Abstract
Increases in weight have been associated with corresponding increases in insulin resistance in postmenopausal women. Although estrogen has significant impact on body fat and body fat distribution, the cellular mechanisms that influence this process are not yet known. We measured adipose tissue fatty acid (FA) storage and FA storage factors in 12 premenopausal and 11 postmenopausal women matched for age and body composition. Postmenopausal women had lower postprandial FA oxidation (indirect calorimetry), greater meal FA, and direct free FA (FFA) storage than premenopausal women, including two-fold greater meal FA storage in the femoral depot. The fed/fasted activities of adipose tissue lipoprotein lipase were not significantly different between premenopausal and postmenopausal women. In contrast, adipocyte acyl-CoA synthetase and diacylglycerol acyltransferase activities in postmenopausal women were significantly upregulated and were positively correlated with direct FFA storage rates. These findings suggest that the propensity for subcutaneous adipose tissue FA storage is increased in postmenopausal women, more so from changes in adipocyte FA storage factors than from adipose tissue lipoprotein lipase activity. Our results suggest that female sex steroids, most likely estrogen, have important effects on adipose tissue FA storage and FA oxidation that could promote fat gain in postmenopausal women.
Increases in body weight are associated with greater risks of type 2 diabetes (1–3), and weight gain in postmenopausal women are of special concern (4). Estrogen has remarkable effects on body fat distribution, and the decreased sex hormone production after menopause is associated with increased total body fat (5,6), especially in the central/abdominal region (7,8). Hormone replacement therapy in early menopause may mitigate these changes in body composition and may decrease central adiposity (9). Despite the strong evidence that female sex steroids have a major influence on total body fat and body fat distribution, the cellular mechanisms mediating these effects are unknown. To address this lack of understanding, we measured the storage of dietary fatty acids (FAs) and circulating free fatty acids (FFAs) into adipose tissue in premenopausal and postmenopausal women carefully matched for age and body composition. These physiological measures were combined with measures of the adipose tissue content of a number of proteins/enzymes required for adipocyte FA storage.
The FA stored in adipose tissue originates primarily from triglyceride-rich lipoproteins (i.e., chylomicrons and VLDL); however, there is also a component of FA redistribution via the direct FFA reuptake pathway. FFAs are the products of adipose tissue lipolysis, which is virtually the sole source of FFA in the postabsorptive state. Triglyceride-rich lipoproteins require lipoprotein lipase (LPL) to liberate the FA from the glycerol backbone of the triglyceride molecule. Regardless of whether FAs are derived from triglyceride-rich lipoproteins or circulating FFA, the FA can enter the adipocyte either via a passive (flip-flop) mechanism or via protein facilitated diffusion (10). Once inside the adipocyte, FA must undergo a series of enzymatic reactions to be stored as triglyceride. Although it is known that estrogen can modulate LPL activity by suppressing gene transcription (11), little else is understood about whether estrogen affects adipocyte storage steps, including protein-facilitated transport and the enzymes needed for triglyceride synthesis.
We performed quantitative measures of meal-derived FA and direct FFA storage in adipose tissue and integrated these physiological assessments with information regarding some key factors that regulate cellular storage of FA as triglycerides. We focused on the FA transport protein (CD36), acyl-CoA synthetase (ACS) activity, and diacylglycerol acyltransferase (DGAT) activity. These FA storage factors are involved in different tiers of adipocyte FA storage. CD36 is a cell-surface glycoprotein that facilitates FA transport into cells (12). The ACS enzymes catalyze the activation of FA to their CoA derivatives (13). The final step in FA storage as triglycerides is catalyzed by DGAT (14,15). By integrating the physiological and cellular information regarding adipocyte FA storage, we are able to provide novel insights into the effects of estrogen on systemic and regional adipose tissue metabolism in humans. The unique differences in FA storage between premenopausal and postmenopausal women we observe contribute toward our understanding of body fat patterning in women.
RESEARCH DESIGN AND METHODS
Subjects.
Eleven women who had undergone menopause naturally (at least 3 years from last menstrual period) or surgically (at least 2 years from salpingo-ophorectomy) participated in the research study. For the purposes of this report, we refer to this group as postmenopausal. To be included in the study, women could not have been using hormone replacement therapy for at least 2 years. Thirteen premenopausal women with normal serum estrogen concentrations (premenopausal) were recruited as age-matched and BMI-matched controls. All participants were healthy and weight was stable (±1.0 kg for > 2 months before the study). Participants were excluded if they had diabetes, anemia, or were using antidepressants or other medications that could affect FA metabolism. Written informed consent was obtained from all participants. The study was approved by the Institutional Review Board of the Mayo Clinic.
Materials.
[1-14C]palmitate and [9,10-3H]triolein were purchased from NEN Life Science Products (PerkinElmer, Boston, MA). 2H2O and [U-13C]palmitate (both 99 atom percent pure) were purchased from Isotec (Miamisburg, OH).
Study design.
All studies were conducted in the Mayo Clinical Research Unit. Before their inpatient study visit, total body water and body composition were measured. Participants were provided with all meals for 5 days immediately before their inpatient study day to ensure that they were in comparable and stable nutritional states (16). Participants then were admitted for their inpatient study stay. The protocol for the inpatient study days has been described elsewhere (16). Briefly, after an overnight stay, volunteers consumed an experimental meal containing [3H]triolein. Blood samples were collected and indirect calorimetry (DeltaTrac, Yorba Linda, CA) was performed hourly. Urine was collected over a 24-h period for nitrogen and 3H2O excretion as part of meal fat oxidation measurements. After a second consecutive overnight visit, FFA tracers were given to measure direct FFA storage rates. Abdominal and femoral adipose tissue biopsies were performed on the first day at 1400 h (1 h after lunch) and on the second day at 30 min after the bolus infusion of [1-14C]palmitate. Blood samples for plasma catecholamine concentrations were collected at 0, 300, and 1,440 min during the study day.
Assays and methods.
Plasma triglyceride concentrations and 3H content in chylomicron and nonchylomicron fractions were measured as previously described (17). Urinary nitrogen was measured using an Analox GM7 Fast Enzymatic Metabolite Analyzer (Analox Instruments, Lunenburg, MA). Plasma glucose was measured using a glucose analyzer (Beckman Instruments, Fullerton, CA) and plasma catecholamine concentrations were measured by reversed phase high-performance liquid chromatography (18). Plasma triglyceride concentrations were measured using a microfluorometric assay (19). Plasma insulin concentrations were measured using chemiluminescent assays on an automated immunoassay system (Assay and DxI, Beckman Instruments, Chaska, MN). Plasma estrogen concentrations were measured via liquid chromatography/mass spectrometry.
Body composition.
Fat-free mass, total body mass, and leg fat mass were measured via dual-energy X-ray absorptiometry (Lunar iDXA, GE Healthcare, Madison, WI) (20). Abdominal subcutaneous and visceral adipose tissue areas were measured by combining data from a single-slice abdominal computed tomography scan at the L2–L3 level with the dual-energy X-ray absorptiometry-measured total abdominal fat content, as previously described (21).
Substrate oxidation.
Participants fasted for 12 h before measuring resting energy expenditure, as previously described (16). Measurements were taken 15 min hourly from 0 to 360 min after the consumption of the experimental meal. Carbohydrate and fat oxidation at each time point were calculated and total oxidation was determined as area under the curve (AUC) (22).
Fatty acid metabolism studies.
Meal FA storage and oxidation were determined as previously described (16). Briefly, 50 µCi [9,10-3H]triolein was sonicated with an Ensure Plus meal that provided 40% of individually measured resting energy expenditure (23). Participants consumed the meal at 0800 h and quadruplicate 50-µL samples of the test meal were counted on a liquid scintillation counter to determine the exact amount of [3H]triolein consumed. Meals with the same macronutrient composition as those provided during the week before admission were consumed at lunch (1300 h) and dinner (1800 h). 3H2O concentration in body water was measured in urine collected after a 24-h void, as previously described (16). A continuous infusion of [U-13C]palmitate to measure FFA flux (24) combined with a bolus infusion of [1-14C]palmitate was administered to measure direct adipose tissue FFA storage rates (25). Abdominal and femoral adipose tissue biopsies were performed using sterile technique and local anesthesia at 6 h and 24 h after experimental meal: the 24-h biopsy was timed to occur 30 min after the 14C bolus (16). After lipid extraction via collagenase digestion (26), adipocyte 3H and 14C lipid-specific activity (dpm/g lipid) were measured as previously described (27).
Adipose tissue analysis.
Fat cell size was measured using photomicrographs (28), LPL activity was measured using the approach of Nilsson-Ehle and Schotz (29), ACS (30) and DGAT (15) activity were determined using enzymatic assays, and CD36 quantification was measured using sandwich ELISA (31).
Calculations, data analysis, and statistics.
Calculations are described in detail elsewhere (16). Because the means of data expression can impact data interpretation (32), we provide two approaches in an attempt to address different perspectives. We use the per unit lipid expression when the question relates to whether one body fat depot competes better for the available FAs than another depot. However, when examining the factors that regulate tissue FA storage rates at a cellular level, we express the data per 1,000 adipocytes. The Shapiro-Wilk test was performed for goodness of fit and data that were not normally distributed were log-transformed. Unpaired t tests were applied for between-group comparisons. A mixed model 2 level and mixed model 3 level ANOVA with post hoc least-squares means tests were used to determine group differences using time and depot as within-group variables. If a relationship was apparent, then a Pearson correlation was used; otherwise, correlations in postmenopausal and premenopausal groups were determined using Spearman rank test. All data are presented as mean ± SEM and were analyzed using JMP 9.0 (SAS Institute, Cary, NC). Statistical significance was defined as P < 0.05.
RESULTS
Subject characteristics.
The two groups of women were matched for age, BMI, and body composition (Table 1). The average and range of BMI were similar in both the postmenopausal and premenopausal groups (21.6–38.3 kg/m2). Abdominal fat cell size was smaller (P < 0.001) than thigh fat cell size in both groups and, by design, serum estrogen concentrations were greater in premenopausal than in postmenopausal women.
TABLE 1.
Subject characteristics
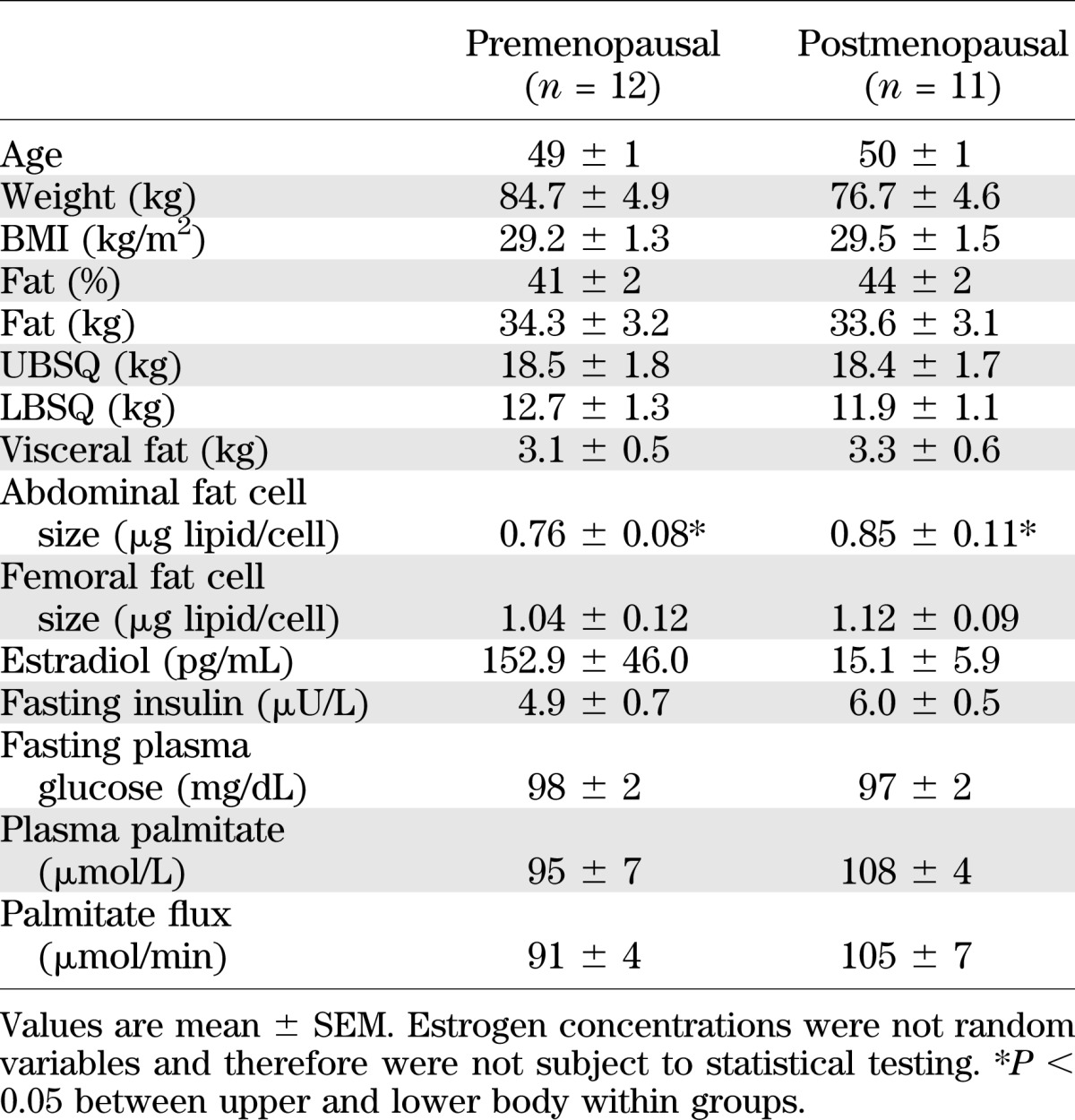
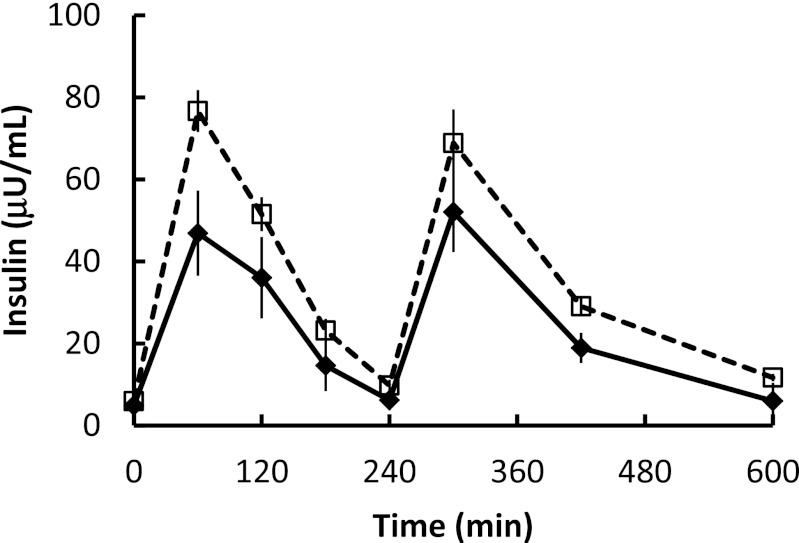
There were no differences in baseline insulin or fasting plasma glucose concentrations between groups. Despite comparable plasma glucose concentrations, the daytime plasma insulin concentrations, as measured by the AUC, was greater (P = 0.008) in postmenopausal women than in premenopausal women (Fig. 1). The average (mean of three samples) plasma epinephrine concentrations were 49 ± 9 and 43 ± 11 pg/mL (postmenopausal versus premenopausal; P = not significant) and plasma norepinephrine concentrations were 219 ± 17 and 176 ± 15 pg/mL (postmenopausal versus premenopausal; P = not significant). Overnight postabsorptive plasma palmitate concentrations and flux were not different in the two groups (Table 1).
FIG. 1.
Daytime plasma insulin concentrations. Plasma insulin concentrations during the first 10 h of the experimental meal day. Black diamonds and solid lines, premenopausal women; white squares and dashed lines, postmenopausal women. AUC was greater (P = 0.008) in postmenopausal women than in premenopausal women.
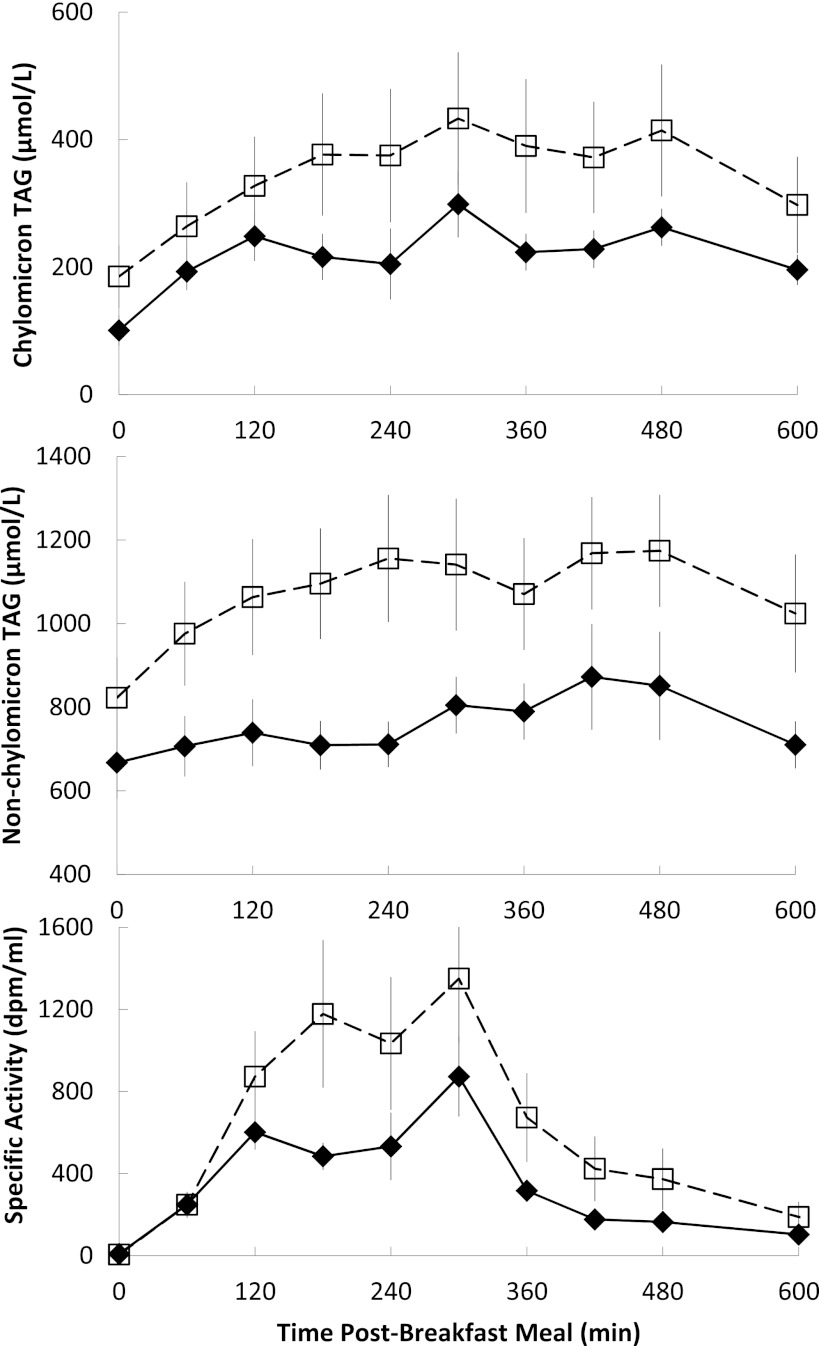
The AUC of nonchylomicron triglyceride concentrations in postmenopausal women tended to be greater (P = 0.05) than in premenopausal women (Fig. 2). There were no significant differences between premenopausal and postmenopausal groups in AUC of chylomicron triglyceride concentrations (P = 0.17) or specific activity (P = 0.15), suggesting that meal FA absorption/transport into the circulation was similar in the two groups.
FIG. 2.
Daytime plasma triglyceride concentrations and specific activity. Plasma chylomicron (top) and nonchylomicron triglyceride concentrations (middle) and chylomicron triglyceride–specific activity (lower) during the first 10 h of the experimental meal day. Black diamonds and solid lines, premenopausal women; white squares and dashed lines, postmenopausal women. AUC of nonchylomicron triglyceride concentrations in postmenopausal tended to be greater (P = 0.05) than in premenopausal women. There were no significant differences between premenopausal and postmenopausal groups in AUC of chylomicron triglyceride concentrations or specific activity.
Substrate and meal FA oxidation.
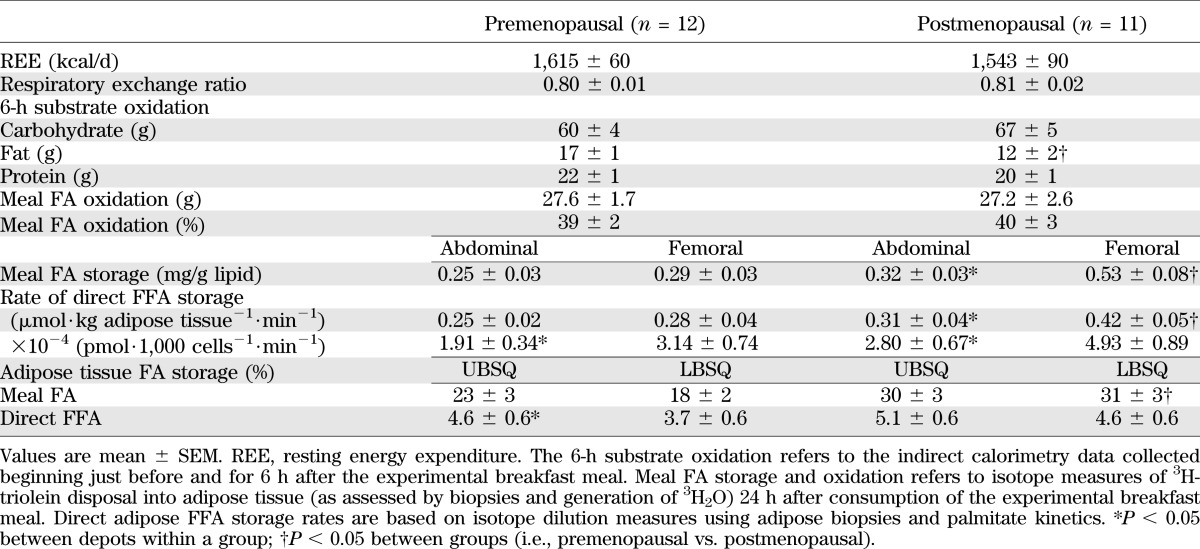
The AUC of total FA oxidation measured by hourly indirect calorimetry over the 6 h after the experimental meal was greater (P = 0.04) in premenopausal women than postmenopausal women (Table 2). In contrast, meal FA oxidation (3H2O generation) over 24 h was not different between groups. Overnight basal metabolic rate and respiratory exchange ratio were similar between groups (Table 2) and within individuals between study days, indicating that the participants were in a metabolically steady state.
TABLE 2.
Energy and FA metabolism
Regional meal FA storage.
There was a group × depot effect (P = 0.01) in 24-h meal FA storage (mg meal FA⋅g lipid−1), with a greater difference between abdominal and femoral meal FA storage in postmenopausal than in premenopausal women. Postmenopausal women stored more (P = 0.0002) meal FA in the femoral than abdominal depot (Table 3). In contrast, meal FA storage in premenopausal women was not significantly different between depots. Postmenopausal women also stored more (P = 0.001) meal FA per gram of lipid in the femoral region than premenopausal women.
TABLE 3.
Adipose tissue characteristics
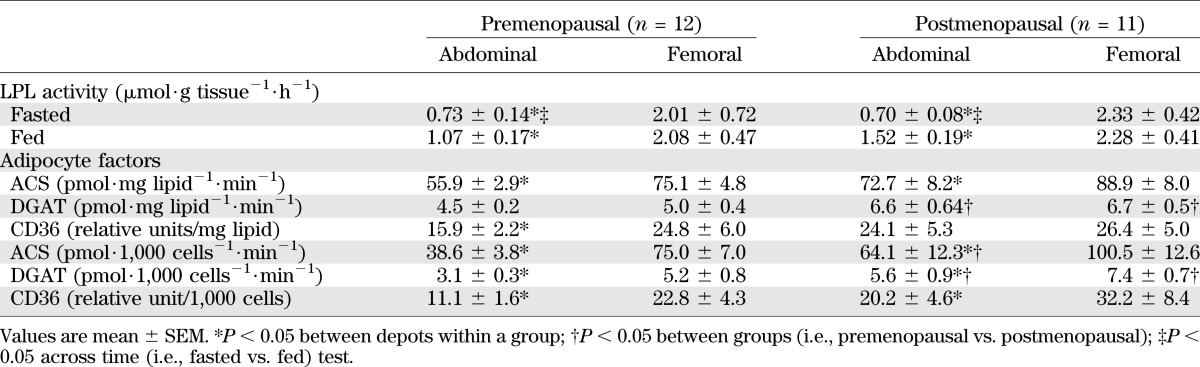
A greater percentage of meal FA was stored in lower body subcutaneous adipose tissue (LBSQ; P = 0.001) in postmenopausal than premenopausal women. There was a trend (P = 0.05) for a greater proportion of meal fat to be stored in upper body subcutaneous adipose tissue (UBSQ) of postmenopausal versus premenopausal women. In total, postmenopausal women stored a greater (P = 0.002) proportion of meal FA in subcutaneous fat than premenopausal women.
Direct adipocyte FFA storage.
Rates of FFA storage are presented as µmol⋅kg adipose tissue−1⋅min−1 for physiological interpretation and as pmol⋅1,000 cells−1⋅min−1 to permit us to address mechanisms at the cellular level. At the physiological level, there was a group × depot effect (P = 0.047) in rate of direct FFA storage (µmol⋅kg adipose tissue−1⋅min−1). Rates of direct FFA storage were greater (P = 0.02) in femoral adipose tissue of postmenopausal than premenopausal women. The rates of direct FFA storage were greater (P = 0.0005) in femoral than abdominal adipose tissue only in postmenopausal women. At the cellular level, rates of FFA storage (pmol⋅1,000 cells−1⋅min−1) were greater in the femoral than abdominal fat for both premenopausal (P = 0.009) and postmenopausal (P = 0.0001) women. Although the proportion of FFA stored in UBSQ and LBSQ depots via the direct pathway was not significantly different in postmenopausal women, premenopausal women stored a greater proportion (P = 0.01) of FFA in UBSQ than in LBSQ.
Adipose-specific effectors of FA storage
LPL activity.
LPL activity was significantly greater in femoral than abdominal fat in both the fasted and fed state in both postmenopausal and premenopausal women (Table 3). However, the difference between abdominal and femoral LPL activity was not as marked in the fed state as indicated by a time × depot effect (P = 0.003). In addition, the change in LPL activity between depots across time was different between groups as indicated by a time × group × depot effect (P = 0.04). This was explained by a greater increase in abdominal LPL activity from the fasted to the fed state in postmenopausal than in premenopausal women (Table 3).
LPL activity as a predictor of meal FA storage.
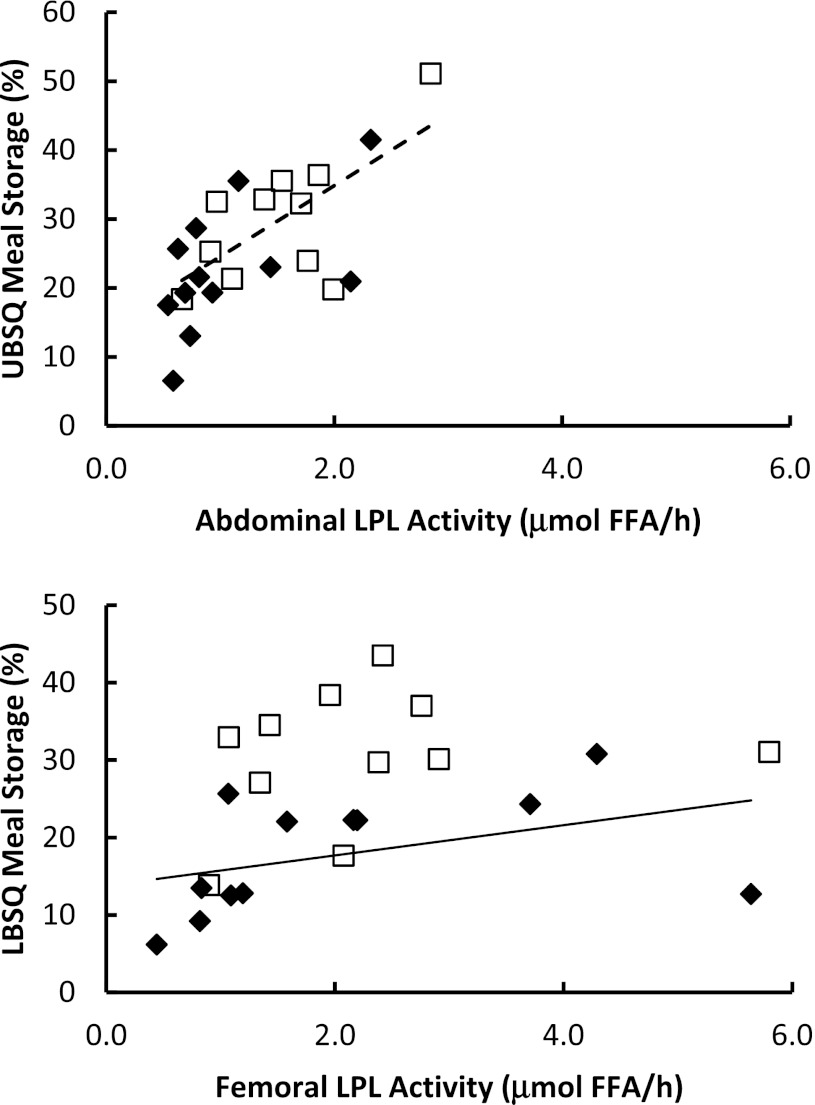
Because fed LPL activity correlates with fractional meal FA storage in young, lean, healthy men and women (33), we examined this relationship in this study (Fig. 3). The correlation between fed abdominal LPL activity and the proportion of meal FA stored in UBSQ was significant (r = 0.66; P = 0.03) in postmenopausal women, and nearly so in premenopausal women (r = 0.57; P = 0.06). Fed femoral LPL activity correlated (P = 0.60; P = 0.04) with the proportion of meal FA stored in LBSQ fat only in premenopausal women.
FIG. 3.
Regional LPL activity compared with regional meal FA storage. Fed LPL activity in the abdominal (top panel) and femoral (lower panel) is plotted compared with the proportion of meal FAs stored in UBSQ and LBSQ adipose tissue. Black diamonds, premenopausal women; white squares, postmenopausal women; dashed line (top panel), a significant correlation in postmenopausal women; and solid line (bottom panel), a significant correlation in the premenopausal women.
Adipocyte FA storage factors.
Overnight postabsorptive adipose tissue CD36 content and ACS and DGAT activity data are presented in Table 3 and are expressed in both physiological (per milligram of lipid) and cellular (per 1,000 cells) terms.
At both the physiological (P = 0.002 for abdominal; P = 0.007 for femoral) and cellular levels (P = 0.005 for abdominal; P = 0.03 femoral), DGAT activity was greater in postmenopausal than in premenopausal women. At the cellular level, abdominal ACS activity was greater (P = 0.04) in postmenopausal than in premenopausal women. There were no significant differences in regional CD36 content (per 1,000 cells) in postmenopausal versus premenopausal women.
At the physiological level, femoral ACS activity was greater (P = 0.01 and P = 0.001, respectively) than abdominal ACS activity in both postmenopausal and premenopausal women. In premenopausal women only, CD36 content per milligram of lipid was greater (P = 0.04) in femoral than in abdominal fat. ACS and DGAT activity and CD36 content per 1,000 cells of fat were greater (P < 0.02 for all) in femoral than in abdominal fat for premenopausal and postmenopausal women.
Adipocyte proteins versus direct FFA storage rates.
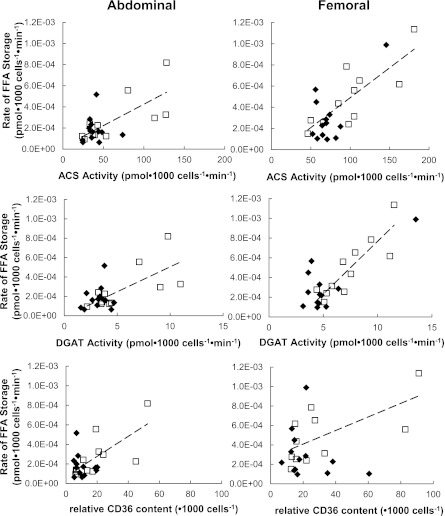
For postmenopausal women, but not for premenopausal women, the activity of ACS, DGAT, and CD36 content were all significantly associated with rate of FFA storage (per 1,000 cells) in abdominal (ρ = 0.75 and P < 0.01 for all) and femoral (ρ = 0.74 and P = 0.01; ρ = 0.85 and P = 0.0008; ρ = 0.63 and P = 0.04, respectively) fat (Fig. 4). No significant associations were found between adipocyte proteins and FFA storage in premenopausal women. There were no significant physiological (per milligram of lipid) relationships between ACS and DGAT activity or between CD36 content and the rates of FFA storage per kilogram of adipose tissue. No significant associations were found between adipocyte proteins and FFA storage in premenopausal women.
FIG. 4.
Relationships between abdominal and femoral adipose tissue FA storage factors compared with direct FFA storage rates expressed relative to adipocytes. Regional rates of FFA storage (pmol⋅1,000 cells−1⋅min−1) compared with lipogenic protein activity expressed per 1,000 cells. Black diamonds, premenopausal women; white squares, postmenopausal women; dash lines, a significant correlation in postmenopausal women.
Relationship between adipocyte FA storage factors.
For abdominal fat, ACS activity, DGAT activity, and CD36 content were correlated with each other in postmenopausal women (ACS versus DGAT: ρ = 0.77 and P = 0.005; ACS versus CD36: ρ = 0.69 and P = 0.02; DGAT versus CD36: ρ = 0.78 and P = 0.005). Similarly, in femoral fat of postmenopausal women, ACS activity was correlated with both CD36 content and DGAT activity (ρ = 0.71 and P = 0.02 for both). There were no relationships between CD36 content and ACS and DGAT activity in the abdominal or femoral depots in premenopausal women.
DISCUSSION
Although there are large amounts of descriptive data regarding the effects of estrogen on body fat distribution, the underlying cellular mechanisms remain unknown. We examined the regulation of fat storage pathways in vivo in postmenopausal and age-matched and body composition–matched premenopausal women. We found that meal FA storage in subcutaneous fat was greater in postmenopausal than in premenopausal women. This difference was especially evident in the femoral depot, where meal FA storage in postmenopausal women was double that of premenopausal women. Rates of direct FFA storage also were greater in postmenopausal versus premenopausal women, although differences were only significant in the femoral region. At the level of the adipocyte, CD36 content and ACS and DGAT activity are highly coregulated in postmenopausal women, and these FA storage factors were significantly related to FFA storage. Our results imply that the sex steroid deficiency of menopause increases CD36 content and ACS and DGAT activity in such a manner as to facilitate greater adipose tissue FA storage.
As expected, we observed that plasma insulin and triglycerides were somewhat greater in postmenopausal women. Although the contribution of changing fat distribution versus hormonal changes with menopause to insulin and triglyceride responses are unclear, other investigators also have observed higher fasting insulin and triglyceride concentrations in postmenopausal women (34–37). Abdominal adipose tissue LPL activity in the fed state was greater in postmenopausal women. The somewhat lower LPL activity in estrogen-sufficient women is consistent with findings from other groups (38,39). Homma et al. (11) found a sequence on the LPL promoter that responds to estrogen by suppressing gene transcription in vitro. Our finding that an association between femoral LPL activity and meal FA storage was present in premenopausal, but not in postmenopausal women, suggests that the upregulated femoral LPL activity in postmenopausal women is no longer rate-limiting for dietary FA storage in this depot, at least at this amount of dietary fat intake.
We also found that postmenopausal women had lower fat oxidation than premenopausal women. Whether estrogen alters fat oxidation is controversial. Although some have found that postmenopausal women treated with estrogen increase fat oxidation (40,41), others have not observed differences in fat oxidation between postmenopausal women who were estrogen-deficient versus estrogen-sufficient (42,43). Our findings of reduced postprandial FA oxidation in postmenopausal women are consistent with the findings of Lwin et al. (41). Regarding mechanism, it has been reported that ovariectomized mice administered estrogen upregulate the skeletal muscle expression of peroxisome proliferator–activated receptor-δ and peroxisome proliferator–activated receptor-α (44,45), which should increase fat oxidation.
To our knowledge, this study is the first to measure a series of adipocyte FA storage factors in premenopausal and postmenopausal women. At the physiological and cellular levels, both abdominal DGAT activity and femoral DGAT activity were most strikingly different between postmenopausal and premenopausal women; DGAT activity was 25–40% higher in the former. Although some adipocyte FA storage factors were not significantly different between groups, all factors we measured were greater in postmenopausal women. In both abdominal and femoral depots, each adipocyte FA storage factor we measured was significantly correlated with direct FFA storage, but only in postmenopausal women. These correlations indicate a global increase in fat storage tendencies that is not specific to abdominal or femoral fat.
We cannot determine from this study whether reduced ability to oxidize fat promotes greater fat storage or whether increases in fat storage limits fat oxidation. The greater FA storage tendencies of postmenopausal women may suggest that adipose tissue diverts fat from oxidative pathways. The greater propensity toward meal and direct FFA storage in the adipose tissue of postmenopausal women was accompanied by decreases in total FA oxidation over a 6-h postprandial interval. We note, however, that meal fat oxidation (from the experimental breakfast meal) was not different between groups. This may point toward a shift of FA oxidation away from FFA and toward meal sources, which would account for the greater adipose storage of dietary fat without reductions in meal FA oxidation. Our finding of greater direct FFA storage, albeit in the postabsorptive state, is consistent with this interpretation. The combination of greater adipose tissue FA storage and greater postprandial carbohydrate oxidation in postmenopausal women theoretically could promote greater food intake to maintain glycogen stores. In this scenario, the slightly greater food intake over time could promote weight gain in the form of excess body fat.
A particular strength of this study is that FA storage is examined in the context of numerous adipocyte FA storage factors, including LPL activity. If we examined only LPL activity, then we may have come to the false conclusion that only abdominal adipocyte FA storage increases with female hypogonadism. By measuring abdominal and femoral ACS and DGAT activity, as well as CD36 content, we were able to document that the overall adipocyte FA storage machinery is increased in postmenopausal women. Because our volunteers were matched for body composition and age, these differences are most likely attributable to differences in sex steroid concentrations, not other factors. However, we cannot be sure whether the differences are caused solely by lower estrogen or by a combination of low estrogen and progesterone concentrations after menopause. Another issue to consider is that differences in insulin concentrations between premenopausal and postmenopausal women may have influenced adipocyte FA storage pathways. Insulin increases the expression of adipose ACS mRNA in vitro (46,47), and human adipose tissue DGAT mRNA expression is greater in those with increased insulin sensitivity (48). Furthermore, DGAT activity is stimulated by insulin and glucose (49). In contrast, insulin does not appear to stimulate CD36 translocation (50). Thus, the greater daytime insulinemia in postmenopausal women may have impacted ACS and DGAT activity. We did not find statistically associations between the 24-h AUC of insulin concentrations and the adipogenic protein activities and content (data not shown), suggesting that the observed differences between premenopausal and postmenopausal women were influenced more so by differences in sex steroids than insulin.
We hypothesize that the effects we documented are more likely made chronic by estrogen deficiency, because premenopausal women had 10-fold greater estrogen concentrations than postmenopausal women. Furthermore, the study was designed to isolate the effects of estrogen from age and the well-described shifts in total and regional body fat distributions that occur with menopause by matching our participants for these variables.
In summary, our findings indicate that overall fat storage tendencies are increased in postmenopausal women. This finding is significant because weight gain is associated with greater risk of diabetes (1–3). The increase in fat storage stems from somewhat greater LPL activity and significantly greater content of adipocyte FA storage factors. It is possible that the upregulation in proteins associated with FA storage capacity in postmenopausal women contributes to the decrease in postprandial total fat oxidation. Whether the differences in FA storage between premenopausal and postmenopausal women are attributable to the effects of estrogen or the combination of estrogen, progesterone, and other factors, such as changing insulin concentrations, remains to be elucidated.
ACKNOWLEDGMENTS
This work was supported by grant NCRR 1UL1 RR024150, National Institutes of Health grants DK-45343 and DK-50456, and by ADA grant 7-06-DCS-03. S.S. received fellowship funding from the Natural Sciences and Engineering Research Council of Canada and incentive funding from the Canadian Diabetes Association.
No potential conflicts of interest relevant to this article were reported.
S.S. contributed to the study design, performed the studies, researched data, and wrote the manuscript. M.D.J. designed and oversaw the study, reviewed the manuscript, and edited the manuscript. M.D.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are indebted to the research volunteers for their participation. The authors also are grateful to Barbara Norby and Carley Vrieze for assistance with nursing care and adipose biopsies. They also thank Christy Allred, Debra Harteneck, Darlene Lucas, and Lendia Zhou for assay development and performance. All persons listed herein are affiliated with the Mayo Clinic, Rochester, Minnesota.
The meal FA metabolism data were presented at the Canadian Obesity Network Meeting, and the direct FFA data were presented at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
REFERENCES
- 1.Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol 1997;146:214–222 [DOI] [PubMed] [Google Scholar]
- 2.Resnick HE, Valsania P, Halter JB, Lin X. Relation of weight gain and weight loss on subsequent diabetes risk in overweight adults. J Epidemiol Community Health 2000;54:596–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colditz GA, Willett WC, Stampfer MJ, et al. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol 1990;132:501–513 [DOI] [PubMed] [Google Scholar]
- 4.Lemay A, Turcot L, Déchêne F, Dodin S, Forest J-C. Hyperinsulinemia in nonobese women reporting a moderate weight gain at the beginning of menopause: a useful early measure of susceptibility to insulin resistance. Menopause 2010;17:321–325 [DOI] [PubMed] [Google Scholar]
- 5.Guo SS, Zeller C, Chumlea WC, Siervogel RM. Aging, body composition, and lifestyle: the Fels Longitudinal Study. Am J Clin Nutr 1999;70:405–411 [DOI] [PubMed] [Google Scholar]
- 6.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr 1992;55:950–954 [DOI] [PubMed] [Google Scholar]
- 7.Gambacciani M, Ciaponi M, Cappagli B, et al. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J Clin Endocrinol Metab 1997;82:414–417 [Erratum appears in J Clin Endocrinol Metab 1997;82:4074] [DOI] [PubMed] [Google Scholar]
- 8.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yüksel H, Odabasi AR, Demircan S, Köseoğlu K, Kizilkaya K, Onur E. Effects of postmenopausal hormone replacement therapy on body fat composition. Gynecol Endocrinol 2007;23:99–104 [DOI] [PubMed] [Google Scholar]
- 10.Hamilton JA, Kamp F. How are free fatty acids transported in membranes? Is it by proteins or by free diffusion through the lipids? Diabetes 1999;48:2255–2269 [DOI] [PubMed] [Google Scholar]
- 11.Homma H, Kurachi H, Nishio Y, et al. Estrogen suppresses transcription of lipoprotein lipase gene. Existence of a unique estrogen response element on the lipoprotein lipase promoter. J Biol Chem 2000;275:11404–11411 [DOI] [PubMed] [Google Scholar]
- 12.Sfeir Z, Ibrahimi A, Amri E, Grimaldi P, Abumrad N. Regulation of FAT/CD36 gene expression: further evidence in support of a role of the protein in fatty acid binding/transport. Prostaglandins Leukot Essent Fatty Acids 1997;57:17–21 [DOI] [PubMed] [Google Scholar]
- 13.Mashek DG, Li LO, Coleman RA. Long-chain acyl-CoA synthetases and fatty acid channeling. Future Lipidol 2007;2:465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yen C-LE, Stone SJ, Koliwad S, Harris C, Farese RV., Jr Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res 2008;49:2283–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou XG, Moser S, Sarr MG, Thompson GB, Que FG, Jensen MD. Visceral and subcutaneous adipose tissue diacylglycerol acyltransferase activity in humans. Obesity (Silver Spring) 2009;17:1129–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santosa S, Jensen MD. Effects of male hypogonadism on regional adipose tissue fatty acid storage and lipogenic proteins. PLoS ONE 2012;7:e31473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in adipose tissue: gender effects in nonobese humans. Am J Physiol Endocrinol Metab 2000;279:E455–E462 [DOI] [PubMed] [Google Scholar]
- 18.Causon RC, Carruthers ME, Rodnight R. Assay of plasma catecholamines by liquid chromatography with electrochemical detection. Anal Biochem 1981;116:223–226 [DOI] [PubMed] [Google Scholar]
- 19.Humphreys SM, Fisher RM, Frayn KN. Micro-method for measurement of sub-nanomole amounts of triacylglycerol. Ann Clin Biochem 1990;27:597–598 [DOI] [PubMed] [Google Scholar]
- 20.Jensen MD, Kanaley JA, Roust LR, et al. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc 1993;68:867–873 [DOI] [PubMed] [Google Scholar]
- 21.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 1995;61:274–278 [DOI] [PubMed] [Google Scholar]
- 22.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 1983;55:628–634 [DOI] [PubMed] [Google Scholar]
- 23.Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in human adipose tissue: technical and experimental design issues. Am J Physiol Endocrinol Metab 2000;279:E447–E454 [DOI] [PubMed] [Google Scholar]
- 24.Persson X-MT, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res 2010;51:2761–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes 2007;56:1369–1375 [DOI] [PubMed] [Google Scholar]
- 26.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509 [PubMed] [Google Scholar]
- 27.Mårin P, Rebuffé-Scrive M, Björntorp P. Uptake of triglyceride fatty acids in adipose tissue in vivo in man. Eur J Clin Invest 1990;20:158–165 [DOI] [PubMed] [Google Scholar]
- 28.Tchoukalova YD, Harteneck DA, Karwoski RA, Tarara J, Jensen MD. A quick, reliable, and automated method for fat cell sizing. J Lipid Res 2003;44:1795–1801 [DOI] [PubMed] [Google Scholar]
- 29.Nilsson-Ehle P, Schotz MC. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res 1976;17:536–541 [PubMed] [Google Scholar]
- 30.Hall AM, Smith AJ, Bernlohr DA. Characterization of the Acyl-CoA synthetase activity of purified murine fatty acid transport protein 1. J Biol Chem 2003;278:43008–43013 [DOI] [PubMed] [Google Scholar]
- 31.Allred CC, Krennmayr T, Koutsari C, Zhou L, Ali AH, Jensen MD. A novel ELISA for measuring CD36 protein in human adipose tissue. J Lipid Res 2011;52:408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanner JM. Fallacy of per-weight and per-surface area standards, and their relation to spurious correlation. J Appl Physiol 1949;2:1–15 [DOI] [PubMed] [Google Scholar]
- 33.Votruba SB, Jensen MD. Sex-specific differences in leg fat uptake are revealed with a high-fat meal. Am J Physiol Endocrinol Metab 2006;291:E1115–E1123 [DOI] [PubMed] [Google Scholar]
- 34.de Aloysio D, Gambacciani M, Meschia M, et al. The Icarus Study Group The effect of menopause on blood lipid and lipoprotein levels. Atherosclerosis 1999;147:147–153 [DOI] [PubMed] [Google Scholar]
- 35.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 2003;88:2404–2411 [DOI] [PubMed] [Google Scholar]
- 36.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med 1989;321:641–646 [DOI] [PubMed] [Google Scholar]
- 37.Stevenson JC, Crook D, Godsland IF. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis 1993;98:83–90 [DOI] [PubMed] [Google Scholar]
- 38.Iverius PH, Brunzell JD. Relationship between lipoprotein lipase activity and plasma sex steroid level in obese women. J Clin Invest 1988;82:1106–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price TM, O’Brien SN, Welter BH, George R, Anandjiwala J, Kilgore M. Estrogen regulation of adipose tissue lipoprotein lipase—possible mechanism of body fat distribution. Am J Obstet Gynecol 1998;178:101–107 [DOI] [PubMed] [Google Scholar]
- 40.dos Reis CM, de Melo NR, Meirelles ES, Vezozzo DP, Halpern A. Body composition, visceral fat distribution and fat oxidation in postmenopausal women using oral or transdermal oestrogen. Maturitas 2003;46:59–68 [DOI] [PubMed] [Google Scholar]
- 41.Lwin R, Darnell B, Oster R, et al. Effect of oral estrogen on substrate utilization in postmenopausal women. Fertil Steril 2008;90:1275–1278 [DOI] [PubMed] [Google Scholar]
- 42.O’Sullivan AJ, Crampton LJ, Freund J, Ho KKY. The route of estrogen replacement therapy confers divergent effects on substrate oxidation and body composition in postmenopausal women. J Clin Invest 1998;102:1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen MD, Martin ML, Cryer PE, Roust LR. Effects of estrogen on free fatty acid metabolism in humans. Am J Physiol 1994;266:E914–E920 [DOI] [PubMed] [Google Scholar]
- 44.D’Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 2005;280:35983–35991 [DOI] [PubMed] [Google Scholar]
- 45.Muoio DM, MacLean PS, Lang DB, et al. Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) alpha knock-out mice. Evidence for compensatory regulation by PPAR delta. J Biol Chem 2002;277:26089–26097 [DOI] [PubMed] [Google Scholar]
- 46.Weiner FR, Smith PJ, Wertheimer S, Rubin CS. Regulation of gene expression by insulin and tumor necrosis factor alpha in 3T3-L1 cells. Modulation of the transcription of genes encoding acyl-CoA synthetase and stearoyl-CoA desaturase-1. J Biol Chem 1991;266:23525–23528 [PubMed] [Google Scholar]
- 47.Kansara MS, Mehra AK, Von Hagen J, Kabotyansky E, Smith PJ. Physiological concentrations of insulin and T3 stimulate 3T3-L1 adipocyte acyl-CoA synthetase gene transcription. Am J Physiol 1996;270:E873–E881 [DOI] [PubMed] [Google Scholar]
- 48.Ranganathan G, Unal R, Pokrovskaya I, et al. The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: effects of obesity, insulin resistance, and TZD treatment. J Lipid Res 2006;47:2444–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meegalla RL, Billheimer JT, Cheng D. Concerted elevation of acyl-coenzyme A:diacylglycerol acyltransferase (DGAT) activity through independent stimulation of mRNA expression of DGAT1 and DGAT2 by carbohydrate and insulin. Biochem Biophys Res Commun 2002;298:317–323 [DOI] [PubMed] [Google Scholar]
- 50.Stahl A, Evans JG, Pattel S, Hirsch D, Lodish HF. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev Cell 2002;2:477–488 [DOI] [PubMed] [Google Scholar]