Abstract
Context
Retrospective, observational studies have reported an association between diabetes treatment with insulin and a higher incidence of cancer.
Objective
Overview the literature for in vitro and in vivo studies of the metabolic and mitogenic properties of basal insulin analogues and assess the implications for clinical use.
Methods
Relevant studies were identified through PubMed and congress abstract database searches; data on metabolic and mitogenic signalling in relation to insulin treatment of diabetes are included in this review.
Results
The balance of evidence shows that although some analogues have demonstrated mitogenic potency in some in vitro studies in cancer cell lines, these findings do not translate to the in vivo setting in animals or to the clinical setting in humans.
Conclusions
The current consensus is that there is no clinical or in vivo evidence to indicate that any commercially available insulin analogue has carcinogenic effects. Large-scale, prospective clinical and observational studies will further establish any potential link.
Keywords: Hyperinsulinaemia, IGF-1, insulin receptor
Introduction
There is growing evidence of an association between diabetes and the incidence of cancer (Giovannucci et al., 2010). Several studies have identified an increased risk of cancer in patients with both type 1 and type 2 diabetes (Saydah et al., 2003; Coughlin et al., 2004; Smith & Gale, 2009; Jalving et al., 2010). The reason behind this association is the subject of much debate and a number of factors could potentially play a role. The link between diabetes and cancer may be indirect and associated with risk factors common to both conditions, such as obesity. Alternatively, there may be a direct causal link due to metabolic disturbances, such as hyperglycaemia, insulin resistance or hyperinsulinaemia, and there are also suggestions that diabetes therapy may be a factor. It is recognized that insulin has dose-related effects on cell proliferation and differentiation (Sandow, 2009) and recent epidemiological studies have also suggested an association between some insulin analogues and an increased risk of developing cancer (Bowker et al., 2006; Currie et al., 2009; Hemkens et al., 2009; Jonasson et al., 2009; Colhoun, 2009; Chang et al., 2011; Morden et al., 2011; Ruiter et al., 2012).
However, much of the evidence for the association between cancer and diabetes treatment comes from retrospective studies with confounding factors that limit interpretation. In principle, retrospective studies are unable to determine a cause-and-effect relationship. One method of progressing the question of a link between diabetes, its treatment and cancer would be to prospectively assess these questions, but there is a dearth of such data at present (Bowker et al., 2006; Farooki & Schneider, 2006; Smith & Gale, 2009). With regard to therapy, it is also important to understand any underlying pathophysiological mechanisms that involve insulin, insulin analogues and cancer. In response, here we review the current literature on pathophysiological mechanisms, including metabolic and mitogenic effects of three long-acting basal insulin analogues, with the aim of assessing the potential link between long-acting insulin analogues and the incidence of cancer.
Methods
Search criteria and analyses
Appropriate studies for inclusion in this review were identified by searching the PubMed database using various combinations of the following terms: cancer, carcinogenic, carcinogenicity, malignancy, malignancies, mitogenic, mitogenicity, metabolic, Type 1 diabetes, Type 2 diabetes, basal insulin, insulin analogues, insulin glargine, insulin detemir, insulin degludec. Congress abstract databases such as the American Diabetes Association and European Association for the Study of Diabetes were also searched. No restrictions on publication date were enforced in order to capture all relevant analyses. Consideration was also given to references detailed in individual publications which did not feature in the original search results. The authors reviewed the resultant studies; data on metabolic and mitogenic signalling in relation to insulin treatment of diabetes are included in this review.
Insulin, insulin-like growth factor-1 and their receptors
The theoretical basis for a biological link between insulin analogues and cancer relates to possible differences in stimulation of insulin and insulin-like growth factor (IGF) receptors by insulin analogues compared with endogenous insulin. The ubiquitous insulin and IGF receptors belong to the receptor tyrosine kinase superfamily and share substantial structural homology. Both insulin receptors (IRs) and IGF-1 receptors (IGF-1Rs) are dimers consisting of two extracellular ligand-binding alpha subunits and two transmembrane beta subunits containing the tyrosine kinase domain. Ligand activation results in transphosphorylation of the kinase domains that triggers shared intracellular signalling pathways for metabolic and mitogenic processes (De Meyts & Wittaker, 2002; De Meyts et al., 2004).
Overview of receptor types
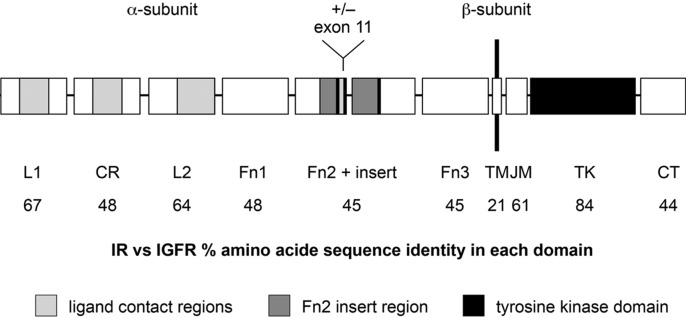
The IRs and IGF-1Rs share more than 50% of their amino acid sequence and have more than 80% homology in their β-subunit tyrosine kinase domains (Ullrich et al., 1986; Chisalita & Arnqvist, 2004). The second highest degree of sequence homology (64–67%) arises in the extracellular α-subunit regions flanking the cysteine-rich subdomains, although similarity is lower within the α-subunit cysteine-rich domain (48%) (Figure 1) (Ullrich et al., 1986). There are two isoforms of human IR, IR-A (short form) and IR-B (long form), which differ from each other by 12 amino acids and are variably expressed in different tissues (Frasca et al., 1999), with IR-A found predominantly in central nervous system and haematopoietic cells and IR-B found predominantly in adipose tissue, liver and muscle (Moller et al., 1989; Mosthaf et al., 1990). IR-A binds both insulin and IGF-2, a growth factor protein similar to IGF-1, which shares homology with insulin (Frasca et al., 1999; Belfiore et al., 2009). The affinity of insulin for IR is approximately 100- to 1000-fold greater than for IGF-1R and the same is true for the affinity of IGF-1 for IGF-1R compared with IR; therefore, at physiological concentrations, little receptor cross-talk occurs (Chisalita & Arnqvist, 2004). Insulin and IGF-1 half-receptors can also heterodimerize to form insulin/IGF-1 hybrid receptors that bind IGF-1 with high affinity (Pandini et al., 2002).
Figure 1. .
Schematic illustration of the sequence homology between the IR and IGF-R (Siddle et al., 2001). IR = insulin receptor; IGF-R = insulin growth factor receptor. Copyright requested.
Receptor expression patterns and function
Most mammalian cells express both IRs and IGF-1Rs. Of the two IR isoforms, IR-A is predominantly expressed during embryogenesis and prenatal development, enhancing the effects of IGF-2, which has been shown to play a role in embryonic development and carcinogenesis (Frasca et al., 1999). However, IR-A expression can also be detected in adult tissues, including the brain, but at a lower degree than IR-B. In contrast, IR-B is predominantly expressed in adult, well-differentiated cells (Belfiore et al., 2009). The IR is mainly involved in mediating metabolic intracellular signalling cascades (protein kinase B (PKB) signalling pathways), whereas the IGF-1R primarily initiates growth and differentiation activities (mitogen-activated protein (MAP) kinase signalling pathways) (LeRoith et al., 1995; Taniguchi et al., 2006). Both the insulin and IGF receptors trigger a complex variety of intracellular signals for metabolism, cell growth and proliferation (Taniguchi et al., 2006). The relative abundance of the IR isoforms affects the intracellular signalling activated by insulin/IGF-1 hybrid receptors, which has important consequences for tissue-specific responses to insulin, IGFs and insulin analogues (Pandini et al., 2002).
Receptor function in cancer cells
Malignant cell growth is often associated with aberrant signalling of both IR and IGF-1R. Overexpression of IR and IGF-1R often coincides with human breast carcinomas, which allows insulin/IGF-1 hybrid receptors to form. These hybrid receptors become tyrosine autophosphorylated when breast cancer cells are exposed to IGF-1 but not insulin and, furthermore, the hybrid receptors mediate growth in response to IGF-1 (Moxham et al., 1989; Soos et al., 1993; Pandini et al., 1999; Belfiore et al., 2009).
IR-A may also play a key role in the development of cancer when activated by IGF-2 in breast cancer cell lines (Sciacca et al., 1999) and thyroid cancer (Vella et al., 2002) in vitro. IR-A activation by IGF-2 leads to the recruitment of different intracellular signalling proteins compared with IR-A activation by human insulin (Frasca et al., 1999). Frasca et al. (1999) showed that when IR-A is activated by insulin, the effects are mainly metabolic, whereas activation by IGF-2 leads to mitogenic effects. The interaction between IR-A and IGF-2 may play a role in both foetal growth and cancer development. IR-A expression is also often aberrant in cancer cells, increasing their responsiveness to IGF-2, which stimulates cell growth; IR-A overexpression also sensitizes cancer cells to circulating insulin, which may explain the cancer-promoting effect of hyperinsulinaemia observed in both obese individuals and those with type 2 diabetes (Belfiore et al., 2009).
In addition, IGF-1R has been implicated in breast cancer development, including proliferation, survival, transformation, differentiation, cell–cell and cell–substrate interactions (Surmacz, 2000). In vitro experiments show that oestrogen receptor-positive cells respond to activation of the IGF-1R/insulin receptor substrate-1 pathway by improving growth and counteracting apoptosis induced by anticancer treatments, whereas breast cancer cells with no or low levels of the oestrogen receptor often express low levels of IGF-1R and fail to respond to IGF-1 with mitogenesis (Bartucci et al., 2001; Bhargava et al., 2011).
As cancer cells have aberrant IR and IGF-1R signalling patterns, it is important to understand how insulin analogues affect both normal and cancerous cells, as this will have implications for diabetes, cancer and cancer treatment. For example, an early investigational insulin analogue, AspB10 insulin, was shown to have not only increased binding affinity for IR, but also an increased occupancy time, which led to prolonged signalling, increasing its metabolic and mitogenic potency (Berti et al., 1998; Kurtzhals et al., 2000).
Long-acting insulin analogues and their metabolites
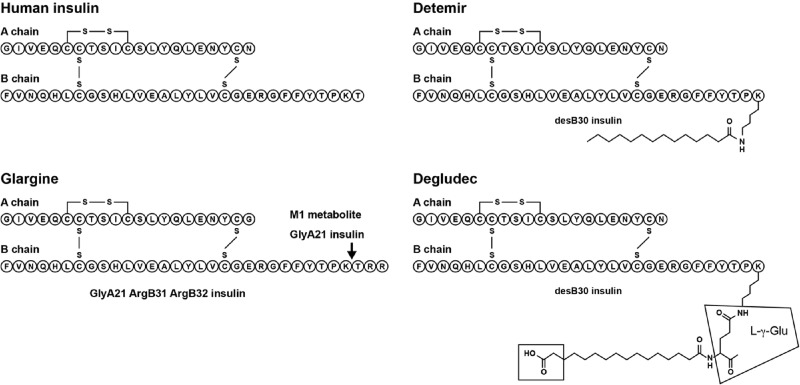
Insulin analogues contain modifications to the insulin structure, primarily to improve their pharmacokinetic profiles compared with human insulin and thus overcome the limitations of traditional insulin preparations (such as the intermediate-acting neutral protamine Hagedorn [NPH] insulin; Novo Nordisk). Conventional human insulin often results in wide glucose fluctuations and adverse effects such as hypoglycaemia, preventing treatment targets being achieved (UKPDS, 1998). Insulin analogues are advantageous compared with human insulin as they have less pharmacokinetic variability and profiles that are better adapted to their specific requirements, either rapid- or long-acting. This translates into improved safety and efficacy (Guerci & Sauvanet, 2005). In particular, insulin analogues are more likely to mimic the physiological pattern of endogenous insulin secretion, reduce the risk of hypoglycaemia and provide greater flexibility for patients compared with human insulin, encouraging better glycaemic control (Bell, 2007). Currently approved long-acting basal insulin analogues are insulin glargine (Lantus®; Sanofi) and insulin detemir (Levemir®, Novo Nordisk) (Figure 2). Insulin degludec (Novo Nordisk), another long-acting insulin analogue, is currently in late-stage clinical development (Figure 2).
Figure 2. .
Schematic diagram showing the modifications to the insulin structure for the long-acting insulin analogues, insulins glargine, detemir and degludec (Agin et al., 2007). Copyright requested.
Given that different insulin analogues have different amino acid residue deletions, substitutions and additions, one can assume that their affinity to IR and IGF-1R may also vary. For example, modifications to amino acid B10 are able to increase the affinity and potency of insulin analogues for both IR and IGF-1R, while addition of residues at B31 or B32 may enhance the binding affinity of an insulin analogue to IGF-1R but not IR, and deletion of B26–B30 residues decreases binding affinity of the insulin analogue to IGF-1R, but only moderately to IR (Slieker et al., 1997).
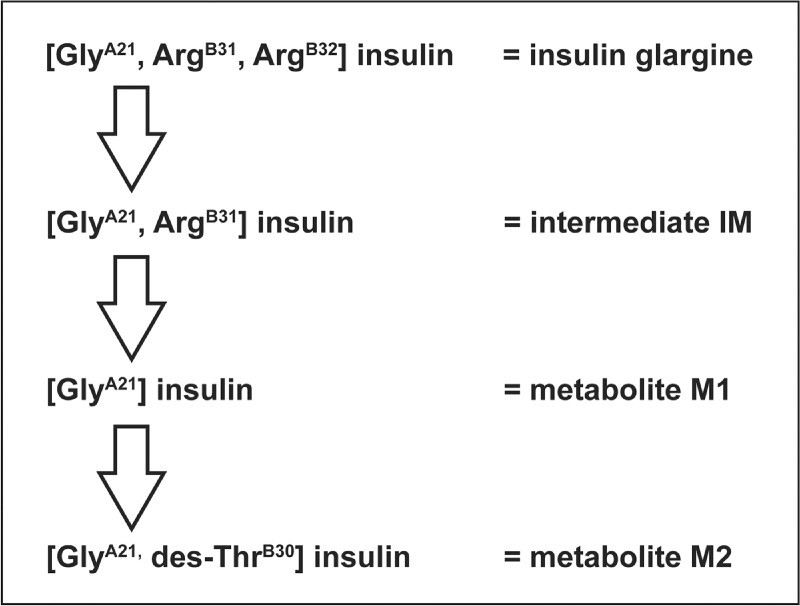
Insulin glargine was the first long-acting insulin analogue to be approved. It differs from human insulin by the substitution of asparagine with glycine at position 21 on the A-chain and the addition of two arginine residues at the carboxy-terminal end of the B-chain (Rosskamp & Park, 1999). The addition of the arginine residues causes a shift in the isoelectric point of the molecule making it less soluble at a neutral pH. As a result, after in vivo subcutaneous injection, insulin glargine precipitates into multi-hexamers in subcutaneous tissue forming a local depot with a slow dissolution into dimers and then monomers (which exert the biological activity of the analogue), thereby extending its duration of action (Berchtold & Hilgenfeld, 1999). The slow release of insulin glargine into solution is accompanied by biotransformation as it undergoes rapid cleavage at the carboxy-terminus of the B-chain forming the active metabolite M1 ([GlyA21] insulin), which lacks the di-arginine residues (Figure 3) (Kuerzel et al., 2003). The threonine at position 30 on the B-chain is also subsequently cleaved to form a second active metabolite M2 ([GlyA21, des-ThrB30] insulin) (Kuerzel et al., 2003). It has been demonstrated consistently in vivo across a range of animals (rats, dogs, diabetic pigs), and in individuals with either type 1 or type 2 diabetes, that almost all administered insulin glargine is rapidly converted into the M1 metabolite (Bolli et al., 2012; Lucidi et al., 2012; Werner et al., 2012), accounting for most (∼90%) of the daily plasma insulin (Lucidi et al., 2012). This has been observed with both subcutaneous and intravenous administration of insulin glargine (Werner et al., 2012). Since it is the predominant metabolite in all species investigated, M1 – as opposed to the insulin glargine molecule itself – is the primary driver of the pharmacodynamic effect and the long-acting time–action profile observed with insulin glargine treatment. The metabolism of insulin glargine is initiated at the site of injection and then continues in the circulatory system (Bolli et al., 2012; Ciaraldi & Sasaoka, 2011; Lucidi et al., 2012; Werner et al., 2012).
Figure 3. .
Schematic diagram showing the metabolism of insulin glargine (Sommerfeld et al., 2010).
Albumin-bound drugs
Insulin detemir is an acylated insulin analogue with the lysine at position 29 on the B-chain modified with a C14 myristic fatty acid group, allowing it to reversibly bind to human serum albumin (HSA) (Olsen & Kaarsholm, 2000). The rate of metabolism of insulin detemir is similar to human insulin with terminal half-lives of 5−7 hours and 4–6 hours, respectively, and all metabolites are inactive (Duckworth et al., 1998; Levemir® Prescribing Information, 2009). After subcutaneous injection, 98% of insulin detemir binds to HSA via its fatty acid side chain, thereby prolonging its duration of action by providing a ‘floating depot’ but also reducing its biological availability (Markussen et al., 1996; Wada et al., 2008; Vigneri et al., 2010). Insulin detemir must be administered at 0.142 mg of the analogue unit instead of 0.036 mg per unit for human insulin or other insulin analogues (3.9-fold higher insulin molecule number per unit) (Vigneri et al., 2010). This four-fold lower potency of insulin detemir relative to that of human insulin is due to the myristic acid moiety being sufficiently close to the receptor recognition site so as to interfere with insulin receptor binding (Levemir: EPAR European Medical Association, 2004). Achieving maximal glucose uptake response with insulin detemir has been shown to require a 10-fold higher plasma insulin concentration than with insulin glargine or human insulin (Wada et al., 2008).
Insulin degludec is an insulin analogue with deletion of ThrB30 and the addition of a 16-carbon fatty di-acid attached to LysB29 via a glutamic acid spacer. It has been proposed that its half-life of ∼25 hours in humans results from the dissolution of multi-hexamer assemblies after subcutaneous injection (Jonassen et al., 2010; 2012). However, data from Jonassen et al. (2012) suggest that, similar to insulin detemir, the molecular design of insulin degludec also results in HSA binding, albeit with a lower affinity (insulin detemir = 1.0, insulin degludec = 2.4). Binding to HSA by insulin degludec is reflected in higher circulating insulin levels compared with human insulin and other insulin analogues as well as reduced bioactivity of in vitro assays.
Although there are no published studies regarding the metabolism of insulin degludec to date, due to their structural similarities (Figure 2), insulin detemir and insulin degludec may be metabolized in a similar manner. Insulin detemir is initially cleaved at the disulphide bridges after which the A- and B-chains are hydrolysed to smaller inactive metabolites that incorporate the lysine residue at B29 and all or part of the myristic acid moiety attached to its ϵ-amino group, which are further degraded (Levemir: EPAR European Medical Association, 2004). It will be interesting to see whether insulin degludec will have similar traits to insulin detemir, for example a potential requirement for a higher concentration at higher BMIs, owing to the structural similarities between these two insulins (Porcellati 2011).
In vitro studies of long-acting insulin analogues
Receptor-binding characteristics and the potential for IGF-1-like activity of the insulin analogues have been studied in vitro using various techniques, including cell lines, primary cell cultures and solubilized receptors. The majority of studies have investigated the IR and IGF-1R binding properties of insulin glargine, but there is a dearth of comparative data with insulins detemir and degludec.
Insulin receptor-binding characteristics of long-acting insulin analogues
In a study by Kurtzhals et al. (2000), insulin glargine was shown to have 86% binding affinity for the IR with an off-rate of 152% (1.5-fold accelerated dissociation) compared with human insulin. In a more recent in vitro study, however, insulin glargine showed about 50% less binding affinity for the two IR isoforms than human insulin, which correlated well with the 40−50% lower metabolic activity (Table 1) (Sommerfeld et al., 2010). The rapid metabolism of insulin glargine into its active metabolites M1 and M2 makes the binding properties of these metabolites of key interest. The M1 insulin glargine metabolite had a similar affinity as insulin glargine for IR (78% relative to human insulin), with an off-rate of 162% (Kurtzhals et al., 2000). Insulin detemir had a relative affinity for the IR of 46% and an off-rate of 204%; the reduced affinity may result from the C14 fatty acid attached to LysB29 making hydrophobic contacts with one or more of the aromatic residues in positions B24−B25, thereby shielding these residues from recognition by the IR (Kurtzhals et al., 2000). So far, there has only been one report on insulin degludec’s affinity for IR, which has a relative affinity of 13−15% compared with human insulin (Nishimura et al., 2010). The dissociation of insulin degludec from IR has not yet been reported.
Table 1. .
Summarized in vitro data for insulin, IGFs and glargine metabolites (Sommerfeld et al., 2010).
| Analogue | IR-A affinity | IR-B affinity | IR-A autophos-phorylation | IR-B autophos-phorylation | Metabolic potency | IGF-1R affinity | IGF-1R autophos-phorylation | Mitogenic potency |
|---|---|---|---|---|---|---|---|---|
| IC50 (nmol/L) | IC50 (nmol/L) | EC50 (nmol/L) | EC50 (nmol/L) | EC50 (nmol/L) | IC50 (nmol/L) | EC50 (nmol/L) | EC50 (nmol/L) | |
| Human insulin | 0.49 ± 0.04 | 0.57 ± 0.02 | 11.0 ± 1.3 | 11.7 ± 1.4 | 0.045 ± 0.003 | 289 ± 53.3 | 447 ± 38.7 | 12.25 ± 0.27 |
| Glargine | 0.83 ± 0.08 | 1.10 ± 0.12 | 18.4 ± 2.4 | 23.6 ± 2.1 | 0.066 ± 0.005 | 63.2 ± 19.9 | 87.5 ± 10 | 1.61 ± 0.26 |
| Glargine IM | 0.78 ± 0.03 | 1.08 ± 0.06 | 23.1 ± 2.7 | 20.9 ± 1.3 | 0.098 ± 0.012 | 80.0 ± 10.2 | 179 ± 19.6 | 3.75 ± 0.31 |
| Glargine M1 | 1.02 ± 0.03 | 1.35 ± 0.20 | 18.6 ± 2.5 | 18.1 ± 1.7 | 0.139 ± 0.009 | 649 ± 31.9 | 644 ± 56.9 | 16.25 ± 2.35 |
| Glargine M2 | 0.93 ± 0.16 | 1.09 ± 0.06 | 19.2 ± 1.6 | 21.4 ± 2.3 | 0.087 ± 0.007 | 427 ± 20.6 | 485 ± 43.6 | 17.90 ± 6.50 |
| [AspB10] insulin | 0.06 ± 0.01 | 0.21 ± 0.03 | 5.1 ± 0.7 | 3.32 ± 0.78 | 0.031 ± 0.005 | 104 ± 12.8 | 72.7 ± 7.6 | 1.52 ± 0.15 |
| IGF-1 | 64.5 ± 5.1 | 171 ± 50 | 449 ± 61.7 | >1000 | 19.01 ± 0.93 | 0.89 ± 0.19 | 2.9 ± 0.4 | 0.22 ± 0.05 |
| IGF-2 | 6.2 ± 0.34 | 46.6 ± 7.8 | 85.4 ± 5.5 | 384 ± 68.4 | − | 6.68 ± 2.24 | − | − |
Data are means ± standard error of the mean. All analogues were tested at least three times on different days. Activity was determined within each experiment and then averaged to yield a single reported mean. IR = insulin receptor; IGF-1R = insulin-like growth factor 1 receptor; IM = intermediate ([GlyA21, ArgB31] insulin); M1 = metabolite 1 ([GlyA21] insulin); M2 = metabolite 2 ([GlyA21,des-ThrB30] insulin); IGF = insulin-like growth factor. Table reproduced from Sommerfeld et al., 2010. In vitro metabolic and mitogenic signaling of insulin glargine and its metabolites. PLoS One 5: e9540.
Structure–function studies have suggested that insulin analogues with a reduced rate of dissociation from the insulin receptor are linked to higher mitogenic potency than metabolic potency versus human insulin (Hansen et al., 1996; Kurtzhals et al., 2000). AspB10, for example, the only analogue with known carcinogenicity, has an off-rate of 14% (Kurtzhals et al., 2000). As described above, insulins glargine and detemir have faster dissociation rates from the insulin receptor than human insulin, suggesting a lower mitogenic potency than human insulin.
Insulin receptor phosphorylation and intracellular signalling stimulated by long-acting insulin analogues
Evidence suggests that insulin analogues have different effects on IR phosphorylation that result in different intracellular signalling properties in various cell types. In fact, consideration of the complexity of the AKT and ERK signalling pathways, primarily responsible for metabolic and mitogenic activities, respectively, helps to explain why even minor conformational differences induced in the receptor molecule by different ligands can alter the signalling cascade (Vigneri et al., 2010). A study by Sciacca et al. (2010) in three engineered cell models (mouse embryonic fibroblasts with a target disruption of the IGF-1R gene [R-] with either human IR-A, IR-B or IGF-1R) reported that long-acting insulin analogues had a phosphorylation pattern of both IR-A and IR-B that was similar to native insulin. However, in contrast, significant differences between native insulin and insulins glargine and detemir were observed in intracellular signalling properties, with an ultimate ERK/AKT activation ratio in favour of ERK for both IR isoforms with the insulin analogues (Sciacca et al., 2010). However, at the concentrations tested (5 nmol/L for insulin glargine and 19 nmol/L for insulin detemir), neither insulin analogue demonstrated increased transforming activity.
In the colorectal cancer cell line HCT116, Weinstein et al. (2009) showed that both insulins glargine and detemir at a concentration of 50 ng/mL were able to phosphorylate IR, while in another study using HCT-116 cells, maximal IR phosphorylation was observed with insulin glargine at 25 ng/mL (Yehezkel et al., 2010). However, in both studies, supraphysiological concentrations of insulin analogues were used that would translate to plasma concentrations that could never be achieved in patients with diabetes. This makes it difficult to determine the clinical relevance of such experiments; besides the fact these insulin analogues are able to activate the IR.
The same holds true for in vitro intracellular signalling experiments. Wada et al, showed that insulin detemir has reduced phosphorylatory effects on intracellular proteins and different signalling effects in various cell types compared with human insulin and insulin glargine (Wada et al., 2008); these differences may be related to differences in the expression of IGF-1R and IR isoforms between the cell lines investigated (Serrano et al., 2005; Sommerfeld et al., 2010). Insulin detemir was less potent than insulin glargine or human insulin with respect to IR phosphorylation, which resulted in a weaker signal transduction via phosphatidylinosital-3-kinase and reduced glucose uptake. Human insulin and insulin glargine (both at the supraphysiological concentration of 50 ng/mL) have been shown to stimulate the phosphorylation of AKT (metabolic signalling pathway) in HCT-116 cells, whereas insulin detemir had a small phosphorylatory effect that was similar to that of IGF-1 (Weinstein et al., 2009; Yehezkel et al., 2010). It is likely that the difference in the amount of phosphorylated intracellular signal cascade proteins produced by human insulin and insulin glargine relative to insulin detemir are not the result of differential kinetic processes but the result of the steric hindrance, caused by the C14 fatty acid attached to the LysB29, in binding to the IR recognition site, resulting in a weaker transduction signal (Kurtzhals et al., 2000).
There is currently no published data regarding receptor activation and intracellular signalling using insulin degludec.
Metabolic properties of long-acting insulin analogues
The metabolic activity of insulin and its analogues is commonly assessed in vitro by studies using glucose uptake, stimulation of lipogenesis and/or inhibition of lipolysis (Sandow, 2009). As already stated, once injected, insulin glargine is rapidly converted into its pharmacologically active metabolites; therefore, in vivo exposure to insulin glargine is likely to be minimal (Lucidi et al., 2012; Bolli et al., 2012; Werner et al., 2012). The main metabolite, M1, accounts for almost all of the pharmacodynamic effects of subcutaneously injected insulin glargine (Lucidi et al., 2012; Bolli et al., 2012; Werner et al., 2012). M1 had similar metabolic potential compared with human insulin as assessed in isolated primary mouse adipocytes (Kurtzhals et al., 2000). In these cells, human insulin was found to stimulate lipogenesis approximately 12-fold over the basal level; insulin glargine, M1 and insulin detemir had metabolic potencies of 60, 88 and ∼27% relative to human insulin, respectively. The measurement for detemir was estimated as it was assumed that only free insulin detemir is biologically active and it was calculated that 93.7% was albumin bound (Kurtzhals et al., 2000). Similar assessments of lipogenesis by Sommerfeld et al. (2010) in primary rat adipocytes support these findings. AspB10 insulin and IGF-1 showed the highest and lowest metabolic activities, respectively (Table 1). The metabolic activity of insulin glargine and its M1 metabolite was lower than that of human insulin as shown by half maximal effective concentration (EC50) values that were 1.4- and 3.0-fold higher, respectively. The metabolic activity of two other insulin glargine metabolites, M2 and IM, were reported for the first time in this study and shown to be less active than human insulin and insulin glargine, but slightly more active than M1 (Table 1).
With respect to glucose uptake, a recent study testing an assay measuring glucose transporter type 4 (GLUT4) translocation in rat myoblasts has shown that insulin glargine behaves much like insulin and not like IGF-1 (Baus et al., 2010). Following an investigation of radioactive glucose uptake, IGF-1 was reported to have an EC50 of 0.3 ± 0.1 nM, while insulin glargine and human insulin had an EC50 of 2.1 ± 0.5 nM and human insulin 2.3 ± 0.6 nM, respectively, with insulin detemir reported as having the lowest potency of 16.9 ± 2.2 nM (Baus et al., 2010).
There is very little data published regarding the metabolic potential of insulin degludec. According to Nishimura et al. (2010) the metabolic responses and maximal effects of insulin degludec were comparable with that of human insulin with respect to lipogenesis in rat adipocytes, glycogen accumulation in rat hepatocytes and glycogen synthesis in rat muscle cells in the absence of albumin.
Insulin-like growth factor receptor-binding characteristics of long-acting insulin analogues
Insulin analogues may have amino acid substitutions and modifications in a domain involved in the interaction with the IGF-1R, and, therefore, may have the potential to display IGF-1-like activities (Werner et al., 2011a). Several independent in vitro studies found higher binding affinity of insulin glargine for the IGF-1R compared with human insulin, i.e. ∼6-fold higher (Kurtzhals et al., 2000), 10-fold higher (Chisalita & Arnqvist, 2004; Chisalita et al., 2006), 4.6-fold higher (Sommerfeld et al., 2010) and at least 100-fold higher binding affinity of insulin glargine for the IGF-1R compared with IGF-1. In particular, the latter study showed that insulin glargine has a 5.1-fold lower EC50 value for autophosphorylation of the receptor. However, the EC50 value of IGF-1 was 154-fold lower than for human insulin (Sommerfeld et al., 2010).
The higher affinity of insulin glargine for IGF-1R in vitro can be attributed to its additional basic arginine residues at positions 31 and 32 of the B-chain, as demonstrated by the fact that di-ArgB31,B32 insulin has an affinity of 2049% for the IGF-1R relative to human insulin. These two arginine residues are also present in human proinsulin and are cleaved during processing to the native insulin molecule. Similarly, these two arginines are rapidly cleaved from insulin glargine once it has been administered in vivo. The principle metabolite of insulin glargine, M1, which lacks the B31 and B32 arginine residues, has a lower affinity for IGF-1R than human insulin (42% affinity relative to human insulin) which correlates well with its significantly lower activation of IGF-1R (Sommerfeld et al., 2010).
Insulin detemir has a low affinity for IGF-1R (16% affinity relative to human insulin) (Kurtzhals et al., 2000). In the absence of albumin, the affinity of insulin degludec for IGF-1R has also been described as low (Nishimura et al., 2010).
Insulin-like growth factor receptor phosphorylation and intracellular signalling stimulated by long-acting insulin analogues
It is not clear if and how insulin analogues affect IGF-1R and subsequent signalling differently from regular human insulin, as there are contradictory results in the literature. In HCT-116 colorectal cancer cells, insulin glargine, but not insulin detemir, (both at 50 ng/mL) was shown to phosphorylate IGF-1R (Weinstein et al., 2009). However, a more recent study has shown that in HCT-116 cells, both insulins glargine and detemir phosphorylated IGF-1R in a dose-dependent manner (Yehezkel et al., 2010). With insulin glargine, the maximal IGF-1R phosphorylation was observed at 5 ng/mL. Maximal IGF-1R phosphorylation with insulin detemir was observed at 100 ng/mL, but initial phosphorylation was observed at 25 ng/mL (Yehezkel et al., 2010). At concentrations of 10–8 M and 10–6 M, insulin glargine was found to phosphorylate the IGF-1R-subunit when tested in human microvascular endothelial cells (Chisalita & Arnqvist, 2004). Thus, supraphysiological concentrations of insulin analogues apparently lead to IGF-1R phosphorylation in vitro. Moreover, in cultured cells supraphysiological concentrations of both insulins glargine and detemir stimulated the phosphorylation of ERK (mitogenic signalling pathway) to a similar extent as IGF-1, but human insulin had a significantly greater effect compared with the other ligands (Weinstein et al., 2009; Yehezkel et al., 2010). Whether or not these reports of mitogenic potential at the IGF-1R with long-acting insulin analogues at supraphysiological concentrations in cancer-derived cell lines are of any clinical relevance remains to be seen. Interestingly, in an analysis of serum samples from patients with Type 2 diabetes treated with metformin plus either insulin glargine or NPH insulin, neither treatment increased IGF-1 signalling; indeed, a decrease in serum IGF-1 phosphorylation was observed (Varewijck et al., 2012). However, the potential influence of metformin on these findings cannot be excluded.
Cell proliferation effects with long-acting insulin analogues
One recombinant insulin analogue, AspB10 insulin, which had already been in clinical development, was associated with carcinomas in rat mammary glands (Ebeling et al., 1996). Therefore, it is important to study the effects of all insulin analogues on the insulin/IGF system that may promote cell proliferation. As AspB10 insulin binds and activates IGF-1R in vitro this effect was linked to its carcinogenic activity in vivo. However, AspB10 insulin also activates IR more potently than human insulin. The mitogenic activity of AspB10 insulin reflects its capacity to activate IR with an increased residence time on the receptor (Berti et al., 1998) as well as potentially activating IGF-1R (Milazzo et al., 1997). Human insulin and the AspB10 insulin analogue cause different patterns of protein phosphorylation when used at physiologically relevant concentrations in MCF-7 breast adenocarcinoma cells, highlighting the importance of understanding the cell proliferation effects of insulin analogues (Oleksiewicz et al., 2011).
Mitogenic activity is often assessed via stimulation of DNA synthesis in proliferation assays (Sandow, 2009). It is important to realize that insulin, as well as IGF-1, belongs to the class of typical growth factors that activate receptor tyrosine kinase signalling pathways in relevant target cells. As a consequence of this, both insulin and IGF-1 trigger cell growth and differentiation, as well as cell-specific metabolism, with insulin having a greater impact on metabolic signalling compared with IGF-1, and IGF-1 being considerably more effective in terms of mitogenic signalling than insulin. However, data regarding the propensity of insulin analogues to promote cell proliferation are heterogeneous and, therefore, difficult to interpret. Some in vitro studies showed mitogenic effects in cancerous cell lines (HCT-166, PC-3 [prostate cancer] and MCF-7) with insulins glargine and detemir, but only at very high concentrations (100 nM), whereas IGF-1 has been shown to stimulate cell proliferation by an average of 21% at all doses tested, without any evident dose–response curve (Weinstein et al., 2009). Other studies showed that unmetabolised insulin glargine at 1.5 and 15 nM concentrations had a significantly higher proliferation effect on MCF-7 cells compared with regular insulin, but not on the benign mammary cell line MCF10A (Mayer et al., 2008). Shukla et al. (2009) observed enhanced growth of the malignant cell line MCF-7 after stimulation with insulin glargine (0.3 nM) compared with other insulin analogues (both long- and short-acting at concentrations of 1.5−15 nM), but all insulin analogues stimulated the proliferation of MCF-10 cells to a similar level. Shukla et al. (2009) also noted that the MCF-7 cells had a higher IGF-1R/IR ratio than the MCF-10 cells. However, another study showed that although insulin glargine stimulated proliferation of both MCF-7 and MCF-10 cells, the level of stimulation was not significantly different from stimulation by human insulin (Staiger et al., 2007). It should be noted that comparisons between these studies should be performed with caution, as different experimental setups and evaluation procedures were used. Insulin-related mitogenic activity is thought to be linked in part to IGF-1R:IR ratio in the cell line studied, with MCF-7 having an IGF-1R:IR ratio of 4:1, whilst the human breast epithelial cell line MCF-10 has a low IGF-1R:IR ratio of 0.8:1 (Müssig et al., 2011).
Neither human insulin nor insulin insulin glargine have been reported to have any effect on the viability and proliferation of human coronary artery endothelial or smooth muscle cells, either at low (0.1 nM) or supraphysiological (100 nM) concentrations (Staiger et al., 2005). Cell proliferation, as assessed by DNA synthesis stimulation, was demonstrated to be similar for primary cultured vascular smooth muscle cells stimulated with equimolar concentrations (1.7, 17 or 170 nM) of human insulin or insulin glargine, and slightly, but not significantly, lower for insulin detemir (Wada et al., 2008). Insulin glargine has also been shown to have a similar mitogenic potency to human insulin in cultured muscle cells and Rat-1 fibroblasts (Ciaraldi et al., 2001; Bähr et al., 1997).
Eckardt et al. (2007) studied the effects of insulin and insulin analogues on primary cultured fibroblasts at supraphysiological concentrations (100 nM), showing that regular insulin stimulated DNA synthesis by exclusively activating IR, whereas it appeared that insulin analogues (AspB10; Lantus®, Sanofi; Humalog®, Eli Lilly and Company; and NovoLog/NovoRapid®, Novo Nordisk) mainly signalled through the IGF-1R pathway.
Gly(A21)-insulin (M1), the main metabolite of insulin glargine, was reported to have lower mitogenic potential than human insulin in a human osteosarcoma cell line (Saos/B10) (Kurtzhals et al., 2000). These cells showed a maximum insulin growth response which was >10-fold increased over basal levels. Stimulation of DNA synthesis in these Saos/B10 cells by insulin glargine was approximately 8-fold higher than by human insulin, whereas M1 showed a 3-fold decrease versus human insulin. Assuming that only free insulin detemir is biologically active, the mitogenic potency of insulin detemir relative to human insulin corrected for albumin binding was approximately 11% (10-fold decrease) (Kurtzhals et al., 2000).
Recent findings in a study by Sommerfeld et al. (2010) strongly support the idea that insulin glargine metabolites contribute to blood glucose control with the same potency as the insulin glargine parent compound, and with a growth-promoting activity comparable with that of human insulin. Insulin glargine had a more potent stimulation of thymidine incorporation (an indirect measure of DNA synthesis and cell proliferation) in Saos-2 cells, whereas the mitogenic activity of its metabolites M1 and M2 were similar to that of human insulin.
Both insulins glargine and detemir exhibited greater anti-apoptotic effects than human insulin in HCT-116 human colorectal cancer cells, but only at the high dose of 100 nM (Weinstein et al., 2009). At a lower dose of 20 nM, human insulin, insulin glargine, insulin detemir and IGF-1 all exhibited a similar anti-apoptotic effect. The mitogenic activity of insulin degludec has been evaluated in myocytes expressing human IR (L6-hIR), human mammary epithelial cells (HMEC), COLO-205 (colorectal adenocarcinoma) and MCF-7 cells, in the absence of albumin, and ranged from 4 to 14% relative to human insulin (Nishimura et al., 2010).
With such a range of evidences available from different cell types, it is difficult to draw definitive conclusions from these in vitro findings. Evidence from studies with insulins detemir and degludec show similar or lower proliferative behaviour compared with human insulin at low doses or in albumin-free conditions, as appropriate. It also appears that the insulin glargine metabolites have a lower mitogenic activity in certain cell lines than both the parent compound and human insulin, and this may be the key point for consideration when attempting to translate these findings into an in vivo or clinical setting.
In vivo studies on insulin, insulin analogues and carcinogenicity
As discussed above, the AspB10 insulin analogue was found to increase the incidence of breast cancer in rats. The in vivo effects of AspB10 insulin, insulin glargine and human insulin (1 IU/kg) on the phosphorylation status of IR, IGF-1R, AKT and ERK1/2 were compared over time in tissue samples taken from rats (Tennagels et al., 2011b; Tennagels et al., 2012). The time courses of the pharmacodynamic effects of human insulin and insulin glargine were found to be distinctively different from that of AspB10 insulin: insulin glargine resulted in phosphorylation levels of IR and AKT that were comparable with that of human insulin. In contrast, injection of AspB10 insulin in rats resulted in at least 2- to 3-fold higher phosphorylation levels and a significantly longer duration of IR and AKT phosphorylation in most of the analysed tissues.
As AspB10 insulin displays higher affinity than human insulin for IGF-1R in vitro, studies were carried out in rats to determine whether insulin analogues with increased IGF-1R affinity in vitro also have increased growth promoting activity in vivo (Tennagels et al., 2011a; Tennagels et al., 2012). After subcutaneous injection of 1–200 U/kg, no increased IGF-1R autophosphorylation in responsive tissues could be observed, either in response to human insulin or insulin glargine, or even to AspB10. However, AspB10 insulin, but not human insulin or insulin glargine, induced an increased and prolonged phosphorylation of IR downstream signalling molecules in various tissues. This led the authors to the conclusion that the in vivo IR signalling pattern of AspB10 insulin is distinctly different from that of both human insulin and insulin glargine, and that the carcinogenic activity of AspB10 insulin in the rat might be based on its altered IR activation profile and, therefore, be independent from its well-documented increased IGF-1R affinity (Werner et al., 2011b).
Observations from animal models lead to the contention that both diet-induced and genetic hyperinsulinaemia/insulin resistance increases the incidence of aberrant crypt foci (Koohestani et al., 1998; Tran et al., 2003) and chemical-induced colon cancer (Weber et al., 2000; Lee et al., 2001). Furthermore, in rat models of insulin resistance (combined hyperinsulinaemia, hyperglycaemia and hypertriglyceridaemia) the incidence of colorectal epithelial proliferation was also increased in vivo (Tran et al., 2006), suggesting that hyperinsulinaemia may augment pro-carcinogenic changes. There is, however, a paucity of conclusive in vivo data investigating insulin analogues and carcinogenicity. Mitogenic effects in vitro were generally only observed at supraphysiological concentrations in cancer-derived cell lines. Doses of insulin or insulin analogues required to achieve comparably high concentrations in vivo would never be applied in clinical practice of as they would result in extreme hypoglycaemia.
Various animal models have been evaluated to determine whether insulin analogues can induce tumours; for example, Stammberger et al. (2002) showed in two species over a 2-year period that there was no association between the incidence of specific tumours and insulin glargine (2, 5 or 12 IU/kg SC in rats and mice) or NPH insulin (12.5 IU/kg in mice and 5 IU/kg in rats). There was no difference in the incidence of mammary tumours in both rats and mice when comparing the insulin glargine treatment with either the sodium chloride vehicle-control or the NPH insulin. Furthermore, no consistent dose-dependent increase in the incidence of tumours was observed in either mice or rats, including hepatocellular adenoma or carcinoma, malignant fibrous histiocytoma, mammary gland adenocarcinoma or malignant mammary gland adenoacanthoma (Stammberger et al., 2002). A study by Nagel et al. (2010a) directly compared the effects of insulin glargine with NPH insulin on epithelial cell proliferation and aberrant crypt foci formation in colons of db/db mice, a commonly used model of type 2 diabetes. In this study, the chronic use (18 weeks) of both insulin glargine and NPH insulin resulted in higher colonic epithelial proliferation and aberrant crypt foci formation compared with a saline control. However, the use of insulin glargine was not associated with increased risk of colonic epithelial proliferation and aberrant crypt foci formation versus NPH insulin. In addition, there was no evidence for lymphadenopathy or spontaneous formation of solid tumours in any treatment group (Nagel et al., 2010a). These results show that suprapharmacological doses of insulin (20−150 IU/kg/day) in the presence of insulin resistance were associated with increased proliferation and aberrant crypt foci formation, irrespective of the type of insulin used, supporting previous evidence in rats showing that hyperinsulinaemia itself enhances colorectal epithelial proliferation in vivo (Tran et al., 2006).
Insulin detemir has also been shown to induce proliferative effects in the mammary glands of young female animals. These proliferative effects were described as modest, however, the longest chronic toxicity study with insulin detemir in rats did not exceed 26-weeks (Levemir: EPAR European Medical Association, 2004).
Taken together, these in vivo studies illustrate that insulin and all insulin analogues in general, with the exception of AspB10, share a similar propensity for inducing cell proliferation at supraphysiological doses, which will not be used in clinical practice, but there is currently little evidence to suggest that this translates into tumour development.
Epidemiological data on insulin analogues and carcinogenicity
The findings in epidemiological studies regarding the carcinogenic potential of basal insulin analogues are not consistent, and the interpretation of these studies is limited by confounding factors. Currie et al. (2000) and Colhoun (2009) concluded that there was no increased risk associated with insulin analogues compared with human insulin.
A German cohort study by Hemkens et al. (2009) reported an association between cancer incidence and insulin dose for all insulin types (human insulin, long-acting insulin analogues and short-acting insulin analogues). Interestingly, the crude incidence rates for cancer and overall mortality were actually lower in the insulin glargine group than in patients treated with human insulin (Hemkens et al., 2009). However, the authors then used an unconventional analysis which found that, after adjusting for dose, the overall incidence of cancer was higher in patients taking insulin glargine compared with human insulin (Hemkens et al., 2009). Nagel et al. (2010b) and Pocock and Smeeth (2009) have subsequently both identified a number of limitations in this study, such as flawed statistical methods (including the unconventional analysis), making the data uninterpretable. Furthermore, despite the large variation in mean daily doses observed for the different types of insulin studied, Hemkens et al. (2009) failed to take into consideration any pathophysiological reasons for these differences (Nagel et al., 2010b; Pocock & Smeeth, 2009). Patients with either type 1 or type 2 diabetes were grouped together in the study, despite the fact that the two diseases show different patterns of malignancy (Nagel et al., 2010b). Nagel et al. (2010b) suggest that data from the German cohort supports previous findings that high doses of insulin, reflecting increased insulin resistance, are associated with an increased risk of cancer and that the data does not warrant safety concerns regarding the use of insulin glargine in diabetic patients.
A nested case-control study showed a possible association between higher insulin glargine doses (≥0.3 IU/kg/day) and cancer incidence in patients with type 2 diabetes (Mannucci et al., 2010). However, in nested case-control studies, the participants from whom the controls were selected may not be fully representative of the original cohort (the control group did not contain those patients who have died from other causes or who have been lost to follow-up); therefore, results from such studies need to be interpreted with caution.
A study by Jonasson et al. (2009) also found that women using insulin glargine alone (i.e. with no other types of insulin) had an increased incidence of breast cancer compared with women using types of insulin other than insulin glargine: the risk ratio (RR) over 2 years (2006–2007) was 1.99 (95% CI 1.31–3.03) after adjustment for age and (when appropriate) sex. However, a follow-up to this study by the same group observed no increase in breast cancer during the third year (2008) in patients receiving insulin glargine, leading the authors to suggest that the increased incidence observed in the original publication may have occurred by chance (Ljung et al., 2011).
A number of other observational analyses support the lack of association between insulin glargine use and cancer risk (van Staa et al., 2012; Blin et al., 2012; Lind et al., 2012; Suissa et al., 2011; Boyle et al., 2012; Autier et al., 2012). A recent meta-analysis of data from epidemiological studies involving a total of 907,008 patients and 2,597,602 person-years of observation which included findings from participating centres in the recent Northern European Study reported that the overall risk of cancer (all forms combined) in addition to any organ-specific type of cancer is not increased among insulin glargine users compared with other insulins (Boyle et al., 2012). Based on independent estimates from 13 studies, summary relative risks (SRR [95% CI]) were 0.90 (0.82, 0.99) for all cancers, and 1.11 (1.00, 1.22) for breast cancer. For new users of insulin glargine (based on six studies) SRR of breast cancer was 1.30 (0.93, 1.81). For colorectal cancer and prostate cancer (based on eight studies), SRRs were 0.84 (0.74, 0.95) and 1.13 (1.00, 1.28), respectively (Boyle et al., 2012). Further data from large database studies expected to be released in 2013 (Northern European Study, Kaiser-Permanent Collaboration, the International Study of Insulin and Cancer and a US database analysis), will further elucidate the potential effects of insulin glargine on cancer risk and on the broader insulin-related cancer risk.
Clinical evidence
As previously discussed, studies in patients with diabetes have established that, when administered, insulin glargine is rapidly converted to the active M1 metabolite (Bolli et al., 2012; Lucidi et al., 2012) which has been demonstrated to have low mitogenic activity (Kurtzhals et al., 2000; Sommerfeld et al., 2010). Serum samples from another clinical study in patients with Type 2 diabetes receiving metformin have demonstrated that addition of therapeutic levels of insulin glargine do not increase IGF-1 activity (Varewijck et al., 2012), further supporting the absence of an increased carcinogenic risk.
Randomization overcomes many of the sources of bias that may be associated with observational trials. A randomized, open-label, long-term safety study, designed to assess ocular complications of diabetes, showed that there was no evidence of a greater risk of the development or progression of diabetic retinopathy with insulin glargine versus NPH insulin treatment in patients with type 2 diabetes (Rosenstock et al., 2009a). Although the study was not designed to investigate the effects of treatment on the frequency of tumour development, its long duration allowed the comparative assessment of the occurrence of malignancies with the two treatments (Rosenstock et al., 2009b). In this study, during a 4.2-year follow-up of 1017 patients, there were 20 and 31 patients with incident cancer in the insulin glargine and NPH insulin groups, respectively, indicating that the overall risk of malignancy appears to be similar for both insulins in patients with type 2 diabetes (Rosenstock et al., 2009b). Clinical evidence from the pooled analysis of 31 randomized controlled trials, including over 10,800 people, showed that there was no increased risk of cancer with insulin glargine versus comparator treatments (other insulin types and anti-diabetic drugs) (Home & Lagarenne, 2009). Another meta-analysis of randomized controlled studies of at least 12 weeks’ duration showed that there was no increased risk of malignancies in patients with diabetes treated with insulin detemir compared with NPH or insulin glargine (Dejgaard et al., 2009). Overall, the incidence rate of cancer was small for all groups, probably reflecting the inclusion/exclusion criteria typical for study populations designed for achieving regulatory approval and the short duration of the follow-up period (Dejgaard et al., 2009).
This is supported by randomized, controlled trial data from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) and ORIGIN (Outcome Reduction with Initial Glargine INtervention) studies. An analysis of 5-year data from 10,251 patients with high cardiovascular risk in the ACCORD study was powered for cardiovascular outcomes but also investigated cancer-related outcomes (Hamaty et al., 2011). The authors concluded that exposure to any insulin, or to basal insulin or insulin glargine specifically, was not associated with increased risk of cancer-related outcomes (hospitalization or death); hazard ratio (HR) related to insulin glargine was 1.00 (95% CI: 0.53, 1.86), p = 0.99 (Hamaty et al., 2011).
The ORIGIN study, which investigated insulin glargine versus placebo treatment in patients with high risk of cardiovascular events and early Type 2 diabetes or pre-diabetes (impaired fasting glucose or impaired glucose tolerance) also included cancer incidence as a secondary outcome (The ORIGIN trial investigators, 2012). Findings from this study of 12,537 patients followed for a median of 6.2 years reported no increased incidence of all cancers combined, any organ-specific type of cancer (including breast, lung, colon, prostate, melanoma) or cancer mortality, in the insulin glargine group versus the standard care group. Hazard ratio (95% CI) for the incidence of all cancers was 1.00 (0.88, 1.13), p = 0.97 and for death from cancer was 0.94 (0.77, 1.15), p = 0.51 (The ORIGIN trial investigators, 2012). To our knowledge, there are no published data regarding the use of insulin degludec and the risk of malignancies. Further analyses from the ACCORD and ORIGIN studies may help elucidate any potential effects of insulin glargine on the incidence of cancer in a randomized, controlled, clinical trial setting.
The increased affinity some insulin analogues may have for IGF-1R raises concerns that if insulin analogues are used during pregnancy, they might lead to increased foetal growth and other mitogenic effects (Pollex et al., 2011). A meta-analysis comparing insulin glargine with NPH insulin in pregnant women showed that there was no increased risk of congenital abnormalities with insulin glargine (risk ratio [RR] 0.97; 95% CI 0.47−1.99), macrosomia (>4 kg; RR 1.28; 95% CI 0.77−2.12) or babies born large for gestational age (>90th centile; RR 1.02; 95% CI 0.80−1.31) (Pollex et al., 2011). However, seven of the eight studies included in this meta-analysis were retrospective in nature and all were observational, thereby limiting the general applicability of the results. No randomized controlled trials of long-acting insulin analogues in pregnant women have yet been conducted. Evidence using an in vitro human placental perfusion model designed to demonstrate the rate of transfer across the human placenta showed that at therapeutic concentrations no insulin glargine was detectable in the foetal circuit (Pollex et al., 2010).
Human IGF-1 has also been tested in clinical studies (including Phase III studies) of type 2 diabetes for potential practical and physiological advantages versus insulin (Zenobi et al., 1992). When given to patients with type 2 diabetes for 5 days, IGF-1 has been shown to improve fasting and postprandial glycaemia and to decrease triglyceride values as well as to increase insulin sensitivity (Zenobi et al., 1992). The risk of hypoglycaemia in type 2 diabetes has also been reported to be significantly lower with IGF-1 than with conventional insulin therapy. Based on these initial reports, it has been suggested that IGF-1 therapy may have a role in treating severely insulin-resistant patients unresponsive to currently available forms of insulin therapy. Relying on these early promising studies, patient safety, with regard to potential IGF-1-mediated proliferation of endothelial and smooth muscle cells, as well as growth-promoting effects on various carcinomas, has been investigated and found to be not significantly affected during IGF-1 treatment.
Conclusions
Although some insulin analogues have demonstrated mitogenic potency in various in vitro studies in cancer cell lines, none of the currently commercially available insulin analogues has yet been proven to be mitogenic in vivo, neither in animals nor in humans. In the case of insulin glargine, this can be attributed to its rapid metabolism into metabolites that have no greater mitogenic activity than human insulin. There is no evidence that either human insulin or insulin analogues have carcinogenic effects at therapeutic doses. Furthermore, in clinical practice, the supraphysiological concentrations required to promote tumour growth in vitro will not be achieved in patients owing to the risk of hypoglycaemia. Taking this into account, there is also no evidence from in vitro studies to support the hypothesis that currently marketed insulin analogues promote tumour growth in humans when used in therapeutic doses. The ORIGINALE (ORIGIN And Legacy Effects) study, in addition to long-term findings from other observational studies, will clearly establish a deeper knowledge of the relationship between diabetes, the incidence of malignancies and the safety of long-acting insulin analogues.
Declaration of interest
Norbert Tennagels and Ulrich Werner are employees of Sanofi-Aventis Deutschland GmbH. Editorial support was provided by Huw Jones, PhD, and Róisín O’Connor, PhD, of Medicus International and funded by Sanofi.
References
- Agin A, Jeandidier N, Gasser F, Grucker D, Sapin R. Glargine blood biotransformation: in vitro appraisal with human insulin immunoassay. Diabetes Metab. (2007);33:205–212. doi: 10.1016/j.diabet.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Autier P, Boniol M, Koechlin A, Robertson C, Rosenstock J, Bolli GB, et al. Diabetes, related factors and breast cancer risk. Diabetes. (2012);61(Suppl 1):A381 (1467-P). [Google Scholar]
- Bähr M, Kolter T, Seipke G, Eckel J. Growth promoting and metabolic activity of the human insulin analogue [GlyA21,ArgB31,ArgB32]insulin (HOE 901) in muscle cells. Eur J Pharmacol. (1997);320:259–265. doi: 10.1016/s0014-2999(96)00903-x. [DOI] [PubMed] [Google Scholar]
- Bartucci M, Morelli C, Mauro L, Andò S, Surmacz E. Differential insulin-like growth factor I receptor signaling and function in estrogen receptor (ER)-positive MCF-7 and ER-negative MDA-MB-231 breast cancer cells. Cancer Res. (2001);61:6747–6754. [PubMed] [Google Scholar]
- Baus D, Yan Y, Li Z, Garyantes T, de Hoop M, Tennagels N. A robust assay measuring GLUT4 translocation in rat myoblasts overexpressing GLUT4-myc and AS160_v2. Anal Biochem. (2010);397:233–240. doi: 10.1016/j.ab.2009.10.036. [DOI] [PubMed] [Google Scholar]
- Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. (2009);30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- Bell DS. Insulin therapy in diabetes mellitus: how can the currently available injectable insulins be most prudently and efficaciously utilised? Drugs. (2007);67:1813–1827. doi: 10.2165/00003495-200767130-00002. [DOI] [PubMed] [Google Scholar]
- Berchtold H, Hilgenfeld R. Binding of phenol to R6 insulin hexamers. Biopolymers. (1999);51:165–172. doi: 10.1002/(SICI)1097-0282(1999)51:2<165::AID-BIP6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Berti L, Kellerer M, Bossenmaier B, Seffer E, Seipke G, Häring HU. The long acting human insulin analog HOE 901: characteristics of insulin signalling in comparison to Asp(B10) and regular insulin. Horm Metab Res. (1998);30:123–129. doi: 10.1055/s-2007-978849. [DOI] [PubMed] [Google Scholar]
- Bhargava R, Beriwal S, McManus K, Dabbs DJ. Insulin-like growth factor receptor-1 (IGF-1R) expression in normal breast, proliferative breast lesions, and breast carcinoma. Appl Immunohistochem Mol Morphol. (2011);19:218–225. doi: 10.1097/PAI.0b013e3181ffc58c. [DOI] [PubMed] [Google Scholar]
- Blin P, Lassalle R, Dureau-Pournin C, Ambrosino B, Bernard MA, Abouelfath A, Gin H, Le Jeunne C, Pariente A, Droz C, Moore N. Insulin glargine and risk of cancer: a cohort study in the French National Healthcare Insurance Database. Diabetologia. (2012);55:644–653. doi: 10.1007/s00125-011-2429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli GB, Hahn AD, Schmidt R, Eisenblaetter T, Dahmen R, Heise T, Becker RH. Plasma exposure to insulin glargine and its metabolites M1 and M2 after subcutaneous injection of therapeutic and subtherapeutic doses of glargine in subjects with type 1 diabetes. Diabetes Care. (2012);35:2626–2630. doi: 10.2337/dc12-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. (2006);29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- Boyle P, Koechlin A, Boniol M, Robertson C, Bolli GB, Rosenstock J. Updated meta-analysis of cancer risk among users of insulin glargine. Diabetes. (2012);61(Suppl 1):A345 (1332-P). [Google Scholar]
- Chang CH, Toh S, Lin JW, Chen ST, Kuo CW, Chuang LM, Lai MS. Cancer risk associated with insulin glargine among adult type 2 diabetes patients–a nationwide cohort study. PLoS ONE. (2011);6:e21368. doi: 10.1371/journal.pone.0021368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisalita SI, Arnqvist HJ. Insulin-like growth factor I receptors are more abundant than insulin receptors in human micro- and macrovascular endothelial cells. Am J Physiol Endocrinol Metab. (2004);286:E896–E901. doi: 10.1152/ajpendo.00327.2003. [DOI] [PubMed] [Google Scholar]
- Chisalita SI, Nitert MD, Arnqvist HJ. Characterisation of receptors for IGF-I and insulin; evidence for hybrid insulin/IGF-I receptor in human coronary artery endothelial cells. Growth Horm IGF Res. (2006);16:258–266. doi: 10.1016/j.ghir.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Ciaraldi TP, Carter L, Seipke G, Mudaliar S, Henry RR. Effects of the long-acting insulin analog insulin glargine on cultured human skeletal muscle cells: comparisons to insulin and IGF-I. J Clin Endocrinol Metab. (2001);86:5838–5847. doi: 10.1210/jcem.86.12.8110. [DOI] [PubMed] [Google Scholar]
- Ciaraldi TP, Sasaoka T. Review on the in vitro interaction of insulin glargine with the insulin/insulin-like growth factor system: potential implications for metabolic and mitogenic activities. Horm Metab Res. (2011);43:1–10. doi: 10.1055/s-0030-1267203. [DOI] [PubMed] [Google Scholar]
- Colhoun HM SDRN Epidemiology Group. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia. (2009);52:1755–1765. doi: 10.1007/s00125-009-1453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. (2004);159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. (2009);52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- De Meyts P, Palsgaard J, Sajid W, Theede AM, Aladdin H. Structural biology of insulin and IGF-1 receptors. Novartis Found Symp. (2004);262:160–71; discussion 171. [PubMed] [Google Scholar]
- De Meyts P, Whittaker J. Structural biology of insulin and IGF1 receptors: implications for drug design. Nat Rev Drug Discov. (2002);1:769–783. doi: 10.1038/nrd917. [DOI] [PubMed] [Google Scholar]
- Dejgaard A, Lynggaard H, Råstam J, Krogsgaard Thomsen M. No evidence of increased risk of malignancies in patients with diabetes treated with insulin detemir: a meta-analysis. Diabetologia. (2009);52:2507–2512. doi: 10.1007/s00125-009-1568-4. [DOI] [PubMed] [Google Scholar]
- Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. (1998);19:608–624. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- Ebeling P, Tuominen JA, Koivisto VA. Insulin analogues and carcinoma of the breast. Diabetologia. (1996);39:124–125. doi: 10.1007/BF00400425. [DOI] [PubMed] [Google Scholar]
- Eckardt K, May C, Koenen M, Eckel J. IGF-1 receptor signalling determines the mitogenic potency of insulin analogues in human smooth muscle cells and fibroblasts. Diabetologia. (2007);50:2534–2543. doi: 10.1007/s00125-007-0815-9. [DOI] [PubMed] [Google Scholar]
- Farooki A, Schneider SH. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin: Response to Bowker et al. . Diabetes Care. (2006);29:1989–1990. doi: 10.2337/dc06-0874. [DOI] [PubMed] [Google Scholar]
- Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. (1999);19:3278–3288. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. CA Cancer J Clin. (2010);60:207–221. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- Guerci B, Sauvanet JP. Subcutaneous insulin: pharmacokinetic variability and glycemic variability. Diabetes Metab. (2005);31:4S7–4S24. doi: 10.1016/s1262-3636(05)88263-1. [DOI] [PubMed] [Google Scholar]
- Hamaty M, Miller ME, Gerstein HC, Olansky L, Probstfield JL, Riddle MC. Is insulin exposure associated with higher risk of cancer-related hospitalization or death? Analysis of 5-year data from the ACCORD trial. Diabetes. (2011);60(Suppl. 1):A99–A100. [Google Scholar]
- Hansen BF, Danielsen GM, Drejer K, Sørensen AR, Wiberg FC, Klein HH, Lundemose AG. Sustained signalling from the insulin receptor after stimulation with insulin analogues exhibiting increased mitogenic potency. Biochem J. (1996);315 (Pt 1):271–279. doi: 10.1042/bj3150271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemkens LG, Grouven U, Bender R, Günster C, Gutschmidt S, Selke GW, Sawicki PT. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. (2009);52:1732–1744. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Home PD, Lagarenne P. Combined randomised controlled trial experience of malignancies in studies using insulin glargine. Diabetologia. (2009);52:2499–2506. doi: 10.1007/s00125-009-1530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalving M, Gietema JA, Lefrandt JD, de Jong S, Reyners AK, Gans RO, de Vries EG. Metformin: taking away the candy for cancer? Eur J Cancer. (2010);46:2369–2380. doi: 10.1016/j.ejca.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. (2012);29:2104–2114. doi: 10.1007/s11095-012-0739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassen I, Havelund S, Ribel U, Hoeg-Jensen T, Steensgaard DB, Johansen T, et al. Insulin degludec: Multi-hexamer formation is the underlying basis for this new generation ultra-long acting basal insulin. Diabetologia. (2010);53:S388. [Google Scholar]
- Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia. (2009);52:1745–1754. doi: 10.1007/s00125-009-1444-2. [DOI] [PubMed] [Google Scholar]
- Koohestani N, Chia MC, Pham NA, Tran TT, Minkin S, Wolever TM, Bruce WR. Aberrant crypt focus promotion and glucose intolerance: correlation in the rat across diets differing in fat, n-3 fatty acids and energy. Carcinogenesis. (1998);19:1679–1684. doi: 10.1093/carcin/19.9.1679. [DOI] [PubMed] [Google Scholar]
- Kuerzel GU, Shukla U, Scholtz HE, Pretorius SG, Wessels DH, Venter C, Potgieter MA, Lang AM, Koose T, Bernhardt E. Biotransformation of insulin glargine after subcutaneous injection in healthy subjects. Curr Med Res Opin. (2003);19:34–40. doi: 10.1185/030079902125001416. [DOI] [PubMed] [Google Scholar]
- Kurtzhals P, Schäffer L, Sørensen A, Kristensen C, Jonassen I, Schmid C, Trüb T. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes. (2000);49:999–1005. doi: 10.2337/diabetes.49.6.999. [DOI] [PubMed] [Google Scholar]
- Lee WM, Lu S, Medline A, Archer MC. Susceptibility of lean and obese Zucker rats to tumorigenesis induced by N-methyl-N-nitrosourea. Cancer Lett. (2001);162:155–160. doi: 10.1016/s0304-3835(00)00635-2. [DOI] [PubMed] [Google Scholar]
- LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. (1995);16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- Levemir® prescribing information. (2009). http://www.levemir-us.com/ http://www.levemir-us.com/ Accessed 26 January, 2011. Prescribing Information.
- Levemir: EPAR European Medical Association. (2004). http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000528/WC500036658.pdf. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000528/WC500036658.pdf Levemir: EPAR – Scientific Discussion. Accessed April 22, 2011.
- Lind M, Fahlén M, Eliasson B, Odén A. The relationship between the exposure time of insulin glargine and risk of breast and prostate cancer: an observational study of the time-dependent effects of antidiabetic treatments in patients with diabetes. Prim Care Diabetes. (2012);6:53–59. doi: 10.1016/j.pcd.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Ljung R, Talbäck M, Haglund B, Jonasson JM, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies - a three-year population-based observation. Acta Oncol. (2011);50:685–693. doi: 10.3109/0284186X.2011.558913. [DOI] [PubMed] [Google Scholar]
- Lucidi P, Porcellati F, Rossetti P, Andreoli AM, Cioli P, Hahn A, Schmidt R, Bolli GB, Fanelli CG. Metabolism of insulin glargine after repeated daily subcutaneous injections in subjects with type 2 diabetes. Diabetes Care. (2012);35:2647–2649. doi: 10.2337/dc12-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannucci E, Monami M, Balzi D, Cresci B, Pala L, Melani C, Lamanna C, Bracali I, Bigiarini M, Barchielli A, Marchionni N, Rotella CM. Doses of insulin and its analogues and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care. (2010);33:1997–2003. doi: 10.2337/dc10-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markussen J, Havelund S, Kurtzhals P, Andersen AS, Halstrøm J, Hasselager E, Larsen UD, Ribel U, Schäffer L, Vad K, Jonassen I. Soluble, fatty acid acylated insulins bind to albumin and show protracted action in pigs. Diabetologia. (1996);39:281–288. doi: 10.1007/BF00418343. [DOI] [PubMed] [Google Scholar]
- Mayer D, Shukla A, Enzmann H. Proliferative effects of insulin analogues on mammary epithelial cells. Arch Physiol Biochem. (2008);114:38–44. doi: 10.1080/13813450801900645. [DOI] [PubMed] [Google Scholar]
- Milazzo G, Sciacca L, Papa V, Goldfine ID, Vigneri R. ASPB10 insulin induction of increased mitogenic responses and phenotypic changes in human breast epithelial cells: evidence for enhanced interactions with the insulin-like growth factor-I receptor. Mol Carcinog. (1997);18:19–25. doi: 10.1002/(sici)1098-2744(199701)18:1<19::aid-mc3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Moller DE, Yokota A, Caro JF, Flier JS. Tissue-specific expression of two alternatively spliced insulin receptor mRNAs in man. Mol Endocrinol. (1989);3:1263–1269. doi: 10.1210/mend-3-8-1263. [DOI] [PubMed] [Google Scholar]
- Morden NE, Liu SK, Smith J, Mackenzie TA, Skinner J, Korc M. Further exploration of the relationship between insulin glargine and incident cancer: a retrospective cohort study of older Medicare patients. Diabetes Care. (2011);34:1965–1971. doi: 10.2337/dc11-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosthaf L, Grako K, Dull TJ, Coussens L, Ullrich A, McClain DA. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J. (1990);9:2409–2413. doi: 10.1002/j.1460-2075.1990.tb07416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxham CP, Duronio V, Jacobs S. Insulin-like growth factor I receptor beta-subunit heterogeneity. Evidence for hybrid tetramers composed of insulin-like growth factor I and insulin receptor heterodimers. J Biol Chem. (1989);264:13238–13244. [PubMed] [Google Scholar]
- Müssig K, Staiger H, Kantartzis K, Fritsche A, Kanz L, Häring HU. Type 2 diabetes mellitus and risk of malignancy: is there a strategy to identify a subphenotype of patients with increased susceptibility to endogenous and exogenous hyperinsulinism? Diabet Med. (2011);28:276–286. doi: 10.1111/j.1464-5491.2010.03132.x. [DOI] [PubMed] [Google Scholar]
- Nagel JM, Staffa J, Renner-Müller I, Horst D, Vogeser M, Langkamp M, Hoeflich A, Göke B, Kolligs FT, Mantzoros CS. Insulin glargine and NPH insulin increase to a similar degree epithelial cell proliferation and aberrant crypt foci formation in colons of diabetic mice. Horm Cancer. 2010a;1:320–330. doi: 10.1007/s12672-010-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel JM, Mansmann U, Wegscheider K, Röhmel J. Insulin resistance and increased risk for malignant neoplasms: confounding of the data on insulin glargine. Diabetologia. 2010b;53:206–208. doi: 10.1007/s00125-009-1535-0. [DOI] [PubMed] [Google Scholar]
- Nishimura E, Sørensen AR, Hansen BF, Stidsen CE, Olsen GS, Schaäffer L, et al. Insulin degludec: A new ultra-long, basal insulin designed to maintain full metabolic effect while minimizing mitogenic potential. Diabetologia. (2010);53:S388–S389. [Google Scholar]
- Oleksiewicz MB, Bonnesen C, Hegelund AC, Lundby A, Holm GM, Jensen MB, Krabbe JS. Comparison of intracellular signalling by insulin and the hypermitogenic AspB10 analogue in MCF-7 breast adenocarcinoma cells. J Appl Toxicol. (2011);31:329–341. doi: 10.1002/jat.1590. [DOI] [PubMed] [Google Scholar]
- Olsen HB, Kaarsholm NC. Structural effects of protein lipidation as revealed by LysB29-myristoyl, des(B30) insulin. Biochemistry. (2000);39:11893–11900. doi: 10.1021/bi001201i. [DOI] [PubMed] [Google Scholar]
- Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. (2002);277:39684–39695. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- Pandini G, Vigneri R, Costantino A, Frasca F, Ippolito A, Fujita-Yamaguchi Y, Siddle K, Goldfine ID, Belfiore A. Insulin and insulin-like growth factor-I (IGF-I) receptor overexpression in breast cancers leads to insulin/IGF-I hybrid receptor overexpression: evidence for a second mechanism of IGF-I signaling. Clin Cancer Res. (1999);5:1935–1944. [PubMed] [Google Scholar]
- Pocock SJ, Smeeth L. Insulin glargine and malignancy: an unwarranted alarm. Lancet. (2009);374:511–513. doi: 10.1016/S0140-6736(09)61307-6. [DOI] [PubMed] [Google Scholar]
- Pollex E, Moretti ME, Koren G, Feig DS. Safety of insulin glargine use in pregnancy: a systematic review and meta-analysis. Ann Pharmacother. (2011);45:9–16. doi: 10.1345/aph.1P327. [DOI] [PubMed] [Google Scholar]
- Pollex EK, Feig DS, Lubetsky A, Yip PM, Koren G. Insulin glargine safety in pregnancy: a transplacental transfer study. Diabetes Care. (2010);33:29–33. doi: 10.2337/dc09-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcellati F, Lucidi P, Rossetti P, Candeloro P, Andreoli AM, Marzotti S, Cioli P, Bolli GB, Fanelli CG. Differential effects of adiposity on pharmacodynamics of basal insulin NPH, glargine, and detemir in type 2 diabetes mellitus. Diabetes Care. (2011);34(12):2521–2523. doi: 10.2337/dc11-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock J, Fonseca V, McGill JB, Riddle M, Hallé JP, Hramiak I, Johnston P, Davis M. Similar progression of diabetic retinopathy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: a long-term, randomised, open-label study. Diabetologia. 2009a;52:1778–1788. doi: 10.1007/s00125-009-1415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock J, Fonseca V, McGill JB, Riddle M, Hallé JP, Hramiak I, Johnston P, Davis M. Similar risk of malignancy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: findings from a 5 year randomised, open-label study. Diabetologia. 2009b;52:1971–1973. doi: 10.1007/s00125-009-1452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosskamp RH, Park G. Long-acting insulin analogs. Diabetes Care. (1999);22 Suppl 2:B109–B113. [PubMed] [Google Scholar]
- Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, et al. Risk of cancer in patients on insulin glargine and other insulin analogues in comparison with those on human insulin: results from a large population-based follow-up study. Diabetologia. (2012);55(1):51–62. doi: 10.1007/s00125-011-2312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow J. Growth effects of insulin and insulin analogues. Arch Physiol Biochem. (2009);115:72–85. doi: 10.1080/13813450902835690. [DOI] [PubMed] [Google Scholar]
- Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Abnormal glucose tolerance and the risk of cancer death in the United States. Am J Epidemiol. (2003);157:1092–1100. doi: 10.1093/aje/kwg100. [DOI] [PubMed] [Google Scholar]
- Sciacca L, Cassarino MF, Genua M, Pandini G, Le Moli R, Squatrito S, Vigneri R. Insulin analogues differently activate insulin receptor isoforms and post-receptor signalling. Diabetologia. (2010);53:1743–1753. doi: 10.1007/s00125-010-1760-6. [DOI] [PubMed] [Google Scholar]
- Sciacca L, Costantino A, Pandini G, Mineo R, Frasca F, Scalia P, Sbraccia P, Goldfine ID, Vigneri R, Belfiore A. Insulin receptor activation by IGF-II in breast cancers: evidence for a new autocrine/paracrine mechanism. Oncogene. (1999);18:2471–2479. doi: 10.1038/sj.onc.1202600. [DOI] [PubMed] [Google Scholar]
- Serrano R, Villar M, Martínez C, Carrascosa JM, Gallardo N, Andrés A. Differential gene expression of insulin receptor isoforms A and B and insulin receptor substrates 1, 2 and 3 in rat tissues: modulation by aging and differentiation in rat adipose tissue. J Mol Endocrinol. (2005);34:153–161. doi: 10.1677/jme.1.01635. [DOI] [PubMed] [Google Scholar]
- Shukla A, Grisouard J, Ehemann V, Hermani A, Enzmann H, Mayer D. Analysis of signaling pathways related to cell proliferation stimulated by insulin analogs in human mammary epithelial cell lines. Endocr Relat Cancer. (2009);16:429–441. doi: 10.1677/ERC-08-0240. [DOI] [PubMed] [Google Scholar]
- Siddle K, Ursø B, Niesler CA, Cope DL, Molina L, Surinya KH, Soos MA. Specificity in ligand binding and intracellular signalling by insulin and insulin-like growth factor receptors. Biochem Soc Trans. (2001);29:513–525. doi: 10.1042/bst0290513. [DOI] [PubMed] [Google Scholar]
- Slieker LJ, Brooke GS, DiMarchi RD, Flora DB, Green LK, Hoffmann JA, Long HB, Fan L, Shields JE, Sundell KL, Surface PL, Chance RE. Modifications in the B10 and B26-30 regions of the B chain of human insulin alter affinity for the human IGF-I receptor more than for the insulin receptor. Diabetologia. (1997);40 Suppl 2:S54–S61. doi: 10.1007/s001250051402. [DOI] [PubMed] [Google Scholar]
- Smith U, Gale EA. Does diabetes therapy influence the risk of cancer? Diabetologia. (2009);52:1699–1708. doi: 10.1007/s00125-009-1441-5. [DOI] [PubMed] [Google Scholar]
- Sommerfeld MR, Müller G, Tschank G, Seipke G, Habermann P, Kurrle R, Tennagels N. In vitro metabolic and mitogenic signaling of insulin glargine and its metabolites. PLoS ONE. (2010);5:e9540. doi: 10.1371/journal.pone.0009540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soos MA, Field CE, Siddle K. Purified hybrid insulin/insulin-like growth factor-I receptors bind insulin-like growth factor-I, but not insulin, with high affinity. Biochem J. (1993);290 (Pt 2):419–426. doi: 10.1042/bj2900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger K, Hennige AM, Staiger H, Häring HU, Kellerer M. Comparison of the mitogenic potency of regular human insulin and its analogue glargine in normal and transformed human breast epithelial cells. Horm Metab Res. (2007);39:65–67. doi: 10.1055/s-2007-957352. [DOI] [PubMed] [Google Scholar]
- Staiger K, Staiger H, Schweitzer MA, Metzinger E, Balletshofer B, Häring HU, Kellerer M. Insulin and its analogue glargine do not affect viability and proliferation of human coronary artery endothelial and smooth muscle cells. Diabetologia. (2005);48:1898–1905. doi: 10.1007/s00125-005-1874-4. [DOI] [PubMed] [Google Scholar]
- Stammberger I, Bube A, Durchfeld-Meyer B, Donaubauer H, Troschau G. Evaluation of the carcinogenic potential of insulin glargine (LANTUS) in rats and mice. Int J Toxicol. (2002);21:171–179. doi: 10.1080/10915810290096306. [DOI] [PubMed] [Google Scholar]
- Suissa S, Azoulay L, Dell’Aniello S, Evans M, Vora J, Pollak M. Long-term effects of insulin glargine on the risk of breast cancer. Diabetologia. (2011);54:2254–2262. doi: 10.1007/s00125-011-2190-9. [DOI] [PubMed] [Google Scholar]
- Surmacz E. Function of the IGF-I receptor in breast cancer. J Mammary Gland Biol Neoplasia. (2000);5:95–105. doi: 10.1023/a:1009523501499. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. (2006);7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Tennagels N, Sommerfeld M, Muller G, Tschank G, Habermann P, Seipke G, et al. Metabolic and mitogenic signaling of AspB10 and insulin glargine in vitro and in vivo [abstract] Diabetes. 2011a;60:0076-OR. doi: 10.1371/journal.pone.0009540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennagels N, Welte S, Jordan H, Hoffman M, Werner U. Characteristics of insulin glargine signalling in rats [abstract] Diabetes. 2011b;60:1562-P. [Google Scholar]
- Tennagels N, Welte S, Jordan H, Hoffmann M, Schmidt R, Werner U. Insulin receptor and IGF-1-receptor signaling by insulin glargine after treatment with therapeutic and supra-pharmacological doses in rats. Diabetes. (2012);61(Suppl 1):A429. [Google Scholar]
- The ORIGIN trial investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. (2012);367:319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- Tran TT, Gupta N, Goh T, Naigamwalla D, Chia MC, Koohestani N, Mehrotra S, McKeown-Eyssen G, Giacca A, Bruce WR. Direct measure of insulin sensitivity with the hyperinsulinemic-euglycemic clamp and surrogate measures of insulin sensitivity with the oral glucose tolerance test: correlations with aberrant crypt foci promotion in rats. Cancer Epidemiol Biomarkers Prev. (2003);12:47–56. [PubMed] [Google Scholar]
- Tran TT, Naigamwalla D, Oprescu AI, Lam L, McKeown-Eyssen G, Bruce WR, Giacca A. Hyperinsulinemia, but not other factors associated with insulin resistance, acutely enhances colorectal epithelial proliferation in vivo. Endocrinology. (2006);147:1830–1837. doi: 10.1210/en.2005-1012. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. (1998);352:837–853. [PubMed] [Google Scholar]
- Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. (1986);5:2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Staa TP, Patel D, Gallagher AM, de Bruin ML. Glucose-lowering agents and the patterns of risk for cancer: a study with the General Practice Research Database and secondary care data. Diabetologia. (2012);55:654–665. doi: 10.1007/s00125-011-2390-3. [DOI] [PubMed] [Google Scholar]
- Varewijck AJ, Janssen JA, Vähätalo M, Hofland LJ, Lamberts SW, Yki-Järvinen H. Addition of insulin glargine or NPH insulin to metformin monotherapy in poorly controlled type 2 diabetic patients decreases IGF-I bioactivity similarly. Diabetologia. (2012);55:1186–1194. doi: 10.1007/s00125-011-2435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella V, Pandini G, Sciacca L, Mineo R, Vigneri R, Pezzino V, Belfiore A. A novel autocrine loop involving IGF-II and the insulin receptor isoform-A stimulates growth of thyroid cancer. J Clin Endocrinol Metab. (2002);87:245–254. doi: 10.1210/jcem.87.1.8142. [DOI] [PubMed] [Google Scholar]
- Vigneri R, Squatrito S, Sciacca L. Insulin and its analogs: actions via insulin and IGF receptors. Acta Diabetol. (2010);47:271–278. doi: 10.1007/s00592-010-0215-3. [DOI] [PubMed] [Google Scholar]
- Wada T, Azegami M, Sugiyama M, Tsuneki H, Sasaoka T. Characteristics of signalling properties mediated by long-acting insulin analogue glargine and detemir in target cells of insulin. Diabetes Res Clin Pract. (2008);81:269–277. doi: 10.1016/j.diabres.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Weber RV, Stein DE, Scholes J, Kral JG. Obesity potentiates AOM-induced colon cancer. Dig Dis Sci. (2000);45:890–895. doi: 10.1023/a:1005560621722. [DOI] [PubMed] [Google Scholar]
- Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev. (2009);25:41–49. doi: 10.1002/dmrr.912. [DOI] [PubMed] [Google Scholar]
- Werner H, Weinstein D, Yehezkel E, Laron Z. Controversies in the use of insulin analogues. Expert Opin Biol Ther. 2011a;11:199–209. doi: 10.1517/14712598.2011.540233. [DOI] [PubMed] [Google Scholar]
- Werner U, Sommerfeld M, Müller G, Tschank G, Habermann P, Seipke G, et al. Differential metabolic and mitogenic signalling of AspB10 and insulin glargine in vitro and in vivo. Diabetologia. 2011b;54 (Suppl1): S237. [Google Scholar]
- Werner U, Schmidt R, Blair E, Renna SM, Tennagels N. The molecular mechanism of insulin glargine metabolism in vivo . Diabetes. 2012;61(Suppl 1):A425. [Google Scholar]
- Yehezkel E, Weinstein D, Simon M, Sarfstein R, Laron Z, Werner H. Long-acting insulin analogues elicit atypical signalling events mediated by the insulin receptor and insulin-like growth factor-I receptor. Diabetologia. (2010);53:2667–2675. doi: 10.1007/s00125-010-1899-1. [DOI] [PubMed] [Google Scholar]
- Zenobi PD, Jaeggi-Groisman SE, Riesen WF, Røder ME, Froesch ER. Insulin-like growth factor-I improves glucose and lipid metabolism in type 2 diabetes mellitus. J Clin Invest. (1992);90:2234–2241. doi: 10.1172/JCI116109. [DOI] [PMC free article] [PubMed] [Google Scholar]