Abstract
According to the dual-mechanisms of cognitive control framework (DMC), older adults rely predominantly on reactive as opposed to proactive control. As a result, we expected elevated response conflict for older relative to younger adults with increasing task difficulty. Response-locked ERP activity was examined separately for fast and slow responses (representing proactive and reactive control, respectively) at low, medium, and high levels of difficulty. Older adults recruited reactive control more often than the young, as reflected by increased behavioral costs and enhanced pre-response negativity (PRN). No age differences in conflict detection (medial frontal negativity, MFN) were evident at low levels of difficulty, but response conflict increased along with difficulty for older adults. These data provide empirical support for the DMC suggesting that aging is associated with a less efficient reactive-control mode of processing.
Descriptors: Aging, Reactive and proactive control, Pre-response negativity, Medial-frontal negativity
Cognitive control refers to the ability to flexibly adjust available cognitive resources to continuously changing external task demands. Without such control, barely any daily activity would be possible in our complex world (Koechlin, Ody, & Kouneiher, 2003). Previous research has shown that aging negatively influences the ability to recruit cognitive control. These deficits are especially obvious when high levels of cognitive control are required, for example, when a prepotent response needs to be suppressed, such as pressing the right button in response to an arrow pointing to the left (e.g., Nessler, Friedman, Johnson, & Bersick, 2007). Such incongruent-response targets cause interference between conflicting response alternatives (see also Mayr, 2001), to which older adults appear particularly susceptible (e.g., Milham et al., 2002;West, 2004; see also Friedman, Nessler, Cycowicz, & Horton, 2009; Nessler et al., 2007). Consequently, the goal of the present study was to investigate age-related changes in the use of cognitive control processes.
Several models have been advanced to describe how increased response conflict is managed by the cognitive system. The conflict-monitoring theory (cf. Botvinick, Braver, Barch, Carter, & Cohen, 2001; Botvinick, Cohen, & Carter, 2004) proposes that at least two processes underlie accurate performance under conditions of heightened response conflict: (a) conflict detection and (b) the upregulation of cognitive control. According to this model, conflict between competing response alternatives is continuously monitored and detected by the anterior cingulate cortex (ACC). The model further proposes that conflict detection in the ACC serves as a trigger to signal the need for an adjustment of cognitive control in the dorsolateral prefrontal cortex (DLPFC) to reduce conflict on subsequent trials (see also Weissman, Warner, & Woldorff, 2004). After response conflict has been reduced, less cognitive control would presumably be required subsequently (Botvinick et al., 2004).
However, the recruitment of cognitive control may not always depend on the detection of response conflict at the time the response is generated, but can sometimes be anticipated. For example, when task preparation is encouraged by the presentation of a valid task cue, anticipatory cognitive resources might be mustered prior to target presentation and, thus, response conflict could be reduced prior to generating the response. According to the dual mechanisms of control (DMC) framework (Braver, Gray, & Burgess, 2007), this so-called proactive control mechanism is very effective (i.e., high accuracy) and allows for rapid, efficient responding. Yet, it can only be used when it is possible to anticipate upcoming task demands and requires that high levels of control be sustained for extended periods of time, for example, over several trials.
Nonetheless, on other occasions, participants might not be able to anticipate, for example, incongruent-response targets. In these instances, task demands will be higher than anticipated and, therefore, response conflict will occur in close proximity to the target. In this case, the detection of conflict at the time of target presentation signals the need for upregulating reactive control processes (Braver & West, 2008), which are time consuming and result in prolonged reaction times (RTs) relative to trials on which proactive control is recruited successfully. In other words, reactive control can be thought of as effective in the sense that it will serve the purpose of selecting the correct response. At the same time, reactive control may not be very efficient compared to proactive control, because the correct decision will be made at the expense of longer RTs, thereby leading to heightened behavioral costs. Moreover, if control processes have to be recruited after target presentation, that is, immediately prior to the response, the urge to respond fast might elicit an incorrect response more often than in situations in which proactive control had been implemented successfully. Thus, proactive control should be associated not only with faster RTs (i.e., more efficient), but also with higher accuracy (i.e., more effective).
Aside from explaining variability in cognitive control processes within each participant, the distinction between proactive and reactive control might also be useful in elucidating age-related changes in cognitive control. Typically, performance decrements are observed in older, relative to younger, adults, especially under demanding task conditions (e.g. Nessler et al., 2007). As a result of their limited processing resources (cf. Park, 2000; Reuter-Lorenz & Lustig, 2005), the effective engagement of proactive control may be problematic for older adults (see also Braver et al., 2001). Thus, the DMC framework proposes that older adults might rely predominantly on reactive control mechanisms, which do not require one to sustain control over extensive time periods (Braver & West, 2008; see also Rabbitt, 1979; Velanova, Lustig, Jacoby, & Buckner, 2007).
In addition, increased trial-by-trial RT fluctuations are one of the hallmarks of advanced age (Hultsch, Hunter, MacDonald, & Strauss, 2005). This increased variability could be a direct consequence of older adults’ difficulties in reliably recruiting proactive control, resulting in a large number of trials in which reactive control is needed, thereby resulting in particularly long RTs. Hence, as a consequence of an age-related deficit in proactive control, we expected that older adults would increasingly recruit reactive control, particularly at higher levels of difficulty.
One way to verify (1) the presence of proactive and reactive control and (2) their differential usage in young and older adults might be to compare fast and slow RTs for congruent and incongruent response targets. Efficient performance, as evident in fast RTs and highly accurate performance, should reflect successful recruitment of proactive control. By contrast, slow trials predominantly reflect “last moment” recruitment of reactive control, and are expected to be associated with decreased accuracy compared to fast trials (cf. Braver et al., 2007). In addition, incongruent-response targets, which cannot be anticipated even though a valid cue has been presented, should increase the need for reactive control, especially in older adults. However, problematic is the fact that incongruent, relative to congruent-response, trials can also be viewed as more difficult. Higher levels of difficulty, in turn, are associated with longer RTs, regardless of the control mechanisms utilized. Consequently, to avoid a confound between the effects of congruence (i.e., interference) and task difficulty, the current study compared interference effects between slow and fast RT distributions at low, medium, and high levels of task difficulty.
As noted above, the DMC framework predicts increased reactive cognitive control processes before the response for older compared to young adults. Hence, in order to understand the temporal pattern of conflict detection and cognitive control, it is essential to employ a technique that can track cognitive processes in a manner consistent with the speed at which they unfold. Because of their high temporal resolution, event-related potentials (ERPs) are particularly well suited to examine these pre-response processes and were, consequently, employed in the present study. In order to ensure accurate response organization and production, reactive control should precede the behavioral decision by a few hundred milliseconds. Previous studies have described a RT-locked negative-going activity around 100–300 ms prior to the response that is enhanced under conditions that require greater amounts of cognitive control. This component is prominent over medial frontal scalp locations (e.g., Friedman et al., 2009; Johnson, Barnhardt, & Zhu, 2004; Nessler et al., 2007) and will be referred to here as the pre-response negativity or PRN. Consistent with the DMC framework, the PRN should be pronounced for slow responses in a high-difficulty condition, reflecting a larger percentage of trials on which reactive control was presumably recruited. Because proactive, relative to reactive, control occurs over longer and perhaps more variable periods of time, a direct ERP measure was not available. Hence, the use of proactive control was inferred based on fast RTs in each condition.
The efficiency of proactive and reactive cognitive control can be measured by the amount of residual response conflict following the RT decision. Response conflict detection has been associated with the medial frontal negativity (MFN) (cf. Bartholow et al., 2005; Friedman et al., 2009; Johnson et al., 2004; Nessler et al., 2007), a response-locked component following a correct response within ~100 ms. Its amplitude is larger for high-compared to low-conflict conditions as defined by RTs and accuracy (cf. Johnson et al., 2004; Nessler et al., 2007). Hence, the MFN has been interpreted as an indicator of the amount of response conflict remaining after a correct response has been generated (Friedman et al., 2009). In addition, dipole localization studies suggest that the MFN originates in or near the ACC (Johnson et al., 2004), which plays a critical role in conflict detection (Botvinick et al., 2004). Because fast trials (in each condition) are expected to reflect predominantly successful recruitment of proactive control, response conflict (reflected by the MFN) should be smaller or perhaps absent for fast relative to slow trials.
Previous studies suggest that aging is associated with either intact (e.g., Nessler et al., 2007) or reduced (e.g., Eppinger, Kray, Mecklinger, & John, 2007; Kray, Eppinger,& Mecklinger, 2005) conflict-detection mechanisms, as reflected by MFN amplitude. For instance, Nessler and colleagues (2007) observed similar increases in MFN amplitude in older and young adults at intermediate levels of conflict, suggesting intact monitoring and detection of response-conflict in older adults. However, when response conflict was greatest (i.e., for post-error, incongruent responses) increased error rates and MFN amplitudes were observed in older relative to young adults, consistent with intact conflict detection, but inefficient upregulation of cognitive control (Nessler et al., 2007, see also Sharp, Scott, Mehta, & Wise, 2006). Hence, age differences in conflict detection and recruitment of cognitive control processes might be especially accentuated at high levels of response conflict (i.e., incongruent-response targets for high levels of task difficulty).
To summarize, the review presented above led us to expect the following general effects for both age groups: 1) more errors on slow relative to fast trials, reflecting the predominance of reactive-relative to proactive-control processes; 2) greater PRN activity for incongruent slow trials at high levels of difficulty, reflecting the recruitment of reactive control prior to RT; and 3) larger MFN amplitudes for slow relative to fast trials, reflecting greater residual response conflict. With respect to aging, we expected 1) that older adults would recruit reactive control to a greater extent than young adults, which would be evident in larger RT and/or accuracy interference costs (i.e., differences between incongruent and congruent-response targets) and larger PRN activity for incongruent relative to congruent trials; and 2) the exertion of relatively inefficient reactive-control processes in older adults would lead to larger residual response conflict. This would be manifested by greater magnitude MFN activity for older relative to younger adults under high but not low difficulty conditions.
Methods
Participants
Twenty young (16 females, mean age 23.3 years, range 20–27) and 20 older (13 females, mean age 71.3 years, range 60–83) adults participated. All participants were native English speakers, right-handed, and had normal or corrected-to-normal vision. Participants reported themselves to be in good physical and mental health and free from medications known to affect the central nervous system. The study was approved by the New York State Psychiatric Institute’s Institutional Review Board, and all participants signed informed consent and were paid for their participation.
Screening Procedures
Prior to electroencephalogram (EEG) recording, all participants were screened with neuropsychological tests. A summary of the demographic information and neuropsychological test results can be found in Table 1. The groups were well matched, and there were no reliable age-related differences for any of the measures. All participants were of at least average intelligence and achieved a score of 50 or better (out of 57) on the Modified Mini-Mental Status (mMMS) examination (Mayeux, Stern, Rosen, & Leventhal, 1981). All older participants also passed a complete medical and neurological examination administered by a board-certified neurologist, and were free from dementia and depression and not limited in the activities of daily living as assessed by a semi-structured interview, the SHORT-CARE (Gurland, Golden, Teresi, & Challop, 1984).
Table 1.
Demographic and Performance Measures (± D) for Young and Older Adults
| Young (n = 20)
|
Older (n = 20)
|
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 23.32 | 2.03 | 71.31 | 7.16 |
| Education (years) | 15.85 | 1.31 | 16.50 | 2.67 |
| Modified MMS | 54.80 | 2.59 | 55.30 | 1.22 |
| Digits forward | 7.45 | 1.36 | 7.60 | 1.10 |
| Digits backward | 5.65 | 1.31 | 6.15 | 1.46 |
| WAIS-III Verbal IQ | 130.75 | 14.53 | 130.08 | 16.82 |
| WAIS-III Performance IQ | 114.50 | 14.50 | 119.46 | 16.02 |
| Depression (SHORT-CARE) | 1.311 | 1.93 | ||
| Dementia (SHORT-CARE) | 0.08 | 0.28 | ||
Notes:
n=13. Modified MMS: modified Mini-Mental Status (Mayeux et al., 1981); maximum score 57. WAIS-III: Wechsler Intelligence Scale III. SHORT-CARE (Gurland et al., 1984); cutoff for depression=6, dementia=7. Excluding age, no reliable age differences were evident in any of these measures (all ps>.26).
Materials and Procedure
The explicit cue task-switching paradigm was based on the design introduced by Cepeda and colleagues (Cepeda, Kramer, & Gonzalez de Sather, 2001), with modifications for ERP recording. Participants were seated comfortably in a sound-damped and electrically shielded room facing a 17-in computer monitor about 100 cm from the screen. They held a small response box on their laps. All stimuli were presented within a grey box (475 × 475 pixels). The rest of the screen was blank.
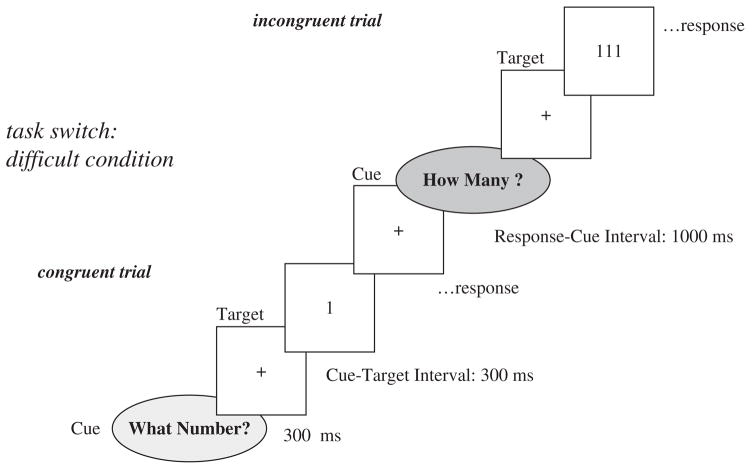
Participants responded to one of four stimulus displays (1, 3, 111, 333), based on one of two tasks: which digit or how many digits were presented. While the stimuli 1 and 333 required the same response regardless of task (congruent-response targets), displays of 3 and 111 required opposite responses for the two tasks (incongruent-response targets). One of two cues, signaling the task to be performed, appeared for 300 ms, followed by a fixation cross for 300ms, resulting in a Cue-Target Interval (CTI) of 600 ms.1 The target stimulus was displayed until a response was made via button press with the left or right index finger. The next cue was presented after a constant 1000 ms response-cue interval, during which the fixation cross appeared. In order to increase the discriminability of the two cues, one of them appeared on a blue, the other on a yellow, background, as shown in Figure 1 in dark and light grey, respectively. All four stimulus displays (i.e., congruent and incongruent) were presented in homogeneous and mixed blocks. In homogeneous blocks, only one task (Which digit? How many?) was performed in each block, preceded by a cue as detailed above. Hence, difficulty was at its lowest level. The task sequence for mixed blocks is illustrated in Figure 1. Again, the task cue indicated which of the two tasks was to be performed next. On some trials (no-switch or stay trials), the cue indicated the same task as in the previous trial, representing a medium level of difficulty, as switching between tasks was not required on these particular trials. After 0, 1, or 2 of these no-switch trials, the cue indicated that a switch to the other task was to be performed, thus representing the highest level of difficulty. Switch and no-switch trials occurred with equal probability during mixed blocks. Each participant first completed one homogeneous block of each task with 36 trials, followed by three mixed blocks with 84 trials each, followed by two homogeneous blocks, with 36 trials each.
Figure 1.
Schematic of the cued task-switching paradigm used to examine the effects of response conflict at low, medium, and high levels of difficulty. The task-switch reflects a high-difficulty trial.
Participants were instructed to respond emphasizing speed and accuracy equally. The assignment of task, cue color, and hands corresponding to a “one” or “three” button press was counterbalanced across participants. Half of the participants responded on the basis of how many digits were presented in the first block, while the remaining participants responded based on which number was presented. Prior to the actual experiment, two homogeneous blocks and one mixed block were included in a practice phase to ensure that participants understood the instructions and were performing adequately.
EEG Recording
EEG activity was recorded from 62 scalp sites, placed according to the extended 10–20 system, with sintered Ag/AgCl electrodes and a ground on the right forehead. Vertical and horizontal electrooculogram (EOG) were recorded from electrodes placed, respectively, above and below the left eye and at the outer can thus of each eye. Electrode impedance was kept below 5 kΩ. The activity of all scalp electrodes was initially referenced to the nose tip and re-referenced offline to averaged mastoids. EEG and EOG were recorded continuously with Synamp amplifiers (DC; 100 Hz high-frequency cutoff; 500 Hz digitization rate). Prior to averaging, trials with visible artifacts (e.g., muscular activity) were rejected and eye movements were corrected (Gratton, Coles, & Donchin, 1983). If single channels showed artifacts, a spherical spline algorithm (Perrin, Pernier, Bertrand, & Echallier, 1989) was used for interpolation on a trial-by-trial basis, with a maximum of four channels interpolated for a given trial.
Data Analysis
The analysis focused on 6 trial types: congruent and incongruent targets for low (i.e., trials in homogeneous blocks), medium (i.e., no-switch trials in mixed blocks) and high levels of difficulty (i.e., switch trials). In a first step, mean RTs and standard deviations (SD) were computed for every participant, and all trials exceeding 2.5 SDs from the individual mean for each of the 6 trial types were excluded from further analysis (young: 2.9% of all trials, older adults: 2.8%). This procedure was used to eliminate exceedingly long RTs, because a response deadline was not imposed. In a second step, trials with RTs longer than the median for each condition were averaged separately from those shorter than the median, resulting in a total of 12 conditions per subject (i.e., the 6 basic trial types above for slow vs. fast RT Bins). An α-level of 0.05 was chosen for all analyses.
Behavioral data
For RTs and percentage of errors, in a first step, interference costs were computed by subtracting the data on congruent trials from those on incongruent trials. Then, these difference scores were further analyzed in between-group ANOVAs with the factors of Age Group (young, older adults),RTB in (slow, fast), and Difficulty (low, medium, high). To parse interactions, planned contrasts were employed to compare low vs. medium and medium vs. high levels of difficulty.
ERP data
Because of our interest in cognitive-control processes leading up to the behavioral decision (reflected in the PRN) and the amount of response conflict remaining after the presumed exertion of control (MFN), RT-locked averages were computed for correct trials only. A 15-Hz low-pass filter was applied to all averages prior to the statistical analyses and is reflected in the figures. For measurement of the PRN and MFN, an EEG epoch extending from 400 ms prior to 300 ms following the response was used. However, to enable the placement of a baseline well before the RT response (e.g., Nessler et al., 2007; Friedman et al., 2009; Johnson et al., 2004; Fiehler, Ullsperger, & von Cramon, 2005), the actual EEG epoch began 1000 ms prior to the response. Using a fixed time period relative to the response as a baseline is problematic because of potential differences in RT between conditions and age groups (e.g., a given time period might co-occur with cue presentation in the high-difficulty condition for older adults, but not for the young who responded ~120–280 ms faster). Therefore, based on the mean RTs for each condition in each group, the 50–100 ms following target onset was chosen as a baseline for the response-locked data. Thus, by keeping the baseline similar with respect to the RT, we ensured that the influence of the stimulus was similar for all conditions and the two groups. During homogeneous blocks, averages were constructed for each participant for congruent (young fast: range=15–37 trials, old fast: 15–37, young slow: 15–35, old slow: 15–35) and incongruent (young fast: 29–36, old fast: 13–36, young slow: 26–35, old slow: 13–35) trials. During mixed-task blocks, averages were constructed for congruent no-switch (young fast: 26–36, old fast: 10–35, young slow: 24–36, old slow 10–34), incongruent no-switch (young fast: 22–32, old fast: 14–33, young slow: 21–31, old slow: 14–32), congruent switch (young fast: 26–33, old fast: 13–32, young slow: 25–33, old slow: 12–31) and incongruent switch (young fast: 20–31, old fast: 13–32, young slow: 20–31, old slow: 12–31) trials.
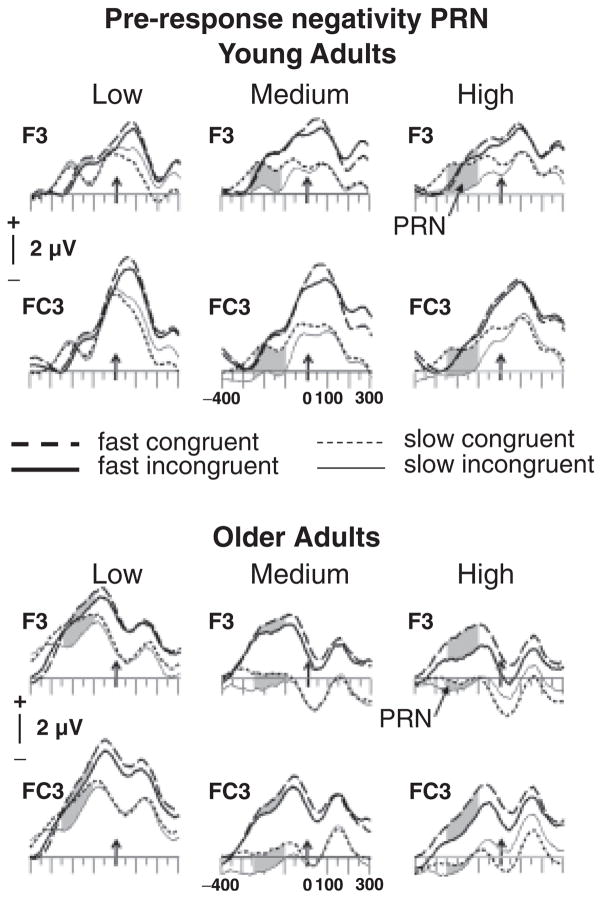
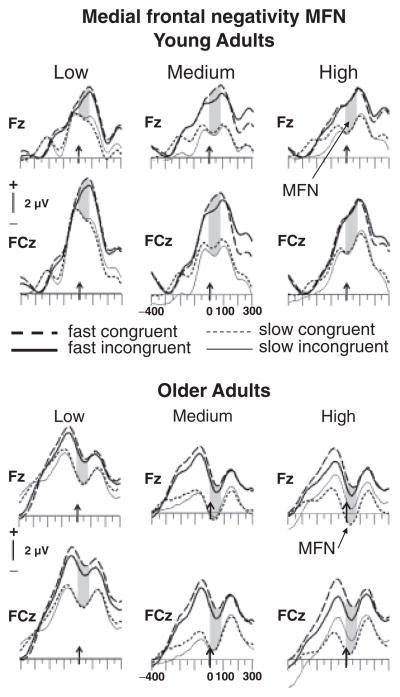
The PRN was measured as an averaged voltage based on the grand-mean ERP data between 250 and 150 ms prior to the response at two left frontal electrode sites, F3 and FC3, where it was maximal (see Figure 2). In accord with previous investigations (Bartholow et al., 2005; Friedman et al., 2009; Gehring & Willoughby, 2002; Johnson et al., 2004; Nessler et al., 2007), and based on the grand-mean ERP data (Figure 4), MFN amplitude was measured as the averaged voltage from 0–80 ms following response onset at two mid-frontal electrode sites, FZ and FCZ, where it was largest (Figure 4). PRN and MFN were evaluated in mixed-model ANOVAs with the factors Age Group, RT Bin (fast, slow), Congruence (congruent, incongruent), Difficulty (low, medium, difficult) and Electrode Site (F3, FC3 for PRN and FZ, FCZ for MFN), and followed up with subsidiary ANOVAs where necessary. Greenhouse-Geisser corrections for the violation of sphericity were used where appropriate, and are reflected in the p-values, which are reported along with uncorrected degrees of freedom and the respective epsilon values (ε). Results are reported only for main effects and interactions involving the between-group factor of Age Group and the within-subjects factors of Difficulty, Congruence, and RT Bin.
Figure 2.
Grand mean response-locked ERPs for young and older adults at left frontal electrode sites for low, medium, and high levels of difficulty. Small arrows mark response onset, with time lines every 50 ms. The time window used for the analyses (250–150 ms pre-RT) is shaded.
Figure 4.
Grand mean response-locked ERPs for young and older adults at mid-frontal electrode sites for easy, medium, and high levels of difficulty. The time window used for the analyses (0–80 ms post RT) is shaded.
Results
Behavioral Data
Error rates
Error rates for congruent and incongruent trials are displayed in Table 2. To assess the effect of interference, error rates for congruent trials were subtracted from those for incongruent trials. A between-group ANOVA with the factors Age Group, RT Bin, and Difficulty confirmed that interference was lower for fast (4.5%) relative to slow responses (6.1%) [F(1,38)=7.84, p<.01]. Interference errors occurred more frequently for young (6.9%) than older adults (3.7%), as indicated by a main effect of Age Group [F(1,38)=6.93, p<.05]. Finally, interference increased from low (2.6%) to medium (4.5%) to high levels of difficulty (8.8%), [F(2,76)=33.20, p<.0001, ε=.83]. The Difficulty main effect was modulated by an interaction with Age Group [F(2,76)=4.32, p<.05, ε=.83]. For the young, planned contrasts revealed that error rates increased from low to medium levels of difficulty (3.1% vs 6.1%) [F(1,19)=10.27, p<.01] and from medium to high difficulty (6.1% vs 11.6%) [F(1,19)=19.80, p<.0001]. For older adults, planned contrasts revealed that error rates did not differ between low (2.2%) and medium (2.9%) levels of difficulty [F<1], but increased from medium to high levels of difficulty (2.9% vs. 6.1%) [F(1,19)=8.59, p<.01]. To summarize, consistent with expectations, error rates were higher for slow relative to fast trials for both groups. Young adults committed more errors than older adults.
Table 2.
Mean Percentage of Errors (± SE) For the Two Age Groups as a Function of RT Bin, Congruence, and Difficulty
| RT bin | Group | Congruence | Difficulty Level
|
||
|---|---|---|---|---|---|
| Low | Medium | High | |||
| Fast | Young | Congruent | 0.88 (0.31) | 0.82 (0.33) | 0.49 (0.27) |
| Incongruent | 3.24 (0.88) | 6.39 (1.26) | 12.05 (1.78) | ||
| Older | Congruent | 0.0 (0.00) | 0.33 (0.23) | 0.37 (0.25) | |
| Incongruent | 2.96 (1.37) | 2.16 (0.61) | 6.11 (1.31) | ||
| Slow | Young | Congruent | 1.55 (0.43) | 1.08 (0.40) | 1.55 (0.36) |
| Incongruent | 5.13 (0.88) | 7.45 (1.31) | 13.30 (1.65) | ||
| Older | Congruent | 0.56 (0.25) | 0.33 (0.23) | 0.52(0.28) | |
| Incongruent | 4.5 (1.33) | 4.27 (0.72) | 8.11 (1.55) | ||
Reaction Times
As shown in Table 3, young adults (411 ms) responded faster than older adults (606 ms) [F(1,38)=39.09, p<.0001]. As expected, the standard deviation (young: 106 ms, old: 201 ms) and the range (young: 558 ms, 270–828 ms, old: 1126 ms, 363–1488 ms) were both considerably larger for older relative to young adults. Although the distribution of mean RTs for all conditions was skewed to the right for both age groups, skewness was larger for older relative to young adults (1.48 vs. 2.03). This latter finding is consistent with the increased prevalence of reactive control in association with particularly slow responses in older adults. In order to account for these age-related differences in the RT distribution and for general age-related slowing, the logarithm of RT was used for all further analyses (Keppel & Wickens, 2004). The untransformed data are displayed in Table 3. RT interference costs were quantified by subtracting the log-transformed RTs to congruent from those to incongruent trials in each difficulty condition. In both groups, RT interference costs were reliably different from 0 in all three difficulty conditions (all ps<.02). An ANOVA with the between-subject factor Age Group and the within-subjects factors of RT Bin and Difficulty revealed that interference costs were larger for older (.05) than young adults (.03) [F(1,38)=5.78, p<.05], suggesting age-related deficits in counteracting response conflict. Interference costs increased with difficulty (low, .02; medium, .04; high, .06) [F(2,76)=16.06, p<.0001, ε=.94] and were larger for slow (.05) relative to fast trials (.03) [F(1,38)=49.72, p<.0001]. The relative increase in RT interference costs differed as a function of Difficulty and RT bin, as indicated by interactions between Difficulty and RT Bin [F(2,76)=5.72, p<.01, ε=.98] and a trend for a triple interaction with Age Group [F(2,76)=2.69, p=.07, ε=.98].
Table 3.
Mean RTs in ms (± SE) for the Two Age Groups as a Function of RT Bin, Congruence, and Difficulty
| RT bin | Group | Congruence | Difficulty Level
|
||
|---|---|---|---|---|---|
| Low | Medium | High | |||
| Fast | Young | Congruent | 315 (6) | 339 (8) | 344 (8) |
| Incongruent | 322 (7) | 352 (10) | 367 (9) | ||
| Older | Congruent | 439 (10) | 489 (20) | 492 (20) | |
| Incongruent | 466 (15) | 529 (27) | 557 (26) | ||
| Slow | Young | Congruent | 408 (10) | 472 (19) | 477 (18) |
| Incongruent | 423 (12) | 537 (30) | 575 (28) | ||
| Older | Congruent | 594 (21) | 713 (49) | 705 (50) | |
| Incongruent | 665 (31) | 806 (54) | 854 (59) | ||
Based on our predictions, separate analyses were performed for young and older adults to parse these interactions. An ANOVA on the young-adult RT interference costs confirmed main effects of Difficulty [F(2,38)=14.85, p<.0001, ε=.93], RT Bin [F(1, 19)=37.46, p<.0001], and an interaction of both factors [F(2,38)=10.18, p<.0001, ε=.95]. The interaction reflected the fact that interference costs were higher for slow relative to fast responses in the medium (.05 vs. .01, [t(19)=3.90, p<.01]) and high conditions (.08 vs. .03, [t(19)=5.92, p<.0001]), but not in the low-difficulty condition (.02 vs. .01, [t(19)=1.24, p>.10]).
For older adults, there were main effects of Difficulty [F(2,38)=5.14, p<.05, ε=.94] and RT Bin [F(1,19)=16.78, p<.01, but no interaction [F<1]. This pattern indicates that interference costs were higher for slow relative to fast responses at all difficulty levels (low: .05 vs. .02 [t(19)=2.86, p<.01], medium: .05 vs. .03 [t(19)=2.35, p<.05], high: .08 vs. .05 [t(19)=2.79, p<.05].
In sum, older adults had larger RT interference costs. Additionally, and by contrast with young adults, the larger interference costs for slow compared to fast trials were also present at the low level of task difficulty.
ERP Data
Pre-response negativity (PRN)
As illustrated in Figures 2 and 3, incongruent relative to congruent trials were associated with more negative-going waveforms ~250 ms prior to response onset (i.e., larger PRNs).2
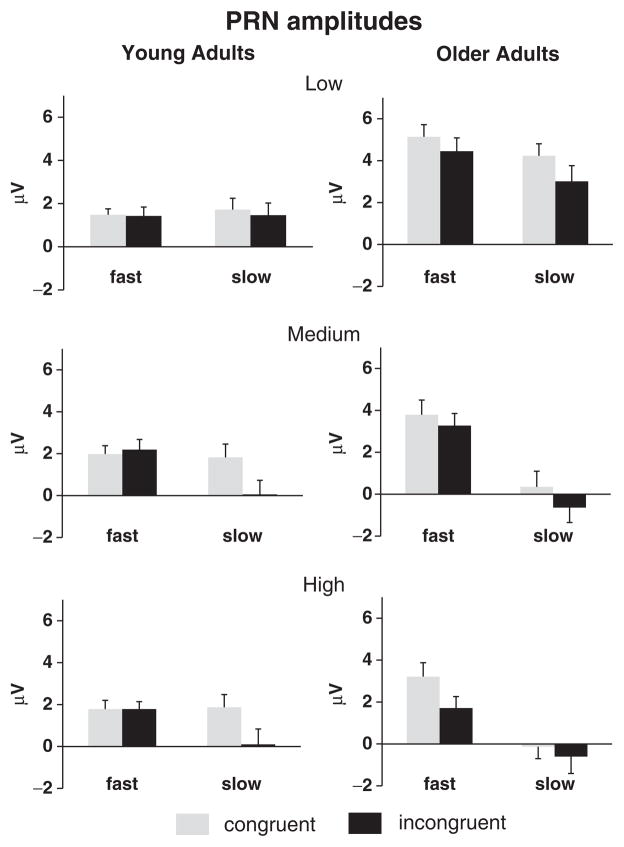
Figure 3.
Illustration of grand mean PRN amplitudes (± SE) as a function of Age Group, RT Bin, Difficulty Level, and Congruence, collapsed across electrode sites F3 and FC3.
The Age Group × Difficulty × RT Bin × Congruence × Electrode Site ANOVA revealed a four-way interaction of Group, Difficulty, RT Bin, and Congruence [F(2,76)=6.03, p<.01, ε=.92]. To parse this interaction, separate ANOVAs were performed for low, medium, and high levels of difficulty. At the low-difficulty level, incongruent trials (2.6 μV) were more negative-going than congruent trials (3.1 μV) [F(1,38)=7.53, p<.01]. Greater PRNs occurred for slow (2.5 μV) relative to fast trials (3.2 μV) [F(1,38)=5.41, p<.05].Young (1.5 μV) relative to older (4.2 μV) adults produced larger PRN activity [F(1,38)=15.14, p<.0001]. Nonetheless, as depicted in Figures 2 and 3, incongruent (3.7 μV) relative to congruent (4.7 μV) trials led to greater PRN activity for older adults only [Age Group × Congruence interaction, F(1,38)=3.85, p=.057]. Similarly, and again for older adults only, slow (3.6 μV) relative to fast (4.8 μV) trials engendered greater PRN activity [Age Group × RT Bin interaction, F(1,38)=3.84, p=.057]. Although both effects just missed the conventional level of significance, these results suggest that older adults may have employed reactive control for slow relative to fast and incongruent relative to congruent trials even in the low-difficulty condition.
At the medium level of difficulty, the PRN was larger for incongruent (1.2 μV) than congruent trials (2.0 μV) [F(1,38)=8.81, p<.01], and slow (0.4 μV) relative to fast (2.8 μV) trials [F(1,38)=45.91, p<.0001]. As illustrated in Figure 3, an interaction of RT Bin and Age Group [F(1,38)=12.63, p<.0001] indicated that the difference between slow and fast trials was more pronounced for older (3.5 μV) than young adults (1.2 μV), suggesting that reactive control was recruited more often by older adults on slow trials. For both age groups, the difference between slow and fast trials was also larger for incongruent (3 μV) than congruent trials (1.8 μV), as indicated by an interaction of RT Bin and Congruence [F(1,38)=6.66, p<.05]. The lack of an interaction between Age Group, RT Bin, and Congruence [F(1, 38)=2.54, p>.1] suggests that reactive control was recruited by both young and older adults, particularly for slow incongruent trials.
In the high-difficulty condition, incongruent (0.7 μV) relative to congruent (1.7 μV) trials were also associated with greater PRN amplitudes [F(1,38)=10.85, p<.01]. Again, as shown in Figures 2 and 3, larger PRNs were observed for slow (0.3 μV) relative to fast trials (2.1 μV) [F(1,38)=24.99, p<.0001]. However, these main effects were modulated by Age Group × RT Bin [F(1,38)=7.84, p<.01] and Age Group × RT Bin × Congruence [F(1,38)=8.75, p<.01] interactions. To parse these interactions, separate analyses were performed for each age group. For young adults, as confirmed by the interaction of RT Bin and Congruence [F(1,19)=8.89, p<. 01], more negative-going PRNs were observed only for slow incongruent trials relative to all other conditions, which did not differ (Figure 3, bottom left). This result is consistent with the selective recruitment of reactive control only on slow incongruent trials. By contrast, for older adults (Figure 3, bottom right), the PRN was larger for slow than fast trials [F(1,19)=53.59, p<.0001] and incongruent than congruent trials [F(1,19)=5.01, p<.05], with no reliable interaction of RT Bin and Congruence (p>.17). This finding presumably reflects the increased recruitment of reactive control for incongruent-response targets on both fast and slow trials.
To summarize, larger PRNs for slow relative to fast trials and for incongruent relative to congruent trials were observed for all conditions in older adults, but only for slow trials at medium and high levels of difficulty for the young. These results suggest increased recruitment of reactive control processes prior to the response in older adults for both slow and fast RTs even in the low-difficulty condition.
Medial-frontal negativity (MFN)
The Age Group × Difficulty × RT Bin × Congruence × Electrode Site ANOVA revealed main effects of Age Group [F(1,38)=4.72, p<.05], Difficulty [F(1,38)=42.92, p<.0001] and RT Bin [F(1,38)=54.23, p<.0001], as well as interactions of RT Bin and Congruence [F(1,38)=6.84, p<.05] and Age Group, Difficulty, and Electrode [F(1,38)=12.05, p<.0001]. To examine the source of these interactions, separate ANOVAs were performed for the low-, medium-, and high-difficulty conditions.
In the low-difficulty condition, slow (5.2 μV) relative to fast trials (8.2 μV) were associated with more negative-going MFN activity [F(1,38)=50.77, p<.0001]. Older (6.0 μV) and young adults (7.4 μV) did not differ reliably [F<1], and there were no reliable interactions with Age Group, consistent with the detection of similar amounts of response conflict in young and older adults. No reliable effects of Congruence were found [Fs<1], as illustrated in Figures 4 and 5.
Figure 5.
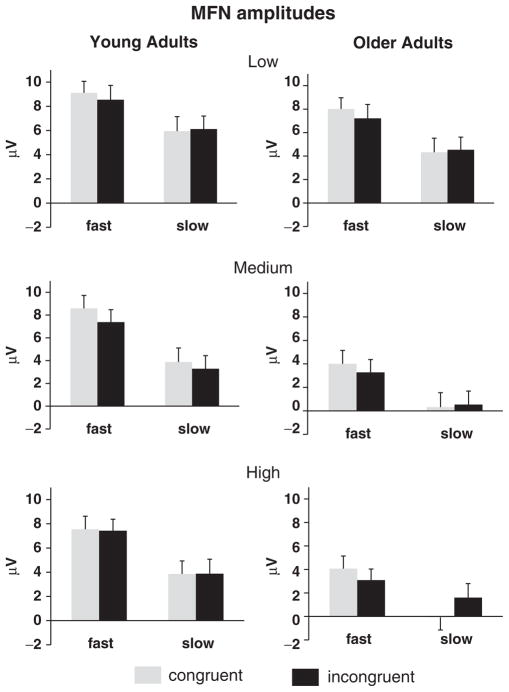
Grand mean MFN amplitudes (± SE) as a function of Age Group, RT Bin, Difficulty Level, and Congruence, collapsed across electrode sites FZ and FCZ.
At the medium level of difficulty, the MFN was more negative-going for older (2.2 μV) than younger adults (5.8 μV) [F(1,38)=6.81, p<.05], suggesting a greater amount of response conflict in older adults. MFN amplitudes for slow responses in young adults (3.88 μV) resembled those for fast responses in older adults (4.01 μV), suggesting that older adults experienced a similar level of residual response conflict for fast responses as did young adults for slow responses. As illustrated in Figure 5, slow (2.0 μV) relative to fast trials (5.8 μV) were associated with greater MFN activity [F(1,38)=42.53, p<.0001], an effect that was of similar size in both age groups [Age Group × RT Bin interaction F(1,38)=1.02, p>.32]. Again, Congruence did not influence MFN magnitude (p>.16).
In the high-difficulty condition, MFN amplitude was larger for older (2.2 μV) than young adults (5.7 μV) [F(1,38)=7.61, p<.01], and slow (2.3 μV) relative to fast trials (5.5 μV) [F(1,38)=28.08, p<.0001]. As for the low- and medium-difficulty levels, neither the main effect of Congruence nor interactions with Age Group were significant [Fs<1].
To summarize, MFN amplitudes were larger for slow than fast trials for both groups whereas, unlike the PRN, Congruence did not seem to influence MFN amplitude. At the low level of difficulty, age-invariant MFN amplitudes were observed. However, at medium and high levels of difficulty, older relative to young adults showed larger MFNs, suggesting that older adults experienced greater amounts of response conflict as difficulty increased.
Discussion
The present study provides evidence for age-related differences in the use of proactive and reactive control as proposed by the DMC framework (Braver et al., 2007). The DMC predicts the recruitment of reactive control preceding the response when proactive control is insufficient, for instance, in response to interference and high levels of difficulty. As will be discussed in detail below, the current behavioral and response-locked ERP results provide empirical evidence for these processes and, moreover, support the increased use of reactive control in older compared to young adults.
The proposed distinction between proactive control processes, supporting fast and accurate responses, and reactive control processes, supporting slower and less accurate responses, was confirmed by the behavioral results. For each age group, large RT differences (~ 100–300 ms) were observed for fast compared to slow trials (see also De Jong, Berendsen, & Cools, 1999), in accord with the prediction of distinct proactive and reactive control processes for each age group. Based on the DMC, it was expected that the recruitment of last-minute reactive control should be larger for slow than fast trials and associated with decreased accuracy. In fact, for both age groups, slow trials were associated with more interference errors than fast trials. The interpretation suggesting the prevalence of proactive control on fast trials and reactive control on slow trials is further supported by the fact that these accuracy results are opposite those predicted by a speed-accuracy tradeoff. That is, if similar types of cognitive control had been recruited for fast and slow trials, then an RT increase should have led to an increase in accuracy. Hence, the data are in accord with the DMC prediction that reactive control is recruited in instances in which proactive control cannot be employed successfully (i.e., on slow trials).
Proactive control processes rely on intact frontal lobe functioning and active maintenance of preparatory and attentional processes over long periods of time, processes that appear deficient in older adults (Braver & West, 2008). Consequently, older adults should be less able to use proactive control than young adults, which should be reflected by age-related increases in RT and decreases in accuracy. However, in the present study, although older adults responded more slowly, they made fewer errors than young adults (cf. Table 2). Previous investigations have indicated that older adults adopt more conservative response criteria and emphasize accuracy over speed (cf. Rabbitt, 1979). Because a response deadline was not imposed in the current study, the age-related strategy of emphasizing accuracy over speed could have counteracted the expected decrease in accuracy.
Aside from the accuracy results, the RT-interference findings also provide evidence for an age-related increase in reactive control. For young adults, RT interference costs were small, particularly for fast responses, consistent with effective proactive control processes. For the low-difficulty condition, young adults apparently employed proactive control processes, even when responding to incongruent-response targets. However, consistent with DMC predictions, the RT results suggest that recruitment of reactive control by young adults increased with heightened difficulty. By contrast, older, relative to young, adults showed large RT interference costs at all levels of difficulty, consistent with an increased reliance on reactive control even at low levels of difficulty. Hence, the behavioral results are in line with the idea that older adults rely more on reactive control than younger adults. The recently proposed load-shift model (Velanova et al., 2007) provides similar predictions as the DMC with respect to aging (Braver et al., 2007). The load-shift model suggests that older adults fail to filter out irrelevant information early in the information- processing sequence. Thus, to enable older adults to respond accurately, additional cognitive resources (i.e., reactive control) need to be recruited at later processing stages relative to the young (Velanova et al., 2007).
The increased use of reactive control as age and difficulty increased was also supported by the ERP results. For young adults, PRN amplitudes were more negative for slow compared to fast trials at medium and high levels of difficulty, but not at the low-difficulty level, consistent with selective recruitment of reactive control processes under demanding task conditions (see also van Veen, Krug, & Carter, 2008). In fact, the PRN results parallel the RT interference costs, supporting the idea that the PRN is an ERP correlate of reactive control.3 Moreover, the left-frontal maximum of the PRN observed in the present study corresponds well with recent hemodynamic evidence. Left ventrolateral PFC activation in association with RT slowing on posterror trials was observed during a stop-signal task in young adults (Li et al., 2008). Although the neural sources of ERP components are difficult to infer based solely on the observed scalp distribution, the left-frontal maximum of the PRN is consistent with the left-ventrolateral PFC activation observed in the Li et al. (2008) investigation, and may reflect the computations of a neural network implementing reactive control.
With respect to aging, PRNs in older adults were larger for slow relative to fast trials and incongruent relative to congruent trials at all three levels of difficulty, compatible with a greater reliance on reactive control in older compared to young adults. In accord with the RT results, older adults recruited reactive control processes for slow responses even at the lowest level of difficulty. Importantly, the time frame for the start of the PRN interval of ~250 ms prior to the response corresponds roughly to the greater RT slowing observed for older relative to younger adults (e.g., older adults were 278 ms slower than younger adults on slow incongruent trials in the difficult condition). While young adults appeared to react more flexibly at the different levels of demand by recruiting both proactive- and reactive-control processes, it is conceivable that reduced frontal resources in older adults (e.g., Reuter-Lorenz & Lustig, 2005) prevented or reduced the recruitment of proactive control processes (Braver & West, 2008). Consistent with this pattern of results, Paxton and colleagues (2008) recently reported dissociations between young and older adults in lateral PFC activations during cue vs. probe presentation in a task requiring the maintenance of goal-relevant information in the interval between these two events. While lateral PFC activation was reduced in older relative to young adults during the cue-probe interval, activation in these regions was enhanced in older adults during probe presentation. This result is consistent with reduced proactive control during task preparation and increased reactive control in close proximity to probe onset (Paxton, Barch, Racine, & Braver, 2008, see also Sharp et al., 2006 for related findings using PET).
Given that the recruitment of reactive control processes occurs in close temporal proximity to the decision, slow, relative to fast, trials should be associated with residual response conflict immediately after the response (MFN activity), as was demonstrated here for both age groups. Interestingly, MFN amplitudes were not modulated by congruence in either group, suggesting that response conflict was successfully reduced by putative reactive- control processes captured by the PRN onsetting ~250 ms earlier. Furthermore, as predicted, age-invariant MFN amplitudes were observed at the low level of difficulty, whereas for medium and high levels of difficulty, older compared to young adults showed larger MFNs, suggesting that older adults experienced more residual response conflict in the latter conditions. To illustrate this point, at medium and high levels of difficulty, similar RTs for fast trials in older adults and slow trials in young adults were found, and associated with comparable levels of response conflict as indexed by MFN amplitudes. This pattern of results may indicate that older adults experienced higher overall levels of response conflict for medium and high levels of difficulty than younger adults, perhaps due to a combination of poorer task preparation and a particular emphasis on accuracy over speed.
Previous results have been mixed with respect to whether or not conflict detection, as indexed by the MFN, is impaired in older adults (e.g., Eppinger et al., 2007; Kray et al., 2005; West, 2004). Nonetheless, our results suggest that older adults are able to detect response conflict (e.g., Nessler et al., 2007, Friedman et al., 2009). One critical difference between these studies and the current investigation might be the placement of the baseline in the response-locked ERPs. For example, while the present study and Nessler et al. (2007) used a baseline well before the response (between −300 to −200 ms prior to the response), Eppinger et al. (2007), Kray et al. (2005), and West (2004) placed their baseline immediately before the response (e.g., Eppinger et al., 2007 used a baseline between −200 ms and response onset). Hence, using a baseline close to the response in these previous studies may have led to inflated age-related differences in MFN magnitudes following the response.
In the current paradigm, older adults were free to take as much time to respond as needed. Consequently, older adults’ tendency to rely increasingly on reactive control was not detrimental to their overall performance. Some experimental paradigms introduce time constraints, precluding sufficient time to rely on reactive control. This is by no means a mere methodological detail, but could help reconcile discrepancies regarding particularly low or elevated errors rates in older adults (e.g., Eppinger et al., 2007; Mager et al., 2007; West, 2004; see also Rabbitt, 1979). Notably, for older adults’ everyday functioning, speed plays only a minor role in most circumstances (with the possible exception of driving a car in heavy traffic). Hence, laboratory tasks emphasizing speed over accuracy do not seem well suited to capture the full range of older adults’ cognitive capacities. In fact, very recent evidence indicates that at least some older adults are capable of changing from a reactive to a more proactive control mode after extensive training (Braver, Paxton, Locke, & Barch, 2009). Based on the DMC framework and older adults’ purported difficulty in employing proactive control, it could be expected that at least some older adults would be very reluctant to respond quickly (Nessler et al., 2007) and, if forced to do so, would most likely generate reduced accuracy rates.
To conclude, in agreement with the DMC, older adults appeared to rely predominantly on reactive control mechanisms just prior to the decision to respond, a finding consistent with a deficit in recruiting proactive control mechanisms. By contrast, young adults were able to recruit both proactive and reactive control processes, thereby flexibly adapting to the entire range of task demands. Age differences in conflict detection were not evident at the low level of task difficulty, whereas response conflict was higher for older adults at medium and high levels of difficulty. As a whole, these results suggest that aging is associated with a less efficient reactive-control mode of processing resulting in a strong emphasis on accuracy over speed.
Acknowledgments
The authors thank Mr. Charles L. Brown III for computer programming and technical assistance. We thank Ms. Rebecca Edelblum and Mr. Cort Horton for their aid in recruitment and data collection, and Dr. Y. M. Cycowicz for her contributions to early phases of this research. This project was supported in part by grants HD14959 (NICHD) and AG005213 (NIA), and the New York State Department of Mental Hygiene.
Footnotes
In a second phase of the experiment, data were also collected following a longer CTI (1200 ms). Because response conflict was maximal in the short CTI, only these data are reported here.
Note that the PRN and the MFN occur primarily above the baseline. Hence, modulations of the waveforms (e.g., by Congruence) are typically manifest as reductions in positivity (i.e., negative-going), as illustrated in Figures 2 and 4 and described below.
However, no reliable correlations were found between PRN amplitudes and RTs or PRN amplitude differences between incongruent and congruent trials with the associated RT costs.
References
- Bartholow BD, Pearson MA, Dickter CL, Sher KJ, Fabiani M, Gratton G. Strategic control and medial frontal negativity: Beyond errors and response conflict. Psychophysiology. 2005;42:33–42. doi: 10.1111/j.1469-8986.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Science. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, et al. Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. Journal of Experimental Psychology General. 2001;130:746–763. [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway A, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in working memory. New York: Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proceedings of the National Academy of Sciences U S A. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, West R. Working memory, executive control, and aging. In: Craik FI, Salthouse T, editors. The handbook of aging and cognition. 3. New York: Psychology Press; 2008. pp. 311–372. [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather JC. Changes in executive control across the life span: Examination of task-switching performance. Developmental Psychology. 2001;37:715–730. [PubMed] [Google Scholar]
- De Jong R, Berendsen E, Cools R. Goal neglect and inhibitory limitations: Dissociable causes of interference effects in conflict situations. Acta Psychologica. 1999;101:379–394. doi: 10.1016/s0001-6918(99)00012-8. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Kray J, Mecklinger A, John O. Age differences in task switching and response monitoring: Evidence from ERPs. Biological Psychology. 2007;75:52–67. doi: 10.1016/j.biopsycho.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Fiehler K, Ullsperger M, von Cramon DY. Electrophysiological correlates of error correction. Psychophysiology. 2005;42:72–82. doi: 10.1111/j.1469-8986.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- Friedman D, Nessler D, Cycowicz YM, Horton C. Development and change in cognitive control: A comparison of children, young and older adults. Cognitive Affective and Behavioral Neuroscience. 2009;9:91–102. doi: 10.3758/CABN.9.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gurland B, Golden RR, Teresi JA, Challop J. The SHORT-CARE: An efficient instrument for the assessment of depression, dementia and disability. Journal of Gerontoly. 1984;39:166– 169. doi: 10.1093/geronj/39.2.166. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hunter MA, MacDonald SWS, Strauss E. Inconsistency in response time as an indicator of cognitive aging. In: Duncan J, Phillips L, McLeod P, editors. Measuring the mind: Speed, control, and age. New York: Oxford University Press; 2005. pp. 33–58. [Google Scholar]
- Johnson R, Jr, Barnhardt J, Zhu J. The contribution of executive processes to deceptive responding. Neuropsychologia. 2004;42:878–901. doi: 10.1016/j.neuropsychologia.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and analysis: A researcher’s handbook. Upper Saddle River, NJ: Prentice-Hall; 2004. [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181– 1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Kray J, Eppinger B, Mecklinger A. Age differences in attentional control: An event-related potential approach. Psychophysiology. 2005;42:407–416. doi: 10.1111/j.1469-8986.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Paliwal P, Constable RT, Sinha R. Neural correlates of post-error slowing during a stop signal task: A functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2008;20:1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager R, Bullinger AH, Brand S, Schmidlin M, Scharli H, Muller-Spahn F, et al. Age-related changes in cognitive conflict processing: An event-related potential study. Neurobiology of Aging. 2007;28:1925–1935. doi: 10.1016/j.neurobiolaging.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Stern Y, Rosen J, Leventhal J. Depression, intellectual impairment, and Parkinson disease. Neurology. 1981;31:645– 650. doi: 10.1212/wnl.31.6.645. [DOI] [PubMed] [Google Scholar]
- Mayr U. Age differences in the selection of mental sets: The role of inhibition, stimulus ambiguity, and response-set overlap. Psychology and Aging. 2001;16:96–109. doi: 10.1037/0882-7974.16.1.96. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, et al. Attentional control in the aging brain: Insights froman fMRI study of the Stroop task. Brain and Cognition. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Nessler D, Friedman D, Johnson R, Jr, Bersick M. ERPs suggest that age affects cognitive control but not response conflict detection. Neurobiology of Aging. 2007;28:1769–1782. doi: 10.1016/j.neurobiolaging.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Park DC. The basic mechanisms accounting for age-related decline in cognitive function. In: Park DC, Schwarz N, editors. Cognitive aging: A primer. New York: Psychology Press; 2000. pp. 3–21. [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex. 2008;18:1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Rabbitt P. How old and young subjects monitor and control responses for accuracy and speed. British Journal of Psychology. 1979;70:305–311. [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Current Opinion in Neurobiology. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Scott SK, Mehta MA, Wise RJS. The neural correlates of declining performance with age: Evidence for age related changes in cognitive control. Cerebral Cortex. 2006;16:1739–1749. doi: 10.1093/cercor/bhj109. [DOI] [PubMed] [Google Scholar]
- van Veen V, Krug MK, Carter CS. The neural and computational basis of controlled speed-accuracy tradeoff during task performance. Journal of Cognitive Neuroscience. 2008;20:1952–1965. doi: 10.1162/jocn.2008.20146. [DOI] [PubMed] [Google Scholar]
- Velanova K, Lustig C, Jacoby LL, Buckner RL. Evidence for frontally mediated controlled processing differences in older adults. Cerebral Cortex. 2007;17:1033–1046. doi: 10.1093/cercor/bhl013. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Warner LM, Woldorff MG. The neural mechanisms for minimizing cross-modal distraction. The Journal of Neuroscience. 2004;24:10941–10949. doi: 10.1523/JNEUROSCI.3669-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R. The effects of aging on controlled attention and conflict processing in the Stroop task. Journal of Cognitive Neuroscience. 2004;16:103–113. doi: 10.1162/089892904322755593. [DOI] [PubMed] [Google Scholar]