapy, including patients who initiate second cART with HIV-1 RNA levels below 400 c/ml. Our results also indicate that work assessing reasons for first cART discontinuation and second cART initiation is needed to inform optimal therapy sequencing, including evaluating the relative contributions of tolerability/toxicity, adherence, and resistance evolution. These analyses will require efforts from large national and international cohort collaborations.
Supplementary Material
Acknowledgments
We would like to thank the patients and research staff at participating HIV clinical cohort studies. Johns Hopkins data management: Li Ming Zhou; Vanderbilt data management: Megan Turner and Sally Bebawy; UNC data management: Brant Stalzer.
Other support for Dr. Moore and the Johns Hopkins HIV Clinical Cohort (National Institutes of Health R01-DA11602, K24-DA00432, R01-AA16893); for Drs. Eron and Napravnik and the UNC CFAR HIV Clinical Cohort (National Institutes of Health P30-AI50410, and the Agency for Healthcare Research and Quality R01-HS018731); and for Dr. Sterling and the Vanderbilt-Meharry CFAR Cohort (National Institutes of Health U01-AI54999 and K24-AI65298).
Author Disclosure Statement
Supported by Bristol Myers Squibb.
References
- 1.Rockstroh JK. Lennox JL. Dejesus E, et al. Long-term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment-naive human immunodeficiency virus-1-infected patients: 156-week results from STARTMRK. Clin Infect Dis. 2011;53:807–816. doi: 10.1093/cid/cir510. [DOI] [PubMed] [Google Scholar]
- 2.Molina JM. Andrade-Villanueva J. Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372:646–655. doi: 10.1016/S0140-6736(08)61081-8. [DOI] [PubMed] [Google Scholar]
- 3.Mills AM. Nelson M. Jayaweera D, et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS. 2009;23:1679–1688. doi: 10.1097/QAD.0b013e32832d7350. [DOI] [PubMed] [Google Scholar]
- 4.Daar ES. Tierney C. Fischl MA, et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med. 2011;154:445–56. doi: 10.1059/0003-4819-154-7-201104050-00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson MA. Aberg JA. Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society–USA panel. JAMA. 2010;304:321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 6.Department of Health and Human Services; [May 15;2012 ]. Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Google Scholar]
- 7.Taiwo B. Yanik E. Napravnik S, et al. Laboratory abnormalities following initiation of modern ART in the US, 2000–2010 among CNICS patients. 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. Mar 5–8;2012 . [Google Scholar]
- 8.Haubrich RH. Riddler SA. DiRienzo AG, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23:1109–1118. doi: 10.1097/QAD.0b013e32832b4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch MS. Gunthard HF. Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society–USA panel. Clin Infect Dis. 2008;47:266–285. doi: 10.1086/589297. [DOI] [PubMed] [Google Scholar]
- 10.Paredes R. Lalama CM. Ribaudo HJ, et al. Pre-existing minority drug-resistant HIV-1 variants, adherence, and risk of antiretroviral treatment failure. J Infect Dis. 2010;201:662–671. doi: 10.1086/650543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waters L. Fisher M. Winston A, et al. A phase IV, double-blind, multicentre, randomized, placebo-controlled, pilot study to assess the feasibility of switching individuals receiving efavirenz with continuing central nervous system adverse events to etravirine. AIDS. 2011;25:65–71. doi: 10.1097/QAD.0b013e328341685b. [DOI] [PubMed] [Google Scholar]
- 12.Althoff KN. Justice AC. Gange SJ, et al. Virologic and immunologic response to HAART, by age and regimen class. AIDS. 2010;24:2469–2479. doi: 10.1097/QAD.0b013e32833e6d14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKinnell JA. Willig JH. Westfall AO, et al. Antiretroviral prescribing patterns in treatment-naive patients in the United States. AIDS Patient Care STDS. 2010;24:79–85. doi: 10.1089/apc.2009.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphreys EH. Chang LW. Harris J. Antiretroviral regimens for patients with HIV who fail first-line antiretroviral therapy. Cochrane Database Syst Rev. 2010:CD006517. doi: 10.1002/14651858.CD006517.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruxrungtham K. Pedro RJ. Latiff GH, et al. Impact of reverse transcriptase resistance on the efficacy of TMC125 (etravirine) with two nucleoside reverse transcriptase inhibitors in protease inhibitor-naive, nonnucleoside reverse transcriptase inhibitor-experienced patients: Study TMC125-C227. HIV Med. 2008;9:883–896. doi: 10.1111/j.1468-1293.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- 16.Mugavero MJ. Napravnik S. Cole SR, et al. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis. 2011;53:927–935. doi: 10.1093/cid/cir526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: The Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(Suppl 1):S38–41. doi: 10.1097/00042560-199801001-00011. [DOI] [PubMed] [Google Scholar]
- 18.Koethe JR. Jenkins CA. Shepherd BE. Stinnette SE. Sterling TR. An optimal body mass index range associated with improved immune reconstitution among HIV-infected adults initiating antiretroviral therapy. Clin Infect Dis. 2011;53:952–960. doi: 10.1093/cid/cir606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Napravnik S. Eron JJ., Jr McKaig RG. Heine AD. Menezes P. Quinlivan E. Factors associated with fewer visits for HIV primary care at a tertiary care center in the Southeastern U.S. AIDS Care. 2006;18(Suppl 1):S45–50. doi: 10.1080/09540120600838928. [DOI] [PubMed] [Google Scholar]
- 20.Cahn P. Fourie J. Grinsztejn B, et al. Week 48 analysis of once-daily vs. twice-daily darunavir/ritonavir in treatment-experienced HIV-1-infected patients. AIDS. 2011;25:929–939. doi: 10.1097/QAD.0b013e328345ee95. [DOI] [PubMed] [Google Scholar]
- 21.Zajdenverg R. Podsadecki TJ. Badal-Faesen S, et al. Similar safety and efficacy of once- and twice-daily lopinavir/ritonavir tablets in treatment-experienced HIV-1-infected subjects at 48 weeks. J Acquir Immune Defic Syndr. 2010;54:143–151. doi: 10.1097/QAI.0b013e3181cbd21e. [DOI] [PubMed] [Google Scholar]
- 22.Moore RD. Bartlett JG. Dramatic decline in the HIV-1 RNA level over calendar time in a large urban HIV practice. Clin Infect Dis. 2011;53:600–604. doi: 10.1093/cid/cir467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanoy E. May M, et al. Antiretroviral therapy cohort collaboration: Prognosis of patients treated with cART from 36 months after initiation, according to current and previous CD4 cell count and plasma HIV-1 RNA measurements. AIDS. 2009;23:2199–2208. doi: 10.1097/QAD.0b013e3283305a00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chene G. Sterne JA. May M, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: Analysis of prospective studies. Lancet. 2003;362:679–686. doi: 10.1016/s0140-6736(03)14229-8. [DOI] [PubMed] [Google Scholar]
- 25.Kitahata MM. Gange SJ. Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egger M. May M. Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: A collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.