Abstract
We previously showed that electroacupuncture (EA) activates medulla-spinal serotonin-containing neurons. The present study investigated the effects of intrathecal 5,7-dihydroxytryptamine creatinine sulfate (5,7-DHT), a selective neurotoxin for serotonergic terminals, the 5-hydroxytryptamine 1A receptor (5-HT1AR) antagonist NAN-190 hydrobromide and the 5-HT2C receptor (5-HT2CR) antagonist SB-242,084 on EA anti-hyperalgesia. EA was given twice at acupoint GB30 after complete Freund’s adjuvant (CFA) injection into hind paw. CFA-induced hyperalgesia was measured by assessing hind paw withdrawal latency (PWL) to a noxious thermal stimulus 30 min post-EA. Serotonin depletion and the 5-HT1AR antagonist blocked EA anti-hyperalgesia; the 5-HT2CR antagonist did not. Immunohistochemical staining showed that spinal 5-HT1AR was expressed and that 5-HT2CR was absent in naive and CFA-injected animals 2.5 hr post-CFA. These results show a correlation between EA anti-hyperalgesia and receptor expression. Collectively, the data show that EA activates supraspinal serotonin neurons to release 5-HT, which acts on spinal 5-HT1AR to inhibit hyperalgesia.
Keywords: acupuncture, serotonin, spinal cord, hyperalgesia, complete Freund’s adjuvant
1. Introduction
Acupuncture has been used in China and other Asian countries for thousands of years and Western countries for over thirty years to treat a variety of diseases and symptoms [1], but its underlying mechanisms are still not fully understood [2–4]. Recent chronic pain electroacupuncture (EA) studies, including our own [5], have shown that EA produces anti-hyperalgesia in ankle sprain and inflammatory pain animal models [6–8]. We also recently demonstrated that, compared to sham control, EA significantly increases withdrawal latency of the inflamed hind paw in sham-treated rats but not in those with dorsolateral funiculus lesions, indicating that lesioning blocks EA-produced anti-hyperalgesia. Additionally, EA has been found to activate serotonin-containing nucleus raphe magnus neurons that project to the spinal cord [9].
These results suggested that EA inhibits hyperalgesia by activating supraspinal serotonin-containing neurons that project to the spinal cord. Previous studies show that serotonin is an important mediator of acupuncture analgesia in naive animals [10, 11]. For example, an intracerebroventricular 5-hydroxytryptamine (5-HT, i.e. serotonin) receptor antagonist blocked EA-produced analgesia in naïve mice [12], a systemic serotonin receptor antagonist prevented EA inhibition of pain in collagen-induced arthritis pain model [13], and intravenous methysergide, a non-selective serotonin antagonist, blocked EA inhibition of tooth pulp stimulation-evoked potential in the superficial layers of the trigeminal nucleus caudalis (Vc-I–II) in rabbits [14]. An additional study showed that spinal serotonin receptors played an important role in EA alleviation of cold allodynia in a neuropathic pain rat model [15].
These studies led us to hypothesize that serotonin is involved in EA inhibition of thermal hyperalgesia in an inflammatory pain rat model and that different serotonin receptor subtypes might have distinct roles in EA action. Since serotonin acts on multiple receptor subtypes, we depleted spinal serotonin in the inflammatory pain rat model, preventing activation of all its subtypes in order to determine its involvement in EA anti-hyperalgesia. The principle 5-HT receptor in the spinal cord, 5-HT1 receptor, accounts for about 50–60% of the total [3H] 5-HT binding sites in the spinal cord of the rat. The 5-HT1A receptor, which is involved in inflammatory pain inhibition in the spinal cord [16], represents approximately 30–50% of all 5-HT1 receptor binding sites [17, 18]. It has been also reported that 5-HT2C receptor mRNA is expressed in the spinal cord [19]. Thus we used specific 5-HT1A and 2C receptor antagonists to investigate the involvement of these two subtypes in EA anti-hyperalgesia.
2. Experimental procedures
Animal Preparation
Male Sprague-Dawley rats weighing 280–320 g (Harlan, Indianapolis, IN) were kept under controlled laboratory conditions (22°C, relative humidity 40% 60%, 12-hour alternate light–dark cycles, food and water ad libitum) and were acclimatized to the environment for 5 days prior to an experimentation. The animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland School of Medicine, Maryland, USA.
Experimental Design
Four sets of experiments were conducted to determine spinal serotonin involvement in EA anti-hyperalgesia: 1) EA plus spinal serotonin depletion, 2) EA plus 5-HT2C receptor antagonists, 3) EA plus 5-HT1A receptor antagonists, and 4) immunostaining of spinal 5-HT1A and 2C receptors to examine their expression in the spinal cord.
Rats were randomly divided into four groups (n=8 per group): a) EA plus intrathecal (i.t.) 5,7-dihydroxytryptamine creatinine sulfate (5,7-DHT; 150 μg in 10 μl, Sigma), a selective neurotoxin for serotonergic terminals; b) sham EA plus 5,7-DHT; c) EA plus vehicle; d) sham EA plus vehicle. Each rat was given 25mg/kg of desipramine intraperitoneally (i.p.) 30 min prior to 5,7-DHT administration to protect noradrenergic neurons from the neurotoxin [20]. The 5,7-DHT was dissolved in sterile saline containing 0.1% ascorbic acid to prevent oxidation and injected at the level of the spinal lumber enlargement [21]. Ten days later, complete Freund’s adjuvant (CFA) was injected into one hind paw of each rat. EA or sham treatment was given immediately after the injection. A paw withdrawal latency (PWL) test was conducted 30 min after EA treatment by an investigator who was blind to the treatment assignments (Fig. 1).
Cannulated rats with CFA -induced inflammation were randomly divided into four groups (n=8 per group): a) EA + SB-242,084, a selective 5-HT2C receptor antagonist (20 μg/10 μl in saline); b) sham EA + the 5-HT2C receptor antagonist; c) EA + saline (10 μl); d) sham EA + saline (10 μl). The SB-242,084 solutions were administered (i.t.) 20 min before the EA treatment. PWL was tested 30 min after EA treatment (Fig. 1).
Cannulated rats with CFA-induced inflammation were randomly divided into four groups (n=7 per group): a) EA plus NAN-190 hydrobromide, a selective 5-HT1A receptor antagonist (20 μg/4 μl in DMSO); b) sham EA plus the 5-HT1A receptor antagonist solution; c) EA plus DMSO (4 μl) and d) sham EA plus DMSO. The NAN-190 hydrobromide solutions were administered (i.t.) 20 min before the EA treatment. PWL was tested 30 min after EA treatment (Fig. 1).
Immunohistochemistry was used in two groups of rats (n=4 per group), CFA-inflamed (2.5 hr post-CFA) and naïve, to determine 5-HT1A and 2C receptor expression in the spinal cord.
Fig. 1.
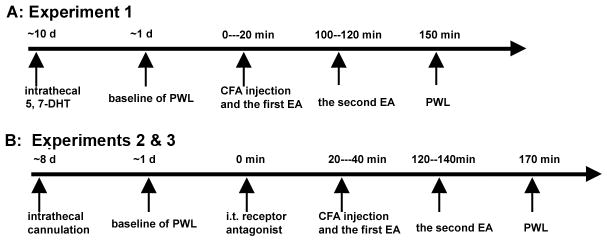
Time course of experiments 1–3.
Intrathecal Cannulation
Rats were prepared for i.t. injection under pentobarbital sodium anesthesia (50 mg/kg, i.p.). The atlanto-occipital membrane at the level between the head and neck (i.e., approximately the obex level) was exposed, and a slit was cut through which a 7 cm length of PE-10 tubing was inserted into the subarachnoid space. The catheter was advanced to the level of the lumbar spinal cord, filled with approximately 7–10 μl of saline, and then plugged at its outer end. The animals were allowed to recover for seven days after the operation before experimentation; those with gross signs of motor impairment were excluded from the study. At the end of the experiments, the location of the distal end of the catheter was verified when the spinal cord was removed.
In the 5,7-DHT study, the chemical was injected i.t., and the tubing was removed immediately after the injection.
Hyperalgesia Testing
Inflammatory hyperalgesia was induced by injecting CFA (Sigma, St Louis, MO) suspended in an 1:1 oil/saline emulsion, 0.08 ml, 40 μg Mycobacterium tuberculosis, subcutaneously into the plantar surface of one hind paw of the rat using a 25-gauge hypodermic needle [5]. Hyperalgesia was determined by a decrease in PWL to a noxious thermal stimulus. PWL was tested with Hargreaves’s method [5, 22]. Each rat was placed under an inverted clear plastic chamber on the glass surface of the Paw Thermal Stimulator System (UCSD, San Diego) and allowed to acclimatize for 30 min before the test. A radiant heat stimulus was applied to the plantar surface of each hind paw from underneath the glass floor with a projector lamp bulb (CXL/CXR, 8 V, 50 W). PWL to the nearest 0.1 sec was automatically recorded when the rat withdrew its paw from the stimulus. Stimulus intensity was adjusted to derive a baseline PWL of approximately 10.0 sec in naive animals. Paws were alternated randomly to preclude order effects; a 20-sec cut-off was used to prevent tissue damage. Four tests were conducted for each hind paw, with a 5-min interval between each test. Mean PWL was established by averaging the tests.
Acupuncture Treatment Procedures
To maximize the anti-inflammatory effect and to treat animals prophylactically, EA treatment was given twice for 20 min each, once right after the administration of CFA and again 2 hr post-CFA. EA parameters of 10 Hz, 3mA, 0.1 ms pulse width, which showed significant anti-inflammatory and anti-hyperalgesic effects in the rat inflammation model in our previous studies [5], were used in the present study.
The equivalent of the human acupoint Huantiao (GB30) was chosen for EA based on traditional Chinese medicine meridian theory [1], on its successful use in our previous studies, and on studies by others [5, 23, 24]. In our previous point-specificity study,[5] EA produced better anti-hyperalgesia at GB30 than at acupoint Waiguan (the fifth acupoint on the Triple Energizer Meridian, TE5) on the forepaw or at two non-specific points, an abdominal point and a point on the quadriceps opposite to GB30. In humans, GB30 is located at the junction of the lateral 1/3 and medial 2/3 of the distance between the greater trochanter and the hiatus of the sacrum; underneath are the sciatic nerve, inferior gluteal nerve, and gluteal muscles [1]. GB30 was located on the rat’s hind limbs using the comparable anatomical landmarks.
GB30 was needled bilaterally. After cleaning the skin with alcohol swabs, a disposable acupuncture needle, 0.25 mm thickness X 0.5 inch length, was inserted obliquely approximately 0.5 inch deep at GB30 on each of the animal’s hind limbs, and a pair of electrodes was attached to the handles of the needles. The needles and electrodes were stabilized with adhesive tape and electrically stimulated with an A300 Pulsemaster (World Precision Instruments) via an A360D Stimulus Isolator (World Precision Instruments) that delivers steady, direct current. This bilateral, cross-limb connection has been used previously by our team and others with no adverse effects [5, 25]. Similar connections are frequently used in clinic, and no adverse effects have been reported [26, 27].
While EA frequency was held constant, intensity was adjusted slowly over the period of approximately 2 min to the designated level of 3 mA, which is the maximum EA current intensity that a conscious animal can tolerate. Mild muscle twitching was observed. During EA treatment, each rat was placed under an inverted clear plastic chamber (approximately 5× 8×11 inches) but was neither restrained nor given anesthetic. The animals remained awake during treatment and showed no signs of distress. For sham treatment control, acupuncture needles were inserted bilaterally into GB30 but without electrical stimulation or manual manipulation. This procedure produced no anti-hyperalgesia in this animal model in our previous study [5]. Since it is comparable to the treatment procedure but lacks therapeutic effect, we used it as sham control in this study.
Immunohistochemistry
Rats were deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and perfused transcardially with 4% paraformaldehyde (Sigma) in 0.1 M phosphate buffer (PB) at pH 7.4. The lumbar4–5 spinal cord was removed, immersed in the same fixative for 2 hr at 4 °C, and transferred to 30% sucrose (w/v) in PB saline (PBS) for overnight cryoprotection. Thirty micron-thick sections were cut on a cryostat, rinsed in PBS, blocked in PBS with 10% normal donkey serum for 60 min, and incubated overnight at room temperature with rabbit polyclonal antibody against the 5-HT2C receptor (1:50, ImmunoStar) or guinea pig polyclonal 5-HT1A receptor (1:50, Millipore). After three 10-min washings in PBS, sections were incubated in CY3-congugated donkey anti-rabbit (1:100, Jackson ImmunoResearch Laboratories) for 5-HT2C, or donkey anti-guinea pig for 5-HT1A receptors. The stained sections were mounted on gelatin-coated slides, coverslipped with aqueous mounting medium (Biomeda Corp., CA), and examined under a Nikon fluorescence microscope for distribution of 5-HT2C or 1A receptor-immunoreactive cells within the spinal dorsal horn. Five sections were randomly selected from each animal for cell counting. Five-HT2C or 1A immunoreactive cells were counted individually under a Nikon microscope using a 20X objective lens on the superficial spinal laminae of each selected section. They were averaged separately for each rat and then for the group. Control sections were similarly processed, except that the primary antisera were omitted, which yielded no staining.
Statistical analyses
Data from the behavioral tests were presented as mean ± SE and analyzed using repeated measures analysis of variance (ANOVA) followed by Bonferroni multiple comparisons (Graphpad Prism). Immunostaining data were analyzed with one-way ANOVA followed by Scheffe’s multiple comparison. P<0.05 was set as the level of statistical significance.
3. Results
Effect of 5-HT depletion on EA-produced inhibition of hyperalgesia
Before 5-HT depletion, PWL to noxious heat stimuli were similar in left (9.51 ± 0.23 sec) and right (9.39 ± 0.20 sec) hind paws (n=16). After the depletion, they were also similar (9.63 ± 0.50 sec and 9.79 ± 0.45 sec respectively, Fig. 2). There were no significant (F(3, 63) =0.29, P= 0.83) PWL differences before and after 5-HT depletion. PWLs were also similar in the left and right hind paws of saline-injected (i.t.) rats before and after saline injection (data not shown).
Fig. 2.
PWLs before and 9 days after 5,7-DHT (n=16) treatment. There were no significant changes between left and right, or before and after 5,7-DHT.
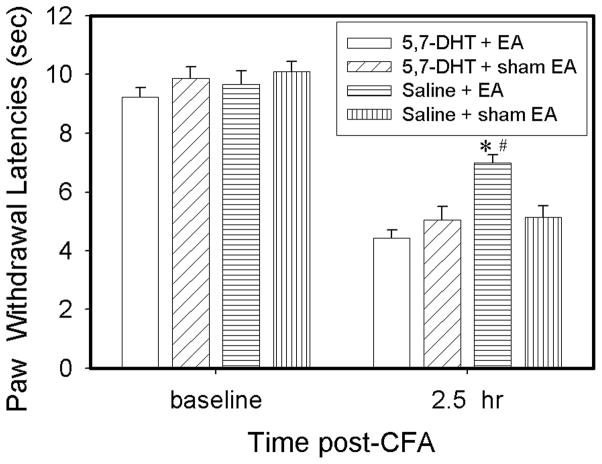
As shown in Fig. 3, before the CFA injection, left hind paw PWL in rats given i.t. saline and those given 5,7-DHT were similar. Following an injection of 0.08 ml CFA in i.t. saline-injected rats, withdrawal latency of the injected paws of sham EA-treated rats was significantly (P<0.05) shorter 2.5 hr post-CFA (5.13 ± 0.39 sec) than at baseline (10.11 ± 0.32 sec). Contralateral PWLs were unchanged from baseline (data not shown). The EA treatment, 10 Hz at bilateral GB 30, significantly (P<0.05) increased PWL of the ipsilateral hind paws compared to sham EA 2.5 hr post-CFA (7.0 ± 0.26 vs 5.13 ± 0.39 sec). This indicates that EA significantly inhibited CFA-induced hyperalgesia in saline-injected rats. EA did not increase withdrawal latency of the contralateral hind paws. Following CFA injection in 5,7-DHT-treated rats, PWL of the injected paws of sham EA also was significantly (P<0.05) shorter at 2.5 hr (5.05 ± 0.46 sec) than at baseline. However, EA treatment did not significantly increase PWL of the injected paws (4.44 ± 0.27 sec) compared to sham EA (5.05 ± 0.46 sec) in 5,7-DHT treated rats (Fig. 3), showing that EA did not inhibit CFA-induced hyperalgesia in rats with depleted spinal 5-HT.
Fig. 3.
Effect of 5,7-DHT treatment on EA-produced anti-hyperalgesia in rats with CFA-induced inflammation (n=8 per group). CFA induced a significant (P<0.05) decrease of ipsilateral PWL in both saline- and 5,7-DHT-treated rats 2.5 hr post-CFA compared to baseline. EA significantly increased PWL in saline- but not 5,7-DHT-treated rats compared to sham EA. #P<0.05 vs saline + sham EA; *P<0.05 vs 5,7-DHT + EA.
Effect of a 5-HT2C receptor antagonist on EA-produced inhibition of hyperalgesia
Figure 4 shows that EA plus vehicle (i.t.) significantly increased PWL compared to sham EA plus vehicle 2.5 hr post-CFA, indicating that EA inhibits hyperalgesia. EA plus the 5-HT2C receptor antagonist SB-242,084, compared to EA plus vehicle, similarly increased PWL at 2.5 hr, indicating that the receptor antagonist did not blocked EA-produced anti-hyperalgesia. Sham EA plus SB-242,084 did not significantly change PWL compared to sham EA plus vehicle, indicating that the antagonist per se had little effect on PWL (Fig. 4).
Fig. 4.
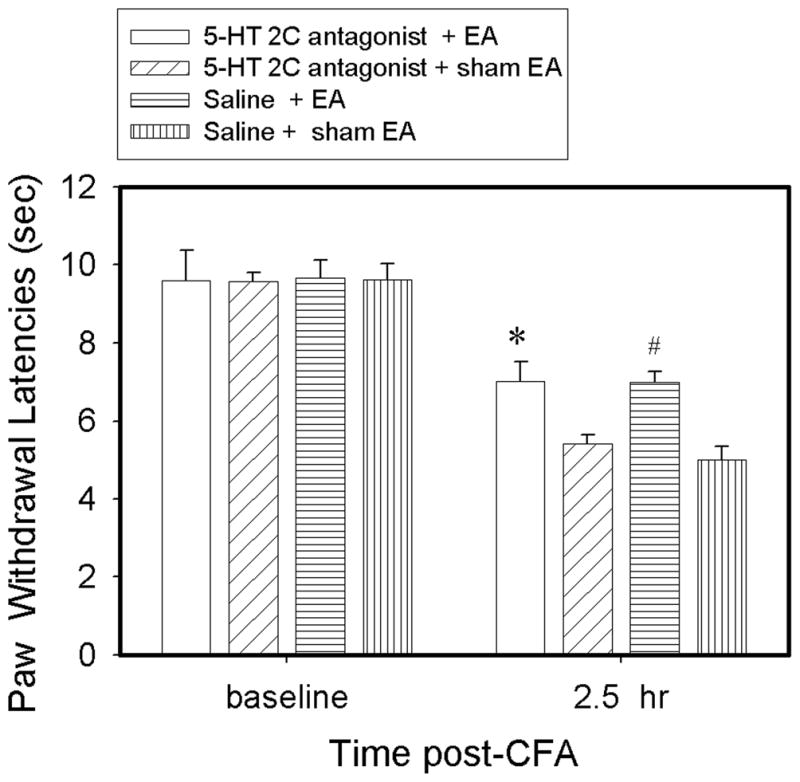
Effect of pretreatment with the 5-HT receptor 2C antagonist SB-242,084 on EA-produced anti-hyperalgesia in rats with CFA-induced inflammation (n=8 per group). CFA induced a significant (P<0.05) decrease of ipsilateral PWL in both saline- and SB-242,084-treated rats 2.5 hr post-CFA compared to baseline. Compared to sham, EA significantly increased PWL at 2.5 hr after either saline or SB-242,084 treatment. #P<0.05 vs saline + sham EA; *P<0.05 vs SB-242,084 + sham EA.
Effect of a 5-HT1A receptor antagonist on EA-produced inhibition of hyperalgesia
EA plus the 5-HT 1A receptor antagonist NAN-190 hydrobromide did not significantly increase PWL 2.5 hr after CFA compared to EA plus vehicle, although EA plus vehicle significantly increased PWL compared to sham plus vehicle. The data indicate that the antagonist blocked EA-produced anti-hyperalgesia. Sham EA plus NAN-190 hydrobromide did not significantly change PWL compared to sham EA plus vehicle, indicating that this antagonist per se had little effect on PWL (Fig. 5).
Fig. 5.
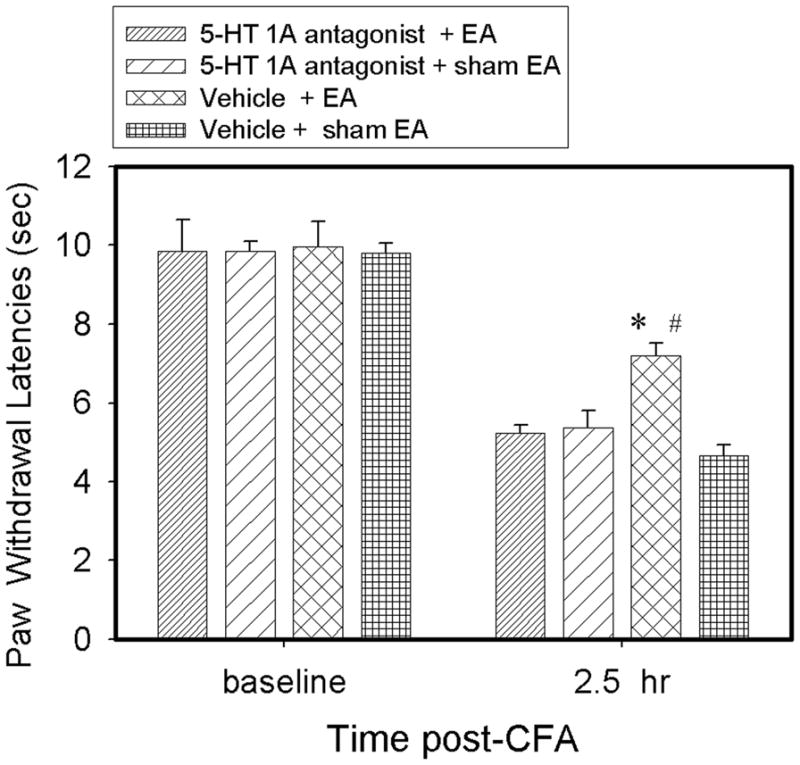
Effect of pretreatment with 5-HT receptor 1A antagonist NAN-190 hydrobromide on EA-produced anti-hyperalgesia in rats with CFA-induced inflammation (n=7 per group). CFA induced significant (P<0.05) decreases of ipsilateral PWL in both saline-and NAN-190 hydrobromide-treated rats 2.5 post-CFA compared to baseline. EA significantly increased PWLs 2.5 hr in vehicle- but not NAN-190 hydrobromide-treated rats compared to sham EA. #P<0.05 vs vehicle + sham EA; *P<0.05 vs NAN-190 hydrobromide + EA.
Expression of 5-HT1A/2C receptors in the spinal cord during pain
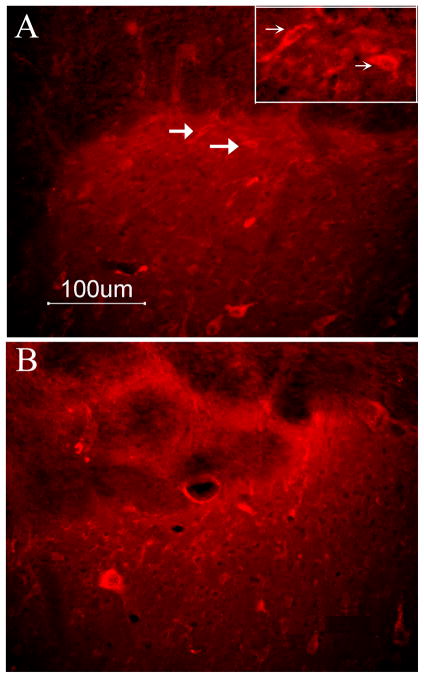
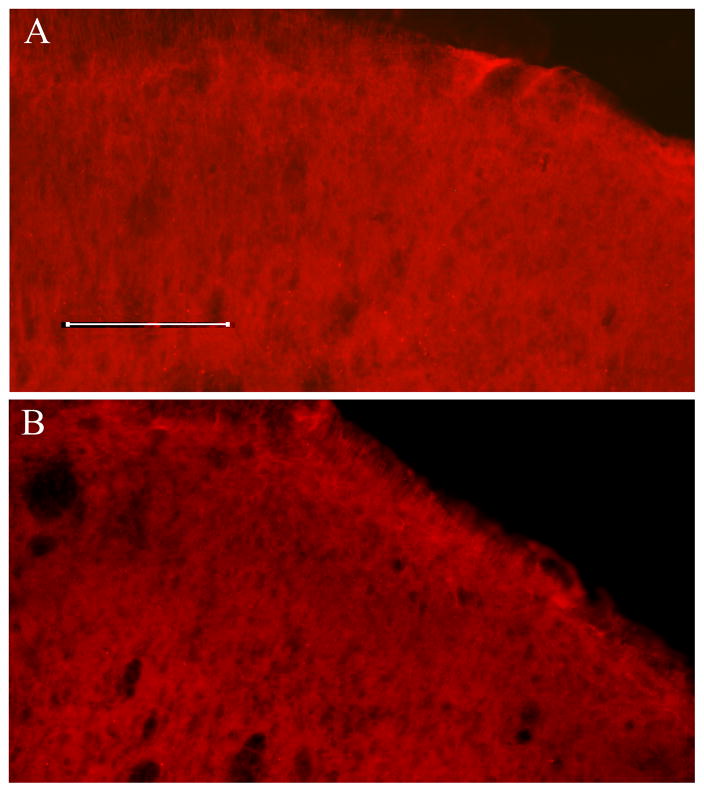
The number of 5-HT1A receptor-immunoreactive neurons in superficial spinal cord laminae was comparable (P> 0.05) in naive (16 ± 3/section) and CFA-injected animals (B, 15± 3) (Fig. 6); 5-HT2C receptor-immunoreactive neurons were absent in naive and CFA-injected animals (Fig. 7).
Fig. 6.
Immunostaining data shows that 5-HT1A receptor expression was similar in naïve animals (A, 16 ± 3) and 2.5 hr post-CFA injection (B, 15± 3). The inset in A is higher magnification of arrow-pointed neurons. Scale is 100 μm.
Fig. 7.
Immunostaining data shows that 5-HT2C receptors were absent in naive animals (A) and 2.5 hr (B) post-CFA injection. Scale is 100 μm.
4. Discussion
In the present study, spinal serotonin depletion completely blocked EA anti-hyperalgesia in the inflammatory pain model, indicating that spinal serotonin is involved in EA anti-hyperalgesia. We previously reported that EA activated serotonin-containing neurons in the nucleus raphe magnus (NRM) that project to the spinal cord [9, 28]. These data suggested that EA may activate supraspinal serotonin-containing neurons and increase serotonin activities in the spinal cord to inhibit pain.
An electrophysiological study demonstrated that NRM stimulation inhibited spinal neuronal activities, 50% of which were suppressed by (±)-8-hydroxy-2-(di-n-propylamino) tetralin hydrobromide (8-OH-DPAT), a 5-HT1A receptor agonist [29]. Interestingly, our present study demonstrates that an i.t. 5-HT1A receptor antagonist blocks EA anti-hyperalgesia. This is consistent with a previous study showing that i.t. injection of a 5-HT1A receptor antagonist significantly blocked the palliative effects of EA on cold allodynia in a neuropathic pain model [15] and on tail flick latency in a rat model of collagen-induced arthritis [13]. Consistent with those behavioral results, 5-HT induced an outward current in the spinal cord dorsal horn, which was mimicked by 8-OH-DPAT but suppressed by the 5-HT1A receptor antagonist WAY-100635 [30]. Similarly, 5-HT induced hyperpolarizing effects in the trigeminal subnucleus caudalis, which was mimicked by 8-OH-DPAT and blocked by WAY-100635. As noted, the hyperpolarization was maintained in the presence of TTX (an Na(+) channel blocker), indicating direct postsynaptic action of 5-HT on trigeminal subnucleus caudalis neurons [31]. In support of these data, our immunostaining study demonstrated that 5-HT1A receptors were consistently expressed in neurons in the spinal cord superficial laminae at baseline and 2.5 hr post CFA. The 5-HT1A receptor represents approximately 30–50% of all 5-HT1 receptor binding sites in the spinal cord [17, 18]. Collectively, these studies show that EA activates serotonin-containing neurons in the NRM to release 5-HT in the spinal cord that act on spinal 5-HT1A receptors to inhibit pain.
It should be noted that the 5-HT1A receptor antagonist blocked EA anti-hyperalgesia, while the 5-HT2C receptor antagonist did not. These results are supported by our immunostaining study, which shows that 5-HT2C receptors were absent both at baseline and 2.5 hr post CFA. Radioimmunoassay showed lower levels of 5-HT2C receptors in the naive rat spinal cord [32]. The insignificant 5-HT2C receptor expression accounts for the lack of 5-HT2C receptor involvement in EA inhibition of hyperalgesia.
It is well known that spinal opioids are involved in EA analgesia in naive animal models [33]. Recent studies have shown that EA produces anti-hyperalgesia through spinal opioid receptors in inflammatory[34] and neuropathic pain [35] models. Our unpublished data showed that i.t. serotonin significantly inhibits inflammation-induced hyperalgesia, which is blocked by mu receptor antagonist pretreatment. Consistent with these results, naloxone inhibited i.t. 5-HT anti-nociception [36, 37]. Collectively, these data suggest that spinal 5-HT and opioids may be serially involved in EA action.
In summary, the present study demonstrates that EA induces the spinal release of 5-HT, which acts on spinal 5-HT1A receptors to inhibit hyperalgesia. EA-induced serotonin may enhance the endogenous endomorphin-mu receptor system to inhibit pain. The findings suggest that acupuncture may have important clinical applications to the treatment of pain, and especially to chronic inflammatory pain conditions such as arthritis.
Acknowledgments
This work was supported by NIH Grant R21AT004113 and P01 AT002605. We would like to thank Dr. Lyn Lowry for her editorial support.
References
- 1.Cheng X. Chinese Acupuncture and Moxibustion. Foreign Languages Press; Beijing: 1999. [Google Scholar]
- 2.Bullock ML, Pheley AM, Kiresuk TJ, Lenz SK, Culliton PD. Characteristics and complaints of patients seeking therapy at a hospital-based alternative medicine clinic. J Altern Complement Med. 1997;3:31–37. doi: 10.1089/acm.1997.3.31. [DOI] [PubMed] [Google Scholar]
- 3.Diehl DL, Kaplan G, Coulter I, Glik D, Hurwitz EL. Use of acupuncture by American physicians. J Altern Complement Med 1997. 1997;3:119–126. doi: 10.1089/acm.1997.3.119. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Z. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85:355–375. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Lao L, Zhang R-X, Zhang G, Wang X, Berman BM, Ren K. A parametric study of electroacupuncture on persistent hyperalgesia and Fos protein expression in rats. Brain Res. 2004;1020:18–29. doi: 10.1016/j.brainres.2004.01.092. [DOI] [PubMed] [Google Scholar]
- 6.Yang E, Koo S, Kim Y, Lee J, Hwang H, Lee M, Choi S. Contralateral electroacupuncture pretreatment suppresses carrageenan-induced inflammatory pain via the opioid-mu receptor. Rheumatol Int. 2010 Feb 4; doi: 10.1007/s00296-010-1364-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y-Q, Ji G-C, Wu G-C, Zhao Z-Q. Excitatory amino acid receptor antagonists and electroacupuncture synergetically inhibit carrageenan-induced behavioral hyperalgesia and spinal fos expression in rats. Pain. 2002;99:525–535. doi: 10.1016/S0304-3959(02)00268-3. [DOI] [PubMed] [Google Scholar]
- 8.Koo ST, Park YI, Lim KS, Chung K, Chung JM. Acupuncture analgesia in a new rat model of ankle sprain pain. Pain. 2002;99:423–431. doi: 10.1016/S0304-3959(02)00164-1. [DOI] [PubMed] [Google Scholar]
- 9.Li A, Wang Y, Xin J, Lao L, Ren K, Berman BM, Zhang R-X. Electroacupuncture suppresses hyperalgesia and spinal Fos expression by activating the descending inhibitory system. Brain Res. 2007;1186:171–179. doi: 10.1016/j.brainres.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang M, Han JS. 5-hydroxytryptamine is an important mediator for both high and low frequency electroacupuncture analgesia. Acupuncture Res. 1985;10:212–215. [PubMed] [Google Scholar]
- 11.Han CS, Chou PH, Lu CC, Lu LH, Yang TH, Jen MF. The role of central 5-hydroxytryptamine in acupuncture analgesia. Sci Sin. 1979;22:91–104. [PubMed] [Google Scholar]
- 12.Chang FC, Tsai HY, Yu MC, Yi PL, Lin JG. The central serotonergic system mediates the analgesic effect of electroacupuncture on ZUSANLI (ST36) acupoints. J Biomed Sci 2004. 2004;11:179–185. doi: 10.1007/BF02256561. [DOI] [PubMed] [Google Scholar]
- 13.Baek YH, Choi DY, Yang HI, Park DS. Analgesic effect of electroacupuncture on inflammatory pain in the rat model of collagen-induced arthritis: mediation by cholinergic and serotonergic receptors. Brain Res. 2005;1057:181–185. doi: 10.1016/j.brainres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Takagi J, Sawada T, Yonehara N. A possible involvement of monoaminergic and opioidergic systems in the analgesia induced by electro-acupuncture in rabbits. Jpn J Pharmacol. 1996;70(1):73–80. doi: 10.1254/jjp.70.73. [DOI] [PubMed] [Google Scholar]
- 15.Kim SK, Park JH, Bae SJ, Kim JH, Hwang BG, Min B-I, Park DS, Na HS. Effects of electroacupuncture on cold allodynia in a rat model of neuropathic pain: Mediation by spinal adrenergic and serotonergic receptors. Exp Neurol. 2005;195:430–436. doi: 10.1016/j.expneurol.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Buritova J, Larrue S, Aliaga M, Besson J-M, Colpaert F. Effects of the high-efficacy 5-HT1A receptor agonist, F 13640 in the formalin pain model: A c-Fos study. Eur J Pharmacol. 2005;514:121–130. doi: 10.1016/j.ejphar.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Huang JC, Peroutka SJ. Identification of 5-hydroxytryptamine1 binding site subtypes in rat spinal cord. Brain Res. 1987;436:173–176. doi: 10.1016/0006-8993(87)91572-1. [DOI] [PubMed] [Google Scholar]
- 18.Marlier L, Teilhac JR, Cerruti C, Privat A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Res. 1991;550:15–23. doi: 10.1016/0006-8993(91)90400-p. [DOI] [PubMed] [Google Scholar]
- 19.Fonseca MI, Ni YG, Dunning DD, Miledi R. Distribution of serotonin 2A, 2C and 3 receptor mRNA in spinal cord and medulla oblongata. Mol Brain Res. 2001;89:11–19. doi: 10.1016/s0169-328x(01)00049-3. [DOI] [PubMed] [Google Scholar]
- 20.Wei F, Dubner R, Ren K, Wei F, Dubner R, Ren K. Nucleus reticularis gigantocellularis and nucleus raphe magnus in the brain stem exert opposite effects on behavioral hyperalgesia and spinal Fos protein expression after peripheral inflammation. Pain. 1999;80:127–141. doi: 10.1016/s0304-3959(98)00212-7. [DOI] [PubMed] [Google Scholar]
- 21.Hung KC, Wu HE, Mizoguchi H, Leitermann R, Tseng LF. Intrathecal treatment with 6-hydroxydopamine or 5,7-dihydroxytryptamine blocks the antinociception induced by endomorphin-1 and endomorphin-2 given intracerebroventricularly in the mouse. J Pharmacol Sci. 2003;93:299–306. doi: 10.1254/jphs.93.299. [DOI] [PubMed] [Google Scholar]
- 22.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 23.Xu R, Guan X, Wang C. Influence of capsaicin treating sciatic nerve on the pain threshold and the effect of acupuncture analgesia of rats. Acupuncture Res. 1993;18:280–284. [PubMed] [Google Scholar]
- 24.Lao L, Zhang G, Wei F, Berman BM, Ren K. Electroacupuncture attenuates behavioral hyperalgesia and selectively reduces spinal Fos protein expression in rats with persistent inflammation. J Pain. 2001;2:111–117. doi: 10.1054/jpai.2001.19575. [DOI] [PubMed] [Google Scholar]
- 25.Iwa M, Matsushima M, Nakade Y, Pappas TN, Fujimiya M, Takahashi T. Electroacupuncture at ST-36 accelerates colonic motility and transit in freely moving conscious rats. Am J Physiol Gastrointest Liver Physiol. 2006;290:G285–292. doi: 10.1152/ajpgi.00068.2005. [DOI] [PubMed] [Google Scholar]
- 26.Lao L, Hamilton GR, Fu J, Berman BM. Is acupuncture safe? A systematic review of case reports. Altern Ther Health Med. 2003;9:72–83. [PubMed] [Google Scholar]
- 27.MacPherson H, Thomas K, Walters S, Fitter M. A prospective survey of adverse events and treatment reactions following 34,000 consultations with professional acupuncturists. Acupunct Med. 2001;19:93–102. doi: 10.1136/aim.19.2.93. [DOI] [PubMed] [Google Scholar]
- 28.Maeda Y, Lisi TL, Vance CGT, Sluka KA. Release of GABA and activation of GABAA in the spinal cord mediates the effects of TENS in rats. Brain Res. 2007;1136:43–50. doi: 10.1016/j.brainres.2006.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zemlan FP, Murphy AZ, Behbehani MM. 5-HT1A receptors mediate the effect of the bulbospinal serotonin system on spinal dorsal horn nociceptive neurons. Pharmacology. 1994;48:1–10. doi: 10.1159/000139156. [DOI] [PubMed] [Google Scholar]
- 30.Abe K, Kato G, Katafuchi T, Tamae A, Furue H, Yoshimura M. Responses to 5-HT in morphologically identified neurons in the rat substantia gelatinosa in vitro. Neuroscience. 2009;159:316–324. doi: 10.1016/j.neuroscience.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Yin H, Park SA, Han SK, Park SJ. Effects of 5-hydroxytryptamine on substantia gelatinosa neurons of the trigeminal subnucleus caudalis in immature mice. Brain Res. 2011;1368:91–101. doi: 10.1016/j.brainres.2010.10.050. [DOI] [PubMed] [Google Scholar]
- 32.Sharma A, Punhani T, Fone KC. Distribution of the 5-hydroxytryptamine2C receptor protein in adult rat brain and spinal cord determined using a receptor-directed antibody: effect of 5,7-dihydroxytryptamine. Synapse. 1997;27:45–56. doi: 10.1002/(SICI)1098-2396(199709)27:1<45::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 33.Han J-S Acupuncture. neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26:17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhang R-X, Lao L, Wang L, Liu B, Wang X, Ren K, Berman BM. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Res. 2004;1020:12–17. doi: 10.1016/j.brainres.2004.05.067. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Min B-I, Na HS, Park DS. Relieving effects of electroacupuncture on mechanical allodynia in neuropathic pain model of inferior caudal trunk injury in rat: mediation by spinal opioid receptors. Brain Res. 2004;998:230–236. doi: 10.1016/j.brainres.2003.11.045. [DOI] [PubMed] [Google Scholar]
- 36.Kellstein DE, Malseed RT, Goldstein FJ. Opioid-monoamine interactions in spinal antinociception: evidence for serotonin but not norepinephrine reciprocity. Pain. 1988;34:85–92. doi: 10.1016/0304-3959(88)90185-6. [DOI] [PubMed] [Google Scholar]
- 37.Yang SW, Zhang ZH, Wang R, Xie YF, Qiao JT, Dafny N. Norepinephrine and serotonin-induced antinociception are blocked by naloxone with different dosages. Brain Res Bull. 1994;35:113–117. doi: 10.1016/0361-9230(94)90090-6. [DOI] [PubMed] [Google Scholar]