Abstract
Here we report the bioactivity-guided isolation of novel galectins from the marine sponge Cinachyrella sp., collected from Iriomote Island, Japan. The lectin proteins, which we refer to as the Cinachyrella galectins (CchGs), were identified as the active principles in an aqueous sponge extract that modulated the function of mammalian ionotropic glutamate receptors. Aggregation of rabbit erythrocytes by CchGs was competed most effectively by galactosides but not mannose, a profile characteristic of members of the galectin family of oligosaccharide-binding proteins. The lectin activity was remarkably stable, with only a modest loss in hemagglutination after exposure of the protein to 100°C for 1 h, and showed little sensitivity to calcium concentration. CchG-1 and -2 appeared as 16 and 18 kDa in sodium dodecyl sulfate–polyacrylamide gel electrophoresis, respectively, whereas matrix-assisted laser desorption ionization-time-of-flight-mass spectrometry indicated broad ion clusters centered at 16,216 and 16,423, respectively. The amino acid sequences of the CchGs were deduced using a combination of Edman degradation and cDNA cloning and revealed that the proteins were distant orthologs of animal prototype galectins and that multiple isolectins comprised the CchGs. One of the isolectins was expressed as a recombinant protein and exhibited physico-chemical and biological properties comparable with those of the natural lectins. The biochemical properties of the CchGs as well as their unexpected activity on mammalian excitatory amino acid receptors suggest that further analysis of these new members of the galectin family will yield further glycobiological and neurophysiological insights.
Keywords: galectin, glutamate receptor, potentiation, sponge
Introduction
Marine organisms are a surprisingly rich source of molecules that alter mammalian neuroactivity. To find new bioactive molecules, we have employed an approach based on complementary bioactivity-guided screening using mouse behavior and highly sensitive electrophysiological assays, which previously facilitated purification of structurally novel glutamate receptor agonists and other small molecules from marine sponges (Sakai et al. 2001, 2006; Sakurada et al. 2010). Proteinaceous molecules with bioactivity also are evident in aqueous extracts of these organisms (Matsunaga et al. 2011). In the current study, one such extract from Cinachyrella sp. that potently modified the functional properties of mammalian ionotropic glutamate receptors (iGluRs) was selected for additional analysis and isolation of the active principles, which resulted in the isolation of new sponge proteins belonging to the galactose-binding lectin, or galectin, family.
Lectins are multivalent sugar-binding proteins that perform a broad range of essential functions in most classes of living organisms, from viruses to humans (Sharon 2007, 2008). They are involved in intrinsic cellular functions such as cell–cell recognition, cell adhesion, pathogen recognition in the innate immune system, biomineralization and symbiosis. Several families of lectins have been differentiated on the basis of their glycan specificity and structural similarities. Galectins are one such family of proteins that bind selectively to galactose- and lactose-containing oligosaccharides and have as a common target the disaccharide N-acetyl-d-lactosamine (β-galactose-1,4-N-acetyl-d-glucosamine, LacNAc), which is commonly attached as repeated units in complex asparagine-linked glycans (Kasai and Hirabayashi 1996; Leffler et al. 2004). Three subfamilies of galectins have been identified; the largest group is denoted as “prototype” galectins, which are functional as noncovalently associated dimers (Kasai and Hirabayashi 1996). We propose that the new sponge lectins from Cinachyrella, CchG-1 and -2, are new prototype galectins based on chemical properties and primary sequence. Furthermore, their unexpected biological activity on iGluRs suggests that members of the structurally related family of animal galectins might play a role in the mammalian central nervous system (CNS).
Results
Isolation and physico-chemical properties of CchGs
In our continuing screening study for neuroactive substances from marine organisms, we found that an intracerebroventricular injection (20 μg/mouse) of a water extract of the sponge Cinachyrella sp., collected in Iriomote Okinawa, elicited robust tonic-clonic seizures. We reasoned that potential targets for the active principle(s) in the extract included critical signaling molecules involved in neurotransmission, including the family of iGluRs that underlie the majority of excitatory neurotransmission in the CNS. Indeed, convulsant marine extracts we isolated in the past contained compounds that altered iGluR signaling (Sakai et al. 2001). For that reason, our in vivo screening was followed by tests of the functional activity of the Cinachyrella extract on two types of recombinant mammalian iGluRs, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) and kainate receptors, which are both known to induce convulsions when activated in the CNS. cDNAs encoding representative members of the AMPA or kainate receptor families, GluA4 and GluK2a, respectively, were transiently transfected into human embryonic kidney cells expressing T-antigen, clone 17 (HEK293-T/17) cells, which were then used in whole-cell patch-clamp recordings following receptor expression to assess sensitivity to the Cinachyrella extract.
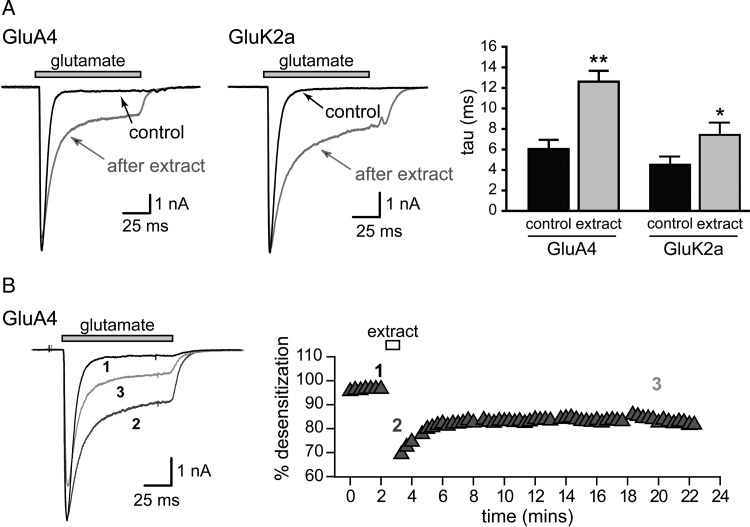
We found that the convulsant sponge extract altered AMPA and kainate receptor gating. Fast application of glutamate to receptor-expressing cells elicits rapidly activating and desensitizing currents under control conditions. The sponge crude extract markedly slowed the desensitization of glutamate-evoked currents and increased steady-state currents (Figure 1). The extract did not directly activate either AMPA or kainate receptors when applied directly in the absence of glutamate (data not shown) and thus seemed to act as a positive allosteric modulator of receptor function. Furthermore, we noted that potentiation of iGluR equilibrium currents was irreversible within the context of our physiology experiments, as shown in the representative example for GluA4 glutamate-evoked currents in Figure 1B. This qualitative effect on iGluR gating was reproducible and stable for many (>5) years while the extract was stored at 4°C.
Fig. 1.
The sponge crude extract slows desensitization of AMPA and kainate receptors. Representative whole-cell currents evoked by glutamate (10 mM) before and after treatment with the Cinachyrella extract (25 μg/mL) for several minutes. (A) Quantitation of the mean tau of exponential fits to the current decay before and after brief applications of sponge extract for either 10 s (GluA4 (flip variant)) or 5 s (GluK2a). N ≥ 3; *, P < 0.05; **, P < 0.01 in paired t-tests. (B) The effects on desensitization are long-lasting. Currents evoked by glutamate from GluA4(i) AMPA receptors are shown before (1), during (2) and 20 minutes after a 1 minute application (3) of extract. An example of the percent desensitization during 100 ms glutamate applications over the course of representative experiment is shown.
We next sought to determine if the in vivo convulsant activity and the in vitro potentiation of iGluRs arose from a shared bioactive principle that co-fractionated on an ion exchange column. The crude extract was separated by anion exchange chromatography with a step gradient from water to 0.5 N acetic acid, finishing with 0.5 N acetic acid containing NaCl (1.0 M). A crude extract (1 g) was eluted with water into 120 samples that were combined into 13 fractions on the basis of UV absorption at 280 nm. Each fraction was tested for convulsant action in mice and alteration of iGluR gating in whole-cell patch-clamp recordings from HEK293-T/17 cells transfected with GluA4 or GluK2 cDNAs. Modulation of iGluR gating was observed in fractions 7–11, while convulsant activities were observed in fractions 12–15 (Table I). Thus, these phenomena clearly arose from separable components in the sponge extract. Subsequent studies reported here focus solely on elucidating the molecule(s) underlying the receptor potentiation activity, because we had already identified a set of molecular targets and defined pharmacological activities in the in vitro electrophysiological assays. The chemical nature of the convulsant principle in the sponge extract remains unknown.
Table I.
Receptor modulation, convulsant and hemagglutinating activities for the sponge extracts and fractions
| Sample | GluK2 |
Mouse | Hemagglutination | ||
|---|---|---|---|---|---|
| Conc. (μg/mL) | Mean % des. | τdes (ms) | |||
| Negative control | 99–100 | 4.5 | |||
| Crude extract | 50 | 48 | 59.1 | Convulsant | 3 |
| Ion exchange fractions | |||||
| 1 (4.5 mg) | 25 | 98 | 6.6 | Inactive | Inactive |
| 2 (6.7) | 25 | 100 | 4.0 | Inactive | Inactive |
| 3 (22.5) | 25 | 100 | 4.3 | Inactive | Inactive |
| 4 (34.1) | 50 | 100 | 4.2 | Inactive | Inactive |
| 5 (59.6) | 50 | 100 | 5.0 | Inactive | Inactive |
| 6 (105.4) | 50 | 99 | 6.5 | Inactive | 2 |
| 7 (85.8) | 50 | 57 | 55.3 | Inactive | 5 |
| 8 (91.2) | 50 | 38 | 51.5 | Inactive | 3 |
| 9 (57.4) | 50 | 73 | 33.1 | Inactive | 5 |
| 10 (13.0) | 50 | 46 | 43.7 | Inactive | 2 |
| 11 (1.5) | 50 | 97 | 8.1 | Convulsant | Inactive |
| 12 (2.4) | 50 | 84 | 12.8 | Convulsant | Inactive |
| Dialyzed fractions | |||||
| Inner solution | 25 | 69 | 55.6 | Inactive | 7 |
| Outer solution | 25 | 99 | 4.0 | Convulsant | – |
The modulatory actions of the crude sponge extract and subsequent anion exchange fractions on iGluR activity were strongly reminiscent of the well-described allosteric activity of the plant mannose-binding lectin concanavalin A (conA); that is, both the marine sponge extract and conA irreversibly enhanced steady-state currents from mammalian kainate receptors (Wong and Mayer 1993). This functional similarity and the large molecular weight of the active sponge compound, consistent with a proteinaceous nature, led us to consider the possibility that the modulatory factors consisted of one or more sponge lectins. To test this hypothesis, we assayed the hemagglutinating activity of the anion exchange-purified fractions against a 4% suspension of rabbit erythrocytes. Each fraction (1 mg/mL) obtained from the ion exchange chromatography was incubated with erythrocytes for 30 min at 37°C. Aggregation of the cells was observed with fractions 7–11, coinciding with the receptor modulating action, thus the result was consistent with the hypothesis that the iGluR activity in the sponge extract was lectin-based. A preliminary screen of glycan selectivity was carried out in the hemagglutination assays using the sugar inhibitors l-alabinose, sucrose, melibiose, maltose, lactose, l-sorbose, d-xylose, l-fucose, d-mannose and d-galactose. Unlike the mannose-selective lectin conA, hemagglutination by the fractionated Cinachyrella extract was inhibited most effectively by lactose and weakly by melibiose and galactose (Gal). No other sugars showed inhibition. Based on this initial sugar selectivity profile, we postulated that the iGluR modulatory activity was conferred by sponge galactose-binding proteins.
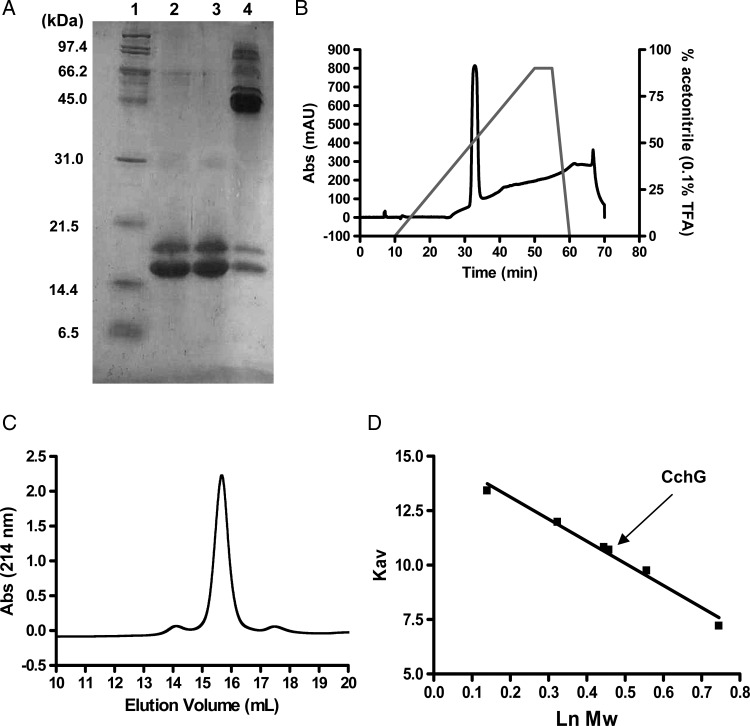
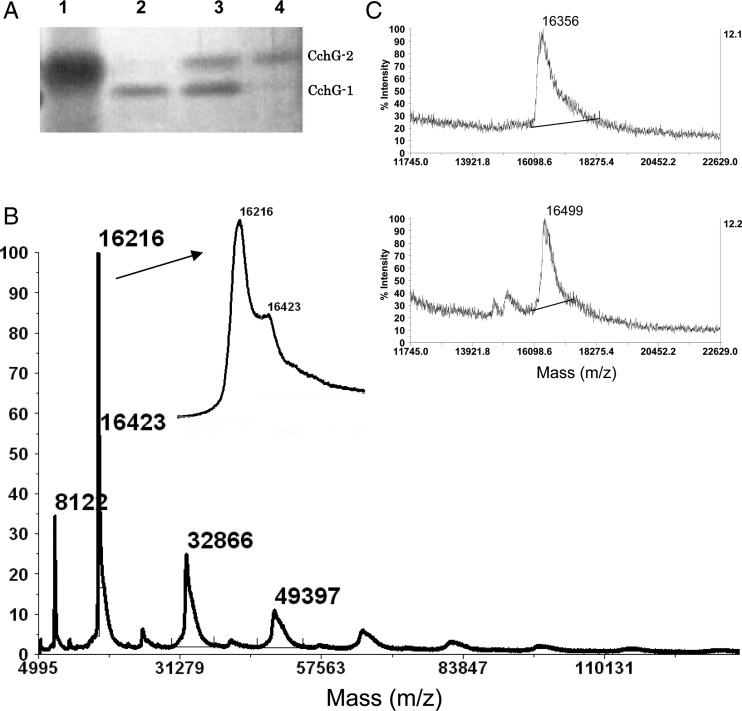
A second, more extensive round of purification was performed in order to isolate the presumed lectins in the sponge extract. The crude extract was first separated by dialysis using a membrane with a 50 Å average pore diameter (∼14 kDa cutoff). The small and large molecular fractions (permeant versus retained during dialysis, respectively) were each tested in the in vivo and in vitro assays. As suggested by the results from initial ion exchange separation, convulsant activity in mice and receptor potentiation activity were clearly distinct. The former appeared in the small molecular fraction, but the latter activity was found in the macromolecular fraction retained in the dialysis tubing (Table I). The macromolecular fraction was again separated on an ion exchange column before the lectin fraction was further purified by affinity chromatography with a lactose-conjugated agarose column. Material retained by the column after an extensive Tris-buffered saline (TBS) wash was eluted with 0.1 M lactose and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). Two distinct protein bands at 16 and 18 kDa were evident following elution and preparation under denaturing conditions. Additional bands at 45, 66 and 90 kDa were observed in nondenaturing preparations that were not subjected to heat treatment (Figure 2A). A gel filtration chromatography of the affinity-purified product gave a mixture of lectin as a single peak (Figure 2C) whose elution volume corresponded to that of a ∼50 kDa protein (Figure 2D). Finally, reversed-phase high pressure (or high performance) liquid chromatography (HPLC) was employed to separate the lactose affinity-purified proteins, but the proteins eluted again as a single peak (Figure 2B). Thus, the nonseparable mixture of proteins was designated as CchGs and used in subsequent experiments.
Fig. 2.
Electrophoretic and chromatographic behaviors of CchGs. SDS–PAGE for CchGs with both heat treatment and mercaptoethanol (line 2), heat treatment but without mercaptoethanol (line 3), no heat treatment without mercaptoethanol (line 4). Standard protein bands (line 1) (A). Protein HPLC chart (B). Gel filtration chromatography analysis (C), and molecular size calibration for standard proteins. Molecular size estimated from the Kav value for CchG is indicated by arrow (D).
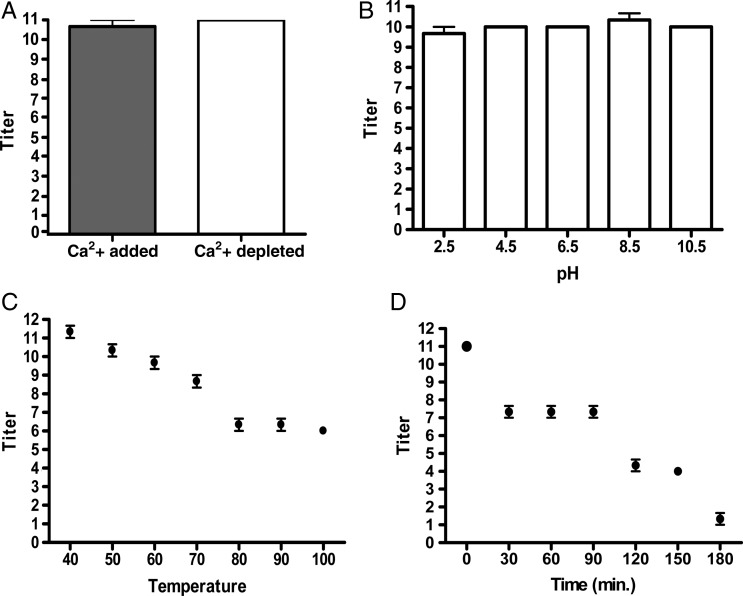
HPLC-purified CchGs exhibited similar agglutinating properties as the fractionated Cinachyrella extract, aggregating erythrocytes at concentrations as low as 0.7 μg/mL. Aggregation was inhibited most efficiently with lactose and to a lesser degree by N-acetyl-d-galactosamine, d-galactose and melibiose (Table II). Other sugars, including mannose and N-acetylneuraminic acid, did not reduce the hemagglutinating activity of CchGs (Table II). Calcium was not required for the lectin activity of CchGs because pretreatment with ethylenediaminetetraacetic acid (EDTA) did not affect hemagglutination (Figure 3A). The lectin activity was also stable after treatment with buffers with various pH values ranging from 4.5 to 10.5 (Figure 3B). Activity of the CchGs was highly heat stable (Figure 3C); >2 h of continuous boiling was required to reduce the lectin activity to half of the maximum titer (Figure 3D).
Table II.
Inhibition of the hemagglutinating activity with various sugars
| Inhibitor compound | Minimum inhibitory concentration (mM) |
|
|---|---|---|
| CchGs | rCchGa | |
| d-galactose | 100 | 50 |
| d-glucose | NI | NT |
| d-mannose | NI | NT |
| d-fructose | NI | NT |
| d-xylose | NI | NT |
| l-fucose | NI | NT |
| l-alabinose | NI | NT |
| l-sorbose | NI | NT |
| Sucrose | NI | NT |
| Lactose | 3.1 | 3.1 |
| Maltose | NI | NT |
| Melibiose | 100 | 100 |
| d-glucuronic acid | NI | NT |
| d-glucosamine | NI | NT |
| N-acetyl-d-glucosamine | NI | NT |
| N-acetyl-d-galactosamine | 25 | 25 |
| N-acetylneuraminic acid | NI | NT |
NI: No inhibition.
Fig. 3.
Hemagglutinating titers for: CchGs and CchGs dialyzed against EDTA (A). CchGs treated with buffers of various pH values (B). CchGs incubated at various temperatures (C) and incubated in boiling water for 30–180 min (D). For all experiments, n = 3, Bars ± SD.
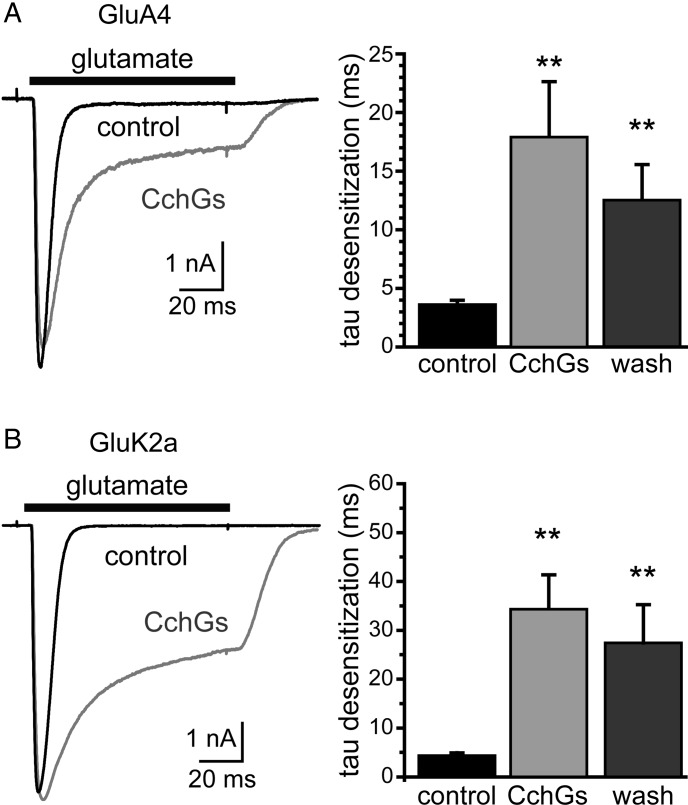
CchGs exhibited the same functional activity on mammalian iGluRs as the original crude sponge extract. In control recordings, fast application of glutamate evoked rapidly desensitizing currents from GluA4 AMPA receptors or GluK2a kainate receptors expressed in HEK293-T/17 cells (Figure 4A and B); a 5-min application of CchGs (10 μg/mL) slowed the time course of desensitization of these currents and increased the amplitude of steady-state currents. The time course of desensitization was quantitated by fitting the currents evoked by glutamate with a two-component exponential decay function that determined relative amplitudes for each component. The weighted τ values calculated from the two-component fit were slowed by 4–8-fold for these representative AMPA and kainate receptors following lectin exposure. We therefore concluded from these data that CchGs accounted for the bioactivity on mammalian iGluRs, just as they were the active hemagglutinating principle in the crude extract.
Fig. 4.
Purified CchGs potentiate currents from GluA4 AMPA and GluK2a kainate receptors expressed in HEK293-T/17 cells. Glutamate (10 mM) was fast-applied to evoke currents before and after a 5 min application of CchGs (10 μg/mL). The graphs show weighted mean tau values derived from two-component fits of the current decays; that currents were slowed by 4–8-fold (**P < 0.01 compared with currents before CchGs application).
For identification purposes, the two distinct protein bands apparent in the CchGs preparation were denoted as CchG-1 and -2. These bands were separated by preparative SDS–PAGE into the ∼16 kDa CchG-1 and the ∼18 kDa CchG-2 proteins (Figure 5A) in advance of spectroscopic analysis. A matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) analysis of the CchGs (containing two protein bands) gave broad molecular ion clusters centered at m/z 16,216 and 16,423 (Figure 5B), whereas MS analysis of the isolated CchG-1 and -2 bands yielded broad but single ion clusters centered at m/z 16,356 and 16,499, respectively (Figure 5C). Divergences in values could be attributable to the relatively low signal-to-noise ratio afforded for those data. It is therefore likely that the two distinguishable molecular ions observed in the whole CchGs preparation correspond to those observed for purified CchG-1 and -2. Of note, the ion for CchG-2 was much smaller than that expected (18 kDa) from the SDS–PAGE result, a divergence that might arise from specific structural features of the lectins (see Discussion). Higher ion clusters were also observed for CchG that corresponded to the molecular weights for a series of putative oligomers as large as a decamer of the ∼16 kDa monomer (Figure 5B).
Fig. 5.
SDS–PAGE for CchG-1 and -2 separated by preparative SDS–PAGE (A), MALDI-TOF-MS of CchGs inset is an expansion of the molecular ion (B), and those of CchG-1 (upper) and 2 (lower) separated by preparative electrophoresis (C).
Determination of CchGs amino acid sequence
The N-terminal amino acid sequences for proteins contained in the bands CchG-1 and -2 were separately determined by automated Edman degradation for up to 40 residues (Table III). We found that dual amino acid signals were detected for each position 1, 2, 3, 6, 8, 12 and 13 of proteins in the CchG-1 band, suggesting that it is a mixture of closely related proteins (Table III). In contrast, Edman sequencing of CchG-2 yielded only single amino acid signals with 85% identity to CchG-1 over the N-terminal 40 amino acids. Neither amino acid sequence showed notable similarity to any known proteins in a BLAST database search.
Table III.
N-terminal amino acid sequences for CchG-1 and -2
| CchG-1 | 1Ta | Ga | P | I | Q | S | I | Ka | V | D | P | Ma | Ka | S | G | G | L | G | V | V | Y | R | S | P | D | K | G | R | V | S | L | Y | L | Y | N | D | G | E | D | I40 |
| V | R | L | K | L | ||||||||||||||||||||||||||||||||||||
| CchG-2 | 1T | R | P | I | Q | S | I | V | V | D | P | M | K | S | G | G | L | G | V | V | Y | R | S | P | N | K | G | R | V | S | L | Y | L | Y | N | E | R | K | D | I40 |
aTwo amino acids were assigned at these positions by an automated sequencer. The minor peak is indicated below the corresponding positions.
To obtain internal amino acid sequences for proteins in CchGs, a tryptic digest of denatured protein was separated by HPLC and peaks were collected and analyzed to obtain their amino acid sequence by automated sequencing. Although reliable information was not obtained for most peaks due to the limited sample quantity, amino acid sequences for three internal peptides were deduced to be SEPLQVVK, DGLPTFIK and SKQIQVTMDANWTDR, respectively. These peptides did not correspond to either of the sequences derived from N-terminal Edman sequencing.
The N-terminal and internal peptide sequences were used to design primer DNAs with the goal of isolating cDNAs encoding the lectin proteins. First-strand synthesis of reverse-transcribed cDNA from sponge RNA was primed with oligo(dT). A cDNA product of 273 bp was then amplified using nested, degenerate primers in two rounds of polymerase chain reaction (PCR). The amplification used an oligo(dT) reverse transcriptase-polymerase chain reaction (RT-PCR) product as the initial template and primers BA-Fwa and BA-Rev-5 (see Table IV), which were designed on the basis of amino acid sequences VVYRSPDKG and QIQTM, respectively. The second round used the first reaction PCR mix as the template and primers BA-Fwb and BA-Rev-6 (degenerate for SLYLYNDGE and DGLPTF, respectively). The resultant 273 bp cDNA predicted a protein fragment of 91 amino acids with the highest homology (∼30%) to residues 51–191 of the sponge Geodia cydonium galectin (GCG) (GenBank accession number CAC38760), consistent with the functional characterization of CchGs as having the highest affinity for lactose and galactose glycans.
Table IV.
Oligonucleotide primers used in this study
| Primer | Nucleotide sequencea |
|---|---|
| BA-Fwa | TNGTNTAYMGNWSNCCNGAYAARGG (VVYRSPDKG) |
| BA-Fwb | TNWSNYTNTAYYTNTAYAAYGAYGG (SLYLYNDGE) |
| BA-Rev5 | CATNGTNACYTGDATYTG (QIQVTM) |
| BA-Rev6 | RAANGTNGGNARNCCRTC (DGLPTF) |
| 3′-Fw1 | GAAGACATCTTGCTTGTTGTTGACGCTC |
| 3′-Fw2 | TTTGATTGGCGTGGCGAGCAGAATGTTC |
| 3′-Fw3 | ACAGGGCCTATTCAATCTATCAAGGTGG (1TGPIQSIKV9) |
| 5′-Rev3 | GCCTACTCTAAACTGCTGCATGCAGTCC |
| 5′-Rev4 | CCGGACGAACTTCAGGTCCCCATTCTCC (66GEWGPEVRP74) |
| NUP | AAGCAGTGGTATCAACGCAGAGT |
| BAex-F1 | AAAGTCGACACAGGGCCTATTCAATCT |
| BAex-R1 | AAAAAGCTTGTAATAAGCGGACAGGGA |
aCorresponding amino acid sequences are shown in parenthesis.
Rapid amplification of cDNA ends (RACEs) was used to isolate the remaining 3′ and 5′ portions of the sponge galectin open-reading frames. The 3′ end of the open-reading frame was obtained by 3′ RACE using the forward primer 3′-Fw1 (Table IV) and a universal primer (UPM) for RACE followed by a nested amplification using a nested universal primer (NUP) and 3′-Fw2 (see Table IV and Methods for details). The resultant 507 bp cDNA was subcloned and sequenced, revealing a polyadenylation signal sequence, AATAAA, and a repeated adenosine sequence (Figure 6), confirming that the open-reading frame encoding the entire C-terminal 99 residues had been deduced. In contrast, 5′-RACE with the primer set UPM and 5′-Rev3 for an initial PCR and NUP and 5′-Rev4 for a nested PCR (Table IV and Experimental procedures) produced cDNAs of ∼500 bp, but an initiation codon and 5′-untranslated region were not identifiable. We found nonetheless that the predicted amino acid sequences from all the cDNAs sequenced were very similar to that obtained from N-terminal analysis of CchG-1 or -2. Therefore, a full-length 525 bp of the galectin cDNA was amplified using primers (3′-Fw3 and 5′-Rev3) designed based on the N-terminal nine residues of protein in CchG-1 and the cloned 3′-untranslated region.
Fig. 6.
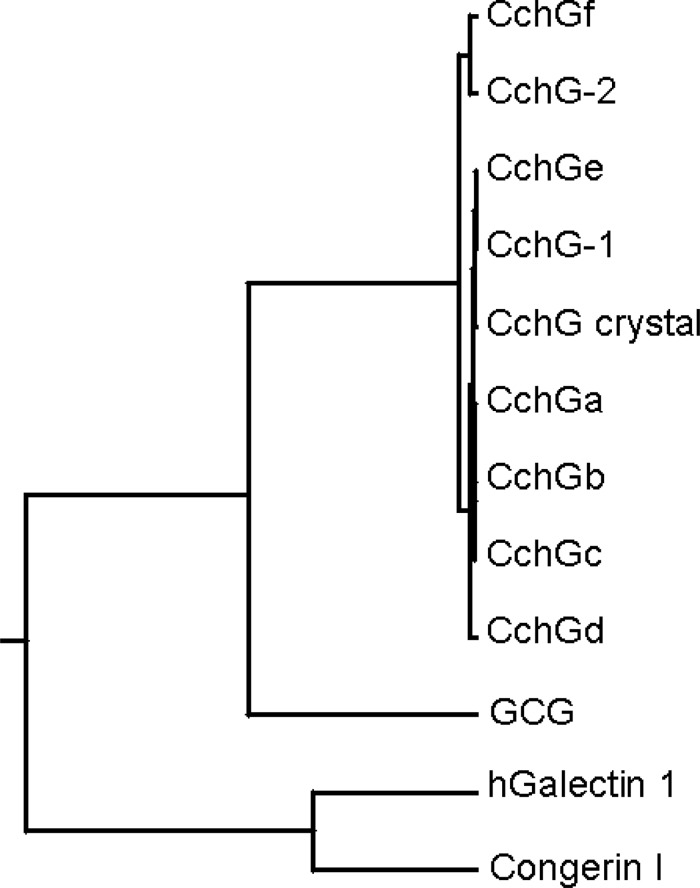
Multiple alignment of amino acid sequences for CchG isolectins and other represented animal galectins. Amino acid sequences for: CchGs deduced from the cDNA sequences (CchGa∼f); determined by Edman degradation (CchG-1 and -2); or X-ray crystallography (CchG crystal), along with other related galectins: Congerin 1 (Conger eel galectins); and human galectin 1 (hGal 1); GCG (51–191 residue, GCG) are aligned. Putative carbohydrate-binding residues are shown by asterisks. Light shadow indicates amino acid residues partly conserved among the proteins. The darker shadow indicates an amino acid residue conserved throughout entire peptides. The alignment of sequences was generated using a software Align X (Life Technologies, Grand Island, NY).
Unexpectedly, sequencing of cDNAs isolated from eight unique colonies were found to diverge at several clustered sites in the open-reading frame, yielding a total of six unique cDNA clones that we have denoted as CchGa-f. The isolated cDNAs all predicted theoretical polypeptides comprising 146 amino acid residues. The N-terminal 40 amino acids for the major protein of CchG-1 determined by Edman degradation was identical to that predicted by CchGe; the protein sequence of CchG-2 coincided with that predicted by the CchGf cDNA sequence with the exception of the second codon for the predicted Gly2, which was clearly an Arg in the Edman degradation data (Table III). The physiological relevance of the divergent cDNA sequences and their predicted proteins is unclear. While they could represent true isoforms arising, for example, from hypervariation in lectin genes (Chen et al. 1993; Ogawa et al. 1999; Wilson et al. 1999; Honda et al. 2000; Stalz et al. 2006; Zhu et al. 2006), it seems at least as likely that the variation in the cDNAs arises from PCR-mediated recombination that produces artificial chimeras when nonidentical but highly similar templates co-exist during amplification (Meyerhans et al. 1990; Brakenhoff et al. 1991). The latter interpretation is supported by the recent X-ray crystallographic resolution of CchG-1, which yielded a primary amino acid sequence that was a hybrid, rather than a precise match, of those predicted by the CchGa-f cDNAs (Figure 6) (Freymann et al. 2012). The crystal structure was identified as CchG-1 based on identity with the N-terminal 40 amino acids obtained from Edman degradation (Table III).
While the cDNA variability introduces a degree of uncertainty into discrete predicted residues, a large proportion of the putative proteins are invariant and match well with the resolved structure of CchG-1. The primary sequence of the CchGe cDNA, for example, predicts peptide identity of 16, 19 and 23% with congerin I from the Conger eel, human galectin 1 and GCG, respectively, and was grouped into the galactose-binding family by Pfam, an online protein family search algorithm. Five residues, 47Arg, 60Asn, 68Trp, 71Glu and 73Arg, which are encoded by all isolated cDNAs (and are present in the crystal structure), contribute to the carbohydrate recognition site in galectins (Leffler et al. 2004), and are conserved among the related proteins, with the exception of the residue corresponding to 73Arg in GCG (Figure 6). However, two carbohydrate-binding residues in human galectin-1, 45His and 47Asn, were replaced by 43Val and 45Asp, respectively, in CchGs (Figure 6). These conserved elements in the primary sequences support the conclusion that CchGs are members of the evolutionarily conserved prototype galectin family.
Expression and biological activity of recombinant lectin
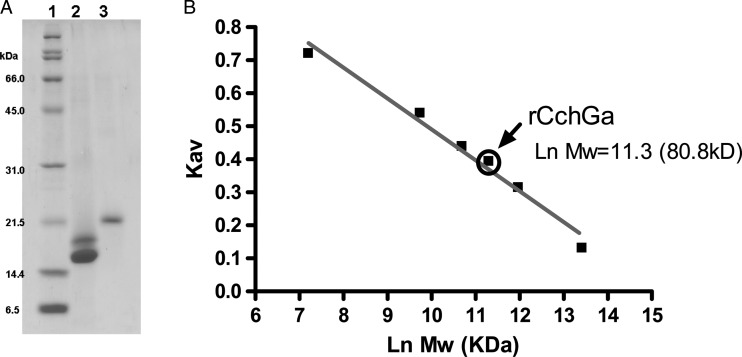
To confirm that isolectins encoded by the isolated cDNAs represented biologically active CchGs, we next prepared recombinant CchGa (rCchGa) protein following expression of the full-length cDNA in Escherichia coli. The recombinant lectin was purified on an Ni affinity column followed by lactose affinity column chromatography. The purified protein rCchGa ran as a single 20 kDa band in SDS–PAGE, as expected (Figure 7A). The molecular size was estimated by gel filtration chromatography to be 80 kDa. The recombinant lectin aggregated rabbit erythrocytes, and this activity was inhibited by lactose as expected (Table II). The hemagglutinating activity was stable after boiling the lectin for 30 min. These data suggested that rCchGa folds and assembles into an active oligomeric lectin with properties analogous to those of the native proteins.
Fig. 7.
SDS–PAGE for standard protein (line 1), CchGs (line 2) and rCchGa (line 3) (A), molecular size calibration for standard proteins and rCchGa in size exclusion chromatography. Molecular size estimated from the Kav value for rCchGa is indicated by an arrow (B).
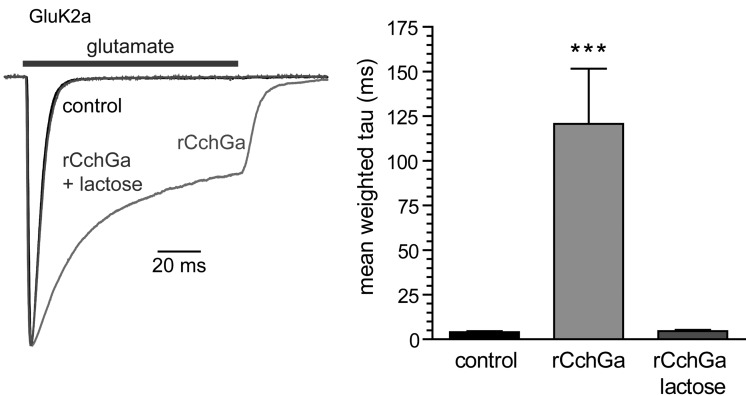
Finally, we confirmed that rCchGa modulated iGluR currents to a similar degree as the natural product isolated from the sponge. Glutamate-evoked currents from homomeric GluK2 kainate receptors expressed in HEK293-T/17 cells were markedly slowed by the application of rCchGa (1 μg/mL) (Figure 8). These modulatory effects on channel gating were completely occluded by the co-application of lactose (20 mM), consistent with the sugar-binding capability of the lectin being the primary determinant of its modulatory activity.
Fig. 8.
rCchGa potentiates currents from GluK2a kainate receptors expressed in HEK293-T/17 cells. Glutamate (10 mM) was fast-applied to evoke currents before and after a 5-min application of rCchGa (1 μg/mL) in the absence or presence of lactose (20 mM). The graph shows weighted mean tau values derived from two-component fits of 1 second current decays (***P < 0.001 compared with currents before rCchGa application).
Discussion
In the present study, we discovered novel galactose-binding lectins from the marine “ball” sponge of Cinachyrella sp. using a bioassay-based approach. The galectins, denoted as CchGs, were in part isolated on the basis of striking and unexpected modulatory activity on mammalian AMPA and kainate iGluRs in the sponge extract. Lectins are known to be distributed in various genera of sponges (Medeiros et al. 2010), a phylum of Porifera, but complete primary sequences are known only for four groups of sponge lectins (Pfeifer, Frank et al. 1993; Gundacker et al. 2001; Funayama et al. 2005; Schroder et al. 2006; Wagner-Hulsmann et al. 1996). These include well-characterized sponge galectins from Geodia cydonium (Pfeifer, Frank et al. 1993; Pfeifer, Haasemann et al. 1993; Stalz et al. 2006; Wagner-Hulsmann et al. 1996), the GCGs, and those isolated more recently from Suberites domuncula (Wiens et al. 2003; Schroder et al. 2006), which have as one important role the formation of spicules that compose the skeleton of these sponges. The primary and secondary structure supports the classification of GCG lectins, the Suberites lectin SDGALEC1 and our CchGs as distant orthologs of mammalian prototype galectins, which typically function as noncovalently associated dimers (Di Lella et al. 2011). In contrast, the second galectin isolated from S. domuncula, SDGALEC2, has two carbohydrate recognition domain motifs in a single peptide chain and thus is classified as a member of the tandem repeat family of galectins (Schroder et al. 2006). It should be noted that lactose-specific lectins have previously been reported from Cinachyrella (Atta et al. 1989; Medeiros et al. 2010). The N-terminal sequence of 23 amino acids determined for one of those lectins, CaL, was unrelated to those of CchGs, but was instead related to silicatein, a protein that catalyzes spicule formation in sponges (Medeiros et al. 2010).
The biologically active lectins we tested were clearly a mixture of isolectins, CchGs. Furthermore, we cloned six cDNAs encoding CchGs, but it is unclear whether the predicted proteins represent galectins actually produced in the sponge given the possibility of artificial chimera formation during PCR amplification. Peptide sequencing of the N-terminal regions, on the other hand, yielded clear evidence for at least two isolectins. Regardless of the precise number of physiologically relevant isolectins in the Cinachyrella sponge extract, however, it is likely that their fundamental biological functions are similar because they exhibit nearly identical predicted carbohydrate-binding domains. This idea was tested by preparing the recombinant lectin rCchGa, which has an amino acid sequence that differs slightly from the proteins CchG-1 and -2 in its N-terminal region. The recombinant and native proteins showed identical physico-chemical properties and biological activities that included modulation on kainate receptors, an activity that was completely inhibited in the presence of lactose. These results unequivocally confirmed that the lectin itself was the active principle in the sponge extract, and iGluR modulation by the lectin was mediated through its interaction with the galactoside chain of the receptor protein.
The CchG preparation possessed remarkable temperature stability in that the lectin exhibited resistance to boiling heat treatment (Figure 3C and D), in comparison with other related prototype galectins, such as that from the Conger eel, congerin I, which forms a stable strand-swap dimer but nonetheless loses hemagglutinating activity completely with milder heat treatment (60°C, 30 min) (Konno et al. 2010). The CchG thermoresistance suggests the formation of higher-order noncovalent oligomers where intermolecular interaction is stable enough to resist the heat treatment.
Other structurally anomalous properties of CchGs included their behavior in gel electrophoresis and MALDI-TOF mass spectrometric analyses. First, although CchG-2 showed a band at 18 kDa in the SDS–PAGE, its actual molecular weight was estimated to be 16,423 by MALDI-TOF-MS and calculated to be 16,318 from its deduced amino acid sequence (for CchGf). The basis for the apparent higher molecular weight in the SDS–PAGE is not clear, but similar “gel shifting” is observed often with membrane proteins and was proposed to result from conformation-dependent differences in the detergent load associated with distinct secondary structures such as helix-loop-helix or hairpin sequences of the peptide (Rath et al. 2009). A similar phenomenon was observed in the case of GCG (Stalz et al. 2006). Secondly, in the MALDI-TOF-MS analysis of CchGs a series of oligomeric ions were observed in addition to the monomeric molecular ion. Similar behavior was reported in the case of GCG (Stalz et al. 2006) and with human galectin-9 (Miyanishi et al. 2007). We conclude from these observations that the CchG sponge galectins have a propensity to aggregate to form stable oligomers in the SDS–PAGE milieu or in vacuo with soft ionization conditions. In the gel filtration analysis, the elution volume of CchGs and rCchG (20 kDa protomers) corresponded with roughly a -50 and -80 kDa standard protein, respectively, consistent with the formation of oligomers in solution. Our recent study indicated that the lectin assembles into a tetramer in the resolved crystal structure of CchG-1, forming a unique toroidal structure with the dimer-of-dimer assemblage (Freymann et al. 2012). These results predict that the tetrameric CchG is likely to be functional in modulation of the mammalian iGluRs.
The amino acid sequence of CchGs resembled not only the other sponge lectin GCG but more distantly other animal galectins, including those from humans (Figure 9). Alignment of the CchGs and the Conger eel galectin congerin I primary sequences identified conserved amino acids within the respective carbohydrate recognition domains (summarized in Table V). Hydrogen bonds between congerin I or galectin-1 and coordinated lactose were taken from the respective crystal structures (Shirai et al. 1999). This analysis suggested that most of the polar interactions and one important hydrophobic interaction between galactose (Gal) moiety-C5 and aromatic moiety of Trp70 (68 in CchGs) are identical between the three lectins. Some differences, however, are apparent: First, most of the direct or indirect hydrogen bonds observed in congerin I or galectin 1 between Gal O1 through O3 are less likely to be found in CchGs based on the corresponding amino acids, with the exception of polar Asp65 for CchGe and f, Arg28 and Asn45 in all CchGs. A conserved residue His44 is missing in CchGs, but Asn46 (Asn45 in CchGs), which is replaced by Asp in congerin I, is present in CchGs. Overall, a total of 12 predicted hydrogen bonds and one hydrophobic interaction can be postulated to occur between CchGs and lactose, which is typical for vertebrate galectins (Shirai et al. 1999). CchGs and galectin-1 also differ in the number of cysteine residues; CchGs have two at Cys81 and 82, whereas galectin-1 has a total of six cysteines. The latter are not involved in intermolecular disulfide bonds; rather, galectin-1 must be reduced in order to exhibit agglutinating activity (Kasai and Hirabayashi 1996). CchG, however, did not require reducing conditions for its lectin activities.
Fig. 9.
Rooted phylogenetic tree with branch length generated by ClustalW (online) for CchGa-f, N-terminal forty residues of Cchg-1 and -2, a sequence from X-ray crystal structure, truncated sponge galectin GCG (51–191 residues), congerin I and human galectin 1.
Table V.
Amino acid residues of congerin I and galectin 1 that participate in intermolecular interactionsa with lactose and corresponding residues for CchG
| Position | Congerin I | Galectin 1 | CchG |
|---|---|---|---|
| Gal-O1 | OW-Tyr51b O | Trp50 | |
| Gal-O2 | OW-Asp67b O | Gly65c/ASP65d | |
| OW-Tyr51b O | Trp50 | ||
| Gal-O3 | Arg29 N1 | Arg28e | |
| Arg29 N2 | Arg28e | ||
| OW-Asp67b O1 | Gly65c/ASP65d | ||
| OW-Asn46b O1 | Asn45 | ||
| Gal-O4 | His44 N2 | His44 N2 | Val43 |
| Arg29 N2 | Arg28e | ||
| Arg48 N1 | Arg48 N1 | Arg47e | |
| Asn46 O1 | Asn45e | ||
| Gal-C5 | Trp70 | Trp70 | Trp68 |
| Gal-O5 | Arg48 N1 | Arg48 N2 | Arg47e |
| Gal-O6 | Asn61 N1 | Asn61 N1 | Asn60e |
| Glu73 O2 | Glu73 O2 | Glu71e | |
| OW-Glu73b O2 | Glu71 | ||
| Glc-O3 | Arg48 N1 | Arg48 N1 | Arg47e |
| Arg48 N2 | Arg48 N2 | Arg47e | |
| Glu73 O2 | Glu73 O2 | Glu71e | |
| Arg75 N2 | Arg75 N2 | Arg73e | |
| Glc-O6 | OW-Glu73b O1 | Glu71 |
aAll interactions are hydrogen bonds except for Gal-C5 and Trp70.
bIndirect hydrogen bond via a water molecule (OW).
cFor CchGa∼d.
dFor CchGe and f.
eDirect hydrogen bond can be expected. Data for congerin I and galectin 1 were taken from Shirai et al., 1999.
Here we used the functional properties of the CchG lectins on iGluRs primarily as a bioassay to inform the separation and identification of the lectins in the current study, but the results clearly have broader implications for a potential role for galectins in the mammalian CNS. Mannose-binding plant lectins like conA have long been used as pharmacological tools to irreversibly potentiate steady-state currents from kainate receptors. The possibility of an endogenous modulatory factor analogous to conA has been postulated previously (Everts et al. 1997), but no such animal lectin has been identified to date. A primary target of galectin binding, N-acetyllactosamine, is a common disaccharide attached to integral membrane proteins during N-glycan processing in the Golgi compartment. The conserved structure and sugar-binding specificity of the CchG proteins suggest that prototype galectins could represent long-sought endogenous modulators with activity analogous to that of conA. If mammalian galectin-1 or other prototype galectins similarly alter the function of iGluRs, they would have a clear impact on fast excitatory transmission, the balance between excitatory and inhibitory tone in neural networks, and potentially learning and memory in the CNS. Moreover, malignant gliomas utilize galectins as a metastatic agent for invasion of new brain areas; certain galectin levels are highly correlated with tumor grade and progression (Camby et al. 2001; Le Mercier et al. 2010). Exposure to glioma-derived galectins therefore could have a profound impact on peritumoral neural processing. In summary, our bioactivity-based screening process of marine sponge extracts, leading to CchG isolation, has unexpectedly led us out of the ocean and into the mammalian brain to explore a novel modulatory role for this family of lectin proteins in neural function.
Experimental procedures
Sponge specimen
Marine sponges of the Cinachyrella sp. were collected at a depth of 15–20 m in Iriomote, Okinawa between 2003 and 2007 using SCUBA. Samples were stored at −20°C until use. A sample of sponge collected in 2007 (1 mm cube) was preserved in RNAlater (QIAGEN, Hilden, Germany) immediately after collection. The preserved sample was stored at −80°C. The specimen was identified as Cinachyrella sp. by Dr. John Hooper at Queensland Museum.
Extraction and purification
The sponge specimen collected in 2007 (173 g) was homogenized with water (330 mL) and lyophilized to give the aqueous extract (9 g). Part of the extract (4 g) was dialyzed against water using a cellulose membrane (∼14 kDa cutoff, Viskase Co., Dadrien, IL). The external and internal solution was lyophilized to give a small molecular (2.1 g) and a macromolecular fraction (1.1 g), respectively. The latter was separated by anion exchange gel (DE52, GE Healthcare, Tokyo, Japan) with a gradient of Tris–HCl (100 mM, pH 8.0) containing NaCl (1.0 M). The elution was monitored with absorbance at 280 nm. The lectin fraction eluted at ∼20–40% of the NaCl-containing buffer and was collected, dialyzed and then lyophilized to give 88 mg of a colorless powder. A portion of the fraction (9 mg) was loaded on a lactose-agarose affinity chromatography (1 mL, Sigma-Aldrich, St. Louis, MO). The unbound fraction was eluted with TBS (0.15 M NaCl/50 mM Tris–HCl pH 7.4) while monitoring absorption at 280 nm. The proteins bound to the column were eluted with lactose (0.1 M) in TBS. The resultant protein was further purified on a reversed-phase HPLC using YMC-Pack Protein-RP (YMC, Kyoto, Japan). A linear gradient elution between 0.1% aqueous trifluoroacetic acid (TFA) and acetonitrile with 0.1% TFA afforded a single peak at Tr of 33 min. Solvents were removed to give 3.4 mg of protein (CchGs).
Animal experiments
The mouse behavioral assays were performed under approval by the ethical committee of experimental animal care at Hokkaido University. The effects of sponge extract on mice behavior were assessed as described earlier (Sakai et al. 2001). Briefly, an aqueous solution of sample (20 μL) was injected intracerebroventricularly in male ddY mice of age 3–4 weeks (Japan SLC Inc., Hamamatsu, Japan) and behaviors were observed; behavioral profiles were observed for 60 min.
Size exclusion chromatography
The apparent molecular size of the native lectin was estimated by size exclusion chromatography using Superdex 200 (1.0 × 30 cm, GE Healthcare, Japan) at a flow rate of 0.25 mL/min. The void volume (V0) of the column was measured to be 7.6 mL by eluting blue dextran 2000 (GE Healthcare, Japan). The standard protein mixture: Thyroglobulin (670 kDa); γ-globulin (158 kDa); ovalbumin (44 kDa); myoglobin (17 kDa); vitamin B12 (1.35 kDa) (BioRad Japan, Tokyo, Japan), was eluted using a phosphate buffer (50 mM (pH 7.0) containing 0.15 M NaCl). The sample was eluted and the major peak was dialyzed and then lyophilized to give 4.8 mg protein.
Gel electrophoresis
SDS–PAGE was performed on a 15% (w/v) polyacrylamide gel and sodium dodecyl sulfate (SDS) 0.1% (v/v) in either denaturing sample buffer (Tris–HCl buffer 62.5 mM (pH6.8), SDS 2%, sucrose 5%, bromophenol blue 2 × 10−3%, mercaptoethanol, 5%) or nondenaturing sample buffer (former buffer without mercaptoethanol) (Laemmli, UK 1970). The protein bands were stained either by 0.2 M imidazole/0.1% SDS followed by 0.2 M ZnSO4, or by 0.25% Coomassie Brilliant Blue. Preparative electrophoresis was performed using AE-6750S Prephoresis S (ATTO, Tokyo, Japan). Sample protein (2 mg) was loaded on a polyacrylamide gel column and separated using an electrophoresis buffer, 25 mM Tris, 192 mM glycine, 0.1% SDS and recovery buffer, 371 mM Tris, 20% sucrose. The recovered sample was dialyzed against water and then lyophilized to give pure protein bands at 16 and 18 kDa.
Hemagglutination assay
Serial 2-fold dilutions of the test solution (1 mg/mL and 20 µL) were made in 96-well plates using 100 mM Tris–HCl. A suspension (4%, 20 µL) of rabbit erythrocytes was added and the mixture was incubated at 37°C for 30 min. The titer of the maximum dilution showing positive agglutination was recorded and the hemagglutination titer was defined as the reciprocal of the highest dilution. Specific activity was defined as the number of hemagglutination units per mg of protein.
Heat and pH stabilities
The lectin sample (1 mg/mL) was dissolved in water and heated at 40–100°C for 30 min or dissolved and heated in boiling water (slightly <100°C) for 30, 60, 90, 120 and 180 min. for heat stability testing. For pH stability, samples (5 mg/mL, 30 μL) were dissolved in buffers of varying pH: Glycine–HCl, pH 2.5; sodium acetate, pH 4.5; phosphate, pH 6.5; Tris–HCl, pH 8.5 or glycine–NaOH, pH 10.5, and was left at room temperature for 1 h. Tris–HCl buffer (pH 8.0, 30 mM and 120 μL) was added to prepare the test solution (1 mg/mL).
Ca2+ requirement for the hemagglutinating activity
A solution of lectin was dialyzed against 50 mM EDTA, 50 mM Tris–HCl (pH 8.5), and then distilled water to remove residual salts. The lyophilized sample was dissolved in 50 mM Tris–HCl (pH 8.5), 150 mM NaCl or that containing 10 mM CaCl2 to prepare 1 mg/mL each test solution.
Inhibition of hemagglutination by sugars
Hemagglutinating titer of the lectin sample was adjusted to 16. Aliquots of the sample solutions (10 µL) were allowed to react with varying concentrations of sugars (10 µL) for 60 min at 20°C and hemagglutination was measured as above.
Enzyme digest
A denatured sample of CchGs in 8 M urea in 50 mM Tris–HCl was digested with trypsin (Worthington, Lakewood, NJ) (substrate/enzyme = 50:1) at 37°C for 8 h or with protease V8 (Worthington) (substrate/enzyme = 50:1) in ammonium bicarbonate (0.1 M) at 37°C for 8 h. Each of the enzyme digests was absorbed on ZipTipC18 (Nihon Millipore, Tokyo, Japan), washed by 0.1% TFA and then eluted off with 80% CH3CN with 0.1% TFA. The digested protein fragments were separated by a reversed-phase Protein & Peptide C18 HPLC column (Vydac) with eluent A (0.1% TFA) and B (CH3CN with 0.1% TFA) using a programmed gradient as follows: 90% A (0–2 min), 90–45% A (2–10 min), 45–10% A (10–25 min), 10% A (25–27.5 min) and 10–90% A (27.5–30 min). Each of the eluents was concentrated in vacuo and the amino acid sequence was directly analyzed by an automated gas-phase amino acid analyzer ABI Procise 492 sequencer (Applied Biosystems, Foster City, CA).
Chemical analyses
MALDI technique was used to measure molecular ions for the lectin using 4700 Proteomics analyzer (Applied Biosystems, Foster City, CA) in a linear high/MS mode. Either α-cyano-4-hydroxycinnamic acid (CHCA) or 2,6-dihydroxyacetophenone was used as a matrix. A 16 kDa sample purified by preparative gel electrophoresis was precipitated by trichloroacetic acid, washed with acetone, dissolved in aqueous acetonitrile (50%) with TFA (0.1% v/v) and then applied on a glass membrane (Applied Biosystems) for automated sequencing (vide supra).
cDNA cloning for CchG
Total RNA was extracted from the sample preserved in RNAlater (Ambion, Inc., TX) with RNeasy Mini Kit (Qiagen, Hilden, Germany). Then, 100 ng of total RNA was utilized for first-strand cDNA synthesis using PrimeScript RTase (Takara, Otsu, Japan) with an oligo(dT) primer, and was used as template for PCR. Degenerate primers, BA-Fwa, BA-Fwb, BARev-5 and BARev-6, were designed based on the internal amino acid sequences of CchG protein as shown in Table IV. The first RT-PCR was performed with a primer set of BA-Fwa and BA-Rev-5 (each 0.5 μM) using KOD Dash DNA polymerase (Toyobo, Osaka, Japan) in a final volume of 20 μL at 94°C, for 2 min, 35 cycles of 94°C for 30 s, 60°C for 30 s and 74°C for 90 s, followed by 74°C for 7 min. Next, 1 μL of 10-fold diluted first RT-PCR mixture was used as a template in the second RT-PCR reaction mixture (20 μL). The PCR conditions were the same as above with the exception of the primer set BA-Fwb and BA-Rev-6 (each 0.5 μM). PCR products were subcloned into the plasmid vector pTAC-1 (BioDynamics Laboratory Inc., Tokyo, Japan), and then nucleotide sequences were determined using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA) and an ABI 3130xl genetic analyzer (Applied Biosystems).
The 3′- or 5′-RACE was performed using a SMARTer™ RACE cDNA Amplification Kit (Clontech Laboratories, Palo Alto, CA). 3′- or 5′-ready cDNA was prepared according to the manufacturer's protocol. Four gene-specific primers, 3′-Fw1 and 3′-Fw2 for 3′-RACE, 5′-Rev3 and 5′-Rev4 for 5′-RACE, were designed (Table IV) based on the nucleotide sequence obtained from RT-PCR products. The initial 3′-RACE reaction was carried out using KOD Dash DNA polymerase with a primer set of a supplied universal primer, UPM (5′-CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT-3′, Clontech) and 3′-Fw1 in a final volume of 20 μL at 94°C, for 2 min, 35 cycles of 94°C for 30 s and 68°C for 90 s followed by 74°C for 7 min. A nested PCR amplification was performed using a nested universal primer, NUP (Table IV) and 3′-Fw2 at the same condition. 5′-RACE was also similarly performed using a primer set of UPM and 5′-Rev3 for an initial PCR and NUP and 5′-Rev4 for a nested PCR. Amplified cDNAs were subcloned and sequenced as described above. Finally, the open-reading frame encoding mature CchG was amplified using a primer set of 3′-Fw3 and 5′-Rev3 (Table IV) with Phusion High-Fidelity DNA polymerase (New England Biolabs, Ipswich, MA). The cycling conditions were 98°C for 2 min, 35 cycles of 98°C for 30 s, 60°C for 30 s and 72°C for 90 s, followed by 72°C for 7 min. After subcloning of PCR products, nucleotide sequences of eight clones were analyzed. Nucleotide sequences for six clones encoding putative amino acid sequences for sponge lectins, CcgGa–f, are available with the following accession numbers: CchGa (AB6994344.1); CchGb (AB6994345.1); CchGc (AB6994346.1); CchGd (AB6994347.1); CchGe (AB6994348.1); CchGf (AB6994349.1).
Expression of recombinant lectin
An insert containing full-length open-reading frame of CchGa with Sal I and HindIII sites was amplified using a set of primers, BAex-F1 and BAex-R1, with Emerald Amp PCR Master Mix (Takara) with the following conditions: CchGa 0.5 µL, BAex-F1[10 µM] 0.4 µL, BAex-R1[10 µM] 0.4 µL, Emerald Amp PCR Master Mix 10 µL, dH2O 8.7 µL (98°C 10 s, 50°C 30 s, 72°C 1 min) × 30 cycles. The PCR product, designated BaeX, was inserted into pEcoli-Nterm-6×HN Vector (Clontech) and transformed into E. coli DH5α Competent Cells (Takara). The cloned plasmid was transformed into C2566H (T7 Express Competent E. coli, New England BioLabs, Tokyo, Japan) by electroporation. The cells were cultured on an Luria broth (LB) agar medium containing ampicillin (100 μg/mL), incubated at 37°C for 16 h. A colony was then transferred into an LB liquid medium and shaken at 37°C until the OD600 became 0.4–0.6. To the medium isopropyl β-d-1-thiogalactopyranoside was added to a final concentration of 0.4 mM and shaken for 2 h. The bacterial cells were harvested by centrifugation (6000 rpm, 10 min), resuspended in a buffer (50 mM Tris–HCl ( pH 7.4),15 mM NaCl), sonicated, frozen/thawed and then centrifuged. The supernatant was affinity purified using Profinity™ IMAC Ni-Charged Resin (10 mL, BioRad, Japan) and then the protein was purified by a lactose affinity column (Sigma-Aldrich) to obtain 1 mg of pure rCchGa.
Electrophysiology
Whole-cell voltage clamp recordings were performed on rat recombinant GluA4 AMPA and GluK2 kainate receptors transiently expressed into HEK293-T/17 cultured at 37°C with 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 100 µg/mL penicillin, 100 µg/mL streptomycin and 10% heat-inactivated fetal bovine serum. One day before transfection, cells were split onto glass coverslips coated with poly-d-lysine and rat collagen (100 mg/μL). TransIT reagent (Mirus Bio, Madison, WI) was used for co-transfection of 50–200 ng of receptor cDNA and 50 ng enhanced green fluorescent protein per well according to the manufacturer's protocol. Patch-clamp recordings were performed using an Axopatch 200B amplifier controlled by the pClamp 9 software (Molecular Devices, Sunnyvale, CA) 24–72 h after addition of the transfection reagent. Glutamate (10 mM) was fast-applied to green receptor-expressing cells through a triple-barrel rectangular glass tool attached to a piezo-bimorph; translation of the bimorph was driven by digital signals from the pClamp 9 software. Extracellular bathing solution consisted of (in mM): 140 NaCl, 10 glucose, 10 Cs-4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) 3 KCl, 2 CaCl2 and 1 MgCl2 (pH 7.3). The patch pipette solution consisted of (in mM): 30 CsF, 110 CsCl, 10 HEPES, 4 NaCl, 5 ethylene glycol bis(2-aminoethyl ether)tetraacetic acid and 0.5 CaCl2 (pH 7.3). Glutamate receptor currents were evoked every 20 s by bimorph-driven solution exchange before, during and after the application of crude Cinachyrella extract or purified CchGs. Tau values shown in Table I correspond to a mean weighted tau derived from biexponential fits to the current decay during 1 sec applications of glutamate to receptor-expressing cells. All analyses of current amplitudes and kinetics were performed off-line using the pClamp 9 software.
Funding
This work was financially supported by the Naito Foundation and a Grant-in-Aid for Scientific Research from Ministry of Education, Culture, Sports, Science and Technology, Japan (15580183 and 17380125 to R.S.), and R01 NS44322 from the NINDS (National Institute of Health) to G.T.S.
Conflict of interest
None declared.
Abbreviations
AMPA, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate; CchGs, Cinachyrella galectins; CHCA, α-cyano-4-hydroxycinnamic acid; CNS, central nervous system; conA, concanavalin A; DMEM: Dulbecco's modified Eagle's medium; EDTA, ethylenediaminetetraacetic acid; Gal, galactose; GCG, Geodia cydonium galectin; HEK293-T/17, human embryonic kidney cells expressing T-antigen, clone 17; HEPES, 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid; HPLC, high pressure (or high performance) liquid chromatography; iGluRs, ionotropic glutamate receptors; LacNAc, N-acetyl-d-lactosamine; LB, Luria broth; MALDI, matrix-assisted laser desorption ionization; mass spectrometry; NUP, nested universal primer; RACEs, rapid amplification of cDNA ends; rCchGa, recombinant CchGa; SDS, sodium dodecyl sulfate; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; TFA, trifluoroacetic acid; TBS, Tris-buffered saline; TOF, time-of-flight; UPM, universal primer.
Acknowledgement
We are grateful to Dr. J. Hooper at the Queensland Museum for the identification of the sponge.
References
- Atta AM, Barral-Netto M, Peixinho S, Sousa-Atta ML. Isolation and functional characterization of a mitogenic lectin from the marine sponge Cinachyrella alloclada. Braz J Med Biol Res. 1989;22:379–385. [PubMed] [Google Scholar]
- Brakenhoff RH, Schoenmakers JG, Lubsen NH. Chimeric cDNA clones: A novel PCR artifact. Nucleic Acids Res. 1991;19:1949. doi: 10.1093/nar/19.8.1949. doi:10.1093/nar/19.8.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camby I, Belot N, Rorive S, Lefranc F, Maurage CA, Lahm H, Kaltner H, Hadari Y, Ruchoux MM, Brotchi J, et al. Galectins are differentially expressed in supratentorial pilocytic astrocytomas, astrocytomas, anaplastic astrocytomas and glioblastomas, and significantly modulate tumor astrocyte migration. Brain Pathol. 2001;11:12–26. doi: 10.1111/j.1750-3639.2001.tb00377.x. doi:10.1111/j.1750-3639.2001.tb00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Ratcliffe NA, Rowley AF. Detection, isolation and characterization of multiple lectins from the haemolymph of the cockroach Blaberus discoidalis. Biochem J. 1993;294(Pt 1):181–190. doi: 10.1042/bj2940181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lella S, Sundblad V, Cerliani JP, Guardia CM, Estrin DA, Vasta GR, Rabinovich GA. When galectins recognize glycans: From biochemistry to physiology and back again. Biochemistry. 2011;50:7842–7857. doi: 10.1021/bi201121m. doi:10.1021/bi201121m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts I, Villmann C, Hollmann M. N-glycosylation is not a prerequisite for glutamate receptor function but is essential for lectin modulation. Mol Pharmacol. 1997;52:861–873. doi: 10.1124/mol.52.5.861. [DOI] [PubMed] [Google Scholar]
- Freymann DM, Nakamura Y, Focia PJ, Sakai R, Swanson GT. Structure of a tetrameric galectin from Cinachyrella sp. (ball sponge) Acta Crystallogr D Biol Crystallogr. 2012;68:1163–1174. doi: 10.1107/S0907444912022834. doi:10.1107/S0907444912022834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama N, Nakatsukasa M, Kuraku S, Takechi K, Dohi M, Iwabe N, Miyata T, Agata K. Isolation of Ef silicatein and Ef lectin as molecular markers for sclerocytes and cells involved in innate immunity in the freshwater sponge Ephydatia fluviatilis. Zoolog Sci. 2005;22:1113–1122. doi: 10.2108/zsj.22.1113. doi:10.2108/zsj.22.1113. [DOI] [PubMed] [Google Scholar]
- Gundacker D, Leys SP, Schroder HC, Muller IM, Muller WE. Isolation and cloning of a C-type lectin from the hexactinellid sponge Aphrocallistes vastus: A putative aggregation factor. Glycobiology. 2001;11:21–29. doi: 10.1093/glycob/11.1.21. doi:10.1093/glycob/11.1.21. [DOI] [PubMed] [Google Scholar]
- Honda S, Kashiwagi M, Miyamoto K, Takei Y, Hirose S. Multiplicity, structures, and endocrine and exocrine natures of eel fucose-binding lectins. J Biol Chem. 2000;275:33151–33157. doi: 10.1074/jbc.M002337200. doi:10.1074/jbc.M002337200. [DOI] [PubMed] [Google Scholar]
- Kasai K, Hirabayashi J. Galectins: A family of animal lectins that decipher glycocodes. J Biochem. 1996;119:1–8. doi: 10.1093/oxfordjournals.jbchem.a021192. doi:10.1093/oxfordjournals.jbchem.a021192. [DOI] [PubMed] [Google Scholar]
- Konno A, Yonemaru S, Kitagawa A, Muramoto K, Shirai T, Ogawa T. Protein engineering of conger eel galectins by tracing of molecular evolution using probable ancestral mutants. BMC Evol Biol. 2010;10:43. doi: 10.1186/1471-2148-10-43. doi:10.1186/1471-2148-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Mercier M, Fortin S, Mathieu V, Kiss R, Lefranc F. Galectins and gliomas. Brain Pathol. 2010;20:17–27. doi: 10.1111/j.1750-3639.2009.00270.x. doi:10.1111/j.1750-3639.2009.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj J. 2004;19:433–440. doi: 10.1023/B:GLYC.0000014072.34840.04. doi:10.1023/B:GLYC.0000014072.34840.04. [DOI] [PubMed] [Google Scholar]
- Matsunaga S, Jimbo M, Gill MB, Wyhe LL, Murata M, Nonomura K, Swanson GT, Sakai R. Isolation, amino acid sequence and biological activities of novel long-chain polyamine-associated peptide toxins from the sponge Axinyssa aculeata. Chembiochem. 2011;12:2191–2200. doi: 10.1002/cbic.201100329. doi:10.1002/cbic.201100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros DS, Medeiros TL, Ribeiro JKC, Monteiro NKV, Migliolo L, Uchoa AF, Vasconcelos IM, Oliveira AS, de Sales MP, Santos EA. A lactose specific lectin from the sponge Cinachyrella apion: Purification, characterization, N-terminal sequences alignment and agglutinating activity on Leishmania promastigotes. Comp Biochem Physiol B Biochem Mol Biol. 2010;155:211–216. doi: 10.1016/j.cbpb.2009.10.016. doi:10.1016/j.cbpb.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Meyerhans A, Vartanian JP, Wain-Hobson S. DNA recombination during PCR. Nucleic Acids Res. 1990;18:1687–1691. doi: 10.1093/nar/18.7.1687. doi:10.1093/nar/18.7.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanishi N, Nishi N, Abe H, Kashio Y, Shinonaga R, Nakakita S, Sumiyoshi W, Yamauchi A, Nakamura T, Hirashima M, et al. Carbohydrate-recognition domains of galectin-9 are involved in intermolecular interaction with galectin-9 itself and other members of the galectin family. Glycobiology. 2007;17:423–432. doi: 10.1093/glycob/cwm001. doi:10.1093/glycob/cwm001. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Ishii C, Kagawa D, Muramoto K, Kamiya H. Accelerated evolution in the protein-coding region of galectin cDNAs, congerin I and congerin II, from skin mucus of conger eel (Conger myriaster) Biosci Biotechnol Biochem. 1999;63:1203–1208. doi: 10.1271/bbb.63.1203. doi:10.1271/bbb.63.1203. [DOI] [PubMed] [Google Scholar]
- Pfeifer K, Frank W, Schroder HC, Gamulin V, Rinkevich B, Batel R, Muller IM, Muller WE. Cloning of the polyubiquitin cDNA from the marine sponge Geodia cydonium and its preferential expression during reaggregation of cells. J Cell Sci. 1993;106(Pt 2):545–553. doi: 10.1242/jcs.106.2.545. [DOI] [PubMed] [Google Scholar]
- Pfeifer K, Haasemann M, Gamulin V, Bretting H, Fahrenholz F, Muller WE. S-type lectins occur also in invertebrates: High conservation of the carbohydrate recognition domain in the lectin genes from the marine sponge Geodia cydonium. Glycobiology. 1993;3:179–184. doi: 10.1093/glycob/3.2.179. doi:10.1093/glycob/3.2.179. [DOI] [PubMed] [Google Scholar]
- Rath A, Glibowicka M, Nadeau VG, Chen G, Deber CM. Detergent binding explains anomalous SDS–PAGE migration of membrane proteins. Proc Natl Acad Sci USA. 2009;106:1760–1765. doi: 10.1073/pnas.0813167106. doi:10.1073/pnas.0813167106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai R, Swanson GT, Sasaki M, Shimamoto K, Kamiya H. Dysiherbaine: A new generation of excitatory amino acids of marine origin. Curr Med Chem Cent Nerv Syst Agents. 2006;6:83–108. doi:10.2174/187152406777441907. [Google Scholar]
- Sakai R, Swanson GT, Shimamoto K, Green T, Contractor A, Ghetti A, Tamura-Horikawa Y, Oiwa C, Kamiya H. Pharmacological properties of the potent epileptogenic amino acid dysiherbaine, a novel glutamate receptor agonist isolated from the marine sponge Dysidea herbacea. J Pharmacol Exp Ther. 2001;296:650–658. [PubMed] [Google Scholar]
- Sakurada T, Gill MB, Frausto S, Copits B, Noguchi K, Shimamoto K, Swanson GT, Sakai R. Novel N-methylated 8-oxoisoguanines from Pacific sponges with diverse neuroactivities. J Med Chem. 2010;53:6089–6099. doi: 10.1021/jm100490m. doi:10.1021/jm100490m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder HC, Boreiko A, Korzhev M, Tahir MN, Tremel W, Eckert C, Ushijima H, Muller IM, Muller WE. Co-expression and functional interaction of silicatein with galectin: Matrix-guided formation of siliceous spicules in the marine demosponge Suberites domuncula. J Biol Chem. 2006;281:12001–12009. doi: 10.1074/jbc.M512677200. doi:10.1074/jbc.M512677200. [DOI] [PubMed] [Google Scholar]
- Sharon N. Lectins: Carbohydrate-specific reagents and biological recognition molecules. J Biol Chem. 2007;282:2753–2764. doi: 10.1074/jbc.X600004200. doi:10.1074/JBC.X600004200. [DOI] [PubMed] [Google Scholar]
- Sharon N. Lectins: Past, present and future. Biochem Soc Trans. 2008;36:1457–1460. doi: 10.1042/BST0361457. doi:10.1042/BST0361457. [DOI] [PubMed] [Google Scholar]
- Shirai T, Mitsuyama C, Niwa Y, Matsui Y, Hotta H, Yamane T, Kamiya H, Ishii C, Ogawa T, Muramoto K. High-resolution structure of the conger eel galectin, congerin I, in lactose-liganded and ligand-free forms: Emergence of a new structure class by accelerated evolution. Structure. 1999;7:1223–1233. doi: 10.1016/s0969-2126(00)80056-8. doi:10.1016/S0969-2126(00)80056-8. [DOI] [PubMed] [Google Scholar]
- Stalz H, Roth U, Schleuder D, Macht M, Haebel S, Strupat K, Peter-Katalinic J, Hanisch FG. The Geodia cydonium galectin exhibits prototype and chimera-type characteristics and a unique sequence polymorphism within its carbohydrate recognition domain. Glycobiology. 2006;16:402–414. doi: 10.1093/glycob/cwj086. doi:10.1093/glycob/cwj086. [DOI] [PubMed] [Google Scholar]
- Wagner-Hulsmann C, Bachinski N, Diehl-Seifert B, Blumbach B, Steffen R, Pancer Z, Muller WE. A galectin links the aggregation factor to cells in the sponge (Geodia cydonium) system. Glycobiology. 1996;6:785–793. doi: 10.1093/glycob/6.8.785-d. doi:10.1093/glycob/6.8.785-d. [DOI] [PubMed] [Google Scholar]
- Wiens M, Mangoni A, D'Esposito M, Fattorusso E, Korchagina N, Schroder HC, Grebenjuk VA, Krasko A, Batel R, Muller IM, et al. The molecular basis for the evolution of the metazoan bodyplan: Extracellular matrix-mediated morphogenesis in marine demosponges. J Mol Evol. 2003;57(Suppl 1):S60–S75. doi: 10.1007/s00239-003-0008-1. doi:10.1007/s00239-003-0008-1. [DOI] [PubMed] [Google Scholar]
- Wilson R, Chen C, Ratcliffe NA. Innate immunity in insects: The role of multiple, endogenous serum lectins in the recognition of foreign invaders in the cockroach, Blaberus discoidalis. J Immunol. 1999;162:1590–1596. [PubMed] [Google Scholar]
- Wong LA, Mayer ML. Differential modulation by cyclothiazide and concanavalin A of desensitization at native alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid- and kainate-preferring glutamate receptors. Mol Pharmacol. 1993;44:504–510. [PubMed] [Google Scholar]
- Zhu Y, Ng PM, Wang L, Ho B, Ding JL. Diversity in lectins enables immune recognition and differentiation of wide spectrum of pathogens. Int Immunol. 2006;18:1671–1680. doi: 10.1093/intimm/dxl101. doi:10.1093/intimm/dxl101. [DOI] [PubMed] [Google Scholar]