Abstract
Cardiovascular disease (CVD) biomarkers were examined in a cohort of HIV-infected and HIV-uninfected adolescents who participated in Adolescent Trials Network study 083 utilizing samples from the Reaching for Excellence in Adolescent Care cohort, a longitudinal study of youth infected through adult risk behavior. Nonfasting blood samples from 97 HIV-infected and 81 HIV-uninfected adolescents infected by adult risk behaviors were analyzed for total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), triglycerides, apolipoprotein A-I, high-sensitivity C-reactive protein (hsCRP), soluble vascular adhesion molecule-1 (sVCAM-1), myeloperoxidase, and neopterin at baseline and 18 months later. Results were analyzed using ANOVA, Wilcoxon signed-rank, and paired t tests. Among infected subjects 67 received antiretroviral therapy and 30 were treatment naive. The HIV-infected and HIV-uninfected subjects were similar in gender, ethnicity, and cardiovascular risk factors such as smoking and obesity. In all groups lipid parameters were within accepted guidelines for cardiovascular risk. Among HIV-infected youth on antiretroviral therapy (ART), HDL and apoprotein A-I were significantly lower when compared to uninfected youth. hsCRP was not elevated and thus not predictive for risk in any group. sVCAM-1 levels were significantly elevated in both HIV-infected groups: 1,435 ng/ml and 1,492 ng/ml in untreated and treated subjects, respectively, and 1,064 ng/ml in the uninfected group (p<0.0001). Across all groups neopterin correlated with sVCAM at 18 months (Spearman correlation coefficient 0.58, p<0.0001). Only 9% of ART-treated subjects fully suppressed virus. Lipid profiles and hsCRP, traditional markers of cardiovascular disease, are not abnormal among HIV-infected youth but elevated sVCAM may be an early marker of atherosclerosis.
Introduction
The long-term consequences of HIV-1 infection involve complex interactions between ongoing viral replication, chronic inflammation, and the effects of antiretroviral therapy (ART).1 It has become increasingly clear that HIV-1 infection results in a high risk for atherosclerosis and may be an additional factor to traditional risks for cardiovascular disease (CVD). HIV-infected adults have a higher CVD risk compared to HIV-uninfected individuals with similar risk factors, especially in women and younger men.2,3 Furthermore, evaluations of carotid artery intima-medial thickness have shown that HIV infection, as a risk factor for preclinical atherosclerosis, is similar to traditional CVD risk factors such as smoking.4 These observations are particularly important for adolescents who become infected through risk behavior and face a lifetime of living with HIV-1 and its consequences.5 Furthermore, HIV-infected youth commonly have coexisting cardiovascular risk factors such as smoking and obesity, which also increase the risk for cardiovascular injury.6 Currently very little longitudinal data exist on markers for atherosclerosis and other forms of subclinical cardiovascular disease among adolescents still early in disease. Identifying biomarkers to help predict future atherosclerotic risk among HIV-infected adolescents is needed in order to predict the complex interplay between the direct effects of viral replication on vascular endothelium, chronic HIV-associated inflammation, and the cumulative adverse effects of ART.7 Biomarkers of endothelial dysfunction and abnormal production of macrophage-derived proinflammatory cytokines are indicators of immune dysregulation and may point to potential mechanisms by which HIV adversely impacts the cardiovascular system.8
In this study we examined changes in established biomarkers of subclinical CVD and predictors of clinical cardiovascular morbidities, including markers of inflammation and endothelial injury, in a prospective cohort of HIV-infected and HIV-uninfected adolescents with similar demographics and risk factors.9 Parameters assessed included lipid profiles and apolipoprotein A-I (Apo 1) levels, which have established normal values in healthy adolescents.10 Inflammatory markers of cardiovascular risk evaluated included high sensitivity C-reactive protein (hsCRP), as well as biomarkers of macrophage-mediated inflammation and endothelial injury such as soluble vascular adhesion molecule-1 (sVCAM-1), myeloperoxidase (MPO), and neopterin.11–14 The results were then evaluated in the context of CVD risk factors including smoking, obesity, substance use, and HIV infection status.
Materials and Methods
Study participants
Study subjects consisted of HIV-infected and HIV-uninfected, at-risk adolescents recruited between 1996 and 2000 from participants from the Reaching for Excellence in Adolescent Care and Health (REACH) cohort. All infected subjects acquired HIV-1 through risk behaviors, primarily sexual transmission. Subjects were balanced for ethnicity, gender, and HIV-acquisition risk factors. The details of the study cohort including timing of evaluations and demographics have been previously described.9 Within the REACH cohort of 578 participants, 178 subjects met inclusion and exclusion criteria that excluded subjects who were pregnant, missed study visits, or who had incomplete sets of specimens. Among the individuals selected, 67 were HIV infected and were receiving ART consisting or two nucleoside reverse transcriptase inhibitors and either a protease inhibitor or a nonnucleoside reverse transcriptase inhibitor.15 There were 30 HIV-infected youth who were untreated over 18 consecutive months and 81 uninfected subjects who remained HIV uninfected throughout the duration of the study based on repeated testing.9
Baseline characteristics of study subjects used in this study are summarized in Table 1. Male and female participants were similar in age and racial distribution. Females had a significantly higher body mass index (BMI) than males (p<0.001). The study involved 15 sites and was approved by all institutional review boards. Sera and plasma were stored in a repository at −70°C from prospectively collected blood samples (Fisher Biosciences Repository, Rockville, MD).
Table 1.
Demographic Characteristics of the REACH Subjects
| Female (n=123) | Male (n=55) | p | |
|---|---|---|---|
| Age (years) | 16.7 (1.2) | 16.9 (1.2) | 0.45 |
| Weight (kg) | 75.6 (23.9) | 70.2 (13.9) | 0.64 |
| Height (m) | 1.62 (0.06) | 1.75 (0.09) | <0.0001 |
| BMI (kg/m2) | 28.6 (8.4) | 23.0 (4.1) | <0.0001 |
| Absolute CD4 count (cells/μl)a | 560 (435, 661) | 472 (370, 597) | 0.0003 |
| Viral load (copies/ml)a | 1,700 (830, 7,800) | 8,500 (3,100, 25,000) | 0.003 |
| Viral load <400 copies/ml, %a | 12.2 | 8.3 | 0.63 |
| Race, % | |||
| Black | 73.2 | 65.5 | 0.57 |
| White | 8.9 | 10.9 | |
| Other | 17.9 | 23.6 | |
| Current smoker (%) | 29.0 | 47.2 | 0.02 |
| Alcohol use, last 3 months (%) | 71.8 | 79.5 | 0.36 |
| Marijuana/illicit drug Use, last 3 months (%) | 50.7 | 55.6 | 0.60 |
These values are given only for HIV-infected adolescents. Values are means (SD) or medians (25th, 75th percentile).
BMI, body mass index.
HIV viral load (VL) and T cell subset monitoring
Quantitative VL in EDTA plasma was measured in a centralized laboratory on frozen specimens using either nucleic acid sequence-based amplification (NASBA) or NucliSens assays (Organon Teknika, Durham, NC).16 The lower limits of detection were 400 and 80 copies/ml, respectively. Immunophenotyping of CD4 and CD8 T lymphocyte cell counts was determined at the individual clinical sites in certified laboratories using AIDS Clinical Trials Group (ACTG) standardized flow cytometry protocols.17
Assessment of lipids and markers of inflammation
The CVD risk markers measured included nonfasting cryopreserved blood samples for lipid profiles [total cholesterol, direct low-density lipoprotein (LDL), high-density lipoprotein (HDL), calculated very low-density lipoprotein (VLDL), triglycerides, Apo AI] and hsCRP. Samples were batch analyzed at Quest Diagnostics (Baltimore, MD).
Assessments of sVCAM-1, MPO, and neopterin were performed at the University of South Florida (USF) using previously frozen (−70°C) plasma samples thawed at room temperature. Samples were diluted and evaluated in duplicate using commercially available enzyme-linked immunosorbent assay (ELISA) kits. Plasma levels of sVCAM-1 were analyzed using an ELISA assay (Invitrogen, Carlsbad, CA) that has a detection range of 0.5–75 ng/ml. Plasma neopterin levels were measured undiluted using ELISA (IBL America, Minneapolis, MN) with a detection range of 0.177–25.3 ng/ml). Plasma MPO levels were measured using samples diluted 1:20 by an ELISA (R & D Systems, Minneapolis, MN) with a range of detection of 0.26–ng/ml.
Statistical analysis
SAS version 9.2 (SAS Institute, Cary, NC) was used for data management and analyses. Comparisons across the three study groups were done using the chi square test for categorical variables and by ANOVA or Kruskal–Wallis test for continuous variables depending on the distribution of the variable. Lipids levels and biomarkers of inflammation at baseline and 18 months were compared using paired t-tests for normally distributed variables (e.g., total cholesterol, LDL, HDL, and Apo AI) and Wilcoxon signed rank test for skewed variables (e.g., triglycerides and biomarkers of inflammation). Values are presented as means (SD) or medians (25th, 75th percentile) for skewed variables.
In secondary analyses using the concentrations of a given biomarker as the dependent variable, ANOVA models that included study group (three categories above), baseline values of the given biomarker, age, race, sex, smoking, and BMI were fitted. Other potential confounders such as use of alcohol were evaluated but not included in the final models if they were not significant. In final analyses, the mean (±SEM) concentration at 18 months for each study group was estimated after adjusting for the covariates above. The means and standard errors were adjusted for multiple comparisons used the Tukey–Kramer test. Differences across groups were considered significant at p≤0.05.
Results
Characteristics of the study groups based on HIV status and treatment
Characteristics of the study groups based on HIV and treatment status are included in Table 2. No differences were found across the groups with respect to BMI, ethnicity, smoking, or age. CD4 T cell counts were significantly higher in the HIV-infected untreated cohort. Overall, 75% of the HIV-infected subjects had CD4 T cells counts >350 cells/μl. Treated and untreated subjects show no differenced in viral load as only 9.1% of treated subjects had undetectable virus. HIV-infected subjects receiving therapy used alcohol, marijuana, and other substance more than their HIV-infected untreated counterparts (p<0.0001).
Table 2.
Characteristics of the REACH Ancillary Study Population by HIV and Treatment Status
| Variable | HIV+, No therapy (n=30) | HIV+, ART (n=67) | HIV uninfected (n=81) | p |
|---|---|---|---|---|
| Age, years | 17.0 (1.1) | 16.9 (1.1) | 16.5 (1.3) | 0.07 |
| BMI at baseline, kg/m2 | 27.29 (6.6) | 26.4 (6.9) | 27.05 (8.9) | 0.84 |
| BMI at 18 months, kg/m2 | 27.8 (5.6) | 27.0 (7.8) | 28.0 (9.1) | 0.73 |
| CD4 count, cells/μla | 595 (475, 831) | 525 (377, 619) | — | 0.03 |
| Viral load, copies/mla | 3,500 (1,300, 7,650) | 5,000 (1,100, 20,500) | — | 0.34 |
| Current smoker, % | 48.3 | 25.0 | 37.2 | 0.08 |
| Alcohol use, % | 90.5 | 48.8 | 86.7 | <0.0001 |
| Marijuana or illicit drug use, % | 91.7 | 47.2 | 84.6 | <0.0001 |
| Race, % | ||||
| Black | 70.0 | 76.1 | 66.7 | 0.81 |
| White | 10.0 | 7.5 | 11.1 | |
| Other | 20.0 | 16.4 | 22.2 | |
These values are given only for HIV-infected adolescents. Values are means (SD) or medians (25th, 75th percentile).
ART, antiretroviral therapy; BMI, body mass index.
Differences in established biomarkers of CVD risk among study groups
Differences in CVD risk biomarkers were measured at baseline and 18 months for several established biomarkers of cardiovascular risk (Table 3). There were no differences in total cholesterol or LDL between the cohorts measured at entry or over time. TG and VLDL levels fell significantly over time only in the HIV-infected untreated subjects. The HIV-infected youth receiving ART had higher triglyceride (TG), VLDL, and total cholesterol to HDL ratios compared to the HIV-infected not receiving therapy and uninfected youth (p<0.05, paired t tests). Interestingly, Apo AI significantly increased in both HIV-infected treated subjects and in the uninfected group overtime. Overall, HDL levels were lower while TG and VLDL levels were higher in the infected compared to the uninfected groups. Compared to HIV-uninfected individuals, HIV-infected subjects had significantly higher total cholesterol-to-HDL ratios.
Table 3.
Changes in Lipids and Biomarkers of Inflammation by HIV and Treatment Status in the REACH Ancillary Study Population
| Time | HIV+ no therapy (n=30) | HIV+, ART (n=67) | HIV uninfected (n=81) | pa | |
|---|---|---|---|---|---|
| Cholesterol, mg/dl | T1 | 148.3 (28.7) | 160.2 (32.8) | 161.0 (35.1) | 0.18 |
| T2 | 147.1 (27.6) | 161.8 (37.3) | 162.7 (33.1) | 0.08 | |
| pb | 0.77 | 0.43 | 0.39 | ||
| Direct LDL, mg/dl | T1 | 90.7 (26.1) | 103.8 (26.2) | 99.0 (31.6) | 0.12 |
| T2 | 90.30 (22.8) | 104.70 (30.1) | 100.16 (31.3) | 0.09 | |
| pb | 0.92 | 0.70 | 0.57 | ||
| HDL, mg/dl | T1 | 43.9 (12.1) | 41.6 (11.1) | 50.8 (13.4) | <0.0001 |
| T2 | 45.2 (12.7) | 41.09 (10.0) | 50.5 (13.2) | <0.0001 | |
| pb | 0.48 | 0.72 | 0.80 | ||
| Triglycerides, mg/dl | T1 | 82.0 (66.0, 127.0) | 98.0 (68.0, 132.0) | 72.0 (52.0, 100.0) | 0.001 |
| T2 | 67.5 (54.0, 105.0) | 90.0 (68.0, 143.0) | 75.00 (57.0, 99.0) | 0.01 | |
| pb | 0.03 | 0.93 | 0.21 | ||
| VLDL, mg/dl | T1 | 19.5 (10.3) | 21.6 (9.5) | 15.6 (6.5) | 0.001 |
| T2 | 16.10 (8.5) | 22.09 (11.3) | 17.09 (8.7) | 0.003 | |
| pb | 0.02 | 0.74 | 0.19 | ||
| TCHOL:HDL ratio | T1 | 3.6 (1.2) | 4.1 (1.1) | 3.3 (0.9) | 0.0003 |
| T2 | 3.5 (1.1) | 4.07 (1.1) | 3.39 (1.0) | 0.0002 | |
| pb | 0.31 | 0.74 | 0.32 | ||
| Apolipoprotein AI, mg/dl | T1 | 121.6 (25.3) | 115.9 (19.6) | 126.6 (20.2) | 0.01 |
| T2 | 125.7 (25.5) | 120.9 (20.7) | 132.94 (25.9) | 0.01 | |
| pb | 0.26 | 0.03 | 0.01 | ||
| hsCRP, mg/liter | T1 | 1.3 (0.6, 3.4) | 1.0 (0.3, 2.8) | 0.9 (0.4, 2.2) | 0.65 |
| T2 | 1.0 (0.4, 3.5) | 1.3 (0.4, 4.7) | 0.7 (0.3, 3.2) | 0.29 | |
| pb | 0.77 | 0.16 | 0.23 | ||
| sVCAM1, ng/ml | T1 | 1436 (1158, 1692) | 1492 (1096, 1850) | 1065 (793, 1391) | <0.0001 |
| T2 | 1467 (1069, 1952) | 1457 (985, 2454) | 969 (671, 1398) | <0.0001 | |
| pb | 0.61 | 0.07 | 0.75 | ||
| Neopterin, ng/ml | T1 | 8.0 (5.7, 10.9) | 7.9 (5.4, 14.1) | 5.6 (4.1, 8.1) | 0.0003 |
| T2 | 6.5 (5.1, 11.7) | 11.4 (6.3, 18.5) | 5.3 (4.0, 7.8) | <0.0001 | |
| pb | 0.96 | 0.01 | 0.42 | ||
| Myeloperoxidase, ng/ml | T1 | 68.2 (45.5, 130.0) | 80.8 (53.1, 122.0) | 60.5 (45.2, 99.2) | 0.19 |
| T2 | 60.2 (45.9, 92.4) | 81.9 (61.4, 149.1) | 62.2 (38.6, 114.8) | 0.03 | |
| pb | 0.26 | 0.09 | 0.77 |
p is from ANOVA or Kruskal–Wallis test comparing the three cohorts at each time point.
p is from the Wilcoxon signed rank test or paired t-test comparing measurements at baseline and 18 months within each cohort.
Values are means (SD) or medians (25th, 75th percentile). T1 and T2 refer to nonfasting laboratory measurements at baseline and 18 months of follow-up. TCHOL, total cholesterol; hsCRP, high-sensitivity C-reactive protein; sVCAM-1, soluble vascular adhesion molecule-1; ART, antiretroviral therapy; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Measurement of endothelial inflammation and macrophage activation within the study groups
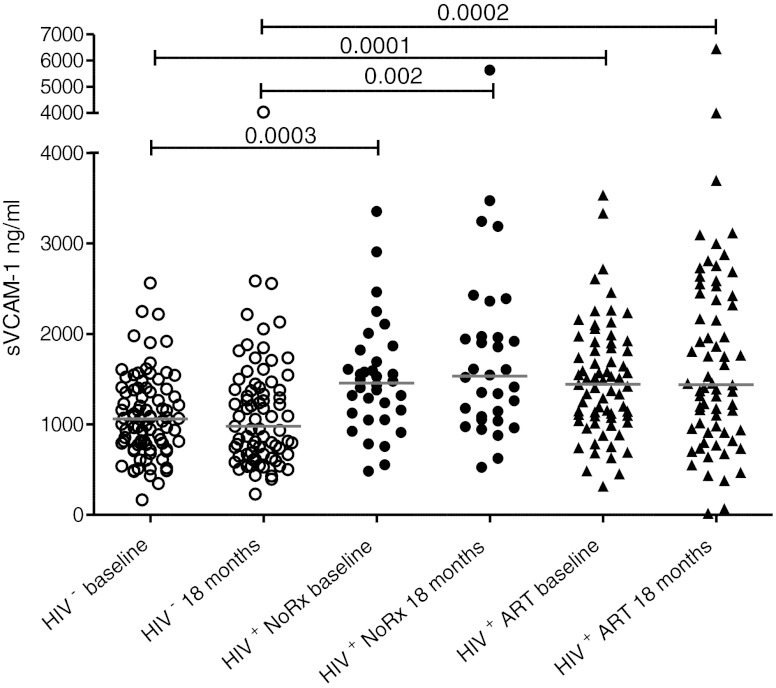
There were no differences in hsCRP either within groups over time or when comparing groups, although hsCRP was elevated across all cohorts when compared to normal levels for age.18,19 Because there were no differences between study groups in hsCRP levels, an established marker of inflammation, we examined sVCAM-1 as a marker of endothelial inflammation. At baseline both HIV-infected groups had significantly higher sVCAM-1 levels compared to the uninfected group (p<0.0001) (Table 3 and Fig. 1). These levels did not change significantly over 18 months and the elevation persisted in the HIV-infected groups.
FIG. 1.
Plasma levels of soluble vascular adhesion molecule (sVCAM) in HIV-infected and uninfected adolescents. Levels of sVCAM, shown on the y-axis in ng/ml, were compared in untreated HIV-infected (HIV+ closed circles), HIV-infected on treatment (HIV+ ART+ closed triangles), and HIV-uninfected (HIV− open circles) adolescents at baseline and after 18 months on study. Significant p values among groups are shown. Comparisons were performed using a Kruskal–Wallis statistic with Dunn's multiple comparison test.
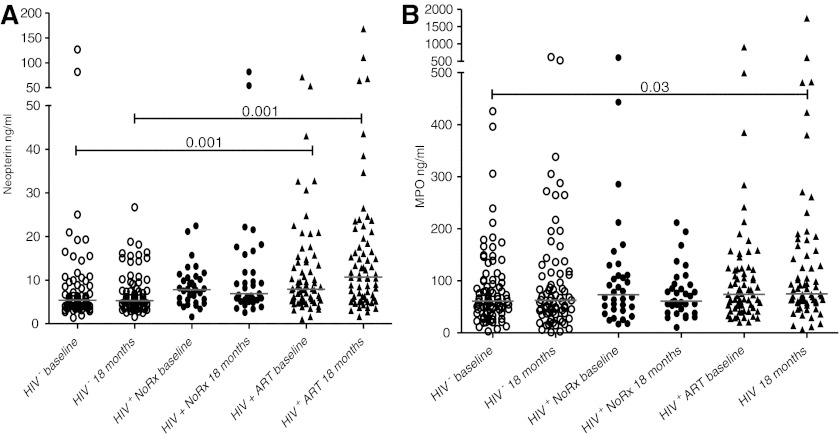
The extent of macrophage activation was assessed by measuring plasma levels of neopterin and MPO. As shown in Table 3 and Fig. 2A, neopterin levels at baseline were significantly higher in HIV-infected groups, both on ART and untreated. Neopterin rose significantly in the HIV-infected, treated group (p=0.01), but did not change in the HIV-infected untreated subjects or the HIV-uninfected subjects. MPO, a biomarker of macrophage activation, did not differ significantly among the study cohorts at baseline but at the 18 month time point MPO was significantly higher in the HIV-infected on therapy compared to the HIV-uninfected group (Fig. 2B and Table 3, MPO).
FIG. 2.
Plasma levels of neopterin and myeloperoxidase (MPO) in HIV-infected and uninfected adolescents. Levels of neopterin (A) (values shown on the y-axis in ng/ml) and MPO (B) levels on the y-axis shown in ng/ml were compared in untreated HIV-infected (HIV+ closed circles), HIV-infected on treatment (HIV+ ART+ closed triangles), and HIV-uninfected (HIV− open circles) adolescents at baseline and after 18 months on study. Significant p values among groups are shown. Comparisons were performed using a Kruskal–Wallis statistic with Dunn's multiple comparison test.
The biomarkers of macrophage activation and endothelial inflammation at baseline were modestly correlated with those at 18 months. The Spearman correlation coefficients were 0.64 for sVCAM-1, 0.58 for neopterin, and 0.56 for MPO, respectively (p<0.0001 for all correlations). The correlations between biomarkers were generally weak, e.g., the highest correlations were between sVCAM-1 and neopterin both at 18 months (r=0.46, p<0.0001) and between sVCAM-1 at baseline and neopterin at 18 months (r=0.37, p<0.0001). There was no correlation (r<0.15, p>0.05 for all) between MPO and neopterin levels at any of the time points.
Multivariate analysis comparing study groups
In analyses that adjusted for age, gender, BMI, smoking, race, and baseline concentration of the respective biomarker, only sVCAM-1 at 18 months showed a significant association (p<0.05) with HIV infection status. In this analysis, the adjusted mean±SEM concentrations of sVCAM-1 were 1,613±107 ng/ml for HIV-infected subjects receiving ART, 1,443±144 ng/ml for HIV-infected subjects not receiving ART, and 1,278±96 ng/ml for uninfected subjects. In these analyses, the difference in concentrations of sVCAM-1 at 18 months were significant only for the HIV-infected group receiving ART and the uninfected groups (p=0.02). Neopterin had a modest correlation with sVCAM-1 levels at baseline (r=0.28, p=0.0002) and 18 months (r=0.48, p<0.0001).
The relationship between viral load and the biomarkers for cardiovascular risk among HIV-infected subjects was examined separately for those receiving, or not receiving, ART. Spearman correlations between viral load and hsCRP, sVCAM, neopterin, and MPO at each time point were weak, r<0.40 for all (data not shown). Further modeling showed some correlation with sVCAM, neopterin, and MPO but no correlation with hsCRP. There were no significant differences based on whether the subjects were receiving or not receiving ART.
The baseline concentration of each biomarker was the strongest predictor of that biomarker's levels at 18 months. In multivariate analyses, smoking and initial VLDL levels were significantly associated with abnormal VLDL at follow-up (p=0.02 and 0.0001, respectively). Similarly, BMI and initial Apo AI were the determinants of Apo AI concentrations at follow-up (p=0.007 and <0.0001, respectively). BMI and initial hsCRP were significant determinants of hsCRP concentrations at follow-up (p<0.05).
Discussion
Studies in HIV-infected adults and vertically infected children indicate significant morphologic abnormalities and dyslipidemia associated with chronic HIV infection that increase CVD risk.20,21 Among these abnormalities were increased TG, LDL, and VLDL, and decreased HDL. More recent studies in behaviorally infected adolescent women have revealed similar patterns of dyslipidemia and obesity as a major cardiovascular risk factor.22 Using the HIV-infected and HIV-uninfected subjects from the REACH cohorts, we extended observations from these earlier studies to further examine biomarkers of cardiovascular risk by comparing HIV-infected and HIV-uninfected groups with similar demographics and CVD risk factors.
Total nonfasting cholesterol, LDL, and VLDL levels among both infected and uninfected youth were in the desirable to borderline high levels.22 As others have shown, HDL levels in HIV-infected subjects were suboptimal and independent of ART treatment.22 Compared to uninfected youth, VLDL levels were higher in the two HIV-infected groups but levels decreased over time. Our results suggest undesirable trends in these lipid levels may not be sensitive biomarkers of cardiovascular risk in adolescents when compared to HIV-infected adults. Similar to HDL, Apo AI levels, another marker of CVD risk, were lower in HIV-uninfected compared to HIV-infected youth and lowest in the treated population. This finding is in contrast to recent studies that have shown that Apo AI increases after initiation of ART, but the degree of improvement is linked to the extent of HIV-associated inflammation.23 There was modest improvement over time among the youth receiving ART even though the majority of youth in the REACH cohort displayed incomplete viral suppression.
A traditional biomarker of inflammation, hsCRP, also predicts risk for CVD in HIV-infected and HIV-uninfected individuals.7,18 However, it remains unclear if ART leads to sustained declines in inflammation, as hsCRPs levels were variable among the cohorts and not significantly different among groups.24 More importantly, hsCRP was generally low (<2.0 mg/liter) and not in the ranges associated with predicting CVD risk.19 These results indicate a need to identify more sensitive measures of cardiovascular risk in adolescents with early HIV-1 infection.
Endothelial inflammation and dysfunction have emerged as major contributors to the pathogenesis of CVD, both in HIV-infected and HIV-uninfected individuals.8,25 Among the biomarkers of endothelial dysfunction, sVCAM has been shown to be highly predictive of CVD risk.26 HIV-1 infection directly contributes to elevated levels of sVCAM-1 and is partially corrected by ART.27 sVCAM was significantly elevated in both cohorts of HIV-infected youth when compared to HIV-uninfected youth. Surprisingly, sVCAM levels among the adolescents were similar to HIV-infected adults with advanced disease and higher than chronically infected children who acquired HIV through maternal-to-child transmission.27,28 However, unlike studies in HIV-infected adults, in our study, ART use did not result in lower sVCAM levels, suggesting that the lack of complete viral suppression may contribute to its sustained elevation.28 Additional studies in fully suppressed HIV-infected adolescents are needed to determine if ART has an impact on sVCAM levels.
Vascular inflammation can occur either by direct interaction of HIV within the endothelial cells inducing a prothrombotic state or by bacterial-induced inflammation of macrophages and subsequent elaboration of proinflammatory cytokines.29,30 Macrophage activation occurs during HIV infection as a consequence of microbial translocation through the gastrointestinal tract due to viral-induced fibrosis that occurs during the initial phase of viremia.31 This process is not completely reversed by ART or reconstitution of CD4 T lymphocytes.32 As a result, macrophage activation persists and contributes to chronic inflammation that raises the risk for both HIV-associated CVD and neurocognitive dysfunction.33,34 Neopterin, a marker of phagocytic activity and monocyte activation, has been linked as a biomarker of inflammation and immune activation during HIV-1 infection.35 It is also associated with atherogenesis in CVD.36 In our study, neopterin levels were elevated in HIV-infected adolescents and displayed significant increases over time among those subjects receiving ART. More importantly, elevations in neopterin levels significantly correlated with elevated sVCAM-1 levels, supporting the potential link between macrophage activation and endothelial dysfunction. This finding suggests that vascular dysfunction resulting from macrophage activation occurs early in disease progression and long before CD4 T cell attrition and susceptibility to opportunistic infections. MPO, another biomarker of leukocyte activation, has also been established as a predictor of CVD and endothelial dysfunction.37 MPO levels have been shown to be elevated in HIV-infected adults, both untreated and receiving ART.38 While MPO levels were higher in our HIV-infected subjects receiving ART, unlike neopterin, we did not find a correlation between sVCAM and MPO levels. These results suggest that MPO may not be as sensitive as sVCAM or neopterin in detecting early HIV-associated vascular injury.
Overall, we were unable to find strong relationships between the biomarkers of CVD and comorbid conditions such as substance use or obesity. However, smoking strongly correlated with VLDL levels and BMI, and along with initial Apo AI level was not associated with abnormal Apo AI levels at the 18-month follow-up visit. The study design was weakened by the lack of inclusion of some other key factors predictive of cardiovascular risk such as hypertension and diabetes. While alcohol use was examined in initial analysis, more detailed analyses were not performed. Baseline values of inflammatory markers were very strongly linked to values measured at 18 months, indicating persistence of these markers over time. Using the biomarkers of VLDL, Apo AI, as well as biomarkers of vascular dysfunction it may be possible to measure short-term gains in reversing CVD risk with interventions targeted at weight loss or smoking cessation.
A major confounding variable in assessing biomarkers of CVD in the HIV-infected adolescents in this study was the high proportion of youth on ART who had incomplete viral suppression. Among the infected subjects on ART, sVCAM, neopterin, and MPO levels were as high or, as in the case of MPO, higher than untreated HIV-infected youth. The significant increase in neopterin levels over time suggests that ART, in combination with incomplete viral suppression, may even accelerate CVD risk. The extent that ART with incomplete virologic suppression reverses or arrests endothelial injury remains unclear. Many studies have shown that macrophage activation driven by ongoing microbial translocation continues in spite of optimal control of viral replication.32,33 Studies are needed to define the optimal timing for the initiation of ART and minimize CVD risk, but it is clear from our study that suboptimal therapy is inadequate in delaying progression of vascular injury.
This study provides a comprehensive assessment of biomarkers of CVD risk among a large cohort of HIV-infected male and female adolescents early in the course of HIV-1 disease. While the study did not directly examine preclinical atherosclerosis using measurements such as carotid intima-medial thickness it examines traditional biomarkers such as lipid abnormalities and vascular injury.2–4 Although dyslipidemia did not emerge as a strong risk indicator, it appears that biomarkers of endothelial dysfunction and macrophage activation may emerge as the earliest sign of atherosclerosis. The observation interval of only 18 months may not be sufficient to adequately evaluate disease progression, especially among young study participants. Longitudinal studies that allow the evaluation of HIV-infected adolescents through adulthood are needed to clarify the clinical value of these markers. Incomplete suppression of viral replication by ART appears to offer no benefit in lowering CVD risk. It is important to consider these factors in the clinical management of HIV-infected adolescents and young adults in order to implement interventions early that would lower CVD risk.
Acknowledgments
This study was supported by the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN), which is supported by the National Institutes of Child Health and Development (5 U01 HD 40533 and 5 U01 HD 40474). REACH was supported by 5 U01 HD32842. The study was also supported by R01 DA031017 and R01 AI47723. The authors thank Drs. Bret Rudy and Kathleen Mulligan for their comments in preparing the manuscript. The contents of this publication are solely the responsibility of the authors and do not necessarily reflect the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Grinspoon SK, et al. State of the science conference: Initiative to decrease cardiovascular risk and increase quality of care for patients living with HIV/AIDS: Executive summary. Circulation. 2008;118(2):198–210. doi: 10.1161/CIRCULATIONAHA.107.189622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Triant VA, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Currier JS, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33(4):506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 4.Grunfeld C, et al. Preclinical atherosclerosis due to HIV infection: Carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23(14):1841–1849. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapogiannis BG, et al. Introduction: Paving the way for biomedical HIV prevention interventions in youth. J Acquir Immune Defic Syndr. 2011;54(Suppl 1):S1–4. doi: 10.1097/QAI.0b013e3181e2cf8f. [DOI] [PubMed] [Google Scholar]
- 6.Kruzich LA, et al. HIV-infected US youth are at high risk of obesity and poor diet quality: A challenge for improving short- and long-term health outcomes. J Am Diet Assoc. 2004;104(10):1554–1560. doi: 10.1016/j.jada.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Hakeem A. Bhatti S. Cilingiroglu M. The spectrum of atherosclerotic coronary artery disease in HIV patients. Curr Atheroscler Rep. 2011;12(2):119–124. doi: 10.1007/s11883-010-0089-4. [DOI] [PubMed] [Google Scholar]
- 8.Crowe SM, et al. The macrophage: The intersection between HIV infection and atherosclerosis. J Leukoc Biol. 2011;87(4):589–598. doi: 10.1189/jlb.0809580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers AS, et al. The REACH Project of the Adolescent Medicine HIV/AID Research Network: Design, methods, and selected characteristics of participants. J Adolesc Health. 1998;22(4):300–311. doi: 10.1016/s1054-139x(97)00279-6. [DOI] [PubMed] [Google Scholar]
- 10.Kavey RE. Daniels SR. Lauer RM. Atkins DL. Hayman LL. Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. J Pediatr. 2003;142(4):368–372. doi: 10.1067/mpd.2003.205. [DOI] [PubMed] [Google Scholar]
- 11.de Gaetano Donati K, et al. Increased soluble markers of endothelial dysfunction in HIV-positive patients under highly active antiretroviral therapy. AIDS. 2003;17(5):765–768. doi: 10.1097/00002030-200303280-00020. [DOI] [PubMed] [Google Scholar]
- 12.Torriani FJ, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52(7):569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Triant VA. Meigs JB. Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51(3):268–273. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf K, et al. Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J Infect Dis. 2002;185(4):456–462. doi: 10.1086/338572. [DOI] [PubMed] [Google Scholar]
- 15.Murphy DA, et al. Antiretroviral medication adherence among the REACH HIV-infected adolescent cohort in the USA. AIDS Care. 2001;13(1):27–40. doi: 10.1080/09540120020018161. [DOI] [PubMed] [Google Scholar]
- 16.Holland CA, et al. Relationship of CD4+ T cell counts and HIV type 1 viral loads in untreated, infected adolescents. Adolescent Medicine HIV/AIDS Research Network. AIDS Res Hum Retroviruses. 2000;16(10):959–963. doi: 10.1089/08892220050058371. [DOI] [PubMed] [Google Scholar]
- 17.Rudy BJ. Crowley-Nowick PA. Douglas SD. Immunology and the REACH study: HIV immunology and preliminary findings. Reaching for Excellence in Adolescent Care and Health. J Adolesc Health. 2001;29(3 Suppl):39–48. doi: 10.1016/s1054-139x(01)00288-9. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 19.Pearson TA, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 20.Riddler SA, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289(22):2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 21.Aldrovandi GM, et al. Morphologic and metabolic abnormalities in vertically HIV-infected children and youth. AIDS. 2009;23(6):661–672. doi: 10.1097/QAD.0b013e3283269dfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulligan K, et al. Obesity and dyslipidemia in behaviorally HIV-infected young women: Adolescent Trials Network study 021. Clin Infect Dis. 2011;50(1):106–114. doi: 10.1086/648728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker JV, et al. Inflammation predicts changes in high-density lipoprotein particles and apolipoprotein A1 following initiation of antiretroviral therapy. AIDS. 2011;25(17):2133–2142. doi: 10.1097/QAD.0b013e32834be088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fichtenbaum CJ. Does antiretroviral therapy increase or decrease the risk of cardiovascular disease? Curr HIV/AIDS Rep. 2011;7(2):92–98. doi: 10.1007/s11904-010-0045-5. [DOI] [PubMed] [Google Scholar]
- 25.Hansson GK. Libby P. The immune response in atherosclerosis: A double-edged sword. Nat Rev Immunol. 2006;6(7):508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 26.Cybulsky MI, et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107(10):1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francisci D, et al. HIV type 1 infection, and not short-term HAART, induces endothelial dysfunction. AIDS. 2009;23(5):589–596. doi: 10.1097/QAD.0b013e328325a87c. [DOI] [PubMed] [Google Scholar]
- 28.Miller T, et al. Metabolic abnormalities and viral replication are associated with biomarkers of vascular dysfunction in HIV-infected children. HIV Med. 2011;13(5):264–275. doi: 10.1111/j.1468-1293.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schecter AD, et al. HIV envelope gp120 activates human arterial smooth muscle cells. Proc Natl Acad Sci USA. 2001;98(18):10142–10147. doi: 10.1073/pnas.181328798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner DD. New links between inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2005;25(7):1321–1324. doi: 10.1161/01.ATV.0000166521.90532.44. [DOI] [PubMed] [Google Scholar]
- 31.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 32.Wallet MA, et al. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. AIDS. 2011;24(9):1281–1290. doi: 10.1097/QAD.0b013e328339e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ancuta P, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3(6):e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandler NG, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baier-Bitterlich G. Wachter H. Fuchs D. Role of neopterin and 7,8-dihydroneopterin in human immunodeficiency virus infection: Marker for disease progression and pathogenic link. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(2):184–193. doi: 10.1097/00042560-199610010-00010. [DOI] [PubMed] [Google Scholar]
- 36.Fuchs D, et al. The role of neopterin in atherogenesis and cardiovascular risk assessment. Curr Med Chem. 2009;16(35):4644–4653. doi: 10.2174/092986709789878247. [DOI] [PubMed] [Google Scholar]
- 37.Brennan ML, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349(17):1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 38.Ross AC, et al. Endothelial activation markers are linked to HIV status and are independent of antiretroviral therapy and lipoatrophy. J Acquir Immune Defic Syndr. 2008;49(5):499–506. doi: 10.1097/QAI.0b013e318189a794. [DOI] [PMC free article] [PubMed] [Google Scholar]