Abstract
The period of in utero development is one of the most critical windows during which adverse intrauterine conditions and exposures may influence the growth and development of the fetus as well as its future postnatal health and behavior. Maternal cigarette smoking during pregnancy remains a relatively common but nonetheless hazardous in utero exposure. Previous studies have associated prenatal smoke exposure with reduced birth weight, poor developmental and psychological outcomes, and increased risk for diseases and behavioral disorders later in life. Researchers are now learning that many of the mechanisms whereby maternal smoke exposure may affect key pathways crucial for proper fetal growth and development are epigenetic in nature. Maternal cigarette smoking during pregnancy has been associated with altered DNA methylation and dysregulated expression of microRNA, but a deeper understanding of the epigenetics of maternal cigarette smoking during pregnancy as well as how these epigenetic changes may affect later offspring health and behavior remain to be elucidated. This review seeks to explore many of the previously described epigenetic alterations associated with maternal cigarette smoking during pregnancy and assesses how such changes may have consequences for both fetal growth and development, as well as later child health, behavior and well-being. We also outline future directions for this new and exciting field of research.
“Developmental psychopathology is an evolving scientific discipline whose predominant focus is elucidating the interplay among the biological, psychological, and social-contextual aspects of normal and abnormal development across the life span” (Cicchetti, 2006). There are few situations during the life course where the interplay between biology and environment (i.e., gene-environment interactions) are more striking than during the period of prenatal development. During this important period, “critical windows” are narrow and certain disturbances may alter fetal growth and development, leading to health and behavioral consequences that manifest and possibly persist across the life course (Maccani & Marsit, 2009). This is indeed suggested by a variety of studies which we summarize below.
INTRAUTERINE DEVELOPMENT: IMPORTANT “CRITICAL WINDOW”
A number of epidemiological studies have explored the links between adverse prenatal conditions and increased risk for diseases, health problems, and psychological outcomes later in development. Historically, such studies have examined the Dutch Famine Birth Cohort, which consists of men and women born as term singletons in Amsterdam, The Netherlands (de Rooij, Wouters, Yonker, Painter, & Roseboom, 2010; Z. Stein, Susser, Saenger, & Marolla, 1972). The formation of the Dutch Famine Birth Cohort, resulting from the Dutch Famine of 1944–45, provided researchers with an opportunity to study one particular adverse exposure – starvation during pregnancy – and a number of health and developmental outcomes potentially associated with this challenging adverse exposure. Associations have been reported between adverse intrauterine environment as influenced by famine and a number of diseases and conditions, including but not limited to increased risk for type 2 diabetes mellitus, cardiovascular disease, other metabolic disorders, and decreased cognitive function later in life (Argente, Mehls, & Barrios, 2010; Barker & Clark, 1997; de Rooij, et al., 2010). In addition, researchers found that maternal weight loss or moderate to low weight gain was significantly associated with infant birth weight, length, and other measures of fetal growth status, as well as with trimester of exposure to famine (A. D. Stein, Ravelli, & Lumey, 1995). In relation to psychological outcomes, increased risk of affective disorders has been found in males exposed to famine during their second trimester of pregnancy (Brown, Susser, Lin, Neugebauer, & Gorman, 1995). Findings such as these further underscore the importance of considering timing of prenatal exposure to adverse conditions, such as famine, as well as potential confounding elements, such as gender of the infant, when looking at outcomes across development. Birth cohorts such as the Dutch Famine Birth Cohort have provided researchers with many of the earliest tools necessary to investigate epidemiological associations between adverse intrauterine conditions and postnatal health and disease.
MATERNAL CIGARETTE SMOKING DURING PREGNANCY
We now move beyond the example of famine to consider one of the most common, potentially hazardous environmental exposures during pregnancy -- maternal cigarette smoking. Studies have shown that there are more than 4,000 chemicals in cigarette smoke including benzo(a)pyrene, nicotine, and carbon monoxide, and more than 40 of these chemicals are known carcinogens (Thielen, Klus, & Muller, 2008; US Department of Health and Human Services, 2010). Nicotine crosses the placenta, and fetal concentrations of nicotine can be 15% higher than maternal concentrations (Lambers & Clark, 1996). Despite a number of studies showing a decrease in the overall prevalence of smoking in women in the past 20 years, key studies have suggested that the prevalence of smoking in young pregnant women under 20 years of age has increased, with prevalence rates of 30–40 percent (Jaakkola, Jaakkola, Gissler, & Jaakkola, 2001; Mohsin & Bauman, 2005; “Smoking during pregnancy--United States, 1990–2002,” 2004). Others have reported that 12–15% of all women of childbearing age smoke while pregnant (Cnattingius, 2004; Goodwin, Keyes, & Simuro, 2007). Considering the fact that women who smoke during pregnancy are more likely to be nicotine dependent, less likely to quit, and importantly, have a partner who smokes (Agrawal et al., 2008; Knopik et al., 2005), it is important to also keep in mind the additional effects of second-hand smoke exposure, which are very difficult to tease apart from direct prenatal primary smoke exposure. That is, women who are pregnant may also be exposed to secondhand smoke in homes, vehicles, the workplace, or public areas. More than 126 million nonsmoking adults continue to be exposed to secondhand smoke and current estimates suggest that almost 60 percent of children, aged 3–11, are exposed to secondhand smoke (U.S. Department of Health and Human Services, 2006). Thus, exposure to cigarette smoke remains a common and hazardous in utero exposure.
Previous work has suggested that maternal cigarette smoking during pregnancy is associated with increased risk for spontaneous abortion (Castles, Adams, Melvin, Kelsch, & Boulton, 1999), preterm delivery (Castles, et al., 1999; Kaddar et al., 2009; Shah & Bracken, 2000), respiratory disease (Cook & Strachan, 1999), immune system difficulties such as asthma and allergies (Prescott & Clifton, 2009), and cancer later in life (Doherty, Grabowski, Hoffman, Ng, & Zelikoff, 2009). Findings also suggest that there are a variety of placental complications linked to prenatal exposure to cigarette smoke, including alterations to the development and function of the placenta (Einarson & Riordan, 2009).
A collection of studies have suggested that prenatal tobacco exposure is associated with a number of serious neurodevelopmental and behavioral consequences in infants, children, and adolescents. For example, studies in infants have shown that maternal smoking during pregnancy is associated with delayed psychomotor and mental developmental scores as measured by the Bayley Scales of Infant Development (Kiechl-Kohlendorfer et al., 2010). Kable and colleagues analyzed the impact of maternal smoking during pregnancy on auditory brainstem responses (ABR) and determined that after controlling for a number of potential confounders, maternal smoking during pregnancy was negatively related to ABR latency in infants (Kable, Coles, Lynch, & Carroll, 2009). Interestingly, since alterations in infants’ auditory processing have been previously found to be highly predictive of future reading and language difficulties, these findings suggest that maternal cigarette smoking during pregnancy may negatively impact a child’s future speech and language development (Benasich & Tallal, 2002; Kable, et al., 2009; Molfese, 2000).
Examples of prenatal smoking exposure research in childhood are many (see Knopik et al., 2009 for a review), perhaps because of more sensitive assessments for this age group, relative to infants. For instance, Olds et al. conducted a study investigating associations between maternal cigarette smoking during pregnancy and offspring intelligence at age 4, finding that children whose mothers smoked 10 or more cigarettes per day during their pregnancy exhibited intellectual impairment relative to children whose mothers did not smoke cigarettes during pregnancy (Olds, Henderson, & Tatelbaum, 1994). Huijbregts et al found associations between maternal cigarette smoking during pregnancy and physical aggression during early childhood (Huijbregts, Seguin, Zoccolillo, Boivin, & Tremblay, 2007, 2008). This is consistent with multiple studies suggesting an association of prenatal smoke exposure and increased externalizing disorders, such as conduct disorder and attention deficit hyperactivity disorder (Abbott & Winzer-Serhan, 2012; Cornelius & Day, 2009; Knopik, 2009). Cognitive function has also been shown to be negatively affected by maternal smoking during pregnancy, with deficits in sustained attention (Fried, O’Connell, & Watkinson, 1992; Fried, Watkinson, & Gray, 1992), response inhibition, memory, and impulsivity, overall cognitive function, receptive language (Fried, O’Connell, et al., 1992), verbal learning and design memory (Cornelius, Ryan, Day, Goldschmidt, & Willford, 2001), problem solving (Cornelius, et al., 2001), speech and language (Makin, Fried, & Watkinson, 1991), school performance (Lambe, Hultman, Torrang, Maccabe, & Cnattingius, 2006), and auditory processing (McCartney, Fried, & Watkinson, 1994). Dose–response relationships, in which the smoking-related relative risk increases with amount smoked, have also been reported for general cognitive ability (Sexton, Fox, & Hebel, 1990), arithmetic, and spelling (Batstra, Hadders-Algra, & Neeleman, 2003), suggesting the presence of vulnerable periods during fetal development (Ernst, Moolchan, & Robinson, 2001).
Recent research has also suggested that second-hand smoke exposure during pregnancy is associated with a decrease in infant mental development index score and an increase in the risk of developmental delay in the infant (B. E. Lee et al., 2011), thereby extending the risk profile related to smoking exposure beyond just direct, primary smoke exposure during pregnancy. Taken collectively, these observations suggest that smoke exposure during pregnancy remains a common exposure that can have major ramifications on the in utero growth and development of the fetus. Further, due to the plethora of scientific data suggesting negative consequences associated with smoking during pregnancy, pregnant women are cautioned against smoking while pregnant (Cornelius & Day, 2009; Shea & Steiner, 2008). However, despite the large literature suggesting undesirable outcomes in children exposed to prenatal smoke exposure and warnings encouraging women to stop smoking while pregnant, the underlying biological processes in humans are not well understood. Thus, we turn our attention now to describing one potential piece of this puzzle – namely, fetal programming and how this may play an important role in future child development.
FETAL PROGRAMMING
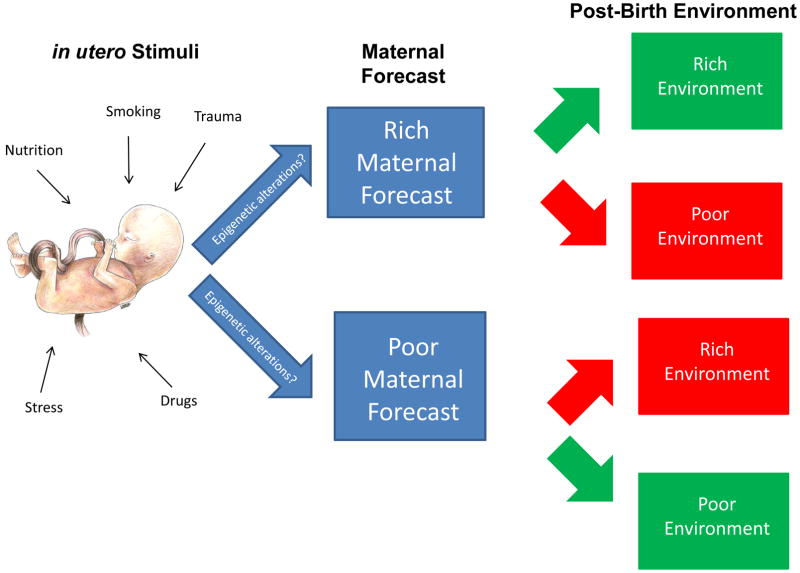
What exactly is meant by “fetal programming”? The theory of “fetal programming” has been described as a model of gene-environment interaction which explains the influence of the in utero environment on the molecular character of development (Barker & Clark, 1997; Hales & Barker, 1992). One of the forefathers of the theory of fetal programming is David Barker who, starting in the 1980s and 1990s, proposed and tested the hypothesis that an adverse fetal nutritional environment but plentiful food in adulthood might be a major factor associated with a number of adult diseases (Schulz, 2010). This “Barker Hypothesis,” as it became known, further hypothesized that adverse intrauterine conditions may result in a negative, or poor, maternal forecast, commonly manifested in small for gestational age status or reduced infant birth weight. This poor maternal forecast in a sense “predicts” that the child will be born into a postnatal environment in which resources are scarce, and thus, the child has been forecasted or programmed to thrive in such a poor environment. Poor maternal forecasts can prove incorrect if a child is born into what is or soon becomes a nutrient-rich environment.
It should be emphasized that current research expands the notion of an adverse intrauterine environment beyond the traditional, nutrient-poor environment first elucidated by Barker and colleagues in their analysis of infants from the Dutch Famine Birth Cohort. Adverse intrauterine conditions may also be the result of exposure to viruses, such as influenza, increased levels of stress during pregnancy, and, most important for this review, maternal cigarette smoking and secondhand (passive) smoke exposure during pregnancy. In this expanded view of adverse intrauterine conditions and resulting effects on the fetus, an exposure such as prenatal exposure to cigarette smoke may lead to a negative maternal forecast for the fetus. While prenatal smoke exposure certainly contributes to this adverse environment, evidence that maternal cigarette smoking during pregnancy is correlated with other potential contributors to adverse in utero environmental conditions can make causal attribution difficult (see Knopik et al., 2009 for a review). This is indeed supported by recent work from our own group and others suggesting that maternal smoking during pregnancy is correlated with many risk factors, such as lower levels of maternal education (D’Onofrio, Singh, Iliadou, Lambe, Hultman, Neiderhiser, et al., 2010), spousal/significant other substance dependence (Knopik et al., 2006; Knopik, et al., 2005), nicotine dependence (Agrawal, et al., 2008), as well as maternal ADHD and other psychopathology (D’Onofrio, Singh, Iliadou, Lambe, Hultman, Neiderhiser, et al., 2010; Huizink & Mulder, 2006; Knopik, 2009; Knopik, Jacob, Haber, Swenson, & Howell, 2009), which may come with a host of additional influences on intrauterine environment that also predict poor offspring performance.
Figure 1 summarizes the concept of fetal programming and the contributions of the variety of stimuli that make up the intrauterine environment on maternal forecasts. As shown in the figure, in utero stimuli include many exposures that may influence the intrauterine environment – such as exposure to nutrients, stress, drugs, trauma, and smoking. A largely positive and healthy intrauterine environment may result in the mother imparting a rich maternal forecast on her developing fetus, predicting a rich post-birth environment where resources are predicted to be plentiful and negative exposures are predicted to be at a minimum level. A relatively negative or adverse intrauterine environment may result in the mother imparting a poor maternal forecast on her developing fetus, sometimes characterized by a “thrifty phenotype” (Hales & Barker, 1992) or small-for-gestational age status, thereby preparing her child to survive in a poor post-birth environment, where resources are predicted to be scarce and/or negative postnatal exposures to be frequent and abundant. Maternal forecasts which do not accurately predict the post-birth environment have been hypothesized to lead to negative consequences for the health of the child over its life course – such as increased risk for metabolic diseases and decreased cognitive functioning in offspring which had been given a poor maternal forecast but were born into a rich environment (Argente, et al., 2010; Barker & Clark, 1997; de Rooij, et al., 2010; Hochberg et al., 2010).
Figure 1. in utero Stimuli-Associated Epigenetic Alterations and Maternal Forecasts.
in utero stimuli comprise the plethora of exposures that may characterize the intrauterine environment – exposure to nutrients, stress, and drugs. A largely positive and healthy intrauterine environment may result in the mother imparting a rich maternal forecast on her developing fetus, predicting a rich post-birth environment where resources are predicted to be plentiful and negative exposures at a minimum level. A relatively negative or adverse intrauterine environment may result in the mother imparting a poor maternal forecast, often characterized by a “thrifty phenotype”, on her developing fetus, thereby preparing her child to survive in a poor post-birth environment where resources are predicted to be scarce and/or negative exposures (e.g., secondhand smoke exposure) to be frequent and abundant. Maternal forecasts which do not accurately predict the post-birth environment have been hypothesized to lead to negative consequences for the health of the child over its life course – such as increased risk for metabolic diseases in offspring born into a rich environment with a poor maternal forecast. in utero stimuli may influence maternal forecasting through epigenetic mechanisms, both directly and indirectly. These epigenetic-mediated maternal forecasts may be accessible to measurement through techniques established for measuring changes to epigenetic modes of regulation. Additionally, these epigenetic marks, such as DNA methylation profiles or miRNA expression, may have utility as diagnostic biomarkers capable of predicting increased risk for diseases or disease progression but also as therapeutic targets. Scientific artwork by Jennifer Z. Joukhadar.
Despite this large literature suggesting undesirable outcomes in children exposed to maternal smoking during pregnancy, the underlying biological processes in human are not well understood. Work to better understand the underlying mechanisms of fetal programming is ongoing, and many researchers have focused on how epigenetic mechanisms may play a role in mediating the effects of environmental exposures on future outcomes, such as the physical and behavioral health of the individual in infancy, childhood, and adulthood (Maccani & Marsit, 2009). The understanding of how fetal programming may lead to future health consequences have caused some to adopt an even more expanded theory of fetal programming, namely the “developmental origins of health and disease” or DoHAD (Barker, 2004; Gillman, 2005).
EPIGENETICS: MECHANISMS BY WHICH FETAL PROGRAMMING MAY OCCUR
The study of epigenetics, or the study of changes in gene expression that are not caused by changes in the sequence of DNA (Bird, 2007), has intrigued scores of scientists over the past few decades. It is these epigenetic mechanisms that can influence whether one’s genes are switched on or off (i.e., gene expression). It may be through epigenetic mechanisms that environmental factors like diet, stress, prenatal nutrition, or prenatal drug exposure can lead to changes in gene expression from one cell to its daughter cells and, in some cases, from one generation to the next. Work in this area has focused on examining four main modes of epigenetic gene regulation: DNA methylation, imprinting, histone modification, and noncoding RNA-mediated gene regulation, especially by microRNA (miRNA). For a more comprehensive review on these four modes of epigenetic regulation, as well as on the technological advances which have made it possible to measure such changes, please see (Maccani & Marsit, 2009).
As hinted at above, new subfields have emerged to explore epigenetic effects in a variety of settings. Described by Reamon-Buettner and colleagues, the field of “environmental epigenetics” studies how environmental exposures affect epigenetic mechanisms (Reamon-Buettner, Mutschler, & Borlak, 2008). Many researchers are interested in uncovering how environmental exposures at sensitive periods of development, such as maternal cigarette smoking during pregnancy, might influence epigenetics and thus affect the developing fetus and fetal programming. Research in both human cohorts and model systems, such as mice, continues to characterize the influence of environmental exposures on epigenetics. Figure 1 describes how certain prenatal exposures may, both directly and indirectly, influence maternal forecasting through epigenetic mechanisms. Additionally, these epigenetic mechanisms, such as DNA methylation profiles or miRNA expression, may have utility not only as diagnostic biomarkers capable of predicting increased risk for behavioral deficits, diseases, or disease progression but also as therapeutic targets. This is similar to recent reports using gene expression differences, identified via a blood test, to predict youth at risk for early onset depression (Pajer, 2012).
As this review will highlight, the research community has placed particular focus on investigating the influence of maternal cigarette smoking during pregnancy on DNA methylation patterns, while work on the effects of maternal cigarette smoking during pregnancy on miRNA has been much less comprehensive. Investigations of specific associations between prenatal smoke exposure and histone modifications or imprinting have yet to be described in the literature, but others have cited these two modes of epigenetic regulation as important future considerations for investigation in the context of in utero smoke exposure (Swanson, Entringer, Buss, & Wadhwa, 2009).
MATERNAL CIGARETTE SMOKING DURING PREGNANCY IS ASSOCIATED WITH ATYPICAL DNA METHYLATION PATTERNS
DNA methylation is one of the more widely studied and well-characterized of the main modes of epigenetic regulation. DNA methylation of cytosine residues is performed by one of a number of DNA methyltransferases which add a methyl group to a specific cytosine residue. These cytosine residues often reside in cytosine- and guanine-rich stretches of DNA called “CpG islands”. Sometimes DNA methylation is also referred to as CpG methylation. Generally, a DNA methylation-regulated gene whose promoter region shows a great degree of methylation will be effectively silenced. When the same gene’s promoter region is not methylated (i.e. the promoter is “hypomethylated”), the gene will likely not be silenced, and thus the gene will be expressed. Research has determined that the blocking of transcription in a methylated gene is not due to the methylation of DNA alone, but rather due to the irregular binding of a variety of proteins. In the presence of DNA methylation, proteins which normally bind DNA and enable transcription to proceed are unable to bind as well, or at all, which effectively limits or stops transcription. Research is continuing to develop a better understanding of how DNA methylation in a gene’s promoter region controls the complex regulatory environment necessary for transcription and thus gene expression.
During the period of embryonic development, methylation patterns of the germline and somatic cell lineages are established (Maccani & Marsit, 2009). During the cleavage phase, or the early cell divisions that occur as a fertilized egg begins to develop into an embryo, methylation in the zygote’s genome is almost completely removed. After implantation, as the cells produced during the cleavage phase begin to organize themselves, a process called gastrulation, the organism’s methylation patterns are reestablished by de novo methylation (Jaenisch, 1997; Kafri et al., 1992; Monk, Boubelik, & Lehnert, 1987; Razin & Shemer, 1995). Such patterning and repatterning of methylation marks also occurs in trophoblast lineages, the various specialized cells comprising the placenta (Jaenisch, 1997). Proper setting and resetting of methylation marks throughout development is key for the proper health and development of the embryo.
Recent studies have even reported initial findings suggesting that an additional mode of epigenetic regulation, namely conversion of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), may play a role in the reprogramming of the zygote (Wossidlo et al., 2011). Further studies will be necessary to further understand the implications of these findings on the patterning of methylation marks during development, as well as the effect of exposures on the underlying mechanisms.
When considering epigenetic research in general, DNA methylation is the most heavily studied mode of epigenetic regulation (Bird, 2007). Thus, it is of no great surprise that the majority of published articles on the epigenetics of maternal cigarette smoking during pregnancy describe associations with DNA methylation. A number of studies have characterized associations between prenatal smoke exposure and aberrant DNA methylation patterns in a variety of tissues. The following sections describe many of these efforts.
DNA methylation and the placenta
One of the most important, functional organs critical to the survival and in utero development of the fetus is the placenta. The placenta provides nutrients, assists in the transfer of waste for ultimate excretion by the mother, and plays an important role in protecting the fetus from maternal immune system attack (Maccani & Marsit, 2009). Another key function of the placenta is to secrete hormones which regulate pregnancy stages and protect the fetus, when possible, from harmful xenobiotic, or foreign, exposures, such as maternal drug use during pregnancy (Sood, Zehnder, Druzin, & Brown, 2006). All of these functions of the placenta, as well as placental gene expression, which is modulated by epigenetic regulation, can be affected by environmental insults (Guo et al., 2008; Sood, et al., 2006). Therefore, many have considered the placenta an important and relatively accessible record or history of in utero exposure and pathology (Maccani & Marsit, 2009).
Important findings using the human placenta have revealed associations between maternal cigarette smoking during pregnancy and DNA methylation in a gene-specific and even global fashion. Suter and colleagues observed that maternal tobacco use is associated with aberrant placental epigenome-wide DNA methylation and gene expression (Suter et al., 2011). Their work also suggested that maternal smoking during pregnancy is associated with altered site-specific CpG methylation which further correlates with important changes in gene expression in pathways crucial for ensuring proper growth and development. In a separate study, Suter and colleagues also noted that maternal tobacco smoking may modify placental CYP1A1 expression by altering methylation at CpG sites proximal to the 5′-xenobiotic response element transcription factor binding site (Suter et al., 2010). CYP1A1 is involved in Phase I of the metabolism of carcinogenic compounds such as polycyclic aromatic hydrocarbons (PAH), which are present in cigarette smoke. Specifically, CYP1A1 converts such compounds in a way that can eventually be excreted in Phase II metabolism, a process modulated in part by other genes (Suter, et al., 2010). Suter and colleagues found that methylation at these sites was significantly lower in the placentas of babies born to mothers who smoked during pregnancy versus non-smoking controls. This downregulation of methylation was also significantly correlated with increased placental CYP1A1 expression, a finding which may have substantial potential implications for future behavior. Wilhelm-Benartzi and colleagues showed that differential methylation of repetitive elements – stretches of DNA exhibiting a large number of repeated bases – in the placenta is associated with birth weight percentile and maternal smoking during pregnancy. More specifically, they found that mean methylation levels of the repetitive element AluYb8 significantly differed by maternal tobacco use during pregnancy (Wilhelm-Benartzi et al., 2011).
Taken collectively, these reports suggest that cigarette smoke may elicit some of its downstream consequences on the placental epigenome in both a global- and site-specific fashion (Suter, et al., 2010; Suter, et al., 2011). Moreover, repetitive elements may have specific methylation patterns influenced by environmental exposures, underscoring the need to carefully consider how “globally-representative” a particular repetitive element’s methylation pattern may be without utilizing a representative genome-wide methylation assessment as comparison (Wilhelm-Benartzi, et al., 2011). These findings in the human placenta build on work described by Breton and colleagues in human buccal cells whereby they described that in utero exposure to tobacco smoke is associated with alterations to global and gene-specific methylation (Breton et al., 2009).
Umbilical cord blood and maternal blood
Others have investigated changes in DNA methylation associated with maternal cigarette smoking during pregnancy in umbilical cord serum. Guerrero-Preston and colleagues tested their hypothesis that global DNA hypomethylation in serum from the umbilical cord is associated with prenatal exposure to maternal smoking and perflouroalkyl compounds (PFCs), which are used in a wide-range of consumer and industrial products, such as stain-resistant coatings (Guerrero-Preston et al., 2010). They determined that global DNA methylation was most reduced in cord blood from newborns with mothers who smoked during pregnancy. Future studies using larger sample sets, as well as utilizing other surrogates of “global” DNA methylation, such as the repetitive elements LINE-1 or AluYb8, or DNA methylation microarrays, are needed in order to assess the possibility of utilizing DNA methylation status in cord blood as a biomarker for prenatal smoke exposure.
Associations between nutrient levels during pregnancy and DNA methylation have also been assessed using blood. A number of previous groups have described importance of folate as a key nutrient involved in the process of establishing and maintaining DNA methylation (Chmurzynska, 2010). Ba and colleagues used both maternal blood and umbilical cord blood to measure specific gene promoter methylation in insulin-like growth factor 2 (IGF2), which is believed to be a major growth factor in the developing fetus (Ba et al., 2011). Amongst their findings, they found that while P2 and P3 methylation in cord blood was not significantly associated with folate levels in either cord blood or maternal blood, P3 methylation was significantly associated with vitamin B12 serum levels in maternal blood. Furthermore, P2 methylation in mother’s blood was associated with vitamin B12 maternal blood serum levels, passive smoke exposure, and maternal weight gain during pregnancy. While not focused on smoking during pregnancy per se, the investigators did consider passive smoke exposure, which is an indication of secondhand smoke exposure during pregnancy. Further, their study underscores the importance of environmental exposures and maternal dietary factors in potentially mediating methylation patterns in specific biological pathways.
Taken collectively, these studies suggest the future utility of using DNA methylation patterns in cord blood or maternal blood as biomarkers of prenatal exposures. Future studies using more agnostic approaches (such as DNA methylation microarrays) may be of important utility in developing comparatively less biased, hypothesis-generating methods capable of further exploring often complex pathways affected by prenatal smoke exposure. Additionally, such technological approaches may discover DNA methylation biomarkers with greater sensitivity and specificity for assessing maternal cigarette smoking during pregnancy.
Leukocyte DNA
Terry and colleagues analyzed DNA methylation profiles in leukocyte, or white blood cell, DNA in a multiethnic birth cohort from New York City (Terry et al., 2008). Multivariable models indicated that overall levels of DNA methylation were significantly associated with maternal smoking during pregnancy and a number of other covariates. Terry and coauthors point out that their data, if replicated in a larger number of samples, suggest that exposures experienced throughout the course of life – from fertilization onward – may be associated with DNA methylation in adulthood. Longitudinal studies capable of measuring within-individual changes in DNA methylation in a variety of tissues over time will yield important data informative of the intragenerational plasticity of DNA methylation.
Brain and neurodevelopmental outcomes
There is a great degree of interest in better understanding the influences of maternal cigarette smoking during pregnancy on the brain and infant neurodevelopment; however, investigations considering epigenetic pathways are only beginning to emerge. Toledo-Rodriguez and colleagues investigated whether prenatal exposure to maternal cigarette smoking is associated with promoter methylation of brain-derived neurotrophic factor (BDNF) in blood samples from adolescents whose mothers smoked during pregnancy. BDNF, which is important for long-term memory, acts on certain neurons of the central nervous system and the peripheral nervous system, helping to support the survival of existing neurons, and encourage the growth and differentiation of new neurons and synapses (Huang & Reichardt, 2001). Toledo-Rodriguez and colleagues found that exposure to maternal cigarette smoking in utero is associated with a higher degree of DNA methylation in the BDNF exon 6 in adolescents whose mothers smoked during pregnancy, further suggesting that exposure to cigarette smoke while in utero may have long-term consequences still measurable into adolescence. The BDNF gene contains several exons, the expression of which is crucial for proper functioning of BDNF protein and which have been shown to be differentially-regulated in an exon-specific manner. One additional preliminary study, for example, has noted that measurement of methylation upstream of BDNF exon I may have promise as a diagnostic blood biomarker for major depression (Fuchikami et al., 2011). Interestingly, exon 6 of the BDNF gene appears to be particularly sensitive to epigenetic modification from environmental factors related to depression. Specifically, chronic stress results in epigenetic changes in histones related to hippocampal BDNF that reduce expression whereas treatment with the antidepressant imipramine results in epigenetic changes of histones associated with the same exon that increase expression rates (Calabrese, Molteni, Racagni, & Riva, 2009). This example highlights the importance the various types of epigenetic modification that may increase (or decrease) risk for psychopathology by modifying the same gene in different ways and how such mechanisms may overlap with prenatal smoke exposure. Abnormal methylation upstream of one BDNF exon may change the ultimate expression of BDNF and thus play a role in altering downstream pathways important for proper brain development and plasticity (Toledo-Rodriguez et al., 2010). Future studies using primary brain tissue may prove challenging but essential in determining the role of BDNF and related biological pathways in brain development and later behavior.
Future analyses focused on assessing epigenetic mechanisms mediating the effects of maternal cigarette smoking during pregnancy on brain, developmental, and behavioral outcomes using both animal models and human cohorts will be essential. Such studies will aid in further defining how in utero exposures may influence neurodevelopmental outcomes and risk for behavioral disorders and diseases.
Summary: DNA methylation
Taken collectively, although studies to date are limited, both in number and in scope, it appears important to consider the role of DNA methylation in the pathway from maternal cigarette smoking during pregnancy to later child and adolescent outcomes. It is clear, however, that we have much to learn. Specifically, a better understanding of the mechanisms involved, or what these associations imply, for later offspring outcomes, such as birth weight, cognitive performance, executive function, externalizing behavior, and other health-related phenotypes, including mental health, will be key. In order to accomplish this, we need to elucidate potential etiological pathways linking prenatal exposure-altered DNA methylation and later behavioral deficits or problems in childhood and adolescence. Finally, there is a need to continue to develop novel techniques for assessing the utility of DNA methylation profiles in accessible tissues, such as placenta and umbilical cord blood, further exploring the potential for DNA methylation as a biomarker for prenatal smoke exposure and other adverse prenatal exposures.
MATERNAL CIGARETTE SMOKING DURING PREGNANCY IS ASSOCIATED WITH DIFFERENTIAL miRNA EXPRESSION
Compared to the growing body of literature characterizing associations between DNA methylation patterns and maternal cigarette smoking during pregnancy, much less is known about associations between maternal cigarette smoking during pregnancy and microRNA (miRNA) expression. miRNA are small, ~22 nucleotide-long noncoding RNA molecules, previously shown to be highly ubiquitous and have conservation across many species (R. C. Lee, Feinbaum, & Ambros, 1993). miRNA control gene expression by base-pairing to the 3′-untranslated region of a target messenger RNA (mRNA). In some cases, there will be an imperfect match, or imperfect complementarity, between the miRNA and its mRNA target, which results in problems translating the mRNA into a protein molecule, through a mechanism called translational repression. In other cases of perfect complementarity between the miRNA and the target mRNA, the result is degradation of the target mRNA. Research has found that since partial or imperfect complementarity of a miRNA to a target mRNA can still result in translational repression, a single miRNA has the capability of regulating a number of genes (Du & Zamore, 2007). Through this mechanism of negative regulation, miRNA have been shown to be involved in regulating a number of key biological processes, including cell proliferation, differentiation, and apoptosis, or cell death (Maccani & Knopik, 2012; Maccani & Marsit, 2009; Miska, 2005).
At the time of this review, one of the only studies investigating maternal cigarette smoking during pregnancy and miRNA expression was conducted by Maccani and colleagues and used a candidate miRNA approach to investigate the changes in four human placental miRNA associated with maternal cigarette smoking during pregnancy (Maccani et al., 2010). The four miRNA were selected for investigation because they had been previously reported to be expressed in the placenta and had been shown to be involved in the regulation of key cell processes. The work identified the association of maternal cigarette smoking during pregnancy with the downregulation of three specific miRNA: miR-16, miR-21, and miR-146a. Based on these findings, this research was expanded to consider three placental cell lines from different stages of placental development. The aim was to further investigate the effects of nicotine and benzo(a)pyrene, two components of cigarette smoke previously suggested to have negative effects on both the placenta and fetus, on miRNA expression in placental cells. Downregulation of miR-146a in one placental cell line, TCL-1 cells, treated with nicotine and benzo(a)pyrene suggested that miR-146a may be especially sensitive to agents of cellular stress, such as prenatal smoke exposure. Moreover, this result suggests that two specific components of cigarette smoke which affect the expression of miR-146a in term placentas may be nicotine and benzo(a)pyrene (Maccani, et al., 2010).
Maccani and colleagues’ observations, however, were limited by a relatively small set of samples (n=25), as well as a lack of data regarding the duration of cigarette smoking during pregnancy, cigarette per day usage, or more extensive environmental exposure information (such as environmental pollutant exposure or secondhand/passive cigarette smoke exposure). Despite these limitations, their data comprise an important first step in determining associations between maternal cigarette smoking during pregnancy and aberrant miRNA expression in the placenta. Furthermore, more work is needed to investigate associations between maternal cigarette smoking during pregnancy and abnormal miRNA expression in a larger sample set with more complete environmental exposure information, as well as potentially using a hypothesis-generating approach, such as using miRNA microarrays (Maccani, et al., 2010; Maccani & Marsit, 2011). Future work assessing associations between exposure-influenced placental miRNA profiles and downstream neurobehavioral and developmental outcomes is also needed. For instance, longitudinal analyses across the lifespan will be critical for elucidating the impact of miRNA-mediated fetal programming on later health, behavior, and disease.
As reviewed by Maccani and Marsit previously (Maccani & Marsit, 2011), future experiments conducted using cigarette smoke condensate (Crane-Godreau et al., 2009) may enable researchers to better understand effects of complex mixtures of components of cigarette smoke on placental cells and cells derived from other tissues. Of course, such experiments also have the caveat of presenting challenges in determining the specific composition of the complex mixture as well as physiological relevance of exposure to such a mixture on a given cell type. Other more extensive experiments aimed at better understanding the more complex effects of organism-wide cigarette smoke exposure might be designed using mouse models, or other animal models, of chronic environmental tobacco smoke exposure as described previously (Maccani & Marsit, 2011; Xiong, Leme, Ray, Shapiro, & Lee, 2011). In such an experimental design, pregnant mice might be exposed to environmental tobacco smoke throughout their entire gestation and euthanized at key timepoints in gestation, with placentas and other key organs harvested for measurement of miRNA expression and for determination of the effects of dysregulated miRNA expression on target gene protein levels and function. Findings from such experiments would elaborate on work done to characterize the association of maternal cigarette smoking during pregnancy with changes in placental miRNA and expand investigations into new tissues. Additionally, such findings using an in vivo model system may provide key mechanistic data that may further strengthen data from previous epidemiologic association studies with findings from a model system experimental paradigm.
INTEGRATION OF EPIGENETIC CHARACTERIZATION INTO EXISTING HUMAN RESEARCH PARADIGMS
As suggested by this review, there is a growing literature on maternal cigarette smoking during pregnancy and epigenetic phenomena, such as DNA methylation and miRNA expression. How do we align these recent advances with the smoking during pregnancy literature at large, particularly the reported associations between smoking during pregnancy and later child/adolescent behavior? There is a large literature suggesting undesirable outcomes in children exposed to maternal smoking during pregnancy. This evidence (in human models), until recently, has been limited by the frequent inability to separate prenatal exposure effects from other confounding environmental and genetic factors. The vast majority of studies have provided very limited control for the fact that prenatal exposures may be correlated with parental behaviors that could act as more proximal risk factors that are, in turn, transmitted to their offspring (Knopik, 2009). In other words, mothers who smoke during their pregnancies share other risk factors with their children and it may be these other risk factors that are associated with the observed adverse outcomes rather than only maternal cigarette smoking during pregnancy per se (Kuja-Halkola, D’Onofrio, Iliadou, Langstrom, & Lichtenstein, 2010). These factors can include unmeasured familial environmental influences, such as secondhand smoke, socio-economic status, and parental education. Additional factors might be genetic influences, such as genetic variation influencing (i) neurocognitive phenotypes through non-exposure contingent mechanisms, (ii) pharmacokinetic and/or pharmacodynamic aspects of nicotinic effects, or (iii) metabolism of the 4000+ other xenobiotics (e.g., foreign substances) found in cigarette smoke. Epigenetic modification has long been considered a possible influence in this regard, but, as indicated by the present review, largely remains to be empirically tested when it comes to behavioral or neurodevelopmental phenotypes.
Because of ethical and practical concerns about research considering prenatal smoke exposure, maternal cigarette smoking during pregnancy in humans cannot be randomized. The consequence of this is that prenatal smoke exposure effects on human offspring outcomes are, by definition, quasi-experimental in nature (Shadish, Cook, & Campbell, 2002). However, innovative designs such as full sibling pairs discordant for prenatal exposure – an extension of the case-crossover design (D’Onofrio, Singh, Iliadou, Lambe, Hultman, Grann, et al., 2010; D’Onofrio, Singh, Iliadou, Lambe, Hultman, Neiderhiser, et al., 2010; D’Onofrio et al., 2008; Knopik, 2009; Knopik, McGeary, Nugent, Francazio, & Heath, 2010; Kuja-Halkola, et al., 2010; Lumley & Levy, 2000; Meyer, Williams, Hernandez-Diaz, & Cnattingius, 2004) and in vitro fertilization (IVF) cross-fostering approaches (Rice et al., 2009; Rice et al., 2010; Thapar et al., 2007; Thapar et al., 2009) have enabled better control for confounding factors. Results from these studies suggest that some associations suggested between maternal cigarette smoking during pregnancy and offspring behavioral outcomes, a common finding in the larger literature, may indeed be influenced by design limitations, specifically an inability to adequately control for shared familial influences, including genetic factors. This has been shown for offspring conduct problems (D’Onofrio, et al., 2008), criminality (D’Onofrio, Singh, Iliadou, Lambe, Hultman, Grann, et al., 2010), academic achievement (D’Onofrio, Singh, Iliadou, Lambe, Hultman, Neiderhiser, et al., 2010; Lambe, et al., 2006), intellectual performance (Lundberg et al., 2009) and ADHD (Knopik, et al., 2010; Thapar, et al., 2009). Thus, the suggestion that maternal cigarette smoking during pregnancy is exclusively responsible for a broad range of later offspring behavioral outcomes must be tempered and reconsidered in light of these recent findings across multiple research groups.
Moreover, preliminary efforts to study the fetal programming hypothesis, as described above, from a phenotypic standpoint (i.e., in the absence of epigenetic data), using low birth weight as a proxy for poor gestational environment, suggest that this mechanism does not wholly explain potential prenatal smoke exposure influences on later psychological functioning (Kuja-Halkola, et al., 2010). Kuja-Halkola and colleagues also suggest that possible effects of low birth weight on later offspring behavior may also be due to familial confounding (Kuja-Halkola, et al., 2010). Thus, further biological characterization, such as specific genetic influences as well as epigenetic modification resulting from pre-pregnancy smoking that has influence irrespective of the decision to smoke during pregnancy may further highlight influences beyond maternal cigarette smoking during pregnancy and add clarity to this complicated story.
The conclusions of this body of quasi-experimental approaches to this research question should not be over-interpreted so as to suggest that maternal cigarette smoking during pregnancy is a benign influence. Substantial evidence exists to suggest prenatal cigarette smoke exposure is associated with numerous adverse outcomes related to birth and infancy, such as birth weight (Cnattingius, 2004; D’Onofrio, Singh, Iliadou, Lambe, Hultman, Grann, et al., 2010; D’Onofrio, Singh, Iliadou, Lambe, Hultman, Neiderhiser, et al., 2010; D’Onofrio et al., 2003; Gilman, Gardener, & Buka, 2008; Johansson, Dickman, Kramer, & Cnattingius, 2009; Rice, et al., 2009; Sexton & Hebel, 1984). Thus, there is a need for continued smoking cessation efforts in pregnant mothers. However, given that smoking during pregnancy is correlated with a host of other behaviors (Agrawal, et al., 2008; D’Onofrio, Singh, Iliadou, Lambe, Hultman, Neiderhiser, et al., 2010; Knopik, 2009; Knopik, et al., 2006; Knopik, et al., 2005), this suggests that treatment and prevention efforts might need to also consider other factors, such as maternal alcohol dependence, maternal nicotine dependence, and maternal ADHD, among other things.
Given the above considerations, the potential influence of maternal cigarette smoking during pregnancy on epigenetic mechanisms which may have consequences for downstream behavior is manifold. First, there is the possibility of intergenerational transmission of smoking-related epigenetic consequences (i.e., grandmaternal smoking influences may affect germline cells or may escape epigenetic reprogramming during development). Second, we can begin to understand the extent to which epigenetic modification may alter critical neurobehavioral circuitry in the developing brain. Third, questions regarding the influence of smoke exposure-induced methylation on the xenobiotic metabolism of cigarette smoke by-products can begin to be answered. These are just a few of the currently unanswered questions for which epigenetic research might begin to provide answers. Fortunately, at least for DNA methylation, existing datasets with collected DNA samples may provide some opportunities to investigate such possibilities. For example, methylomic characterization of samples collected in sibling pairs discordant for prenatal exposure might provide initial evidence of longer lasting impacts of pre-pregnancy smoking that may make maternal smoking during pregnancy per se less important than overall maternal smoking history. Use of animal models in mutually informative translational research (especially in inbred lines where genetic background is held constant) may further triangulate our ability as a field to investigate these tantalizing hypotheses.
Preliminary evidence already points to important genetic differences in xenobiotic metabolism with downstream impacts on intrauterine growth (Price, Grosser, Plomin, & Jaffee, 2010) and behavior (Knopik, McGeary, Nugent, Francazio, & Heath, in press) Further characterization of these samples from an epigenetic standpoint could explain additional variance in behavioral outcomes while simultaneously identifying potentially targetable mechanisms to reduce such impacts.
CONCLUSION & FUTURE DIRECTIONS
Important advances in understanding DNA methylation patterns and altered miRNA expression associated with maternal cigarette smoking during pregnancy have laid the groundwork for a future of exciting research in this domain. Research is ongoing to better understand the reported associations reviewed in this report and the mechanisms underlying the effects of maternal cigarette smoking during pregnancy. It will also be important to expand the current literature pertaining to the epigenetic mechanisms mediating the effects of maternal cigarette smoking during pregnancy on neuropsychiatric and neurobehavioral outcomes across development. Additionally, more work needs to be done to explore the role of maternal cigarette smoking during pregnancy on histone modification and imprinting, two crucial epigenetic mechanisms which play key roles in development and health and which have been vastly understudied in the context of maternal cigarette smoking during pregnancy.
One research focus yet to be fully explored is determining epigenetic mechanisms underlying psychological disorders associated with maternal cigarette smoking during pregnancy. This could, perhaps, be described through mechanisms described in the theory of behavioral teratogenicity, such as fetal brain exposure to nicotine and the pathological activation of acetylcholine nicotinic receptors during early stages of brain development (Paz, Barsness, Martenson, Tanner, & Allan, 2007). Because there does exist a high degree of comorbidity between nicotine dependence and neuropsychiatric conditions (Maughan, Taylor, Caspi, & Moffitt, 2004), researchers have struggled to determine how influential exposure to cigarette smoke is on risk for developing psychological disorders.
A variety of previous studies suggest that there is an increased risk for developing attention deficit disorder (ADHD), major depressive disorder (MDD), and substance abuse in adolescents and children who had been exposed to prenatal nicotine (Fergusson, Woodward, & Horwood, 1998; Linnet et al., 2005; Maughan, et al., 2004; Mick, Biederman, Faraone, Sayer, & Kleinman, 2002; Milberger, Biederman, Faraone, Chen, & Jones, 1996; Paz, et al., 2007; Thapar et al., 2003; Weissman, Warner, Wickramaratne, & Kandel, 1999). However, others suggest that these associations are due less to prenatal nicotine and more to shared familial influences, including genetics (D’Onofrio, Singh, Iliadou,Lambe, Hultman, Grann, et al., 2010; D’Onofrio, Singh, Iliadou, Lambe, Hultman, Neiderhiser, et al., 2010; D’Onofrio et al., 2008; Knopik, 2009; Knopik, McGeary, Nugent, Francazio, & Heath, 2010; Kuja-Halkola, et al., 2010; Lumley & Levy, 2000; Rice et al., 2009; Rice et al., 2010; Thapar et al., 2007; Thapar et al., 2009). In their manuscript, Paz and colleagues used a self-administered maternal nicotine consumption model to assess how prenatal nicotine exposure might influence a variety of neuropsychiatric disorders in mice (Paz, et al., 2007). They found that prenatal nicotine exposure had a significant effect on increasing latency to escape in a learned helplessness paradigm, the degree of spontaneous locomotion, and an increase in addictive behavior as assessed by preference for a place previously associated with cocaine. Paz and colleagues’ findings suggest that prenatal nicotine exposure may be significantly influencing the increased prevalence of MDD, ADHD, and substance abuse found in children and adolescents whose mothers smoked cigarettes during pregnancy, a finding which underscores the behavioral teratogenic effects of maternal cigarette smoking during pregnancy. Moreover, they speculate that this may be due to direct interaction between nicotine and acetylcholine nicotine receptors in the fetal brain. Variations in the genes coding for such receptors may indeed affect the entire biological pathway from prenatal exposure to later development.
Important work still needs to be done to determine the mechanisms affected by such exposures which may lead to neurodevelopmental and neurobehavioral outcomes. DNA methylation patterns in both candidate gene promoters as well as on more genomewide levels should be assessed using both animal models of maternal cigarette smoking during pregnancy as well as human cohorts. Additional studies which may be of special note might include an investigation of associations of maternal cigarette smoking during pregnancy on DNA methylation of the promoter of the glucocorticoid receptor (GR). This receptor is known to be a mediator of glucocorticoid signaling (a major component of the stress-response system that is closely linked to depression and anxiety psychopathology), and previously shown to be regulated, in part, by DNA methylation (Filiberto et al., 2011; Herbeck, Gulevich, Amelkina, Plyusnina, & Oskina, 2010; Ke et al., 2010; Oberlander et al., 2008; Weaver et al., 2004; Weaver et al., 2001). While previous studies have controlled for maternal cigarette smoking during pregnancy and did not find significant associations between maternal cigarette smoking during pregnancy and aberrant methylation at the 13 CpG sites in exon 1F of the GR gene (Filiberto, et al., 2011), a more comprehensive analysis of the promoter region controlling expression of GR may reveal CpG loci more responsive to and influenced by maternal cigarette smoking during pregnancy. Future work might be conducted using animal models and human cohorts and should utilize a variety of tissues for analysis, including but not limited to placenta, specific brain regions, saliva, and blood. A more comprehensive investigation of altered DNA methylation of the GR gene promoter associated with maternal cigarette smoking during pregnancy might better describe effects of environmental toxicant exposure during pregnancy on mediators of the hypothalamic-pituitary-adrenal (HPA) axis, which may contribute, in part, to an increased risk for developing psychological disorders in childhood or later in life. Investigation of the epigenetic mechanisms which may underlie a suite of psychological disorders, including but not limited to post-traumatic stress disorder (PTSD), depression, schizophrenia, and ADHD, may provide researchers with a greater understanding of the potential epigenetic nature of these disorders while also providing novel therapeutic and pharmacokinetic targets for treatment. Both candidate and genome-wide association studies should be employed to investigate how maternal cigarette smoking during pregnancy might influence risk for neuropsychiatric disease through epigenetic mechanisms.
Even though brain researchers have limited access to the tissue of interest compared to other areas of medicine, progress in the areas of brain-based neurological illness provides evidence of the importance of this understanding. The literature related to the epigenetics of Alzheimer’s disease and Parkinson’s disease, more specifically miRNA which may be involved in regulating genes described to be key for disease progression, has grown quite substantially over the past decade. Meza-Sosa and colleagues have extensively reviewed recent findings demonstrating that miRNA may play important roles in the development of the central nervous system (CNS) as well as in a variety of neuropathologies, including Alzheimer’s and Parkinson’s (Meza-Sosa, Valle-Garcia, Pedraza-Alva, & Perez-Martinez, 2011). Future studies in both human cohorts and animal models investigating epigenetic mechanisms altered by maternal cigarette smoking during pregnancy should also consider exploring exposure-associated epigenetic links to Alzheimer’s and Parkinson’s disease. Such links may both further the understanding of these complex etiologies and may reveal novel therapeutic targets for future treatment options.
In summary, the utilization of a variety of approaches will allow researchers to better investigate changes in DNA methylation, miRNA expression, histone modification, and imprinting which may be associated with in utero exposures, behavior, and even disease development. Profiles of epigenetic regulation in a variety of tissues associated with maternal cigarette smoking during pregnancy will inform researchers of the impact of maternal cigarette smoking during pregnancy on the epigenetic mechanisms in a particular tissue and may also prove to be powerful biomarkers of exposure, disease susceptibility or progression, and even pharmacokinetic efficacy. In such ways, translational epigenetics may be brought from benchtop-to-bedside and may prove advantageous in aiding clinicians in the diagnosis of exposure, disease, risk for mental health and behavioral problems, as well as treatment.
Lastly, as mentioned above, a consistent body of research stresses the need for more careful examination of maternal cigarette smoking during pregnancy within the context of genetic background. While prenatal smoke exposure-induced epigenetic modification is consistently found to impact physical health phenotypes, the examination of epigenetic modification as a moderator of later neurobehavioral effects is in its infancy. The same biological systems investigated in studies of genetic variation (e.g., nicotinic and dopaminergic systems, xenobiotic metabolism pathways) remain viable candidates for epigenetic influences on neurobehavioral outcomes in exposed offspring. Indeed, the co-modeling of genetic and epigenetic variation as they relate to these outcomes has the potential to bring new clarity to the field. Although epigenetic patterns change over time and with environmental exposures (and thus are not as static as genetic variation), DNA samples in existing datasets may be used to provide an informative cross-sectional view of this phenomenon, provided that there is careful attention given to the time of sample collection. Promising findings can be followed up with longitudinal studies with sequential sampling designed to assess the relative impact of environmental exposures. For example, in the context of prenatal smoke exposure research, sampling at the beginning and the end of pregnancy in smokers who did and did not decide to quit smoking while pregnant would allow examination of the extant methylation resulting from pre-pregnancy smoking. This would also allow for investigation of whether or not smoking cessation during pregnancy has an impact on epigenetic mechanisms.
In sum, we are on the verge of a new and more complete understanding of the biological impact of maternal cigarette smoking during pregnancy that, when coupled with advances in the field of prenatal exposure research to date, holds great promise of increased understanding of offspring consequences of such behavior. Such measures will be essential in understanding and addressing the effects of maternal cigarette smoking during pregnancy on fetal programming and child development.
Acknowledgments
This work was supported by R01DA023134 (VSK) and 2 T32 AA 07459-26 (MAM). The authors also wish to thank Jennifer Z. Joukhadar for her contribution of scientific artwork for Figure 1.
References
- Abbott LC, Winzer-Serhan UH. Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models. Critical Reviews in Toxicology. 2012;42(4):279–303. doi: 10.3109/10408444.2012.658506. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Knopik VS, Pergadia ML, Waldron M, Bucholz KK, Martin NG, Madden PA. Correlates of cigarette smoking during pregnancy and its genetic and environmental overlap with nicotine dependence. Nicotine & Tobacco Research. 2008;10(4):567–578. doi: 10.1080/14622200801978672. 792239248 [pii] [DOI] [PubMed] [Google Scholar]
- Argente J, Mehls O, Barrios V. Growth and body composition in very young SGA children. Pediatric Nephrology. 2010;25(4):679–685. doi: 10.1007/s00467-009-1432-2. [DOI] [PubMed] [Google Scholar]
- Ba Y, Yu H, Liu F, Geng X, Zhu C, Zhu Q, Zhang Y. Relationship of folate, vitamin B12 and methylation of insulin-like growth factor-II in maternal and cord blood. European Journal of Clinical Nutrition. 2011;65(4):480–485. doi: 10.1038/ejcn.2010.294. ejcn2010294 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. Developmental origins of adult health and disease. Journal of Epidemiology & Community Health. 2004;58(2):114–115. doi: 10.1136/jech.58.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Clark PM. Fetal undernutrition and disease in later life. Reviews of Reproduction. 1997;2(2):105–112. doi: 10.1530/ror.0.0020105. [DOI] [PubMed] [Google Scholar]
- Batstra L, Hadders-Algra M, Neeleman J. Effect of antenatal exposure to maternal smoking on behavioural problems and academic achievement in childhood: prospective evidence from a Dutch birth cohort. Early Human Development. 2003;75(1–2):21–33. doi: 10.1016/j.earlhumdev.2003.09.001. S0378378203001452 [pii] [DOI] [PubMed] [Google Scholar]
- Benasich AA, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behavioural Brain Research. 2002;136(1):31–49. doi: 10.1016/s0166-4328(02)00098-0. S0166432802000980 [pii] [DOI] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396–398. doi: 10.1038/nature05913. nature05913 [pii] [DOI] [PubMed] [Google Scholar]
- Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. American Journal of Respiratory and Critical Care Medicine. 2009;180(5):462–467. doi: 10.1164/rccm.200901-0135OC. 200901-0135OC [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Susser ES, Lin SP, Neugebauer R, Gorman JM. Increased risk of affective disorders in males after second trimester prenatal exposure to the Dutch hunger winter of 1944–45. British Journal of Psychiatry. 1995;166(5):601–606. doi: 10.1192/bjp.166.5.601. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Racagni G, Riva MA. Neuronal plasticity: a link between stress and mood disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S208–216. doi: 10.1016/j.psyneuen.2009.05.014. S0306-4530(09)00181-4 [pii] [DOI] [PubMed] [Google Scholar]
- Castles A, Adams EK, Melvin CL, Kelsch C, Boulton ML. Effects of smoking during pregnancy. Five meta-analyses. American Journal of Preventative Medicine. 1999;16(3):208–215. doi: 10.1016/s0749-3797(98)00089-0. S0749-3797(98)00089-0 [pii] [DOI] [PubMed] [Google Scholar]
- Chmurzynska A. Fetal programming: link between early nutrition, DNA methylation, and complex diseases. Nutrition Reviews. 2010;68(2):87–98. doi: 10.1111/j.1753-4887.2009.00265.x. NURE265 [pii] [DOI] [PubMed] [Google Scholar]
- Cicchetti D. Development and psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology Vol. 1: Theory and Method. 2. New York: Wiley; 2006. [Google Scholar]
- Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Research. 2004;6(Suppl 2):S125–140. doi: 10.1080/14622200410001669187RUXN36F9J24X6H0N. [pii] [DOI] [PubMed] [Google Scholar]
- Cook DG, Strachan DP. Health effects of passive smoking-10: Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54(4):357–366. doi: 10.1136/thx.54.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Day NL. Developmental consequences of prenatal tobacco exposure. Current Opinions in Neurology. 2009;22(2):121–125. doi: 10.1097/WCO.0b013e328326f6dc00019052-200904000-00002. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Ryan CM, Day NL, Goldschmidt L, Willford JA. Prenatal tobacco effects on neuropsychological outcomes among preadolescents. Journal of Developmental & Behavioral Pediatrics. 2001;22(4):217–225. doi: 10.1097/00004703-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Crane-Godreau MA, Maccani MA, Eszterhas SK, Warner SL, Jukosky JA, Fiering S. Exposure to Cigarette Smoke Disrupts CCL20-Mediated Antimicrobial Activity in Respiratory Epithelial Cells. The Open Immunology Journal. 2009;2:86–93. doi: 10.2174/1874226200902010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Singh AL, Iliadou A, Lambe M, Hultman CM, Grann M, Lichtenstein P. Familial confounding of the association between maternal smoking during pregnancy and offspring criminality: a population-based study in Sweden. Archives of General Psychiatry. 2010;67(5):529–538. doi: 10.1001/archgenpsychiatry.2010.33. 67/5/529 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Singh AL, Iliadou A, Lambe M, Hultman CM, Neiderhiser JM, Lichtenstein P. A quasi-experimental study of maternal smoking during pregnancy and offspring academic achievement. Child Development. 2010;81(1):80–100. doi: 10.1111/j.1467-8624.2009.01382.x. CDEV1382 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Turkheimer EN, Eaves LJ, Corey LA, Berg K, Solaas MH, Emery RE. The role of the children of twins design in elucidating causal relations between parent characteristics and child outcomes. The Journal of Child Psychology and Psychiatry. 2003;44(8):1130–1144. doi: 10.1111/1469-7610.00196. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, Lahey BB. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Development and Psychopathology. 2008;20(1):139–164. doi: 10.1017/S0954579408000072. S0954579408000072 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij SR, Wouters H, Yonker JE, Painter RC, Roseboom TJ. Prenatal undernutrition and cognitive function in late adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(39):16881–16886. doi: 10.1073/pnas.1009459107. 1009459107 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty SP, Grabowski J, Hoffman C, Ng SP, Zelikoff JT. Early life insult from cigarette smoke may be predictive of chronic diseases later in life. Biomarkers. 2009;14(Suppl 1):97–101. doi: 10.1080/13547500902965898. [DOI] [PubMed] [Google Scholar]
- Du T, Zamore PD. Beginning to understand microRNA function. Cell Research. 2007;17(8):661–663. doi: 10.1038/cr.2007.67. cr200767 [pii] [DOI] [PubMed] [Google Scholar]
- Einarson A, Riordan S. Smoking in pregnancy and lactation: a review of risks and cessation strategies. European Journal of Clinical Pharmacology. 2009;65(4):325–330. doi: 10.1007/s00228-008-0609-0. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40(6):630–641. doi: 10.1097/00004583-200106000-00007. S0890-8567(09)60466-4 [pii] [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Archives of General Psychiatry. 1998;55(8):721–727. doi: 10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]
- Filiberto AC, Maccani MA, Koestler D, Wilhelm-Benartzi C, Avissar-Whiting M, Banister CE, Marsit CJ. Birthweight is associated with DNA promoter methylation of the glucocorticoid receptor in human placenta. Epigenetics. 2011;6(5):566–572. doi: 10.4161/epi.6.5.15236. 15236 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA, O’Connell CM, Watkinson B. 60- and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: cognitive and language assessment. Journal of Developmental and Behavioral Pediatrics. 1992;13(6):383–391. [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicology and Teratology. 1992;14(5):299–311. doi: 10.1016/0892-0362(92)90036-a. 0892-0362(92)90036-A [pii] [DOI] [PubMed] [Google Scholar]
- Fuchikami M, Morinobu S, Segawa M, Okamoto Y, Yamawaki S, Ozaki N, Terao T. DNA methylation profiles of the brain-derived neurotrophic factor (BDNF) gene as a potent diagnostic biomarker in major depression. PLoS One. 2011;6(8):e23881. doi: 10.1371/journal.pone.0023881. PONE-D-11-04668 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman MW. Developmental origins of health and disease. The New England Journal of Medicine. 2005;353(17):1848–1850. doi: 10.1056/NEJMe058187. 353/17/1848 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Gardener H, Buka SL. Maternal smoking during pregnancy and children’s cognitive and physical development: a causal risk factor? American Journal of Epidemiology. 2008;168(5):522–531. doi: 10.1093/aje/kwn175. kwn175 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Keyes K, Simuro N. Mental disorders and nicotine dependence among pregnant women in the United States. Obstetrics & Gynecology. 2007;109(4):875–883. doi: 10.1097/01.AOG.0000255979.62280.e6. 109/4/875 [pii] [DOI] [PubMed] [Google Scholar]
- Guerrero-Preston R, Goldman LR, Brebi-Mieville P, Ili-Gangas C, Lebron C, Witter FR, Sidransky D. Global DNA hypomethylation is associated with in utero exposure to cotinine and perfluorinated alkyl compounds. Epigenetics. 2010;5(6):539–546. doi: 10.4161/epi.5.6.12378. 12378 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Choufani S, Ferreira J, Smith A, Chitayat D, Shuman C, Weksberg R. Altered gene expression and methylation of the human chromosome 11 imprinted region in small for gestational age (SGA) placentae. Developmental Biology. 2008;320(1):79–91. doi: 10.1016/j.ydbio.2008.04.025. S0012-1606(08)00312-6 [pii] [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- Herbeck YE, Gulevich RG, Amelkina OA, Plyusnina IZ, Oskina IN. Conserved methylation of the glucocorticoid receptor gene exon 1(7) promoter in rats subjected to a maternal methyl-supplemented diet. International Journal of Developmental Neuroscience. 2010;28(1):9–12. doi: 10.1016/j.ijdevneu.2009.10.004. S0736-5748(09)00163-4 [pii] [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Feil R, Constancia M, Fraga M, Junien C, Carel JC, Albertsson-Wikland K. Child Health, Developmental Plasticity, and Epigenetic Programming. Endocrine Reviews. 2010 doi: 10.1210/er.2009-0039. er.2009-0039 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annual Review of Neuroscience. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677 24/1/677. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts SC, Seguin JR, Zoccolillo M, Boivin M, Tremblay RE. Associations of maternal prenatal smoking with early childhood physical aggression, hyperactivity-impulsivity, and their co-occurrence. Journal of Abnormal Child Psychology. 2007;35(2):203–215. doi: 10.1007/s10802-006-9073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts SC, Seguin JR, Zoccolillo M, Boivin M, Tremblay RE. Maternal prenatal smoking, parental antisocial behavior, and early childhood physical aggression. Development and Psychopathology. 2008;20(2):437–453. doi: 10.1017/S0954579408000217. S0954579408000217 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neuroscience & Biobehavioral Reviews. 2006;30(1):24–41. doi: 10.1016/j.neubiorev.2005.04.005. S0149-7634(05)00095-3 [pii] [DOI] [PubMed] [Google Scholar]
- Jaakkola N, Jaakkola MS, Gissler M, Jaakkola JJ. Smoking during pregnancy in Finland: determinants and trends, 1987–1997. American Journal of Public Health. 2001;91(2):284–286. doi: 10.2105/ajph.91.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. DNA methylation and imprinting: why bother? Trends in Genetics. 1997;13(8):323–329. doi: 10.1016/s0168-9525(97)01180-3. [DOI] [PubMed] [Google Scholar]
- Johansson AL, Dickman PW, Kramer MS, Cnattingius S. Maternal smoking and infant mortality: does quitting smoking reduce the risk of infant death? Epidemiology. 2009;20(4):590–597. doi: 10.1097/EDE.0b013e31819dcc6a. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Lynch ME, Carroll J. The impact of maternal smoking on fast auditory brainstem responses. Neurotoxicology and Teratology. 2009;31(4):216–224. doi: 10.1016/j.ntt.2009.02.002. S0892-0362(09)00016-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddar T, Rouault JP, Chien WW, Chebel A, Gadoux M, Salles G, Magaud JP. Two new miR-16 targets: caprin-1 and HMGA1, proteins implicated in cell proliferation. Biology of the Cell. 2009;101(9):511–524. doi: 10.1042/BC20080213. BC20080213 [pii] [DOI] [PubMed] [Google Scholar]
- Kafri T, Ariel M, Brandeis M, Shemer R, Urven L, McCarrey J, Razin A. Developmental pattern of gene-specific DNA methylation in the mouse embryo and germ line. Genes & Development. 1992;6(5):705–714. doi: 10.1101/gad.6.5.705. [DOI] [PubMed] [Google Scholar]
- Ke X, Schober ME, McKnight RA, O’Grady S, Caprau D, Yu X, Lane RH. Intrauterine growth retardation affects expression and epigenetic characteristics of the rat hippocampal glucocorticoid receptor gene. Physiological Genomics. 2010;42(2):177–189. doi: 10.1152/physiolgenomics.00201.2009. physiolgenomics.00201.2009 [pii] [DOI] [PubMed] [Google Scholar]
- Kiechl-Kohlendorfer U, Ralser E, Pupp Peglow U, Reiter G, Griesmaier E, Trawoger R. Smoking in pregnancy: a risk factor for adverse neurodevelopmental outcome in preterm infants? Acta Paediatrica. 2010;99(7):1016–1019. doi: 10.1111/j.1651-2227.2010.01749.x. APA1749 [pii] [DOI] [PubMed] [Google Scholar]
- Knopik VS. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Developmental Neuropsychology. 2009;34(1):1–36. doi: 10.1080/87565640802564366. 907760348 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Jacob T, Slutske WS, Bucholz KK, Madden PA, Martin NG. Maternal alcohol use disorder and offspring ADHD: disentangling genetic and environmental effects using a children-of-twins design. Psychological Medicine. 2006;36(10):1461–1471. doi: 10.1017/S0033291706007884. S0033291706007884 [pii] [DOI] [PubMed] [Google Scholar]
- Knopik VS, Jacob T, Haber JR, Swenson LP, Howell DN. Paternal alcoholism and offspring ADHD problems: a children of twins design. Twin Research and Human Genetics. 2009;12(1):53–62. doi: 10.1375/twin.12.1.53 10.1375/twin.12.1.53. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, McGeary JE, Nugent N, Francazio S, Heath AC. Smoking during pregnancy, maternal xenobiotic metabolism genes, and child externalizing behavior: A case-crossover design. Behavior Genetics. 2010;40:799–800. [Google Scholar]
- Knopik VS, McGeary JE, Nugent N, Francazio S, Heath AC. Child ADHD: Maternal xenobiotic metabolism genes and smoking during pregnancy. Behavior Genetics in press. [Google Scholar]
- Knopik VS, Sparrow EP, Madden PA, Bucholz KK, Hudziak JJ, Reich W, Heath AC. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychological Medicine. 2005;35(5):625–635. doi: 10.1017/s0033291704004155. [DOI] [PubMed] [Google Scholar]
- Kuja-Halkola R, D’Onofrio BM, Iliadou AN, Langstrom N, Lichtenstein P. Prenatal smoking exposure and offspring stress coping in late adolescence: no causal link. International Journal of Epidemiology. 2010;39(6):1531–1540. doi: 10.1093/ije/dyq133. dyq133 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe M, Hultman C, Torrang A, Maccabe J, Cnattingius S. Maternal smoking during pregnancy and school performance at age 15. Epidemiology. 2006;17(5):524–530. doi: 10.1097/01.ede.0000231561.49208.be. [DOI] [PubMed] [Google Scholar]
- Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Seminars in Perinatology. 1996;20(2):115–126. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- Lee BE, Hong YC, Park H, Ha M, Kim JH, Chang N, Ha EH. Secondhand smoke exposure during pregnancy and infantile neurodevelopment. Environmental Research. 2011;111(4):539–544. doi: 10.1016/j.envres.2011.02.014. S0013-9351(11)00069-7 [pii] [DOI] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. 0092-8674(93)90529-Y [pii] [DOI] [PubMed] [Google Scholar]
- Linnet KM, Wisborg K, Obel C, Secher NJ, Thomsen PH, Agerbo E, Henriksen TB. Smoking during pregnancy and the risk for hyperkinetic disorder in offspring. Pediatrics. 2005;116(2):462–467. doi: 10.1542/peds.2004-2054. 116/2/462 [pii] [DOI] [PubMed] [Google Scholar]
- Lumley T, Levy D. Bias in the case-crossover design: Implications for studies of air pollution. Environmetrics. 2000;11:689–704. [Google Scholar]
- Lundberg F, Cnattingius S, D’Onofrio B, Altman D, Lambe M, Hultman C, Iliadou A. Maternal smoking during pregnancy and intellectual performance in young adult Swedish male offspring. Paediatrics and Perinatal Epidemiology. 2009;24(1):79–87. doi: 10.1111/j.1365-3016.2009.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccani MA, Avissar-Whiting M, Banister CE, McGonnigal B, Padbury JF, Marsit CJ. Maternal cigarette smoking during pregnancy is associated with downregulation of miR-16, miR-21, and miR-146a in the placenta. Epigenetics. 2010;5(7):583–589. doi: 10.4161/epi.5.7.12762. 12762 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccani MA, Knopik VS. Cigarette smoke exposure-associated alterations to non-coding RNA. Frontiers in Genetics. 2012;3:53. doi: 10.3389/fgene.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccani MA, Marsit CJ. Epigenetics in the placenta. American Journal of Reproductive Immunology. 2009;62(2):78–89. doi: 10.1111/j.1600-0897.2009.00716.x. AJI716 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccani MA, Marsit CJ. Exposure and fetal growth-associated miRNA alterations in the human placenta. Clinical Epigenetics. 2011;2(2):401–404. doi: 10.1007/s13148-011-0046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin J, Fried PA, Watkinson B. A comparison of active and passive smoking during pregnancy: long-term effects. Neurotoxicology and Teratology. 1991;13(1):5–12. doi: 10.1016/0892-0362(91)90021-n. [DOI] [PubMed] [Google Scholar]
- Maughan B, Taylor A, Caspi A, Moffitt TE. Prenatal smoking and early childhood conduct problems: testing genetic and environmental explanations of the association. Archives of General Psychiatry. 2004;61(8):836–843. doi: 10.1001/archpsyc.61.8.83661/8/836. [pii] [DOI] [PubMed] [Google Scholar]
- McCartney JS, Fried PA, Watkinson B. Central auditory processing in school-age children prenatally exposed to cigarette smoke. Neurotoxicology and Teratology. 1994;16(3):269–276. doi: 10.1016/0892-0362(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Meyer KA, Williams P, Hernandez-Diaz S, Cnattingius S. Smoking and the risk of oral clefts: exploring the impact of study designs. Epidemiology. 2004;15(6):671–678. doi: 10.1097/01.ede.0000142148.51230.60. 00001648-200411000-00005 [pii] [DOI] [PubMed] [Google Scholar]
- Meza-Sosa KF, Valle-Garcia D, Pedraza-Alva G, Perez-Martinez L. Role of microRNAs in central nervous system development and pathology. Journal of Neuroscience Research. 2011 doi: 10.1002/jnr.22701. [DOI] [PubMed] [Google Scholar]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(4):378–385. doi: 10.1097/00004583-200204000-00009. S0890-8567(09)60864-9 [pii] [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? The American Journal of Psychiatry. 1996;153(9):1138–1142. doi: 10.1176/ajp.153.9.1138. [DOI] [PubMed] [Google Scholar]
- Miska EA. How microRNAs control cell division, differentiation and death. Current Opinion in Genetics & Development. 2005;15(5):563–568. doi: 10.1016/j.gde.2005.08.005. S0959-437X(05)00134-6 [pii] [DOI] [PubMed] [Google Scholar]
- Mohsin M, Bauman AE. Socio-demographic factors associated with smoking and smoking cessation among 426,344 pregnant women in New South Wales, Australia. BMC Public Health. 2005;5:138. doi: 10.1186/1471-2458-5-138. 1471-2458-5-138 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfese DL. Predicting dyslexia at 8 years of age using neonatal brain responses. Brain and Language. 2000;72(3):238–245. doi: 10.1006/brln.2000.2287S0093-934X(00)92287-9. [pii] [DOI] [PubMed] [Google Scholar]
- Monk M, Boubelik M, Lehnert S. Temporal and regional changes in DNA methylation in the embryonic, extraembryonic and germ cell lineages during mouse embryo development. Development. 1987;99(3):371–382. doi: 10.1242/dev.99.3.371. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. 6034 [pii] [DOI] [PubMed] [Google Scholar]
- Olds DL, Henderson CR, Jr, Tatelbaum R. Intellectual impairment in children of women who smoke cigarettes during pregnancy. Pediatrics. 1994;93(2):221–227. [PubMed] [Google Scholar]
- Pajer K, Andrus BM, Gardner W, Lourie A, Strange B, Campo J, Bridge J, Blizinsky K, Dennis K, Vedell P, Churchill GA, Redel EE. Discovery of blood transcriptomic markers for depression in animal models and pilot validation in subjects with early-onset major depression. Translational Psychiatry. 2012;2:e101. doi: 10.1038/tp.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Barsness B, Martenson T, Tanner D, Allan AM. Behavioral teratogenicity induced by nonforced maternal nicotine consumption. Neuropsychopharmacology. 2007;32(3):693–699. doi: 10.1038/sj.npp.1301066. 1301066 [pii] [DOI] [PubMed] [Google Scholar]
- Price TS, Grosser T, Plomin R, Jaffee SR. Fetal genotype for the xenobiotic metabolizing enzyme NQO1 influences intrauterine growth among infants whose mothers smoked during pregnancy. Child Development. 2010;81(1):101–114. doi: 10.1111/j.1467-8624.2009.01383.x. CDEV1383 [pii] [DOI] [PubMed] [Google Scholar]
- Razin A, Shemer R. DNA methylation in early development. Human Molecular Genetics. 1995;4(Spec No):1751–1755. doi: 10.1093/hmg/4.suppl_1.1751. [DOI] [PubMed] [Google Scholar]
- Reamon-Buettner SM, Mutschler V, Borlak J. The next innovation cycle in toxicogenomics: environmental epigenetics. Mutation Research. 2008;659(1–2):158–165. doi: 10.1016/j.mrrev.2008.01.003. S1383-5742(08)00025-2 [pii] [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Boivin J, Hay DF, van den Bree M, Thapar A. Disentangling prenatal and inherited influences in humans with an experimental design. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(7):2464–2467. doi: 10.1073/pnas.0808798106. 0808798106 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F, Harold GT, Boivin J, van den Bree M, Hay DF, Thapar A. The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychological Medicine. 2010;40(2):335–345. doi: 10.1017/S0033291709005911. S0033291709005911 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz LC. The Dutch Hunger Winter and the developmental origins of health and disease. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(39):16757–16758. doi: 10.1073/pnas.1012911107. 1012911107 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton M, Fox NL, Hebel JR. Prenatal exposure to tobacco: II. Effects on cognitive functioning at age three. International Journal of Epidemiology. 1990;19(1):72–77. doi: 10.1093/ije/19.1.72. [DOI] [PubMed] [Google Scholar]
- Sexton M, Hebel JR. A clinical trial of change in maternal smoking and its effect on birth weight. Journal of the American Medical Association. 1984;251(7):911–915. [PubMed] [Google Scholar]
- Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. Belmont, CA: Wadsworth, Cengage Learning; 2002. [Google Scholar]
- Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. American Journal of Obstetrics & Gynecology. 2000;182(2):465–472. doi: 10.1016/s0002-9378(00)70240-7. S0002937800645337 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea AK, Steiner M. Cigarette smoking during pregnancy. Nicotine & Tobacco Research. 2008;10(2):267–278. doi: 10.1080/14622200701825908. 790124430 [pii] [DOI] [PubMed] [Google Scholar]
- Smoking during pregnancy--United States 1990–2002. MMWR Morbidity and Mortality Weekly Report. 2004;53(39):911–915. mm5339a1 [pii] [PubMed] [Google Scholar]
- Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(14):5478–5483. doi: 10.1073/pnas.0508035103. 0508035103 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein AD, Ravelli AC, Lumey LH. Famine, third-trimester pregnancy weight gain, and intrauterine growth: the Dutch Famine Birth Cohort Study. Human Biology. 1995;67(1):135–150. [PubMed] [Google Scholar]
- Stein Z, Susser M, Saenger G, Marolla F. Nutrition and mental performance. Science. 1972;178(62):708–713. doi: 10.1126/science.178.4062.708. [DOI] [PubMed] [Google Scholar]
- Suter M, Abramovici A, Showalter L, Hu M, Shope CD, Varner M, Aagaard-Tillery K. In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism. 2010;59(10):1481–1490. doi: 10.1016/j.metabol.2010.01.013. S0026-0495(10)00028-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter M, Ma J, Harris AS, Patterson L, Brown KA, Shope C, Aagaard-Tillery KM. Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics. 2011;6(11) doi: 10.4161/epi.6.11.17819. 17819 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Entringer S, Buss C, Wadhwa PD. Developmental origins of health and disease: environmental exposures. Seminars in Reproductive Medicine. 2009;27(5):391–402. doi: 10.1055/s-0029-1237427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MB, Ferris JS, Pilsner R, Flom JD, Tehranifar P, Santella RM, Susser E. Genomic DNA methylation among women in a multiethnic New York City birth cohort. Cancer Epidemiology, Biomarkers, & Prevention. 2008;17(9):2306–2310. doi: 10.1158/1055-9965.EPI-08-0312. 17/9/2306 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Fowler T, Rice F, Scourfield J, van den Bree M, Thomas H, Hay D. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. The American Journal of Psychiatry. 2003;160(11):1985–1989. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- Thapar A, Harold G, Rice F, Ge X, Boivin J, Hay D, Lewis A. Do intrauterine or genetic influences explain the foetal origins of chronic disease? A novel experimental method for disentangling effects. BMC Medical Research Methodology. 2007;7:25. doi: 10.1186/1471-2288-7-25. 1471-2288-7-25 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Rice F, Hay D, Boivin J, Langley K, van den Bree M, Harold G. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: evidence from a novel design. Biological Psychiatry. 2009;66(8):722–727. doi: 10.1016/j.biopsych.2009.05.032. S0006-3223(09)00704-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielen A, Klus H, Muller L. Tobacco smoke: unraveling a controversial subject. Experimental and Toxicologic Pathology. 2008;60(2–3):141–156. doi: 10.1016/j.etp.2008.01.014. S0940-2993(08)00013-4 [pii] [DOI] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Lotfipour S, Leonard G, Perron M, Richer L, Veillette S, Paus T. Maternal smoking during pregnancy is associated with epigenetic modifications of the brain-derived neurotrophic factor-6 exon in adolescent offspring. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2010;153B(7):1350–1354. doi: 10.1002/ajmg.b.31109. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [Google Scholar]
- US Department of Health and Human Services. A Report of the Surgeon General: How Tobacco Smoke Causes Disease: What It Means To You. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7(8):847–854. doi: 10.1038/nn1276nn1276. [pii] [DOI] [PubMed] [Google Scholar]
- Weaver IC, La Plante P, Weaver S, Parent A, Sharma S, Diorio J, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Molecular and Cellular Endocrinology. 2001;185(1–2):205–218. doi: 10.1016/s0303-7207(01)00635-9. S0303720701006359 [pii] [DOI] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(7):892–899. doi: 10.1097/00004583-199907000-00020. S0890-8567(09)66539-4 [pii] [DOI] [PubMed] [Google Scholar]
- Wilhelm-Benartzi CS, Houseman EA, Maccani MA, Poage GM, Koestler DC, Langevin SM, Marsit CJ. In Utero Exposures, Infant Growth, and DNA Methylation of Repetitive Element and Developmentally Related Genes in Human Placenta. Environmental Health Perspectives. 2011 doi: 10.1289/ehp.1103927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wossidlo M, Nakamura T, Lepikhov K, Marques CJ, Zakhartchenko V, Boiani M, Walter J. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nature Communications. 2011;2:241. doi: 10.1038/ncomms1240. ncomms1240 [pii] [DOI] [PubMed] [Google Scholar]
- Xiong Z, Leme AS, Ray P, Shapiro SD, Lee JS. CX3CR1+ Lung Mononuclear Phagocytes Spatially Confined to the Interstitium Produce TNF-{alpha} and IL-6 and Promote Cigarette Smoke-Induced Emphysema. Journal of Immunology. 2011 doi: 10.4049/jimmunol.1003221. jimmunol.1003221 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]