Abstract
This review provides a basis for substantiating both kinetically and pathologically the differences between chrysotile and amphibole asbestos. Chrysotile, which is rapidly attacked by the acid environment of the macrophage, falls apart in the lung into short fibers and particles, while the amphibole asbestos persist creating a response to the fibrous structure of this mineral. Inhalation toxicity studies of chrysotile at non-lung overload conditions demonstrate that the long (>20 µm) fibers are rapidly cleared from the lung, are not translocated to the pleural cavity and do not initiate fibrogenic response. In contrast, long amphibole asbestos fibers persist, are quickly (within 7 d) translocated to the pleural cavity and result in interstitial fibrosis and pleural inflammation. Quantitative reviews of epidemiological studies of mineral fibers have determined the potency of chrysotile and amphibole asbestos for causing lung cancer and mesothelioma in relation to fiber type and have also differentiated between these two minerals. These studies have been reviewed in light of the frequent use of amphibole asbestos. As with other respirable particulates, there is evidence that heavy and prolonged exposure to chrysotile can produce lung cancer. The importance of the present and other similar reviews is that the studies they report show that low exposures to chrysotile do not present a detectable risk to health. Since total dose over time decides the likelihood of disease occurrence and progression, they also suggest that the risk of an adverse outcome may be low with even high exposures experienced over a short duration.
Keywords: Amphibole asbestos, cement products, chrysotile, epidemiology, health risk, inhalation toxicology, mining
Introduction
Recent scientific studies have contributed to a more complete understanding of the health risk from chrysotile asbestos as used today in high-density products. Key to understanding this is the differentiation of exposure, dose and response of the serpentine mineral chrysotile in comparison to the amphibole asbestos types such as crocidolite, tremolite and amosite. This paper reviews scientific studies identified as chrysotile only or predominately chrysotile and discusses how the newer toxicological and epidemiological data provide a convergence in the understanding of the risk from chrysotile.
The association of asbestos exposure with disease dates from the turn of the twentieth century (McDonald & McDonald, 1996). The report by Wagner et al. (1960), reporting on 33 cases of mesothelioma, which the authors stated were primarily from the crocidolite mining area in the North West Cape Province of South Africa (18 out of 33 cases), was instrumental in establishing a relationship to asbestos exposure. While the relationship Wagner et al. (1960) described concerned individuals working primarily in crocidolite mining, there was virtually no quantification of exposure at this time. Subsequently, Selikoff et al. (1984), reported on 632 insulation workers exposed to asbestos who entered the trade before 1943 and were traced through 1962; 45 died of cancer of the lung or pleura, whereas only 6.6 such deaths were expected. Three of the pleural tumors were mesotheliomas; there was also one peritoneal mesothelioma. The use of the generic term “asbestos” to describe both minerals, the serpentine chrysotile and members of the amphibole family (amosite, crocidolite, tremolite, anthophyllite and actinolite, of which only the first two were industrially important) and the lack of complete occupational histories are significant limitations in the early epidemiology studies, resulting in improper characterization of fiber-specific exposure. These factors further confused and effectively prevented differentiation in the association of disease to fiber mineral type. In addition, because of the common use of the name “asbestos” for either of the two mineral types and their similar uses, it was conceivable to imagine that all asbestos types could have similar potency. In essence, because the same name was used for these two very different minerals, the impetus was to equate rather than differentiate the two.
As a result of the frequent use of the all-inclusive term asbestos and the limitations in analysis and identification, most studies through the late 1990s provided little quantitative scientific basis for distinguishing between the effects of chrysotile as compared to those of amphibole asbestos. NIOSH (2011) in their Asbestos Roadmap, stated that “Imprecise terminology and mineralogical complexity have affected progress in research. ‘Asbestos’ and ‘asbestiform’ are two commonly used terms that lack mineralogical precision. ‘Asbestos’ is a term used for certain minerals that have crystallized in a particular macroscopic habit with certain commercially useful properties”. And, “The use of non-standard terminology or terms with imprecise definitions when reporting studies makes it difficult to fully understand the implications of these studies or to compare the results to those of other studies”.
The differences in serpentine and amphibole asbestos
The physical and chemical properties which differentiate chrysotile which is a serpentine mineral from the amphibole asbestos types such as amosite and crocidolite have only recently been factored into the understanding of the toxicology and epidemiology of these minerals. The use of the common name asbestos for both of these mineral types further obscured the important differences between the serpentine and amphibole fibers. In addition, some of the earlier methods of characterization of the fibers were rudimentary in that length and width were generally not addressed, even if the fiber type was reported.
Chrysotile was first described by von Kobell (1834). The name chrysotile was derived by combining the Greek words for golden and fibrous. von Kobell described that chrysotile is distinguished by its behavior of being decomposed by acid. The curved structure of the Mg-analog of kaolinite was suggested by Pauling (1930) because of the misfit between the octahedral and tetrahedral sheets. The crystal structure of chrysotile asbestos was first determined by Warren & Bragg (1930). Subsequently, Noll & Kircher (1951) and Bates et al. (1950) published electron micrographs showing cylindrical and apparently hollow chrysotile fibers. Chrysotile is one of the three different polymorphs of serpentine (antigorite, lizardite and chrysotile) that are thought to be the result of different structural mechanisms which reduce strain in the formations (Evans, 2004; Veblen & Wylie, 1993; Wicks & O’Hanley, 1988).
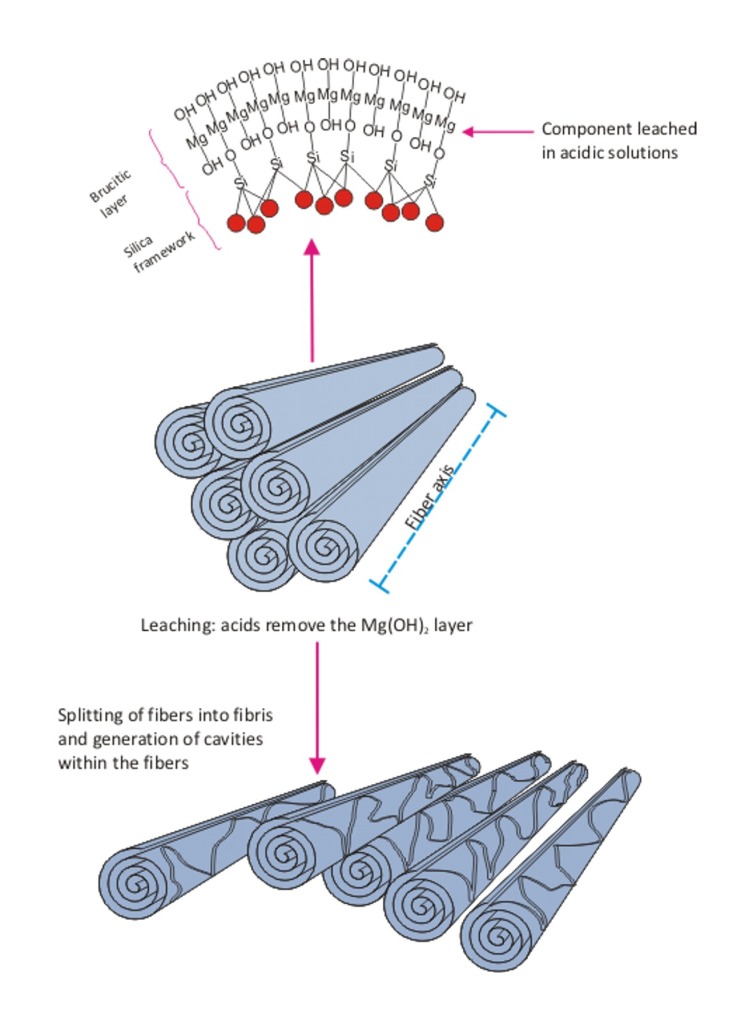
Chrysotile has the approximate composition Mg3Si205(OH)4 and is a sheet silicate composed of silicate and brucite layers. The silica layer is a tetrahedra in a pseudohexagonal network. Joined to this is a sheet of magnesium hydroxide octahedra, in which on one side, two out of every three hydroxyls are replaced by apical oxygens of the silica tetrahedral (Cressey & Whittaker, 1993). The different dimensions of these two components result in a structural mismatch in which the layers curl, concentrically or spirally. The fiber walls are made up of approximately 12–20 of these layers in which there is some mechanical interlocking. However, there is no chemical bonding as such between the layers. Each layer is about 7.3 Å. thick, with the magnesium hydroxide part of each layer closest to the fiber surface and the silicon–oxygen tetrahedra “inside” the curl (Whittaker, 1963, 1957; Tanji, 1985. Titulaer et al. (1993, Table 2) reported on the porous structure of chrysotile by transmission electron microscopy (TEM). Based upon a number of samples, the authors determined that the thickness of the chrysotile wall in the fibers ranged from 8 to 15 nm, with from 11 to 21 sheets in each tube wall.
Table 2. .
Epidemiological studies characterized as predominately chrysotile exposure by Hodgson & Darnton (2000).
The structure of chrysotile is shown in Figure 1 (as a rolled sheet although concentric sheets also occur). The cylinders are chrysotile fibrils which bunch together to form a chrysotile fiber. The magnesium is on the outside of the roll and, as discussed below, the magnesium layer is soluble in biological systems. The magnesium is readily attacked by the acid milieu inside the macrophage (pH 4–4.5), and dissociates from the crystalline structure, leaving the now unstable silicate sheet. This process causes the rolled sheet of the chrysotile fiber to break apart and decompose into smaller pieces. These pieces can then be readily cleared from the lung by macrophages through mucociliary and lymphatic clearance. Fibers cleared on the mucociliary escalator are cleared to the gut where they are attacked by the even stronger acid environment (hydrochloric acid, pH 1.2, Oze & Solt (2010)) of the stomach.
Figure 1. .
Schematic illustration of the chrysotile fiber. Chrysotile is a rolled sheet or concentrate rings of silicate with the magnesium on the outside of the sheet and the silica on the inside. The chrysotile fiber is acid soluble. Chrysotile has the formula Mg3Si2O5(OH)4. The fiber consists of magnesium hydroxide layers condensed onto silicon·oxygen tetrahedra. The fiber walls are made up of 11 to 21 such layers in which there is some mechanical interlocking. There is not any chemical bonding as such between the layers, however. Each layer is about 7.3 Å thick. The Mg(OH)2 part of the molecule layers is closest to the fiber surfaces; the silicon–oxygen tetrahedra are inside. Under the acid conditions associated with the macrophage, the fiber structure is weakened and the long fibers break into short pieces which can be engulfed and cleared by the macrophages.
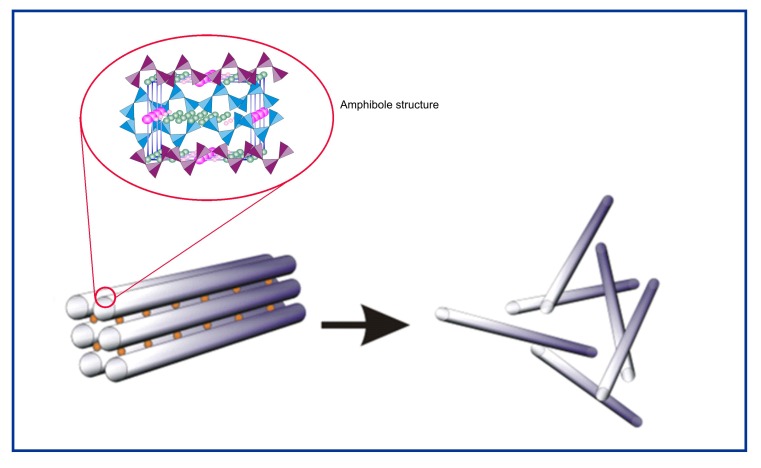
In contrast, the amphibole asbestos class of fibers is formed as solid rods/fibers (Skinner et al., 1988; Whittaker, 1960). The structure of an amphibole is a double chain of tetrahedral silicate with the silica on the outside of the fiber which makes it very strong and durable (Figure 2). There are five asbestiform varieties of amphiboles: anthophyllite asbestos, grunerite asbestos (amosite), riebeckite asbestos (crocidolite), tremolite asbestos and actinolite asbestos. Of these, crocidolite and amosite were the only amphiboles with significant industrial uses (Virta, 2002). Tremolite, while not used commercially, has been found as a contaminant in other fibers or in other industrial minerals (e.g. chrysotile and talc). The chemical composition of the amphiboles fibers is more complex and the idealized chemical formulae of the five amphiboles are shown below. Although their structures are the same, this variability in composition is a direct consequence of the fact that the silicate framework can accommodate a mixture of many different ions (as determined by the host rock) in the space between the silicate ribbons which form the fibers (Speil & Leineweber, 1969).
Figure 2. .
With amphiboles, the soluble cations shown as small circles are located between the fibers which are formed with double chain silicate. When the soluble cations dissolve as can happen in the lung, the amphibole fibers in these bundles are released as individual fibers. The double chain silicate amphibole fibers themselves are highly insoluble in both the lung fluids and in the macrophages.
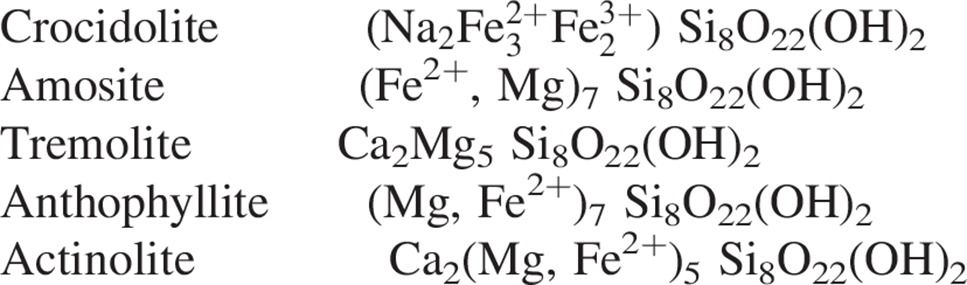 |
The crystalline structure common to amphibole minerals consists of two ribbons of silicate tetrahedra placed back to back (Virta, 2002).
Due to the structural matrix of amphibole fibers, they have negligible solubility at any pH that might be encountered in an organism (Speil & Leineweber, 1969). Some associated surface contaminating metals such as iron can become ionized and can then be released from the fiber (Aust et al., 2011).
In-vitro biodurability
The magnesium hydroxide part of each layer being closest to the fiber surface is reflected in the chemical characteristics of chrysotile, which has poor acid resistance compared to other asbestiform substances. The amphiboles, for example, in which the silicate oxygens are on the “outside” of the layers and the hydroxides are masked within, have better resistance to acids. Hargreaves & Taylor (1946) reported that if fibrous chrysotile is treated with dilute acid, the magnesia can be completely removed. The hydrated silica which remains, though fibrous in form, had completely lost the elasticity characteristic of the original chrysotile and had a structure that was “amorphous” or “glassy” in type. Wypych et al. (2005) examined what happens to natural chrysotile fibers when acid-leached under controlled conditions. The authors reported that the leached products consisted of layered hydrated disordered silica with a “distorted” structure resembling the silicate layer existing in the original minerals. Extensive characterization techniques confirmed the removal of the brucite-like sheets, leaving silica with an eminently amorphous structure. Suquet (1989) reported on the assessment of the structural damage produced by grinding or acid leaching of chrysotile. The author reported that “Acid leaching transformed chrysotile into porous, non-crystalline hydrated silica, which easily fractured into short fragments. If the acid attack was too severe, these fragments converted into shapeless material”.
Seshan (1983) reported that following exposure to water, strong acids and simulated gastric juices, chrysotile asbestos underwent changes in the physical, chemical and surface properties. The authors reported that the surface becomes silica-like and that upon exposure to water and acid the magnesium is lost from the fibers. The authors also reported that upon acid exposure, the magnesium ions are leached out, leaving a magnesium-free silica network. In addition, the acid treatment also destroyed the X-ray diffraction pattern of chrysotile and changed its refractive index. In contrast, crocidolite asbestos remained unchanged.
Larsen (1989) evaluated different types of natural and synthetic fibers which had been subjected to systematic solubility tests in vitro in a physiological solution at 37 °C. Included in this evaluation were chrysotile and crocidolite. Solubility was evaluated by the measurement of silicon in a Gamble’s solution similar in composition to lung fluid (without the organic components) using atomic absorption spectrophotometry. The authors reported that the dissolution values ranged from a few nanograms of silicon dissolved per cm2 (chrysotile and crocidolite) to several thousands of ng/cm2 silicon dissolved (glass wools) and that aramide and carbon fibers proved to be practically insoluble. For chrysotile, the authors reported that after a 6-week shaking-table experiment (closed system) that 6 ng/cm2 silicon and 160 ng/cm2 magnesium had dissolved.
Oze & Solt (2010) investigated the biodurability of chrysotile and tremolite asbestos in simulated lung and gastric fluids. The simulated gastric fluid (SGF) was composed of HCl and NaCl solution at a pH 1.2 and the simulated lung fluid (SLF) was a modified Gamble’s solution at pH 7.4 at 37 °C. The studies were performed under batch conditions using 0.01, 0.1 and 1 g of ground fiber in a 50 ml vial over 720 h in apparently static conditions. There was no discussion of the influence of the large number of fibers present in such quantities on fluid contact and whether the suspensions settled over time. The relative biodurabilities determined under these conditions were (from most to least) tremolite (SLF) > chrysotile (SLF) > tremolite (SGF) > chrysotile (SGF) when accounting for the greater surface area of chrysotile per mass or per fiber compared to tremolite. Silica release from chrysotile was 30–66 times greater under acid conditions as compared to neutral pH. The authors estimated that a chrysotile fiber will dissolve ∼200 × faster in SLF and ∼2.5 × faster in SGF compared to tremolite asbestos. The authors calculated that a 1 × 10 μm chrysotile fiber will completely dissolve in neutral pH in ∼19 months while a tremolite fiber of equal shape will dissolve in ∼4 years. At acid pH, a chrysotile fiber of the same dimensions will dissolve in ∼33 h and a tremolite fiber will dissolve in ∼9 months. The authors pointed out that these values represent approximate fiber lifetimes and do not account for changes in the surface area with respect to time, or for preferential dissolution sites such as crystal defects or edges. In addition, these times do not take into account the inflammatory processes in the lung that have been shown to occur with tremolite and their influence on dissolution rates.
In another study using a Gambles solution, Osmon-McLeod et al. (2011) assessed the durability of a number of fibers including long fiber amosite and long fiber chrysotile. In this study, the pH of the Gambles solution was adjusted to 4.5 to mimic that inside macrophage phagolysosomes, which the authors described as “potentially the most degradative environment that a particle should encounter following lung deposition and macrophage uptake”. Fiber durability was assessed from the loss of mass of the fiber. The chrysotile was recovered with ∼30% of original weight after the 24-week incubation. The amosite asbestos was recovered with ∼75% of original weight. None of the carbon nano-tube samples included in the study showed a significant loss of mass by week 24 with one exception which was recovered at only ∼70% of its original weight at all time-points from week 3 onward. The authors stated that for chrysotile, the percent recoveries reflect true mass loss, whereas the small mass loss for amosite asbestos over the 24-week period may be due to the loss of small fibers in the sample. The chrysotile showed no difference in average fiber width with incubation, but did show a marked decrease in length. At 0 weeks the chrysotile sample comprised a mixture of fibrils and ropes of fibrils, while at 10 weeks only small fibrils remained. The authors commented that it is probable that the measured loss of length accurately reflects fiber shortening in addition to the breaking up of large fiber bundles. Pathogenicity of these samples was also evaluated in vivo using a mouse model sensitive to inflammogenic effects of fibers. Osmon-McLeod et al. (2011) found that the data indicate that long fiber chrysotile showed ∼70% mass loss and a marked decrease in length with long-term incubation in the Gambles solution, with a concomitant mitigation of the pathogenicity seen in mice injected with 0 weeks samples. Long fiber amosite that had been incubated for 10 weeks, however, also showed a loss of mass comparable to one of the long carbon nano-tubes at the same time-point, but no fiber shortening, and did not lose its pathogenicity.
These studies illustrate the differences in dissolution rates between chrysotile and amphibole asbestos under both neutral and acidic conditions and provide support for understanding the results of the inhalation studies discussed below.
The relevance of early inhalation toxicology studies
The early inhalation toxicology studies of asbestos are often difficult to interpret. While they used rudimentary techniques to quantify concentrations and in general were unable to measure the dimension of fibers, the early inhalation toxicology studies should not be completely disregarded as they did give some, although limited, information on possible worker exposures. Exposure concentration was determined using gravimetric techniques without consideration of fiber number or fiber length and diameter, and little consideration was given to the length and diameter distribution of the fibers to which the animals were exposed. To fluidize the fibers to facilitate aerosol generation, the fibers were usually ground extensively which shortened the length and produced a very large number of particles and shorter fibers (Timbrell et al., 1968).
In early inhalation studies, such as those reported by Vorwald et al. (1951), fiber dust concentrations in the exposure chamber were produced using a rotating paddle in a dust hopper. Aerosol concentrations were reported based upon light microscopy in the range of 30–50 million particles and fibers per cubic foot. This corresponds to approximately 500 000 particles and fibers/cm3 if it were measured by TEM (Breysse et al., 1989). Subsequent studies such as those by Gross et al. (1967) based exposure on gravimetric concentration and reported a mean gravimetric concentration of 86 mg/m3 (range 42–146 mg/m3). There was no further characterization of the aerosol in this study. Following this, Wagner et al. (1974) reported on studies of UICC Canadian and Rhodesian chrysotile performed at a nominal concentration of 10 mg/m3. This gravimetric concentration of 10 mg/m3 became the standard concentration for subsequent studies by Wagner and other investigators through the 1980s with some investigators still reporting on studies at this exposure concentration more recently (e.g. Morris et al., 2004).
The historical chrysotile chronic inhalation studies are presented in Table A1 (Appendix). The exposure concentrations in all studies were based upon gravimetric determination. Of the 16 studies, six did not report the fiber concentration, eight reported estimates by phase contrast optical microscopy (PCOM) and three by scanning electron microscopy (SEM).
The two chrysotile samples used most often in these studies were either the UICC (Timbrell et al., 1968; Timbrell & Rendall, 1972) chrysotile or the NIEHS (Pinkerton et al., 1983) chrysotile. Both samples were ground extensively using large-scale milling machines.
The UICC chrysotile sample was milled using a “Classic Mill designed by R. F. Bourne, at The Asbestos Grading Equipment Company, Johannesburg, South Africa” (Timbrell et al., 1968). Timbrell & Rendall (1972) describe “The Classic mill is an air swept attrition mill fitted with a disc rotor (16 inch diameter) which carries four beaters and is mounted on a horizontal shaft driven by an electric motor at speeds up to 5000 rpm”. The patent (Patent number GB 3,490,704) on the mill provides greater detail.
The characteristics of the NIEHS chrysotile can be obtained from the publication by Pinkerton et al. (1983). They refer to an NTIS report by Campbell et al. (1980) concerning the actual preparation of the sample. The NIEHS chrysotile was prepared from a grade 4 chrysotile used in the plastics industry, which was prepared by passing the material through a hurricane pulverizer. The hurricane pulverizer is an industrial high-speed impact hammer mill with a size classifier which recycled larger fibers/particles back into the device for continued milling (Perry & Chilton, 1973; Work, 1963).
Suquet (1989) assessed the structural damage produced by grinding and acid leaching of chrysotile and the surface state of ground and leached products. The author reported that “Severe dry grinding converted chrysotile fibers into fragments cemented by a shapeless, non-crystalline material”. This comminution treatment apparently broke atomic bonds and produced strong potential reaction sites, which were able to adsorb CO2 and H2O molecules from the atmosphere.
The number of fibers that would have been present in a chrysotile aerosol with a gravimetric concentration of 10 mg/m3 has been estimated based upon a chronic inhalation study using NIEHS chrysotile (Hesterberg et al., 1993; Mast et al., 1995). In this study total fiber aerosol exposure was reported by SEM as 100 000 (World Health Organization) WHO* fibers/cm3. If measured by TEM, this would have likely been more than 1 000 000 fibers/cm3 (Breysse et al., 1989).
Exposure of rats to high aerosol concentrations of fibers creates a very different dose profile in the lung in comparison to human exposures. Rats are considerably smaller than humans and correspondingly rat lungs are more than 300 times smaller than human lungs. While the rat inhales proportionally less air per minute, the doses administered in some toxicology studies can result in unrealistic fiber lung burdens as compared to human exposure. In addition, for the rat which is a mandatory nasal breather, alveolar deposition is largely limited to fibers less than approximately 1 µm in diameter, while in humans this limit is approximately 3 µm (Morgan, 1995). For most asbestos fiber types, however, this difference is less important than for MMVF. The total chrysotile lung burden following 24 months of exposure in the Mast et al. (1995) study was 5.5 × 1010 fibers/lung as measured by SEM (Bernstein, 2007). With extrapolation to that which would have been observed by TEM, the lung burden would have been 9.4 × 1011 fibers/lung. This would correspond to an average of 2300 fibers per alveoli (assuming 10% deposition).
The gravimetric exposure concentrations ranged from 2 to 86 mg/m3, which based upon the extrapolation described above (Breysse et al., 1989; Mast et al., 1995), corresponds to between 200 000 and 8 600 000 fibers/cm3. The large majority of these earlier studies targeted 10 mg/m3. The single study performed at the lowest concentration of 2 mg/m3 had a comparative concentration group of 10 mg/m3. In this study, the author’s reported “With a 2 mg/m3 cloud the percentage retention of chrysotile is almost double that for a 10 mg/m3 cloud”, which reflects the difficulty of evaluating dose response at these overload conditions.
This is illustrated in Wagner et al.’s (1974) study which had five exposure periods at the same exposure concentration of 10 mg/m3. The exposure periods were (7 h/d, 5 wk) for either 1 d, 3, 6, 12 and 24 months with the animals maintained their lifetime. In the crocidolite exposed groups, the number of mesothelioma were 1 (1d grp), 1 (3m grp), 0 (6m grp), 2 (12m grp) and 0 for (24m grp). Thus, the 1 d of exposure produced more mesothelioma than the 24-month exposure most likely due to the effect of the high-exposure concentration, resulting with continued exposure in lung overload.
An asbestos exposure concentration of 10 mg/m3 corresponds to more than 10 million times the American Conference of Industrial Hygienists (ACGIH) threshold limit value (TLV) of 0.1 fiber/cm3 for asbestos.
The fiber size distribution and the ratio of longer fibers to shorter fibers and non-fibrous particle content are essential in determining the dose–response relationship to these fibers. Thus, it can become very difficult to use these studies for human risk assessment or even to compare the effects of one study with those of another.
The issue of using an equivalent fiber number for exposure was approached in a study reported by Davis et al. (1978) where chrysotile, crocidolite and amosite were compared on an equal mass and equal number basis. However, the fiber number was determined by phase contrast optical microscopy (PCOM) and thus the actual number, particularly of the chrysotile fibers, was probably greatly underestimated.
At such high exposure concentrations, it would be reasonable to expect that the number of particles and short fibers present in the exposure would be sufficient to overload the lung through impairment of macrophage function. These conditions which occurred in the earlier high gravimetric dose studies of ground chrysotile would be sufficient based upon studies with insoluble particles (Bolton et al., 1983; Morrow, 1988, 1992; Muhle et al., 1988; Oberdörster, 1995) to severely reduce the normal clearance of the chrysotile fibers from the lung and initiate a non-specific inflammatory and proliferative response which has been shown to lead for innocuous dusts to fibrosis and cancer. The following section discuses studies at several orders of magnitude above regulatory levels but without approaching the extremes discussed above.
The correlation of fiber length and biopersistence to chronic toxicity
The association that long fibers (20–50 µm) have with both lung and peritoneal disease, as opposed to shorter ball-milled fibers (3 µm or less), was reported as early as 1951 (Vorwald et al., 1951).
The importance of fiber length in the pathogenicity of fibers in the pleural cavity was investigated by Stanton (1972, 1973) in a series of studies on the relationship of fiber length and characteristics to their pathogenicity in on the pleural surface. The fibers were evaluated using a highly artificial exposure by implantation in gelatin, and placing them on the pleural mesothelial surface. The authors reported that in this system, carcinogenicity was related to “durable” fibers longer than 10 μm.
Davis et al. (1986) evaluated the toxicological response in chronic inhalation and interperitoneal injection studies to samples of either short (∼ < 5 µm) or long (∼ > 10 µm) amosite asbestos with equal airborne mass concentration. The authors reported that in the inhalation study with LFA the long fiber caused the development of widespread pulmonary fibrosis and one-third of the animals developed pulmonary tumors that were mesotheliomas. In the group with short fiber amosite no fibrosis or pulmonary or mesothelioma tumors were found in any animal.
Poland et al. (2008) reported on a study in which carbon nanotubes were compared with short fiber and long fiber amosite asbestos following intraperitoneal injection. The amosite samples were prepared by Davis et al. (1986) for use in the studies discussed above. 50 mg of each material was injected into the peritoneal (abdominal) cavity of mice and the cavity systematically lavaged at 24 h or 7 d post exposure with physiological saline. The long fiber amosite developed inflammatory and granulomatous changes while the short fiber amosite did not.
In a study investigating the biopersistence of synthetic mineral fibers (SMFs), Hammad et al. (1988) found that fibers <5 µm in length had the longest retention following short-term inhalation, with longer fibers clearing more rapidly and fibers >30 µm in length clearing very rapidly. He proposed that clearance of mineral wools is a result of biological clearance and the elimination of fibers by dissolution and subsequent breakage. However, there was no relationship between these phenomena and long-term toxicological effects.
Adamson (1993, 1994) exposed mice to long and short crocidolite asbestos and found that long fibers (>20 µm), which were deposited in bronchiolar regions induced fibrosis and a proliferative response while short fibers (<1 µm), which reached the alveoli did not induce fibrosis and a proliferative response.
Lippmann (1990), McClellan et al. (1992), WHO (1988), and Goodglick & Kane (1990) reviewed as well the importance of fiber length to the potential of a fiber to induce a pathogenic effect.
In an analysis that provided the basis for the European Commission’s directive on synthetic vitreous fibers (SVF), Bernstein et al. (2001a,b) reported that a good correlation exists for SVFs between the biopersistence of fibers longer than 20 µm and the pathological effects following either chronic inhalation or chronic intraperitoneal injection studies. This analysis showed that it was possible using the clearance half-time of the fibers longer than 20 µm as obtained from the inhalation biopersistence studies to predict the number of fibers longer than 20 µm remaining after 24 months of chronic inhalation exposure (Bernstein et al., 2007). These studies, however, only included SVFs.
Berman et al. (1995) statistically analyzed the results of 13 separate animal inhalation studies, which exposed animals to nine different asbestos types. Due to limitations in the characterization of asbestos structures in the original studies, new exposure measures were developed from samples of the original dusts, which were regenerated and analyzed by TEM. The authors reported that while no univariate model was found to provide an adequate description of the lung tumor responses in the inhalation studies, the measure most highly correlated with tumor incidence was the concentration of structures (fibers) ≥20 µm in length. However, using multivariate techniques, measures of exposure were identified which adequately described the lung tumor responses. The authors reported that
Structures contributing to lung tumor risk appear to be long (≥5 µm) thin (0.4 µm) fibers and bundles, with a possible contribution by long and very thick (≥5 µm) complex clusters and matrices. Potency appears to increase with increasing length, with structures longer than 40 µm being about 500 times more potent than structures between 5 and 40 µm in length. Structures <5 µm in length do not appear to make any contribution to lung tumor risk.
This analysis found no difference in the potency of chrysotile and amphibole regarding the induction of lung tumors. However, the authors stated that the mineralogy appears to be important in the induction of mesothelioma, with chrysotile being less potent than amphibole. These results, however, should be viewed in the context of the inhalation toxicology studies evaluated by Berman et al. (1995, Table 1), the majority of which were performed at very high concentrations (10 mg/m3). As discussed above, the overload effect from these very high exposure concentrations would be expected to produce similar tumorigenic response in the lung for chrysotile and amphibole.
Table 1. .
Capabilities and limitations of analytical techniques used for asbestos measurements (reproduced from Berman & Crump, 2003)†.
| Parameter | Midget impinger | Phase contrast microscopy | Scanning electron microscopy | Transmission electron microscopy |
|---|---|---|---|---|
| Range of magnification | 100 | 400 | 2000–10 000 | 5000–20 000 |
| Particles counted | All | Fibrous structures‡ | Fibrous structures‡ | Fibrous Structures‡,§ |
| Minimum diameter (size) | 1 µm | 0.3 µm | 0.1 µm | <0.01 µm |
| Visible | ||||
| Resolve internal structure | No | No | Maybe | Yes |
| Distinguish mineralogy¶ | No | No | Yes | Yes |
†The capabilities and limitations in this table are based primarily on the physical constraints of the indicated instrumentation. Differences attributable to the associated procedures and practices of methods in common use over the last 25 years are highlighted in Table 2.
‡Fibrous structures are defined here as particles exhibiting aspect ratios (the ratio of length to width) greater than 3 (Walton, 1982).
§TEM counts frequently resolve individual fibrous structures within larger, complex structures. Based on internal structure, several different counting rules have been developed for handling complex structures. See the discussion of methods presented below.
¶Most SEM and TEM instruments are equipped with the capability to record selected area electron diffraction (SAED) spectra and perform energy dispersive X-ray analysis (EDXA), which are used to distinguish the mineralogy of structures observed.
Recent studies on the serpentine asbestos, chrysotile, have shown that it is not very biopersistent in the lung (Bernstein et al., 2003, 2004, 2005a,b, 2011). As serpentine is a naturally occurring mined fiber, there appear to be some differences in biopersistence depending upon from where it is mined. However, chrysotile lies on the soluble end of this scale and ranges from the least biopersistent fiber to a fiber with biopersistence in the range of glass and stonewools. It remains less biopersistent than refractory ceramic fibers and special purpose glasses and more than an order of magnitude less biopersistent than amphibole asbestos (Bernstein, 2007). A 90 d sub-chronic inhalation toxicity study of chrysotile in rats showed that at an exposure concentration 5000 times greater than the US-ACGIH TLV of 0.1 f(WHO)/cm3, chrysotile produced no significant pathological response or sustained inflammatory response (Bernstein et al., 2006).
Some earlier studies have shown chrysotile to clear less rapidly than in the studies performed using the EC protocol. An example is the study by Coin et al. (1992) in which rats were exposed for 3 h to a NIEHS chrysotile aerosol of 10 mg (respirable)/m3 and then followed for a period of 29 d. The authors reported that through 3 weeks after cessation of exposure, fibers greater than 16 µm in length were cleared slowly, if at all.
While a brief description is provided, the details of the aerosol exposure to the NIEHS chrysotile which was used in the Coin et al. (1992) study are not described directly in the publication. However, the characteristics of the exposure aerosol and the preparation methods can be derived from an earlier publication by Pinkerton et al. (1983) referenced by Coin and a non-published report by Campbell et al. (1980) referenced by Pinkerton et al.
These publications describe that the chrysotile used by Coin et al. (1992) was prepared from a grade 4 chrysotile used in the plastics industry which was prepared by passing the material through a hurricane pulverizer. The hurricane pulverizer is an industrial high-speed impact hammer mill with a size classifier which recycled larger fibers/particles back into the device for continued milling (Perry & Chilton, 1973; Work, 1962).
The aerosol used in the Coin et al. (1992) study was generated from this ground material as described by Pinkerton et al. (1983) using a Timbrell generator (Timbrell, 1968). The stainless steel blades of this generator are known to further pulverize fiber samples. While the original chrysotile sample had 13.9% fibers longer than 19.9 µm (Campbell et al., 1980), the final aerosolized sample used in the Coin et al. (1992) study had 1.8% fibers longer than 19.9 µm (Pinkerton et al., 1983). For fibers ≥ 16 µm in length, Coin et al., only present the data graphically. Visual extrapolation from Figure 5 of Coin et al. indicates that there were approximately 2, 2, 5 and 4 × 105 fibers L ≥ 16 µm (measured by SEM) present at 1, 8, 15 and 29 d post-exposure, respectively, (no error bars were indicated and no tables of the values given). In addition, the Coin et al. (1992) study used a single exposure and examined sub-groups on animals for 3 weeks. The mean number of fibers found in the control animals was 7 × 105 WHO fibers per animal and 3 × 103 fibers ≥ 16 µm per animal, indicating contamination. No standard deviation is given, however, so the extent of this contamination remains unknown. Coin does not state how this contamination occurred. In the chrysotile studies performed following the EC protocol, animals were exposed for 5 d and then followed for 1 year post-exposure. In the EC protocol studies, no WHO fibers (including fibers with L > 20 µm) were observed in the lungs of any of the control animals.
Non-overload studies that evaluate the toxicity of chrysotile
As discussed above, the early toxicology studies were difficult to interpret. Concentration was determined using gravimetric techniques without consideration of fiber number or fiber length and diameter and little consideration was given to the dose, and the length and diameter distribution of the fibers to which the animals were exposed.
Chronic inhalation toxicity studies
While well-designed chronic inhalation toxicology studies limiting particle overload effects of SVFs have been performed, few chronic inhalation toxicology studies of asbestos have been performed taking this into account.
Davis et al. (1986) reported on the only chronic inhalation study that evaluated the pathogenicity of long versus short amosite asbestos. The short fiber amosite sample was produced so that almost all fibers were less than 5 µm in length with 70 WHO fibers/cm3 in the exposure atmosphere. The LFA had 2060 WHO fibers/cm3 with approximately half of this longer than 10 µm. The mass concentration of both groups was similar. The authors reported that following 12 months of exposure that significantly more short fiber amosite was present in the lung as compared to long fibers. The long fibers caused the development of widespread fibrosis, however, with the short fibers no fibrosis was found in any animal. In addition, one-third of the animals treated with long fibers developed pulmonary tumors or mesothelioma while no pulmonary neoplasms were found in the animals treated with short fibers. In parallel intraperitoneal injection studies also reported by Davis et al. (1986), the long fiber amosite produced mesothelioma in 95% of the animals treated while the short fiber amosite produced one mesothelioma over the same period.
McConnell et al. (1999) reported on a chronic inhalation study on amosite asbestos in hamsters in which the number of particles and shorter fibers were reduced while maintaining the number of fibers longer than 20 µm in the test atmosphere. The amosite aerosol concentration ranged from 10 to 69 long fibers (>20 µm)/cm3 with exposure levels selected based upon a previous, multi-dose 90 d sub-chronic inhalation study (Hesterberg et al., 1999). At the high-dose amphibole amosite asbestos exposure of 263 WHO fibers/cm3 (69 fibers L > 20 µm/cm3) 20% of the animals developed mesotheliomas with 82% of the animals developing mesothelial hyperplasia.
Sub-chronic inhalation toxicity studies
The 90 d sub-chronic inhalation toxicity study has been used extensively in regulatory evaluation. The use of this and other shorter term studies for the evaluation of the toxicity and potential carcinogenicity of fibers was reviewed by an ILSI Risk Science Institute Working Group (Washington, DC) (Bernstein et al., 2005c). This working group was sponsored by the ILSI Risk Science Institute and the US Environmental Protection Agency Office of Pollution Prevention and Toxics(Washington, DC). The working group stated that current short-term testing methods, defined as 3 months or less in exposure duration, evaluate a number of endpoints that are considered relevant for lung diseases induced by fibers such as asbestos. Sub-chronic studies to assess biomarkers of lung injury (e.g. persistent inflammation, cell proliferation and fibrosis) are considered to be more predictive of carcinogenic potential than in vitro measures of cellular toxicity. Of particular importance in the evaluation of fiber toxicity using the 90 d sub-chronic inhalation toxicity study is the association reported by the Working Group based upon the available inhalation toxicology studies that:
All fibers that have caused cancer in animals via inhalation have also caused fibrosis by 3 month. However, there have been fibers that have caused fibrosis but not cancer. Therefore, in vivo studies that involve short-term exposure of rat lungs to fibers and subsequent assessment of relevant endpoints, notably fibrosis, are probably adequately conservative for predicting long-term pathology – that is, will identify fibers that have a fibrogenic or carcinogenic potential (Bernstein et al., 2005c).
Bellmann et al. (2003) reported on a calibration study which compared the toxicity of a range of SVFs with different biosolubilities in a 90 d sub-chronic inhalation toxicity study. One of the SVFs tested was a calcium–magnesium-silicate (CMS) fiber, a relatively biosoluble fiber, for which the stock preparation had a large concentration of non-fibrous particles in addition to the fibers. In this study, due to the method of preparation, the aerosol exposure concentration for the CMS fiber was 286 fibers/cm3 length < 5 µm, 990 fibers/cm3 length > 5 µm and 1793 particles/cm3, a distribution which is not observed in the commercial product. The total CMS exposure concentration was 3069 particles & fibers/cm3. The authors pointed out that “The particle fraction of CMS that had the same chemical composition as the fibrous fraction seemed to cause significant effects”. For the CMS fiber, the authors reported that the number of polymorphonuclear leukocytes in the bronchoalveolar lavage fluid was higher and interstitial fibrosis was more pronounced than had been expected on the basis of biopersistence data. In addition, the interstitial fibrosis persisted through 14 weeks after cessation of the 90 d exposure. This effect was attributed to the large number of non-fibrous particles in the exposure aerosol – 50% of the aerosol was composed of non-fibrous particles and short fibers.
By comparison, after chronic inhalation exposure of rats to another CMS fiber, X607 fiber, which had considerably fewer non-fibrous particles present (particles with an aspect ration of < 3:1), no lung tumors or fibrosis was detected (Hesterberg et al., 1998). This provides support for the argument that it was the large non-fibrous component of the CMS used in the Bellmann study and the resulting lung overload that caused the pathogenicity observed with this relatively biosoluble fiber. A similar overload mechanism might explain the results of earlier chrysotile inhalation studies, in which animals were exposed to much higher levels of non-fibrous particles and short (<5 µm) fibers.
Bernstein et al. (2006) reported on the toxicological response of a commercial Brazilian chrysotile following exposure in a multi-dose sub-chronic 90 d inhalation toxicity study, which was performed according to the protocols specified by the US EPA (2001) and the European Commission (EUR 18748 EN, 1999).
In this study, male Wistar rats were exposed to two chrysotile levels at mean fiber aerosol concentrations of 76 fibers with L >20 μm/cm3 (3 413 total* fiber/cm3 and 536 WHO fiber/cm3) or 207 fibers L > 20 μm/cm3 (8941 total fiber/cm3; 1429 WHO fiber/cm3). The animals were exposed using a flow-past, nose-only exposure system for 5 d per week, 6 h/d, during 13 consecutive weeks followed by a subsequent non-exposure period of 92 d. Animals were sacrificed after cessation of exposure and after 50 and 92 d of non-exposure recovery. At each sacrifice, the following analyses were performed on sub-groups of rats: lung burden; histopathological changes; cell proliferation; inflammatory cells in the broncho–alveolar lavage; clinical biochemistry and confocal microscopic analysis.
Exposure to chrysotile for 90 d followed by 92 d of recovery, at a mean exposure of 76 fibers with L > 20 μm/cm3 (3413 total fiber/cm3) resulted in no fibrosis (Wagner score 1.8–2.6) at any time-point. At an exposure concentration of 207 fibers L > 20 μm/cm3 (8941 total fiber/cm3), slight fibrosis was observed. In comparison with other studies, the lower dose of chrysotile produced less inflammatory response than the biosoluble synthetic vitreous CMS fiber referred to above, and considerably less than amosite asbestos (Bellmann et al., 2003).
These similarly designed 90 d inhalation toxicity studies show that the pathological response from exposure to chrysotile is similar or less than that of SVFs.
Shorter term inhalation toxicity studies
In a short-term exposure study in rats (6 h/d, 5 d) with the amphibole tremolite asbestos at an exposure concentration of 100 long fibers (>20 µm)/cm3 and 2016 total fiber/cm3, extensive inflammatory response was observed immediately after the end of the 5 d exposure and interstitial fibrosis developed within 28 d after cessation of the 5 d exposure (Bernstein et al., 2005b).
In a recent study by Bernstein et al. (2010, 2011), the pathological response and translocation of a commercial chrysotile product similar to that which was used through the mid-1970s in a joint compound intended for sealing the interface between adjacent wall boards was evaluated in comparison to amosite asbestos. This study was unique in that it presented a combined real-world exposure and was the first study to investigate whether there were differences between chrysotile and amosite asbestos fibers in time course, size distribution and pathological response in the pleural cavity. Rats were exposed by inhalation for 5 d (6 h/d) to either sanded joint compound consisting of both chrysotile fibers and sanded joint compound particles or amosite asbestos.
The mean fiber number was 295 fibers/cm3 for chrysotile and 201 fibers/cm3 for amosite. The mean number of WHO fibers in the chrysotile fibers and sanded joint compound particle atmosphere was 1496 fibers/cm3, which was more than 10 000 times the OSHA occupational exposure limit of 0.1 fibers/cm3. The amosite exposure atmosphere had fewer shorter fibers, resulting in a mean of 584 WHO fibers/cm3.
An important part of the Bernstein et al. (2010, 2011) study was to design procedures for evaluation of the pleural space while limiting procedural artifacts. These methods included examination of the diaphragm as a parietal pleural tissue and the in situ examination of the lungs and pleural space obtained from freeze-substituted tissue in deeply frozen rats. The diaphragm was chosen as a representative parietal pleural tissue because at necropsy it could be removed within minutes of sacrifice with minimal alteration of the visceral lung surface. The area of the diaphragm chosen for examination included an important lymphatic drainage site (stomata) on the diaphragmatic surface. The use of both confocal microscopy and SEM enabled the identification of fibers as well as examination of the pleural space, in situ, for possible inflammatory response. The examination of the pleural space in situ including the lung, visceral pleura and parietal pleura in rats deeply frozen immediately after termination provided a non-invasive method for determining fiber location and inflammatory response.
No pathological response was observed at any time-point in the chrysotile fibers and sanded joint compound particles exposure group. The long chrysotile fibers (L > 20 μm) cleared rapidly (T 1/2 of 4.5 d) and were not observed in the pleural cavity. In contrast, a rapid inflammatory response occurred in the lung following exposure to amosite resulting in Wagner grade 4 interstitial fibrosis within 28 d and which persisted through 90 d (histopathology was evaluated through 90 d post exposure as the animals were allocated to the confocal analyses from 181 to 365 d post exposure). Long amosite fibers had a biopersistence of T 1/2 > 1000 d in the lung and were observed in the pleural cavity within 7 d post exposure. By 90 d, the long amosite fibers were associated with a marked inflammatory response on the parietal pleura. This study provides support that in contrast to amosite asbestos, exposure to chrysotile fibers and joint compound particles following short-term inhalation would not initiate an inflammatory response in the lung, and that the chrysotile fibers present following this exposure do not migrate to, or cause an inflammatory response in the pleural cavity, the site of mesothelioma formation.
These studies provide further confirmation of the differences between exposure to chrysotile alone and to chrysotile mixed in a joint compound and amphibole asbestos.
What do the toxicology studies indicate?
The more recent toxicology studies summarized above demonstrate that chrysotile asbestos has a relatively short biopersistence and does not result in pathological response even through 90 d of exposure (Bernstein et al., 2006). These studies also confirm the difference between chrysotile and amphibole asbestos which is highly persistent in the lung and results in a fibrotic response even after 5 d of exposure (Bernstein et al., 2005b, 2010, 2011).
This is mirrored in pathological response to chrysotile and amphibole asbestos following both short-term (5 d of exposure) (Bernstein et al., 2005b, 2010, 2011) and long-term (90 d of exposure) repeated dose inhalation exposure to well-defined chrysotile aerosols in the rat (Bernstein et al., 2006) and following chronic exposure to amosite in the hamster (McConnell et al., 1999).
Following such exposures, chrysotile asbestos produces neither a pathological response in the lung nor in the pleural cavity at doses up to 5000 times the US TLV for chrysotile. In the 90 d exposure study (Bernstein et al, 2006), at an exposure concentration more than 14 000 times the TLV, slight fibrosis was observed. In addition, the chrysotile fibers clear rapidly from the lung and are not observed at the visceral pleural surface, neither in the pleura nor on the parietal pleural surface.
The amphibole asbestos fibers tremolite and amosite have thus far been evaluated. In the lung, immediately following a 5 d exposure, the amphibole fibers have been shown to produce extensive inflammation with granuloma formation. With 28 d after cessation of exposure, interstitial fibrosis (Wagner grade 4) was observed with both tremolite and amosite. Both of these fibers were poorly cleared from the lung with the fibers longer than 20 µm persisting through the end of the study (365 d post exposure) (Bernstein et al., 2005b, 2010, 2011).
The pleural transfer was also evaluated for amosite asbestos. Within 2 weeks following cessation of the 5 d exposure, amphibole fibers were observed at the visceral pleural surface and were associated with extensive inflammation and fibrotic development. Amphibole fibers were observed penetrating the visceral pleura and extending in the pleural cavity. Inflammation was also observed on the parietal pleural surface (Bernstein et al., 2010, 2011).
The study by Osmon-McLeod et al. (2011), which reported that long fiber chrysotile showed ∼70% mass loss and a marked decrease in length with long-term incubation in a Gamble’s solution which was adjusted to mimic that inside macrophage phagolysosomes provides a basis for understanding the rapid clearance of chrysotile.
These studies strongly suggest that even short exposures to amphibole can influence the pathological development in the lung and pleural cavity and provide a new perspective in understanding and differentiating the results presented in epidemiology studies of chrysotile and amphibole asbestos exposed cohorts.
Epidemiology studies
While chrysotile is currently used largely in high-density cement products, the epidemiological and regulatory evaluation of chrysotile is based upon a cross section of all uses in the past. Of particular importance for understanding the implications of the current use of chrysotile are those studies characterized as chrysotile only. Those studies characterized as chrysotile only are reviewed below in light of the toxicological studies, which indicate the importance of even short-term exposure to amphibole asbestos in causing disease.
The early case-control studies of mesothelioma provided relationships of occupational exposure to asbestos (Ashcroft, 1973; Elmes & Wade, 1965; Hain et al., 1974; McDonald et al., 1970; McEwen et al., 1970; Newhouse & Thompson, 1965; Rubino, 1972; Zielhuis et al., 1975). However, due to the state of occupational hygiene measurements at the time, none of the studies were able to use exposure measurements which included fiber number or fiber type. The associations to disease were attributed to the fiber most used without consideration of the criteria that have been understood more recently to determine fiber potency: biopersistence and fiber length. In addition, the lack of complete occupational histories is a significant limitation in the early epidemiology studies, resulting in improper characterization of fiber-specific exposure.
Berman & Crump (2003) summarized the various limitations that likely influence the epidemiological evaluations and that had to be addressed in order to assess the uncertainty in the available epidemiology studies. These included:
limitations in air measurements and other data available for characterizing historical exposures;
limitations in the manner that the character of exposure (i.e. the mineralogical types of fibers and the range and distribution of fiber dimensions) was delineated;
limitations in the accuracy of mortality determinations or incompleteness in the extent of tracing of cohort members;
limitations in the adequacy of the match between cohort subjects and the selected control population and
inadequate characterization of confounding factors, such as smoking histories for individual workers.
In addition, the capabilities and limitations of the analytical techniques used for determining the asbestos exposure measurements in these epidemiological studies were summarized as shown in Table 1. Midget impinger (MI) and phase contrast microscopy (PCM) were the two analytical techniques used to derive exposure estimates in the majority of epidemiology studies from which the existing risk factors were derived. However, the MI and PCM measurements did not determine fiber length which has been shown to be related to biological activity.
With few exceptions, little to no quantitative sampling was conducted prior to the 1960s when exposure concentrations were generally considered to be higher than those monitored more recently, due to lack of use of dust control equipment at the time and procedures to reduce dust levels that were introduced only later. For most studies, therefore, early exposures had to be estimated by extrapolation from later measurements (Berman & Crump, 2003).
In particular, as a result of the measurement techniques, there was often little quantitative exposure information on the types of fibers to which workers were exposed. The nature of the industrial process may have suggested the type of fiber used. However, in the past there was little attempt to differentiate serpentine from amphibole asbestos, and as a result amphibole was often substituted or mixed with serpentine without detailed documentation. The use of amphibole in place of serpentine resulted from such factors as availability, cost and effectiveness in the process. In addition, work histories of employees were not always as well documented as might occur today (Berman & Crump, 2003).
While all uncertainty factors are important in assessing the difference between chrysotile and amphiboles, the differentiation of the fiber type in the exposure atmosphere is obviously critical in determining possible effects associated with each type of fiber. Of equal importance is the number of fibers in the exposure atmosphere with length greater than approximately 20 µm, that is, those fibers which are not readily phagocytized and removed from the lung by macrophages and which therefore have greatest potential in producing disease if they do not readily break apart or dissolve in the lung fluids.
An additional issue which is often not well addressed is that of possible exposures to asbestos either prior to employment or concurrent to employment in the industry under study and consequently the fiber types to which the individuals were exposed.
Evaluation of epidemiology studies considered in earlier evaluations
Hodgson & Darnton (2000) reviewed asbestos exposed cohorts which gave information on exposure levels from which (as a minimum) a cohort average cumulative exposure could be estimated. In another review, Berman & Crump (2008) also assessed the health risks associated with “asbestos” exposure also using the cohorts in which they determined that there was sufficient information to estimate exposure.
In both of these evaluations, the authors classified the cohorts by asbestos fiber type based on what was reported in the cited publications. That is whether they considered the cohort exposed to chrysotile alone, a mixture of chrysotile with amphibole asbestos, or to amphibole asbestos alone. These assessments were made from the then currently available literature and presented potential biases based upon the published data.
These studies are reviewed here in light of current data and the information learned from the toxicology studies on the importance of fiber type and fiber length in producing a pathological response in the lung and the pleural cavity.
Studies characterized as predominately chrysotile exposure
It is interesting to note that the authors of very few of the epidemiology studies on asbestos were able to state that there was no amphibole exposure present in the cohort. Hodgson & Darnton (2000) considered the following studies which were characterized as predominately chrysotile exposure (Table 2) and stated that very small quantities of amphibole fiber were ignored as being important to the findings in some cohorts (South Carolina, New Orleans plant 2, CT).
Similarly, Berman & Crump (2008) considered the same cohorts as being exposed to chrysotile and considered other possible exposure either within the plant in question, or before or concurrent to employment as not important.
At the time the exposures took place, in none of these cohorts were the type of fibers to which workers were exposed actually determined from air samples, and in none of these studies were the fiber length distributions of the fibers determined in the workplace. While some investigators have attempted to recreate the work environment, experience with fiber aerosol generation in animal toxicology studies strongly indicates that accurately recreating all the factors which influence fiber size and distribution would be very difficult.
The results from Hodgson & Darnton (2000) for these studies for lung cancer and mesothelioma are presented in Table 3.
Table 3. .
Studies characterized as predominately chrysotile exposure (Hodgson & Darnton, 2000).
| Study | Exposure estimates | Smoking histories | Fiber specificity | Lung cancer risk (% expected lung cancer per fiber/cm3 year)† RL (95% CI) | Mesothelioma risk (% total expected mortality per fiber/cm3 year)† RM age adjusted 95%CI |
|---|---|---|---|---|---|
| South Carolina: Dement et al. (1994) and McDonald et al. (1983) | MI measurements 1930–1975 In 1968 and 1971, both impinger and PCM samples were collected (a total of 986 samples) | Based on two surveys conducted by the U S Public Health Service in 1964 and 1971 and on data collected by the company | Chrysotile textile plant. Crocidolite yarn was used in small quantities to make tape or braided packing from 1950s until 1975 | Women 6.7 (3.6, 11) Men 4.6 (2.9, 6.7) | Women 0 (0.0, 0.35) Men 0.013 (0.0016, 0.047) |
| Balangero: Piolatto et al. (1990) | Fiber levels were measured by PCM in 1969. In order to estimate earlier exposures, information on daily production, equipment changes, number of hours worked per day, etc. were used to create conditions at the plant during earlier years. PCM samples were obtained under these simulated conditions and combined with work histories to create individual exposure histories | No information on smoking | Chrysotile mine and mill with presence of Balangeroite fiber | 0.03 (−0.11, 0.24) | 0.0025 (0.0003, 0.009) |
| Quebec: Liddell et al. (1997) | MI measurements Conversions between dust levels and PCM concentrations were derived from side-by-side samples | Smoking history was obtained in 1970 by a questionnaire administered to current workers, and to proxies of those who had died after 1950 | 1. Chrysotile mine and mill at the town of Asbestos 2. Factory at the town of Asbestos that, in addition to processing chrysotile, had also processed some crocidolite 3. Chrysotile mining and milling company complex near Thetford Mines (evidence of greater amounts of tremolite in the ore at Thetford Mines) 4. Number of smaller mines and mills also in the vicinity of Thetford Mines | 0.06 (0.042, 0.079) | 0.0009 (0.0006, 0.0013) |
| New Orleans (plant 2): Hughes et al. (1987) | MI measurements initiated in the early 1950s Levels estimated from initial samples in the 1950s were also assumed to hold for all earlier periods because no major dust control measures had been introduced prior to that time In plant 2, the revised estimates tended to be about one-third of the previous estimates through the 1940s and about one-half the previous estimates thereafter | Based upon a cross sectional study of over 95% of workers employed in these plants in 1969. Information concerning the smoking habits of earlier workers in these plants is not available | Plant 1: Some amosite was used from the early 1940s until the late 1960s, constituting about 1% of some products, and crocidolite was used occasionally for approximately 10 years beginning in 1962 Plant 2: Utilized only chrysotile, except that pipe production, which began in 1946 and was housed in a separate building, produced a final product that contained about 3% crocidolite | 0.81 (0.21, 1.6) | 0 (0, 0.033) |
| Connecticut: McDonald et al. (1984) | Dust levels from impinger measurements were available for the years 1930, 1935, 1936 and 1939. There was little other exposure information available until the 1970s. No conversion from MPPCF to fiber/cm3 value was suggested by the authors | No information on smoking | Plant that manufactured asbestos friction products. The plant began operation in 1913 and used only chrysotile until 1957, when a little anthophyllite was used. Also, a small amount of crocidolite (about 400 pounds) was handled experimentally between 1964 and 1972 | 0.80 (0.029, 1.8) | 0 (0, 0.016) |
†Risk estimates as determined by Hodgson & Darnton (2000).
Fiber lung burdens: Charleston, South Carolina, and Quebec
The analysis of the types and numbers of fibers found in lung tissue of individuals exposed to asbestos provides the most robust indicator of past exposure. While in general, such analyses were not performed, in two of the above-mentioned studies, fiber lung burdens were analyzed to determine the type and quantity of fibers present in the samples analyzed.
The lung burden analyses provide an indication to which fibers the workers were exposed. The samples were usually taken from lung biopsy sections or at necropsy and were often from paraffin blocks. As an example, in the Sebastien et al. (1989) study, the samples analyzed were around 1 g (personal communication, P. Sebastien). As such, only a small portion of the lung was analyzed.
Sebastien et al. (1989) reported in the analysis of 161 lung tissue samples taken at necropsy from asbestos textile workers in Charleston, South Carolina and Quebec miners and millers, both exposed to chrysotile. The authors reported that while chrysotile, tremolite, amosite, crocidolite, talc-anthrophyllite and other fiber types (included rutile, micas, iron, silica and unidentified silicates) fibers were found in both cohorts tremolite predominated. Non-trivial concentrations (>0.1 f/µg) of amosite and crocidolite were measured in 32% of specimens from Charleston, SC and 9% from Thetford, VT. The analysis indicted that in Charleston, commercial amphiboles were detected only in cases hired before 1940; no crocidolite was detected in cases hired after 1940. In Thetford, concentrations greater than 0.1 f/µg were measured in five cases.
Churg et al. (1984) analyzed the fiber lung content from six cases with mesothelioma derived from a series of approximately 90 autopsies of long-term workers in the Quebec chrysotile industry. These six cases represented all the mesotheliomas present in the series of 90 cases. The authors reported that the patients with mesothelioma having only chrysotile ore components had a much higher ratio of tremolite group amphiboles (9.3) than chrysotile fibers (2.8) compared to the control group. This was not true for one patient in whom amosite was found.
Pooley & Mitha (1986) in reporting on the determination and interpretation of the levels of chrysotile in lung tissue included result from the South Carolina textile workers in their Table 2 which compared the calculated mean values mass per 1000 fibers of asbestos obtained from lung tissue extracts. They reported that South Carolina textile plant cases had 0.032 ng/103 fibers of chrysotile compared with 1.19 ng/103 fibers crocidolite and 2.098 ng/103 fibers amosite. In addition, the South Carolina control lung tissues had 0.015 ng/103 fibers chrysotile and 0.725 ng/103 fibers amosite.
Case et al. (2000) evaluated asbestos fiber type and length in lungs of fibers longer than 18 µm in length in chrysotile textile from the South Carolina cohort and chrysotile miners/milers from the Thetford Mines portion of the Quebec cohort. Lung samples were obtained from either deparaaffinized paraffin blocks or formalin fixed tissues and were chemically digested in commercial bleach. The authors stated that the lung retained fiber measurements were limited in inference as the results represented only the fraction of internal dose that was retained until death. In addition, they could not be certain to what degree the groups of chrysotile miners/millers and textile workers were representative of the cohorts from which they were derived. The results obtained closely paralleled those reported by Sebastian et al. (1989). The Case et al. (2000) results indicated that the “chrysotile only” textile workers had a high proportion of individuals with lung tissue containing amosite and/or crocidolite. The results did not support a role of the fiber length alone in explaining the greater lung cancer risk in textile workers. The authors concluded that “this subset of the Charleston textile workers does not support the hypothesis that this is a pure chrysotile cohort” (WHO, 1998). In addition, they stated that “the exposure experience of textile workers is clearly unique and should not be used to assess risk of lung cancer in miners, cement workers or friction products workers, regardless of fiber type”.
In these two cohorts, the hypothesis that exposure was to chrysotile only is not supported from the lung burden measurements.
Discussion of the predominately chrysotile epidemiology studies
In addition to the analysis of lung burden in the two studies presented above, each of the studies characterized as predominately chrysotile have been examined for the presence of amphibole asbestos in the exposure and the evaluation of other factors in the study design which could have influenced the results.
South Carolina cohort
In the analyses presented by Hodgson & Darnton (2000) and Berman & Crump (2008), the South Carolina cohort stands out as the study which reports a carcinogenic potential attributed to the use of “chrysotile” in the textile plant. The South Carolina cohort (Dement & Brown, 1994; Hein et al., 2007) is very interesting because it involved the use of textile grade chrysotile fibers. The authors acknowledge that small quantities of crocidolite (approximately 2000 pounds) were used in the plant in separate processes and concluded that this use was isolated and did not influence possible exposures in the textile plant. Dement et al. (1982) reported on a study of this factory and observed a large excess of lung cancer corresponding to an standardized mortality ratio (SMR) of 500 at 100 fiber-years/cm3 which was reported as statistically significant as compared to the control cohort This study is in pronounced contrast to any other study where there was exposure only to chrysotile. As presented in the above section, the lung burden measurements on workers from this cohort indicate that both amosite and crocidolite were present in the workers’ lungs.
In reviewing this study, the following important factors which would influence the results are apparent:
Very close proximity to US Navy base which used large amounts of amosite
Close proximity to other facilities using potentially toxic materials
Possible prior use of amphiboles
Very close proximity to US Navy base which used large amounts of amosite
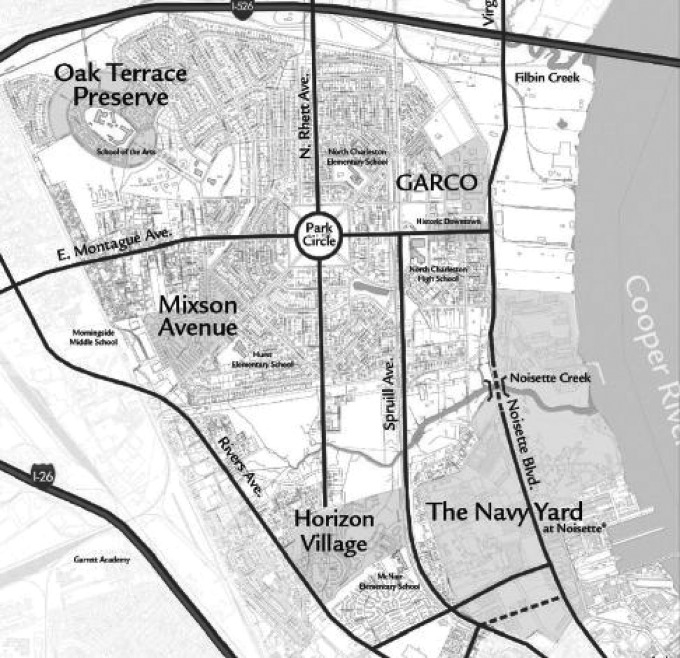
The plant (General Asbestos & Rubber Co. known as GARCO) was located in North Charleston within a few hundred meters of the US Navy base in Charleston (Figure 3). This base was very active leading up to and during WWII and as Dement mentions employed 29 000 people building and repairing military ships. The Navy base opened in 1909 and during the war years, 1359 vessels were worked at the shipyard: damaged ships were repaired, combat vessels overhauled and 253 warships were constructed and launched. Nearly every military ship at the time was insulated using large quantities of amphibole asbestos (Balzer & Cooper, 1968; Bowles & Barsigian, 1954; Bowles & Stoddard, 1933; Virta, 2005). This process also involved the use of potentially toxic substances* in addition to the extensive use of amphibole asbestos. Dement et al. do not consider this important and do not factor into the analysis the possible influence of the emissions from the base nor the industrial area immediately adjacent to the GARCO plant.
Close proximity to other facilities using potentially toxic materials
Figure 3. .
Map of North Charleston showing the location of the Textile plant (GARCO) and the US Navy Yard. The distance from GARCO to the Navy Yard is a few hundred meters. The width of the map is approximately 3.5 km.
Close proximity to other facilities using potentially toxic materials is of importance as the predominate finding in the Dement et al. study is lung cancer with a potential of other substances contributing to possible causality.
There is no consideration of the Naval Weapons Station Charleston which occupies 17 000 acres of land – seven times larger than the Naval Shipyard site which was commissioned in 1941 and located on the western shore of the Cooper River just north of the GARCO plant. The Naval Weapons Station Charleston had a production capacity for more than 60 million pounds of conventional ordnance. Among other industries that could affect the health of the Charleston workers was the Rollins Chemical Company established in 1914 in South Charleston. Adjoining the Rollins plant on the west was the Warner–Klipstein plant, starting in 1915 as a producer of chlorine and chlorine products. This plant, reorganized in 1928 as the Westvaco Chlorine Products Corporation, became an important manufacturer of caustic, chlorine and chlorinated compounds. The Carbide and Carbon Chemicals Company moved to South Charleston from Clendenin in 1925 and began operations in buildings acquired from the Rollins Chemical Company. Currently it is a division of Union Carbide Corporation, the company was a producer of more than 400 chemicals, plastics and fibers from derivatives of natural gas and petroleum.
Amphibole asbestos exposure in the cohort population
In a report predating Dement et al. (1994), Dreesen et al. (1938) stated that “Approximately 90% of the asbestos used in these plants is obtained from Canada. The remaining 10% comes from Arizona or South Africa, and, infrequently, from Russia and Australia”. While no specifics on fiber type were provided, South Africa was a large supplier of the blue and brown amphibole asbestos, crocidolite and amosite asbestos while Australia supplied crocidolite asbestos.
As presented above, the environment within Charleston had unique sources of pollutants from industrial and military operations that would very likely influence the cancer and mortality incidence of the region. This is reflected in the much higher mortality rate in Charleston compared to the US average.
Dement et al. supports the use of the US mortality rates stating “it is difficult to estimate the exact number of persons ever employed at this plant; however, this is likely to exceed 10 000 prior to 1965”. They do not consider the larger number of persons that worked just a short distance from the plant at the Naval ship yard.
The US mortality rate was reported by the authors as 39 per 100 000 over the period 1950–1969. The US National Cancer Institute (Devesa et al., 1999) provides the mortality rate for Charleston over the period 1950–1969 as 101.5 which is 2.6 times the rate used in Dement et al. (1982). As GARCO provided housing for its employees in North Charleston and considering the proximity of this neighborhood to the Navy base and other installations, it is likely that the local mortality rate was even higher than 101.5. While the issue of which rate would be most appropriate is difficult to reconstruct, the available information indicates that the rate used underestimates the control background level.
Another issue which is not addressed in the Dement et al. (1982) study is that of prior and or concurrent exposures or exposures through family members. It would not be unreasonable to expect that GARCO employees and or family members had prior work experience in the military or in other industries. A brief internet search of recently published death summaries (The Post and Courier, Charleston, SC) shows individuals such as:
Marine Corps and Merchant Marines veteran and retired supervisor for GARCO.
Long term employee of GARCO Mill and a retired owner/operator of – Garage for 26 years. He also served his country in the US Army. He was an automobile enthusiast and loved racing and working on vehicles.
Army veteran, retired employee of GARCO
Occupation: GARCO, retired Contractor, self-employed military: US Merchant Marine, WW II veteran
Formerly worked at GARCO, the Charleston Navy Exchange and the former Geer Drug Company
Machinist with GARCO and a retired employee with the Charleston Naval Shipyard
Navy veteran, retired employee with GARCO
Hein et al. (2007) stated that in addition to a lack of smoking histories for all of the cohort members that the findings reported were subject to additional limitations including incomplete lifetime work histories and high rates of loss to follow-up, especially among female workers. The idea that the population studied worked uniquely at GARCO is neither supported in the Dement et al. (1982) nor the Hein et al. (2007) publications.
Other factors influencing lung cancer incidence
Dement et al. (1982) state that one of the most important factors which need to be considered in evaluating the occupational contribution to observed mortality patterns are cigarette smoking patterns among the cohort. They showed in Table 9 that the prevalence of cigarette smoking among 292 out of the 768 asbestos study cohort members was similar to that of the US white adult males (1965). For the other 475 cohort members, no information on smoking was provided. This was based upon a classification of current smoker, past smoker or non-smoker. However, no information was provided on the smoking incidence in the asbestos cohort and how this compares to the US white adult males. For those workers who had also been in the military, the military rates of tobacco and alcohol use have been reported as higher than those found in comparable civilian sectors (Ballweg & Brey, 1989; Bray et al., 1989, 1991; Conway et al., 1989; US DHHS, 1989).
The authors determined a conversion from the MI measurements in millions of particles per cubic foot of air (MPPCF), to membrane filter counts, measured as fibers longer than 5 µm/cm3 using concurrent samples by these two methods in plant operations collected during 1968–1971. The authors reported that for textile operations, except preparation, a conversion of 3 fiber/cm3 for 1 MPPCF was used while for preparation a conversion of 8 fiber/cm3 was used. The 95% confidence limits on these conversions were estimated as 3 fiber/cm3 (CI 2.5–3.5) and as 8 fiber/cm3 (CI 5–9).
In subsequent analyses of occasional samples of air filters from the South Carolina plant the authors reported that, “Only two fibers of the 18 840 fiber structures (0.01%) were found to be amphiboles and the remainder were chrysotile based on morphology” (Stayner et al., 2008). As presented above, several studies have analyzed the fiber content of lungs from workers and have shown the presence of significant quantities of amphiboles. Stayner et al. (2008) did not report the presence of even tremolite fibers, this was perhaps due to using a physical morphology based analysis rather than chemical based identification techniques (EDAX, or the Addison & Davies, 1990).
Green et al. (1997) examined pulmonary fiber burdens in a necropsy population in 39 former workers from the South Carolina textile plant and 31 controls. The authors reported that the grade of pulmonary fibrosis correlated better with the tremolite asbestos concentration than the chrysotile concentration. They also found that the geometric mean concentrations for amosite and crocidolite asbestos were higher in the textile plant workers than in the controls. They reported that 28% of the textile asbestos workers and 13% of the controls had values of crocidolite or amosite asbestos in their lungs which exceeded 1 million fibers per g dry lung [a value considered above background for that lab at that time]. These amphibole concentrations could easily explain the small number of mesotheliomas which occurred in the cohort.
The above information strongly suggests that The South Carolina textile workers were exposed to amphiboles and other causative agents (pollutants, smoking) either directly or indirectly which confounds the understanding of what exposure produced the lung cancer and mesothelioma.
Based upon the more recent inhalation toxicology studies of amphiboles, that even short exposures to amphibole asbestos in the South Carolina textile plant or through prior or para-occupational exposure could have significantly impacted the results. The recent work by Bernstein et al. (2010) has confirmed that amphibole asbestos fiber types are much more potent than chrysotile asbestos and that with such a differential in response, even small amphibole exposure could have had a significant influence on the findings reported in the South Carolina cohort. McDonald et al. (1983) attributed the cancer incidence to the small amount of tremolite present in the mine. Analyses have shown that the tremolite was present in quantities of less than 1% and showed that the amphibole accumulated with time in the lung while the chrysotile did not. With a larger potential for exposure to amphibole asbestos and other pollutants than originally perceived in the South Carolina cohort, it is clear that the South Carolina cohort was not a pure chrysotile cohort as originally postulated.
Piolatto et al. (1990)
Piolatto et al. (1990) reported on the analyses of a cohort of asbestos workers from the Balangero mine in Italy. The authors reported that
examination of several samples of chrysotile from the mine ruled out the presence of contamination with fibrous amphiboles at detectable concentrations. A fibrous silicate (balangeroite) was characterised, however, consisting of brown, rigid and brittle xyloid fibers with a complex structure similar to gageite, usually intergrown with chrysotile.
The Balangeroite fiber was reported as accounting for 0.2–0.5% of the total mass of samples of chrysotile as commercialized from the Balangero mine. There is no mention of the actual concentration in the mine pit. The authors stated as well that “Nothing is at present known about its adverse effects, although they can be suspected on the basis of its fiber dimensions being similar to those of amphiboles”.
Silvestri et al. (2001) summarized information on work practice, fiber concentration and health-related effects in the workers at the Balangero mine and in the population of the surrounding area. The authors stated that in addition to chrysotile, Balangeroite, a fibrous magnesium-iron silicate first discovered at Balangero is present in the ore and that it is very similar, from a morphological point of view, to amphiboles. From its opening in 1930 there were no exposure controls at the mine until the 1960s and no standard was imposed until 1986 when the European directive was implemented in Italy. The authors cited a report from the 1940s that “The damage is not so bad for the trees and plants, but rather for the cows, as the dust is often so deep on the grass that they can't pasture”. Estimated exposure concentrations in the mine exceeded 50 fiber/ml; in the crushing area 120 fibers/ml; in the fiber selection area 235 fibers/ml and in the bagging area 80 fibers/ml. By 1989 with controls, they were 0.19 fibers/ml in the mine; 0.54 fibers/ml in the crushing area; 0.93 fibers/ml in the fiber selection area and 0.78 fibers/ml in the bagging area.
The percentage of Balangeroite fiber was similar to that of tremolite in Quebec. The difference however, is that the tremolite occurs in separate veins in Quebec (Williams-Jones et al., 2001) while as reported above the Balangeroite fiber was “usually intergrown with chrysotile”. Balangeroite has been classed as an “iron-rich asbestiform” fiber with structural, biochemical, and perhaps most important biodurability characteristics similar to crocidolite (Gazzano et al., 2005; Groppo et al., 2005; Turci et al., 2005). There is no report of lung-retained fiber analyses from workers at the Balangero mine (Case & McDonald, 2008).
Liddell et al. (1997)
Liddell et al. (1997) reported on the mortality experience of a cohort of ∼11 000 workers from Quebec chrysotile miners and millers. The cohort extended over a long period of observations (a birth cohort 1891–1920) and the several updates reported at different intervals since 1971. In the last update published, Liddell et al. (1997) reported that high exposures have led to excesses, increasing with degree of exposure, of mortality from all causes, and from lung cancer and stomach cancer. However, at exposures below 300 (million particles per cubic foot) × years, (mpcf.y), equivalent to roughly 1000 (fibers/cm3) × years (which is equivalent to an exposure of 80 fibers/cm3 over a period of 10 years such as might have occurred in the 1940s) the findings were as follows: there were no discernible associations of degree of exposure and SMRs, whether for all causes of death or for all the specific cancer sites examined. The authors concluded that from the viewpoint of mortality that exposure in this industry to less than 300 mpcf.y has been essentially innocuous.
The issue of the possible presence and impact of contamination of the chrysotile ore with tremolite had been addressed by McDonald & McDonald (1995) in which preliminary investigations had suggested as important in the aetiology of mesothelioma. In the area of Thetford Mines, there were some 15 geographically dispersed mines and mills falling into two clearly definable groups: 5 in a circumscribed central area and 10 located in a peripheral area. Lung burden analysis (Sebastien et al., 1989) of 58 members of the cohort in the central area and 25 in the peripheral area had shown that the geometric mean concentration of tremolite was almost four times higher in the central area than in the peripheral area.
Hughes et al. (1987)
The plants in this study started operation in the 1920s and produced asbestos cement building materials. There is little exposure data prior to the 1950s. Starting in 1952, air sampling data was collected using MIs (with measurements made in MPPCF). In plant 2, totally 248 measurements were made during the 1950s, and more than 1100 during the 1960s. Weill et al. (1979) reported that the original study population consisted of workers who were employed continuously in the months before January 1970 in either of the two asbestos cement building materials plants in New Orleans, LA. These plants opened in the early 1920s and were in operation at the time of the study. The authors reported that the predominant fiber used was chrysotile. In addition, crocidolite was used in the pipe department of the second plant (where it constituted 3% of the product). In the first plant, amosite was used (1% of various products), and crocidolite was used infrequently in the manufacture of corrugated bulkheads. In addition, they stated that “silicate” was used in both plants. Hughes et al. (1987) reported that plant 2 consisted of four separate buildings, each one manufacturing different products. Pipe production, which opened in 1946, used crocidolite in addition to chrysotile. The authors stated that all other areas used chrysotile only. Amosite was never used. Jones et al. (1989) stated that there was “a systematic use of crocidolite in the pipe production area of plant 2, although chrysotile was the primary fiber in both plants”. There are no lung burden measurements available from workers in the study.
McDonald et al. (1984)
McDonald et al. (1984) reported that this factory was established in 1913 and manufactured a number of asbestos-related products over the years. The authors reported that chrysotile from mainly Canada was used until 1957, when some anthophyllite was added in making paper discs and bands. In addition, they reported that approximately 400 lb of crocidolite was used experimentally on a few occasions in the laboratory during 1964 and 1972. The overall quality of anthophyllite and crocidolite used within the factory was not specified further. In addition, the authors reported that the situation was complicated by the fact that the plant under study developed from an earlier asbestos textile plant some 10 miles away which manufactured woven brake linings from 1905 until 1939. Effort was made from the work history records found to eliminate from the cohort people who worked in certain numbered departments (28–50) in the woven brake lining plant. Prior to the 1970s, the few measurements available on exposure were made by impinger and reported in mpcf. Subsequently, measurements were made using membrane filters (without identification of fiber type on the filter). There was no report of lung burden measurements in this study.
Chrysotile epidemiological cohort studies
This section provides an evaluation of epidemiological studies of workers exposed to chrysotile which provided as well differentiation when amphibole asbestos exposure also occurred.
Chrysotile high-density cement studies
Weill et al. (1979) reported on an investigation on 5645 asbestos-cement manufacturing workers. Dust exposures were based on total airborne particulate measurements using the MI at various locations throughout both plants and were recorded in MPPCF. No excess mortality was observed following exposure for 20 years to chrysotile asbestos at exposure levels equal to or less than 100 MPPCF years (corresponding to approximately 15 fibers/cm3 × years). The authors stated:
… However, the demonstration that low cumulative and short-term exposures did not produce a detectable excess risk for respiratory malignancy may be of assistance in the development of regulatory policy, because a scientifically defensible position based on these data is that there are low degrees of exposure not associated with a demonstrable excess risk
The authors also assessed the influence of fiber type on the risk of respiratory malignancy. Workers with exposure to chrysotile only (n = 4201) were compared with two groups of workers exposed to crocidolite asbestos in addition to chrysotile: those with steady employment in the pipe plant (n = 1004) and those with intermittent exposure to crocidolite through occasional maintenance work in that area (n = 235). Persons with exposure to amosite asbestos (n = 205) were excluded from analysis. The authors observed that the additional exposure to crocidolite asbestos enhanced the risk for respiratory malignancy, particularly for those workers exposed intermittently in maintenance jobs which were characterized by high exposure concentrations of dust for short periods of time.
Thomas et al. (1982) reported on a cohort within an asbestos-cement factory that used chrysotile. Some crocidolite was used in the factory prior to 1936 and thereafter only chrysotile was used. A total of 1970 workers were traced, and their mortality experience was examined. No information was available on smoking habits. Dust measurements were not made prior to 1968. Pre-1968 exposure concentrations were estimated as ranging from 0.1 fiber/cm3 at the cement machine to 20+ fiber/cm3 on the beater floor and at hard waste grinding. Since 1968 dust controls reduced exposure to below 2 fibers/cm3. The authors reported that there was no appreciably raised SMR for the causes of death investigated, including all causes, all neoplasms, cancer of the lung and pleura and cancers of the gastrointestinal tract (standard errors were not reported). The authors indicate: “Thus the general results of this mortality survey suggest that the population of the chrysotile asbestos-cement factory studied are not at any excess risk in terms of total mortality, all cancer mortality, cancers of the lung and bronchus or gastrointestinal cancers”. Two pleural mesotheliomas were observed in men who had worked at the factory before 1936 and had been exposed to crocidolite.
Gardner et al. (1986) reported on a cohort study carried out on 2167 subjects employed between 1941 and 1983 at an asbestos cement factory in England. The production process used chrysotile asbestos only, except for a small amount of amosite asbestos during 4 months in 1976. No excess of lung cancers or other asbestos-related excess death was reported, at mean fiber concentrations below 1 fiber/cm3, although higher levels had probably occurred in certain areas of the asbestos-cement factory. One death was observed from pleural mesothelioma and one with asbestosis mentioned as an associated cause on the death certificate, however, neither was considered by the authors to be linked to asbestos exposure at the factory.
Ohlson & Hogstedt (1985) reported on a cohort study of 1176 asbestos cement workers in a Swedish plant using chrysotile asbestos. Only a few exposure measurements were available for the 1950s and 1960s. These indicated a dust level of 10 mg/m3 before the 1970s and half that amount during the 1970s. The fiber concentrations averaged 1 fiber/cm3 based on several hundred samples from five sets of measurements between 1970 and 1976. The fiber concentration at earlier times was estimated to have been twice that level, 2 fiber/cm3 in accordance with the total dust measurements. The highest value was 8 fibers/cm3 recorded during 45 min in 1970 in the asbestos bag barn. The vast majority of asbestos used was chrysotile although 630 tons of amosite were used between 1949 and 1951 and 400 tons of crocidolite in 1962. Smoking habits were not known for the entire cohort. In a sub-sample of the cohort 40% were smokers, 24% never-smokers and 36% ex-smokers. The authors stated that while the distribution was close to the national average, the participants in a voluntary health survey may not have been representative of the whole cohort. No excess work-related mortality was observed at cumulative exposures estimated at about 10–20 fibers/cm3 years.
Yano et al. (2001) reported on cancer mortality among workers exposed to amphibole-free chrysotile asbestos in China. The plant studied opened in 1939 and since 1958 greatly expanded in the size and variety of products with 6000 tons of raw asbestos used in 1996. The authors stated that in the 1970s, the products were classified into textiles, asbestos cement products, friction materials, rubber products and heat resistant materials. This study is included in this section as it included cement products even though other products were manufactured as well. The authors reported that the adjusted relative risk of lung cancer was 8.1 (95% confidence interval (CI): 1.8, 36.1) for workers exposed to high versus low levels of asbestos. The authors stated that they “compared the various sections of the asbestos plant for three groups of workers exposed to high, intermediate and low levels of asbestos fibers”. The few aerosol measurements performed are presented in Table 4 reproduced from Yano et al. (Table 1). The authors point out that there was an apparent discordance between the concentrations of airborne dust and fibers.
Table 4. .
Concentrations of fiber and dust for workers in major sections of the Chongqin, China, asbestos plant, by job category, 1999. (Reproduced from Yano et al’s)*.
| Job category | Fiber (fibers/ml (range)) | Dust (mg/m3 (range)) |
|---|---|---|
| Raw material (opening) | 6.5 (5.8–7.5) | 8.8 (6.1–12.3) |
| Raw material (bagging) | 12.6 (5.2–58.4) | 18.2 (14.5–22.4) |
| Rubber plate† | 2.8 (2.6–3.1) | 237.5 (176.0–320.5) |
| Textile | 4.5 (0.7–17.0) | 22.4 (15.8–35.5) |
| Asbestos cement‡ | 0.1 | 22.3 |
*Geometric mean (range) for three to five workers exposed in each asbestos plant section.
†In the rubber plate section, workers were engaged mainly in dumping mica and various raw materials into a pit in a small room without ventilation.
‡In the asbestos cement section, the number of workers in the dusty environment was small, and only one worker who was engaged in dumping raw materials into the pit was monitored.
The authors also reported that there were two cases of malignant mesothelioma, one pleural and the other peritoneal, in the asbestos cohort which are discussed below. They concluded that these results suggest that heavy exposure to pure chrysotile asbestos alone, with negligible amphibole contamination, can cause lung cancer and malignant mesothelioma in exposed workers, however, they do not define further the exposure characteristics. There are considerable inconsistencies in this study. The authors report that there are no consistent industrial hygiene measurements over the history of the study. They state that the respirable dust concentration was measured once every 4 years. Yano et al. do not present any information on what fiber types were on these filters and more importantly, the fiber concentration measurements (0.1–58 fibers/cm3) account for a very small part of the 6.1–320 mg/m3 dust burden. In a recent animal inhalation toxicology study, a chrysotile exposure of 1500 fiber/cm3 has a gravimetric weight of 2.6 mg/m3 (Bernstein et al., 2010). In the Yano et al. (2001) paper, the highest fiber concentration was 58.4 fiber/cm3, which would correspond to approximately 0.1 mg/m3. Even assuming in Table 5 that the “Raw material (opening)” category was pure chrysotile (which has not been verified in the publication), 1 fiber/cm3 would weigh 1.35 mg/m3 assuming no other particulate matter present. For the rubber plate category, fibers accounted for 3.8 out of 238 mg/m3; for the textile category, fibers accounted for 6 out of 22 mg/m3; for the asbestos cement category, fibers accounted for 0.14 out of 22.3 mg/m3. Other than stating that in the rubber plate section, workers were engaged mainly in dumping mica and various raw materials into a pit in a small room without ventilation, there is no discussion about composition of the “dust” which ranged in mass concentration from 6.1 to 320.5 mg/m3. To put these exposures in perspective, the ACGIH TLV for nuisance dusts is 10 mg/m3 (total dust), 3 mg/m3 (respirable fraction), for mica 3 mg/m3, and for latex rubber 0.0001 mg/m3. There is no indication that the control cohort had similar exposures, as there is no presentation of what the control was exposed to. Considering that the dose makes the poison, this very high unaccounted dose, which was clearly not chrysotile, should be of major concern. This study is clearly not a pure chrysotile exposure as based upon the mass concentration presented in Table 5, 99.9% of the exposure was to something else. Even on the small biopsy samples there is no lung burden analysis, which has always been the bottom line in determining the fibers present to which workers were exposed. Yano et al. (2001) state that a pleural mesothelioma death was reported which occurred 13.8 years after first exposure. This would suggest some prior exposure. If the exposure occurred prior to employment, as suggested by Yano et al. (2009), then this case should not have been included in this study. In a follow-up to this study Wang et al. (2012) reported that “asbestos dust concentrations were measured periodically in the different workshops, but fiber concentrations and personal samples were not available until 1999”. Additionally, Yano et al. (2009) stated that the analysis of the lungs indicated that the vast majority of these asbestos fibers present were tremolite with some occasional chrysotile fibers. This would clearly suggest that small asbestos fiber component of the exposure was not to pure chrysotile but to chrysotile contaminated with tremolite.
The purity of Chinese chrysotile was evaluated by Tossavainen et al. (2001) who reported on the analysis for amphibole fibers in 10 chrysotile bulk samples originating from six Chinese chrysotile mines. In addition, the asbestos fiber content in lung tissue from seven deceased workers of the Shenyang asbestos plant using these raw materials was determined. The authors reported that all of the bulk samples contained amphibole fibers as an impurity in concentrations ranging from 0.002 and 0.310 wt%. Tremolite fibers were detected in every sample but anthophyllite fibers were present only in the sample originating from the dolomite-hosted deposit. In the lung, anthophyllite (71%), tremolite (9%) and chrysotile (10%) were found as the main fiber types. The authors noted that all except one of the mines studied were located in western China, and that nearly all of the bulk Chinese chrysotile comes from mines in this region. Yano et al. (2001) reported on a mine that was West/South West China.
Sichletidis et al. (2009) reported on an investigation into the mortality rate among workers exposed to relatively “pure chrysotile” in an asbestos cement factory in Greece. The asbestos cement plant was opened in 1968 and the investigation covered all 317 workers. The plant used 2000 tons of chrysotile annually. Regular asbestos fiber measurements were made and the day and cause of death were recorded among active and retired workers. Asbestos fiber concentrations were always below permissible levels. Fifty-two workers died during the study. The cause was cancer in 28 subjects, with 16 of those cases diagnosed as lung cancer. No case of mesothelioma was reported. The overall mortality rate was significantly lower than that of the Greek general population, SMR was 0.71 (95% CI 0.53–0.93). Mortality due to cancer was increased (SMR: 1.15, 95% CI 0.77–1.67), mainly due to lung cancer mortality (SMR: 1.71, 95% CI 0.98–2.78), but not significantly. The authors stated that the SMR for lung cancer of 1.71 was attributed almost exclusively to cigarette smoking. The authors concluded that occupational exposure to relatively pure chrysotile within permissible levels was not associated with a significant increase in lung cancer or with mesothelioma. Decreased overall mortality of workers indicates a healthy worker effect, which – together with the relatively small cohort size – could have prevented the detection of small risks.
Chrysotile studies not specifically of cement products
Berry & Newhouse (1983) reported on a mortality (1942–1980) study carried out in a factory manufacturing friction material. Chrysotile was the only type of asbestos used except during two well-defined periods before 1945 when crocidolite asbestos was used as well, and over 99% of the population was traced. Compared with national death rates, there was no detectable excess of deaths due to lung cancer, gastrointestinal cancer or other cancers. The exposure levels were relatively low, with only 5% of men having a cumulative exposure of 100 fiber-years/ml. This was due, in part, to the inclusion of several short-term workers but was also a consequence of good environmental control in the factory during the past 30 years. The authors state: “The experience at this factory over a 40-year period showed that chrysotile asbestos was processed with no detectable excess mortality”. The authors also reported on a case control study that was carried out on the 11 deaths due to mesothelioma which showed that eight of the workers had been exposed to crocidolite asbestos and another was possibly exposed intermittently to crocidolite asbestos. The other two had been employed for most of their working lives outside the factory, and their mesotheliomas could not be definitely attributed to exposure to chrysotile.
Newhouse & Sullivan (1989) reported on a further analysis of the Berry & Newhouse (1983) cohort though an additional seven years. The authors confirmed that there were no excess deaths from lung cancer or other asbestos related cancers, or from chronic respiratory disease. After 1950, hygienic control was progressively improved at this factory, and from 1970, the authors reported that the levels of asbestos did not exceeded 0.5–1.0 fiber/cm3. The authors stated: “It is concluded that with good environmental control, chrysotile asbestos may be used in manufacture without causing excess mortality”. At this time there were 13 deaths attributed to mesothelioma and of these, 11 had known contact with crocidolite asbestos. Of the remaining two, one had an uncertain diagnosis and in the other the occupational history was not well established.
The importance of tremolite asbestos contamination in chrysotile dust and talc was evaluated by Roggli et al. (2002a) who examined the association of the development of mesothelioma to contaminating tremolite fibers present in chrysotile dust and talc. The authors examined 312 cases of mesothelioma, for which fiber burden analyses of lung parenchyma had been performed by means of SEM. The amount of tremolite asbestos, non-commercial amphibole asbestos, talc and chrysotile was determined. Of the 312 cases, 166 had tremolite asbestos with 81 of these above background levels. Fibrous talc was identified in 193 cases with a strong correlation to the tremolite content (p < 0.0001). Chrysotile was identified in only 32 cases, but still correlated strongly with the tremolite content (p < 0.0001). Non-commercial amphibole fibers (tremolite, actinolite and/or anthophyllite) were the only fiber types found above background in 14 cases. The authors concluded that tremolite asbestos in lung tissue samples from mesothelioma victims derived from both talc and chrysotile and that tremolite asbestos accounts for a considerable fraction of the excess fiber burden in end-users of asbestos products.
In another study, Roggli et al. (2002b) evaluated the type of occupational exposure in correlation with asbestos fiber content and type in 1445 cases of mesothelioma with known exposure history. Of these, 268 cases had lung fiber burden analysis. Fiber analyses were performed on formalin-fixed or paraffin embedded lung tissue specimens by using techniques described in Roggli et al. (1992). The authors stated that samples usually included lung parenchyma abutting against the visceral pleura, with each sample typically weighing 0.25 to 0.35 gm (wet weight) and as little as 0.1 gm or less of wet tissue. Lung tissue was processed for digestion by using the sodium hypochlorite technique. Asbestos bodies were determined by light microscopy and fiber analysis by SEM with fiber morphology as determined by SEM and elemental composition assessed by EDXA. The cases were classified into 23 exposure categories which included occupational as well as non-occupational exposures although there was a substantial overlap in exposure types. The authors reported that all but one of the occupational categories analyzed had above-background levels of commercial amphiboles and that commercial amphiboles are responsible for most of the mesothelioma cases observed in the USA.
Carel et al. (2007), a study led by the International Agency for Research on Cancer, examined the risk of lung cancer following occupational exposure to asbestos and man-made vitreous fibers in a multicenter case-control study in Europe. Two regions were studied in this program, six Central and Eastern European countries and the UK, during the period 1998–2002. Comprehensive occupational and socio-demographic information was collected from 2205 newly diagnosed male lung cancer cases and 2305 frequency matched controls. Adjustment was made in the odds ratios (OR)* to take into account other relevant occupational exposures and tobacco smoking. The OR for asbestos exposure was 0.92 (95% CI 0.73–1.15) in Central and Eastern Europe and 1.85 (95%CI 1.07–3.21) in the UK. Similar ORs were found for exposure to amphibole asbestos. The OR for MMVF exposure was 1.23 (95%CI 0.88–1.71) with no evidence of heterogeneity by country. The Central and Eastern European asbestos industry had been reliant upon Russia for supplying asbestos in the 30–50 years prior, when exposure would have been important for determining this outcome. Russia, then as now, uses chrysotile asbestos commercially. While not discussed directly in this publication, the differences in the ORs are readily understood by the fact that the UK was the largest importer and user of amphibole per capita in the world. Commercial (non-military) asbestos production in the Soviet Union was of chrysotile alone (Kashansky et al., 2001). Carel et al.’s (2007) study clearly demonstrated that when chrysotile alone was used as in Central and Eastern Europe, there is no measurable excess of lung cancer risk.
South Africa, like Australia, represents a very particular situation in the history of asbestos use. Both countries have historically been the major sources of amphiboles (crocidolite and amosite in South Africa), and have used these varieties of asbestos locally along with chrysotile, which was also mined in both South Africa and Australia. In both these countries, the number of mesothelioma cases has been much higher than anywhere else in the world. White et al. (2008) have indicated that 23% of cases in South Africa were found in persons never employed in mining. These cases, however, were found associated with living in neighborhoods close to amphibole mining facilities, predominately one area with crocidolite mines, thus associated with environmental exposure. The authors concluded that:
No cases [of mesothelioma] were associated with South African chrysotile. Consequently, in the vast majority of cases of mesothelioma, environmental exposure to asbestos occurred in the Northern Cape Province, in proximity to mines, mills and dumps where crocidolite was processed. Crocidolite appears more mesotheliomagenic than amosite, and chrysotile has not been implicated in the disease. This is true for both occupationally and environmentally exposed individuals.
The association of amphibole asbestos with lung disease was evaluated by Schneider et al. (2010) who reported on the measurement of asbestos fiber content of the lungs as it was associated with diffuse interstitial fibrosis (DPF). The asbestos fiber burden was determined in patients with DPF who had a history of asbestos exposure in which their biopsies did not meet established criteria for asbestosis. This was compared to the fiber burden in confirmed asbestosis cases. The fiber burden analysis was performed using SEM and EDXA of lung parenchyma from 86 patients with DPF and 163 patients with asbestosis. The correlation of the number of asbestos fibers found for a quantitative degree of fibrosis was reported. Schneider et al. (2010) reported that the fibrosis scores of the asbestosis cases correlated best with the number of uncoated commercial amphibole fibers.
Chrysotile epidemiological reviews
As reviewed above, most exposures in the past even when characterized as pure chrysotile would be more accurately described as predominantly chrysotile exposure. Pierce et al. (2008) have analyzed the cumulative exposure-response data reported for predominantly chrysotile-exposed cohorts in the published literature to identify an actual “no-effect” exposure level for chrysotile-related lung cancer and mesothelioma. From over 350 published studies, 14 were found to meet the inclusion criteria in which lung cancer risk was stratified by cumulative chrysotile exposure and four studies were found for mesothelioma. The authors reported that
The preponderance of the cumulative “no-effects” exposure levels for lung cancer and mesothelioma fall in a range of approximately 25–1000 fibers per cubic centimeter per year (f/cc-yr) and 15–500 f/cc-yr, respectively, and a majority of the studies did not report an increased risk at the highest estimated exposure.
The authors detailed as well that a number of sources of uncertainty affected these no-effect levels. These included uncertainty in the cumulative exposure estimates, conversion of dust counts to fiber data and use of national age-adjusted mortality rates. The authors also explained that there were numerous potential biases in the data including, for example, smoking was rarely controlled for and amphibole exposure did in fact occur in a majority of the studies, which would bias many of the reported “no-effect” exposure levels toward lower values.
Paustenbach et al. (2004) reviewed the potential environmental and occupational health hazards associated with the presence of chrysotile asbestos in brake linings and pads. This review, covering studies and observations published over several decades, demonstrated that in general, exposures have been minimal and did not show any demonstrable risk when chrysotile was used in brake linings and pads. The authors reported that only the friction materials manufacturing workers in the UK who were exposed to crocidolite while making railroad engine brake linings were found to have an increased relative risk of mesothelioma. In addition, the authors reviewed 20 published studies evaluating asbestos exposure or asbestos-related health effects in friction product manufacturing workers. The authors found that these studies indicated that friction product manufacturing workers were historically exposed to concentrations of chrysotile fibers perhaps 10–50 times greater than those of brake mechanics, however, the risk of asbestosis, mesothelioma and lung cancer, if any, was not apparent, except for those friction materials manufacturing workers who had some degree of exposure to amphibole asbestos during their careers.
Kanarek (2011) presented a review of asbestos and associated mesothelioma including case series, case-control and cohort epidemiology in which he stated that chrysotile is the “exclusive or overwhelming fiber exposure”. However, the presentation of each case presents little if any data in support of this view. In the discussion, he states that “This review sought to search the world epidemiology literature on mesothelioma to catalogue the case-series, cohort and case-control studies in which the asbestos exposure appeared to be overwhelmingly to the chrysotile type”. However, if the individual studies are examined closely, they appear not to be exclusively of chrysotile exposure. As an example, one of the studies cited in support is by Aguilar-Madrid et al. (2010) that reported on a study in which they carried out a case-control study of malignant pleural mesothelioma in 472 workers insured by the Mexican Institute of Social Security, all Valley of Mexico residents, with 119 incident cases and 353 controls. Unfortunately, in the study there was no measure of exposure in any work environment in which asbestos was used. The authors “estimated” exposure in four categories based upon comparison with other studies. As a result there was no knowledge available on which fibers were used in the work environments. However, for “asbestos” workers, the use of amphibole types (especially crocidolite, or mixtures containing amphiboles) was widespread in Mexico up to the 1990s, particularly in the manufacture of fibro-cement pipes. As it is well known that clinical diagnosis of mesothelioma can be some 40–45 years after onset of exposure, mesothelioma cases that are diagnosed in 2010 may well relate to exposure conditions prevailing back in the 1970s. For this reason, it is almost certain that more new cases will be diagnosed in the near future. Because there was no measure of which fibers were used and their concentrations, in this study it is impossible to distinguish effects from chrysotile versus those from amphibole asbestos. In addition, the recent confirmation of mesothelioma cases following exposure to naturally occurring erionite, which outcrops over an area of central Mexico, will produce difficulties in attributing cause to occupational cases (Ilgren et al., 2008a,b; Kliment et al., 2009). In another example, Mancuso et al. (1983, 1988) is cited stating that exposure of railroad workers was exclusively to chrysotile. However, as explained by Gibbs & Pooley (2008), subsequent tissue analyses have shown the presence of amosite and crocidolite in the rail workers lungs.
Similarly, Smith & Wright (1996) also postulated that chrysotile asbestos is the main cause of pleural mesothelioma. In the studies cited, the authors often state that exposure was predominately to chrysotile without providing specific data as to how much amphibole was present. As discussed above, more recent inhalation toxicology studies demonstrate that even short 5 d exposures to amphiboles can result in significant pathological response in the lung and pleura.
Yarborough (2006) reviewed all available epidemiological studies to determine if chrysotile was a cause of mesothelioma. This review was prompted by the long-standing debate over the potential contribution of chrysotile to mesothelioma risk. Yarborough undertook an extensive review of the epidemiological cohort studies in order to evaluate the extent of the evidence related to free chrysotile fibers, with particular attention to confounding by other fiber types, job exposure concentrations, and consistency of findings. A total of 71 asbestos cohorts exposed to free asbestos fibers were reviewed. The authors concluded that the data “does not support the hypothesis that chrysotile, uncontaminated by amphibolic substances, cause mesothelioma”.
Use and exposures in the past and today
Historically, the two minerals groups, chrysotile and amphibole asbestos, were often used interchangeably in industrial applications. In some situations one was preferential to the other in terms of process. Often cost and availability were the overriding factors in determining which mineral was used. Additionally, industrial associations were often instrumental in determining which fiber was used. As an example, in the UK many of the mining operations in South Africa were either owned or associated with a UK company and as such, the UK became the largest importer of amphibole asbestos in the world.
Dust levels were not well controlled in the mines, and some applications for which the minerals were used, such as open spraying, also resulted in very high exposure concentrations (Esmen & Corn, 1998; Gibbs, 1994).
A review of the epidemiological studies described as chrysotile only show that implementation of workplace controls reduce the exposure concentration in these applications to low levels. As an example: Silvestri et al. (2001) summarized information on work practice, fiber concentration and health-related effects in the workers at the Balangero mine reported that by 1989 with controls, exposure concentrations were 0.19 fibers/ml in the mine; 0.54 fibers/ml in the crushing area; 0.93 fibers/ml in the fiber selection area and 0.78 fibers/ml in the bagging area.
Concerning the Quebec miners and millers, Liddell et al. (1998) stated that “On the other hand, modern dust conditions are well below the average even of dust category one and so there can be considerable confidence that the risk of lung cancer as a result of such exposure has become vanishingly small”.
Today the situation is remarkably different. Only chrysotile is used commercially. In the past, some chrysotile mines had veins of tremolite running through the ore body, which were excavated with the chrysotile. Today, the tremolite veins when present are easily differentiated from chrysotile because they are of a different color and can be identified and avoided in those few mines that have such veins (Williams-Jones et al., 2001).
The Cana Brava chrysotile mine in Brazil routinely has the chrysotile analyzed to assess the presence of amphiboles. The reports from the Institute of Occupational Medicine in Edinburgh (Karbownik & Clark, 1997, 2005, 2006, 2007, 2008, 2009, 2012) as well as a laboratory in Brazil (Zamataro & Franzini 2012) have shown that there is no detectable amphibole asbestos in the chrysotile.
The chrysotile from the Calidria (New Idria, CA) chrysotile mine has also been assessed for the presence of amphibole asbestos (Coleman, 1996; Pooley, 2003). Ilgren (2004) summarized these results stating that “Only very rarely have non-asbestiform ‘non-friable’ amphibole (so-called cleavage fragment) minerals been found in the New Idria serpentine body but away from the ore zone”.
Two reports (Kashansky et al., 2001; Tossavainen et al., 1996) found no tremolite in air samples from the Uralasbest mine in Asbest, Russia, which is the largest mine currently in production. Tossavainen et al. (2000) reported on the pulmonary mineral fibers concentrations in 24 chrysotile miners, millers, and product manufacturers from workers at the Uralasbest mine. The authors reported that while “the mean and range of pulmonary chrysotile concentrations were about the same as reported previously from the Canadian mining and milling industry. In the Russian samples, the mean concentration of tremolite fibers was less by at least one order of magnitude”. The authors also reported that no amosite or crocidolite fibers were detected in any tissue sample with coated ferruginous bodies relatively rare (<1% of counted fibers).
Finley et al. (2012) reported on the evaluation of tremolite asbestos exposures associated with the use of chrysotile-containing commercial products. The authors conservatively estimated the cumulative tremolite asbestos exposures as: career auto mechanic: 0.0279 f/cc-year; non-occupational use of joint compound: 0.0006 f/cc-year; non-occupational use of vermiculite-containing gardening products: 0.0337 f/cc-year; home-owner removal of Zonolite insulation: 0.0002 f/cc-year. They also reported that these exposures are far below the lowest-observed-adverse-effect level that they determined for tremolite.
In the past even when no effort was made to avoid mining the tremolite veins, the percentage of tremolite was very small and measurements in one study showed it never amounted to more than 0.24% found in one out of eight chrysotile samples analyzed, while the other seven samples contained no tremolite (detection limit of 0.002 < 0.0002% using SEM, the most sensitive of the analytical methods used) (Addison & Davies, 1990).
Such levels of tremolite asbestos would be important if the chrysotile exposure was at very high concentrations and included a significant number of longer fibers which persisted over many years. In actual practice in the past, even when exposures to chrysotile were very high in chrysotile mining and milling, much of the tremolite asbestos has short length and low aspect ratio; with effects from exposure to tremolite asbestos only reported following long-term exposures at very high concentrations (McDonald et al., 1997).
Studies have reported that chrysotile as mined in the past without differentiation of the possible tremolite asbestos exposure, will not produce mesotheliomas in those exposed to current or recently regulated exposure concentrations, and certainly not in those exposed at environmental levels (Churg, 1988). With the awareness of industry of the tremolite issue specific measures have been introduced to avoid any tremolite veins in those few mines in which they occur.
In addition, in mines today, the use of water control spraying technology has greatly limited ambient dust levels to which the workers are exposed during mining and closed-circuit systems greatly reduce dust levels during milling (Bragg, 2001) and (Safe Use of Chrysotile Asbestos: A Manual on Preventive and Control Measures, 1993 and The Basics of Chrysotile Asbestos Dust Control, 2008. 4th edition, Published by the Chrysotile Institute, Montreal, QC, Canada (jmarcleblond@2011ica.com).
Today, the vast majority of chrysotile is used in high-density cement products (Virta, 2006). In these products, chrysotile is integrally bound into the cement particles and matrix with little or no opportunity for release as individual fibers. The industry also has instituted extensive training and educational programs on how to limit dust levels to assure personal protection not only in the mining sectors, but also in use (installation, maintenance, repair and disposal) in the construction trades.
Discussion
While the safe use of asbestos mandates that exposures be controlled, the extensive literature base clearly differentiates the dose response of chrysotile as compared to amphibole asbestos and demonstrates that controlled use of chrysotile is not associated to a significant risk while even short exposure to amphibole asbestos can produce cancer.
The studies by Dement et al. (1982) and Yano et al. (2001) which have been interpreted as studies on chrysotile asbestos are, after careful review and understanding of the conditions and data presented, not representative of chrysotile exposure alone but rather have numerous other elements as described above which were not fully taken into consideration.
The importance of amphibole point sources, either industrial or environmental to the incidence of mesothelioma has been documented in a number of studies. The studies by Musti et al. (2009) and Barbieri et al. (2012) show the relationship of increased mesothelioma risk in individuals without occupational or domestic or household exposure who lived near an asbestos plant in an urban area that had documented use of amphibole asbestos over 50 years. Kurumatani & Kumagai (2008) investigated the magnitude of the risk among residents who lived near a former large asbestos cement pipe plant that used crocidolite and chrysotile. The authors reported that residents, who had lived within a 300 m radius of the plant, had a SMR for mesothelioma of 13.9 (5.6–28.7) for men and 41.1 (15.2–90.1) for women. Case & Abraham (2009) examined the mesothelioma risk in two American counties, Jefferson Parish, Louisiana and El Dorado County, California. Jefferson Parish, LA, was chosen as the prototype of legacy exposures on the basis of historical evidence of crocidolite use in asbestos plants with known mesotheliomas in the workforce, known shipyards with amosite use in the same area, and the presence of crocidolite-containing scrap in over 1400 properties. El Dorado, CA, was chosen due to the presence of naturally occurring amphibole exposures. The authors reported that the industrial use legacy exposure area was high in mesothelioma incidence and mortality in Jefferson Parish as a result of crocidolite and amosite exposure, while a clear increase in incidence or mortality was not observed in the naturally occurring asbestos area of El Dorado County. Pan et al. (2005) examined the mesothelioma incidence of people living near ultramafic rock deposits which are the principal source of asbestos. The authors reported that some occupations such as shipyard worker, boilermaker, insulator, plumber, pipefitter and steamfitter, and industries such as shipping, construction and Navy had higher occupational exposure to asbestos and were strongly associated with an increased risk of malignant mesothelioma. They also reported that residential proximity to ultramafic rock deposits shows an independent and dose–response association with mesothelioma risk.
The world production of asbestos in 1960 was around 2 million tons, and remained at 2 million tons in 2010 (Virta, 2006, 2011). However, while in the early 1960s production included all major types (chrysotile, crocidolite and amosite), due to their recognized toxicity, the United States has not imported amosite since 1985 and has not imported crocidolite since about 1995 (Virta, 2006). The mining of crocidolite and amosite in South Africa ended in 1997 and 1992, respectively, and the mining of crocidolite in Australia and Bolivia ended in 1983 and 1968 (Virta, 2006). Ilgren et al. (2012) have reported on plants in which crocidolite asbestos is still used in Bolivia. The authors reported that there was no increase in the incidence of mesothelioma in associated populations. Ilgren et al. attributed this to the specific characteristics of the Bolivian crocidolite which has a larger fiber width distribution than other crocidolite asbestos, with considerably fewer Stanton fibers (longer than 8 µm and thinner than 0.25 µm) (Stanton et al., 1981; van Orden et al., 2012).
Unfortunately, because of procrastination by some governments in implementing regulation of amphiboles (e.g. France, Décret n°94-645 du 26 juillet 1994), the remaining amphiboles inventories were allowed to be used in some factories up to the mid-1990s. In addition, due to the large use in past years of amphiboles by some countries and their relative insolubility, a significant background level of amphibole asbestos remains (in the environment, buildings and devices). With the characteristic long latency associated with onset of asbestos-related cancer, especially with mesothelioma, a high incidence of this particular cancer of the pleura may be expected in those countries for the next two or three decades due to the extended use of amphiboles. As observed in both the recent inhalation toxicology studies and in the epidemiology studies, even a short exposure to amphiboles can result in lung cancer and or mesothelioma.
The carcinogenic potency of amphibole asbestos has been established both epidemiologically and toxicologically, leading to it being no longer used in commerce. In 1989, a group of international experts convened by the WHO in Oxford (UK) had recommended that these asbestos varieties should be prohibited immediately, and that the use of chrysotile should be controlled and regulated at a permissible exposure limit (PEL) of 1 fiber/cm3 in the workplace. The workplace PEL has since been lowered in some countries to 0.1 fiber/cm3 (e.g. ACGIH TLV 0.1 f/cm3; European OEL 0.1 f/cm3; Pohanish, (2008)).
Today, the remaining practical concern is whether chrysotile can be produced and used safely, and if indeed this regulation carries a reasonable assurance that workers are adequately protected. Based upon the current science reviewed above, in absence of amphibole asbestos, the use of chrysotile at current Québec PELs in the workplace has not been associated with a statistically detectable increase in risk as observed epidemiologically. From these published studies, it can be seen that chrysotile can be used safely in the manufacturing of cement high-density applications. The International Labour Organization has issued a Code of Practices entitled “Safety in the Use of Asbestos” (ILO, 1984), which addresses all pertinent issues regarding the modern and responsible use of asbestos.
Erosion of surface deposits over millennia means that chrysotile is a ubiquitous component of the particulate matter in the air. The WHO (1985) estimates the background exposure to chrysotile as between 0.01 and 0.001 fiber per milliliter of air. The risk to health from exposure to chrysotile at this background level based upon the toxicology and epidemiology studies is certainly not significant. Industrial and other exposure at the high end of this range has been labeled acceptable by the Ontario Royal on Asbestos, not significant by the WHO, and “… further control not justified” by the Royal Society in London (UK).
In the area of occupational health, and specifically with regard to the use of chrysotile asbestos, regulatory agencies in all countries have the responsibility to set workplace exposure limits that will reduce the risk to workers to the lowest possible level. That this exercise should be based on the most recent scientific assessment available would seem obvious.
Conclusion
This review provides an important basis for substantiating both kinetically and pathologically the differences between chrysotile and amphibole asbestos. Chrysotile which is rapidly attacked by the acid environment of the macrophage, falls apart in the lung into short fibers and particles, while the amphibole asbestos persist creating a response to the fibrous structure of this mineral.
Chrysotile is mineralogically distinct from the amphiboles with a very different chemical structure. The thin rolled or concentric sheets that form the chrysotile fiber leads to the ability of the lung/macrophage system to decompose the chrysotile fibers once inhaled as seen in the biopersistence studies of commercial chrysotiles. This effect is substantiated by both mineralogical and in-vitro studies.
The short-term inhalation toxicity studies of chrysotile that have been performed at non-lung overload conditions demonstrate that the long (>20 µm) fibers are rapidly cleared from the lung, are not translocated to the pleural cavity and do not initiate any fibrogenic response. This is in marked contrast to the long amphibole asbestos fibers which persist through the rat’s lifetime, are quickly (within 7 d) translocated to the pleural cavity and result in interstitial fibrosis and pleural inflammation. Following sub-chronic inhalation at a mean exposure of 76 fibers L > 20 µm/cm3 (3413 total fibers/cm3) resulted in no fibrosis at any time point and no difference with controls in BrdU response or biochemical and cellular parameters. The long chrysotile fibers were observed to break apart into small particles and smaller fibers.
Recent quantitative reviews of epidemiological studies of mineral fibers have determined the potency of chrysotile and amphibole asbestos for causing lung cancer and mesothelioma in relation to fiber type and have also differentiated between these two minerals. The most recent analyses also concluded that it is the longer, thinner fibers that have the greatest potency as has been reported in animal inhalation toxicology studies. The epidemiology studies on chrysotile have been reviewed and effects are evaluated in light of the frequent use of amphibole asbestos.
The studies reporting on the use of chrysotile alone in high-density cement products as well as other applications and the implementation of controls in mining and manufacturing provide a framework for establishing safe use.
As with other respirable particulates, there is evidence that heavy and prolonged exposure to chrysotile can produce lung cancer. The importance of the present and other similar reviews is that the studies they report show that low exposures to chrysotile do not present a detectable risk to health. Since total dose over time decides the likelihood of disease occurrence and progression, they also suggest that the risk of an adverse outcome may be low with even high exposures experienced over a short duration.
Declaration of interests
The preparation of this review was supported by a grant from the International Chrysotile Association, Washington, DC, USA, in cooperation with The Canadian Chrysotile Association, Montréal, QC, Canada. The affiliation of the authors is as shown on the cover page and includes university, government institute, hospital and corporate affiliations as well as independent toxicology consultants. The review is the professional work product of the authors and may not necessarily represent the views of the corporate sponsors. Two of the authors, David Bernstein and Allen Gibbs have appeared as expert witnesses in litigation concerned with alleged health effects of exposure to chrysotile. Jacques Dunnigan has served as an expert witness on the health effects of chrysotile before the Commission de la santé et sécurité du travail du Quebec/Workers Compensation Board of Québec.
Appendix
Table A1. .
Chronic inhalation studies with chrysotile.
| Fiber type | ExposureTime (h/d, d/wk, total months) | Fiber concentration (f/cm3) | Fiber mass concentration (mg/m3) | Equivalent fiber concentration/ cm3 TEM | Type, total number of rats | Number of pulmonary tumors | % of Pulmonary tumors | Number of mesotheliomas | References |
|---|---|---|---|---|---|---|---|---|---|
| Chrysotile Canadian (nickel, cobalt, chromium and lead contamination) | 6, 5, 14 | Nd | 86 mg/m3 42–106 mg/m3 first year67–146 mg/m3 second year | 8 600 000 | NS,41 | 10 | 24 | 1 | Gross et al. (1967) |
| Chrysotile UICC Canadian | 7, 5, 24 | Nd | 9.7 mg/m3 | 970 000 | W, 21 | 10 | 48 | 1 | Wagner et al., (1974) |
| Chrysotile UICC Rhodesian | 7, 5, 24 | Nd | 14.7 mg/m3 | 1 470 000 | W, 17 | 11 | 65 | 0 | Wagner et al., (1974) |
| Chrysotile Canadian 714-7D (friction linings) | 5, 5, 24 | 1.7 × 105 SEM 9978 > 5 µm | 15 mg/m3 Total 5.2 mg/m3 response | 1 500 000 | W, 45 | 9 | 20 | 0 | Le Bouffant et al. (1987) |
| SFA chrysotile | 7, 5, 24 | 430 > 5 µm PCOM 669 particles PCOM | 10.8 mg/m3 | 1 080 000 | W, 22 | 8 | 36 | 1 | Wagner et al., (1980) |
| Grade 7 chrysotile | 7, 5, 24 | 1020 > 5 µm PCOM 745 particles PCOM | 10.8 mg/m3 | 1 080 000 | W, 24 | 3 | 13 | 0 | Wagner et al., (1980) |
| UICC chrysotile | 7, 5, 24 | 3750 > 5 µm PCOM 338 particles PCOM | 10.8 mg/m3 | 1 080 000 | W, 23 | 5 | 22 | 0 | Wagner et al., (1980) |
| Chrysotile Calidria | 5, 5, 12 | 241 by SEM 131>5 µm reported as “thick bundles” | 6 mg/m3 | 600 000 | W, 50 | 0 | 0 | 0 | Muhle et al. (1987) |
| Chrysotile long | 7, 5, 12 | 1170 > 5 µm PCOM 33 > 20 µm PCOM 12% > 5 µm SEM | 10 mg/m3 0.5% > 20 µm SEM | 1 000 000 | W, 40 | 20 | 50 | 2 | Davis et al. (1988) |
| Chrysotile short | 7, 5, 12 | 5510 > 5 µm PCOM 670 > 20 µm PCOM 7% > 5 µm SEM | 10 mg/m3 0.2% > 20 µm SEM | 1 000 000 | W, 40 | 7 | 17 | 1 | Davis et al. (1988) |
| Chrysotile UICC A | 7, 5, 12 | 2560 > 5 µm PCOM | 10 mg/m3 | 1 000 000 | Included for comparative fiber numbers without animal exposure | Davis et al. (1988) | |||
| Chrysotile NIEHS | 6, 5, 24 | 1.02 × 105 SEM 1.06 × 104 > 5 µm | 10 mg/m3 | 1 000 000 | F, 69 | 13 | 18 | 1 | Mast et al. (1995) and Hesterberg et al. (1993) |
| Chrysotile | 7, 5, 12 | 1950 > 5 µm PCOM 360 > 20 µm PCOM | 10 mg/m3 | 1 000 000 | W, 40 | 15 | 38 | 0 | Davis et al. (1978) |
| Chrysotile | 7, 5, 12 | 390 > 5 µm PCOM 72 > 20 µm PCOM | 2 mg/m3 | 200 000 | W, 42 | 8 | 19 | 1 | Davis et al. (1978) |
| Author’s reported “With a 2 mg/m3 cloud the percentage retention of chrysotile is almost double that for a 10 mg/m3 cloud”. | |||||||||
| Chrysotile Calidria | 7, 5, 12 | Nd | 7.78 mg/m3 | 778 000 | F, 51 | 2 | 4 | 0 | Ilgren & Chatfield (1997, 1998) and Pinkerton et al. (1983) |
| Chrysotile Jeffrey | 7, 5, 12 | Nd | 11.36 mg/m3 | 1 136 000 | F, 49 | 11 | 22 | 0 | Ilgren & Chatfield (1997, 1998) and Pinkerton et al. (1983) |
| Chrysotile UICC/B | 7, 5, 12 | Nd | 10.99 mg/m3 | 1 099 000 | F, 54 | 13 | 24 | 0 | Ilgren & Chatfield (1997, 1998) and Pinkerton et al. (1983) |
Footnotes
*WHO fibres: defined as fibers >5 μm long, <3 μm wide and with length:width ratios >3:1; WHO (1985).
*Total fibers: all objects with a length:diameter aspect ratio greater than 3:1
*OSHA 29 CFR Part 1915: coal tar pitch volatile, 4-nitrobiphenyl, alpha-naphthylamine, methyl chloromethyl ether, 3,3′-dichlorobenzidine (and its salts), bis-chloromethyl ether, beta-naphthylamine, benzidine, 4-aminodiphenyl, ethyleneimine, beta-propiolactone, 2-actylaminofluorene, 4-dimethylaminoazobenzene, nitrosodimethylamine, vinyl chloride, inorganic arsenic, lead, benzene, acrylonitrile, ethylene oxide, formaldehyde, asbestos.
*Odds Ratio (OR): The odds ratio is a relative measure of risk, telling us how much more likely it is that someone who is exposed to the factor under study will develop the outcome as compared to someone who is not exposed; an OR of 1 or less indicates no effect. Even if the OR is greater than 1, if the lower bound of the 95 % confidence interval (CI) is 1 or less then the OR is not different statistically from 1.
References
- Adamson IY, Bakowska J, Bowden DH. Mesothelial cell proliferation after instillation of long or short asbestos fibers into mouse lung. Am J Pathol. 1993;142:1209–16. [PMC free article] [PubMed] [Google Scholar]
- Adamson IY, Bakowska J, Bowden DH. Mesothelial cell proliferation: a nonspecific response to lung injury associated with fibrosis. Am J Respir Cell Mol Biol. 1994;10:253–8. doi: 10.1165/ajrcmb.10.3.7509611. [DOI] [PubMed] [Google Scholar]
- Addison J, Davies LS. Analysis of amphibole asbestos in chrysotile and other materials. Ann Occup Hyg. 1990;34:159–75. doi: 10.1093/annhyg/34.2.159. [DOI] [PubMed] [Google Scholar]
- Aguilar-Madrid G, Robles-Pérez E, Juárez-Pérez CA, et al. Case-control study of pleural mesothelioma in workers with social security in Mexico. Am J Ind Med. 2010;53:241–51. doi: 10.1002/ajim.20780. [DOI] [PubMed] [Google Scholar]
- Ashcroft T. Epidemiological and quantitative relationships between mesothelioma and asbestos on Tyneside. J Clin Pathol. 1973;26:832–40. doi: 10.1136/jcp.26.11.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aust AE, Cook PM, Dodson RF. Morphological and chemical mechanisms of elongated mineral particle toxicities. J Toxicol Environ Health B Crit Rev. 2011;14:40–75. doi: 10.1080/10937404.2011.556046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballweg JA, Bray RM. Smoking and tobacco use by US military personnel. Mil Med. 1989;154:165–8. [PubMed] [Google Scholar]
- Balzer JL, Cooper WC. The work environment of insulating workers. Am Ind Hyg Assoc J. 1968;29:222–7. doi: 10.1080/00028896809342992. [DOI] [PubMed] [Google Scholar]
- Barbieri PG, Mirabelli D, Somigliana A, et al. (2012). Asbestos fibre burden in the lungs of patients with mesothelioma who lived near asbestos-cement factories. Ann Occup Hyg. 2012. [Epub ahead of print]. doi:10.1093/annhyg/mer126. [DOI] [PubMed]
- Bates TF, Sand LB, Mink JF. Tubular crystals of chrysotile asbestos. Science. 1950;111:512–3. doi: 10.1126/science.111.2889.512. [DOI] [PubMed] [Google Scholar]
- Bellmann B, Muhle H, Creutzenberg O, et al. Calibration study on subchronic inhalation toxicity of man-made vitreous fibers in rats. Inhal Toxicol. 2003;15:1147–77. doi: 10.1080/08958370390229843. [DOI] [PubMed] [Google Scholar]
- Berman DW, Crump KS. (2003). Draft technical support document for a protocol to assess asbestos-related risk [report]. Washington (DC): Office of Solid Waste and Emergency Response US Environmental Protection Agency.
- Berman DW, Crump KS. A meta-analysis of asbestos-related cancer risk that addresses fiber size and mineral type. Crit Rev Toxicol. 2008;38:49–73. doi: 10.1080/10408440802273156. [DOI] [PubMed] [Google Scholar]
- Berman DW, Crump KS, Chatfield EJ, et al. The sizes, shapes, and mineralogy of asbestos structures that induce lung tumors or mesothelioma in AF/HAN rats following inhalation. Risk Anal. 1995;15:181–95. doi: 10.1111/j.1539-6924.1995.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Bernstein DM. Synthetic vitreous fibers: a review toxicology, epidemiology and regulations. Crit Rev Toxicol. 2007;37:839–86. doi: 10.1080/10408440701524592. [DOI] [PubMed] [Google Scholar]
- Bernstein D, Castranova V, Donaldson K, et al. Testing of fibrous particles: short-term assays and strategies. Inhal Toxicol. 2005c;17:497–537. doi: 10.1080/08958370591001121. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Chevalier J, Smith P. Comparison of Calidria chrysotile asbestos to pure tremolite: final results of the inhalation biopersistence and histopathology following short term exposure. Inhal Toxicol. 2005b;17:427–49. doi: 10.1080/08958370591002012. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Riego-Sintes JM, Ersboell BK, et al. Biopersistence of synthetic mineral fibers as a predictor of chronic inhalation toxicity in rats. Inhal Toxicol. 2001a;13:823–49. doi: 10.1080/089583701752378133. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Riego-Sintes JM, Ersboell BK, et al. Biopersistence of synthetic mineral fibers as a predictor of chronic intraperitoneal injection tumour response in rats. Inhal Toxicol. 2001b;13:851–75. doi: 10.1080/089583701752378142. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Rogers R, Chevalier J, et al. The toxicological response of Brazilian chrysotile asbestos: a multidose sub-chronic 90 d inhalation toxicology study with 92 day recovery to assess cellular and pathological response. Inhal Toxicol. 2006;18:313–32. doi: 10.1080/08958370500515871. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Rogers RA, Sepulveda R, et al. The pathological response and fate in the lung and pleura of chrysotile in combination with fine particles compared to amosite asbestos following short term inhalation exposure – interim results. Inhal Toxicol. 2010;22:937–62. doi: 10.3109/08958378.2010.497818. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Rogers RA, Sepulveda R, et al. Quantification of the pathological response and fate in the lung and pleura of chrysotile in combination with fine particles compared to amosite-asbestos following short-term inhalation exposure. Inhal Toxicol. 2011;23:372–91. doi: 10.3109/08958378.2011.575413. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Rogers R, Smith P. The biopersistence of Canadian chrysotile asbestos following inhalation. Inhal Toxicol. 2003;15:101–28. doi: 10.1080/08958370390241713. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Rogers R, Smith P. The biopersistence of Brazilian chrysotile asbestos following inhalation. Inhal Toxicol. 2004;16:745–61. doi: 10.1080/08958370490490176. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Rogers R, Smith P. The biopersistence of Canadian chrysotile asbestos following inhalation: final results through 1 year after cessation of exposure. Inhal Toxicol. 2005a;17:1–14. doi: 10.1080/08958370590885663. [DOI] [PubMed] [Google Scholar]
- Berry G, Newhouse ML. Mortality of workers manufacturing friction materials using asbestos. Br J Ind Med. 1983;40:1–7. doi: 10.1136/oem.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton RE, Vincent JH, Jones AD, et al. An overload hypothesis for pulmonary clearance of UICC amosite fibres inhaled by rats. Br J Ind Med. 1983;40:264–72. doi: 10.1136/oem.40.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles O, Barsigian FM. (1954). Asbestos, In: McGann, Paul W, ed. Minerals yearbook 1951, Bureau of Mines. United States Government Printing Office, 167–176. Available from: http://digital.library.wisc.edu/1711.dl/EcoNatRes.MinYB1951.
- Bowles O, Stoddard BH. (1933). Asbestos, In: Kiessling OE, ed. Minerals yearbook 1932–33, United States Government Printing Office, 1933, 745–752. Available from: http://digital.library.wisc.edu/1711.dl/EcoNatRes.MinYB193132.
- Bragg GM. Fiber release during the handling of products containing chrysotile asbestos using modern control technology. In: Nolan RP, Langer AM, Ross M, et al., editors. eds. The health effects of chrysotile asbestos: contribution of science to risk-management decisions. Ottawa, Canada: Canadian Mineralist; 2001. Spec. Publ. 5, 111–14. [Google Scholar]
- Bray RM, Guess LL, Marsden ME. Prevalence, trends, and correlates of alcohol use, nonmedical drug use, and tobacco use among US military personnel. Mil Med. 1989;154:1–11. [PubMed] [Google Scholar]
- Bray RM, Marsden ME, Peterson MR. Standardized comparisons of the use of alcohol, drugs, and cigarettes among military personnel and civilians. Am J Public Health. 1991;81:865–9. doi: 10.2105/ajph.81.7.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breysse PN, Cherrie JW, Addison J, et al. Evaluation of airborne asbestos concentrations using TEM and SEM during residential water tank removal. Ann. Occup Hyg. 1989;33:243–56. [Google Scholar]
- Campbell WJ, Huggins CW, Wylie AG. (1980). Chemical and physical characterization of amosite, chrysotile, crocidolite, and nonfibrous tremolite for oral ingestion studies by the national institute of environmental health sciences [report of investigation 8452]. Avondale (MD): United States Department of The Interior, US Bureau of Mines.
- Carel R, Olsson AC, Zaridze D, et al. Occupational exposure to asbestos and man-made vitreous fibers and risk of lung cancer: a multicentre case-control study in Europe. Occup Environ Med. 2007;64:502–8. doi: 10.1136/oem.2006.027748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case BW, Abraham JL. Heterogeneity of exposure and attribution of mesothelioma: trends and strategies in two American counties. J Phys: Conf Ser. 2009;151:012008. [Google Scholar]
- Case BW, Dufresne A, McDonald AD, et al. Asbestos fibre type and length in lungs of chrysotile textile and production workers: fibers longer than 18 mum. Inhal Toxicol. 2000;12:411–8. doi: 10.1080/08958378.2000.11463253. [DOI] [PubMed] [Google Scholar]
- Case BW, McDonald C. Chrysotile, tremolite, balangeroite and mesothelioma: similar situations? Occup Environ Med. 2008;65:815–9. [Google Scholar]
- Churg A. Chrysotile, tremolite and malignant mesothelioma. Chest. 1988;93:621–8. doi: 10.1378/chest.93.3.621. [DOI] [PubMed] [Google Scholar]
- Churg A, Wiggs B, Depaoli L, et al. Lung asbestos content in chrysotile workers with mesothelioma. Am Rev Respir Dis. 1984;130:1042–5. doi: 10.1164/arrd.1984.130.6.1042. [DOI] [PubMed] [Google Scholar]
- Coin PG, Roggli VL, Brody AR. Deposition, clearance, and translocation of chrysotile asbestos from peripheral and central regions of the rat lung. Environ Res. 1992;58:97–116. doi: 10.1016/s0013-9351(05)80207-5. [DOI] [PubMed] [Google Scholar]
- Coleman RG. New Idria serpentinite: a land management dilemma. Environ Eng Geoscience. 1996;2:9–22. [Google Scholar]
- Conway TL, Trent LK, Conway SW. (1989). Physical readiness and lifestyle habits among US navy personnel during 1986, 1987, and 1988. Technical Report No 89-24. San Diego, California: Naval Health Research Center.
- Cressey BA, Whittaker EJW. Five-fold symmetry in chrysotile asbestos revealed by transmission electron microscopy. Mineral Mag. 1993;57:729–32. [Google Scholar]
- Davis JM, Addison J, Bolton RE, et al. The pathogenicity of long versus short fibre samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br J Exp Pathol. 1986;67:415–30. [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Beckett ST, Bolton RE, et al. Mass and number of fibres in the pathogenesis of asbestos-related lung disease in rats. Br J Cancer. 1978;37:673–88. doi: 10.1038/bjc.1978.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Jones AD. Comparisons of the pathogenicity of long and short fibres of chrysotile asbestos in rats. Br J Exp Pathol. 1988;69:717–37. [PMC free article] [PubMed] [Google Scholar]
- Dement JM, Brown DP. Lung cancer mortality among asbestos textile workers: a review and update. Ann Occup Hyg. 1994;38:525–32, 412. doi: 10.1093/annhyg/38.4.525. [DOI] [PubMed] [Google Scholar]
- Dement JM, Harris RL, Jr, Symons MJ, et al. Estimates of dose-response for respiratory cancer among chrysotile asbestos textile workers. Ann Occup Hyg. 1982;26:869–87. [PubMed] [Google Scholar]
- Devesa SS, Grauman DJ, Blot WJ, et al. (1999). Atlas of cancer mortality in the United States 1950–1994 [NIH Publication No. 99-4564]. Bethesda (MD): National Institutes of Health, National Cancer Institute.
- Dreessen WC, Dallavalle JM, Edwards TI, et al. (1938). A study of asbestosis in the asbestos textile industry [Public Health Bulletin No. 211]. Bethesda (MD): National Institute of Health, 91–125.
- Elmes PC, Wade OL. Relationship between exposure to asbestos and pleural malignancy in Belfast. Ann N Y Acad Sci. 1965;132:549–57. doi: 10.1111/j.1749-6632.1965.tb41135.x. [DOI] [PubMed] [Google Scholar]
- Esmen NA, Corn M. Airborne fiber concentrations during splitting open and boxing bags of asbestos. Toxicol Ind Health. 1998;14:843–56. doi: 10.1177/074823379801400606. [DOI] [PubMed] [Google Scholar]
- European Commission Joint Research Centre, Institute for Health and Consumer Protection, Unit: Toxicology and Chemical Substances, European Chemicals Bureau. (1999). Methods for the determination of the hazardous properties for human health of man made mineral fibers (MMMF). Bernstein DM, Riego-Sintes JMR, eds, Vol. EUR 18748 EN, April 93. Available from: http://ecb.ei.jrc.it/DOCUMENTS/Testing- Methods/mmmfweb.pdf.
- Evans BW. The serpentinite multisystem revisited: chrysotile is metastable. Int Geol Rev. 2004;46:479–506. [Google Scholar]
- Finley BL, Pierce JS, Phelka AD, et al. Evaluation of tremolite asbestos exposures associated with the use of commercial products. Crit Rev Toxicol. 2012;42:119–46. doi: 10.3109/10408444.2011.636028. [DOI] [PubMed] [Google Scholar]
- Gardner MJ, Winter PD, Pannett B, et al. Follow up study of workers manufacturing chrysotile asbestos cement products. Br J Ind Med. 1986;43:726–32. doi: 10.1136/oem.43.11.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzano E, Riganti C, Tomatis M, et al. Potential toxicity of nonregulated asbestiform minerals: balangeroite from the western Alps. Part 3: depletion of antioxidant defenses. J Toxicol Environ Health Part A. 2005;68:41–9. doi: 10.1080/15287390590523957. [DOI] [PubMed] [Google Scholar]
- Gibbs GW. The assessment of exposure in terms of fibres. Ann Occup Hyg. 1994;38:477–87. doi: 10.1093/annhyg/38.4.477. 409–10. [DOI] [PubMed] [Google Scholar]
- Gibbs AR, Pooley F. Mineral fibers analysis and asbestos related diseases. In: Craighead JE, Gibbs AR, editors. Asbestos and its diseases. New York: Oxford University Press; 2008. pp. 299–316. [Google Scholar]
- Goodglick LA, Kane AB. Cytotoxicity of long and short crocidolite asbestos fibers in vitro and in vivo. Cancer Res. 1990;50:5153–63. [PubMed] [Google Scholar]
- Green FHY, Harley R, Vallyathan V, et al. Exposure and mineralogic correlates of pulmonary fibrosis in chrysotile asbestos workers. Occup Environ Med. 1997;54:549–59. doi: 10.1136/oem.54.8.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppo C, Tomatis M, Turci F, et al. Potential toxicity of nonregulated asbestiform minerals: balangeroite from the western Alps. Part 1: identification and characterization. J Toxicol Environ Health Part A. 2005;68:1–19. doi: 10.1080/15287390590523867. [DOI] [PubMed] [Google Scholar]
- Gross P, Cralley LJ, DeTreville RT. “Asbestos” bodies: their nonspecificity. Am Ind Hyg Assoc J. 1967;28:541–2. doi: 10.1080/00028896709342681. [DOI] [PubMed] [Google Scholar]
- Hain E, Dalquen P, Bohlig H, et al. [Retrospective study of 150 cases of mesothelioma in Hamburg area (author’s transl)] Int Arch Arbeitsmed. 1974;33:15–37. [PubMed] [Google Scholar]
- Hammad Y, Simmons W, Abdel-Kader H, et al. Effect of chemical composition on pulmonary clearance of man-made mineral fibers. Ann Occup Hyg. 1988;22:769–79. [Google Scholar]
- Hargreaves A, Taylor WH. An X-ray examination of decomposition products of chrysotile asbestos and serpentine. Mineral Mag. 1946;27:204–16. [Google Scholar]
- Hein MJ, Stayner LT, Lehman E, et al. Follow-up study of chrysotile textile workers: cohort mortality and exposure-response. Occup Environ Med. 2007;64:616–25. doi: 10.1136/oem.2006.031005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterberg TW, Axten C, McConnell EE, et al. Studies on the inhalation toxicology of two fiberglasses and amosite asbestos in the Syrian golden hamster. Part I. Results of a subchronic study and dose selection for a chronic study. Inhal Toxicol. 1999;11:747–84. doi: 10.1080/089583799196745. [DOI] [PubMed] [Google Scholar]
- Hesterberg TW, Hart GA, Chevalier J, et al. The importance of fiber biopersistence and lung dose in determining the chronic inhalation effects of X607, RCF1, and chrysotile asbestos in rats. Toxicol Appl Pharmacol. 1998;153:68–82. doi: 10.1006/taap.1998.8522. [DOI] [PubMed] [Google Scholar]
- Hesterberg TW, Miiller WC, McConnell EE, et al. Chronic inhalation toxicity of size-separated glass fibers in Fischer 344 rats. Fundam Appl Toxicol. 1993;20:464–76. doi: 10.1006/faat.1993.1057. [DOI] [PubMed] [Google Scholar]
- Hodgson JT, Darnton A. The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann Occup Hyg. 2000;44:565–601. [PubMed] [Google Scholar]
- Hughes JM, Weill H, Hammad YY. Mortality of workers employed in two asbestos cement manufacturing plants. Br J Ind Med. 1987;44:161–74. doi: 10.1136/oem.44.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilgren EB. Coalinga chrysotile a short fibre, amphibole free, chrysotile Part V – lack of amphibole asbestos contamination. Indoor Built Environ. 2004;13:375–82. [Google Scholar]
- Ilgren EB, Breña MO, Larragoitia JC, et al. A reconnaissance study of a potential emerging Mexican mesothelioma epidemic due to fibrous zeolite exposure. Indoor Built Environ. 2008a;17:496–515. [Google Scholar]
- Ilgren E, Chatfield E. Coalinga fibre – a short, amphibole-free chrysotile. Part 1: Evidence for lack of fibrogenic activity. Indoor Built Environ. 1997;6:264–76. [Google Scholar]
- Ilgren E, Chatfield E. Coalinga fibre – a short, amphibole-free chrysotile. Part 2: Evidence for lack of tumorigenic activity. Indoor Built Environ. 1998;7:18–31. [Google Scholar]
- Ilgren EB, Pooley FD, Larragoitia JC, et al. First confirmed erionite related mesothelioma in North America. Indoor Built Environ. 2008b;17:567–8. [Google Scholar]
- Ilgren E, Ramirez R, Claros E, et al. (2012). Fiber width as a determinant of mesothelioma induction and threshold Bolivian crocidolite: epidemiological evidence from Bolivia mesothelioma demography and exposure pathways. Ann Respir Med. Available from: www.slm-respiratory.com .
- ILO. Safety in the use of asbestos: an ILO code of practice. Geneva: International Labour Office; 1984. [Google Scholar]
- Jones RN, Diem JE, Hughes JM, et al. Progression of asbestos effects: a prospective longitudinal study of chest radiographs and lung function. Br J Ind Med. 1989;46:97–105. doi: 10.1136/oem.46.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanarek MS. Mesothelioma from chrysotile asbestos: update. Ann Epidemiol. 2011;21:688–97. doi: 10.1016/j.annepidem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Karbownik J, Clark S. (1997, 2005, 2006, 2007, 2008, 2009, 2012). Determination of presence of amphibole asbestos fibres in four bulk samples of chrysotile [report to client, project no: 610]. Edinburgh, UK: IOM Consulting Limited.
- Kashansky SV, Domnin SG, Kochelayev VA, et al. Retrospective view of airborne dust levels in workplace of a chrysotile mine in Ural, Russia. Ind Health. 2001;39:51–6. doi: 10.2486/indhealth.39.51. [DOI] [PubMed] [Google Scholar]
- Kliment CR, Clemens K, Oury TD. North American erionite-associated mesothelioma with pleural plaques and pulmonary fibrosis: a case report. Int J Clin Exp Pathol. 2009;2:407–10. [PMC free article] [PubMed] [Google Scholar]
- Kobell F. Ueber den schillernden Asbest von Reichenstein in Schlesien. J Prakt Chemie. 1834;2:297–8. [Google Scholar]
- Kurumatani N, Kumagai S. Mapping the risk of mesothelioma due to neighborhood asbestos exposure. Am J Respir Crit Care Med. 2008;178:624–9. doi: 10.1164/rccm.200801-063OC. [DOI] [PubMed] [Google Scholar]
- Larsen G. Experimental data on in vitro fibre solubility. IARC Sci Publ. 1989;90:134–9. [PubMed] [Google Scholar]
- LeBouffant L, Daniel H, Henin JP, et al. Experimental study on long-term effects of inhaled MMMF on the lung of rats. Ann Occup Hyg. 1987;31:765–90. doi: 10.1093/annhyg/31.4b.765. [DOI] [PubMed] [Google Scholar]
- Legifrance. (1994). Décret no 94-645 du 26 juillet 1994 modifiant le décret no 88-466 du 28 avril 1988 relatif aux produits contenant de l'amiante, JORF n°173 du 28 juillet 1994 page 10907. Available from: http://www.legifrance.gouv.fr .
- Liddell FD, McDonald AD, McDonald JC. The 1891–1920 birth cohort of Quebec chrysotile miners and millers: development from 1904 and mortality to 1992. Ann Occup Hyg. 1997;41:13–36. doi: 10.1016/S0003-4878(96)00044-0. [DOI] [PubMed] [Google Scholar]
- Liddell FDK, McDonald AD, McDonald JC. Dust exposure and lung cancer in Quebec chrysotile miners and millers. Ann Occup Hyg. 1998;42:7–20. doi: 10.1016/s0003-4878(97)00053-7. [DOI] [PubMed] [Google Scholar]
- Lippmann M. Effects of fiber characteristics on lung deposition, retention, and disease. Environ Health Perspect. 1990;88:311–7. doi: 10.1289/ehp.9088311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso TF. Mesothelioma among machinists in railroad and other industries. Am J Ind Med. 1983;4:501–13. doi: 10.1002/ajim.4700040404. [DOI] [PubMed] [Google Scholar]
- Mancuso TF. Relative risk of mesothelioma among railroad machinists exposed to chrysotile. Am J Ind Med. 1988;13:639–57. doi: 10.1002/ajim.4700130604. Erratum in: (1989). Am J Ind Med, 15, 125. [DOI] [PubMed] [Google Scholar]
- Mast RW, McConnell EE, Anderson R, et al. Studies on the chronic toxicity (inhalation) of four types of refractory ceramic fiber in male Fischer 344 rats. Inhal Toxicol. 1995;7:425–67. doi: 10.3109/08958379509015208. [DOI] [PubMed] [Google Scholar]
- McClellan RO, Miller FJ, Hesterberg TW, et al. Approaches to evaluating the toxicity and carcinogenicity of man-made fibers: summary of a workshop held November 11–13, 1991, Durham, North Carolina. Regul Toxicol Pharmacol. 1992;16:321–64. doi: 10.1016/0273-2300(92)90011-w. [DOI] [PubMed] [Google Scholar]
- McConnell EE, Axten C, Hesterberg TW, et al. Studies on the inhalation toxicology of two fiberglasses and amosite asbestos in the Syrian golden hamster. Part II. Results of chronic exposure. Inhal Toxicol. 1999;11:785–835. doi: 10.1080/089583799196754. [DOI] [PubMed] [Google Scholar]
- McDonald AD, Case BW, Churg A, et al. Mesothelioma in Quebec chrysotile miners and millers: epidemiology and aetiology. Ann Occup Hyg. 1997;41:707–19. doi: 10.1016/S0003-4878(97)00020-3. [DOI] [PubMed] [Google Scholar]
- McDonald AD, Fry JS, Woolley AJ, et al. Dust exposure and mortality in an American factory using chrysotile, amosite, and crocidolite in mainly textile manufacture. Br J Ind Med. 1983;40:368–74. doi: 10.1136/oem.40.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AD, Fry JS, Woolley AJ, et al. Dust exposure and mortality in an American chrysotile asbestos friction products plant. Br J Ind Med. 1984;41:151–7. doi: 10.1136/oem.41.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AD, Harper A, McDonald JC, et al. Epidemiology of primary malignant mesothelial tumors in Canada. Cancer. 1970;26:914–19. doi: 10.1002/1097-0142(197010)26:4<914::aid-cncr2820260427>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- McDonald JC, Liddell FD. Mortality in Canadian miners and millers exposed to chrysotile. Ann NY Acad Sci. 1979;330:1–9. doi: 10.1111/j.1749-6632.1979.tb18704.x. [DOI] [PubMed] [Google Scholar]
- McDonald JC, McDonald AD. Chrysotile, tremolite and mesothelioma. Science. 1995;267:775–6. doi: 10.1126/science.267.5199.776-a. [DOI] [PubMed] [Google Scholar]
- McDonald JC, McDonald AD. The epidemiology of mesothelioma in historical context. Eur Respir J. 1996;9:1932–42. doi: 10.1183/09031936.96.09091932. [DOI] [PubMed] [Google Scholar]
- McEwen J, Finlayson A, Mair A, et al. Mesothelioma in Scotland. Br Med J. 1970;4:575–8. doi: 10.1136/bmj.4.5735.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. Deposition of inhaled asbestos and man-made mineral fibres in the respiratory tract. Ann Occup Hyg. 1995;39:747–58. doi: 10.1016/0003-4878(95)00050-o. [DOI] [PubMed] [Google Scholar]
- Morris GF, Notwick AR, et al. Development of lung tumors in mutant p53-expressing mice after inhalation exposure to asbestos. Chest. 2004;125:85S–6S. doi: 10.1378/chest.125.5_suppl.85s. [DOI] [PubMed] [Google Scholar]
- Morrow PE. Possible mechanisms to explain dust overloading of the lung. Fundam Appl Toxicol. 1988;10:369–84. doi: 10.1016/0272-0590(88)90284-9. [DOI] [PubMed] [Google Scholar]
- Morrow PE. Dust overloading of the lungs: update and appraisal. Toxicol Appl Pharmacol. 1992;113:1–12. doi: 10.1016/0041-008x(92)90002-a. [DOI] [PubMed] [Google Scholar]
- Muhle H, Bellman B, Heinrich U. Overloading of lung clearance during chronic exposure of experimental animals to particles. Ann Occup Hyg. 1988;32:141–7. [Google Scholar]
- Muhle H, Pott F, Bellmann B, et al. Inhalation and injection experiments in rats to test the carcinogenicity of MMMF. Ann Occup Hyg. 1987;31:755–64. doi: 10.1093/annhyg/31.4b.755. [DOI] [PubMed] [Google Scholar]
- Musti M, Pollice A, Cavone D, et al. The relationship between malignant mesothelioma and an asbestos cement plant environmental risk: a spatial case-control study in the city of Bari (Italy) Int Arch Occup Environ Health. 2009;82:489–97. doi: 10.1007/s00420-008-0358-5. [DOI] [PubMed] [Google Scholar]
- Newhouse ML, Sullivan KR. A mortality study of workers manufacturing friction materials: 1941–86. Br J Ind Med. 1989;46:176–9. doi: 10.1136/oem.46.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse ML, Thompson H. Mesothelioma of pleura and peritoneum following exposure to asbestos in the London area. Br J Ind Med. 1965;22:261–9. doi: 10.1136/oem.22.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIOSH. (2011). Asbestos fibers and other elongate mineral particles: state of the science and roadmap for research [revised April 2011, publication number 2011-159, current intelligence bulletin 62]. Washington (DC): Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health.
- Noll W, Kircher H. Über die Morphologie von Asbesten und ihren Zusammenhang mit der Kristallstruktur. Neues Jb. Mineral., Mh. 1951;1951:219–40. [Google Scholar]
- Oberdörster G. Lung particle overload: implications for occupational exposures to particles. Regul Toxicol Pharmacol. 1995;21:123–35. doi: 10.1006/rtph.1995.1017. [DOI] [PubMed] [Google Scholar]
- Ohlson CG, Hogstedt C. Lung cancer among asbestos cement workers. A Swedish cohort study and a review. Br J Ind Med. 1985;42:397–402. doi: 10.1136/oem.42.6.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond-McLeod MJ, Poland CA, Murphy F, et al. Durability and inflammogenic impact of carbon nanotubes compared with asbestos fibres. Part Fibre Toxicol. 2011;8:15. doi: 10.1186/1743-8977-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oze C, Solt K. Biodurability of chrysotile and tremolite asbestos in simulated lung and gastric fluids. Am Mineral. 2010;95:825–31. [Google Scholar]
- Pan XL, Day HW, Wang W, et al. Residential proximity to naturally occurring asbestos and mesothelioma risk in California. Am J Respir Crit Care Med. 2005;172:1019–25. doi: 10.1164/rccm.200412-1731OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L. (1930). The structure of the chlorites. Proc Nat Acad Sci USA, 16, 578–82. [DOI] [PMC free article] [PubMed]
- Paustenbach DJ, Finley BL, Lu ET, et al. Environmental and occupational health hazards associated with the presence of asbestos in brake linings and pads (1900 to present): A ‘state-of-the-art review’. J Toxicol Environ Health, Part B. 2004;7:33–110. doi: 10.1080/10937400490231494. [DOI] [PubMed] [Google Scholar]
- Perry RH, Chilton CH, eds. (1973). Chemical engineers’ handbook. 5th ed. New York (NY): McGraw-Hill. TIC: 242591.
- Pierce JS, McKinley MA, Paustenbach DJ, et al. An evaluation of reported no-effect chrysotile asbestos exposures for lung cancer and mesothelioma. Crit Rev Toxicol. 2008;38:191–214. doi: 10.1080/10408440701845609. [DOI] [PubMed] [Google Scholar]
- Pinkerton KE, Brody AR, McLaurin DA, et al. Characterization of three types of chrysotile asbestos after aerosolization. Environ Res. 1983;31:32–53. doi: 10.1016/0013-9351(83)90060-9. [DOI] [PubMed] [Google Scholar]
- Piolatto G, Negri E, La Vecchia C, et al. An update of cancer mortality among chrysotile asbestos miners in Balangero, northern Italy. Br J Ind Med. 1990;47:810–4. doi: 10.1136/oem.47.12.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohanish RP, ed. (2008). Sittig’s handbook of toxic and hazardous chemicals and carcinogens. 5th ed. Norwich (NY): William Andrews, 272.
- Poland CA, Duffin R, Kinloch I, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–8. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- Pooley F. Personal communication of a report prepared under contract to KCAC of the examination of chrysotile asbestos samples from the asbestos mine and processing plant of KCAC, Inc., 1991. Cited in Bernstein DM, Chevalier J, Smith P. (2005). Comparison of calidria chrysotile asbestos to pure tremolite: Final results of the inhalation biopersistence and histopathology examination following short-term exposure. Inhal Toxicol. 2003;17:427–49. doi: 10.1080/08958370591002012. [DOI] [PubMed] [Google Scholar]
- Pooley FD, Mitha R. (1986). Determination and interpretation of the levels of chrysotile asbestos in lung tisue. In: Wagner JC, ed. Biological effects of chrysotile. Accomplishments in Oncology Vol. 1. No. 2. Philadelphia: Lippincott, 12–18.
- Roggli VL, Pratt PC, Brody AR. (1992). Analysis of tissue mineral fiber content. In: Roggli VL, Greenberg SD, Pratt PC, eds. Pathology of asbestos-associated diseases. Chap. 11. Boston (MA): Little, Brown, 299–345.
- Roggli VL, Sharma A, Butnor KJ, et al. Malignant mesothelioma and occupational exposure to asbestos: a clinicopathological correlation of 1445 cases. Ultrastruct Pathol. 2002b;26:55–65. doi: 10.1080/01913120252959227. [DOI] [PubMed] [Google Scholar]
- Roggli VL, Vollmer RT, Butnor KJ, et al. Tremolite and mesothelioma. Ann Occup Hyg. 2002a;46:447–53. [PubMed] [Google Scholar]
- Rubino GF, Scansetti G, Donna A, et al. Epidemiology of pleural mesothelioma in North-western Italy (Piedmont) Br J Ind Med. 1972;29:436–42. doi: 10.1136/oem.29.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Sporn TA, Roggli VL. Asbestos fiber content of lungs with diffuse interstitial fibrosis: An analytical scanning electron microscopic analysis of 249 cases. Arch Pathol Lab Med. 2010;134:457–61. doi: 10.5858/134.3.457. [DOI] [PubMed] [Google Scholar]
- Sebastien P, McDonald JC, McDonald AD, et al. Respiratory cancer in chrysotile textile and mining industries: exposure inferences from lung analysis. Br J Ind Med. 1989;46:180–7. doi: 10.1136/oem.46.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selikoff IJ, Churg J, Hammond EC. Landmark article April 6, 1964: Asbestos exposure and neoplasia. By Irving J. Selikoff, Jacob Churg, and E. Cuyler Hammond. JAMA. 1984;252:91–5. doi: 10.1001/jama.252.1.91. [DOI] [PubMed] [Google Scholar]
- Seshan K. How are the physical and chemical properties of chrysotile asbestos altered by a 10-year residence in water and up to 5 days in simulated stomach acid? Environ Health Perspect. 1983;53:143–8. doi: 10.1289/ehp.8353143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sichletidis L, Chloros D, Spyratos D, et al. Mortality from occupational exposure to relatively pure chrysotile: a 39–year study. Respiration. 2009;78:63–8. doi: 10.1159/000163443. [DOI] [PubMed] [Google Scholar]
- Silvestri S, Magnani C, Calisti R, et al. The experience of the Balangero chrysotile asbestos mine in Italy: health effects among workers mining and milling asbestos and the health experienceof persons living nearby. In: Nolan RP, Langer AM, Ross M, Wicks FJ, Martin RF, editors. The health effects of chrysotile asbestos: contribution of science to risk-management decisions. Vol. 5. Ottawa, Canada: Canadian Mineralogist, Spec. Publ.; 2001. pp. 177–86. [Google Scholar]
- Skinner HCW, Ross M, Frondel C. Asbestos and other fibrous materials – mineralogy, crystal chemistry, and health effects. New York (NY): Oxford University Press; 1988. p. 204. [Google Scholar]
- Smith AH, Wright CC. Chrysotile asbestos is the main cause of pleural mesothelioma. Am J Ind Med. 1996;30:252–66. doi: 10.1002/(SICI)1097-0274(199609)30:3<252::AID-AJIM2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Speil S, Leineweber JP. Asbestos minerals in modern technology. Environ Res. 1969;2:166–208. doi: 10.1016/0013-9351(69)90036-x. [DOI] [PubMed] [Google Scholar]
- Stanton MF. Some etioligical considerations of fibre carcinogenesis. In: Bogovski P, Gilson JC, Timbrell V, Wagner JC, editors. Biological effects of asbestos. Lyon: WHO IARC; 1973. pp. 289–94. [Google Scholar]
- Stanton MF, Layard M, Tegeris A, et al. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J Natl Cancer Inst. 1981;67:965–75. [PubMed] [Google Scholar]
- Stanton MF, Wrench C. Mechanisms of mesothelioma induction with asbestos & fiberous glass. J Natl Cancer Inst. 1972;48:797–821. [PubMed] [Google Scholar]
- Stayner L, Kuempel E, Gilbert S, et al. An epidemiological study of the role of chrysotile asbestos fiber dimensions in determining respiratory disease risk in exposed workers. Occup Environ Med. 2008;65:613–9. doi: 10.1136/oem.2007.035584. [DOI] [PubMed] [Google Scholar]
- Suquet H. Effects of dry grinding and leaching on the crystal structure of chrysotile. Clays Clay Miner. 1989;37:439–45. [Google Scholar]
- Thomas HF, Benjamin IT, Elwood PC, et al. Further follow-up study of workers from an asbestos cement factory. Br J Ind Med. 1982;39:273–6. doi: 10.1136/oem.39.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbrell V, Hyett AW, Skidmore JW. A simple dispenser for generating dust clouds from standard reference samples of asbestos. Ann Occup Hyg. 1968;11:273–81. doi: 10.1093/annhyg/11.4.273. [DOI] [PubMed] [Google Scholar]
- Timbrell V, Rendall REG. Preparation of the UICC standard reference samples of asbestos. Powder Technol. 1972;5:279–87. [Google Scholar]
- Titulaer MK, van Miltenburg JC, Jansen JBH, et al. Characterization of tubular chrysotile by thermoporometry, nitrogen sorption, drifts, and TEM. Clays Clay Miner. 1993;41:496–513. [Google Scholar]
- Tossavainen A, Kotilainen M, Takahashi K, et al. Amphibole fibres in Chinese chrysotile asbestos. Ann Occup Hyg. 2001;45:145–52. [PubMed] [Google Scholar]
- Tossavainen A, Kovalevsky E, Vanhala E, et al. Pulmonary mineral fibers after occupational and environmental exposure to asbestos in the Russian chrysotile industry. Am J Ind Med. 2000;37:327–33. doi: 10.1002/(sici)1097-0274(200004)37:4<327::aid-ajim1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Tossavainen A, Riala R, Kamppi R, et al. Dust measurements in the chrysotile mining and milling operations of Uralasbest Company, Asbest, Russia [summary report] Helsinki: Institute of Occupational Health; 1996. p. 220. [Google Scholar]
- Turci F, Tomatis M, Gazzano E, et al. Potential toxicity of nonregulated asbestiform minerals: balangeroite from the western Alps. Part 2: oxidant activity of the fibers. J Toxicol Environ Health Part A. 2005;68:21–39. doi: 10.1080/15287390590523911. [DOI] [PubMed] [Google Scholar]
- US DHHS. (1989). Reducing the health consequences of smoking: 25 years of progress. A report of the Surgeon General, 1989 [DHHS Publication No (CDC) 89-8411]. Rockville (MD): Public Health Service, Centers for Disease Control, Office on Smoking and Health.
- US EPA. (2001). US EPA health effects test guidelines OPPTS 870.8355 guideline for combined chronic toxicity/carcinogenicity testing of respirable fibrous particles [EPA 712-C-01-352, July]. Washington (DC): US Environmental Protection Agency.
- Van Orden DR, Lee RL, Sanchez MS, et al. (2012). The size distribution of airborne Bolivian crocidolite fibers [case report]. The Annals of Respiratory Medicine. Monroeville (PA): RJ Lee Group, Inc. Available from: www.slm-respiratory.com. [last accessed 26 Jul 2012]
- Veblen DR, Wylie AG. Mineralogy of amphiboles and 1:1 layer silicates. In: Guthrie Jr GD, Mossman BT, editors. Health effects of mineral dusts. Washington (DC): Mineralogical Society of America; 1993. Reviews in Mineralogy, Vol. 28, 61–137. [Google Scholar]
- Virta RL. (2002). Asbestos: geology, mineralogy, mining, and uses. Prepared in cooperation with Kirk-Othmer encyclopedia of chemical technology. USGS Open file 02-149. Online Edition. New York (NY): Wiley-Interscience, a division of John Wiley & Sons, Inc.
- Virta RL. (2005). Mineral commodity profiles - asbestos [US Geological Survey circular 1255-KK]. Reston (VA): US Geological Survey.
- Virta RL. (2006). Worldwide asbestos supply and consumption trends from 1900 through 2003 [circular 1298]. Reston (VA): US Geological Survey.
- Virta RL. (2011). 2010 minerals yearbook – asbestos. US Geological Survey Minerals Yearbook – 2010. Reston (VA): US Geological Survey.
- von Kobell F. Ueber den schillernden Asbest von Reichenstein in Schlesian: Jour. Prakt. Chemie. 1834;2:297–8. [Google Scholar]
- Vorwald AJ, Durkan TM, Pratt PC. Experimental studies of asbestosis. AMA Arch Ind Hyg Occup Med. 1951;3:1–43. [PubMed] [Google Scholar]
- Wagner JC, Berry G, Skidmore JW, et al. The effects of the inhalation of asbestos in rats. Br J Cancer. 1974;29:252–69. doi: 10.1038/bjc.1974.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JC, Berry G, Skidmore JW, et al. The comparative effects of three chrysotiles by injection and inhalation in rats. In: Wagner JC, editor. Biological Effects of Mineral Fibers. IARC Publication 30. Lyon: International Agency Research on Cancer; 1980. 363–73. [PubMed] [Google Scholar]
- Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med. 1960;17:260–71. doi: 10.1136/oem.17.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton WH. The nature, hazards, and assessment of occupational exposure to airborne asbestos dust: a review. Ann Occup Hyg. 1982;25:117–247. doi: 10.1093/annhyg/25.2.117. [DOI] [PubMed] [Google Scholar]
- Wang X, Yano E, Qiu H, et al. A 37-year observation of mortality in Chinese chrysotile asbestos workers. Thorax. 2012;67:106–10. doi: 10.1136/thoraxjnl-2011-200169. [DOI] [PubMed] [Google Scholar]
- Warren BE, Bragg WL. The structure of chrysotile, H4Mg3Si2O9 . Z Krystallographie. 1930;76:201–10. [Google Scholar]
- Weill H, Hughes J, Waggenspack C. Influence of dose and fiber type on respiratory malignancy risk in asbestos cement manufacturing. Am Rev Respir Dis. 1979;120:345–54. doi: 10.1164/arrd.1979.120.2.345. [DOI] [PubMed] [Google Scholar]
- White N, Nelson G, Murray J. South African experience with asbestos related environmental mesothelioma: is asbestos fiber type important? Regul Toxicol Pharmacol. 2008;52:S92–6. doi: 10.1016/j.yrtph.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Whittaker EJW. The structure of chrysotile. V. Diffuse reflexions and fibre texture. Acta Cryst. 1957;10:149–56. [Google Scholar]
- Whittaker EJW. The Structure of Chrysotile. V. Diffuse Reflexions and Fibre Texture. Acta Cryst. 1957;10:149–56. [Google Scholar]
- Whittaker EJW. The crystal chemistry of the amphiboles. Acta Cryst. 1960;13:291–98. [Google Scholar]
- Whittaker EJW. in Research report: Chrysotile Fibers --Filled or Hollow Tubes? Mathematical interpretation may resolve conflicting evidence. Chem. Eng. News. 1963;41:34–35. , September 30, 1963. [Google Scholar]
- WHO. (1985). Reference methods for measuring airborne man-made mineral fibers (MMMF): WHO/EURO MMMF reference scheme. Copenhagen: WHO.
- WHO. (1988). Environmental health criteria 77: man-made mineral fibres. Vol. 77. Geneva: WHO.
- Wicks FJ, O’Hanley DS. Serpentine minerals: structures and petrology. In: Bailey S, editor. Hydrous phyllosilicates (exclusive of micas) Washington, DC: Mineralogical Society of America; 1988. Reviews in Mineralogy, Vol. 19, 91–167. [Google Scholar]
- Williams-Jones AE, Normand C, Clark JR, et al. Controls of amphibole formation in chrysotile deposits: evidence from the Jeffrey Mine, Asbestos, Quebec. In: Nolan RP, Langer AM, Ross M, Wicks FJ, Martin RF, editors. The health effects of chrysotile asbestos: contribution of science to risk-management decisions. Ottawa, Canada: Canadian Mineralogist; 2001. Spec. Publ. 5, 89–104. [Google Scholar]
- Work LT. Size reduction gets a new stature. Ind Eng Chem. 1962;54:52–4. [Google Scholar]
- Wypych F, Adad LB, Mattoso N, et al. Synthesis and characterization of disordered layered silica obtained by selective leaching of octahedral sheets from chrysotile. J Colloid Interface Sci. 2005;283:107–12. doi: 10.1016/j.jcis.2004.08.139. [DOI] [PubMed] [Google Scholar]
- Yano E, Wang ZM, Wang XR, et al. Cancer mortality among workers exposed to amphibole-free chrysotile asbestos. Am J Epidemiol. 2001;154:538–43. doi: 10.1093/aje/154.6.538. [DOI] [PubMed] [Google Scholar]
- Yano E, Wang ZM, Wang XR, et al. Mesothelioma in a worker who spun chrysotile asbestos at home during childhood. Am J Ind Med. 2009;52:282–7. doi: 10.1002/ajim.20675. [DOI] [PubMed] [Google Scholar]
- Yarborough CM. Chrysotile as a cause of mesothelioma: an assessment based on epidemiology. Crit Rev Toxicol. 2006;36:165–87. doi: 10.1080/10408440500534248. [DOI] [PubMed] [Google Scholar]
- Zamataro RSI, Franzini MJ. (2012). Verificar qualitativamente a tipologia das amostras de amianto extraídas na SAMA, por análise difratométrica de raios X [Relatório N° 021E-12]. São Paulo – SP, Brazil: Projecontrol Cons. Empresarial e Serviços Ltda.
- Zielhuis RL, Versteeg JP, Planteijdt HT. (1975). Pleura mesothelioma and exposure to asbestos. A retrospective case-control study in the Netherlands. Int Arch Occup Environ Health, 36, 1–18. [DOI] [PubMed]