Abstract
High levels of plasma homocysteine are implicated in the pathogenesis of cardiovascular diseases especially if accompanied by sleep apnea, but a direct pathogenetic link between plasma homocysteine levels and obstructive sleep apnea is debatable. This association can have far-reaching public health implications considering the inverse association between folate and plasma homocysteine. We used data from the 2005-2006 cycle of the National Health and Nutrition Examination Survey (NHANES) to test the hypothesized associations. Of the 4490 subjects included in analysis, 177 reported sleep apnea. Age-standardized and design-effect-corrected prevalence rates were differential across gender, plasma homocysteine, and red cell folate status. Plasma homocysteine was positively correlated with age (r = 0.38, P < 0.0001). Multivariate analyses using sociodemographic and clinical covariates demonstrated that plasma homocysteine levels retained their respective associations with self-reported sleep apnea in all models except when age was included as a covariate. Our results demonstrate that the claimed association of plasma homocysteine with sleep apnea may be confounded by age.
1. Introduction
Obstructive sleep apnea (OSA)—a disorder in which a person frequently stops breathing during sleep—results from an obstruction of the upper airway that occurs because of inadequate motor tone of the tongue and/or airway dilator muscles. In the United States, the prevalence of OSA is estimated to be 3–7% in men and 2–5% in women [1]. In addition, up to 93% of women and 82% of men may already have an undiagnosed moderate to severe OSA [2]. Further, the comorbid occurrence of OSA with obesity is well-recognized: prevalence of OSA is reported to be 41% among patients with a body mass index (BMI) greater than 28 Kg/m2 and as high as 78% in morbidly obese patients who present for bariatric surgery [3, 4]. Of greater interest and importance, however, is the association of OSA with cardiovascular disorders [5]. OSA has been identified as a crucial intermediate factor in the pathophysiology of hypertension, ischemic heart disease, arrhythmias, stroke and diabetes. It has been shown that habitual snorers are at a 2 times higher likelihood of developing type 2 diabetes independently of other covariates [6]. Also, treatment of sleep-disordered breathing is known to improve outcomes after congestive heart failure and stroke [7, 8].
A possible mechanism for the strong correlation between OSA and cardiovascular risk factors is the concomitant association of plasma homocysteine levels with both these disorders [9–11]. Homocysteine—a homologue of cysteine is a biosynthesized amino acid in the metabolism of methionine. Its production correlates with the deficiency of vitamins B6, B12, and folic acid. Indeed, plasma homocysteine levels are considered a good indicator of the deficiency of these vitamins [12]. The importance of homocysteine metabolism in the initiation or precipitation of cardiovascular diseases can be appreciated by the fact that the attributable risk of hyperhomocysteinemia in the epidemiology of cardiovascular diseases is nearly 25% and competes with that of other well-known factors like smoking and hyperlipidemia [13]. In contrast, the association of plasma homocysteine levels with the risk, severity and long-term complications of OSA is less clear. Over the last decade, 15 epidemiological studies [14–28] have examined the potential association of plasma homocysteine levels with OSA under varying scenarios (Table 1). Of these, nine studies [4–6, 12, 16, 17, 21, 24, 26] have reported overall or subgroup-specific association while six studies [3, 8, 9, 13, 20, 23] have not found any association. Some elegant reviews [9, 10] in this area have also not been fully conclusive about such an association.
Table 1.
Summary of evidence for and against the association of plasma homocysteine with sleep apnea.
| No. | Authors [Ref] | Year | Type of study | N | Results |
|---|---|---|---|---|---|
| 1 | Chen et al. [17] | 2011 | Cross-sectional | 102 | Severity of OSA is associated with increased homocysteine levels in subjects with ischemic heart disease |
| 2 | Basoglu et al. [14] | 2011 | Case control | 36 cases, 34 controls | No association between plasma homocysteine and OSA in obese patients |
| 3 | Cintra et al. [18] | 2011 | Matched case control | 75 cases, 75 controls | Cysteine but not homocysteine is differentially distributed across OSA and non-OSA patients |
| 4 | Wang et al. [27] | 2010 | Cross-sectional | 83 patients with OSA, 52 without OSA | Oxidative stress might induce high plasma homocysteine levels in elderly patients with OSA |
| 5 | Cerbo et al. [16] | 2010 | Case report | 1 | Early onset homocystinuria is associated with apneic spells |
| 6 | Sariman et al. [25] | 2010 | Cross-sectional | 38 cases of OSA | Plasma homocysteine levels correlate with the severity of OSA |
| 7 | Yavuz et al. [28] | 2008 | Cross-sectional | 62 patients of OSA, 12 controls | Plasma homocysteine levels are elevated in patients with OSA |
| 8 | Ozkan et al. [23] | 2008 | Cross-sectional | 34 OSA patients, 15 controls | Plasma homocysteine levels are raised in patients with severe OSA |
| 9 | Ryan et al. [24] | 2007 | Cross-sectional | 80 patients with OSA, 30 controls | Plasma homocysteine levels are not associated with either the risk or severity of OSA |
| 10 | Kumor et al. [21] | 2006 | Cross-sectional | 47 patients of OSA, 12 controls | Plasma homocysteine levels are not differentially distributed across patients and controls of OSA |
| 11 | Hachul de Campos et al. [19] | 2006 | Cross-sectional | 38 insomniac postmenopausal women | Plasma homocysteine levels are not associated with risk of apnea |
| 12 | Can et al. [15] | 2006 | Cross-sectional | 30 OSA patients, 32 controls | Serum homocysteine levels are significantly higher in OSA patients |
| 13 | Kokturk et al. [20] | 2006 | Cross-sectional | 72 OSA patients, 42 controls | Serum homocysteine is significantly increased in patients with OSA |
| 14 | Svatikova et al. [26] | 2004 | Case control | 22 OSA patients, 20 controls | Plasma homocysteine levels exhibit diurnal variation and are not differentially distributed across patients and controls of OSA |
| 15 | Lavie et al. [22] | 2001 | Case control | 237 cases of OSA, 108 controls | Patients with ischemic heart disease and OSA have elevated plasma homocysteine levels |
In concept, this association—if statistically and truly existent–proffers an enticing opportunity for simple public health measures like vitamin supplementation for the prevention and control of OSA as well as its cardiovascular implications [29]. However, as can be gleaned from Table 1, most of the studies in this area have been based on relatively small sample sizes which somewhat limits the public health implication of these results. We therefore analyzed a large nationally representative sample which was collected during the National Health and Nutrition Examination Survey (NHANES) in the 2005-2006 cycle. Here we report the results of our investigation into the association of plasma homocysteine, and folate levels in the plasma as well as the red blood cell (RBC) with the risk of self-reported sleep apnea in an epidemiological context.
2. Methods
2.1. The NHANES 2005-2006 Dataset
The National Health and Nutrition Examination Survey (NHANES) is an annual survey conducted by the National Center of Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA. It comprises a combination of interviews, physical examination and laboratory tests to assess the health and nutritional status of adults and children in the United States. The NHANES 2005-2006 and 2007-2008 cycles contain a questionnaire to identify subjects with diagnosed sleep disorders. Preliminary results from this questionnaire and the relationship of sleep apnea with obesity in the 2005-2006 dataset have been described elsewhere [30]. Even though both the 2005-2006 and 2007-2008 datasets contain data on the sleep questionnaire, currently the plasma homocysteine levels are available for the 2005-2006 cycle only. Therefore, we included this dataset for our analysis. The NHANES 2005-2006 survey was approved by the NCHS Ethics Review Board, and all participants or parents (for minors) provided written consent. Total plasma homocysteine levels were determined using the fully automated fluorescence polarization immunoassay (Abbott Laboratories). The RBC and plasma folate estimations were conducted using the Quantaphase II folate/vitamin B12 radioassay (Bio-Rad Laboratories, Hercules, CA, USA) using 125I and 57Co as tracers. Detailed description of the NHANES 2005-2006 survey can be found online at http://www.cdc.gov/nchs/nhanes.htm.
2.2. Outcomes and Predictors
Our primary outcome of interest was presence of self-reported sleep apnea. Although the association of plasma homocysteine has been predominantly examined in the context of OSA, the NHANES sleep questionnaire did not explicitly probe into the type of sleep apnea. We therefore used the diagnosed, self-reported sleep apnea as our outcome of interest. Our primary predictors were plasma homocysteine levels and RBC and plasma folate. However, as described previously using this dataset, there were additional variables that were (or could have been) associated with altered risk of sleep apnea. These variables were age, gender, race, country of birth, obesity, hypertension, ever smoking, and ever alcohol use. We examined the potential association of these sociodemographic and clinical variables with the reported diagnosis of sleep apnea and included the significant variables as secondary covariates in multivariate models. For these analyses, we dichotomized plasma homocysteine, RBC folate, and age based on the basis of the receiver operating characteristic (ROC) curves. The optimum cut-off points for these variables were obtained at ≥8.02 μmol/L, ≥279 ng/mL, and ≥47 years, respectively. Education status was dichotomized as high (high school or above) or low (up to and including 11th grade), marital status as married or other, obesity was defined as BMI > 28 Kg/m2, and hypertension was defined as an average (mean of three readings) systolic blood pressure > 140 mmHg and/or a diastolic blood pressure > 90 mmHg. Ever smoking was defined as having smoked at least 100 cigarettes in lifetime while ever alcohol use was defined as having had 12 alcoholic drinks in life time.
2.3. Statistical Analysis
Descriptive statistics included the means and standard errors (for continuous variables) and proportions (for discrete variables). Statistical significance for difference across study groups was assessed using the Student's t test (for continuous variables) or chi-square test (for categorical variables). For binarizing continuous variables, we made use of the ROC and selected the optimum cut-off point by finding the shortest distance from the upper-left corner of the ROC plot. The distance of a point on the ROC from the upper left corner was estimated as , where Sn is the sensitivity and Sp is the specificity of the binarized variable to predict self-reported sleep apnea. We estimated two important effect measures: age-standardized, design-effect-corrected prevalence rates of self-reported sleep apnea in various subgroups and the design-effect-adjusted multivariate association of the above-mentioned predictors with the risk of self-reported sleep apnea.
To determine the potentially independent association of high plasma homocysteine with self-reported sleep apnea, we decomposed the observed total plasma homocysteine levels into age-independent and age-dependent components using the following approach. We fitted a linear regression model HCY = b * age + c, where HCY is the plasma homocysteine level, b is the regression coefficient, and c is constant. Using the results of this model, we generated the two components as HCYdep = b * age and HCYind = HCY − HCYdep, where the suffixes dep and ind indicate the age-dependent and -independent components, respectively. We then conducted multivariate logistic regression analysis to examine the association of the HCYind and HCYdep components on the risk of self-reported sleep apnea. For all analyses, we used the svy commands contained in the Stata 12.0 statistical software (Stata Corp, College Station, TX, USA). These commands help account for the survey design effect. The survey was a single-stage, 30-cluster design, and we used the procedure described by the Centers for Disease Control to set the survey data in Stata. To calculate the prevalence rates, we used the svy: mean command with the stdize option while, to conduct the multivariate analyses, we used the svy: estimate command. Statistical significance was evaluated at a type I error rate of 0.05.
3. Results
3.1. Characteristics of Study Subjects
Plasma homocysteine levels, RBC folate levels, sleep questionnaire responses, and demographic information were available on 4490 subjects in the NHANES 2005-2006 dataset of whom 177 (3.94%) reported past diagnosis of sleep apnea. The distribution of the sociodemographic and clinical variables in subjects with and without reported sleep apnea is shown in Table 2. We found that the subjects with self-reported sleep apnea were on an average over 8 years older than subjects who did not report sleep apnea. Interestingly, the proportion of males, non-Hispanic Whites, and subjects born in the US were ~18% higher than the respective proportions in subjects with self-reported sleep apnea as compared to those without it. Also, a higher proportion of subjects with self-reported apnea were more educated and married. We also observed that the persons with self-reported sleep apnea had a higher body mass index, a higher percentage of ever smokers, and ever alcohol users as compared to those without sleep apnea. There was no significant difference in the economic status of the families as indicated by the poverty income ratio.
Table 2.
Sociodemographic, clinical, and relevant biochemical characteristics of the population included in this study (total n = 4490).
| Characteristic | Sleep apnea (n = 177) | No sleep apnea (n = 4313) | P |
|---|---|---|---|
| Age (yrs); mean(SD) | 56.5 (15.2) | 48.1 (19.0) | <0.001 |
| Males; n (%) | 116 (65.5) | 2036 (47.2) | <0.001 |
| Race/ethnicity; n (%) | <0.001 | ||
| Mexican American | 11 (6.2) | 899 (20.8) | |
| Other Hispanic | 2 (1.1) | 136 (3.12) | |
| Non-Hispanic White | 120 (67.8) | 2144 (49.7) | |
| Non-Hispanic Black | 40 (22.6) | 962 (22.3) | |
| Others | 4 (2.3) | 172 (4.0) | |
| Country of birth; n (%) | <0.001 | ||
| United States | 168 (94.9) | 3345 (77.6) | |
| Mexico | 4 (2.3) | 583 (13.5) | |
| Elsewhere | 5 (2.8) | 385 (8.9) | |
| Education; n (%) | 0.039 | ||
| Less than 9th grade | 10 (5.7) | 543 (12.6) | |
| 9–11th grade | 20 (11.3) | 670 (15.5) | |
| High school | 48 (27.1) | 1018 (23.6) | |
| Some college education | 53 (29.9) | 1228 (28.5) | |
| College graduate | 46 (26.0) | 849 (19.7) | |
| Refused/do not know | 0 (0.0) | 5 (0.1) | |
| Marital status; n (%) | 0.016 | ||
| Married | 121 (68.4) | 2340 (54.3) | |
| Widowed | 11 (6.2) | 388 (9.0) | |
| Divorced | 15 (8.5) | 412 (9.6) | |
| Separated | 5 (2.8) | 130 (3.0) | |
| Never married | 14 (7.9) | 675 (15.7) | |
| Living with partner | 11 (6.2) | 365 (8.5) | |
| Refused to answer | 0 (0.0) | 3 (0.1) | |
| Poverty income ratio; mean (SD) | 2.84 (1.64) | 2.68 (1.59) | 0.194 |
| Body mass index (Kg/m2); mean (SD) | 34.90 (0.62) | 28.55 (0.10) | <0.001 |
| Ever smoking; n (%) | 101 (57.06) | 2026 (46.97) | 0.008 |
| Ever alcohol use; n (%) | 41 (23.16) | 682 (15.81) | 0.009 |
| Plasma homocysteine (μmol/L); mean (SD) | 9.49 (3.75) | 8.44 (4.62) | 0.003 |
| RBC folate (ng/mL); mean (SD) | 326.7 (180.4) | 297.0 (135.4) | 0.005 |
| Plasma folate (ng/mL); mean (SD) | 14.65 (15.4) | 13.8 (9.5) | 0.255 |
3.2. Prevalence of Self-Reported Sleep Apnea Based on Plasma Homocysteine and RBC Folate Levels
We observed that the mean plasma homocysteine and mean RBC folate levels were significantly higher in subjects with self-reported sleep apnea (Table 2), but the mean plasma folate levels were comparable in subjects with or without self-reported sleep apnea. Yet, we found that there was a negative correlation of the plasma homocysteine levels with both the RBC and plasma folate levels (r = −0.08, P < 0.0001 and r = −0.03, P = 0.0321, resp.).
Considering the several observed associations of the sociodemographic, clinical, and biochemical variables with self-reported sleep apnea, we first estimated the prevalence of self-reported sleep apnea in various subgroups defined by these variables. We observed (Figure 1) that the overall age-standardized prevalence rate of 4.3% was significantly differential across gender; males had a prevalence rate of ~6% while females had a prevalence of ~2.9%. Thus, we next estimated age-standardized prevalence rates for other subgroups separately for males and females. Since we had observed significant differences in the mean plasma homocysteine and RBC folate (but not plasma folate) across self-reported sleep apnea status, we first categorized these two variables into four groups based on the respective quartiles. The quartiles were generated for all subjects irrespective of the self-reported sleep apnea status. The quartiles for plasma homocysteine were as follows: Q1, <6.23 μmol/L; Q2, 6.23–7.76 μmol/L; Q3, 7.77–9.75 μmol/L; Q4, >9.75 μmol/L. The quartiles for RBC folate were Q1, <207 ng/mL; Q2, 207–268 ng/mL; Q3, 269–354 ng/mL and Q4, ≥355 ng/mL.
Figure 1.
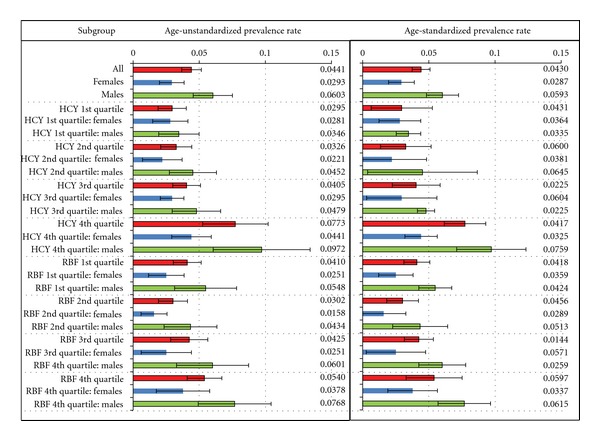
Prevalence of diagnosed, self-reported sleep apnea in the NHANES 2005-2006 dataset based on gender and quartiles of plasma homocysteine and RBC folate. The plot on the left is not standardized for age while the plot on the right shows age-standardized rates. Prevalence rates are shown as horizontal bars, and the estimates are indicated by the value at the right side of the plots. Error bars indicate the 95% confidence interval for the prevalence rates. Prevalence rates are shown for all subjects (red bars), females only (blue bars) and males only (green bars). Range of values for plasma homocysteine and RBC folate represented by their respective quartiles is described in text. HCY: plasma homocysteine; RBF: RBC folate.
We observed that there was a consistent increase in the age-standardized prevalence of self-reported sleep apnea over the four quartiles of plasma homocysteine levels in both males and females albeit the prevalence rates were always lower in females. However, a similar consistent trend was not observed for the quartiles of RBC folate. With the exception of the lowest quartile for RBC folate, the remaining three quartiles demonstrated a consistent increase in the age-standardized prevalence of self-reported sleep apnea. Again, this trend was observed in males as well as females with consistently lower rates in females.
We next estimated the age-standardized prevalence rates for a combination of the plasma homocysteine and RBC folate quartiles (Figure 2). We found some interesting patterns in these analyses. First, females with very low levels of plasma homocysteine as well as RBC folate had a very high prevalence of self-reported sleep apnea. Second, low levels of both plasma homocysteine and RBC folate were associated with a very low prevalence of self-reported sleep apnea in males. Third, highest prevalence of self-reported apnea in the males was found in those who had high level of both plasma homocysteine and RBC folate. Fourth, the mechanistically expected high prevalence of self-reported apnea in the low RBC folate/high plasma homocysteine subgroup was observed in males only but not in females. Together these findings indicated that there existed a complex and surprising combinatorial association of plasma homocysteine and RBC folate levels with self-reported sleep apnea.
Figure 2.
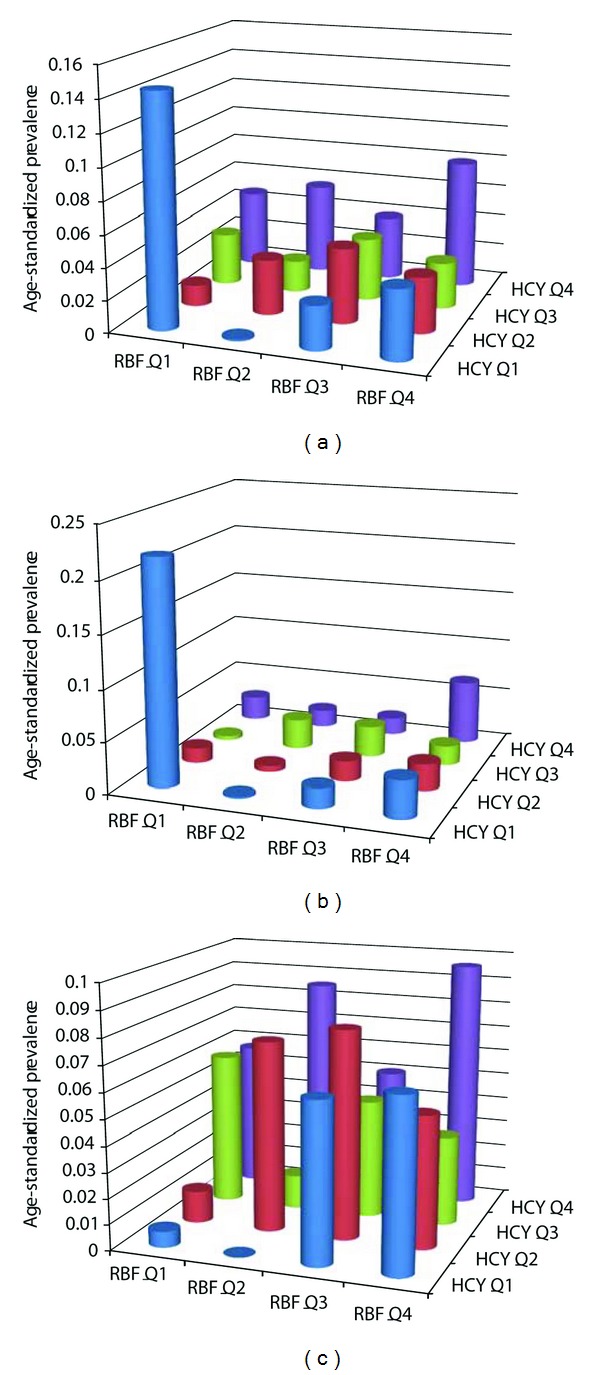
Age-standardized prevalence rates of diagnosed, self-reported sleep apnea based on combinations of quartiles of plasma homocysteine and RBC folate levels. Plots are for all subjects (a), females only (b), and males only (c). HCY: plasma homocysteine; RBF: RBC folate; Q: quartile.
3.3. Multivariate Association of Plasma Homocysteine and RBC Folate with Self-Reported Sleep Apnea
With the use of a series of 20 nested multivariate logistic regression models, we determined if inclusion of epidemiologically important significant covariates influenced the association of plasma homocysteine and RBC folate with self-reported sleep apnea (Figure 3). When no covariates were included along with the two primary predictors, we found that both were independently associated with the risk of self-reported sleep apnea (Model 1, Figure 3). The addition of other important variables like gender, non-Hispanic White race, birth in the United States, education, marital status, obesity, ever smoking, and ever alcohol use somewhat decreased the strength of the associations of plasma homocysteine and RBC folate with self-reported sleep apnea, however, in most instances, the associations retained their statistical significance. Interestingly however, when age was added to the multivariate model (Models 10 and 18, Figure 3), the significance of both plasma homocysteine and RBC folate reduced drastically. The results of the full model (Model 18 in Figure 3) are shown in detail in Table 3.
Figure 3.
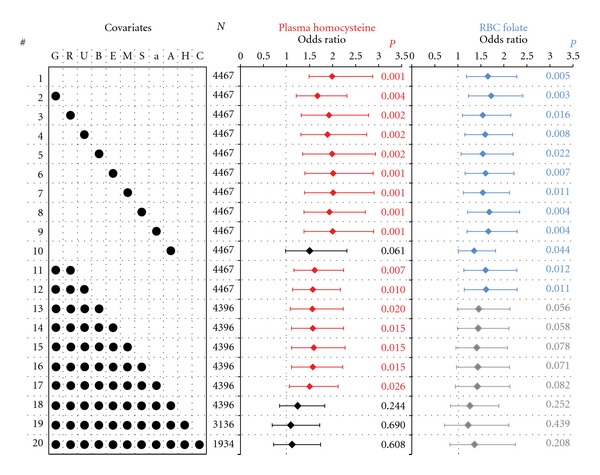
Multivariate association of high plasma homocysteine and RBC folate levels with the risk of self-reported sleep apnea. Results are shown as point (diamonds) and 95% confidence intervals (error bars) for odds ratios estimated through a series of nested logistic regression commands. Twenty logistic regression models (indicated by # on the left) were run with varying combinations of covariates. The covariates included were G: male gender; R: non-Hispanic white race; U: birth in the United States; B: body mass index > 28 Kg/m2; E: high education; M: married; S: ever smoker; a: ever alcohol use; A: age > 46 years; H: hypertension; C: cardiovascular disease. Model 1 contained only high plasma homocysteine and high RBC folate as the independent variables. The results from models 19 and 20 cannot be directly compared with the remaining 18 models since the information for hypertension and cardiovascular disease was not available for a large number of subjects (shown under column titled N). Statistically significant associations (when the error bars did not straddle unity indicated by dashed vertical lines) are shown in red color for high plasma homocysteine and in blue color for high RBC folate. Statistically nonsignificant associations are shown in black color for plasma homocysteine and gray color for RBC folate. Statistical significance is shown on individual plots as color-coded P values.
Table 3.
Results from the full multivariate logistic regression analysis (Model 18 in Figure 3) for the outcome of self-reported sleep apnea.
| Covariate | OR | 95% CI | P |
|---|---|---|---|
| High plasma homocysteine | 1.24 | 0.85–1.83 | 0.244 |
| High RBC folate | 1.26 | 0.84–1.89 | 0.252 |
| Male gender | 2.22 | 1.45–3.4 | 0.001 |
| Non-Hispanic White race | 1.21 | 0.74–1.96 | 0.420 |
| Birth in the United States | 1.89 | 0.93–3.82 | 0.073 |
| BMI > 28 Kg/m2 | 7.56 | 4.14–13.79 | <0.001 |
| High education | 1.71 | 1.21–2.41 | 0.005 |
| Married | 1.60 | 1.07–2.39 | 0.025 |
| Ever smoker | 1.28 | 0.76–2.14 | 0.334 |
| Ever alcohol use | 2.01 | 1.3–3.11 | 0.004 |
| Age > 46 years | 2.19 | 1.39–3.44 | 0.002 |
OR: odds ratio; CI: confidence interval; P: significance value.
It is noteworthy that high BMI, male gender, age > 46 years, alcohol use, high education, and married status continued to demonstrate independent association with self-reported sleep apnea in a multivariate context, but the association of high plasma homocysteine and high RBC folate became statistically insignificant (Table 3). Indeed, all the multivariate models (Models 18, 19, 20) that contained age as a covariate demonstrated a nonsignificant association of plasma homocysteine and folate with self-reported sleep apnea. Since the information of hypertension and cardiovascular disease was not available on large number of study subjects, we added these variables to the full model (Model 18) and observed that the strength as well as significance of the association of plasma homocysteine and RBC folate with self-reported sleep apnea was further diminished (Models 19 and 20).
The results presented thus far indicated a strong potential influence of age on the association of plasma homocysteine with self-reported sleep apnea. Considering the disposition of the survey data that we analyzed, we further refined our analyses. We examined if the strong positive correlation of age with plasma homocysteine levels (r = 0.38, P < 0.0001) masked the potentially true, independent association of plasma homocysteine with self-reported sleep apnea. We found that the odds ratio associated with a unit increase in the HCYdep levels was 1.51 (95% confidence interval 1.36–1.68), P < 0.0001 while that for a unit increase in HCYind was 1.02 (95% confidence interval 0.99–1.05), P = 0.189. These results demonstrated that the observed overall association of the plasma homocysteine levels with the risk of self-reported sleep apnea was indeed due to age.
4. Discussion
In this large, nationally representative sample of noninstitutionalized US subjects, we observed that plasma homocysteine is not independently associated with an altered risk of self-reported sleep apnea. Further, we found that age was the most important confounding factor that could have contributed to an apparent association of plasma homocysteine with the risk of self-reported sleep apnea. It has been shown by other investigators in differing contexts [31–34] that plasma homocysteine levels increase with age. It is also very well recognized that the risk of cardiovascular morbidity rises with age as well as plasma homocysteine levels. However, this concomitant elevation of homocysteine with age appears to be associative rather than causal in the context of sleep apnea. As identified by Lavie et al. [22], Svatikova et al. [26] and Winnicki and Palatini [9] sleep apnea and high plasma homocysteine levels can independently and additively increase the risk of subsequent cardiovascular morbidity; however, a potentially causal link between elevated plasma homocysteine and sleep apnea does not seem to be operative from our results.
Interestingly and intriguingly, we observed a positive association of the RBC folate levels with the risk of self-reported sleep apnea in spite of a mild negative correlation between RBC folate and plasma homocysteine levels. Further, despite a strong positive correlation between plasma and RBC folate (r = 0.49, P < 0.0001; data not shown), we observed that plasma folate levels were not associated with an altered risk of self-reported sleep apnea. These observations have two important implications. First, these findings indicate that initial enthusiasm generated by the possibility of vitamin supplementation aimed at reducing the prevalence of sleep apnea may be an oversimplification of the apnea challenge. For example, even though folate fortification of grains has been attributed with a decrease in homocysteine levels as well as cardiovascular morbidity [35], such a decrease has not been observed in the prevalence of sleep apnea in the United States. Second, the National Pathology Alliance benchmarking guidelines [36] stipulate that RBC and plasma folate can act as surrogates of each other and that no additional insights are gained by screening samples by both these methods. However, our results demonstrate that these methods may not be redundant and that differential insights can be obtained by a careful examination of the results. Thus, the issue deserves a closer investigation.
Although recruiting a large, representative sample, the current study was an observational study, and, therefore, all limitations implicit in such study designs and compounded by the survey sample selection should be considered while interpreting these results. In addition, it should be noted that the NHANES survey methodology did not use established apnea screening questionnaires but rather recorded self-reported sleep apnea. Therefore, there exist possibilities of missed diagnoses and a consequent misclassification bias. Despite these limitations, our results indicate that increased plasma homocysteine levels, augmented cardiovascular morbidity, and enhanced risk and prevalence of self-reported sleep apnea may actually be representing a syndromic constellation of the aging phenomenon. Consequently, more involved and intense efforts to understand the genetic and environmental contribution and interaction need to be undertaken before a mechanistic understanding, and the public health implications thereof can be fully realized.
Authors Contribution
T. P. Thakre and M. Mamtani have contributed equally to this paper.
Conflict of Interests
The authors have no conflict of interests to declare.
References
- 1.Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea concise review for clinicians. Mayo Clinic Proceedings. 2011;86(6):549–555. doi: 10.4065/mcp.2010.0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye L, Pien GW, Weaver TE. Gender differences in the clinical manifestation of obstructive sleep apnea. Sleep Medicine. 2009;10(10):1075–1084. doi: 10.1016/j.sleep.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Chilukuri K, Dalal D, Gadrey S, et al. A prospective study evaluating the role of obesity and obstructive sleep apnea for outcomes after catheter ablation of atrial fibrillation. Journal of Cardiovascular Electrophysiology. 2010;21(5):521–525. doi: 10.1111/j.1540-8167.2009.01653.x. [DOI] [PubMed] [Google Scholar]
- 4.Yeh PS, Lee YC, Lee WJ, et al. Clinical predictors of obstructive sleep apnea in Asian bariatric patients. Obesity Surgery. 2010;20(1):30–35. doi: 10.1007/s11695-009-9854-2. [DOI] [PubMed] [Google Scholar]
- 5.Kuniyoshi FHS, Pusalavidyasagar S, Singh P, Somers VK. Cardiovascular consequences of obstructive sleep apnoea. Indian Journal of Medical Research. 2010;131(2):196–205. [PubMed] [Google Scholar]
- 6.Al-Delaimy WK, Manson JE, Willett WC, Stampfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study. American Journal of Epidemiology. 2002;155(5):387–393. doi: 10.1093/aje/155.5.387. [DOI] [PubMed] [Google Scholar]
- 7.Lanfranchi PA, Somers VK. Sleep-disordered breathing in heart failure: characteristics and implications. Respiratory Physiology and Neurobiology. 2003;136(2-3):153–165. doi: 10.1016/s1569-9048(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 8.Iranzo A, Santamaría J, Berenguer J, Sánchez M, Chamorro A. Prevalence and clinical importance of sleep apnea in the first night after cerebral infarction. Neurology. 2002;58(6):911–916. doi: 10.1212/wnl.58.6.911. [DOI] [PubMed] [Google Scholar]
- 9.Winnicki M, Palatini P. Obstructive sleep apnoea and plasma homocysteine: an overview. European Heart Journal. 2004;25(15):1281–1283. doi: 10.1016/j.ehj.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Phillips BG, Somers VK. Sleep disordered breathing and risk factors for cardiovascular disease. Current Opinion in Pulmonary Medicine. 2002;8(6):516–520. doi: 10.1097/00063198-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Bananian S, Lehrman SG, Maguire GP. Cardiovascular consequences of sleep-related breathing disorders. Heart Disease. 2002;4(5):296–305. doi: 10.1097/00132580-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Ientile R, Curro' M, Ferlazzo N, Condello S, Caccamo D, Pisani F. Homocysteine, vitamin determinants and neurological diseases. Frontiers in Bioscience. 2010;2:359–372. doi: 10.2741/s70. [DOI] [PubMed] [Google Scholar]
- 13.Stanger O, Herrmann W, Pietrzik K, et al. DACH-LIGA homocystein (German, Austrian and Swiss homocysteine society): consensus paper on the rational clinical use of homocysteine, folic acid and B-vitamins in cardiovascular and thrombotic diseases: guidelines and recommendations. Clinical Chemistry and Laboratory Medicine. 2003;41(11):1392–1403. doi: 10.1515/CCLM.2003.214. [DOI] [PubMed] [Google Scholar]
- 14.Basoglu OK, Sarac F, Sarac S, Uluer H, Yilmaz C. Metabolic syndrome, insulin resistance, fibrinogen, homocysteine, leptin, and C-reactive protein in obese patients with obstructive sleep apnea syndrome. Annals of Thoracic Medicine. 2011;6(3):120–125. doi: 10.4103/1817-1737.82440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Can M, Açikgöz S, Mungan G, et al. Serum cardiovascular risk factors in obstructive sleep apnea. Chest. 2006;129(2):233–237. doi: 10.1378/chest.129.2.233. [DOI] [PubMed] [Google Scholar]
- 16.Cerbo RM, Cabano R, Lombardi G, Bollani L, Colombo R, Stronati M. From apneic spells to the development of hypertensive hydrocephalus: a case report of homocystinuria with early onset. Journal of Child Neurology. 2010;25(3):368–370. doi: 10.1177/0883073809336877. [DOI] [PubMed] [Google Scholar]
- 17.Chen M, Wu B, Ye X, et al. Association between plasma homocysteine levels and obstructive sleep apnoea in patients with ischaemic stroke. Journal of Clinical Neuroscience. 2011;18(11):1454–1457. doi: 10.1016/j.jocn.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 18.Cintra F, Tufik S, D'Almeida V, et al. Cysteine: a potential biomarker for obstructive sleep apnea. Chest. 2011;139(2):246–252. doi: 10.1378/chest.10-0667. [DOI] [PubMed] [Google Scholar]
- 19.Hachul De Campos H, Brandão L, D’Almeida V, et al. Sleep disturbances, oxidative stress and cardiovascular risk parameters in postmenopausal women complaining of insomnia. Climacteric. 2006;9(4):312–319. doi: 10.1080/13697130600871947. [DOI] [PubMed] [Google Scholar]
- 20.Kokturk O, Ciftci TU, Mollarecep E, Ciftci B. Serum homocysteine levels and cardiovascular morbidity in obstructive sleep apnea syndrome. Respiratory Medicine. 2006;100(3):536–541. doi: 10.1016/j.rmed.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Kumor M, Rubinsztajn R, Byśkiniewicz K, Bielicki P, Chazan R. Serum concentration of homocysteine and the risk of atherosclerosis in patients with obstructive sleep apnea syndrome. Pneumonologia i Alergologia Polska. 2006;74(1):117–121. [PubMed] [Google Scholar]
- 22.Lavie L, Perelman A, Lavie P. Plasma homocysteine levels in obstructive sleep apnea: association with cardiovascular morbidity. Chest. 2001;120(3):900–908. doi: 10.1378/chest.120.3.900. [DOI] [PubMed] [Google Scholar]
- 23.Ozkan Y, Firat H, Şimşek B, Torun M, Yardim-Akaydin S. Circulating nitric oxide (NO), asymmetric dimethylarginine (ADMA), homocysteine, and oxidative status in obstructive sleep apnea-hypopnea syndrome (OSAHS) Sleep and Breathing. 2008;12(2):149–154. doi: 10.1007/s11325-007-0148-4. [DOI] [PubMed] [Google Scholar]
- 24.Ryan S, Nolan GM, Hannigan E, Cunningham S, Taylor C, McNicholas WT. Cardiovascular risk markers in obstructive sleep apnoea syndrome and correlation with obesity. Thorax. 2007;62(6):509–514. doi: 10.1136/thx.2006.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sariman N, Levent E, Aksungar FB, Soylu AC, Bektaş O. Homocysteine levels and echocardiographic findings in obstructive sleep apnea syndrome. Respiration. 2009;79(1):38–45. doi: 10.1159/000210429. [DOI] [PubMed] [Google Scholar]
- 26.Svatikova A, Wolk R, Magera MJ, Shamsuzzaman AS, Phillips BG, Somers VK. Plasma homocysteine in obstructive sleep apnoea. European Heart Journal. 2004;25(15):1325–1329. doi: 10.1016/j.ehj.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Li J, Xie Y, Zhang XG. Association between serum homocysteine and oxidative stress in elderly patients with obstructive sleep apnea/hypopnea syndrome. Biomedical and Environmental Sciences. 2010;23(1):42–47. doi: 10.1016/S0895-3988(10)60030-X. [DOI] [PubMed] [Google Scholar]
- 28.Yavuz Z, Ursavaş A, Ege E, et al. Homocysteine levels in patients with obstructive sleep apnea syndrome. Tüberküloz ve Toraks. 2008;56(1):37–42. [PubMed] [Google Scholar]
- 29.Shih JL, Malhotra A. Could vitamins be helpful to patients with sleep apnea? Chest. 2011;139(2):237–238. doi: 10.1378/chest.10-2017. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Ford ES, Zhao G, Croft JB, Balluz LS, Mokdad AH. Prevalence of self-reported clinically diagnosed sleep apnea according to obesity status in men and women: National Health and Nutrition Examination Survey, 2005-2006. Preventive Medicine. 2010;51(1):18–23. doi: 10.1016/j.ypmed.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Budge MM, De Jager C, Hogervorst E, Smith AD. Total plasma homocysteine, age, systolic blood pressure, and cognitive performance in older people. Journal of the American Geriatrics Society. 2002;50(12):2014–2018. doi: 10.1046/j.1532-5415.2002.50614.x. [DOI] [PubMed] [Google Scholar]
- 32.El-Sammak M, et al. Elevated plasma homocysteine is positively associated with age independent of C677T mutation of the methylenetetrahydrofolate reductase gene in selected Egyptian subjects. International Journal of Medical Sciences. 2004;1(3):181–192. doi: 10.7150/ijms.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norlund L, Grubb A, Fex G, et al. The increase of plasma homocysteine concentrations with age is partly due to the deterioration of renal function as determined by plasma cystatin C. Clinical Chemistry and Laboratory Medicine. 1998;36(3):175–178. doi: 10.1515/CCLM.1998.032. [DOI] [PubMed] [Google Scholar]
- 34.Spotila LD, Jacques PF, Berger PB, Ballman KV, Ellison RC, Rozen R. Age dependence of the influence of methylenetetrahydrofolate reductase genotype on plasma homocysteine level. American Journal of Epidemiology. 2003;158(9):871–877. doi: 10.1093/aje/kwg234. [DOI] [PubMed] [Google Scholar]
- 35.Lavie L, Lavie P, Svatikova A, et al. Obstructive sleep apnoea and plasma homocysteine. European Heart Journal. 2005;26(20):2210–2211. [PubMed] [Google Scholar]
- 36.Galloway M, Rushworth L. Red cell or serum folate? Results from the National Pathology Alliance benchmarking review. Journal of Clinical Pathology. 2003;56(12):924–926. doi: 10.1136/jcp.56.12.924. [DOI] [PMC free article] [PubMed] [Google Scholar]


