Abstract
Background
Chemokines likely play multiple roles in HIV-1 and SIV pathogenesis. To examine potential associations between chemokine expression levels and apoptosis of cells in lymphoid tissues during SIV infection, we measured chemokine and cytokine mRNA levels in multiple lymphoid tissues compartments from uninfected and SIV-infected cynomolgus macaques (Macaca fascicularis).
Methods
Real-time RT-PCR was used to measure host mRNA levels in macaque lymphoid tissues. Proliferating or apoptotic cells were identified in lymphoid tissues by immunohistochemistry.
Results
We found that CXCL12 and CCL25 mRNAs in SIV-infected lymphoid tissues were decreased and their levels were negatively correlated with the numbers of proliferating and apoptotic cells. In vitro analyses revealed that CXCL12 and CCL25 were capable of reducing apoptosis induced by SIV infection.
Conclusions
These findings suggest that increased apoptosis in lymphoid tissues due to reduced levels of anti-apoptotic chemokines might be a mechanism that contributes to loss of immune function following pathogenic SIV infection.
Keywords: activated caspase-3, apoptosis, chemokine, SIV
Introduction
Chemokines are small chemoattractant cytokines involved in normal and pathological immune processes [25]. Increasing evidence indicates that chemokines also have other diverse functions such as lymphoid organ development, T-lymphocyte maturation, and inhibition of viral replication, in addition to their role in cellular trafficking [4, 25]. A potentially important chemokine function is the recently identified anti-apoptotic activity of CCL25 and CXCL12, which are CCR9 and CXCR4 ligands, respectively. For example, CXCL12 can enhance survival of murine embryonic stem cells [11] and promote CD4+ T cell survival by post-translational inactivation of the cell death machinery and increased transcription of cell survival-related genes [29]. Similarly, CCL25 does not induce apoptosis of malignant T cells and prevents malignant T cells from undergoing chemotherapy-induced apoptosis [23]. In addition, CCL21 has been found to act as an anti-apoptotic factor for mesangial cells possibly by affecting activation of caspase-3 [1]. In contrast, the type 1 chemokine CXCL10 induces cell death in neurons [28]. These studies indicate that chemokines can have disparate apoptotic and anti-apoptotic effects on cells.
Because simian immunodeficiency virus (SIV) infection can lead to increased apoptosis in macaques [6, 9, 18, 31], we hypothesized that SIV infections in nonhuman primate model systems might broadly alter chemokine networks in lymph node tissues in ways that contribute to SIV-associated cell death. To address this issue, we used the SIV-infected cynomolgus macaque (Macaca fascicularis) model to investigate the relationships between the apoptosis of lymph node cells and chemokine expression. In this study, we found that CXCL12, CCL25 and homeostatic lymphoid chemokine CCL21 mRNAs were decreased after SIV infection, whereas the numbers of proliferating and apoptotic cells were increased in lymphoid tissues during acute infection and AIDS. Correlation analyses revealed that CXCL12 and CCL25 mRNA levels were negatively correlated with the numbers of proliferating and apoptotic cells in these important tissue compartments. In vitro analyses revealed that CXCL12 and CCL25 can reduce apoptosis induced by SIV infection. Taken together, these findings expand our understanding of the effects of SIV infection on lymphoid tissue environments and define a viral pathogenic mechanism contributing to increased apoptosis and potentially to T cell loss in these tissues.
Materials and methods
Animals and tissues
These studies were performed under the approval and guidance of the University of Pittsburgh Institutional Animal Care and Use Committee. They included 12 cynomolgus macaques (M. fascicularis) infected intra-rectally with the pathogenic SIV/DeltaB670 isolate and sacrificed at different times after infection. Of these animals, six were sacrificed during acute infection (2 weeks post-infection [PI]), five were sacrificed upon progression to AIDS, and five served as uninfected controls. In the macaques with acute SIV infection or AIDS, plasma SIV viral loads ranged from 3.6 × 105 to 3.8 × 108 copies/ml. Among animals with AIDS, in addition to weight loss and CD4+ T-lymphocyte loss, one animal had watery diarrhoea and one animal harboured widespread infection with P. carinii in the lungs. Details regarding tissue processing and fixation have been described [22].
RNA isolation and real-time RT-PCR
Total RNAs from axillary, mesenteric and hilar LNs were isolated, treated with DNase (Ambion, Austin, TX, USA) and further purified with RNeasy columns (Qiagen, Germantown, MD, USA) as described [27]. Four hundred ng of RNA from each specimen was reverse transcribed with reverse transcriptase-negative controls included in parallel for each RNA sample. Primers and probes used for real-time RT-PCR were either purchased (Applied Biosystem, Foster City, CA, USA) or were designed using the Primer Express (Applied Biosystems) software package. Real-time RT-PCR was used to measure relative mRNA expression levels by the comparative threshold cycle (Ct) method of relative quantitation. The Ct values for each gene were normalized to the endogenous control mRNA β-glucuronidase and then to an uninfected LN calibrator sample. Quantitation of tissue-associated SIV viral loads was performed as described previously [27].
In situ hybridization (ISH) and immunohistochemistry (IHC)
ISHs for cellular RNAs were performed as described [24]. Autoradiographic exposure times, following ISH with antisense and control sense 35S-labelled riboprobes specific for CCL25 and CXCL12 RNAs were 7–10 days. IHC for activated caspase-3 and Ki67 were performed with rabbit polyclonal sera (R&D Systems, Minneapolis, MN, USA and Vector Laboratories Inc., Burlington, CA, USA respectively). IHC was performed on cryosections that were post-fixed in 4% paraformaldehyhde (PF)/PBS, washed in absolute ethanol, and microwaved in 0.01 m sodium citrate (pH 6.0) or Retrievagen B buffer (pH 9.5) for a total of 10 minutes in 2 minute increments. Tissue sections were incubated with primary antibodies for 1 hr at room temperature and the primary antibodies were detected using the SuperPicTure Kit (Zymed Laboratories Inc., South San Francisco, CA), according to the manufacturer’s recommendations. Counterstaining was performed with hematoxylin (Fisher Chemicals, Fairlawn, NJ). Combined ISH/IHC was performed with a rabbit anti-CD3 polyclonal antibody (Dako) or murine monoclonal antibodies specific for CD68 (clone KP1, Dako, Carpenteria, CA) or CD209/DC-SIGN (clone DCN46, BD Pharmingen, San Jose, CA) in tissue sections and subjected to IHC immediately following ISH, with subsequent autoradiographic exposure times of 5–7 days. The numbers of activated caspase-3+ cells or Ki67+ cells were determined by counting total cells and caspase-3+ cells or Ki67+ cells in five high powered microscopic fields (×600).
Anti-apoptotic activity of CXCL12 and CCL25 on CEMx174 cells infected with SIV
The CEMx174 cell line was cultured in 24 well-plates (1 × 105 per well) with SIV/DeltaB670 (5 × 103 TCID50/ml) alone and with CXCL12 (0.1 µg/ml) or CCL25 (0.5 µg/ml; Peprotech Inc., Rocky Hill, NJ) for 96 hours at 37°C/5% CO2. Fresh CXCL12 or CCL25 were added to the cultures at the same concentration every 24 hours. After 96 hours of culture, the cells were harvested and spotted onto slides. The cells were fixed with 4% PF/PBS, dehydrated in graded ethanols, and then stained for activated caspase-3 by immunocytochemistry.
Statistical analyses
All statistical analyses were performed using the Minitab software package (State College, PA). Real-time RT-PCR and IHC data were analyzed using the two sample t-test to compare differences between disease states and Pearson’s correlation analyses were used to measure associations between relative mRNA expression levels and the numbers of apoptotic cells or proliferation cells. A P-value of <0.05 was considered significant.
Results
SIV infection leads to downregulation of CXCL12 and CCL25 mRNAs in macaque lymph nodes
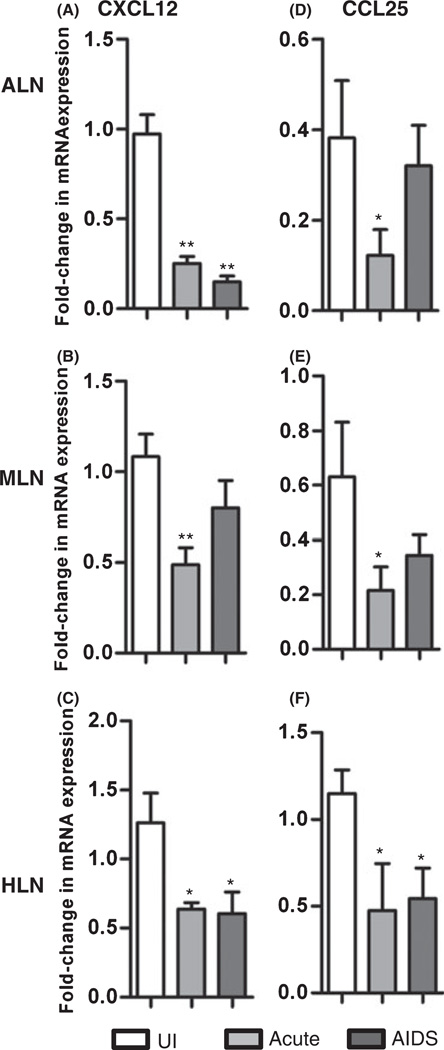
To measure the effects of SIV infection on chemokine expression profiles in lymph node tissues, we used a cynomolgus macaque (M. fascicularis) model and examined the tissues at different stages after intrarectal infection with the pathogenic SIV/DeltaB670 isolate. Lymph nodes (LNs), including axillary LNs (ALNs), mesenteric LNs (MLNs) and hilar LNs (HLNs) were examined from acutely infected (2 weeks PI) and AIDS-developing animals, as well as uninfected controls. We performed real-time RT-PCR to detect 18 different chemokine mRNAs and found that CXCL12 and CCL25 mRNA levels were reduced in SIV-infected LNs relative to uninfected controls (Fig. 1). These differences were statistically significant in all instances between the group of acutely SIV-infected animals and uninfected controls (Fig. 1). During AIDS, CXCL12 mRNA was significantly decreased in ALN and HLN, whereas CCL25 was significantly decreased only in HLN (Fig. 1). Previous analyses of type 2 chemokines CCL17 and CCL22 and homeostatic chemokine CCL21 mRNAs have revealed that they are also decreased following SIV-infection in LN tissues [22]. In contrast, expression of type 1 chemokine mRNAs (CXCL9, CXCL10 and CXCL11) were increased in animals with acute SIV infection or developing AIDS compared to uninfected macaques [22]. Correlation analyses revealed that CXCL12 and CCL25 mRNA levels were positively correlated with the levels of TGF-β, regulatory T cell (Treg) marker FOXP3, type 2 chemokines CCL17 and CCL22, and homeostatic chemokine CCL21, but were negatively correlated with the levels of IFN-γ, TNF-α, type 1 chemokines CXCL9-11 and CCL4-5, and local SIV viral loads (Table 1). These findings indicate that SIV infection leads to downregulation of CXCL12 and CCL25 mRNAs in macaque lymph nodes, simultaneous with increased SIV replication and alterations in other chemokine expression levels.
Fig. 1.
Decreased expression of CXCL12 and CCL25 mRNAs in LNs during SIV infection. Real-time RT-PCR was used to measure CXCL12 and CCL25 mRNA expression in LNs from uninfected macaques or macaques in the acute or AIDS stages of infection (mean ± SEM). (A–C) CXCL12 mRNA levels in ALN, MLN and HLN, respectively; (D–F) CCL25 mRNA levels in ALN, MLN and HLN, respectively; *P < 0.05 and **P < 0.01, compared with uninfected controls.
Table 1.
Correlation analyses between CXCL12 and CCL25 mRNA levels, activated caspase-3+ cell Ki67+ cell numbers, and other parameters in macaque axillary LNs
| Dependent variable | Comparison parameter |
Pearson’s Correlation coefficient |
P-value1 |
|---|---|---|---|
| CXCL12 mRNA levels2 | CCL21 | 0.711 | 0.001 |
| FOXP3 | 0.646 | 0.005 | |
| TGF-β1 | 0.603 | 0.010 | |
| CCL22 | 0.556 | 0.020 | |
| CCL17 | 0.549 | 0.022 | |
| SIV | −0.844 | <0.001 | |
| TNF-α | −0.796 | <0.001 | |
| Ki67+ cells/mm2 | −0.766 | 0.004 | |
| Activated caspase-3+ cells/mm2 | −0.756 | 0.004 | |
| CXCL9 | −0.643 | 0.005 | |
| IFN-γ | −0.614 | 0.009 | |
| CXCL11 | −0.593 | 0.012 | |
| CXCL10 | −0.549 | 0.005 | |
| CCL25 mRNA levels2 | CCL17 | 0.815 | <0.001 |
| CCL22 | 0.773 | <0.001 | |
| IL-10 | 0.724 | 0.001 | |
| FOXP3 | 0.695 | 0.002 | |
| TGF-β1 | 0.619 | 0.008 | |
| CCL21 | 0.563 | 0.023 | |
| CCL4 | −0.796 | <0.001 | |
| Activated caspase-3+ cells/mm2 | −0.732 | 0.025 | |
| IFN-γ | −0.662 | 0.005 | |
| CCL5 | −0.643 | 0.005 | |
| SIV | −0.552 | 0.027 | |
| Activated | SIV | 0.912 | <0.001 |
| caspase-3+ cells/(mm2)3 | IFN-γ | 0.908 | 0.001 |
| CXCL11 | 0.823 | 0.001 | |
| CXCL10 | 0.776 | 0.003 | |
| CXCL9 | 0.687 | 0.014 | |
| Ki67+ cells/mm2 | 0.606 | 0.037 | |
| TNF-α | 0.582 | 0.047 | |
| CCL21 | −0.835 | 0.001 | |
| FOXP3 | −0.795 | 0.002 | |
| CCL22 | −0.794 | 0.022 | |
| IL-2 | −0.788 | 0.002 | |
| TGF-β1 | −0.785 | 0.003 | |
| CXCL12 | −0.756 | 0.004 | |
| CCL25 | −0.732 | 0.025 | |
| Ki67+ cells/(mm2)3 | TNF-α | 0.716 | 0.009 |
| CCL5 | 0.701 | 0.011 | |
| SIV | 0.680 | 0.015 | |
| IFN-γ | 0.628 | 0.029 | |
| Activated caspase-3+ cells/mm2 | 0.606 | 0.037 | |
| CCL21 | −0.836 | 0.001 | |
| CXCL12 | −0.766 | 0.004 | |
| FOXP3 | −0.722 | 0.008 | |
| CCL22 | −0.649 | 0.022 | |
| TGF-β1 | −0.629 | 0.029 | |
| IL-2 | −0.623 | 0.031 |
All pairwise comparisons for which P < 0.05 are shown.
Measured using real-time RT-PCR.
Measured using IHC.
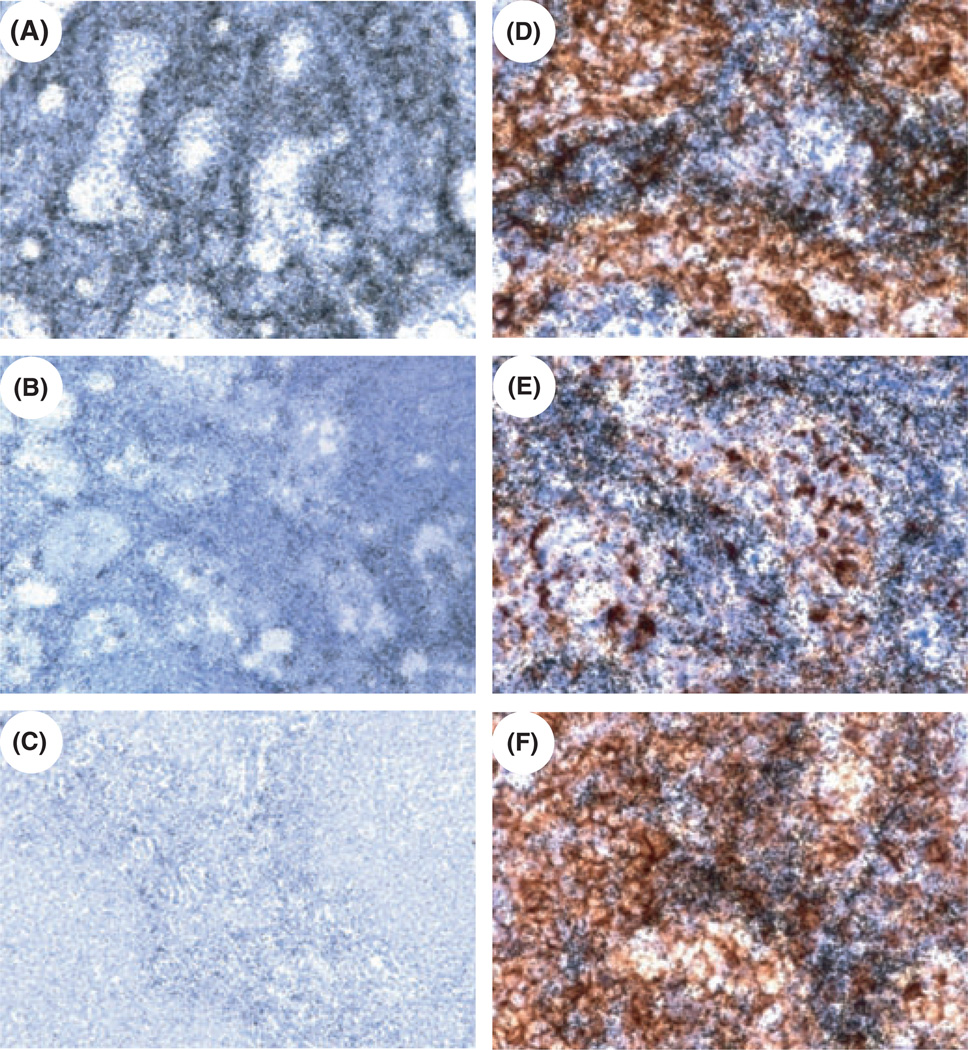
The localization of cells expressing CXCL12 mRNA was examined in ALN tissue sections using in situ hybridization (ISH). Expression of CXCL12 mRNA was highest in uninfected macaques, and was lower in SIV-infected macaques, especially in animals with AIDS (Fig. 2A–C), consistent with the real-time RT-PCR data. To identify the cellular sources of CXCL12 in ALN we combined IHC for cellular markers with ISH for CXCL12 mRNA. The CXCL12 ISH signal co-localized with CD209+ dendritic cells (DC) or macrophages, CD68+ macrophages, and CD3+ T-lymphocytes (Fig. 2D–F), with only limited co-localization with CD20+ B cells (not shown). This mRNA distribution pattern in LN tissues suggested that multiple cell types are producing CXCL12, although the focal yet somewhat diffuse signal for CXCL12 mRNA limited the ability to fully define the cells within the dense network that expressed the mRNA. Unexpectedly, we were unable to detect CCL25 mRNA in LN tissues by ISH, despite readily detecting it in macaque jejunal epithelium (not shown). In summary, these findings indicate that among the changes in lymphoid tissues caused by SIV infection there is decreased CXCL12 and CCL25 mRNA expression.
Fig. 2.
CXCL12 mRNA expression in ALN tissues of cynomolgus macaques during SIV infection. (A–C) ISH was performed for CXCL12 mRNA in axillary LN tissue sections from uninfected (A), acutely SIV-infected (B) and AIDS-developing macaques (C). (D–F) ISH and immunohistochemistry staining were performed simultaneously to detect the cellular sources of CXCL12 in axillary LN tissue sections; (D) CXCL12 ISH and DC-SIGN IHC; (E) CXCL12 ISH and CD68 IHC; (F) CXCL12 ISH and CD3 IHC. Parallel hybridization of tissue sections with the cognate sense control probe provided no specific ISH signal and parallel staining of tissue sections with the cognate isotype control provided no specific IHC signal (not shown). Original magnifications, ×200 (A–C) and ×600 (D–F).
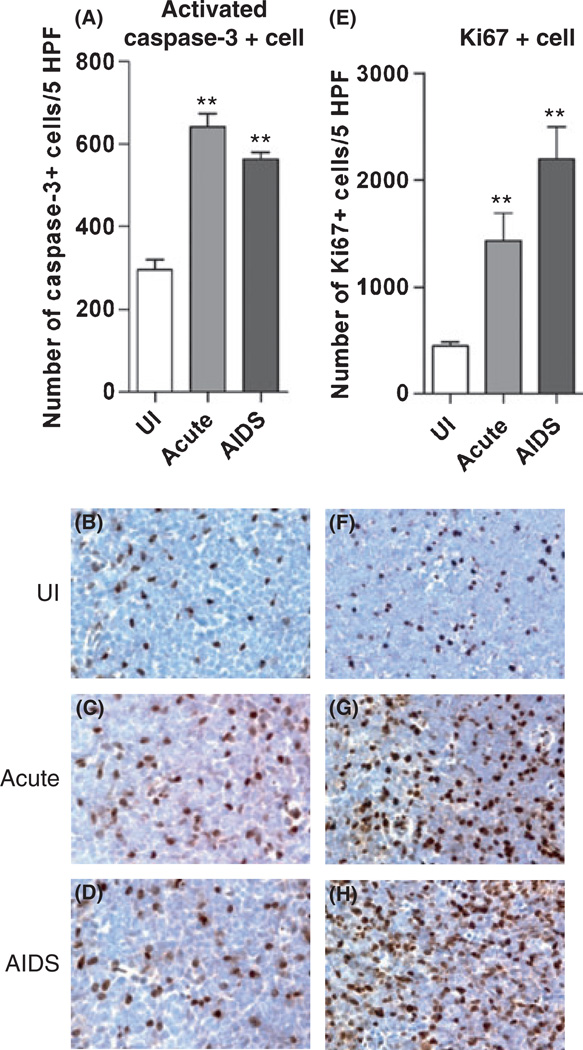
Increased apoptosis in lymph nodes during SIV infection
To measure the effects of pathogenic SIV infection on apoptosis in these LNs, we performed IHC for activated caspase-3. The numbers of activated caspase-3+ cells increased 2-fold in SIV-infected ALNs compared to uninfected controls (Fig. 3A–D). There were similar increases in the levels of activated caspase-3+ cells in MLNs and HLNs in the same animals (not shown). Correlation analyses revealed that the numbers of apoptotic cells in ALN sections were negatively correlated with CXCL12, CCL25, IL-2, TGF- β1, FOXP3, type 2 chemokines CCL22 and homeostatic chemokine CCL21 mRNA levels, but positively correlated with local SIV viral loads, IFN-γ, TNF-α, CXCL9-11 mRNA levels and the numbers of Ki67+ cells (Table 1). These findings indicate that the apoptosis of cells in macaque LNs during SIV infection is associated with multiple changes in the expression of chemokines (CXCR3 ligands, CXCL12 and CCL25) and cytokines (TGF-β1, IFN-γ and TNF-α), and with the levels of local SIV replication.
Fig. 3.
SIV infection leads to increases in levels of apoptosis and proliferation in ALN tissues. Immunohistochemistry was performed to detect (B–D) and quantitate (A) activated caspase-3+ cells and to detect (F–H) and quantitate (E) Ki67+ cells in ALN tissue sections. Parallel staining of tissue sections with the cognate isotype control provided no specific IHC positive reaction (data not shown). The mean (±SEM) values are shown from analyses performed on cells from four different animals in each disease stage group. Original magnifications, ×600 (B–D), ×400 (F–H). **P < 0.01.
Increased cellular proliferation in lymph nodes during SIV infection
One hallmark of HIV-1 infection is immune activation [12]. To examine the extent of cellular proliferation and activation in the context of changing chemokine levels and the relationship between the extent of LN apoptosis and the level of immune activation [13], we performed IHC for detection of Ki67+ cells in LN tissues. The proportions of total cells that were Ki67+ significantly increased 3- to 5-fold after SIV infection (Fig. 3E–H). Consistent with these findings, we previously reported that the immunofluorescence detection and enumeration of CD3+ cells also positive for Ki67 in macaque LNs revealed that the percentage of CD3+/Ki67+ cells increased during SIV infection [7, 22]. Correlation analyses revealed that the numbers of Ki67+ cells in LN sections were positively correlated with the numbers of apoptotic cells, local SIV viral loads, IFN-γ, TNF-α, type 1 chemokine, and CCL5 mRNA levels, but negatively correlated with IL-2, TGF-β1, FOXP3, CXCL12, type 2 chemokines CCL17 and CCL22, and homeostatic chemokine CCL21 mRNA levels (Table 1). These data indicated that local cellular activation levels increased during SIV infection and were associated with sustained viral replication and increased cell death. These changes were associated with decreased CXCL12 and CCL25 expression, implicating their loss in the pathogenesis of SIV.
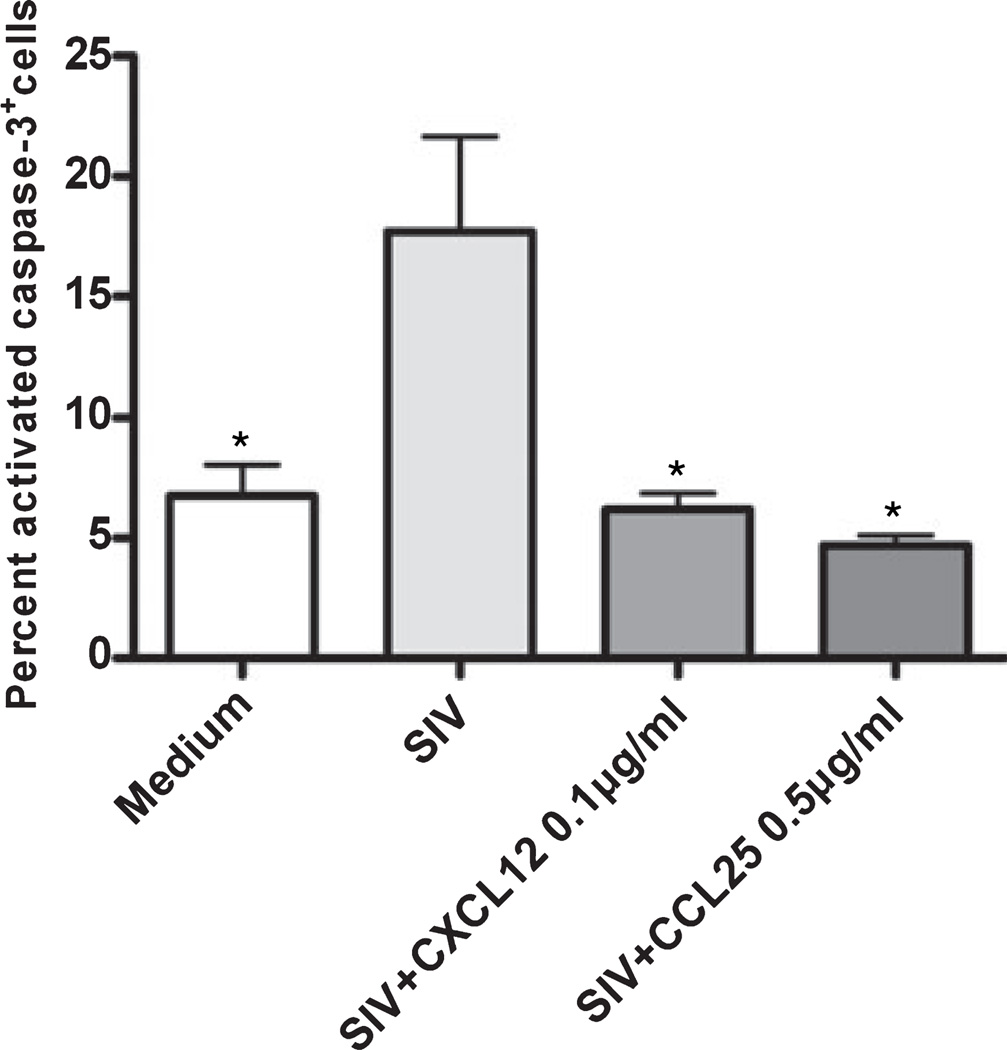
CXCL12 and CCL25 reduce CEMx174 cell apoptosis induced by SIV in vitro
To examine further the association between loss of CXCL12 or CCL25 expression and SIV-associated apoptosis, we infected CEMx174 cells with SIV to induce apoptosis and then treated with CXCL12 or CCL25 to measure the anti-apoptotic activities of these chemokines. Using real-time RT-PCR to profile chemokine receptor expression in CEMx174 cells, we found that these cells express chemokine receptors CXCR4 and CCR9, the receptors for CXCL12 and CCL25, respectively, as well as CCR5, the major co-receptor for SIV entry. Infected CEMx174 cells cultured with or without CXCL12 and CCL25 were stained for activated caspase-3 using immunocytochemistry. The results revealed that SIV-induced CEMx174 cell apoptosis was abrogated by co-incubation with CXCL12 or CCL25 (Fig. 4). These data indicated that CXCL12 and CCL25 have anti-apoptotic effects on cultures containing SIV-infected cells.
Fig. 4.
CXCL12 and CCL25 reduce CEMx174 apoptosis induced by SIV. The CEMx174 cell line was infected with SIV/DeltaB670 for 4 days either with or without co-incubation with CXCL12 or CCL25. Immunocytochemical staining was performed to detect and quantitate activated caspase-3+ cells in cell populations fixed onto slides. Parallel staining of cells with the cognate isotype control provided no specific signal. The mean (± SEM) values are shown from analyses performed on cells from three independent experiments. *P < 0.05, compared to untreated, SIV-infected cells.
Discussion
Lymphoid tissues are critical locations for the generation of cellular and humoral immune responses, and chemokines are major regulators of the cell trafficking necessary for such responses. Using the well-established SIV/macaque model, we have further defined the infection-associated changes to the chemokine networks in this immune compartment as well as the association of these changes with activation and apoptosis of cells during the course of SIV infection. We found that CXCL12 and CCL25 mRNAs were down-regulated coincident with increased proportions of apoptotic and proliferating cells in LNs during SIV infection. Mechanistically, in vitro analyses revealed that CXCL12 and CCL25 were capable of reducing apoptosis induced by SIV infection. These findings suggest that the increased apoptosis that we and others [6, 9, 13, 19] have observed in lymphoid tissues following SIV infection could result, in part, from decreased expression of anti-apoptotic chemokines.
There is a growing body of evidence that chemokines can have anti- or pro-apoptotic functions on different cell types. Chemokines that have exhibited anti-apoptotic function include CCL25 [23, 33, 34], CXCL12 [17], CCL21 and CCL19 [3, 26, 32], CXCL13[3], and CCL5 [10]. In contrast, chemokines that have exhibited proapoptotic function include CXCL12 [5], CXCL9 [32], and CXCL10 [28, 32]. The data presented here have revealed that CXCL12 and CCL25 are decreased in expression in LNs during pathogenic SIV infection, and that they can have protective effects on model cells undergoing SIV-induced apoptosis. In addition, we have found that CCL21 expression was decreased in LNs (Table 1 and [22]) and negatively correlated with the numbers of apoptotic cells and activated cells following SIV infection. CCL21 is a CCR7 ligand involved in homeostatic trafficking of lymphocytes and DCs during immune surveillance that also has anti-apoptotic activity on mesangial cells [1], DCs [26], and T-lymphocytes [14]. Interestingly, chemokines CXCL9 and CXCL10, which are consistently upregulated in LNs during SIV infection [22, 24], are potentially pro-apoptotic [28]. Collectively, these changes in chemokine expression could create an environment that not only leads to altered homing of immune cells to lymphoid tissues, but also leads to increased apoptosis and immune cell loss. Although the induction of the inflammatory chemokines CXCL9 and CXCL10 are closely linked with induction of IFN-γ [24], the mechanisms responsible for decreased CXCL12 and CCL25 expression are not yet known.
CXCL12, also known as stromal cell-derived factor (SDF-1), is a highly conserved ligand for the CXCR4 receptor [16], a major co-receptor for HIV-1 viral entry [8], and has important roles in hematopoesis and cell trafficking in the lymphoreticular system. CCL25, also known as thymus expressed chemokine (TECK [30]), is expressed in thymus and in the small intestinal epithelium, contributing to the CCR9-dependent trafficking of mucosal homing immune cells to intestinal compartments [2]. The potentially different roles played by these chemokines in immunologic and pathologic events in lymphoid tissues during health and SIV-associated disease are not clear. First, most SIVs use CCR5 as an entry co-receptor, so CXCL12 expression levels would not be expected to have major effects on virus entry. In addition, CCL25 expression has been thought to be reasonably restricted to thymus and small intestine, although using standard and real-time RT-PCR we were able to detect CCL25 mRNA in macaque lymphoid tissues. To be certain these PCRs were amplifying CCL25 the products were cloned and sequenced and determined to be CCL25 (not shown). It should be noted, however, that by ISH we were unable to detect CCL25 mRNA, which must be expressed below the limits of sensitivity of the assay. Further analyses are required to better understand the function of CCL25 expression in lymphoid tissues as well as the tissue distribution of CCL25 expression, in particular, but also CXCL12 expression.
A major hallmark of pathogenic HIV-1 or SIV infection is immune activation [6, 12, 20], and there is evidence that this increased activation, as well as increased apoptosis, contribute to the eventual development of immunodeficiency following pathogenic SIV infection [15, 21]. Previously, we have proposed that chemokines contribute through multiple mechanisms to the loss of immunosuppressive regulatory Treg and therefore to increased immune activation [22]. In the macaque LNs studied here, we found that the proportions of presumably proliferating Ki67+ cells were increased in SIV-infected LNs and positively correlated with the numbers of apoptotic cells. In addition, the numbers of Treg (FOXP3+ cells) were decreased [22] and negatively correlated with the number of apoptotic cells and proliferating cells (Table 1).
Therefore, these findings expand our understanding of the effects of SIV infection on lymphoid tissue environments and suggest that changes in chemokine expression might contribute to increased apoptosis in these tissues. The data presented here on the reduction in CXCL12 and CCL25 expression in lymphoid tissues, coupled with previous findings on CXCR3 ligand induction and CCR4 ligand loss, suggest that chemokines can affect local apoptosis in multiple ways. These mechanisms include reduced protection from apoptosis through loss of anti-apoptotic chemokines, stimulation of apoptosis through induction of pro-apoptotic chemokines, and/or altered recruitment of immunosuppressive cells that in turn control the local levels of activation and apoptosis. Modulation of chemokine/chemokine receptor function(s) in HIV-1 infected individuals might represent a therapeutic approach that targets multiple functional activities of chemokines that collectively could improve overall immunologic function.
Acknowledgments
This work was supported by NIH PHS grant AI060422 (TAR).
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
References
- 1.Banas B, Wornle M, Berger T, Nelson PJ, Cohen CD, Kretzler M, Pfirstinger J, Mack M, Lipp M, Grone HJ, Schlondorff D. Roles of SLC/CCL21 and CCR7 in human kidney for mesangial proliferation, migration, apoptosis, and tissue homeostasis. J Immunol. 2002;168:4301–4307. doi: 10.4049/jimmunol.168.9.4301. [DOI] [PubMed] [Google Scholar]
- 2.Campbell DJ, Butcher EC. Intestinal attraction: CCL25 functions in effector lymphocyte recruitment to the small intestine. J Clin Invest. 2002;110:1079–1081. doi: 10.1172/JCI16946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chunsong H, Yuling H, Li W, Jie X, Gang Z, Qiuping Z, Qingping G, Kejian Z, Li Q, Chang AE, Youxin J, Jinquan T. CXC chemokine ligand 13 and CC chemokine ligand 19 cooperatively render resistance to apoptosis in B cell lineage acute and chronic lymphocytic leukemia CD23+CD5+ B cells. J Immunol. 2006;177:6713–6722. doi: 10.4049/jimmunol.177.10.6713. [DOI] [PubMed] [Google Scholar]
- 4.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 5.Colamussi ML, Secchiero P, Gonelli A, Marchisio M, Zauli G, Capitani S. Stromal derived factor-1 alpha (SDF-1 alpha) induces CD4+ T cell apoptosis via the functional up-regulation of the Fas (CD95)/Fas ligand (CD95L) pathway. J Leukoc Biol. 2001;69:263–270. [PubMed] [Google Scholar]
- 6.Cumont MC, Diop O, Vaslin B, Elbim C, Viollet L, Monceaux V, Lay S, Silvestri G, Le Grand R, Muller- Trutwin M, Hurtrel B, Estaquier J. Early divergence in lymphoid tissue apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J Virol. 2008;82:1175–1184. doi: 10.1128/JVI.00450-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fallert BA, Poveda S, Schaefer TM, Pfeifer ME, Sanghavi SK, Watkins SC, Murphey-Corb MA, Tarwater PM, Kirschner DE, Reinhart TA. Virologic and immunologic events in hilar lymph nodes during simian immunodeficiency virus infection: development of polarized inflammation. J Acquir Immune Defic Syndr. 2008;47:16–26. doi: 10.1097/QAI.0b013e31815cea8b. [DOI] [PubMed] [Google Scholar]
- 8.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seventransmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 9.Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 10.Grayson MH, Holtzman MJ. Chemokine signaling regulates apoptosis as well as immune cell traffic in host defense. Cell Cycle. 2006;5:380–383. doi: 10.4161/cc.5.4.2427. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, Hangoc G, Bian HM, Pelus LM, Broxmeyer HE. SDF-1/CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells. 2005;23:1324–1332. doi: 10.1634/stemcells.2005-0085. [DOI] [PubMed] [Google Scholar]
- 12.Hazenberg MD, Otto SA, van Benthem BHB, Roos MTL, Coutinho RA, Lange JMA, Hamann D, Prins M, Miedema F. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. Aids. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 13.Hurtrel B, Petit F, Arnoult D, Muller-Trutwin M, Silvestri G, Estaquier J. Apoptosis in SIV infection. Cell Death Differ. 2005;12(Suppl. 1):979–990. doi: 10.1038/sj.cdd.4401600. [DOI] [PubMed] [Google Scholar]
- 14.Kim JW, Ferris RL, Whiteside TL. Chemokine C receptor 7 expression and protection of circulating CD8+ T lymphocytes from apoptosis. Clin Cancer Res. 2005;11:7901–7910. doi: 10.1158/1078-0432.CCR-05-1346. [DOI] [PubMed] [Google Scholar]
- 15.Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, Poaty-Mavoungou V, Rouquet P, Estaquier J, Mortara L, Desoutter JF, Butor C, Le Grand R, Roques P, Simon F, Barre-Sinoussi F, Diop OM, Muller-Trutwin MC. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115:1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 17.Lataillade JJ, Clay D, Bourin P, Herodin F, Dupuy C, Jasmin C, Le Bousse-Kerdiles MC. Stromal cell-derived factor 1 regulates primitive hematopoiesis by suppressing apoptosis and by promoting G(0)/G(1) transition in CD34(+) cells: evidence for an autocrine/paracrine mechanism. Blood. 2002;99:1117–1129. doi: 10.1182/blood.v99.4.1117. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Estes JD, Duan L, Jessurun J, Pambuccian S, Forster C, Wietgrefe S, Zupancic M, Schacker T, Reilly C, Carlis JV, Haase AT. Simian immunodeficiency virus-induced intestinal cell apoptosis is the underlying mechanism of the regenerative enteropathy of early infection. J Infect Dis. 2008;197:420–429. doi: 10.1086/525046. [DOI] [PubMed] [Google Scholar]
- 19.Monceaux V, Estaquier J, Fevrier M, Cumont MC, Riviere Y, Aubertin AM, Ameisen JC, Hurtrel B. Extensive apoptosis in lymphoid organs during primary SIV infection predicts rapid progression towards AIDS. Aids. 2003;17:1585–1596. doi: 10.1097/00002030-200307250-00002. [DOI] [PubMed] [Google Scholar]
- 20.Muro-Cacho CA, Pantaleo G, Fauci AS. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J Immunol. 1995;154:5555–5566. [PubMed] [Google Scholar]
- 21.Pandrea I, Sodora DL, Silvestri G, Apetrei C. Into the wild: simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 2008;29:419–428. doi: 10.1016/j.it.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin S, Sui Y, Soloff AC, Junecko BA, Kirschner DE, Murphey-Corb MA, Watkins SC, Tarwater PM, Pease JE, Barratt-Boyes SM, Reinhart TA. Chemokine and cytokine mediated loss of regulatory T cells in lymph nodes during pathogenic simian immunodeficiency virus infection. J Immunol. 2008;180:5530–5536. doi: 10.4049/jimmunol.180.8.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiuping Z, Jei X, Youxin J, Wei J, Chun L, Jin W, Qun W, Yan L, Chunsong H, Mingzhen Y, Qingping G, Kejian Z, Zhimin S, Qun L, Junyan L, Jinquan T. CC chemokine ligand 25 enhances resistance to apoptosis in CD4+ T cells from patients with T-cell lineage acute and chronic lymphocytic leukemia by means of livin activation. Cancer Res. 2004;64:7579–7587. doi: 10.1158/0008-5472.CAN-04-0641. [DOI] [PubMed] [Google Scholar]
- 24.Reinhart TA, Fallert BA, Pfeifer ME, Sanghavi S, Capuano S, III, Rajakumar P, Murphey-Corb M, Day R, Fuller CL, Schaefer TM. Increased expression of the inflammatory chemokine CXC chemokine ligand 9/ monokine induced by interferon-gamma in lymphoid tissues of rhesus macaques during simian immunode .ciency virus infection and acquired immunodeficiency syndrome. Blood. 2002;99:3119–3128. doi: 10.1182/blood.v99.9.3119. [DOI] [PubMed] [Google Scholar]
- 25.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Sanchez N, Riol-Blanco L, de la RG, Puig-Kroger A, Garcia-Bordas J, Martin D, Longo N, Cuadrado A, Cabanas C, Corbi AL, Sanchez-Mateos P, Rodriguez-Fernandez JL. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood. 2004;104:619–625. doi: 10.1182/blood-2003-11-3943. [DOI] [PubMed] [Google Scholar]
- 27.Sanghavi SK, Reinhart TA. Increased expression of TLR3 in lymph nodes during simian immunodeficiency virus infection: implications for inflammation and immunodeficiency. J Immunol. 2005;175:5314–5323. doi: 10.4049/jimmunol.175.8.5314. [DOI] [PubMed] [Google Scholar]
- 28.Sui Y, Potula R, Dhillon N, Pinson D, Li S, Nath A, Anderson C, Turchan J, Kolson D, Narayan O, Buch S. Neuronal apoptosis is mediated by CXCL10 overexpression in simian human immunodeficiency virus encephalitis. Am J Pathol. 2004;164:1557–1566. doi: 10.1016/S0002-9440(10)63714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki Y, Rahman M, Mitsuya H. Diverse transcriptional response of CD4(+) T cells to stromal cellderived factor (SDF)-1: cell survival promotion and priming effects of SDF-1 on CD4(+) T cells. J Immunol. 2001;167:3064–3073. doi: 10.4049/jimmunol.167.6.3064. [DOI] [PubMed] [Google Scholar]
- 30.Vicari AP, Figueroa DJ, Hedrick JA, Foster JS, Singh KP, Menon S, Copeland NG, Gilbert DJ, Jenkins NA, Bacon KB, Zlotnik A. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity. 1997;7:291–301. doi: 10.1016/s1074-7613(00)80531-2. [DOI] [PubMed] [Google Scholar]
- 31.Viollet L, Monceaux V, Petit F, Ho Tsong FR, Cumont MC, Hurtrel B, Estaquier J. Death of CD4+ T cells from lymph nodes during primary SIVmac251 infection predicts the rate of AIDS progression. J Immunol. 2006;177:6685–6694. doi: 10.4049/jimmunol.177.10.6685. [DOI] [PubMed] [Google Scholar]
- 32.Wornle M, Schmid H, Merkle M, Banas B. Effects of chemokines on proliferation and apoptosis of human mesangial cells. BMC Nephrol. 2004;5:8. doi: 10.1186/1471-2369-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youn BS, Kim YJ, Mantel C, Yu KY, Broxmeyer HE. Blocking of c-FLIP(L)–independent cycloheximideinduced apoptosis or Fas-mediated apoptosis by the CC chemokine receptor 9/TECK interaction. Blood. 2001;98:925–933. doi: 10.1182/blood.v98.4.925. [DOI] [PubMed] [Google Scholar]
- 34.Youn BS, Yu KY, Oh J, Lee J, Lee TH, Broxmeyer HE. Role of the CC chemokine receptor 9/TECK interaction in apoptosis. Apoptosis. 2002;7:271–276. doi: 10.1023/a:1015320321511. [DOI] [PubMed] [Google Scholar]