Abstract
Antigen-presenting cells (APCs) sense the microenvironment through several types of receptors that recognize pathogen-associated molecular patterns. In particular, C-type lectins receptors (CLRs), which are expressed by distinct subsets of dendritic cells (DCs) and macrophages (MØs), recognize and internalize specific carbohydrate antigens in a Ca2+-dependent manner. The targeting of these receptors is becoming an efficient strategy for parasite recognition. However, relatively little is known about how CLRs are involved in both pathogen recognition and the internalization of parasites. The role of CLRs in parasite infections is an area of considerable interest because this research will impact our understanding of the initiation of innate immune responses, which influences the outcome of specific immune responses. This paper attempts to summarize our understanding of the effects of parasites' interactions with CLRs.
1. Introduction
Lectins are a diverse group of mono- and multivalent proteins and glycoproteins of nonimmune origin that have selective affinity for a carbohydrate or a group of carbohydrates [1]. These proteins are widely distributed in plants, animals, and microorganisms. In animals, lectins have been identified in a great number of cells. Lectins are either embedded in intracellular or cell surface membranes or are present in a soluble form in the plasma. Inside cells, lectins are also found in the cytosol and in the nucleus. Animal lectins play a crucial role in both physiological and pathological processes. Specific interactions between lectins and complex carbohydrates (glycoproteins, glycolipids, polysaccharides, or proteoglycans) are involved in numerous basic phenomena, such as embryonic development, intracellular trafficking, cell-cell and cell-matrix recognition, cell homing, endocytosis, phagocytosis, inflammation, and the metastatic spread of cancer cells (Table 1) [2].
Table 1.
Summary of structural and functional properties of the lectin family receptors.
| Group | Molecules structure | Family members | Ligands | Expression | Function | Reference |
|---|---|---|---|---|---|---|
| MR | Mannose, fucose, and N-acetylglucosamine |
MoPh, retina DCs, LCs, Fbls, and kidney | Pathogen recognition, antigen presentation, clearance of endogenous cytopathic molecules, and regulation of circulating hormones | [3–14] | ||
| DC-SIGN | Mannose, ICAM-3 | Mesangial cells and CMs, MØ, and DCs | Pathogen recognition, antigen presentation, cell migration, and DC-T-cell interactions | |||
| C-type | Type-I Type-II (Tm) |
SIGNR-1 | Zymosan, mannans, and dextran | iDCs spleen MZ, lymph node, and pMØ | Clearance of blood borne antigens | |
| Dentin1 | β-glucans | DCs, neutrophils, and splenic T cells | Antifungal host defense, induction of TNF-α, and regulation of T-cell proliferation | |||
| Dectin2 | α-mannans | MØ, DCs | Impairment of UV-induced tolerance | |||
| mMGL1 | Gal | MØ, DCs | Internalization and antigen presentation, bind to CD45 to inhibit T cells | |||
| mMGL2 | Sructure Lex | MØ, DCs | Anti-inflammatory response | |||
| L-SIGN | Structure Le(a,b,y) | Liver sinusoidal endothelial cells | Antigen receptor | |||
|
| ||||||
| P-type | Type-I (Tm) |
CD-MPR CI-MPR |
Man-P-GlcNAc Man-6-P |
Lysosomal hydrolases | Transport Man-6-P containing acid hydrolases from the Golgi to endosomal/lysosomal compartments | [15–17] |
|
| ||||||
| F-type | AAA MsaFBP32 |
Fucose | Liver and kidney | Modulation of cell functions | [18–22] | |
|
| ||||||
| I-type | Type-I (Tm) |
Siglec-1 Siglec-2 Siglec-4 Siglec-15 |
Sialic acids with N- and O-linked glycosylations | Myeloid and lymphoid cells | Regulation of cell signaling from leucocytes | [23, 24] |
| Siglec-3 10 members humans (3, 5, 6, 7, 8, 9, 10, 11, 14, 16) Rodents Siglec-3, E, F, G, H |
Endocytic receptors | |||||
Abbreviations: Tm: transmenbrane; MØ: macrophages; pMØ: peritoneal macrophages; Dcs: dendritic cells; iDCs: immature dendritic cells; MoPh: mononuclear phagocytes; Fbls: fibroblasts; LCs: langerhans cells; CMs: cardiomyocytes; Lex: Lewis x, a, b, and y structures; Gal: galactose; MR: mannose receptor; DC-SIGN: dendritic cell-specific ICAM-3-grabbing nonintegrin; SIGNR-1: SIGN-related 1; homologe DC-SIGN; mMGL: macrophage galactose type c-lectin; L-SIGN: liver/lymph node-specific ICAM-3 grabbing nonintegrin; CD-MPR: cation-dependent mannose 6-phosphate receptor; CI-MPR: cation-independent mannose 6-phosphate receptor; Man-6-P: mannose 6-phosphate; Man-P-GlcNAc: mannose 6-phisphate N-acetylglucosamine ester; AAA: Anguilla anguilla agglutinin; MsaFBP32: F-lectin present in striped bass (Morone saxatilis).
2. Structural Characteristics of C-Type Lectin Receptors
The CLRs constitute a superfamily of more than 1,000 proteins classified into 17 groups based on their phylogeny and domain organization. Most CLRs possess one or more carbohydrate recognition domains (CRDs) or C-type lectin-like domains (CTLDs). The CTLD is a conserved structural motif containing as two protein loops stabilized by two disulfide bridges at the base of each loop. The second loop is more flexible than the first and generally contains the ligand binding site. Most CLRs are membrane-associated receptors that are involved in antigen capture and presentation [25, 26]. Endocytosis mediated by CLRs is guided by their intracellular internalization motifs, whereas some CLRs contain ITIM (immunoreceptor tyrosine-based inhibitory motif)-or ITAM (immunoreceptor tyrosine-based activation motif)-like motifs in their cytoplasmic domains, illustrating the potential immune-suppression or immune-activation functions of these receptors (Figure 1) [27].
Figure 1.
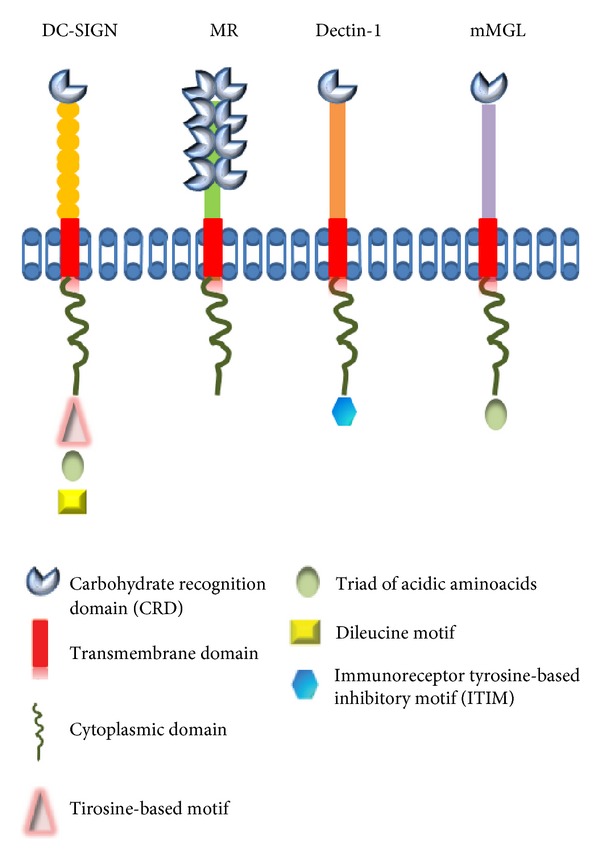
Structure of members of the C-type lectin (DC-SIGN, MR, Dectin1, and MGL). These receptors contain one or more carbohydrate-recognition domain (CRD), transmembrane domain, and cytoplasmic domain may contains tyrosine-based motif, triad of acidic amino acids, dileucine motif or immunoreceptor tyrosine-based inhibitory motif.
Based on the primary structure of their CRDs, their folding patterns, and their cation requirements, animal lectins can be classified into several families, including C-, F-, P-, and I-type lectins, galectin, pentraxin, and others (Table 1) [18]. However, the most important molecules from the CLR family include macrophage galactose-type C-type lectin (MGL), dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN), the mannose receptor (MR), DEC205, and Dectin-1 (Figure 1).
3. Role of C-Type Lectin Receptors in the Immune Response
The initial recognition of an invading pathogen by antigen-presenting cells APCs, such as macrophages (MØs) or dendritic cells (DCs), is crucial in determining the type of effector T cell that subsequently mediates an immune response [1, 2]. APCs are equipped with highly specialized receptors, including an array of pattern recognition receptors (PRRs), such as C-type receptors (CLRs) and Toll-like receptors (TLRs). These receptors play an important role in the activation/maturation of APCs upon binding with conserved pathogen structures known as pathogen-associated molecular patterns (PAMPs). In contrast to TLRs, CLRs recognize and internalize specific carbohydrate antigens expressed by pathogens and host tissues in Ca2+-dependent manner [18, 25–27].
Protein-carbohydrate interactions have important roles in two distinct aspects of the immune response. These interactions are involved both in pathogen recognition and in the cellular interactions that lead to pathogen neutralization [28]. Lectin receptors play an important role in the innate immune response by recognizing and binding specific carbohydrate moieties (usually a nonreducing terminal monosaccharide or oligosaccharide) on the surface of potential pathogens through CRDs [1, 2]. CRDs, in combination with other domains, can recognize carbohydrate moieties and induce agglutination, immobilization, complement-mediated opsonization and lysis [25].
In this review, we focus on integral membrane C-type lectins and their participation in the recognition of glycosylated parasite antigens. Despite the presence of a highly conserved domain, C-type lectins are functionally diverse and have been implicated in various processes, including cell adhesion, tissue integration and remodeling, platelet activation, complement activation, pathogen recognition, endocytosis, and phagocytosis [3, 29, 30].
The importance of C-type lectins is highlighted by the fact that several pathogens and tumor antigens take advantage of these receptors to escape intracellular degradation and to suppress the generation of an efficient immune response [31]. Several studies have demonstrated that some C-type lectins may function as adhesion, signaling, or antigen-uptake receptors [32–35], and these results are consistent with the fact that some CLRs are present on MØs and DCs, which play a role in the initial step of capturing the antigens containing carbohydrates [36].
Several CLRs have been shown to contribute to the loading of endocytosed antigens on MHC class I and class II, thereby facilitating effective antigen-specific CD4+ and CD8+ T-cell responses [37, 38]. There are evidence that some CLRs (like DC-SING, DC205, and Dectin-1) are able to trigger distinct signaling pathways that modulate cell functions through the expression of specific molecules and cytokines, in most cases promote the antigen presentation and determining the polarizations of T cells [4, 39]. However, most evidence about CLR trigger signaling pathways have emerged using virus, bacterial pathogens, fungus, or peptides. There are not evidence about the interaction of parasites with CLRs and activation of a signaling pathway.
The signaling through MGL is emerging recently, using DCs, has been demonstrated that MGL engagement to anti-MGL antibody or MUC19Tn triggered the phosphorylation of ERK1,2 and the activation of NF-kB signal promoting DC activation and increase in antigen-specific CD8+ T-cell activation; however, the effects of this activation are strongly dependent on the type of stimulus added to the cells [5].
Moreover, several studies suggest that CLRs may also modulate immune reactions through cross-talk with other receptors, especially TLRs. These results indicate that the outcome of an immune response is determined by the balance between triggering the two receptors families [5, 40]. Many transmembrane C-type lectins belonging to groups II, V, and VI are expressed primarily by myeloid cells. Although many are “orphan” receptors, others have been shown to promote the phagocytosis of nonopsonized microbes and to induce cytokine production in MØs and DCs, leukocytes that play critical roles in innate immunity and in the subsequent modulation of adaptive immune responses [41]. These properties make the C-type lectin family an optimal tool for APCs to target parasites.
4. C-Type Lectins in Parasitic Infection (Table 2)
Table 2.
C-type lectins in parasitic infection.
| Parasite | Receptor | Model | In vivo/in vitro | Role | Reference |
|---|---|---|---|---|---|
| Protozoo | |||||
| L. donovani | MR | BALB/c mice | in vivo/ in vitro | Uptake of mannose containing glycoconjugates | [48] |
| MR | Swiss albino mice | in vitro | Binding promastigotes | [36] | |
| MR | hmDMØ | in vitro | Attachment and ingestion promastigotes | [49] | |
| L. amazonensis | MR | Skin Fbls | in vitro | Uptake of mannosylated ligands | [6] |
| L. major | MR | BMDMs MR-KO mice |
in vivo | Recognizes mannose residues on the surface Leishmania, but it's not essential for host defense | [45] |
| L. pifanoi | DC-SIGN | MDDCs cell line K562 |
in vitro | Binding and internalization of amastigotes | [50] |
|
L. pifanoi
L. infantum |
DC-SIGN | IMDDCs cell lineK562 |
in vitro | Receptor for promastigotes and amastigote infective stages from both visceral and cutaneous leishmaniasis | [51] |
|
T. cruzi
Tulahuen strain |
MR | BALB/c mice Cell line J774 (MØ) |
in vivo/in vitro | Bind to Cz, increasing MR recycling which leads to arginase activity | [46] |
| Y and DM strains | MR | CM and MØ | in vitro | Adhesion and uptake of parasites | [52] |
| T. brucei | MGL | C57BL/6 mice BALB/c mice |
in vivo | Marker of aaMØ | [53] |
| Nematodes | |||||
| T. muris | MR | C57BL/6 MR-KO mice | in vivo/in vitro | Recognized components E/S of parasites | [47] |
| Trematodes | |||||
| S. mansoni | MGL | Cell lines SW948, SKBR3, ZR75-1 CHO, BLM, FM3.29 FM6, SK23mel |
in vitro | Recognized LDN and LDNF glycans | [54] |
| MGL | Human DCs | in vitro | Internalization of glycolipids of SEA | [55] | |
| DC-SIGN | Human DCs | in vitro | Adhesion to glycolipids of SEA | [7] | |
| DC-SIGN | Human DCs | in vitro | Recognize glycans of SEA | [56] | |
| L-SIGN | Cell line K562 | in vitro | Binds to structures Lea,b,y of SEA | [8] | |
| L-SING | Cell line K562 | in vitro | Binds and internalization of SEA | [57] | |
| SIGNR1 | BALB/c WT or SIGNR1-KO |
in vivo/in vitro | Recognize antigens of AWA and SEA | [9] | |
| Dectin-2 | C57BL/6 | in vivo/in vitro | Binds SEA component | [58] | |
| MR | C57BL/6 WT or MR-KO |
in vivo/in vitro | Internalization E/S material by schistosome larvae | [59] | |
| Cestodes T. crassiceps |
MGL DC-SIGN |
Human DCs | in vitro | TcES positively modulated the expression of MGL but negatively modulated DC-SIGN | [60] |
Abbreviations: MR: mannose receptor; DC-SIGN: dendritic cell-specific ICAM-3 grabbing nonintegrin; SIGNR-1: SIGN-related 1; homologe DC-SIGN; mMGL: macrophage galactose type c-lectin; L-SIGN: liver/lymph node-specific ICAM-3 grabbing nonintegrin; Fbls: fibroblasts; BMDMs: bone marrow-derived macrophages; MDDCs: monocyte-derived dendritic cells; IMDDCs: immature monocyte-derived DCs; MØ: macrophages; CM: cardiomyocyte; Cz: cruzipaina; E/S: excretory/secretory; LDN: [GalNAcβ1–4GlcNAc-R]; LDNF: [GalNAcβ1–4(Fucα1–3)GluNAc-R]; SEA: soluble egg antigens; Le: structures of Lewis; AWA: adult worm antigen; TcES: Taenia crassiceps excreted-secreted antigens.
A number of glycan moieties have been identified in most parasites that potentially bind various CLRs, which act as sensors of the innate immune system.
4.1. Protozoa
4.1.1. Leishmania
The trypanosomatid flagellates of the genus Leishmania cause diverse diseases with varying clinical symptoms and underlying pathologies. These diseases include visceral leishmaniasis (Kala-azar), mucocutaneous leishmaniasis, cutaneous leishmaniasis, and post-Kala-azar dermal leishmaniasis (PKDL) [42].
These diseases cause significant morbidity and mortality in the 98 countries or territories, where they are endemic [43]. Leishmania have two developmental stages: the promastigote, which is an extracellular flagellated form that is transmitted by insect vectors, and the amastigote, which is an intracellular multiplicative form that multiplies within the phagocytes of the vertebrate host, a process that involves different ligand-receptor systems [44]. The repetitive structure and glycan modifications associated with many Leishmania cell surface molecules suggest that these parasites may interact with CLRs, for example, MR and DC-SIGN [6, 45].
Mannose Receptor (MR). MR is a C-type lectin. It is a transmembrane glycoprotein (175 kDa) with eight C-type-lectin-like domains (or carbohydrate-recognition domains, CRDs) that is expressed on the surface of several cell types, such as MØs, DCs, and some epithelial cells. MR mediates the binding and internalization of mannosylated glycoproteins and participates in the endocytosis of different pathogens bearing mannose residues on their surfaces [6, 46, 47].
Previous studies both in vivo and in vitro have demonstrated the involvement of MR during the recognition and internalization of promastigotes of different Leishmania species (donovani, amazonensis). Mouse peritoneal MØs infected with L. donovani exhibited a decrease in MR activity, with a loss of 50% of original binding activity after 4 days of infection. A possible explanation for this decrease in the expression of MR is the direct correlation with the number of amastigotes within MØs and the recovery of MR activity after the elimination of parasites from MØs after treatment with methotrexate/mL conjugated with bovine serum albumin modified with mannose (Man-BSA) for 3 h [48]. Competition assays with different MR ligands (Man-BSA or D-mannose) revealed an important decrease in the activity of MR, with a loss between 50% to 80% in phagocytic capacity, demonstrating the participation of MR during parasite recognition and the upregulation of MR expression during the initial steps of the infection [6, 35, 48, 49].
A recent study showed that bone marrow-derived macrophages (BMDMs) infected with L. major metacyclic promastigotes exhibit TNF-α and IL-12 production levels similar to those in MR-wild-type (MR-WT) mice and MR-knockout (MR-KO) mice. The clinical course of L. major and L. donovani infections was slightly different with respect to the area covered by lesions between the MR-WT and MR-KO mice at week 7. However, the levels of ulcer healing and the resolution of the lesions were equivalent. Moreover, assays measuring the activation of MAPKs (ERK1/2, p38, and JNK) revealed that MR is not necessary for the inhibition of ERK and p38 activation. In addition, immunohistochemical analysis of cutaneous lesions from MR-KO and MR-WT mice revealed no differences in lesion architecture or cell components. Together, these data suggest that MR is not essential for host resistance against Leishmania infections and that either redundant MØ receptors compensate for the lack of MR or MR does not play a role in parasite attachment [45].
Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Nonintegrin (DC-SIGN). Also known as CD209, DC-SIGN is a type II transmembrane CLR that is expressed on DCs and involved in cell-cell interactions through its capacity to bind ICAM-3 and ICAM-2 [50, 61]. This receptor is used by protozoan parasites of the genus Leishmania. Previous studies have investigated possible Leishmania/DC-SIGN interactions through the use of fluorescence-labeled parasites in combination with blocking agents such as anti-DC-SIGN antibodies and soluble mannan. These studies showed that DC-SIGN is a receptor for the promastigotes and amastigotes of both the visceral (L. infantum) and cutaneous (L. pifanoi) forms but not for Leishmania major metacyclic promastigotes, suggesting that DC-SIGN is a broad Leishmania receptor that exhibits variable affinity for distinct infective forms and species of the parasite [50, 51]. There is no doubt that these findings are important; however, it remains to be determined whether this recognition influences the immune response to Leishmaniasis.
4.1.2. Trypanosoma cruzi
The protozoan parasite Trypanosoma cruzi (T. cruzi), the etiological agent of human Chagas disease, is endemic in Latin America, where 18–20 million of people are infected [62]. Infection leads to an acute phase that may last between 2 and 4 months and is characterized by high numbers of parasites in the bloodstream and tissues. The control of parasite replication leads to chronic, often life-long disease. Most individuals in the chronic phase have a silent, asymptomatic clinical form of Chagas' disease and are classified as indeterminate patients [63]. However, approximately 30% of chronically infected individuals develop a severe clinical form in which digestive and/or cardiac alterations often lead to death [64–67].
During the process of parasite internalization, the interaction between receptors expressed in the host cell and the parasite is important because these receptors are responsible for recognizing the major antigens of T. cruzi. This parasite expresses mucin-like glycoproteins (TcMUCs) on its membrane. These proteins are highly glycosylated glycoconjugates (approximately 60% of their weight is carbohydrates) and are threonine-rich, serine- and proline-rich polyanionic molecules that are anchored to the plasma membrane through glycosylphosphatidylinositol [64, 68, 69].
Furthermore, T. cruzi contains a major lysosomal cysteine proteinase called cruzipain (Cz), one of the immunodominant antigens of T. cruzi. Cz is a glycoprotein of approximately 52–58 kDa and has both high mannose and complex type-N-linked glycans in the C-terminal domain. It is expressed in all stages of the parasite and is highly immunogenic in humans. Moreover, it has been shown that Cz induces the alternative activation of MØs in vitro and upregulates arginase activity. This activation profile was shown to be associated with the functional ability of these cells to promote the intracellular growth of T. cruzi [46].
Mannose Receptor (MR). Enzyme binding assays using HRP (horseradish peroxidase) as the mannosylated ligand, which were used to characterize the cardiomyocyte mannose receptor (CM-MR) and its involvement in T. cruzi invasion, demonstrated that after the infection of cardiomyocytes (CM) with T. cruzi, a considerable reduction in HRP binding was noticed. Binding was almost completely restored by treating the infected cultures with the trypanocidal drug nifurtimox [52]. These results showed that CM-MR participated in the adhesion and uptake of T. cruzi by CM.
Another study found that T. cruzi-infected MØs pre-incubated with mannose-bovine serum albumin (Man-BSA, MR specific ligand) exhibited high levels of urea, increased intracellular amastigote growth, the downregulation of JNK and p44/p42 phosphorylation, and an increase in p38 MAPK phosphorylation relative to control cells. In addition, MØs incubated with Cz or Man-BSA exhibited enhanced MR recycling. However, T. cruzi-infected peritoneal MØs incubated with an MR-blocking antibody showed reductions in arginase activity and intracellular parasite growth. Moreover, the level of MR on peritoneal cells from T. cruzi-infected BALB/c mice at 13 and 15 days after-infection has been evaluated, and flow cytometry analysis revealed an increase in F4/80+ MR+ cells as the infection progressed. Together, these results showed that the interaction with MR on MØs may be a mechanism by with T. cruzi evades the innate immune response both in vitro and in vivo [46].
4.1.3. Trypanosoma brucei
The protozoan parasite Trypanosoma brucei (T. brucei) is the causative agent of the human and animal African trypanosomiasis, which is frequently fatal if not treated. This parasite has a digenetic life cycle, replicating in the alimentary canal of its vector, the tsetse fly, and in the bloodstream of mammals. In the mammalian host, the bloodstream form of T. brucei lives and divides extracellularly in the blood, lymph, and interstitial fluids [70, 71]. The bloodstream form of T. brucei is rich in galactose-containing glycoproteins, most notably the abundant variant surface glycoprotein (VSG), which protects the parasite from the complement pathway and undergoes antigenic variation to evade specific immune responses [72].
Macrophage Galactose Type C-Lectin (MGL). MGL is a member of the type II family of C-type lectins and has an approximate molecular mass of 42 kDa. MGL is expressed on immature human and mouse DCs and MØs in the skin and lymph nodes [27, 73]. Mice contain two functional copies of the MGL gene, mMGL1 and mMGL2 [74], which are both expressed by dermal DCs and MØs [53, 75], whereas in humans, only one MGL gene is found [76]. mMGL1 and mMGL2 have different carbohydrate specificities: mMGL1 is specific for Lewis X (Lex) and LewisA structures, whereas mMGL2, similar to hMGL, recognizes α/β-GalNAc structures and galactose, including O-linked Tn-antigen, TF-antigen, and core 2 structure [54, 77]. In the skin, MGL is a marker for CD1a+ dermal DCs, a cell type with enhanced ability to stimulate naive T cells relative to other dermal APC subsets.
Raes et al. report that mMGL1 and mMGL2 are induced in peritoneal MØs during in vivo infection with T. brucei, correlating with a switch from a type I cytokine environment in the early stage of infection to a type II cytokine environment in the late and chronic phases. In addition, it has been demonstrated that the incubation of thioglycolate-elicited peritoneal MØs with IL-4 o IL-13 moderately induced mMGL1 expression and strongly induced mMGL2 expression, but IFN-γ did not [53]. The results presented in this paper suggest that the mMGL1 and mMGL2 receptors are novel markers for type II cytokine-dependent alternatively activated macrophages (aaMØ) both in vitro and in the chronic phase of infection with T. brucei. These findings are important, but the possible interaction between antigens of T. brucei and mMGL remains to be defined, as does the role of mMGL in the immune response.
4.2. Nematodes
4.2.1. Trichuris muris
Several gastrointestinal nematodes have been reported to express ligands for MR on their surface. Trichuris muris (T. muris) is a natural mouse model of the gastrointestinal nematode parasite Trichuris trichiura (T. trichiura), one of the most prevalent human helminth infections. Studies of the role of cells in immune responses to T. muris and the mechanisms of immune expulsion of these worms from mice have demonstrated that B cells and antibodies are required for resistance to thisparasite. The evasion of the immune response by T. muris causes chronic infection, which has the ability to manipulate the host immune system. T. muris excretory/secretory (E/S) products from a heterogeneous solution of worm proteins contain substances that have been shown to bear mannose and N-acetylglucosamine residues; therefore, these substances are potential ligands for C-type lectin receptors such as MR [78].
Deschoolmeester et al. showed in vitro that MR-KO-derived bone-marrow-derived MØs (BMDMs) expressed similar levels of several cytokines when exposed to T. muris E/S. The only difference observed was a reduction in the production of IL-6 by alternatively activated BMDMs in the absence of MR, and the infection of MR-KO mice revealed the expulsion of T. muris with the same kinetics as observed for WT animals and a similar cytokine response in the draining mesenteric lymph nodes. Moreover, there were no differences in MØ recruitment, the ability of MØs to become alternatively activated, goblet cell hyperplasia, or gross crypt pathology during infection. In summary, MR binds to components of T. muris, but it is not required for the development of an immune response leading to the expulsion of T. muris [47].
4.3. Helminths: Trematodes
4.3.1. Schistosoma mansoni
Parasitic helminths express various carbohydrates containing glycoproteins on their surface and release glycan-rich E/S products that can potentially bind to various CLRs [59]. The parasite helminth Schistosoma mansoni (S. mansoni) is the causative agent of the chronic disease schistosomiasis, which is the second most prevalent human parasitic disease, affecting ∼300 million people worldwide, particularly in tropical countries [55, 79]. Immunologically, S. mansoni infection is dominated by two distinct Th phases: an initial Th1 (IFN-γ) response, which switches to a stronger Th2 (IL-10, IL-5, and IL-13) response [58]. One of the most striking features of schistosomiasis is that the worms are experts in modulating and evading the host immune response, enabling their survival, migration, and development in different host tissues. Schistosomal glycoconjugates (glycoproteins and glycolipids) have shown to play important roles in host-parasite interactions. These glycoconjugates are often developmentally regulated antigens that are expressed during different life cycle stages. Some studies have indicated that LewisX antigens Galβ1,4(Fucα1–3)GlcNAc have important roles in host-schistosome interactions. LewisX (Lex) antigens have been found in glycoconjugates from all life cycle stages, including the membrane-bound glycoproteins of adult schistosomes and secreted egg and gut glycoproteins [7].
Macrophage Galactose Type C-Lectin (MGL). Human MGL has an exclusive specificity for terminal GalNAc residues, such as those found in the glycoproteins of the helminth parasite S. mansoni, in filoviruses, and in tumor-associated antigens [80].
Binding assays revealed that MGL recognizes both terminal β-GalNAc residues of LDN [GalNAcβ1–4GlcNAc-R] and LDNF [GalNAcβ1–4(Fucα1–3)GlcNAc-R] glycans present in SEA of S. mansoni. The specific interaction between MGL and SEA glycoproteins containing LDN and LDNF demonstrates that MGL functions as a pattern recognition receptor for S. mansoni [54].
In another study using binding assays and blocking antibodies reported that SEA of S. mansoni is internalized by human DCs through MGL. Moreover, the confocal laser scanning microscopy reveals colocalization of SEA with MHC-II in the lysosomal compartments suggests that Ag processing and presentation can occur. Certainly these findings are important, however remains to be answered if this recognition leads to antigen presentation and modulation of the immune response to S. mansoni [55].
DC-SIGN. It has been demonstrated that the blockade with monoclonal antibodies against the carbohydrate antigens Lex and LDNF inhibit the binding of DC-SIGN to soluble egg antigens (SEAs). The glycoproteins several SEAs from different schistosome species (S. mansoni, S. haematobium, and S. japonicum) contain ligands for DC-SIGN. It has also been demonstrated that a specific mutation in the carbohydrate-recognition domain (CDR) of DC-SIGN abrogates binding to either SEAs or Lex [56].
Structural characterization of the glycolipids and the study of cellular binding revealed that DC-SIGN binds to the carbohydrate moieties of glycosphingolipids with Lex and Ley structure [Fucα1-2Galβ14(Fucα1–3)GlcNAc] moieties. DC-SIGN recognizes not only the self-glycan ligand Lex within cercarial glycolipids, but also glycolipids carrying pseudo-Ley, a nonself-structure that to date has been found within Schistosome cercarial (S. cercarial) glycolipids and ES products [7]. These results show that DC-SIGN recognizes Lex and Ley antigens present in the SEAs and glycolipids of S. cercarial. Thus, DCs likely interact with Schistosomes early during infection through this lectin. However, more studies are needed to determine whether the recognition of glycosylated antigens through DC-SIGN is involved in resistance or susceptibility to S. mansoni infection in vivo.
L-SIGN. Liver/lymph node-specific ICAM-3-grabbing nonintegrin (LSIGN/CD209L/DC-SIGN-R) is a human homolog of DC-SIGN. L-SIGN shares 77% amino acid sequence identity with DC-SIGN and is expressed on liver sinusoidal endothelial cells (LSECs), which function as antigen-presenting cells in the liver [81].
L-SIGN, a highly related homolog of DC-SIGN, can bind both schistosome egg antigens (SEAs) and glycosphingolipids and can mediate the internalization of SEAs. However, binding assays showed that L-SIGN recognizes a glycoprotein fraction different from that recognized by DC-SIGN. It has been demonstrated that L-SIGN does not bind to neoglycoconjugates carrying Lex but does recognize other fucosylated glycans, that is, Le(a,b and y). Other studies have demonstrated that the glycosylation of schistosome antigens plays an important role in immunological process during schistosome infection [8, 57]. Those studies confirmed that L-SIGN recognizes both oligomannosidic N-glycans and multiply fucosylated carbohydrate motifs within SEAs. In addition, these studies demonstrated that L-SIGN can recognize a broad but specific glycan profile.
SIGNR1. Also called CD-209b, is one of the eight mouse homologs of human DC-SIGN and is expressed on particular MØ subsets in the marginal zone of the spleen and the medulla of the lymph nodes and on the peritoneal MØs. SIGNR1 recognizes glycans from different pathogens and has been shown to bind Lewisx/y and Lewisa/b-containing carbohydrates [82, 83].
An in vitro studyusing cells transfected with SIGNR1 showed that glycans from both SEAs and schistosome worm antigens were bound by SIGNR1 in a dose-dependent manner, demonstrating the ability of SIGNR1 to recognize and bind to two different stages of the parasite. However, the in vivo infection of SIGR1-deficient BALB/c mice (SIGNR-KO) with 25 cercariae of Schistosoma revealed that SIGNR1 has no role in primary or secondary pulmonary granuloma induced by schistosome eggs. SIGNR-KO mice exhibited unaltered worm fecundity, and the fecal eggs and the size and eosinophil content of the granulomas surrounding eggs in the liver were comparable, as were the levels of hepatic fibrosis. Moreover, no differences in the cytokine the production by spleen cells were observed. In conclusion, although SIGNR1 can recognize S. mansoni antigens in vitro, this receptor does not have a functional role in vivo during infection [9].
Dectin-2. Dectin-2 is a member of the C-type lectin family and has single complementarity-determining region (CRD). This protein expressed mainly in MØs and DCs. Dectin-2 recognizes α-mannans and transduces the signal through an association with the ITAM-containing Fc receptor γ chain [84, 85].
In vitro restimulation assays using spleen and MLN cells with SEA (20 μg/mL) have demonstrated that SEA associates with Dectin-2 and Fc receptor γ-chain (FcRγ) receptors. Moreover, SEA-mediated IL-1β production was significantly inhibited when BMDCs were pretreated with Dectn-2-specific antibodies or when Dectin-2-deficient BMDCs were used. In contrast, TNF-α production was not impaired. Thus, different components within SEAs mediate different immune reactions. These observations suggest that SEA triggers the Dectin-2 receptor, which couples with FcRγ chain, to activate the Syk-kinase signaling pathway, which controls IL-1β release in an ROS- and potassium efflux-dependent manner, the Nlrp3 inflammasome activation, and IL-1β release. However, even though these findings are important, it is necessary to determine whether this receptor plays a key role during infection in vivo [58].
Mannose Receptor (MR). It has been demonstrated that infective larvae of the parasitic helminth S. mansoni contain a large number of glycosylated components specific for MR. MR ligands are particularly rich in excretory/secretory (E/S) material released during the transformation of cercariae into schistosomula, a process that is critical for infection of the host. E/S material from carboxyfluorescein diacetate succinimidyl ester (CFDA-SE)-labeled cercariae showed enhanced binding by Chinese hamster ovary cells (CHO) lines transduced to express MR and by an MØ cell line that overexpresses MR (J774E) relative to the level of binding by WT CHO cells. Conversely, uptake was significantly lower by bone marrow-derived macrophages (BMDM) from MR-KO mice, although these cells were more active as judged by the enhanced proinflammatory cytokine production and CD40 expression. After natural percutaneous infection of MR-KO mice with CFDA-SE-labeled parasites, there were fewer cells in the skin and draining lymph nodes that were CFDA-SE(+) relative to the numbers in WT mice, indicating that there was reduced uptake and presentation of larval parasite antigens. However, the antigen-specific proliferation of skin-draining lymph node cells was significantly enhanced, and these cells secreted markedly elevated levels of IFN-γ but decreased levels of IL-4. These results demonstrated that MR on mononuclear phagocytic cells plays a significant role in internalizing E/S material released by the invasive stages of the parasite, which in turn modulates the production of proinflammatory cytokines. In the absence of MR, antigen-specific CD4+ cells are Th1 biased, suggesting that the ligation of MR by glycosylated E/S material released by schistosome larvae modulates the production of IFN-γ by CD4+ cells [59].
4.4. Helminths: Cestodes
4.4.1. Taenia crassiceps
Taenia crassiceps (T. crassiceps) is a tapeworm that is found in wild and domestic animals but does not cause clinical disease in nonimmunocompromised humans. This parasite has been used as an experimental model for cysticercosis [86]. Previous studies demonstrated that soluble antigens from T. crassiceps are highly glycosylated and are responsible for Th2 polarization in vivo [87, 88]. One study found that the excretory/secretory products of the cestode T. crassiceps (TcESs) do not induce the maturation of human DCs, as demonstrated by the lack of increase in the expression levels of CD83, HLA-DR, CD80, and CD86. TcESs enhanced the production of IL-10, positively modulated the expression of mMGL and negatively modulated the expression of DC-SIGN, although the source of these antigens is not a human parasite. These results showed that TcESs induce a tolerogenic-like phenotype in human DCs and modulate the expression of PRRs involved in key functions of DCs such as mMGL and DC-SIGN. This modulation is a possible mechanism used by T. crassiceps to modify the phenotype and hence the functions of human DCs, directing the balance toward immune suppression and allowing the survival of this parasite [60].
5. Conclusion
All studies described above demonstrate that CLRs are essential to the recognition of different carbohydrates present on surface or in the excretory/secretory products of different parasites. This recognition can promote the uptake, internalization and processing of parasite antigens that can influence the immune response. However, little is known about the role of CLRs in the immune response to parasitic infections. Future studies are needed to understand the immune mechanisms underlying the interaction of parasite antigens with CLRs.
Acknowledgments
The authors thank M. S. Imelda Juarez for collecting the items required for this paper. National Council of Science and Technology (CONACYT)-Mexico supported PhD fellowship for A. Vázquez-Mendoza and this review is a requirement to obtain his degree in Biomedical Sciences, UNAM. The author's research was supported by the Grants ICYT-DF-317-2010, UNAM-DGAPA-PAPIIT-IN212412, PAPCA-23-2012 and by the CONACYT-152224.
References
- 1.West I, Goldring O. Lectin affinity chromatography. Methods in Molecular Biology. 2004;244:159–166. doi: 10.1385/1-59259-655-x:159. [DOI] [PubMed] [Google Scholar]
- 2.Duverger E, Lamerant-Fayel N, Frison N, Monsigny M. Carbohydrate-lectin interactions assayed by SPR. Methods in Molecular Biology. 2010;627:157–178. doi: 10.1007/978-1-60761-670-2_10. [DOI] [PubMed] [Google Scholar]
- 3.Kerrigan AM, Brown GD. C-type lectins and phagocytosis. Immunobiology. 2009;214(7):562–575. doi: 10.1016/j.imbio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geijtenbeek TBH, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nature Reviews Immunology. 2009;9(7):465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Napoletano C, Zizzari IG, Rughetti A, et al. Targeting of macrophage galactose-type C-type lectin (MGL) induces DC signaling and activation. European Journal of Immunology. 2012;42:936–945. doi: 10.1002/eji.201142086. [DOI] [PubMed] [Google Scholar]
- 6.Hespanhol RC, Soeiro MDNC, Meuser MB, Meirelles MDNSL, Côrte-Real S. The expression of mannose receptors in skin fibroblast and their involvement in Leishmania (L.) amazonensis invasion. Journal of Histochemistry and Cytochemistry. 2005;53(1):35–44. doi: 10.1177/002215540505300105. [DOI] [PubMed] [Google Scholar]
- 7.Meyer S, Van Liempt E, Imberty A, et al. DC-SIGN mediates binding of dendritic cells to authentic pseudo-Lewis Y glycolipids of Schistosoma mansoni cercariae, the first parasite-specific ligand of DC-SIGN. Journal of Biological Chemistry. 2005;280(45):37349–37359. doi: 10.1074/jbc.M507100200. [DOI] [PubMed] [Google Scholar]
- 8.Van Liempt E, Imberty A, Bank CMC, et al. Molecular basis of the differences in binding properties of the highly related C-type lectins DC-SIGN and L-SIGN to Lewis X trisaccharide and Schistosoma mansoni egg antigens. Journal of Biological Chemistry. 2004;279(32):33161–33167. doi: 10.1074/jbc.M404988200. [DOI] [PubMed] [Google Scholar]
- 9.Saunders SP, Walsh CM, Barlow JL, et al. The C-type lectin SIGNR1 binds Schistosoma mansoni antigens in vitro, but SIGNR1-deficient mice have normal responses during schistosome infection. Infection and Immunity. 2009;77(1):399–404. doi: 10.1128/IAI.00762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGreal EP, Martinez-Pomares L, Gordon S. Divergent roles for C-type lectins expressed by cells of the innate immune system. Molecular Immunology. 2004;41(11):1109–1121. doi: 10.1016/j.molimm.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Geijtenbeek TBH, Van Vliet SJ, Engering A, ’T Hart BA, Van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annual Review of Immunology. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 12.van Vliet SJ, Saeland E, van Kooyk Y. Sweet preferences of MGL: carbohydrate specificity and function. Trends in Immunology. 2008;29(2):83–90. doi: 10.1016/j.it.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Sabatte J, Faigle W, Ceballos A, et al. Semen clusterin is a novel DC-SIGN ligand. Journal of Immunology. 2011;187:5299–5309. doi: 10.4049/jimmunol.1101889. [DOI] [PubMed] [Google Scholar]
- 14.Sakakura M, Oo-Puthinan S, Moriyama C, et al. Carbohydrate binding mechanism of the macrophage galactose-type C-type lectin 1 revealed by saturation transfer experiments. Journal of Biological Chemistry. 2008;283(48):33665–33673. doi: 10.1074/jbc.M804067200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahms NM, Hancock MK. P-type lectins. Biochimica et Biophysica Acta. 2002;1572(2-3):317–340. doi: 10.1016/s0304-4165(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 16.Song X, Lasanajak Y, Olson LJ, et al. Glycan microarray analysis of P-type lectins reveals distinct phosphomannose glycan recognition. Journal of Biological Chemistry. 2009;284(50):35201–35214. doi: 10.1074/jbc.M109.056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JJP, Olson LJ, Dahms NM. Carbohydrate recognition by the mannose-6-phosphate receptors. Current Opinion in Structural Biology. 2009;19(5):534–542. doi: 10.1016/j.sbi.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Xiao S, Yu Z. F-type lectin involved in defense against bacterial infection in the pearl oyster (Pinctada martensii) Fish and Shellfish Immunology. 2011;30(2):750–754. doi: 10.1016/j.fsi.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 19.Park HJ, Kim JW, Kim EG, et al. Molecular cloning and expression analysis of two distinct F-type lectins from the rock bream, Oplegnathus fasciatus. Developmental and Comparative Immunology. 2012;36:230–235. doi: 10.1016/j.dci.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Odom EW, Vasta GR. Characterization of a binary tandem domain F-type lectin from striped bass (Morone saxatilis) Journal of Biological Chemistry. 2006;281(3):1698–1713. doi: 10.1074/jbc.M507652200. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa T, Watanabe M, Naganuma T, Muramoto K. Diversified carbohydrate-binding lectins from marine resources. Journal of Amino Acids. 2011;2011:20 pages. doi: 10.4061/2011/838914.838914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianchet MA, Odom EW, Vasta GR, Amzel LM. Structure and specificity of a binary tandem domain F-Lectin from striped bass (Morone saxatilis) Journal of Molecular Biology. 2010;401(2):239–252. doi: 10.1016/j.jmb.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends in Pharmacological Sciences. 2009;30(5):240–248. doi: 10.1016/j.tips.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jandus C, Simon HU, Von Gunten S. Targeting Siglecs-A novel pharmacological strategy for immuno- and glycotherapy. Biochemical Pharmacology. 2011;82(4):323–332. doi: 10.1016/j.bcp.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Sancho D, Reis e Sousa C. Signaling by myeloid C-Type lectin receptors in immunity and homeostasis. Annual Review of Immunology. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dam TK, Fred Brewer C. Lectins as pattern recognition molecules: the effects of epitope density in innate immunity. Glycobiology. 2009;20(3):270–279. doi: 10.1093/glycob/cwp186.cwp186 [DOI] [PubMed] [Google Scholar]
- 27.van Kooyk Y. C-type lectins on dendritic cells: key modulators for the induction of immune responses. Biochemical Society Transactions. 2008;36(6):1478–1481. doi: 10.1042/BST0361478. [DOI] [PubMed] [Google Scholar]
- 28.Weis WI, Taylor ME, Drickamer K. The C-type lectin superfamily in the immune system. Immunological Reviews. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 29.Kilpatrick DC. Animal lectins: a historical introduction and overview. Biochimica et Biophysica Acta. 2002;1572(2-3):187–197. doi: 10.1016/s0304-4165(02)00308-2. [DOI] [PubMed] [Google Scholar]
- 30.Singh RS, Wang HH. Timing of repeat thyroid fine-needle aspiration in the management of thyroid nodules. Acta Cytologica. 2011;55(6):544–548. doi: 10.1159/000334214. [DOI] [PubMed] [Google Scholar]
- 31.Engering A, Geijtenbeek TBH, Van Kooyk Y. Immune escape through C-type lectins on dendritic cells. Trends in Immunology. 2002;23(10):480–485. doi: 10.1016/s1471-4906(02)02296-2. [DOI] [PubMed] [Google Scholar]
- 32.Engering A, Geijtenbeek TBH, Van Vliet SJ, et al. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. Journal of Immunology. 2002;168(5):2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- 33.Brown GD, Herre J, Williams DL, Willment JA, Marshall ASJ, Gordon S. Dectin-1 mediates the biological effects of β-glucans. Journal of Experimental Medicine. 2003;197(9):1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and toll-like receptor 2. Journal of Experimental Medicine. 2003;197(9):1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakraborty P, Ghosh D, Basu MK. Modulation of macrophage mannose receptor affects the uptake of virulent and avirulent Leishmania donovani promastigotes. Journal of Parasitology. 2001;87(5):1023–1027. doi: 10.1645/0022-3395(2001)087[1023:MOMMRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.Oo-puthinan S, Maenuma K, Sakakura M, et al. The amino acids involved in the distinct carbohydrate specificities between macrophage galactose-type C-type lectins 1 and 2 (CD301a and b) of mice. Biochimica et Biophysica Acta. 2008;1780(2):89–100. doi: 10.1016/j.bbagen.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Švajger U, Anderluh M, Jeras M, Obermajer N. C-type lectin DC-SIGN: an adhesion, signalling and antigen-uptake molecule that guides dendritic cells in immunity. Cellular Signalling. 2010;22(10):1397–1405. doi: 10.1016/j.cellsig.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lloyd DH, Viac J, Werling D, Rème CA, Gatto H. Role of sugars in surface microbe-host interactions and immune reaction modulation. Veterinary Dermatology. 2007;18(4):197–204. doi: 10.1111/j.1365-3164.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 39.Kingeter LM, Lin X. C-type lectin receptor-induced NF-kappaB activation in innate immune and inflammatory responses. Cellular & Molecular Immunology . 2012;9:105–112. doi: 10.1038/cmi.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Vliet SJ, García-Vallejo JJ, Van Kooyk Y. Dendritic cells and C-type lectin receptors: coupling innate to adaptive immune responses. Immunology and Cell Biology. 2008;86(7):580–587. doi: 10.1038/icb.2008.55. [DOI] [PubMed] [Google Scholar]
- 41.Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Sousa CR. Myeloid C-type lectins in innate immunity. Nature Immunology. 2006;7(12):1258–1265. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- 42.Dorlo TP, Balasegaram M, Beijnen JH, de Vries PJ. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. Journal of Antimicrobial Chemotherapy. 2012;67(11):2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- 43.Alvar J, Velez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0035671.e35671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beattie L, Kaye PM. Leishmania-host interactions: what has imaging taught us? Cell Microbiol. 2011;13:1659–1667. doi: 10.1111/j.1462-5822.2011.01658.x. [DOI] [PubMed] [Google Scholar]
- 45.Akilov OE, Kasuboski RE, Carter CR, McDowel MA. The role of mannose receptor during experimental leishmaniasis. Journal of Leukocyte Biology. 2007;81(5):1188–1196. doi: 10.1189/jlb.0706439. [DOI] [PubMed] [Google Scholar]
- 46.Garrido VV, Dulgerian LR, Stempin CC, Cerban FM. The increase in mannose receptor recycling favors arginase induction and Trypanosoma cruzi survival in macrophages. International Journal of Biological Sciences. 2011;7:1257–1272. doi: 10.7150/ijbs.7.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deschoolmeester ML, Martinez-Pomares L, Gordon S, Else KJ. The mannose receptor binds Trichuris muris excretory/secretory proteins but is not essential for protective immunity. Immunology. 2009;126(2):246–255. doi: 10.1111/j.1365-2567.2008.02893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basu N, Sett R, Das PK. Down-regulation of mannose receptors on macrophages after infection with Leishmania donovani . Biochemical Journal. 1991;277(2):451–456. doi: 10.1042/bj2770451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson ME, Pearson RD. Evidence that Leishmania donovani utilizes a mannose receptor on human mononuclear phagocytes to establish intracellular parasitism. Journal of Immunology. 1986;136(12):4681–4688. [PubMed] [Google Scholar]
- 50.Colmenares M, Puig-Kröger A, Pello OM, Corbí AL, Rivas L. Dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN, CD209), a C-type surface lectin in human DCs, is a receptor for Leishmania amastigotes . Journal of Biological Chemistry. 2002;277(39):36766–36769. doi: 10.1074/jbc.M205270200. [DOI] [PubMed] [Google Scholar]
- 51.Colmenares M, Corbí AL, Turco SJ, Rivas L. The dendritic Cell receptor DC-sign discriminates among species and life cycle forms of Leishmania . Journal of Immunology. 2004;172(2):1186–1190. doi: 10.4049/jimmunol.172.2.1186. [DOI] [PubMed] [Google Scholar]
- 52.Soeiro MDNC, Paiva MM, Barbosa HS, Meirelles MDNSL, Araújo-Jorge TC. A cardiomyocyte mannose receptor system is involved in Trypanosoma cruzi invasion and is down-modulated after infection. Cell Structure and Function. 1999;24(3):139–149. doi: 10.1247/csf.24.139. [DOI] [PubMed] [Google Scholar]
- 53.Raes G, Brys L, Dahal BK, et al. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. Journal of Leukocyte Biology. 2005;77(3):321–327. doi: 10.1189/jlb.0304212. [DOI] [PubMed] [Google Scholar]
- 54.van Vliet SJ, van Liempt E, Saeland E, et al. Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. International Immunology. 2005;17(5):661–669. doi: 10.1093/intimm/dxh246. [DOI] [PubMed] [Google Scholar]
- 55.van Liempt E, van Vliet SJ, Engering A, et al. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Molecular Immunology. 2007;44(10):2605–2615. doi: 10.1016/j.molimm.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 56.van Die I, van Vliet SJ, Nyame AK, et al. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology. 2003;13(6):471–478. doi: 10.1093/glycob/cwg052. [DOI] [PubMed] [Google Scholar]
- 57.Meyer S, Tefsen B, Imberty A, Geyer R, van die I. The C-type lectin L-SIGN differentially recognizes glycan antigens on egg glycosphingolipids and soluble egg glycoproteins from Schistosoma mansoni . Glycobiology. 2007;17(10):1104–1119. doi: 10.1093/glycob/cwm073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ritter M, Gross O, Kays S, et al. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(47):20459–20464. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paveley RA, Aynsley SA, Turner JD, et al. The Mannose Receptor (CD206) is an important pattern recognition receptor (PRR) in the detection of the infective stage of the helminth Schistosoma mansoni and modulates IFNgamma production. International Journal for Parasitology. 2011;41:1335–1345. doi: 10.1016/j.ijpara.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 60.Terrazas CA, Sanchez-Munoz F, Mejia-Dominguez AM, et al. Cestode antigens induce a tolerogenic-like phenotype and inhibit LPS inflammatory responses in human dendritic cells. International Journal of Biological Sciences. 2011;7:1391–1400. doi: 10.7150/ijbs.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garg R, Trudel N, Tremblay MJ. Consequences of the natural propensity of Leishmania and HIV-1 to target dendritic cells. Trends in Parasitology. 2007;23(7):317–324. doi: 10.1016/j.pt.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 62.Terrazas CA, Huitron E, Vazquez A, et al. MIF synergizes with Trypanosoma cruzi antigens to promote efficient dendritic cell maturation and IL-12 production via p38 MAPK. International Journal of Biological Sciences. 2011;7:1298–1310. doi: 10.7150/ijbs.7.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodrigues MM, Oliveira AC, Bellio M. The immune response to Trypanosoma cruzi: role of toll-like receptors and perspectives for vaccine development. Journal of Parasitology Research. 2012;2012:12 pages. doi: 10.1155/2012/507874.507874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campos MA, Gazzinelli RT. Trypanosoma cruzi and its components as exogenous mediators of inflammation recognized through Toll-like receptors. Mediators of Inflammation. 2004;13(3):139–143. doi: 10.1080/09511920410001713565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ropert C, Ferreira LRP, Campos MAS, et al. Macrophage signaling by glycosylphosphatidylinositol-anchored mucin-like glycoproteins derived from Trypanosoma cruzi trypomastigotes. Microbes and Infection. 2002;4(9):1015–1025. doi: 10.1016/s1286-4579(02)01609-x. [DOI] [PubMed] [Google Scholar]
- 66.Souza PEA, Rocha MOC, Menezes CAS, et al. Trypanosoma cruzi infection induces differential modulation of costimulatory molecules and cytokines by monocytes and T cells from patients with indeterminate and cardiac Chagas' disease. Infection and Immunity. 2007;75(4):1886–1894. doi: 10.1128/IAI.01931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobs T, Erdmann H, Fleischer B. Molecular interaction of Siglecs (sialic acid-binding Ig-like lectins) with sialylated ligands on Trypanosoma cruzi . European Journal of Cell Biology. 2010;89(1):113–116. doi: 10.1016/j.ejcb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 68.Acosta-Serrano A, Almeida IC, Freitas-Junior LH, Yoshida N, Schenkman S. The mucin-like glycoprotein super-family of Trypanosoma cruzi: structure and biological roles. Molecular and Biochemical Parasitology. 2001;114(2):143–150. doi: 10.1016/s0166-6851(01)00245-6. [DOI] [PubMed] [Google Scholar]
- 69.Oliveira AC, Peixoto JR, De Arrada LB, et al. Expression of functional TLR4 confers proinflammatory responsiveness to Trypanosoma cruzi glycoinositolphospholipids and higher resistance to infection with T. cruzi . Journal of Immunology. 2004;173(9):5688–5696. doi: 10.4049/jimmunol.173.9.5688. [DOI] [PubMed] [Google Scholar]
- 70.Beschin A, Brys L, Magez S, Radwanska M, De Baetselier P. Trypanosoma brucei infection elicits nitric oxide-dependent and nitric oxide-independent suppressive mechanisms. Journal of Leukocyte Biology. 1998;63(4):429–439. doi: 10.1002/jlb.63.4.429. [DOI] [PubMed] [Google Scholar]
- 71.Atrih A, Richardson JM, Prescott AR, Ferguson MAJ. Trypanosoma brucei glycoproteins contain novel giant poly-N- acetyllactosamine carbohydrate chains. Journal of Biological Chemistry. 2005;280(2):865–871. doi: 10.1074/jbc.M411061200. [DOI] [PubMed] [Google Scholar]
- 72.Urbaniak MD, Turnock DC, Ferguson MAJ. Galactose starvation in a bloodstream form Trypanosoma brucei UDP-glucose 4′-epimerase conditional null mutant. Eukaryotic Cell. 2006;5(11):1906–1913. doi: 10.1128/EC.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumamoto Y, Higashi N, Denda-Nagai K, et al. Identification of sialoadhesin as a dominant lymph node counter-receptor for mouse macrophage galactose-type C-type lectin. Journal of Biological Chemistry. 2004;279(47):49274–49280. doi: 10.1074/jbc.M409300200. [DOI] [PubMed] [Google Scholar]
- 74.Tsuiji M, Fujimori M, Ohashi Y, et al. Molecular cloning and characterization of a novel mouse macrophage C-type lectin, mMGL2, which has a distinct carbohydrate specificity from mMGL1. Journal of Biological Chemistry. 2002;277(32):28892–28901. doi: 10.1074/jbc.M203774200. [DOI] [PubMed] [Google Scholar]
- 75.Kumamoto Y, Denda-Nagai K, Aida S, Higashi N, Irimura T. MGL2+ dermal dendritic cells are sufficient to initiate contact hypersensitivity in vivo . PLoS One. 2009;4(5) doi: 10.1371/journal.pone.0005619.e5619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Vliet SJ, Aarnoudse CA, Broks-van den Nerg VCM, Boks M, Geijtenbeek TBH, van Kooyk Y. MGL-mediated internalization and antigen presentation by dendritic cells: a role for tyrosine-5. European Journal of Immunology. 2007;37(8):2075–2081. doi: 10.1002/eji.200636838. [DOI] [PubMed] [Google Scholar]
- 77.Singh SK, Streng-Ouwehand I, Litjens M, et al. Characterization of murine MGL1 and MGL2 C-type lectins: distinct glycan specificities and tumor binding properties. Molecular Immunology. 2009;46(6):1240–1249. doi: 10.1016/j.molimm.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 78.Gaherwal S, Prakash MM. Lymphocyte migration inhibition response in Trichuris muris infected and vaccinated mice. Iranian Journal of Parasitology. 2011;6(1):34–40. [PMC free article] [PubMed] [Google Scholar]
- 79.van Stijn CMW, Meyer S, van den Broek M, et al. Schistosoma mansoni worm glycolipids induce an inflammatory phenotype in human dendritic cells by cooperation of TLR4 and DC-SIGN. Molecular Immunology. 2010;47(7-8):1544–1552. doi: 10.1016/j.molimm.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 80.Meevissen MHJ, Yazdanbakhsh M, Hokke CH. Schistosoma mansoni egg glycoproteins and C-type lectins of host immune cells: molecular partners that shape immune responses. Experimental Parasitology. 2011;132(1):14–21. doi: 10.1016/j.exppara.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 81.Bashirova AA, Geijtenbeek TBH, Van Duijnhoven GCF, et al. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. Journal of Experimental Medicine. 2001;193(6):671–678. doi: 10.1084/jem.193.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choi HJ, Choi WS, Park JY, et al. SIGN-R1, a C-type lectin, binds to Bip/GRP78 and this interaction mediates the regurgitation of T-cell-independent type 2 antigen dextran through the endoplasmic reticulum. Immunobiology. 2011;216(4):437–446. doi: 10.1016/j.imbio.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 83.Takahara K, Tokieda S, Nagaoka K, Inaba K. Efficient capture of Candida albicans and zymosan by SIGNR1 augments TLR2-dependent TNF-α production. International Immunology. 2012;24(2):89–96. doi: 10.1093/intimm/dxr103. [DOI] [PubMed] [Google Scholar]
- 84.Saijo S, Iwakura Y. Dectin-1 and Dectin-2 in innate immunity against fungi. International Immunology. 2011;23(8):467–472. doi: 10.1093/intimm/dxr046. [DOI] [PubMed] [Google Scholar]
- 85.Sainz J, Lupiáñez CB, Segura-Catena J, et al. Dectin-1 and DC-SIGN polymorphisms associated with invasive pulmonary aspergillosis infection. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0032273.e32273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Terrazas LI, Bojalil R, Govezensky T, Larralde C. Shift from an early protective TH1-type immune response to a late permissive TH2-type response in murine cysticercosis (Taenia crassiceps) Journal of Parasitology. 1998;84(1):74–81. [PubMed] [Google Scholar]
- 87.Gómez-García L, López-Marín LM, Saavedra R, Reyes JL, Rodríguez-Sosa M, Terrazas LI. Intact glycans from cestode antigens are involved in innate activation of myeloid suppressor cells. Parasite Immunology. 2005;27(10-11):395–405. doi: 10.1111/j.1365-3024.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 88.Reyes JL, Terrazas CA, Vera-Arias L, Terrazas LI. Differential response of antigen presenting cells from susceptible and resistant strains of mice to Taenia crassiceps infection. Infection, Genetics and Evolution. 2009;9(6):1115–1127. doi: 10.1016/j.meegid.2009.05.011. [DOI] [PubMed] [Google Scholar]


