First described in 1987 by Winter et al. as “atypical diabetes” (1), A−β+ ketosis-prone diabetes (KPD) describes a group of primarily middle-aged individuals brought to medical attention by an episode of spontaneous ketoacidosis (i.e., without evidence of infection, alcohol abuse, etc.) and subsequently diagnosed with diabetes (2,3). Although not exclusive to a particular ethnic group, KPD has primarily been described in individuals of African descent, Hispanics, and groups traditionally classified as ethnic minorities. Experience from our county hospital in Dallas, Texas, suggests that among those who present with new-onset diabetes and ketoacidosis, 60% meet criteria for the diagnosis of KPD (4). These patients generally lack evidence of endocrine pancreas autoimmunity (A−) and experience improved insulin secretory capacity (β+) and sensitivity after near-normalization of glycemia (5). Patients with KPD tend to be male and obese, and they typically have a family history of diabetes (3). Over time, this condition is characterized by a period of near-normoglycemia followed by sustained deterioration in glucose control and episodes of ketoacidosis. The metabolic perturbations that lead to transient β-cell dysfunction and spontaneous ketoacidosis in individuals with KPD remain unclear. Insights on the extreme metabolic phenotype of KPD have the potential to improve our understanding of more subtle metabolic derangements of type 2 diabetes.
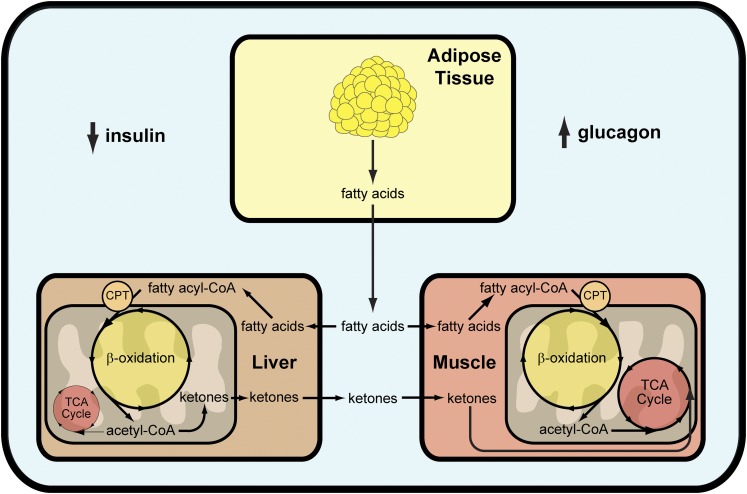
The fluctuating β-cell function that is characteristic of KPD suggests that acute glucose and fatty acid toxicity may play a role in its pathogenesis. However, recent studies of KPD patients during near-normoglycemic remission demonstrated that a 48-h exposure to excess fatty acids does not appreciably alter β-cell function (6) and that exposure to excess dextrose may (7) or may not (8) lead to impaired β-cell function depending on the magnitude and duration of the hyperglycemic stimulus. Although interesting, these data do not address the most striking phenotypic characteristic of these patients who more closely resemble type 2 diabetic patients—spontaneous ketoacidosis. Insulin deficiency alone is not sufficient to cause ketoacidosis (9). The majority of ketoacids (primarily β-hydroxybutyrate and acetoacetate) originate from the β-oxidation of fatty acids in liver (10) (Fig. 1). During insulin deficiency, peripheral lipolysis is not suppressed, which ultimately leads to increased fatty acid release. Data from Gosmanov et al. (8) suggest that during the recovery period of KPD, defects in fatty acid handling persist despite improved β-cell function. In liver, fatty acids are either esterified or shuttled into mitochondria via carnitine palmitoyl transferase to undergo β-oxidation (11). The entry of fatty acids into mitochondria is highly regulated; however, critically important to mitochondrial fatty acid oxidation is an excess of glucagon (or glucagon-like counterregulatory hormones such as cortisol and epinephrine) (9–11). As a result, ketoacidosis can only occur if insulin deficiency and glucagon excess occur simultaneously. Just this year, Choukem et al. (7) reported that patients with KPD have higher basal concentrations of glucagon and attenuated suppression of glucagon release, suggesting that the α-cell may also be important in the pathophysiology of KPD.
FIG. 1.
The physiology of ketosis and ketogenesis in humans. CPT, carnitine palmitoyl transferase.
In the current issue of Diabetes, Patel et al. (12) studied KPD patients with metabolomic approaches and then quantified metabolic fluxes using in vivo stable isotope tracers. This approach harnessed the high-throughput and broad information content of a metabolomic platform to generate hypotheses without the bias of a priori assumptions about disease-related metabolic derangements. However, static metabolite measurements usually lack the information content necessary to identify the activity of metabolic pathways, especially for metabolites that can be produced and consumed by multiple pathways. On the other hand, the metabolic flux through targeted pathways can be measured using stable isotope tracers to provide definitive evidence of a metabolic mechanism. Unfortunately, flux measurements are notoriously labor intensive, complex, expensive, and simply impractical for unbiased approaches. Patel et al. (12) exploited the strengths of both approaches by first applying metabolomics to identify candidate pathways and then testing the function of these pathways using in vivo stable isotope tracer methods.
Using this tactic, Patel et al. (12) studied banked samples from 20 obese, near-normoglycemic KPD patients 4–8 weeks after their index episode of ketoacidosis. Data from these patients were compared with those of 19 obese, nondiabetic control subjects. Metabolomic differences identified fatty acid, ketone, and amino acid pathways that were then functionally quantified by infusion of stable isotope tracers in a group of newly recruited subjects (9 KPD and 7 control subjects). Together these data revealed somewhat unexpected findings. Despite a “diabetic” phenotype, both the rate of release of peripheral fatty acids and their conversion to β-hydroxybutyrate was modestly lower in KPD patients. Surprisingly, ketosis occurred in these individuals in association with decreased fatty acid and ketone utilization, rather than the increased lipolysis and ketone production putatively associated with ketosis/ketoacidosis. Even more intriguing was a disturbance in branched-chain amino acid metabolism, similar to that observed in glycogen storage disease type V (McArdle syndrome). Patients with KPD demonstrated increased catabolic flux of leucine, a purely ketogenic branched-chain amino acid, toward ketogenesis as well as increased conversion of glutamine to glutamate without evidence of increased glutamate oxidation. As with McArdle syndrome, the authors suggest that the observed pattern of metabolic changes is indicative of defective oxidative metabolism and tricarboxylic acid (TCA) cycle anaplerosis. While this is possible, it is difficult to invoke an intrinsic defect such as this when KPD generally manifests in middle age. Likewise, the contribution of leucine to the pool of plasma ketones relative to fatty acids has historically been considered modest, primarily because of the marked difference in blood concentrations of the two precursors. Indeed, the contribution of leucine to ketone production is 2–5 and 4–10% in starved rats and dogs, respectively (13,14). However, given similar precursor concentrations, there is no metabolic barrier to prevent ketogenesis from leucine and fatty acids from occurring at similar rates (15). Although the development of ketoacidosis in the absence of elevated ketogenesis may be surprising, it is not unprecedented: the saturability of ketone uptake by peripheral tissues contributes significantly to ketosis during the onset of starvation (16). As a result, it is highly likely that the attenuated disposal of ketones in patients with KPD contributes to both ketosis and ketoacidosis. It is also interesting to note that many of the changes observed in amino acid metabolism by the authors appear similar to those observed during sustained administration of glucagon (17).
The findings of Patel et al. (12) advance our understanding of the metabolic basis of KPD; however, some experimental limitations should be considered while interpreting the data. The arduous process of recruiting KPD subjects was amplified by difficult and time-consuming study protocols, which may have contributed to the drop-out of several subjects before completion of the study. As a result, not all subjects underwent all tracer studies and, therefore, the data may not be reflective of those with KPD as a whole. The significance of the majority of the findings, however, would argue that this is a minor concern. Additionally, an equally important comparison group of ketosis-resistant patients with diabetes should be the focus of future studies to ensure that these findings are unique to patients with KPD. Overall, the elegant approaches used in this study yielded novel and unexpected results that improve our understanding of the pathophysiology of KPD and will stimulate future studies to confirm and expand these findings. Dissecting the metabolic dysregulation particular to KPD is a promising strategy toward understanding diabetes in general.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 912.
REFERENCES
- 1.Winter WE, Maclaren NK, Riley WJ, Clarke DW, Kappy MS, Spillar RP. Maturity-onset diabetes of youth in black Americans. N Engl J Med 1987;316:285–291 [DOI] [PubMed] [Google Scholar]
- 2.Maldonado M, Hampe CS, Gaur LK, et al. Ketosis-prone diabetes: dissection of a heterogeneous syndrome using an immunogenetic and β-cell functional classification, prospective analysis, and clinical outcomes. J Clin Endocrinol Metab 2003;88:5090–5098 [DOI] [PubMed] [Google Scholar]
- 3.Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med 2006;144:350–357 [DOI] [PubMed] [Google Scholar]
- 4.Piñero-Piloña A, Raskin P. Idiopathic Type 1 diabetes. J Diabetes Complications 2001;15:328–335 [DOI] [PubMed] [Google Scholar]
- 5.Banerji MA, Chaiken RL, Lebovitz HE. Long-term normoglycemic remission in black newly diagnosed NIDDM subjects. Diabetes 1996;45:337–341 [DOI] [PubMed] [Google Scholar]
- 6.Umpierrez GE, Smiley D, Robalino G, Peng L, Gosmanov AR, Kitabchi AE. Lack of lipotoxicity effect on β-cell dysfunction in ketosis-prone type 2 diabetes. Diabetes Care 2010;33:626–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choukem SP, Sobngwi E, Boudou P, et al. β- and α-cell dysfunctions in Africans with ketosis-prone atypical diabetes during near-normoglycemic remission. Diabetes Care 2013;36:118–123 [DOI] [PMC free article] [PubMed]
- 8.Gosmanov AR, Smiley D, Robalino G, et al. Effects of intravenous glucose load on insulin secretion in patients with ketosis-prone diabetes during near-normoglycemia remission. Diabetes Care 2010;33:854–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerich JE, Lorenzi M, Bier DM, et al. Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N Engl J Med 1975;292:985–989 [DOI] [PubMed] [Google Scholar]
- 10.McGarry JD. Lilly Lecture 1978. New perspectives in the regulation of ketogenesis. Diabetes 1979;28:517–523 [DOI] [PubMed] [Google Scholar]
- 11.McGarry JD, Foster DW. Ketogenesis and its regulation. Am J Med 1976;61:9–13 [DOI] [PubMed] [Google Scholar]
- 12.Patel SG, Hsu JW, Jahoor F, et al. Pathogenesis of A−β+ ketosis-prone diabetes. Diabetes 2013;62:912–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas LK, Ittmann M, Cooper C. The role of leucine in ketogenesis in starved rats. Biochem J 1982;204:399–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulaylat MN, Frexes-Steed M, Geer R, Williams PE, Abumrad NN. The role of leucine in hepatic ketogenesis. Surgery 1988;103:351–360 [PubMed] [Google Scholar]
- 15.Krebs HA, Lund P. Aspects of the regulation of the metabolism of branched-chain amino acids. Adv Enzyme Regul 1976;15:375–394 [DOI] [PubMed] [Google Scholar]
- 16.Reichard GA, Jr, Owen OE, Haff AC, Paul P, Bortz WM. Ketone-body production and oxidation in fasting obese humans. J Clin Invest 1974;53:508–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battezzati A, Simonson DC, Luzi L, Matthews DE. Glucagon increases glutamine uptake without affecting glutamine release in humans. Metabolism 1998;47:713–723 [DOI] [PubMed] [Google Scholar]