Abstract
Objectives
This study sought to explore the therapeutic potential of platelet gel for the treatment of myocardial infarction.
Background
Cardiac dysfunction after acute myocardial infarction is a major cause of heart failure. Current therapy relies on prompt reperfusion and blockage of secondary maladaptive pathways by small molecules. Platelet gels are biomaterials rich in cytokines and growth factors, which can be manufactured in an autologous manner and are effective in various models of wound healing. However, the potential utility of platelet gel in cardiac regeneration has yet to be tested.
Methods
Platelet gel was derived from syngeneic rats and its morphology, biocompatibility, secretion of beneficial factors, and in vivo degradation profile were characterized.
Results
After delivery into infarcted rat hearts, the gel was efficiently infiltrated by cardiomyocytes and endothelial cells. Gel-treated hearts exhibited enhanced tissue protection, greater recruitment of endogenous regeneration, higher capillary density, and less compensatory myocyte hypertrophy. The cardiac function of control-injected animals deteriorated over the 6-week time course, while that of platelet gel-injected animals did not. In addition, the gel did not exacerbate inflammation in the heart.
Conclusions
Intramyocardial injection of autologous platelet gel ameliorated cardiac dysfunction after myocardial infarction. The striking functional benefits, the simplicity of manufacturing, and the potentially autologous nature of this biomaterial provide impetus for further translation.
Keywords: biomaterials, endogenous recruitment, myocardial infarction
The emerging field of tissue engineering and biomaterials has begun to provide promising alternatives or adjuncts to cellular cardiomyoplasty (1). Injectable biomaterial gels are particularly appealing as they are amenable to minimally invasive delivery (2); moreover, they can be injected alone or spiked with cells (3). Various injectable biomaterials have been studied for cardiac regeneration, including fibrin glue (4,5), collagen (6), alginate (7), matrigel (8), self-assembling peptides (9), extracellular matrix emulsion (10), synthetic polymer hydrogel (11), and microspheres (12), with generally positive effects. An ideal biomaterial for cardiac regeneration should be biodegradable, biocompatible, cause little or no foreign body reaction, and provide both mechanical and functional support to the injured heart.
Platelet gel (also known as platelet fibrin scaffold [13]) is an appealing choice for therapeutic development as it can be easily manufactured, whether as an allogeneic or autologous product. The regenerative potential of platelet gel in other tissues (e.g., cartilage repair, bone repair, wound healing, spinal cord repair) has been well documented (14 –16) but potential utility in cardiac regeneration has yet to be tested. We hypothesize that platelet gel may preserve cardiac function and attenuate adverse ventricular remodeling after myocardial infarction (MI), based on the following reasoning: 1) the injected biomaterial can provide mechanical support to the heart by increasing left ventricular (LV) wall thickness and reducing wall tension (Laplace’s law); and 2) unlike plain fibrin glue scaffolds (4,5), platelet gel can provide biological support by secreting various beneficial factors that may facilitate angiogenesis and adaptive myocardial healing. In the present study, we investigated the regenerative potential of platelet gel in a rat model of acute MI and delineated the underlying mechanisms of functional benefit.
Methods
Derivation of platelet gel
Platelet gel was derived from venous blood of Wistar-Kyoto (WKY) or Sprague-Dawley rats according to previously reported methods (15). To enable histological detection of injected platelet gel in vivo, we labeled the fibrin components by incubation with Texas Red-X succinimidyl ester (1 mg/ml [Invitrogen, Carlsbad, California]).
In vitro cytokine release
To study sustained release of cytokines and growth factors, the conditioned media was collected at various times (days 2, 5, 9, and 14), and fresh media was added back into the well to be conditioned for the next time point. The concentrations of vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF)-1, and hepatocyte growth factor (HGF) in the conditioned media were measured by rat-specific enzyme-linked immunosorbent assay kits.
Animal model
Animal care was in accordance with Cedars-Sinai Medical Center Institutional Animal Care and Use Committee guidelines. Platelet gel was derived from male WKY rats and then intramyocardially injected into female WKY rats (n = 78 total) through a left thoracotomy under general anesthesia, and myocardial infarction (MI) was produced by permanent ligation of the left anterior descending coronary artery. Immediately after MI, the animals were subjected to intramyocardial injections with a 29G needle at 4 points in the infarct zone, under 1 of 2 randomly assigned conditions: 1) control group—injection of 150 μl vehicle (Dulbecco’s Modified Eagle’s Medium [DMEM]); and 2) gel group—injection of 150 μl platelet gel. For platelet gel injection, 75 μl host plasma were mixed with 75 μl pre-warmed DMEM.
Morphometric analysis
For morphometric analysis, animals were euthanized at 3 weeks, and the hearts were harvested and frozen in optimal cutting temperature compound. Sections every 100 μm (10-μm thickness) were prepared. Masson’s trichrome staining (6 sections per heart, collected at 400-μm intervals) was performed as described (17).
Cardiac function assessment
To assess global cardiac function and LV size, echocardiography was performed with the Vevo 770 system (Visual Sonics, Toronto, Ontario, Canada) on day 0 (baseline, i.e., 4 h post-MI), and 3 and 6 weeks afterward. Blinded reading of echocardiograms was conducted independently by an experienced echocardiographer (D.S.).
Microvascular perfusion assessment with lectin angiography
To ensure that newly formed microvessels are functionally perfused, a subgroup of animals underwent lectin microvascular angiography, as described (18). The green fluorescence was quantified with National Institutes of Health ImageJ software, and the values were normalized to those from noninfarcted normal hearts to generate the relative blood flow data.
Enzymatic isolation of cardiomyocytes for hypertrophy assessment
Cardiomyocytes were isolated from WKY rats 3 weeks after control or platelet-gel treatment by enzymatic dissociation of the whole heart on a Langendorff apparatus, as described (19). The sizes of myocytes were evaluated in cells cytospun onto 22-mm cover glasses by fluorescent immunocytochemistry (anti–alpha-sarcomeric actin) in combination with confocal microscopy.
Statistical analysis
Results are presented as mean ± SD unless specified otherwise. Statistical significance between baseline and 3-week left ventricular ejection fraction (LVEF) was determined using 2-tailed paired Student’s t test. All the other comparisons between any 2 groups were performed using 2-tailed unpaired Student’s t test. Differences were considered statistically significant when p < 0.05.
Results
Characterization of platelet gel in vitro
Citration of blood immediately after exsanguination reduces free calcium, thus preventing immediate clotting while preserving coagulability. The platelet gel is easily derived and is available within ~15 min of venous sampling. Mixing host plasma with cell culture media (e.g., DMEM) containing a high concentration of free calcium results in gelation starting in <30 s, and is complete within 120 s (Fig. 1A). Hematoxylin-eosin staining revealed a fibrous structure (Fig. 1B) containing platelets (Fig. 1B, blue arrows). To test whether the gel is biocompatible with cardiomyocytes, neonatal rat cardiac myocytes (NRCM) were cultured in platelet gel derived from the same rat strain. Fourteen days after incorporation, NRCM exhibited normal morphology (Fig. 1C) and viability (Online Fig. 1). In addition, NRCM continued to beat spontaneously within the gel (Online Video 1). To confirm the sustained release of beneficial paracrine factors from the platelet gel, we measured the concentrations of VEGF, IGF-1, and HGF in gel-conditioned media. Enzyme-linked immunosorbent assay revealed robust and sustained release of those factors from the platelet gel (without cells) for at least 14 days (Figs. 1D to 1F).
Figure 1. Characterization of Platelet Gel In Vitro.
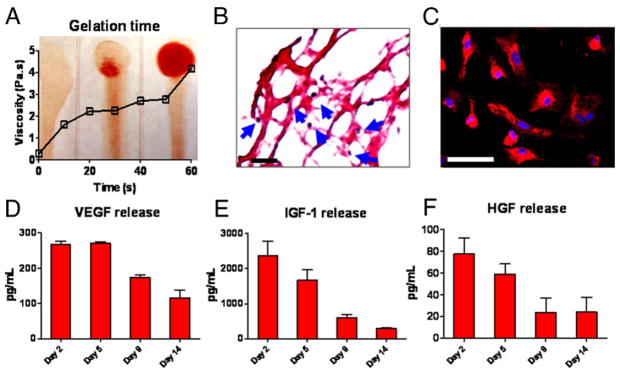
(A) Mixture of platelet-containing plasma and Dulbecco’s Modified Eagle’s Medium was immediately dispensed onto coverslips, and gel formation was monitored over time. At 0 s, tilting the coverslip resulted in a liquid smear, indicating no gel formation. Gel formation started at 30 s, and at 60 s, only a little smear was seen when tilting the coverslip, indicating gelation. Viscosity was measured by a rheometer and plotted against time. The increase of viscosity over time was consistent with the progress of gelation. (B) Hematoxylin-eosin staining revealed a fibrous structure of the platelet gel and the presence of platelets (blue arrows). Bar = 100 um. (C) Neonatal rat cardiac myocytes (NRCM) cultured in the platelet gel for 14 days and stained with CM-DiI. Bar = 50 um. (D to F) Concentrations of vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF)-1, and hepatocyte growth factor (HGF) from platelet gel-conditioned media at various time points (n = 3 per time point) measured by enzyme-linked immunosorbent assay.
Degradation of injected platelet gel in post-MI hearts
We then studied the degradation of platelet gel in post-MI hearts. To enable histological detection, the gel was labeled with Texas Red-X succinimidyl ester (Invitrogen). The dye bonds to the amine groups in the fibrin matrix and disappears as the gel is degraded in vivo (20). The platelet gel was intramyocardially injected into WKY rat hearts with MI, and animals were sacrificed 1, 7, and 14 days later. Cryosections of the hearts enabled ready identification of the platelet gel by Texas Red-X epifluorescence (Figs. 2A to 2C, red areas). Hematoxylin-eosin staining revealed penetration of local cells into the gel (Fig. 2D) at day 7. We evaluated gel degradation by measuring the myocardium area occupied by the gel. By day 7, the area occupied by the gel decreased to 40% of that at day 1, whereas only rare (<5%) weak Texas Red-X staining spots were identified at 2 weeks (Fig. 2E). Thus, the injected platelet gel is largely resorbed within 14 days, presumably by fibrinolysis and/or physical washout (20).
Figure 2. Degradation of Injected Platelet Gel in Infarcted Rat Hearts.
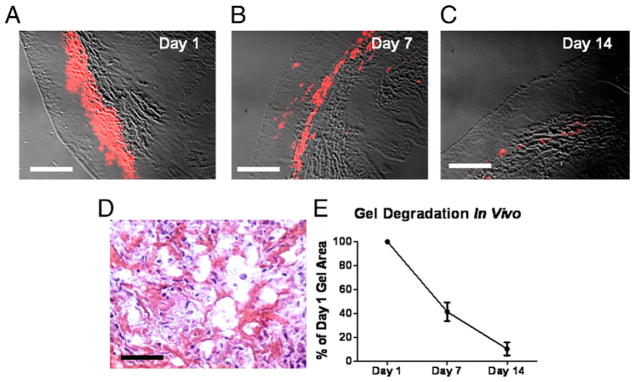
Platelet gel labeled with Texas Red-X succinimidyl ester was intramyocardially injected into Wistar-Kyoto rat hearts with myocardial infarction (MI), and heart sections were studied histologically at various time points. (A to C) The areas of platelet gel could be readily identified with the detection of Texas Red fluorescence. Bars = 500 um. (D) Hematoxylin-eosin staining revealed penetration of endogenous cells into the gel. Bar = 50 um. (E) To calculate the percentage of degradation over time, the gel area at days 7 and 14 was measured and then normalized to the day 1 gel area.
Cardiomyocytes and endothelial cells populate injected platelet gel in vivo
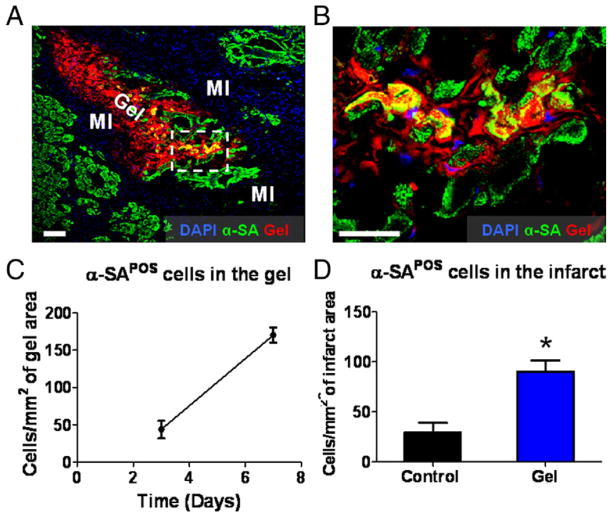
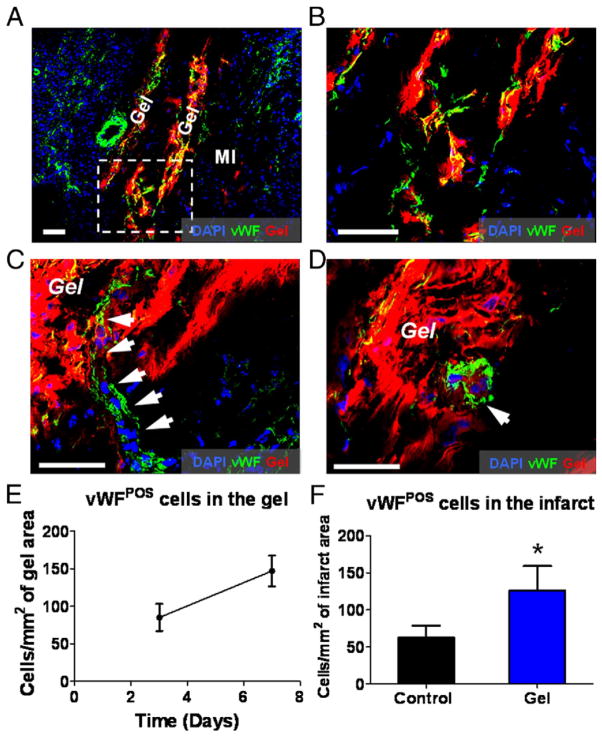
Histological analysis at day 7 identified the infarct area by its paucity of cardiomyocytes and endothelial cells but richness of inflammatory cells (Figs. 3A and 4A, marked as “MI”). Interestingly, the majority of surviving cardiomyocytes in the infarct were adjacent to the injected platelet gel (Fig. 3A). Higher magnification revealed integration of cardiomyocytes into the gel (Fig. 3B), an effect also seen with endothelial cells (Figs. 4A and 4B). The numbers of myocytes and endothelial cells in the gel increased from day 3 to day 7 (Figs. 3C and 4E), indicating their gradual invasion into the platelet gel. At day 7, more cardiomyocytes and endothelial cells were detected in the gel-treated infarct than in controls (intramyocardial injection of the same volume of DMEM media) (Figs. 3D and 4F). Moreover, mature blood vessels could be detected within the platelet gel 7 days after treatment, indicating de novo angiogenesis (Figs. 4C and 4D).
Figure 3. Cardiomyocytes Populate Injected Platelet Gel In Vivo.
Animals were sacrificed at days 3 and 7, and heart cryosections were stained with 4′,6-diamidino-2-phenylindole (DAPI) for nuclei and alpha-sarcomeric actin (α-SA) for cardiomyocytes. (A) Representative confocal microscopic images. The infarct area could be identified by the paucity of cardiomyocytes and endothelial cells but with infiltration by nuclei belonging to inflammatory cells (marked as “MI”). Platelet gel exhibited Texas Red fluorescence (marked as “Gel”). (B) High-magnification image of the area in the white box in A, showing the penetration of cardiomyocytes into the platelet gel. (C) Quantitation of myocytes residing in the platelet gel at day 3 and day 7, respectively. (D) Quantitation of cardiomyocytes in the infarct area from gel- or control-treated hearts on day 7 after treatment. Bars = 100 um. *p < 0.0001 when compared to control. POS = positive.
Figure 4. Endothelial Cells Populate Injected Platelet Gel In Vivo and Contribute to De Novo Angiogenesis.
Animals were sacrificed at days 3 and 7, and heart cryosections were stained with 4′,6-diamidino-2-phenylindole (DAPI) for nuclei and von Willebrand factor (vWF) for endothelial cells. (A) Representative confocal microscopic images. The infarct area could be identified by the paucity of endothelial cells but with infiltration by nuclei belonging to inflammatory cells (marked as “MI”). Platelet gel exhibited Texas Red fluorescence (marked as “Gel”). (B) High-magnification image of the area in the white box in A, showing the penetration of endothelial cells into the platelet gel. Bars = 100 um. (C, D) Confocal microscopy images showing blood vessel-like structures (white arrows) in the platelet gel 7 days after injection into the injured myocardium. Bars = 20 um. (E) Quantitation of endothelial cells residing in the platelet gel at day 3 and day 7, respectively. (F) Quantitation of endothelial cells in the infarct area from gel- or control-treated hearts on day 7 after treatment. *p < 0.05 when compared to control. POS = positive.
Platelet gel attenuates tissue apoptosis and recruits endogenous repair
In addition to tissue regeneration, tissue preservation may be a salutary component of biomaterial therapy for acute MI. To quantify tissue preservation, apoptotic cells at day 7 after vehicle or gel injection were terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-stained (Roche, Indianapolis, Indiana). Fewer apoptotic nuclei (green) were detected in the gel-treated hearts than in controls (Online Figs. 2A to 2C). It has been reported that some platelet secretory cytokines such as stromal cell-derived factor-1 can stimulate the recruitment of endogenous stem cells (e.g., c-kit+ cells) to the injured site (18). The c-kit (also known as CD117) is a marker for cardiac stem cells, and such cells can undergo multilineage differentiation into cardiomyocytes, smooth muscle cells, and endothelial cells (21,22). Therefore, we compared the numbers of c-kit+ cells in and around the infarct in gel-treated and control hearts. The number of c-kit+ cells nearly tripled in the gel-injected hearts (Online Figs. 2D to 2F). Although most c-kit+ cells were adjacent to but outside the gel, some cells had penetrated into the gel (Online Fig. 2E, white arrows). To exclude exacerbation of inflammation by the gel, we compared the numbers of CD68+ macrophages in gel-treated and control hearts. Macrophage number was slightly reduced in the gel-treated hearts (Online Figs. 2G to 2I); in addition, no macrophage infiltration into the gel was evident. Thus, not only did the gel not exacerbate inflammation, but also apparently blunted it somewhat, most likely because of reduced apoptosis and accelerated healing. Moreover, tissue Western blot analysis revealed similar myocardial expression of inflammatory cytokine tumor necrosis factor-alpha in the 2 treatment groups (Online Fig. 3), verifying that platelet gel injection did not elicit inflammation.
Platelet gel reduces myocyte hypertrophy and promotes angiogenesis
Smaller myocyte cross-sectional areas were seen in the platelet gel-treated hearts than in untreated infarcted hearts (Online Figs. 4A to 4C), indicating that the injected platelet gel indeed prevents myocyte hypertrophy. Reduction of myocyte hypertrophy was also confirmed in enzymatically isolated cardiomyocytes 3 weeks after platelet gel treatment (Online Fig. 5). Immunostaining revealed that capillary density 3 weeks after treatment was higher in platelet gel-treated hearts than in controls (Online Figs. 4D to 4F), indicative of an angiogenic effect. To further confirm that newly formed capillaries are functionally perfused, lectin microvascular angiography was performed, and it confirmed improved relative blood flow in the platelet gel-treated hearts (Online Fig. 6). Angiogenesis pathway-focused real-time polymerase chain reaction array revealed up-regulation of several angiogenesis genes (e.g., HGF, basic fibroblast growth factor, alanyl aminopeptidase), extracellular matrix genes (e.g., fibronetin, collagen type XVIII alpha 1), and cardiac protection genes (e.g., sphingosine kinase 1) in the platelet gel-injected hearts (Online Fig. 7). Interestingly, some genes related to remodeling (e.g., MMP-3, MMP-9) are down-regulated in gel-injected hearts. Although the results are preliminary (n = 2), the polymerase chain reaction data suggested injection of platelet gel may regulate angiogenesis pathways in the post-MI heart.
Platelet gel attenuates LV remodeling and preserves cardiac function
Quantitative morphometry showed severe LV chamber dilation and infarct wall thinning in the nontreated hearts at 3 weeks (Figs. 5A and 5C, left). In contrast, platelet gel-treated hearts exhibited attenuated LV remodeling and less abnormal heart morphology (Figs. 5B and 5C, right), with thicker infarcted walls (Fig. 5E), smaller LV cavities (Fig. 5D) and shorter infarct perimeters (Fig. 5F). These effects are notable, as one of the most important predictors of mortality in patients after MI is the degree of LV dilation (23).
Figure 5. Morphometry Analysis.
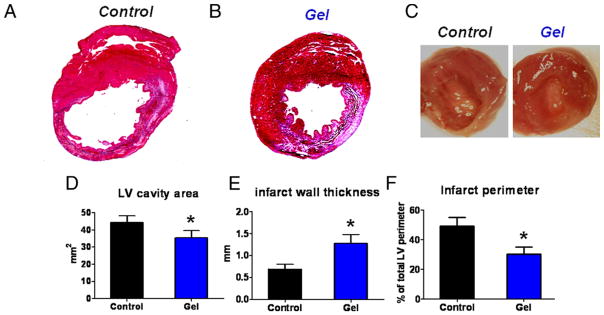
(A, B) Representative Masson’s trichrome-stained myocardial sections 3 weeks after treatment. Scar tissue and viable myocardium are identified by blue and red, respectively. (C) White light photos of excised hearts from control- and gel-treated animals. (D to F) Quantitative analysis and left ventricular (LV) morphometric parameters of the Masson’s trichrome images (n = 5 animals per group).
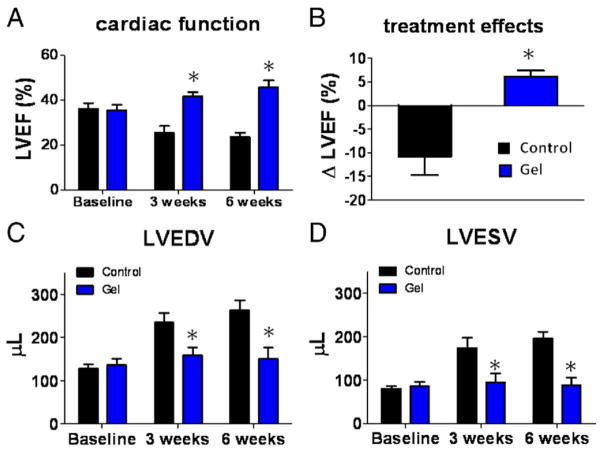
The most meaningful indicator of therapeutic benefit, in clinical practice, is the ability to preserve or improve function in the injured heart. The LVEF at baseline did not differ between the 2 groups, indicating a comparable degree of initial injury (Fig. 6A). Over the next 6 weeks, LVEF declined progressively in the untreated group, but not in the platelet gel-treated hearts. Both 3 and 6 weeks after treatment, LVEF in platelet gel-treated animals was higher than in untreated hearts (Fig. 6A). To facilitate comparisons, we calculated the treatment effect, namely, the change in LVEF at 3 weeks relative to baseline, in each group (Fig. 6B). Untreated controls had a negative treatment effect, as LVEF decreased over time; in contrast, the platelet gel-treated group exhibited robust LVEF preservation. Echo-enabled volumetric analysis revealed smaller end-diastolic volume (Fig. 6C) and end-systolic volume (Fig. 6D) in the platelet gel-treated hearts, confirming the beneficial role of the gel in preventing maladaptive chamber remodeling post-MI.
Figure 6. Cardiac Function and Chamber Dimensions.
(A) Left ventricular ejection fraction (LVEF) measured by echocardiography at baseline (post-myocardial infarction), 3 weeks and 6 weeks afterward in control group (black bars) or gel-treatment group (blue bars) (n = 8 animals per group). Baseline LVEFs were indistinguishable in the 2 groups. (B) Changes of LVEF from baseline to 3 weeks in each group. (C) Left ventricular end-diastolic volumes (LVEDV) measured by echocardiography. (D) Left ventricular end-systolic volumes (LVESV) measured by echocardiography. *Indicates p < 0.05 when compared to control.
Discussion
The past decade witnessed a burst of new biomaterial and tissue engineering approaches for the treatment of ischemic cardiac disease (3). We are interested in biomaterials derived from an entirely endogenous source that will cause minimal or no foreign body reaction and immunological response. As a natural wound healing process, the coagulation cascade is triggered by tissue damage to form a fibrin matrix at the injured site. The fibrin matrix provides mechanical support to the wound and recruits endogenous repair processes. Here, we demonstrated the regenerative potential of platelet gel for the treatment of acute MI. This study differs fundamentally from previous studies using fibrin glue (4,5) for cardiac repair. Instead of using xenogeneic fibrin glue purchased from commercial sources, we used platelet gel derived from the blood of syngeneic animals. Platelet-rich plasma has been used to treat experimental ischemic cardiomyopathy (24,25), but platelet-rich plasma is a liquid solution with questionable ability to form stable gels in situ. Also, the mechanisms underlying functional benefits were unexplored. The present study described the regenerative potential of syngeneic platelet gel injection in an experimental animal model of acute MI.
On the basis of the reinitiation of the coagulation cascade, a hydrogel forms spontaneously upon mixing platelet-rich plasma with any calcium-containing media (Fig. 1A). As a scaffold for cardiac regeneration, the fibrous matrix with interconnected pores (Fig. 1B) is permissive of cell penetration for endogenous cardiomyocytes (Fig. 3), endothelial cells (Fig. 4), and other progenitor cell types such as c-kit+ stem cells (Online Figs. 2D to 2F). Coinciding with its structural features is the biological propensity of platelet gel to release various beneficial growth factors (Figs. 1D through 1F). We postulate that these factors are secreted by the live platelets in situ, although some could be a carry-over from the blood. The concentrations of these factors were comparable to those secreted by cardiosphere-derived cells (now being tested in the CADUCEUS [Cardiosphere-Derived Autologous Stem Cells to Reverse Ventricular Dysfunction] trial; NCT00893360), which robustly produce angiogenic, anti-apoptotic, and chemotactic factors (26). These factors, among many secreted by platelets, are essential for cardiac repair: VEGF is well known for its pro-angiogenic effects (27); IGF-1 is a small peptide hormone that stimulates mitogenesis, promotes differentiation, and inhibits apoptosis in many organs, including the heart (28); and HGF stimulates cell proliferation, motility, morphogenesis and angiogenesis and also provides tissue protection after injury (29).
The in vivo degradation of platelet gel is fast, likely occurring over ~2 weeks in the heart (Fig. 2), possibly due to a combination of fibrinolysis and wash-out. Although the biomaterials are gone, the therapeutic benefit is sustained: endogenous cells gradually populate the injected scaffold (Figs. 3 and 4), create their own extracellular matrixes, and mediate endogenous repair. The fast biodegradability, together with the harmless degradation by-products (mainly D-dimers) that cause no incremental myocardial inflammation (Online Figs. 2G to 2I and 3), makes platelet gel superior to most synthetic polymer scaffolds, whose degradation is generally slow and productive of potentially pro-inflammatory by-products (12). Future work should be focused on custom designing the degradation and mechanical properties of the platelet gel to match the healing of the post-infarct heart. That can be achieved by altering the processing protocol and concentrations of key components.
The potentiation of angiogenesis (Online Figs. 4D to 4F, 6, and 7) likely shares contributions from both the matrix-bonded and soluble portions of the injected platelet gel. On the one hand, the porous structure along with the extracellular matrix motifs present a “warm bed” for the recruitment of endothelial cells and/or progenitor cells (Online Figs. 2D to 2F), which can contribute to de novo angiogenesis. On the other hand, soluble factors secreted by the platelet gel can also contribute to angiogenesis. In addition, it is notable that the enhancement of overall vascularization may not exclusively come from regeneration but also from protecting pre-existing vascular cells. In fact, injected platelet gel is anti-apoptotic after MI (Online Figs. 2A to 2C). Our experiments cannot elucidate the mechanism underlying the increase of c-kit+ stem cells in platelet gel-treated hearts. That may arise from the proliferation and/or migration of resident c-kit+ cells, from homing of circulating c-kit+ cells, or from both.
The attenuation of adverse LV remodeling (Fig. 5) and preservation of cardiac function (Fig. 6) from injected platelet gel are notable, but not totally surprising given the observations of tissue protection and regeneration from platelet gel injection. We postulate that synergizing the biological support of platelet gel is its physical support to post-MI myocardium (Laplace law). Although we did not measure stress directly, the injected biomaterials did reduce myocyte hypertrophy (Online Figs. 4A to 4C) and prevent chamber dilation (Figs. 6C and 6D), which can be caused by excessive myocardial stress during LV remodeling.
Both allogeneic and autologous applications are feasible. An allogeneic application scenario will involve using cryo-banked and precharacterized platelet-rich plasma lots. Simple ABO blood type matching can be employed to eliminate safety concerns from the remnant blood cells in the gel. Autologous applications are also possible. We can get approximately 2× ml of platelet gel from × ml of venous blood. Given we used 150 μl of platelet gel to treat an ~150 g rat (1 ml/kg of body weight) and saw functional efficacy, we postulate that a 80-kg human would need 80 ml of platelet gel; that could be derived from 40 ml (<10% of 1 U) of venous blood, a clinically tolerable amount. In any case, the dose and timing of injection remains to be optimized.
Study limitations
Although we used open-chest surgery and direct muscle injection in the animal model, minimally invasive injection catheter delivery methods are more favorable for translation. Certainly, the gelation time needs to be optimized for catheter-based delivery, although simple loading of catheters with citrated plasma may suffice, as gelation will occur soon after injection into calcium-rich interstitium in vivo.
Conclusions
Given the simplicity of manufacturing this biomaterial and its potentially autologous nature, our findings provide impetus for further translation of platelet gel therapy for the treatment of myocardial infarction. The present proof-of-concept study does not teach precisely how clinical delivery might take place, but our evidence for the safety and efficacy of platelet gel injection motivates further development of this simple therapeutic paradigm.
Supplementary Material
Acknowledgments
This work was supported by research grants from the National Institutes of Health, the California Institute of Regenerative Medicine, and the Cedars-Sinai Board of Governors Heart Stem Cell Center. Dr. Malliaras has relationships with Capricor Inc. Dr. Marbán is a founder and equity holder in Capricor Inc. Capricor provided no funding for the present study. Dr. Marbán is the Mark S. Siegel Family Professor of the Cedars-Sinai Medical Center.
The authors thank Kolja Wawrowsky, Pirouz Kavehpour, and Pooria Kashani for technical assistance; and Rachel Smith, Linda Marbán, and Tao-Sheng Li for helpful discussions.
Abbreviations and Acronyms
- DMEM
Dulbecco’s Modified Eagle’s Medium
- HGF
hepatocyte growth factor
- IGF
insulin-like growth factor
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- SDF
stromal cell-derived factor
- VEGF
vascular endothelial growth factor
APPENDIX
For an expanded Methods section, supplemental figures, and a video, please see the online version of the article.
Footnotes
All other authors have reported they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Engelmayr GC, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. 2008;7:1003–10. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson DM, Ma Z, Fujimoto KL, Hashizume R, Wagner WR. Intra-myocardial biomaterial injection therapy in the treatment of heart failure: materials, outcomes and challenges. Acta Biomater. 2010;7:1–15. doi: 10.1016/j.actbio.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48:907–13. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654– 60. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 5.Ryu JH, Kim IK, Cho SW, et al. Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials. 2006;26:319–26. doi: 10.1016/j.biomaterials.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 6.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–9. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 7.Landa N, Miller L, Feinberg MS, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–96. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 8.Kofidis T, de Bruin JL, Hoyt G, et al. Injectable bioartificial myocardial tissue for large-scale intramural cell transfer and functional recovery of injured heart muscle. J Thorac Cardiovasc Surg. 2004;128:571– 8. doi: 10.1016/j.jtcvs.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Davis ME, Motion JP, Narmoneva DA, et al. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–50. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Z-Q, Puskas JD, Xu D, et al. Improvement in cardiac function with small intestine extracellular matrix is associated with recruitment of c-kit cells, myofibroblasts, and macrophages after myocardial infarction. J Am Coll Cardiol. 2010;55:1250– 61. doi: 10.1016/j.jacc.2009.10.049. [DOI] [PubMed] [Google Scholar]
- 11.Madden LR, Mortisen DJ, Sussman EM, et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A. 2010;107:15211– 6. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sy JC, Seshadri G, Yang SC, et al. Sustained release of a p38 inhibitor from non-inflammatory microspheres inhibits cardiac dysfunction. Nat Mater. 2008;7:863– 8. doi: 10.1038/nmat2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dozza B, Di Bella C, Lucarelli E, et al. Mesenchymal stem cells and platelet lysate in fibrin or collagen scaffold promote non-cemented hip prosthesis integration. J Orthop Res. 2011;29:961– 8. doi: 10.1002/jor.21333. [DOI] [PubMed] [Google Scholar]
- 14.Driver VR, Hanft J, Fylling CP, Beriou JM Autologel diabetic foot ulcer study group. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52:68–70. 72, 74. [PubMed] [Google Scholar]
- 15.Bryan N, Rhodes NP, Hunt JA. Derivation and performance of an entirely autologous injectable hydrogel delivery system for cell-based therapies. Biomaterials. 2009;30:180– 8. doi: 10.1016/j.biomaterials.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Johnson PJ, Tatara A, McCreedy DA, Shiu A, Sakiyama-Elbert SE. Tissue-engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI. Soft Matter. 2010;6:5127–37. doi: 10.1039/c0sm00173b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng K, Li TS, Malliaras K. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ Res. 2010;106:1570– 81. doi: 10.1161/CIRCRESAHA.109.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frederick JR, Fitzpatrick JR, III, McCormick RC, et al. Stromal cell-derived factor-1alpha activation of tissue-engineered endothelial progenitor cell matrix enhances ventricular function after myocardial infarction by inducing neovasculogenesis. Circulation. 2010;122 (Suppl):S107–17. doi: 10.1161/CIRCULATIONAHA.109.930404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Li TS, Lee ST, Wawrowsky KA, et al. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS ONE. 2010;5:e12559. doi: 10.1371/journal.pone.0012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam CK, Yoo T, Hiner B, Liu Z, Grutzendler J. Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature. 2010;465:478– 82. doi: 10.1038/nature09001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 23.Migrino RQ, Young JB, Ellis SG, et al. End-systolic volume index at 90 to 180 minutes into reperfusion therapy for acute myocardial infarction is a strong predictor of early and late mortality. The Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO)-I Angiographic Investigators. Circulation. 1997;96:116–21. doi: 10.1161/01.cir.96.1.116. [DOI] [PubMed] [Google Scholar]
- 24.Li X-H, Zhou X, Zeng S, et al. Effects of intramyocardial injection of platelet-rich plasma on the healing process after myocardial infarction. Coron Artery Dis. 2008;19:363–70. doi: 10.1097/MCA.0b013e3282fc6165. [DOI] [PubMed] [Google Scholar]
- 25.Mishra A, Velotta J, Brinton TJ, et al. RevaTen platelet-rich plasma improves cardiac function after myocardial injury. Cardiovasc Revasc Med. 2010;12:158– 63. doi: 10.1016/j.carrev.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Chimenti I, Smith RR, Li TS, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971– 80. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–5. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 28.Angoulvant D, Ivanes F, Ferrera R, Matthews PG, Nataf S, Ovize M. Mesenchymal stem cell conditioned media attenuates in vitro and ex vivo myocardial reperfusion injury. J Heart Lung Transplant. 2010;30:95–102. doi: 10.1016/j.healun.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 29.Yan L, Zhu TB, Wang LS, et al. Inhibitory effect of hepatocyte growth factor on cardiomyocytes apoptosis is partly related to reduced calcium sensing receptor expression during a model of simulated ischemia/reperfusion. Mol Biol Rep. 2011;38:2695–701. doi: 10.1007/s11033-010-0412-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.