Abstract
Toxoplasma gondii is a leading cause of neurological birth defects and a serious opportunistic pathogen. The authors and others have found that Toxoplasma uses a unique nucleosome composition supporting a fine gene regulation together with other factors. Post-translational modifications in histones facilitate the establishment of a global chromatin environment and orchestrate DNA-related biological processes. Histone acetylation is one of the most prominent post-translational modifications influencing gene expression. Histone acetyltransferases and histone deacetylases have been intensively studied as potential drug targets. In particular, histone deacetylase inhibitors have activity against apicomplexan parasites, underscoring their potential as a new class of antiparasitic compounds. In this review, we summarize what is known about Toxoplasma histone acetyltransferases and histone deacetylases, and discuss the inhibitors studied to date. Finally, the authors discuss the distinct possibility that the unique nucleosome composition of Toxoplasma, which harbors a nonconserved H2Bv variant histone, might be targeted in novel therapeutics directed against this parasite.
Keywords: acetylation, HATs, HDACi, HDACs, histone, therapy, Toxoplasma gondii
Apicomplexan parasites are important human and animal pathogens that cause diseases of far-reaching impact on global health [1]. In this varied phylum, there are important pathogens like Plasmodium, Toxoplasma, Cryptosporidium and Babesia genus, all of which have obligate intracellular lifestyles that involve multiple proliferative and latent stages that promote transmission. Toxoplasma is distinct from most members of the large coccidian family contained in the Apicomplexa phylum owing to the exceptional number of animals that are able to serve as hosts, including virtually all warm-blooded vertebrates. Toxoplasma gondii completes the definitive life cycle in a single animal host (feline); the capacity of oocysts (shed from the feline host) as well as tissue cysts to infect multiple intermediate hosts has enabled T. gondii to dramatically increase presence worldwide [1–4].
Toxoplasmosis is of medical health importance because T. gondii can cause opportunistic disease in immunocompromised individuals because of reactivation of latent infection. Toxoplasma can also cause spontaneous abortion or congenital birth defects in newborns if the mother contracts the parasite for the first time during pregnancy [5]. The active form of the parasite can cause encephalitis and neurologic diseases, but also can affect the heart, liver, inner ears and eyes (chorioretinitis). Recently, toxoplasmosis has been linked with brain cancer, attention-deficit hyperactivity disorder, obsessive compulsive disorder and schizophrenia [6–9]. Despite the existence of effective drug regimens, in some cases either the therapy is not well tolerated by the patient or drug-resistant parasites develop. In patients under immunosuppressive therapy and particularly in those with AIDS, the treatment with sulfonamides and dihydrofolate reductase inhibitors can cause side effects despite the preventive administration of folinic acid [10]. Moreover, failure in therapy against T. gondii has been observed after the treatment of toxoplasmic encephalitis, chorioretinitis and congenital toxoplasmosis [11–13]. In addition, most of the anti-T. gondii drugs do not kill the latent stage [14]. Therefore, intense research is currently focused on the development of new drugs against T. gondii (reviewed in [15]). In this regard, it is expected that parasite-specific proteins, which present important or essential roles in T. gondii pathogenesis, allow the generation of novel therapies that can overcome some of the mentioned situations.
Toxoplasma is able to differentiate from the rapidly replicating tachyzoite stage (active form) into a latent cyst form (brady-zoite stage). The tachyzoite–bradyzoite interconversion includes important morphological and physiological changes that require modulation of stage-specific gene expression [16]. Epigenetics, which includes post-translational modifications (PTMs) of histones as well as histone variant replacement [17], has arisen as a central player in the regulation of gene expression in apicomplexan parasites. The goal of this review is to summarize the knowledge about the modulation of histone acetylation and deacetylation in Toxoplasma, and how the inhibition of these processes alters parasite biology. Finally, the authors discuss the rationale of using drugs that target enzymes regulating these processes as a novel therapy against this important parasite.
Toxoplasma has a unique nucleosome composition
Cellular development, differentiation, growth and survival would not be possible without tight control of gene expression, replication and DNA repair, processes that are linked to nucleosome organization. Core histones are the building blocks of the nucleosome. They have been classified into two groups: canonical histones and histone variants [18]. In every protist parasite analyzed so far, the four core histones (H2A, H2B, H3 and H4) have been identified, but the linker histone H1 appears to be absent in some cases, including Apicomplexa [18]. Histone variants are paralogs of the canonical histones that assemble into nucleosomes with specialized functions [19,20]. H4 and H2B lineages are practically invariant, whereas H2A and H3 have extensively specialized variants (H2AZ, H2AX, macroH2A, H2ABbd, H3.3, cenH3) for many roles in transcription, DNA organization and repair [21]. To date, macroH2A and H2ABbd have been detected only in vertebrates, whereas the other histone variants have been identified in almost all of the protozoan species. Interestingly, apicomplexan parasites contain a novel variant of H2B family named H2Bv [22]. In T. gondii, H2Bv forms dimers mainly with H2AZ, but not with H2AX [23]. Moreover, H2AZ and H2AX are not present in the same nucleosome. It is interesting that acetylated H3 can be present in the same nucleosome with these three variants but mainly with H2Bv and H2AZ, also linking H2AZ and H2Bv with transcriptional activity [23]. These findings reveal that the nucleosomal arrangement is not random in protozoa and may exhibit intriguing differences relative to their mammalian counterparts.
T. gondii H2Bv, a unique hyperacetylated histone variant
Canonical histone H2B is often present in a number of nearly identical subtypes in higher eukaryotes and there is currently no evidence of specific functions for each of the subtypes [19]. Very few functional variants of H2B have been reported in nature and those that are reported completely replace the canonical H2B and have very specialized functions related to gametogenesis [24–26]. There are also reports of a highly divergent H2B variant located on the X-chromosome of human testis (TH2B-175) that may have telomere-associated functions and participate in the telomere-binding complex in human sperm [25,27].
By contrast, trypanosomatids and apicomplexan parasites possess two lineages of H2B, one resembling canonical H2B and the other harboring characteristics of variant histones (Figure 1A) [22,28–30]. Plasmodium falciparum and T. gondii H2Bvs are constitutively transcribed, in contrast to canonical H2Bs that exhibit replication-associated transcription [22,29]. H2Bvs differ from canonical H2Bs mainly in their long N-terminal sequence [18]. Recently, it has been determined that this longer N-tail is hyperacetylated on K3, K8, K13, K14 and K18 in P. falciparum (Figure 1B) [31]. Lysine acetylation is widespread on proteins of diverse function and localization in the protozoan parasite T. gondii, and also there exists extensive histone acetylation among the unusual H2A and H2B variants expressed in Toxoplasma [32]. Lysine acetylation was detected at four positions (K8, K13, K14 and K18) within the N-terminal of the H2B variant [32], and also in lysines 107, 108, 113 and 117 [Jeffers V, Unpublished Data].. By contrast, no acetylation of canonical H2B has been observed in P. falciparum [31] or in the N-terminal of T. gondii H2Ba/H2Bb [32], but new data indicate that some lysines of T. gondii canonical H2B are acetylated at the core and C-terminal region of this histone [Jeffers V, Unpublished Data]. In addition, a preliminary western blot analysis using antiacetylated H2B confirms that T. gondii H2Bv is acetylated [Vanagas L, Unpublished Data].. The observation of two fairly distinct H2B subtypes suggests a novel divergence of H2B function in T. gondii and P. falciparum. Considering the unique features of H2Bv in parasites, one might expect that its acetylation is important for chromatin modulation. The presence of such hyperacetylated histone variants unique for T. gondii (and api-complexans), linked to active promoters, opens the possibility to find unique remodeler enzymes whose inactivation could have a relevant effect on parasite development and survival. If this is the case, we could hypothesize that unique and specific inhibitors could also be developed against H2Bv-associated remodelers.
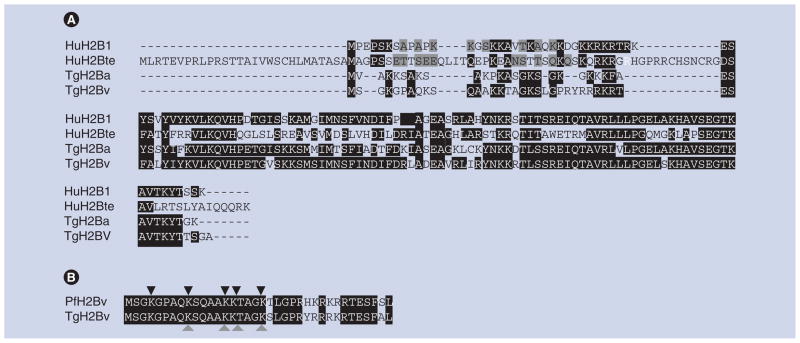
Figure 1. Human and Toxoplasma H2B family.
(A) Sequence alignment of human and Toxoplasma gondii H2Bs. Comparison by multiple alignment (Clustal W). Identical amino acids are denoted by a black shadow and similar amino acids are denoted by gray shadow. (B) N-tail of TgH2Bv and PfH2Bv (Plasmodium falciparum H2B variant, Gene ID: 2655098 PF07_0054). Comparison by multiple alignment (Clustal W). Identical amino acids are denoted by a black shadow and similar amino acids are denoted by gray shadow. Black triangles denote the lysine residues found to be acetylated in P. falciparum, whereas gray triangles denote the lysine residues found to be acetylated in T. gondii.
AN: Accession number; HuH2B1: human canonical H2B1 (AN: AAN06695.1); HuH2Bte: Human testis H2B (AN:AAH38109.1);
TgH2Ba: Canonical T. gondii H2Ba (Gene ID: TGME49_105160); TgH2Bv: T. gondii H2B variant (AN: AAL01371).
Histone acetylases & deacetylases as drug targets
Acetylation is one of several PTMs that occur in canonical and variant histones, and is widely studied as one of the most prominent modifications influencing gene expression [33–35]. Other PTMs include methylation, phosphorylation, ubiquitination, poly-ADP-ribosylation and sumoylation, most of which are located on the N-terminal tails [36]. These modifications can alter the chromatin structure directly by modulating the interactions of proteins with DNA or allowing the recruitment of specific effector proteins. The different PTMs on canonical histones and variants generate different marks on the chromatin that are read by numerous transcriptional regulators as a unique histone code [37].
The role of histone acetylation in the regulation of chromatin structure in higher eukaryotes involves neutralization of the positive charge of the histone N-terminal tails. This attenuation between the histone proteins and the DNA leads to chromatin decondensation, thereby enhancing transcriptional activity. On the other hand, histone hypoacetylation restores the positive charge of histone N-tails, which tightens the binding of DNA and histones, leading to condensed chromatin and gene silencing [38]. The level of histone acetylation is controlled by the concerted activity of histone acetylases (HATs) and deacetylases (HDACs). In addition to weakening the interaction between DNA and nucleosomes, acetylation contributes to the histone code by virtue of recruiting bromodomain proteins. Acetylated lysines can be recognized by bromodomains, a domain that is found on several transcriptional effectors [39].
HATs are divided into two classes depending on their subcellular localization. Type A HATs include a number of heterogenic enzymes that share nuclear localization and global functional similarity due to catalysis of transcriptional-related processes [40,41]. Within this group, HAT members can be classified into five distinct families based on structural homology in the primary sequence as well as the biochemical mechanism of acetyl transfer: GNATs (GCN5 N-acetyltransferases), MYSTs (MOZ, Ybf1/Sas3, Sas2 and Tip60), p300/CBP (CREB-binding protein), general transcription factor HATs and nuclear hormone-related HATs [41–43]. By contrast, Type B HATs, like human HAT1, are cytoplasmic and responsible for acetylation of newly synthesized histone proteins, but not those that are deposited. Type B HATs are also required for the transport of de novo-translated histones through the nuclear membrane for subsequent replacement into newly replicated DNA [44].
HDACs in eukaryotes have been divided into four classes [45–47], with classes I and II comprising enzymes that share similar catalytic domains and a Zn2+ -dependent catalytic mechanism. HDAC class I is represented in mammals by HDACs 1, 2, 3 and 8 and are generally smaller than the class II HDACs with relatively short N- and C-terminal domains surrounding the catalytic domain. The apicomplexan Cryptosporidium parvum HDAC3 was identified as a member of a distinct group of Rpd3-like class I histone deacetylase proteins, and it has also been identified in other apicomplexan parasites, including Toxoplasma and Plasmodium spp, and in photosynthetic eukaryotes as well [48]. In mammals, class II HDACs include HDAC4–7, HDAC9 and HDAC10 [47]. Class III Sir2-related protein (sirtuin) HDACs contain seven members (SIRT1–7) that share homology with yeast Sir2 [47]. Sirtuins (Sirt) 3, 4 and 5 are localized in the mitochondria, Sirts 6 and 7 are exclusively nuclear, Sirt 1 has a dual nuclear/cytosolic localization and Sirt 2 is cytosolic [49]. Unlike other known protein deacetylases, which simply hydrolyze acetyllysine residues, sirtuins can act as mono-ADP-ribosyltransferases and the sirtuinmediated deacetylation reaction couples lysine deacetylation to NAD+ hydrolysis [50]. Mammalian HDAC11 is in a separate class (IV), the only member in this group to date [45].
As central factors in the regulation of chromatin and gene expression, histone remodelers are often associated with cancer, prompting an increasing interest in HATs and HDACs as targets for chemotherapy [51–54]. Inhibition of HDAC activity has been largely validated as a viable therapeutic strategy for cancer treatment, with one hydroxamate-based HDAC inhibitor (HDACi), suberoylanilide hydroxamic acid (vorinostat) [55], approved for the treatment of persistent or refractory T-cell lymphoma [56]. It is widely accepted that HDACis perform their antiproliferative activity through perturbation of chromatin remodeling and altering the acetylation states of key nonhistone proteins [57]. HDACis are also capable of inducing apoptosis via several pathways, including death receptors, the mitochondrial pathway, selective activation of BH3-only proteins, or via the regulation of the production of reactive oxygen species (ROS) [58]. Many HDACis induce cell-cycle arrest at G1/S [59]. As more HDACis become approved and enter the market, it becomes increasingly important to test their efficacy against infectious diseases like toxoplasmosis. If HDACis exhibit potent antiparasite effects in vivo, this could lead to a rapid new advancement in therapy against Toxoplasma and related pathogens. In this sense, an important goal in this field is to identify aspects of the acetylation/deacetylation machinery that contain significant differences between human and T. gondii, as these differences may form the basis for novel and highly specific antiparasitic drugs.
Toxoplasma histone acetylases
Toxoplasma possesses homologues of the type A GCN5 and MYST family HATs, but not the p300/CBP families (Figure 2) [60]. In addition, Toxoplasma possesses an Elp3 homologue, the catalytic component of the elongator complex [60], and TAF250 [61]. A noteworthy feature regarding the Toxoplasma GCN5 family is the presence of two members (TgGCN5-A and -B) that exhibit different H3 acetylation activities and differentially associate with two Toxoplasma ADA2 homologues in vitro [62]. In higher eukaryotes, recombinant GCN5 was found to acetylate histone H3 strongly and H4 weakly in a free histone mixture. The primary sites of acetylation were lysine 14 on histone H3, and lysines 8 and 16 on histone H4 [43]. In contrast to other GCN5s, TgGCN5-A exhibits an unusual bias to preferentially acetylate lysine 18 of histone H3 (H3K18) [63]. In humans, this modification is mediated by the metazoan HAT CBP/p300 and facilitates recruitment of an arginine methylase, coactivator-associated arginine methyltransferase 1, to activate transcription [64]. It is important to note that, despite having nearly identical catalytic domains, TgGCN5-A and -B display different substrate specificity in HAT assays [62]. When individual lysine residues were examined for acetylation, TgGCN5-B was found to be more like a prototypical GCN5 HAT because it is capable of targeting H3 [K9], [K14] and [K18] [62]. TgGCN5-A appears to be dispensable for normal tachyzoite growth, but it has been detected by chromatin immunoprecipitation (ChIP) at the promoters of genes upregulated during alkaline stress, which induces tachyzoite to bradyzoite differentiation. Supporting a role in alkaline-induced bradyzoite development, the T. gondii gcn5-A null mutant fails to upregulate 74% of the genes normally activated in response to alkaline pH, including key bradyzoite-specific genes [65]. Similar attempts to disrupt TgGCN5-B have been unsuccessful, suggesting that in contrast to TgGCN5-A, TgGCN5-B may be essential in tachyzoites.
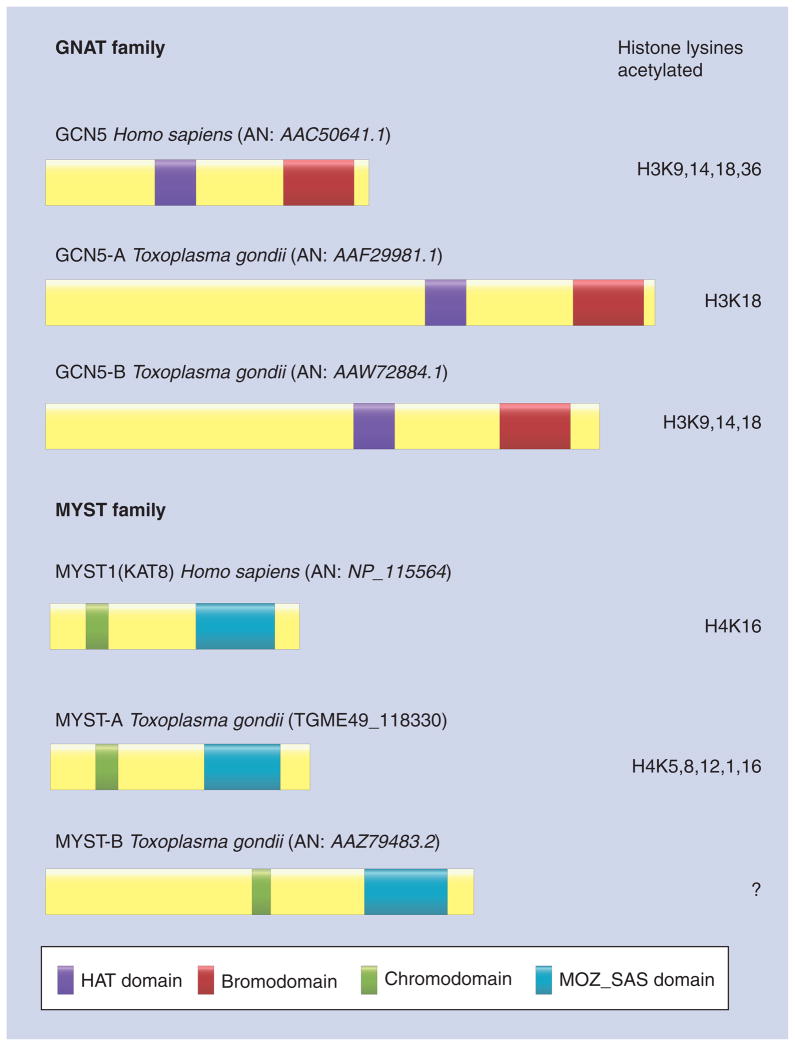
Figure 2. Human and Toxoplasma histone acetylase remodelers.
The figure shows examples of the nuclear type A HAT families from human and Toxoplasma. The AN or gene ID are indicated in brackets. Domains of these proteins are illustrated. On the right, there are indicated the lysine position and/or histone that are acetylated by the remodeler. MOZ_SAS domain: MOZ_SAS family, proteins with this region have been suggested to be homologous to acetyltransferases.
?: Unknown; AN: Accession numbers; HAT: Histone acetyltransferase.
It is interesting to note that the related apicomplexan parasite P. falciparum contains only a single GCN5 member, PfGCN5, which preferentially acetylates H3K9 and K14 in vitro [66]. PfGCN5 has an unusually long N-terminal extension, which appears to harbor the nuclear localization signal, like in the T. gondii GCN5-A and -B [67–69].
GCN5 HATs function in multisubunit complexes and the substrate profile could vary in vivo. The coactivator ADA2 is a subunit of the GCN5 HAT complexes. While most lower eukaryotes have one ADA gene, Toxoplasma contains two independent ADA2 homologues (TgADA2-A and -B) [62]. The ADA2 domain of each T. gondii homologue contains the trademark regions present in other species, including the zinc finger ZZ domain, SANT domain and ADA3-binding domain [62]. In the light of yeast two-hybrid tests, it was observed that TgGCN5-A only interacted with TgADA2-B, whereas TgGCN5-B could interact with both TgADA2s [62].
Undoubtedly, TgGCN5-A and -B are not the only histone-modifying proteins involved in regulating Toxoplasma gene expression. Toxoplasma has a couple of novel MYST family HATs (TgMYST-A and -B), each possessing a chromodomain (Figure 2) [70]. In Toxoplasma, recombinant TgMYST-A has been verified to preferentially acetylate histone H4 in vitro, a modification identified by ChIP to be a mark of gene activation pertinent to parasite differentiation [17]. Further characterization of TgMYST-A reveals that its transcript gives rise to a long and short version of the HAT protein, both of which are more abundant in the tachyzoite stage than in the bradyzoite stage. Attempts to knock out TgMYST-A or TgMYST-B have failed, and overexpression of either TgMYST HAT severely attenuates growth unless the HAT domain is rendered inactive by mutation, suggesting that the expression levels of these HATs require precise regulation [70,71].
Collectively, these unique features of Toxoplasma GCN5 and MYST remodelers and their essential role in parasite viability make these proteins attractive targets that could be used against toxoplasmosis.
Toxoplasma histone deacetylases
Based on data retrieved by mining the T. gondii genome project it could be observed that the parasite genome encodes genes for five class I/II HDAC homologues as well as a homologue to the Sir2 (class III) subtype (Figure 3) [63]. A Sir2 orthologue in the apicomplexan P. falciparum has been noted to have a critical role in mediating var gene expression [72,73]. It has been proposed that PfSir2A and B could be potential targets for antimalarial therapies because it is essential for P. falciparum growth in vitro [74], probably due to the role in regulating virulence gene expression associated with erythrocyte cytoadhesion [73,75]. PfSir2A, which has both histone deacetylase and ADP-ribosyltransferase activity, and PfSir2B, are classified as type III and type IV sirtuins, respectively [75–77]. An analogous form of antigenic variation does not appear to occur in Toxoplasma, and the characterization of subtelomeric region remains unstudied. Our preliminary data suggest that the subtelomeric region in Toxoplasma is a gene-free region that is silenced with heterochromatin markers and a group of uncharacterized Toxoplasma specific gene family [DalmassoMC, Unpublished Data]. It will be of interest to elucidate if TgSir2 has a role similar to PfSir2.
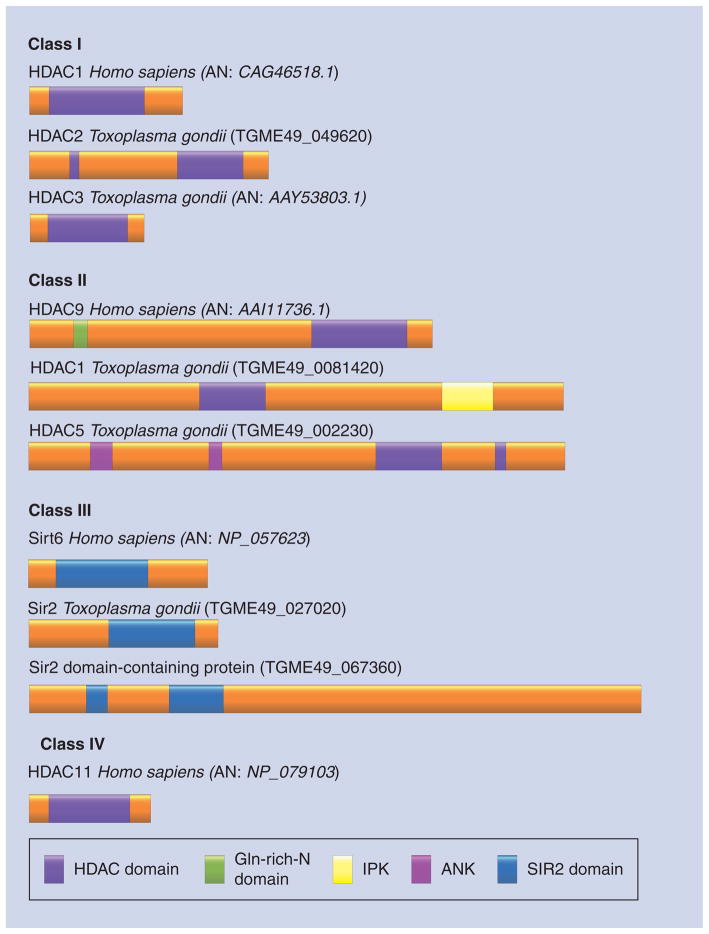
Figure 3. Human and Toxoplasma histone deacetylase remodelers.
The figure shows examples of the human class I (HDAC1), class II (HDAC9), class III (Sirt6) and class IV (HDAC11) HDACs and Toxoplasma HDACs. The accession numbers or gene ID are indicated between brackets. Domains of these proteins are illustrated.
AN: Accession number; ANK: Ankyrin repeats (ankyrin repeats mediate protein–protein interactions in very diverse families of proteins); Gln-rich N-domain: Glutamine-rich N-terminal helical domain of HDAC9. This domain confers responsiveness to calcium signals and mediates interactions with transcription factors and cofactors; HDAC: Histone deacetylase; IPK: Inositol polyphosphate kinase.
By using ChIP analysis, it has been possible to deduce which histone-modifying proteins may be controlling certain genes. For example, TgGCN5-A and TgHDAC3 work in concert to activate or repress stage-specific genes, respectively [63]. TgHDAC3 has been shown to localize in the nucleus [63] and is part of a large multiprotein complex termed the T. gondii corepressor complex (Tg-CRC) [74]. This complex includes TgHDAC3, TgTBL1, actin, HSP70-like proteins, subunits of a T. gondii TCP-1 ring complex (TRiC), and AP2 factors [78]. AP2 domain proteins may represent a major lineage of transcription factors in apicomplexan parasites [79]. Homologues of the human HDAC3-containing complexes are present in the TgHDAC3 complex, allowing us to predict that TgHDAC3 removes acetyl groups from H4K5, H4K8 and H4K12 [80].
Remarkably, TgHDAC3 has been shown to pull down with a slicer-deficient Toxoplasma Argonaute (Tg-AGO), an enzyme associated with RNAi silencing [78]. T. gondii has a vast and complex repertoire of sRNA, including rdsRNA, satRNA, miRNA and siRNA [78]. It is known that rdsRNA and satRNA could be associated to maintain a repressive state of heterochromatin [81,82]. In fact, T. gondii satellite regions are enriched in H3K9met1 and H4K20met1, both marks of hetero-chromatin [78]. Based on that, Braun et al. [78] hypothesized that TgAGO-rdsRNA and TgAGO-satRNA complexes recruit TgHDAC3 to guide this deacetylase to the heterochromatin region of the parasite genome, spreading chromatin silencing.
Histone acetylation & Toxoplasma biology
A key report regarding the importance of epigenetics on Toxoplasma physiology was described in 2005, when researchers showed that histone-modifying complexes regulate gene expression pertinent to brady-zoite differentiation [63]. The study was the first to use ChIP assays to establish that bradyzoite-specific promoters are hypoacetylated in tachyzoites but become acetylated under differentiation conditions. The authors extended this analysis to show that TgGCN5-A was present at tachyzoite-specific promoters acetylating lysine residues in amino-terminal histone tails at the tachyzoite SAG1 promoter to allow transcription. In addition, TgHDAC3 was present at bradyzoite-specific promoters, inhibiting transcription at brady-zoite-induced BAG1 and LDH2 promoters [63,83]. As expected, promoters of constitutively expressed genes had acetylated histones in both life cycle stages. Overall, these data indicate that there is a correlation between the patterns of histone acetylation at gene promoters and the expression patterns of the respective gene, which supports that acetylation is a mark of gene activation in Toxoplasma [17]. Microarray analysis of Toxoplasma treated with an HDACi further supported an important role for histone acetylation in life cycle stage conversion [84].
By using the ChIP-chip method, Kim and colleagues were able to verify that acetylation of H3 at lysine 9 (H3K9), H4, and trimethylation of H3K4 occurs at promoters of actively expressed genes [85]. Similar methods were used to identify that trimethylation of H3K9 and H4K20 is found at repressed genes localized at heterochromatic domains. It should be noted that perfect correlations between acetylation levels and gene activation are not always observed. For example, nucleosomes in the promoters of bradyzoite-specific genes bag1 and ldh2 have been found to be H3-acetylated in low-passage tachyzoites from strains that differentiate more readily, whereas long-term strains (RH), which present a low efficient rate of differentiation, did not show acetylated H3 in bradyzoite-specific genes [86]. These results seem to suggest that in low passage tachyzoite strains these genes are pre-activated or poised for expression but not necessarily expressed yet in the tachyzoite stage.
Histone acetylase and histone deacetylase inhibitors & T. gondii
Intense research is being conducted to explore the application of HDACi/HAT inhibitor (HATi) to diseases other than cancer, where chromatin remodeling and protein acetylation states may play significant roles [87]. Parasites can be likened to tumors in that they undergo intense metabolic activity that is outside the control of the host. In 1996, Darkin–Rattray et al. made the notable discovery that a novel antiprotozoal agent called apicidin (API) inhibited a parasite HDAC [88]. The antiparasitic spectrum of API is unique in that it appears to be specific for the api-complexan protozoa but is inactive against several of the flagellated protozoa, including Giardia lamblia, Tritrichomonas foetus, Leishmania major, Trypanosoma cruzi and Trypanosoma brucei [88]. Table 1 summarizes the HDACis/HATis that have been examined in parasites to date, including T. gondii, and the specificity and effectiveness demonstrated.
Table 1.
Inhibitors of histone acetylases and deacetylases tested in parasites.
| Class | Compound | Range | Parasites tested | HDAC/HAT specificity | Ref. |
|---|---|---|---|---|---|
| Short-chain fatty acid | Butyrate | mM | Poor or modest activity against Toxoplasma gondii, Plasmodium falciparum and Schistosoma mansoni | HDAC I, II | [89,99–101] |
| Valproic acid | mM | Poor or modest activity against T. gondii, P. falciparum and S. mansoni | HDAC I, II | [89,99–101] | |
|
| |||||
| Hydroxamate | TSA | nM | Inhibits T. gondii proliferation and P. falciparum growth | HDAC I, II | [89,102–104] |
| SAHA, Vorinostat | nM | Inhibits T. gondii proliferation | HDAC I, II | [89] | |
| ABHA | μM | Antimalarial activity against P. falciparum | NA | [105,106] | |
| SBHA | μM | Antimalarial activity against P. falciparum, cytostatic against Plasmodium berghei in mice | NA | [105,106] | |
| Scriptaid | μM | T. gondii tachyzoites (more sensitive than TSA and SAHA) | NA | [89] | |
|
| |||||
| Cyclictetrapeptide | Apicidin | nM | Specific for the apicomplexans | HDAC1, HDAC3 | [88,102–104] |
| Nicotinamide | mM | Inhibits P. falciparum Sir2. Inactive against T. gondii tachyzoites | HDAC III (Sir2) | [76,77,107] | |
| Sirtinol | NA | Not sensitive in P. falciparum. Inhibits the in vitro growth of Leishmania infantum (apoptosis) | HDAC III (Sir2) | [77,108] | |
| FR235222 | nM | Active against a wide range of Apicomplexa | TgHDAC3 | [90,91,93] | |
|
| |||||
| Natural products | Anacardic acid, curcumin | μM | Antiproliferative activity against Plasmodium | PfGCN5, p300/ CBP, MYST | [96,97,109,110] |
| Quinoline derivative MC1626 | μM | No effect on T. gondii | GCN5 | [95] | |
HDAC and HAT inhibitors, indicating the parasites where they were tested, their effect and range, and the specificity.
ABHA: Azelaicbishydroxamic acid; HAT: Histone acetyltransferase; HDAC: Histone deacetylase; NA: Not applicable.; SAHA: Suberoylanilidehydroxamic acid; SBHA: Suberohydroxamic acid; TSA: Trichostatin A.
Histone deacetylase inhibitors
It is now recognized that HDACs control gene activation as well as gene repression [89], and participate in many reactions that might impact tachyzoite proliferation and survival. Comparing the impact of different HDACis on T. gondii proliferation shows that cyclic tetrapeptides (e.g., API, HC-toxin and FR235222) are more effective than other HDACis, such as trichostatin A, and the clinically relevant compound pyrimethamine (Table 1) [88,90]. The use of T. gondii cDNA arrays to analyze parasites exposed to the HDACi API showed that transcriptional activity during bradyzoite development is largely affected, perhaps because of the importance of gene-specific acetylation and deacetylation of histones in parasite development [84]. The HDACi FR235222 is a novel cyclic tetrapeptide that selectively targets TgHDAC3; it can cause induction of bradyzoite differentiation at sublethal concentrations [90]. FR235222 also blocks the growth and differentiation of P. falciparum and P. berghei parasites in red blood cells [90]. Sonda et al. found that exposure of parasites to the HDACi FR235222 increased the levels of histone acetylation, altered gene transcription and inhibited Giardia lamblia encystation, thus providing evidence that epigenetic mechanisms are involved in stage differentiation in other parasitic protozoa [91].
The inhibitory activity of FR235222 depends on a two-residue insertion within the catalytic site of TgHDAC3, which is present exclusively in the HDAC3 family of proteins in Apicomplexa; this insertion is absent from all other HDACs identified so far in other organisms [90]. Using ChIP-chip assays, Bougdour et al. were able to identify 369 T. gondii genes that contained hypera-cetylated nucleosomes upon FR235222 treatment, one-third of which are mainly expressed in the sporozoite and/or bradyzoite stage of the parasite [90]. In the presence of 40 nM FR235222, growth was inhibited and approximately 80% of the parasitic vacuoles expressed the bradyzoite-specific surface antigen glyco-protein-related sequence 9/P36 at levels comparable to alkaline-stressed parasites, a stress that is known to induce stage conversion. Inhibition of TgHDAC3 also prevents the formation of the daughter cells. This is consistent with observations that a transient slowing of S phase leads to mature bradyzoites, which possess a uniform genome content (1N DNA) consistent with cell-cycle arrest in G1/G0 [92]. Taken together, these findings support that histone acetylation plays an important role in the control of parasite differentiation and that TgHDAC3 participates in the regulatory pathway leading to bradyzoites [90]. In a subsequent study, FR235222 and derivatives were able to affect bradyzoites differentiated in vitro [93]. Interestingly, some FR235222 derivatives have shown a significant increase in selectivity and a complete absence of cysts in infected mice upon treatment. This study also showed the ability of FR235222 to permeate cell membranes, which is an important advantage because anti-Toxoplasma therapeutics should be able to cross the blood–brain barrier to access the central nervous system tissue where cysts are frequently located [93]. These studies suggest new approaches to develop drugs that are selective against T. gondii, especially in patients with cysts who are at risk of reactivating acute toxoplasmosis (patients with HIV infection, hematological malignancies or undergoing organ transplantation).
Histone acetylase inhibitors
In contrast to the growing number of HDACi, only a few HATis have been described. This is a strange disparity since the modulation of HAT activities might contribute to the treatment of cancer, AIDS, pulmonary diseases, Alzheimer’s disease, neurodegenerative disorders or fungal infections [94]. Up to now, there are no studies analyzing the effect of HATis in toxoplasmo-sis, largely because of the paucity of specific HATis. The quinolone derivative MC1626, a putative GCN5 HATi, slowed parasite growth but did not appear to have activity against TgGCN5-A or -B enzymes [95]. There are some examples where HATis have been used in the related parasite P. falciparum. The treatment of different P. falciparum strains with the polyphenolic compound curcumin inhibited the growth of parasites [96]. Another effect observed was a retardation of cell cycle and changes in parasite morphology. The cytotoxic effect on P. falciparum was attributed to its pro-oxidant activity resulting in the generation of ROS [96]. The analysis also showed that curcumin inhibited PfGCN5 and reduced the acetylation level of H3, a process that could be partially reversed by ROS scavengers, suggesting that curcumin’s effect on HAT enzymatic activity could be through oxidation of its essential residues [96]. Another HATi studied against malaria was anacardic acid (AA). AA inhibited the HAT activity of recombinant PfGCN5 directly in a reversible manner [97]. In addition, AA showed inhibitory effects on parasite growth in vitro. Further analysis showed that AA inhibited PfGCN5 in the parasite, resulting in a reduced acetylation of H3K9 and H3K14, but not H4 and H3K9. As expected, the downstream effect of AA is an important decompensation in global gene expression, mainly in genes associated with cell signaling, stress response and detoxification [97]. It will be of interest to examine additional HATis for antiprotozoal activity as they become available. Given the genetic data to date, it seems clear that most parasite HATs are essential and would serve as excellent candidate drug targets.
Acetylation of nonhistone proteins
HDACis/HATis may have further mechanisms of action beyond the dysregulation of gene expression. It is now understood that protein acetylation represents an additional level of regulation for metabolic enzymes [98] and, considering the prevalence of this modification on many other proteins [34], could dictate the flux of many other cellular processes. In Toxoplasma, a number of HATs have been observed to localize predominantly in the parasite cyto-sol, suggesting that they may also target nonhistone substrates [70]. To determine the extent of protein acetylation in Toxoplasma, a recent study utilizing high-resolution liquid chromatography-mass spectrometry in combination with enrichment of acetylated peptides by acetyllysine immunopurification, reported the ‘acetylome’ of intracellular tachyzoites [32]. This approach successfully identified over 400 novel acetylation sites on a wide variety of proteins throughout the parasite cell, including those with roles in transcription, translation, metabolism and stress responses. Intriguingly, an extensive set of parasite-specific proteins are acetylated in the parasite, including those found in organelles unique to Apicomplexa such as the apicoplast and rhoptries. With protein acetylation emerging as a regulatory process analogous to phosphorylation, HDACis/HATis could conceivably disrupt not only gene expression patterns, but also critical cellular and enzymatic processes in the parasites. The expanding relevance of lysine acetylation beyond histone proteins should spur further efforts to identify specific inhibitors of parasite HDAC and HAT enzymes.
Expert commentary
T. gondii is an obligate intracellular protozoan parasite that can cause severe infection in humans and livestock. The pathology of toxoplasmosis is due to repeated cycles of host cell invasion and lysis by actively dividing tachyzoites, but slowly dividing brady-zoite forms can remain latent for years, capable of reactivating the acute infection [16]. The present chemotherapy for toxoplasmosis is not ideal; there are significant shortcomings to current treatments that target tachyzoites, creating an urgent need for newer, better-tolerated therapies to target this proliferative stage. Besides, new anti-T. gondii drugs, active against the bradyzoite stage in vivo, are also urgently needed to overcome the possibility of reactivation of acute illness in immunosuppressed patients. Therefore, the need to develop new, safer drugs based on the knowledge of the biochemistry and physiology of this pathogen is underscored.
In the last few years, attention has been drawn to PTMs that occur in canonical and variant histones as one of the major factors influencing gene expression. Acetylation is one of several PTMs, and HATs and HDACs are crucial organizers of chromatin structure and gene transcription. HATis and HDACis have emerged as new potential therapeutic agents for a variety of pathologies, including infectious diseases like toxoplasmosis. Up to now, the experience in cancer treatment with HDACis is based only on vorinostat for treatment of a very specific white blood cell cancer [55,58]. The fact that some T. gondii HATs and HDACs present unique features could favor the development of effective inhibitors, which could be used as alternative therapies or in combination with existing ones.
One of the goals to develop new anti-T. gondii drugs relies on the ability to be active against the bradyzoite stage in vivo. Research in this field has shown that histone acetylation plays an important role in the control of parasite differentiation. Although inhibitors like FR235222 have shown to induce cyst formation at sublethal concentrations, it is clear that other HDACis (or higher concentrations) are parasiticidal, as can be read from Table 1. Moreover, FR235222 proved to prevent bradyzoite to tachyzoite stage conversion, and was also able to eliminate the brain cysts in vitro [93]. Besides, other drugs like API have been shown to affect transcriptional activity during bradyzoite development. In our opinion, the analysis of inhibitors of proteins or enzymes involved in gene regulation, like epigenetic mechanisms, are always of relevance because of their potential to attack the differentiation of the parasite and therefore affect its pathological process. We consider that the T. gondii HAT/HDAC system is unique and interesting enough to explore more drugs that could be tested to be specific to control or even eliminate bradyzoites, which is an unresolved subject in the control of toxoplasmosis.
There are some important considerations in the development of new antiparasitic drugs. Compounds must:
Demonstrate high potency and selectivity for the parasite;
Have high safety profile, so as to be used in children and pregnant women;
Avoid the generation of drug-resistant organisms;
Have the ability to cross the blood–brain barrier, especially in the case of T. gondii.
The unusual nucleosome composition, expression of unique histone variants like H2Bv, and the presence of unique HAT/HDAC remodelers, represent a promising opportunity to find compounds that cover all these criteria. We have highlighted recent findings that suggest the parasite epigenetic machinery is an exciting new area for therapeutic exploitation.
Five-year view
The next few years are likely to shed new light on the role of epigenetics in parasite physiology, which in turn will reveal important ways that these mechanisms could be exploited for therapeutic value. For example, epigenetic mechanisms are involved in modulating stage-specific gene activation and parasite differentiation in T. gondii. The tachyzoite–bradyzoite interconversion includes important morphological and physiological changes that require modulation of stage-specific gene expression. Learning the mechanisms of interconversion is important to develop new treatments for chronic toxoplasmosis.
A better understanding of the parasite nucleosome composition and the PTMs that coordinate gene expression is required. With the development of histone variant-specific antibodies and the use of ChIP-chip, it should be possible to map the Toxoplasma epigenome in the context of these unusual nucleosomes. This information should also lead to a better understanding of how HATs and HDACs fit into the overall gene expression network.
The HATis/HDACis studied to date have been demonstrated to target acetylation modulation of histones H3 and H4. Although canonical H2B also presented some acetylation, the hyperacetylation of H2Bv, together with its association to active chromatin, suggests an important role for this PTM in the parasite biology. Future research will reveal which HATs and HDACs modulate H2Bv histone, and the analysis of the biological role of this hyperacetylated state of H2Bv as well as the identification of the remodelers will open a promising source of new chemotherapies.
While a number of HDACis have been characterized and even show activity against protozoan parasites, there are few HAT-specific inhibitors. Given the unique features of Toxoplasma GCN5 and MYST HATs and their essential role in parasite viability, efforts should be made to identify small molecules that interfere with HAT activity.
Key issues.
Toxoplasmosis is a medically important disease, especially in immunocompromised individuals and congenitally infected newborns.
The present chemotherapy for toxoplasmosis suffers serious shortcomings, highlighting the need to develop new, safer drugs.
Histone acetylase and histone deacetylase (HDAC) inhibitors are being investigated as anticancer agents and may be adapted to treat parasitic diseases like toxoplasmosis.
Epigenetic mechanisms are involved in parasite gene regulation and stage differentiation, both vital processes of Toxoplasma gondii pathogenesis and transmission.
T. gondii exhibits novel nucleosome composition and chromatin remodelers with divergent properties that could be exploited as specific drug targets.
The histone H2Bv is a unique H2B variant of Apicomplexa associated with gene activation and is highly acetylated in contrast with the canonical H2B.
The HDAC inhibitor FR235222 and its derivatives have been shown to be specific for T. gondii HDAC3, affecting gene regulation, cell cycle and bradyzoite viability.
Given the great importance of chromatin biology on parasite viability and stage conversion, targeting these systems with novel therapeutics holds significance promise.
Acknowledgments
We thank Maria Lis Alomar and Andres Alonso for their help with the figures.
Footnotes
Financial & competing interests disclosure
SO Angel (Researcher), MC Dalmasso, SS Bogado and L Vanagas (Fellows) are members of National Research Council of Argentina (CONICET). SO Angel and L Vanagas are also professors of UNSAM. This work was supported by NIH-NIAID 1R01AI083162 (to SO Angel and WJ Sullivan) and NIH R01 AI077502 (to WJ Sullivan). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010;26(4):190–196. doi: 10.1016/j.pt.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Su C, Evans D, Cole RH, Kissinger JC, Ajioka JW, Sibley LD. Recent expansion of Toxoplasma through enhanced oral transmission. Science. 2003;299(5605):414–416. doi: 10.1126/science.1078035. [DOI] [PubMed] [Google Scholar]
- 3.Isaac-Renton J, Bowie WR, King A, et al. Detection of Toxoplasma gondii oocysts in drinking water. Appl Environ Microbiol. 1998;64(6):2278–2280. doi: 10.1128/aem.64.6.2278-2280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi WY, Nam HW, Kwak NH, et al. Foodborne outbreaks of human toxoplasmosis. J Infect Dis. 1997;175(5):1280–1282. doi: 10.1086/593702. [DOI] [PubMed] [Google Scholar]
- 5.Carlier Y, Truyens C, Deloron P, Peyron F. Congenital parasitic infections: a review. Acta Trop. 2012;121(2):55–70. doi: 10.1016/j.actatropica.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Brynska A, Tomaszewicz-Libudzic E, Wolanczyk T. Obsessive–compulsive disorder and acquired toxoplasmosis in two children. Eur Child Adolesc Psychiatry. 2001;10(3):200–204. doi: 10.1007/s007870170027. [DOI] [PubMed] [Google Scholar]
- 7.Miman O, Mutlu EA, Ozcan O, Atambay M, Karlidag R, Unal S. Is there any role of Toxoplasma gondii in the etiology of obsessive-compulsive disorder? Psychiatry Res. 2010;177(1–2):263–265. doi: 10.1016/j.psychres.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Yolken RH, Dickerson FB, Fuller Torrey E. Toxoplasma and schizophrenia. Parasite Immunol. 2009;31(11):706–715. doi: 10.1111/j.1365-3024.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- 9.Vittecoq M, Elguero E, Lafferty KD, et al. Brain cancer mortality rates increase with Toxoplasma gondii seroprevalence in France. Infect Genet Evol. 2012;12(2):496–498. doi: 10.1016/j.meegid.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15(2):211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 11.Baatz H, Mirshahi A, Puchta J, Gümbel H, Hattenbach LO. Reactivation of toxoplasma retinochoroiditis under atovaquone therapy in an immunocompetent patient. Ocul Immunol Inflamm. 2006;14(3):185–187. doi: 10.1080/09273940600659740. [DOI] [PubMed] [Google Scholar]
- 12.Petersen E. Prevention and treatment of congenital toxoplasmosis. Expert Rev Anti Infect Ther. 2007;5(2):285–293. doi: 10.1586/14787210.5.2.285. [DOI] [PubMed] [Google Scholar]
- 13.Dannemann B, McCutchan JA, Israelski D, et al. Treatment of toxoplasmic encephalitis in patients with AIDS. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine The California Collaborative Treatment Group. Ann Intern Med. 1992;116(1):33–43. doi: 10.7326/0003-4819-116-1-33. [DOI] [PubMed] [Google Scholar]
- 14.Weiss LM, Kim K. The development and biology of bradyzoites of Toxoplasma gondii. Front Biosci. 2000;5:D391–D405. doi: 10.2741/weiss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez JB, Szajnman SH. New antibacterials for the treatment of toxoplasmosis; a patent review. Expert Opin Ther Pat. 2012;22(3):311–333. doi: 10.1517/13543776.2012.668886. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan WJ, Jr, Jeffers V. Mechanisms of Toxoplasma gondii persistence and latency. FEMS Microbiol Rev. 2012;36(3):717–733. doi: 10.1111/j.1574-6976.2011.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan WJ, Jr, Hakimi MA. Histone mediated gene activation in Toxoplasma gondii. Mol Biochem Parasitol. 2006;148(2):109–116. doi: 10.1016/j.molbiopara.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 18•.Dalmasso MC, Sullivan WJ, Jr, Angel SO. Canonical and variant histones of protozoan parasites. Front Biosci. 2012;17:2086–2105. doi: 10.2741/3841. State of knowledge about canonical and variant histones of protozoan parasites like Plasmodium falciparum, Trypanosoma cruzi and Toxoplasma gondii based on literature and database searching. [DOI] [PubMed] [Google Scholar]
- 19.Kamakaka RT, Biggins S. Histone variants: deviants? Genes Dev. 2005;19(3):295–310. doi: 10.1101/gad.1272805. [DOI] [PubMed] [Google Scholar]
- 20.Yuan G, Zhu B. Histone variants and epigenetic inheritance. Biochim Biophys Acta. 2012;1819(3–4):222–229. doi: 10.1016/j.bbagrm.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Malik HS, Henikoff S. Phylogenomics of the nucleosome. Nat Struct Biol. 2003;10(11):882–891. doi: 10.1038/nsb996. [DOI] [PubMed] [Google Scholar]
- 22•.Dalmasso MC, Echeverria PC, Zappia MP, Hellman U, Dubremetz JF, Angel SO. Toxoplasma gondii has two lineages of histones 2b (H2B) with different expression profiles. Mol Biochem Parasitol. 2006;148(1):103–107. doi: 10.1016/j.molbiopara.2006.03.005. Discovery of a novel H2B variant histone in T. gondii. Demonstration of its expression in every stage by reverse transcription-PCR. Using triton, acetic acid and urea polyacrylamide gel electrophoresis, they show this histone variant as the one more expressed between H2Bs. [DOI] [PubMed] [Google Scholar]
- 23•.Dalmasso MC, Onyango DO, Naguleswaran A, Sullivan WJ, Jr, Angel SO. Toxoplasma H2A variants reveal novel insights into nucleosome composition and functions for this histone family. J Mol Biol. 2009;392(1):33–47. doi: 10.1016/j.jmb.2009.07.017. Characterization of three T. gondii H2A histones and H2B variants. T. gondii nucleosome composition and chromatin immunoprotector analysis show that H2AZ and H2Bv are enriched at active genes whereas H2AX is enriched in silenced genome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aul RB, Oko RJ. The major subacrosomal occupant of bull spermatozoa is a novel histone H2B variant associated with the forming acrosome during spermiogenesis. Dev Biol. 2001;239(2):376–387. doi: 10.1006/dbio.2001.0427. [DOI] [PubMed] [Google Scholar]
- 25.Churikov D, Siino J, Svetlova M, et al. Novel human testis-specific histone H2B encoded by the interrupted gene on the X chromosome. Genomics. 2004;84(4):745–756. doi: 10.1016/j.ygeno.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Churikov D, Zalenskaya IA, Zalensky AO. Male germline-specific histones in mouse and man. Cytogenet Genome Res. 2004;105(2–4):203–214. doi: 10.1159/000078190. [DOI] [PubMed] [Google Scholar]
- 27.Zalensky AO, Siino JS, Gineitis AA, et al. Human testis/sperm-specific histone H2B (hTSH2B). Molecular cloning and characterization. J Biol Chem. 2002;277(45):43474–43480. doi: 10.1074/jbc.M206065200. [DOI] [PubMed] [Google Scholar]
- 28.Alsford S, Horn D. Trypanosomatid histones. Mol Microbiol. 2004;53(2):365–372. doi: 10.1111/j.1365-2958.2004.04151.x. [DOI] [PubMed] [Google Scholar]
- 29.Miao J, Fan Q, Cui L, Li J, Li J, Cui L. The malaria parasite Plasmodium falciparum histones: organization, expression, and acetylation. Gene. 2006;369:53–65. doi: 10.1016/j.gene.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 30.Lowell JE, Kaiser F, Janzen CJ, Cross GA. Histone H2AZ dimerizes with a novel variant H2B and is enriched at repetitive DNA in Trypanosoma brucei. J Cell Sci. 2005;118(Pt 24):5721–5730. doi: 10.1242/jcs.02688. [DOI] [PubMed] [Google Scholar]
- 31•.Trelle MB, Salcedo-Amaya AM, Cohen AM, Stunnenberg HG, Jensen ON. Global histone analysis by mass spectrometry reveals a high content of acetylated lysine residues in the malaria parasite Plasmodium falciparum. J Proteome Res. 2009;8(7):3439–3450. doi: 10.1021/pr9000898. Detection of post-translational modifications in Plasmodium falciparum analyzed by reversed-phase liquid chromatography–mass spectrometry, showing a novel differentiation in the modification pattern of the two histone H2B variants (H2B and H2Bv), suggesting divergent functions of the two H2B variants in the parasite. [DOI] [PubMed] [Google Scholar]
- 32••.Jeffers V, Sullivan WJ., Jr Lysine acetylation is widespread on proteins of diverse function and localization in the protozoan parasite Toxoplasma gondii. Eukaryotic Cell. 2012;11(6):735–742. doi: 10.1128/EC.00088-12. First proteome-wide analysis of acetylation for a protozoan organism, T. gondii. Lysine acetylation is abundant in the actively proliferating tachyzoite form of the parasite, including histones and α-tubulin, and a wide variety of additional proteins, including those with roles in transcription, translation, metabolism and stress responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basu A, Rose KL, Zhang J, et al. Proteome-wide prediction of acetylation substrates. Proc Natl Acad Sci USA. 2009;106(33):13785–13790. doi: 10.1073/pnas.0906801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choudhary C, Kumar C, Gnad F, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325(5942):834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 35.Feng Y, Wang J, Asher S, et al. Histone H4 acetylation differentially modulates arginine methylation by an in Cis mechanism. J Biol Chem. 2011;286(23):20323–20334. doi: 10.1074/jbc.M110.207258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 37••.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. First to propose that post-translational modifications on tail domains of histones act sequentially or in combination to form a ‘histone code’ that is read by other proteins to bring about distinct downstream events. [DOI] [PubMed] [Google Scholar]
- 38.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 40.Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103(2):263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 41.Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8(4):284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 42.Carrozza MJ, Utley RT, Workman JL, Côté J. The diverse functions of histone acetyltransferase complexes. Trends Genet. 2003;19(6):321–329. doi: 10.1016/S0168-9525(03)00115-X. [DOI] [PubMed] [Google Scholar]
- 43.Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000;64(2):435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campos EI, Fillingham J, Li G, et al. The program for processing newly synthesized histones H3.1 and H4. Nat Struct Mol Biol. 2010;17(11):1343–1351. doi: 10.1038/nsmb.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338(1):17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Carey N, La Thangue NB. Histone deacetylase inhibitors: gathering pace. Curr Opin Pharmacol. 2006;6(4):369–375. doi: 10.1016/j.coph.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Xu K, Dai XL, Huang HC, Jiang ZF. Targeting HDACs: a promising therapy for Alzheimer’s disease. Oxid Med Cell Longev. 2011;2011:143269. doi: 10.1155/2011/143269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rider SD, Jr, Zhu G. An apicomplexan ankyrin-repeat histone deacetylase with relatives in photosynthetic eukaryotes. Int J Parasitol. 2009;39(7):747–754. doi: 10.1016/j.ijpara.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16(10):4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guarente L. Sir2 links chromatin silencing, metabolism, and aging. Genes Dev. 2000;14(9):1021–1026. [PubMed] [Google Scholar]
- 51.Copeland RA, Solomon ME, Richon VM. Protein methyltransferases as a target class for drug discovery. Nat Rev Drug Discov. 2009;8(9):724–732. doi: 10.1038/nrd2974. [DOI] [PubMed] [Google Scholar]
- 52.Chung D. Histone modification: the ‘next wave’ in cancer therapeutics. Trends Mol Med. 2002;8(4 Suppl):S10–S11. doi: 10.1016/s1471-4914(02)02303-1. [DOI] [PubMed] [Google Scholar]
- 53••.Kristeleit R, Stimson L, Workman P, Aherne W. Histone modification enzymes: novel targets for cancer drugs. Expert Opin Emerg Drugs. 2004;9(1):135–154. doi: 10.1517/eoed.9.1.135.32947. Current knowledge about the use of compounds that inhibit histone deacetylase (HDAC) activity and also have anti-tumor properties as anticancer drugs. [DOI] [PubMed] [Google Scholar]
- 54.Wigle TJ. Promoting illiteracy in epigenetics: an emerging therapeutic strategy. Curr Chem Genomics. 2011;5(Suppl 1):48–50. doi: 10.2174/1875397301005010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grant S, Easley C, Kirkpatrick P. Vorinostat. Nat Rev Drug Discov. 2007;6(1):21–22. doi: 10.1038/nrd2227. [DOI] [PubMed] [Google Scholar]
- 56.Rangwala S, Zhang C, Duvic M. HDAC inhibitors for the treatment of cutaneous T-cell lymphomas. Future Med Chem. 2012;4(4):471–486. doi: 10.4155/fmc.12.6. [DOI] [PubMed] [Google Scholar]
- 57.Frew AJ, Johnstone RW, Bolden JE. Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett. 2009;280(2):125–133. doi: 10.1016/j.canlet.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 58.Xargay-Torrent S, López-Guerra M, Saborit-Villarroya I, et al. Vorinostat-induced apoptosis in mantle cell lymphoma is mediated by acetylation of proapoptotic BH3-only gene promoters. Clin Cancer Res. 2011;17(12):3956–3968. doi: 10.1158/1078-0432.CCR-10-3412. [DOI] [PubMed] [Google Scholar]
- 59.Noh JH, Jung KH, Kim JK, et al. Aberrant regulation of HDAC2 mediates proliferation of hepatocellular carcinoma cells by deregulating expression of G1/S cell cycle proteins. PLoS ONE. 2011;6(11):e28103. doi: 10.1371/journal.pone.0028103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60•.Dixon SE, Stilger KL, Elias EV, Naguleswaran A, Sullivan WJ., Jr A decade of epigenetic research in Toxoplasma gondii. Mol Biochem Parasitol. 2010;173(1):1–9. doi: 10.1016/j.molbiopara.2010.05.001. State of knowledge about epigenetic research in T. gondii. They discuss how features like unusual histone variants and the existence of plant-like transcription factors impact on parasite physiology and potential therapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jammallo L, Eidell K, Davis PH, et al. An insertional trap for conditional gene expression in Toxoplasma gondii: identification of TAF250 as an essential gene. Mol Biochem Parasitol. 2011;175(2):133–143. doi: 10.1016/j.molbiopara.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Bhatti MM, Livingston M, Mullapudi N, Sullivan WJ., Jr Pair of unusual GCN5 histone acetyltransferases and ADA2 homologues in the protozoan parasite Toxoplasma gondii. Eukaryotic Cell. 2006;5(1):62–76. doi: 10.1128/EC.5.1.62-76.2006. Discovery of T. gondii second GCN5 (TgGCN5-B), which acetylates multiple lysines in the H3 tail. Identification of two ADA2 homologues that interact differently with the TgGCN5s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Saksouk N, Bhatti MM, Kieffer S, et al. Histone-modifying complexes regulate gene expression pertinent to the differentiation of the protozoan parasite Toxoplasma gondii. Mol Cell Biol. 2005;25(23):10301–10314. doi: 10.1128/MCB.25.23.10301-10314.2005. Purification of the first HDAC complex from apicomplexans. Toxoplasma possesses extensive chromatin remodeling machinery that modulates gene expression relevant to differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daujat S, Bauer UM, Shah V, Turner B, Berger S, Kouzarides T. Crosstalk between CARM1 methylation and CBP acetylation on histone H3. Curr Biol. 2002;12(24):2090–2097. doi: 10.1016/s0960-9822(02)01387-8. [DOI] [PubMed] [Google Scholar]
- 65•.Naguleswaran A, Elias EV, McClintick J, Edenberg HJ, Sullivan WJ., Jr Toxoplasma gondii lysine acetyltransferase GCN5-A functions in the cellular response to alkaline stress and expression of cyst genes. PLoS Pathog. 2010;6(12):e1001232. doi: 10.1371/journal.ppat.1001232. Establishment of TgGCN5-A as a major contributor to the alkaline stress response (a common stress used to induce bradyzoite differentiation in vitro) in RH strain Toxoplasma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fan Q, An L, Cui L. PfADA2, a Plasmodium falciparum homologue of the transcriptional coactivator ADA2 and its in vivo association with the histone acetyltransferase PfGCN5. Gene. 2004;336(2):251–261. doi: 10.1016/j.gene.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 67.LaCount DJ, Vignali M, Chettier R, et al. A protein interaction network of the malaria parasite Plasmodium falciparum. Nature. 2005;438(7064):103–107. doi: 10.1038/nature04104. [DOI] [PubMed] [Google Scholar]
- 68.Bhatti MM, Sullivan WJ., Jr Histone acetylase GCN5 enters the nucleus via importin-alpha in protozoan parasite Toxoplasma gondii. J Biol Chem. 2005;280(7):5902–5908. doi: 10.1074/jbc.M410656200. [DOI] [PubMed] [Google Scholar]
- 69.Dixon SE, Bhatti MM, Uversky VN, Dunker AK, Sullivan WJ., Jr Regions of intrinsic disorder help identify a novel nuclear localization signal in Toxoplasma gondii histone acetyltransferase TgGCN5-B. Mol Biochem Parasitol. 2011;175(2):192–195. doi: 10.1016/j.molbiopara.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Smith AT, Tucker-Samaras SD, Fairlamb AH, Sullivan WJ., Jr MYST family histone acetyltransferases in the protozoan parasite Toxoplasma gondii. Eukaryotic Cell. 2005;4(12):2057–2065. doi: 10.1128/EC.4.12.2057-2065.2005. Description of the first MYST family histone acetyltransferases in Apicomplexa (TgMYST-A and -B). Further characterization of TgMYST-A, including activity and localization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.Vonlaufen N, Naguleswaran A, Coppens I, Sullivan WJ., Jr MYST family lysine acetyltransferase facilitates ataxia telangiectasia mutated (ATM) kinase-mediated DNA damage response in Toxoplasma gondii. J Biol Chem. 2010;285(15):11154–11161. doi: 10.1074/jbc.M109.066134. Characterization of the second MYST family lysine acetyltransferase in the protozoan parasite T. gondii (TgMYST-B) and involvement with ataxia telangiectasia mutated kinase. First to show that a MYST lysine acetyltransferase contributes to ataxia telangiectasia mutated kinase gene expression (responding to DNA damage) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Freitas-Junior LH, Hernandez-Rivas R, Ralph SA, et al. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell. 2005;121(1):25–36. doi: 10.1016/j.cell.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 73.Duraisingh MT, Voss TS, Marty AJ, et al. Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium falciparum. Cell. 2005;121(1):13–24. doi: 10.1016/j.cell.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 74•.Andrews KT, Haque A, Jones MK. HDAC inhibitors in parasitic diseases. Immunol Cell Biol. 2012;90(1):66–77. doi: 10.1038/icb.2011.97. Current state of knowledge of HDAC inhibitors targeted to the parasitic diseases malaria, schistosomiasis, trypanosomiasis, toxoplasmosis and leishmaniasis. [DOI] [PubMed] [Google Scholar]
- 75.Tonkin CJ, Carret CK, Duraisingh MT, et al. Sir2 paralogues cooperate to regulate virulence genes and antigenic variation in Plasmodium falciparum. PLoS Biol. 2009;7(4):e84. doi: 10.1371/journal.pbio.1000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chakrabarty SP, Saikumari YK, Bopanna MP, Balaram H. Biochemical characterization of Plasmodium falciparum Sir2, a NAD+-dependent deacetylase. Mol Biochem Parasitol. 2008;158(2):139–151. doi: 10.1016/j.molbiopara.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 77.Merrick CJ, Duraisingh MT. Plasmodium falciparum Sir2: an unusual sirtuin with dual histone deacetylase and ADP-ribosyltransferase activity. Eukaryotic Cell. 2007;6(11):2081–2091. doi: 10.1128/EC.00114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Braun L, Cannella D, Ortet P, et al. A complex small RNA repertoire is generated by a plant/fungal-like machinery and effected by a metazoan-like Argonaute in the single-cell human parasite Toxoplasma gondii. PLoS Pathog. 2010;6(5):e1000920. doi: 10.1371/journal.ppat.1000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Painter HJ, Campbell TL, Llinás M. The Apicomplexan AP2 family: integral factors regulating Plasmodium development. Mol Biochem Parasitol. 2011;176(1):1–7. doi: 10.1016/j.molbiopara.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bougdour A, Braun L, Cannella D, Hakimi MA. Chromatin modifications: implications in the regulation of gene expression in Toxoplasma gondii. Cell Microbiol. 2010;12(4):413–423. doi: 10.1111/j.1462-5822.2010.01446.x. [DOI] [PubMed] [Google Scholar]
- 81.Matzke M, Kanno T, Daxinger L, Huettel B, Matzke AJ. RNA-mediated chromatin-based silencing in plants. Curr Opin Cell Biol. 2009;21(3):367–376. doi: 10.1016/j.ceb.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 82.Verdel A, Moazed D. RNAi-directed assembly of heterochromatin in fission yeast. FEBS Lett. 2005;579(26):5872–5878. doi: 10.1016/j.febslet.2005.08.083. [DOI] [PubMed] [Google Scholar]
- 83.Rooney PJ, Neal LM, Knoll LJ. Involvement of a Toxoplasma gondii chromatin remodeling complex ortholog in developmental regulation. PLoS ONE. 2011;6(5):e19570. doi: 10.1371/journal.pone.0019570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boyle JP, Saeij JP, Cleary MD, Boothroyd JC. Analysis of gene expression during development: lessons from the Apicomplexa. Microbes Infect. 2006;8(6):1623–1630. doi: 10.1016/j.micinf.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 85••.Gissot M, Kelly KA, Ajioka JW, Greally JM, Kim K. Epigenomic modifications predict active promoters and gene structure in Toxoplasma gondii. PLoS Pathog. 2007;3(6):e77. doi: 10.1371/journal.ppat.0030077. Characterization of the epigenetic organization and transcription patterns of T. gondii genome using custom oligonucleotide microarrays. Histone methylation and acetylation ‘activation’ marks that are strongly associated with gene expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Behnke MS, Radke JB, Smith AT, Sullivan WJ, Jr, White MW. The transcription of bradyzoite genes in Toxoplasma gondii is controlled by autonomous promoter elements. Mol Microbiol. 2008;68(6):1502–1518. doi: 10.1111/j.1365-2958.2008.06249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patil V, Guerrant W, Chen PC, et al. Antimalarial and antileishmanial activities of histone deacetylase inhibitors with triazole-linked cap group. Bioorg Med Chem. 2010;18(1):415–425. doi: 10.1016/j.bmc.2009.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Darkin–Rattray SJ, Gurnett AM, Myers RW, et al. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc Natl Acad Sci USA. 1996;93(23):13143–13147. doi: 10.1073/pnas.93.23.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89••.Strobl JS, Cassell M, Mitchell SM, Reilly CM, Lindsay DS. Scriptaid and suberoylanilide hydroxamic acid are histone deacetylase inhibitors with potent anti-Toxoplasma gondii activity in vitro. J Parasitol. 2007;93(3):694–700. doi: 10.1645/GE-1043R.1. Compares the activity of hydroxamic acid, suberic bishydroxamic acid, scriptaid and trichostatin A HDAC inhibitors against the RH strain of T. gondii. Nicotinamide, an inhibitor of NAD+-dependent class III HDAC, had minimal activity against T. gondii. [DOI] [PubMed] [Google Scholar]
- 90••.Bougdour A, Maubon D, Baldacci P, et al. Drug inhibition of HDAC3 and epigenetic control of differentiation in Apicomplexa parasites. J Exp Med. 2009;206(4):953–966. doi: 10.1084/jem.20082826. First time that the HDAC inhibitor FR235222 was used on T. gondii. Demonstration that FR235222 treatment induces differentiation of the tachyzoite (replicative) into the bradyzoite (nonreplicative) stage, as well as the induction of some bradyzoite genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sonda S, Morf L, Bottova I, et al. Epigenetic mechanisms regulate stage differentiation in the minimized protozoan Giardia lamblia. Mol Microbiol. 2010;76(1):48–67. doi: 10.1111/j.1365-2958.2010.07062.x. [DOI] [PubMed] [Google Scholar]
- 92.Jerome ME, Radke JR, Bohne W, Roos DS, White MW. Toxoplasma gondii bradyzoites form spontaneously during sporozoite-initiated development. Infect Immun. 1998;66(10):4838–4844. doi: 10.1128/iai.66.10.4838-4844.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93••.Maubon D, Bougdour A, Wong YS, et al. Activity of the histone deacetylase inhibitor FR235222 on Toxoplasma gondii: inhibition of stage conversion of the parasite cyst form and study of new derivative compounds. Antimicrob Agents Chemother. 2010;54(11):4843–4850. doi: 10.1128/AAC.00462-10. Demonstration of the action of FR235222 on in vitro-converted cysts and bradyzoites and also on ex vivo T. gondii cysts. This compound is able to cross the T. gondii cystic cell wall, which is important for the therapeutics not only of acute but also chronic toxoplasmosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Furdas SD, Kannan S, Sippl W, Jung M. Small molecule inhibitors of histone acetyltransferases as epigenetic tools and drug candidates. Arch Pharm (Weinheim) 2012;345(1):7–21. doi: 10.1002/ardp.201100209. [DOI] [PubMed] [Google Scholar]
- 95.Smith AT, Livingston MR, Mai A, Filetici P, Queener SF, Sullivan WJ., Jr Quinoline derivative MC1626, a putative GCN5 histone acetyltransferase (HAT) inhibitor, exhibits HAT-independent activity against Toxoplasma gondii. Antimicrob Agents Chemother. 2007;51(3):1109–1111. doi: 10.1128/AAC.01256-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cui L, Miao J, Cui L. Cytotoxic effect of curcumin on malaria parasite Plasmodium falciparum: inhibition of histone acetylation and generation of reactive oxygen species. Antimicrob Agents Chemother. 2007;51(2):488–494. doi: 10.1128/AAC.01238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cui L, Miao J, Furuya T, et al. Histone acetyltransferase inhibitor anacardic acid causes changes in global gene expression during in vitro Plasmodium falciparum development. Eukaryotic Cell. 2008;7(7):1200–1210. doi: 10.1128/EC.00063-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao S, Xu W, Jiang W, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327(5968):1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62(3):237–244. doi: 10.1016/s0920-9964(02)00357-2. [DOI] [PubMed] [Google Scholar]
- 100.Azzi A, Cosseau C, Grunau C. Schistosoma mansoni: developmental arrest of miracidia treated with histone deacetylase inhibitors. Exp Parasitol. 2009;121(3):288–291. doi: 10.1016/j.exppara.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 101.Dubois F, Caby S, Oger F, et al. Histone deacetylase inhibitors induce apoptosis, histone hyperacetylation and up-regulation of gene transcription in Schistosoma mansoni. Mol Biochem Parasitol. 2009;168(1):7–15. doi: 10.1016/j.molbiopara.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 102.Colletti SL, Myers RW, Darkin-Rattray SJ, et al. Broad spectrum antiprotozoal agents that inhibit histone deacetylase: structure-activity relationships of apicidin. Part 1. Bioorg Med Chem Lett. 2001;11(2):107–111. doi: 10.1016/s0960-894x(00)00604-1. [DOI] [PubMed] [Google Scholar]
- 103.Colletti SL, Myers RW, Darkin-Rattray SJ, et al. Broad spectrum antiprotozoal agents that inhibit histone deacetylase: structure-activity relationships of apicidin. Part 2. Bioorg Med Chem Lett. 2001;11(2):113–117. doi: 10.1016/s0960-894x(00)00605-3. [DOI] [PubMed] [Google Scholar]
- 104.Chaal BK, Gupta AP, Wastuwidyaningtyas BD, Luah YH, Bozdech Z. Histone deacetylases play a major role in the transcriptional regulation of the Plasmodium falciparum life cycle. PLoS Pathog. 2010;6(1):e1000737. doi: 10.1371/journal.ppat.1000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Andrews KT, Walduck A, Kelso MJ, Fairlie DP, Saul A, Parsons PG. Anti-malarial effect of histone deacetylation inhibitors and mammalian tumour cytodifferentiating agents. Int J Parasitol. 2000;30(6):761–768. doi: 10.1016/s0020-7519(00)00043-6. [DOI] [PubMed] [Google Scholar]
- 106.Andrews KT, Tran TN, Lucke AJ, et al. Potent antimalarial activity of histone deacetylase inhibitor analogues. Antimicrob Agents Chemother. 2008;52(4):1454–1461. doi: 10.1128/AAC.00757-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Prusty D, Mehra P, Srivastava S, et al. Nicotinamide inhibits Plasmodium falciparum Sir2 activity in vitro and parasite growth. FEMS Microbiol Lett. 2008;282(2):266–272. doi: 10.1111/j.1574-6968.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- 108.Vergnes B, Vanhille L, Ouaissi A, Sereno D. Stage-specific antileishmanial activity of an inhibitor of SIR2 histone deacetylase. Acta Trop. 2005;94(2):107–115. doi: 10.1016/j.actatropica.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 109.Singh N, Misra K. Computational screening of molecular targets in Plasmodium for novel non resistant anti-malarial drugs. Bioinformation. 2009;3(6):255–262. doi: 10.6026/97320630003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martinelli A, Rodrigues LA, Cravo P. Plasmodium chabaudi: efficacy of artemisinin + curcumin combination treatment on a clone selected for artemisinin resistance in mice. Exp Parasitol. 2008;119(2):304–307. doi: 10.1016/j.exppara.2008.02.011. [DOI] [PubMed] [Google Scholar]