Physical inactivity and overeating leading to obesity and diabetes are both linked to increased risk of age-related chronic diseases. By contrast, caloric restriction and physical activity promote health. However, the cellular mechanisms that link the metabolic state to long-term health outcomes have remained unclear. Damage to mitochondrial DNA (mtDNA), which accumulates with aging in diseased human tissues and with diabetes complications, has been shown in animal models to recapitulate several features of aging. Importantly, mitochondrial morphology, function, and the integrity of mtDNA directly respond to the metabolic state. The oversupply of cells with excess lipids and glucose (i.e., hyperglycemia) fragments mitochondria, increases mitochondrial reactive oxygen species production, and promotes the accumulation of mtDNA damage. In turn, the limited supply of energy substrates promotes fusion and elongation of mitochondria and limits accumulation of mtDNA damage. Here we propose a model in which mitochondrial dynamics (fusion/fission) integrate systemic metabolic information and control the stability of the mitochondrial genome, thus helping to mediate the effects of physical activity, inactivity, and calorie intake on health outcomes.
It is well established both epidemiologically and clinically that physical inactivity and behaviors leading to weight gain are associated with elevated risk of most age-related diseases as well as mortality (1). However, the underlying mechanisms mediating these effects are not fully explained. Conversely, the health promoting and life-extending effects of caloric restriction and physical activity are well described (2,3). Nonetheless, the exact mechanisms underlying the health benefits of these interventions, including the reduced risk of most age-related metabolic diseases and diabetes complications, have not been fully elucidated. Here we present a common mechanism that may account for the combined long-term effects of physical activity/inactivity and diet on health outcomes and aging. Improved understanding of the acute cellular events initiated by fluctuations in the metabolic state may facilitate development of targeted therapies and could be leveraged to nurture behavior change, thereby providing new opportunities to promote metabolic health.
Mitochondria are cellular organelles that transform energetic substrates (e.g., lipids and glucose) and oxygen into energy. These organelles retain several remnant characteristics of their past lives as aerobic bacteria, including a double membrane, circular DNA molecules, and the ability to interact with each other (4). Constant mitochondrion-mitochondrion interactions take place through dynamic processes of membrane fusion and fission. These interactions alter mitochondrial morphology and simultaneously modulate the organelles’ function (5). Importantly, mitochondrial morphology and function undergo significant transitions in response to changes in the cellular metabolic state, which are defined here as the balance between the supply of energetic substrates and cellular energy demand. In turn, mitochondrial fusion and fission control the integrity of mtDNA whereby normal mitochondrial fusion promotes stability of the mitochondrial genome and excessive mitochondrial fission leads to preferential accumulation of mutated mtDNA (6,7).
Increased amounts of mutated mtDNA in postmitotic tissues are a feature of aging and of age-related neurodegenerative diseases (8). Because the mitochondrial genome encodes essential elements of the electron transport chain that fuels ATP synthesis, damage to mtDNA causes severe bioenergetic failure. Evidence from mouse models of mtDNA diseases caused by a mutated proofreading domain in the gene encoding the mitochondrial polymerase γ (PolG) has demonstrated that high levels of mutated mtDNA generate signs consistent with premature aging. This is observed across organ systems and reduces life span in mice by up to 50% (9,10). The clonal expansion of mutated mtDNA copies toward high heteroplasmy levels leads to respiratory chain deficiency and impacts mitochondrial function. This causes cell loss via apoptosis and reduction of whole-body exercise tolerance (8), both features of normal aging. Thus, along with other putative mechanisms such as cellular quality control processes (autophagy/mitophagy) (11,12), telomere dynamics (13), and antioxidant/anti-inflammatory mechanisms (14), chronic metabolic imbalances causing prolonged alterations in mitochondrial morphology could influence both mitochondrial function and the integrity of the mitochondrial genome. In turn, these alterations may modify disease susceptibility and long-term health outcomes.
METABOLIC OVERSUPPLY, CHRONIC DISEASE, AND MORTALITY
Weight gain and obesity are among the most potent biological risk factors for development of age-related chronic diseases such as diabetes, cancer, and cardiovascular and neurodegenerative diseases. Relative to normal BMI, obesity and its associated metabolic state incur an average 5- to 20-year reduction in life span (1,15) (Fig. 1). The health consequences of physical inactivity are remarkably similar (and perhaps more detrimental to health) than those associated with overeating. Sedentary individuals are at greater risk of cardiovascular disease and mortality than individuals with physically active lifestyles (16), making obesity and inactivity two strong and often independent predictors of morbidity and mortality (1). But what are the underlying physiological mechanisms causing these effects on human health?
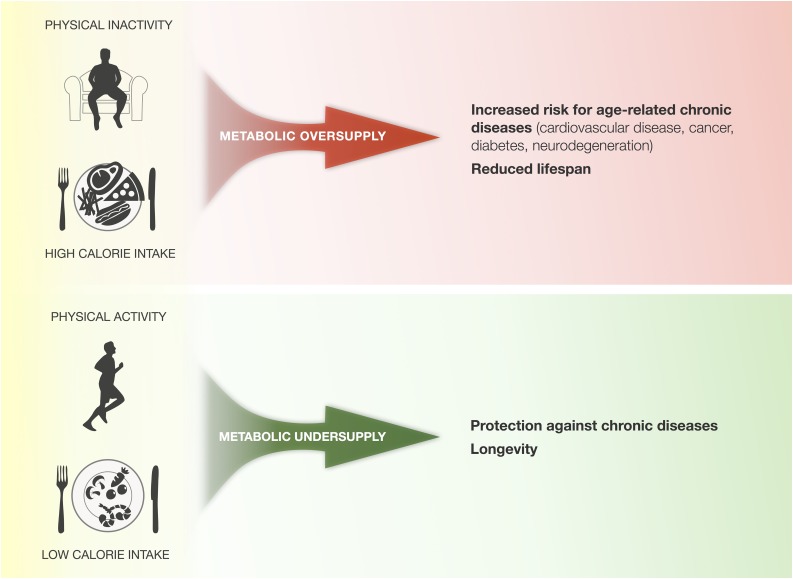
FIG. 1.
Effect of physical activity/inactivity and calorie intake on health outcomes. Chronic physical inactivity and overeating create a state of metabolic oversupply that predispose to age-related chronic diseases and reduce life span. In contrast, physical activity/exercise and limited calorie intake create a state of metabolic undersupply that improve health and resilience of most organ systems, and can hereby promote longevity.
A physiological state common to both weight gain and physical inactivity is the overabundance, relative to tissue demand, of energetic substrates such as lipids and glucose. The corollary physiological state is a chronic overabundance of energetic substrates in the systemic circulation (17). In these settings, tissues are exposed to excessive supply of energetic substrates relative to their metabolic needs. This “metabolic oversupply” is a systemic and potentially cytotoxic state that damages mitochondria (18). A recent meta-analysis showed that fasting plasma glucose levels above 6 mmol/L (or >93 mg/dL) are associated in a dose-response manner with increased risk of developing age-related diseases including cardiovascular disease, cancer, and all-cause mortality (19). This risk is magnified in diabetes, where glycemia is elevated in both the fasting and postprandial states. In turn, diabetes is associated with greater health risk than the general population for several age-related diseases, and it reduces life span as a result of this association (19,20). Postprandial hyperglycemia is a major—if not the strongest—determinant of diabetes complications (21). Likewise, excessive parenteral nutrition in the critically ill is associated with delayed recovery and more complications (22), whereas adequate glycemic control with intensive insulin therapy prevents mitochondrial damage (23) and reduces morbidity (24).
A primary consequence of metabolic oversupply is oxidative damage to molecules including mtDNA. As a result, ongoing accumulation of mutated mtDNA in tissues (brain, skeletal muscle, intestine), which is a hallmark of aging, occurs at increased levels in patients with diabetic hyperglycemia (25–27). Experimentally induced diabetes (28) or the natural predisposition to hyperglycemia and hyperlipidemia in animal models (29) results in increased accumulation of mtDNA mutations, demonstrating that chronic metabolic oversupply is damaging to mtDNA. In turn, mtDNA defects may accelerate telomere shortening. Telomeres are repetitive DNA sequences and associated proteins located at the end of chromosomes that generally get shorter with time (30) and are another hallmark of cellular aging and senescence (31). Interestingly, telomeres erode prematurely in the tissues of obese individuals (32) and those with diabetes (33), and telomere shortening is inversely correlated with the number of diabetes complications (34). This indicates that metabolic oversupply may promote cellular senescence, possibly through a mitochondria-telomere signaling axis (35). Overall, there is evidence suggesting a link between metabolic oversupply and mitochondrial genome insult. In this model, exposure to chronic hyperglycemia accelerates the accumulation of mutated mtDNA and cellular senescence, possibly as a result of excessive mitochondrial fragmentation (see discussion below).
In contrast, physical activity increases substrate utilization by active muscles, reduces circulating levels of lipids (36), and can even prevent the onset and modify the course of diabetes complications, ultimately reducing mortality risk (37). Dietary restriction alone can also reverse hyperglycemia and restore hepatic function (38). Nonetheless, physical activity/inactivity and the resulting fitness levels appear to be more strongly associated with health outcomes than calorie intake and/or BMI (39). This notion may be attributable to the fact that energy expenditure by working muscles can increase up to 100-fold during maximal strenuous activity (40), whereas calorie intake alone generally influences circulating substrate levels by no more than two- to fivefold. As a result, the deleterious biological impact that results from overeating or high-calorie diets can be prevented through increased physical activity and weight loss, indicating that the systemic balance (supply vs. demand) of energy substrates is a key mediating factor of the health-damaging effects of metabolic oversupply.
METABOLIC UNDERSUPPLY AND HEALTH PROMOTION
A long-standing conundrum in the biological and clinical sciences involves the mechanisms by which caloric restriction extends life span and health span (disease-free years). Reducing caloric intake by 20–40% in a variety of living organisms in the laboratory setting consistently increases median and maximal life span (41). More importantly for this discussion, in both animals and humans, caloric restriction reduces risk of age-related diseases including diabetes, cancer, cardiovascular, as well as neurodegenerative diseases and immune deficiencies (42,43). In animal models, lifelong calorie restriction compared with ad libidum feeding reduces oxidative damage to mtDNA (44), delays disease onset, and reduces mortality (45). Several downstream mechanisms have been proposed to explain the effects of caloric restriction on health and life span (41) but it is clear that caloric restriction acts through a primary reduction in the systemic availability of energetic substrates to somatic tissues (46). This occurs at least in part by increasing insulin sensitivity in peripheral tissues, an action that potentiates glucose removal from the systemic circulation (47). The resultant physiological state is one in which systemic supply of substrates is lower than the metabolic demand, a state termed “metabolic undersupply.”
Physical activity has similar health-promoting effects as caloric restriction (3,48). Weight loss in obese individuals—a process involving a shift toward metabolic undersupply—reduces the risk of type 2 diabetes; coronary heart disease; dementia; cancer of the colon, prostate, breast, and uterus; and it also reduces mortality risk (49,50). Physically active or exercise-trained individuals also have longer telomeres than their sedentary counterparts (51,52) suggesting that physical activity may mitigate telomere shortening in response to stress (53). Increased energy expenditure associated with physical activity promotes metabolic undersupply both locally and systemically (36,54), thereby lowering the metabolic status in the direction of undersupply. Thus, metabolic oversupply—such as occurs in diabetes—promotes ill health, whereas metabolic undersupply promotes good health. A candidate cellular mechanism that could simultaneously explain the health effects of metabolic oversupply and undersupply must 1) be inherently responsive to fluctuations in the metabolic state (both oversupply and undersupply) and 2) be capable of protecting and predisposing to chronic aging-related diseases. Mitochondrial dynamics, via their effects on mtDNA integrity and in concert with other possible mechanisms including autophagy (12), fulfill both of these roles. Changes in mitochondrial dynamics in response to the metabolic state constitute an integrative concept that may help both clinicians and patients to appreciate the immediate benefits of preserving an adequate metabolic balance.
METABOLIC STATUS, MITOCHONDRIAL DYNAMICS, AND mtDNA
The metabolic state has a direct impact on mitochondrial dynamics of fusion/fission and impacts the integrity of mtDNA. In vitro, metabolic oversupply causes fragmentation (fission) of the mitochondrial network (55–60) (Fig. 2). In tissues and cells from diabetic humans and in animals with hyperglycemia, mitochondria morphology, gene expression, and fission/fusion protein abundance profiles are consistent with enhanced mitochondrial fission (61,62). Expression and protein abundance of the mitochondrial fission proteins 1 (Fis1) and dynamin-related protein 1 (Drp1) increase with hyperglycemia (55,63), whereas hyperglycemia reduces both the expression and abundance of the fusion proteins mitofusin 2 (Mfn2) and optic atrophy 1 (OPA1) in cells and tissues (61,63,64). As a result, metabolic oversupply induces molecular damage resulting from mitochondrial fragmentation, including oxidative stress and mtDNA damage (26,65,66). On the other hand, metabolic undersupply causes mitochondrial fusion and elongation (67–69). Exercise training and interventions that directly increase energy expenditure upregulate Mfn1 and Mfn2 (61,70–72) and can prevent accumulation of mtDNA mutations in genetically susceptible PolG mice, thus counteracting their accelerated aging phenotype (73). These findings suggest that tilting the energy balance toward metabolic undersupply (during and/or after exercise) prevents accumulation of mtDNA damage and may preserve optimal mitochondrial function.
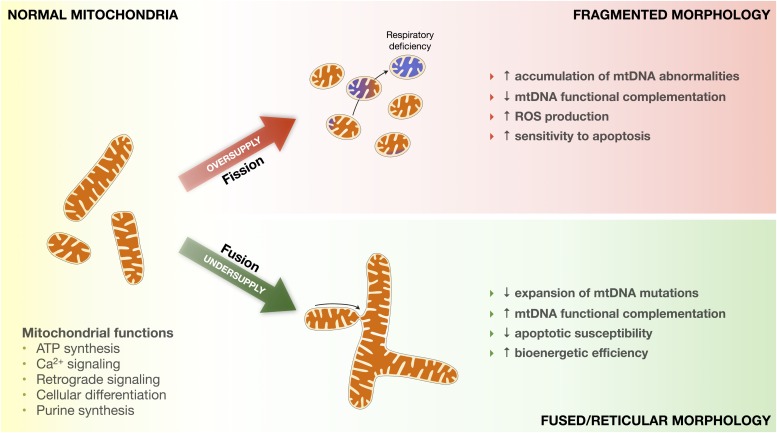
FIG. 2.
Impact of metabolic oversupply and undersupply on mitochondrial morphology and function. Mitochondria regulate several key aspects of cellular function. Metabolic oversupply induces mitochondrial fission and fragmentation, transitioning from normal to fragmented morphology. Chronically fragmented mitochondria more rapidly accumulate mutated mtDNA, become respiratory chain deficient (blue mitochondria), produce more reactive oxygen species (ROS), and are sensitized to apoptotic signaling. In contrast, metabolic undersupply induces mitochondrial fusion and promotes the formation of a fused/reticular mitochondrial network. Fused mitochondria accumulate fewer mutated mtDNA, can functionally complement mtDNA defects, emit less ROS and are less susceptible to proapoptotic signaling, and possibly exhibit greater bioenergetic efficiency.
Competent fusion of mitochondria is vital to prevent excessive accumulation of abnormal mtDNA and subsequent bioenergetic failure (6,74). Ablation of fusion proteins in both mouse models and in humans with pathogenic mutations in genes encoding Mfn2 and OPA1 leads to accumulation of mutated mtDNA, severe respiratory chain deficiency, and multisystemic disease (6,75). Although the underlying mechanisms remain to be identified, smaller population size of mtDNA (in smaller organelles) (76), increased reactive oxygen species production (57), absent functional complementation of mtDNA transcriptional products (74), and impaired mitochondrial quality control caused by unbalanced mitochondrial fragmentation (12) may help explain the deleterious effects of excessive fission. This suggests that chronic exposure to high levels of energetic substrates—oversupply—compromises bioenergetic function via chronic mitochondrial fragmentation. In turn, this could accelerate accumulation of mtDNA damage, thus promoting age-related functional decline across a variety of tissues. Conversely, phases of scarcity of energetic substrate—undersupply—could preserve mitochondrial bioenergetics in part by favoring mitochondrial fusion that preserves the integrity of the mitochondrial genome (Fig. 3).
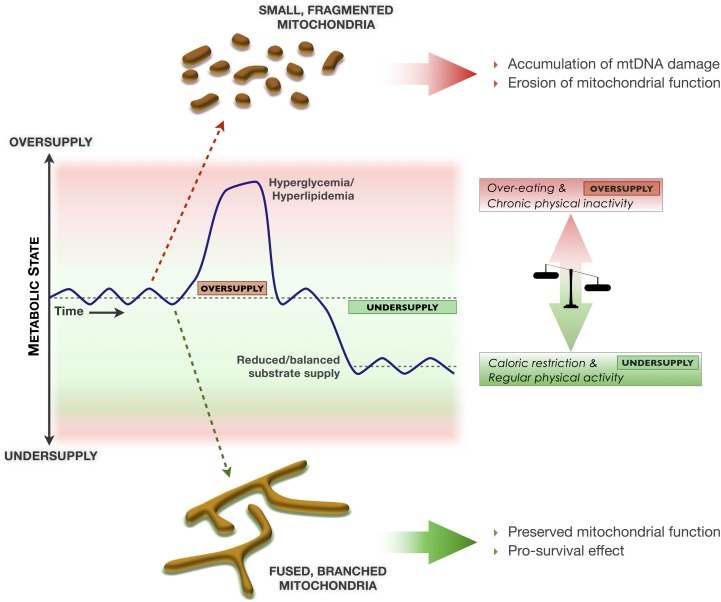
FIG. 3.
Model linking fluctuations in the metabolic state, mitochondrial morphology, integrity of mtDNA and long-term health outcomes. Normal modest fluctuations in the metabolic state between periods of relative oversupply and undersupply contribute to mitochondrial health. Chronic episodes of metabolic oversupply may fragment the mitochondrial network and as a result compromise mtDNA and mitochondrial function, thus predisposing to age-related diseases and premature functional decline. On the other hand, conditions that favor metabolic undersupply induce mitochondrial fusion and as a result preserve mtDNA integrity and optimal function, which may promote optimal physiological adaptation and healthy aging. Red vs. green shadings represent damaging and protective metabolic states, respectively.
TESTING THE HYPOTHESIS
In keeping with the concept of metabolic balance, the importance of normal periodic fluctuations of the metabolic state from settings of moderate oversupply (meals, physical rest, satiety) to those characterized by undersupply (fasted periods between meals, physical activity and exercise, hunger) should be emphasized. For example, the benefits of lifelong calorie restriction may principally arise from preventing prolonged episodes of metabolic oversupply, rather than placing the organism in a constant state of undersupply. Although mitochondrial fusion appears to establish a favorable state of cellular coherence that limits expansion of mtDNA abnormalities, periodic fission events are essential to the mitochondrial quality control process (12,77). This provides a potential molecular basis for the health benefits of maintaining healthy daily variations in energy supply to most tissues.
It must also be acknowledged that whether mtDNA mutations are caused by or are only associated with normal human aging remains a contentious issue (78). An explicit assumption of the model presented in this report is that accumulation of mutant mtDNA causes cellular dysfunctions that precipitate age-related functional decline. As noted above, this notion is supported by the fact that 1) mtDNA mutations normally accumulate within aging human tissues, 2) inherited and acquired mtDNA defects result in multisystemic pathological defects, and 3) accumulation of mtDNA mutations in the PolG mouse model causes progeroid phenotypes. Nevertheless, other putative causes of aging that may also be affected by the metabolic state (and by mitochondrial dysfunction) include telomere shortening, accumulation of intracellular macromolecular damage, and possibly epigenetic drift (79,80).
Another relevant consideration to our model relates to the existence of insulin resistance. In diabetes, the downregulation of GLUT4-mediated glucose import in insulin-sensitive tissues could conceivably limit energetic substrate (glucose) flux into cells, thereby acting as a protective mechanism against mitochondrial overload (81). Nonetheless, hyperglycemia or hyperlipidemia still expose cells to damaging intracellular levels of substrates within tissues harboring noninsulin-dependent glucose transport mechanisms (GLUT1 and GLUT3). Tissues expressing these constitutive glucose transporters include endothelial cells, the blood-brain barrier, immune cells, kidneys, and colon (82). As a consequence, these tissues are vulnerable to glucotoxicity and thus are frequently implicated in diabetes complications (microvascular damage, strokes, impaired wound healing, nephropathies). Therefore, although insulin resistance may confer relative protection against metabolic oversupply in insulin-dependent tissues, the majority of other tissues are not protected from the deleterious effects of metabolic oversupply on mitochondria. Diabetes—even after insulin resistance develops—is thus a bona fide source chronic metabolic oversupply.
Although there are several studies showing the in vitro effects of metabolic over- and undersupply on cells and tissues, we need to pursue comprehensive studies of tissues in both animal models and humans that have undergone changes in either diet or exercise regimen. In these studies, it will be crucial to determine if the mitochondrial morphology changes occur across different tissues, how sensitive these changes are to long-term changes in behavior, and most importantly, if the changes can be prospectively and causally linked to impaired mitochondrial function and mtDNA mutations. Also, the degree to which mitochondrial dysfunction contributes to cellular senescence under different metabolic conditions, as well as the underlying pathways associated with this dysfunction, need to be further established. Assuming these studies prove the hypothesis, understanding the cell signaling or epigenetic pathways causing these changes may lead to new approaches to the management of patients with metabolic oversupply and/or physical inactivity.
SUMMARY AND PERSPECTIVES
Chronological aging and its associated physiological vulnerability to disease are undoubtedly influenced by the metabolic state with diabetes being an exemplar of this relationship. Metabolic oversupply and undersupply are determined by the interactive forces of energy expenditure through physical activity and caloric intake through diet and possibly by hormonal mediators of stress (83,84), thereby offering several potential windows of opportunity to intervene clinically and behaviorally. The innate sensitivity of mitochondria to energetic substrate levels and their recently discovered ability to dynamically undergo function-defining morphology transitions that influence the integrity of the mitochondrial genome constitutes a novel potential mechanism to explain long-term modulations of health and disease.
The typical 21st century sedentary lifestyle favors chronic metabolic oversupply (18), a physiological state that promotes mitochondrial fission and fragmentation. This can alter mtDNA and compromise cellular function. In contrast, caloric restriction and exercise induce a state of metabolic undersupply that triggers mitochondrial fusion. This establishes a life-sustaining intracellular state that promotes cell survival and maintains the integrity of mtDNA. A healthy balance of both sides of mitochondrial dynamics (fusion and fission) promotes healthy cellular adaptation (77). Mitochondrial dynamics are therefore emerging as a potential new link mediating the impact of calorie intake and physical activity/inactivity on health. More research is needed to test this hypothesis, but if proven it would open new therapeutic avenues to prevent complications of hyperglycemia and provide a model (Fig. 3) for patient education aimed at lifestyle changes. The dynamic changes in mitochondrial morphology in response to metabolic oversupply and undersupply have the potential to link behavior, physiology, mitochondrial biology, and genetics in health and disease.
ACKNOWLEDGMENTS
This work is funded by the Newcastle University Centre for Brain Ageing and Vitality (supported by the Biotechnology and Biological Sciences Research Council, the Engineering and Physical Sciences Research Council, the Economic and Social Research Council, and the Medical Research Council), the Wellcome Trust Centre for Mitochondrial Research, and the National Institute for Health Research Newcastle Biomedical Research Centre based at Newcastle upon Tyne Hospitals National Health Service Foundation Trust and Newcastle University. M.P. is supported by a Canadian Institutes of Health Research Postdoctoral Fellowship from the Institute of Neurosciences, Mental Health and Addiction as part of the Canadian Epigenetics, Environment and Health Research Consortium.
No potential conflicts of interest relevant to this article were reported.
M.P. originally conceived the model and figures, and both D.M.T. and M.P. were closely involved in their refinement and in the preparation of the manuscript.
The authors are grateful to Bob Lightowlers (Newcastle University), Michael Trenell (Newcastle University), and Richard Godin (Université de Montréal) for providing critical feedback on earlier versions of the manuscript, and to Aldine Calveyrac (Université de Montréal) and Orian Shirihai (Boston University) for insightful discussions.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-1203/-/DC1.
REFERENCES
- 1.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med 2004;351:2694–2703 [DOI] [PubMed] [Google Scholar]
- 2.Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA 2007;297:986–994 [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services Physical activity and health: a report of the Surgeon General. Atlanta, Georgia, U.S. Department of Health and Human Services, Public Health Service, CDC, National Center for Chronic Disease Prevention and Health Promotion, 1996, p. 1–300 [Google Scholar]
- 4.Braschi E, McBride HM. Mitochondria and the culture of the Borg: understanding the integration of mitochondrial function within the reticulum, the cell, and the organism. Bioessays 2010;32:958–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 2010;11:872–884 [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Vermulst M, Wang YE, et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 2010;141:280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bess AS, Crocker TL, Ryde IT, Meyer JN. Mitochondrial dynamics and autophagy aid in removal of persistent mitochondrial DNA damage in Caenorhabditis elegans. Nucleic Acids Res 2012;40:7916–7931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 2005;39:359–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004;429:417–423 [DOI] [PubMed] [Google Scholar]
- 10.Kujoth GC, Hiona A, Pugh TD, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005;309:481–484 [DOI] [PubMed] [Google Scholar]
- 11.He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 2012;481:511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 2008;27:433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shammas MA. Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care 2011;14:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 2008;454:463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 2005;352:1138–1145 [DOI] [PubMed] [Google Scholar]
- 16.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 2007;56:2655–2667 [DOI] [PubMed] [Google Scholar]
- 17.Björntorp P. Metabolic implications of body fat distribution. Diabetes Care 1991;14:1132–1143 [DOI] [PubMed] [Google Scholar]
- 18.Bremer AA, Mietus-Snyder M, Lustig RH. Toward a unifying hypothesis of metabolic syndrome. Pediatrics 2012;129:557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seshasai SR, Kaptoge S, Thompson A, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonker JT, De Laet C, Franco OH, Peeters A, Mackenbach J, Nusselder WJ. Physical activity and life expectancy with and without diabetes: life table analysis of the Framingham Heart Study. Diabetes Care 2006;29:38–43 [DOI] [PubMed] [Google Scholar]
- 21.Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008;57:1349–1354 [DOI] [PubMed] [Google Scholar]
- 22.Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med 2011;365:506–517 [DOI] [PubMed] [Google Scholar]
- 23.Vanhorebeek I, De Vos R, Mesotten D, Wouters PJ, De Wolf-Peeters C, Van den Berghe G. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet 2005;365:53–59 [DOI] [PubMed] [Google Scholar]
- 24.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006;354:449–461 [DOI] [PubMed] [Google Scholar]
- 25.Liang P, Hughes V, Fukagawa NK. Increased prevalence of mitochondrial DNA deletions in skeletal muscle of older individuals with impaired glucose tolerance: possible marker of glycemic stress. Diabetes 1997;46:920–923 [DOI] [PubMed] [Google Scholar]
- 26.Suzuki S, Hinokio Y, Komatu K, et al. Oxidative damage to mitochondrial DNA and its relationship to diabetic complications. Diabetes Res Clin Pract 1999;45:161–168 [DOI] [PubMed] [Google Scholar]
- 27.Nomiyama T, Tanaka Y, Hattori N, et al. Accumulation of somatic mutation in mitochondrial DNA extracted from peripheral blood cells in diabetic patients. Diabetologia 2002;45:1577–1583 [DOI] [PubMed] [Google Scholar]
- 28.Kakimoto M, Inoguchi T, Sonta T, et al. Accumulation of 8-hydroxy-2′-deoxyguanosine and mitochondrial DNA deletion in kidney of diabetic rats. Diabetes 2002;51:1588–1595 [DOI] [PubMed] [Google Scholar]
- 29.Fukagawa NK, Li M, Liang P, Russell JC, Sobel BE, Absher PM. Aging and high concentrations of glucose potentiate injury to mitochondrial DNA. Free Radic Biol Med 1999;27:1437–1443 [DOI] [PubMed] [Google Scholar]
- 30.Chan SR, Blackburn EH. Telomeres and telomerase. Philos Trans R Soc Lond B Biol Sci 2004;359:109–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oexle K, Zwirner A. Advanced telomere shortening in respiratory chain disorders. Hum Mol Genet 1997;6:905–908 [DOI] [PubMed] [Google Scholar]
- 32.Niemann B, Chen Y, Teschner M, Li L, Silber RE, Rohrbach S. Obesity induces signs of premature cardiac aging in younger patients: the role of mitochondria. J Am Coll Cardiol 2011;57:577–585 [DOI] [PubMed] [Google Scholar]
- 33.Monickaraj F, Gokulakrishnan K, Prabu P, et al. Convergence of adipocyte hypertrophy, telomere shortening and hypoadiponectinemia in obese subjects and in patients with type 2 diabetes. Clin Biochem 2012;45:1432–1438 [DOI] [PubMed] [Google Scholar]
- 34.Testa R, Olivieri F, Sirolla C, et al. Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabet Med 2011;28:1388–1394 [DOI] [PubMed] [Google Scholar]
- 35.Sahin E, Colla S, Liesa M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011;470:359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med 2002;347:1483–1492 [DOI] [PubMed] [Google Scholar]
- 37.Colberg SR, Sigal RJ, Fernhall B, et al. American College of Sports Medicine. American Diabetes Association Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care 2010;33:2692–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011;54:2506–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 2000;132:605–611 [DOI] [PubMed] [Google Scholar]
- 40.Hochachka PW, Matheson GO. Regulating ATP turnover rates over broad dynamic work ranges in skeletal muscles. J Appl Physiol 1992;73:1697–1703 [DOI] [PubMed] [Google Scholar]
- 41.Guarente LP, Patridge L, Wallace DC. Molecular Biology of Aging. New York, Cold Springs Harbor, 2008 [Google Scholar]
- 42.Everitt AV, Le Couteur DG. Life extension by calorie restriction in humans. Ann N Y Acad Sci 2007;1114:428–433 [DOI] [PubMed] [Google Scholar]
- 43.Mattison JA, Roth GS, Beasley TM, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 2012;489:318–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamilton ML, Van Remmen H, Drake JA, et al. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci USA 2001;98:10469–10474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009;325:201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab 2010;21:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halberg N, Henriksen M, Söderhamn N, et al. Effect of intermittent fasting and refeeding on insulin action in healthy men. J Appl Physiol 2005;99:2128–2136 [DOI] [PubMed] [Google Scholar]
- 48.Sattelmair JR, Pertman JH, Forman DE. Effects of physical activity on cardiovascular and noncardiovascular outcomes in older adults. Clin Geriatr Med 2009;25:677–702, viii-ix [DOI] [PubMed]
- 49.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab 2004;89:2583–2589 [DOI] [PubMed] [Google Scholar]
- 50.Bray GA. The missing link - lose weight, live longer. N Engl J Med 2007;357:818–820 [DOI] [PubMed] [Google Scholar]
- 51.Werner C, Fürster T, Widmann T, et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation 2009;120:2438–2447 [DOI] [PubMed] [Google Scholar]
- 52.LaRocca TJ, Seals DR, Pierce GL. Leukocyte telomere length is preserved with aging in endurance exercise-trained adults and related to maximal aerobic capacity. Mech Ageing Dev 2010;131:165–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puterman E, Lin J, Blackburn E, O’Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS ONE 2010;5:e10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helge JW, Stallknecht B, Richter EA, Galbo H, Kiens B. Muscle metabolism during graded quadriceps exercise in man. J Physiol 2007;581:1247–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shenouda SM, Widlansky ME, Chen K, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 2011;124:444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molina AJ, Wikstrom JD, Stiles L, et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes 2009;58:2303–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res 2008;79:341–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leinninger GM, Backus C, Sastry AM, Yi YB, Wang CW, Feldman EL. Mitochondria in DRG neurons undergo hyperglycemic mediated injury through Bim, Bax and the fission protein Drp1. Neurobiol Dis 2006;23:11–22 [DOI] [PubMed] [Google Scholar]
- 59.Paltauf-Doburzynska J, Malli R, Graier WF. Hyperglycemic conditions affect shape and Ca2+ homeostasis of mitochondria in endothelial cells. J Cardiovasc Pharmacol 2004;44:423–436 [DOI] [PubMed] [Google Scholar]
- 60.Men X, Wang H, Li M, et al. Dynamin-related protein 1 mediates high glucose induced pancreatic beta cell apoptosis. Int J Biochem Cell Biol 2009;41:879–890 [DOI] [PubMed] [Google Scholar]
- 61.Bach D, Naon D, Pich S, et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes 2005;54:2685–2693 [DOI] [PubMed] [Google Scholar]
- 62.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002;51:2944–2950 [DOI] [PubMed] [Google Scholar]
- 63.Zhong Q, Kowluru RA. Diabetic retinopathy and damage to mitochondrial structure and transport machinery. Invest Ophthalmol Vis Sci 2011;52:8739–8746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bach D, Pich S, Soriano FX, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem 2003;278:17190–17197 [DOI] [PubMed] [Google Scholar]
- 65.Picard M, Jung B, Liang F, et al. Mitochondrial dysfunction and lipid accumulation in the human diaphragm during mechanical ventilation. Am J Respir Crit Care Med 2012;186:1140–1149 [DOI] [PubMed] [Google Scholar]
- 66.Medikayala S, Piteo B, Zhao X, Edwards JG. Chronically elevated glucose compromises myocardial mitochondrial DNA integrity by alteration of mitochondrial topoisomerase function. Am J Physiol Cell Physiol 2011;300:C338–C348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 2011;13:589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci USA 2011;108:10190–10195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tolstonog GV, Belichenko-Weitzmann IV, Lu JP, et al. Spontaneously immortalized mouse embryo fibroblasts: growth behavior of wild-type and vimentin-deficient cells in relation to mitochondrial structure and activity. DNA Cell Biol 2005;24:680–709 [DOI] [PubMed] [Google Scholar]
- 70.Soriano FX, Liesa M, Bach D, Chan DC, Palacín M, Zorzano A. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes 2006;55:1783–1791 [DOI] [PubMed] [Google Scholar]
- 71.Cartoni R, Léger B, Hock MB, et al. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol 2005;567:349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ding H, Jiang N, Liu H, et al. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim Biophys Acta 2010;1800:250–256 [DOI] [PubMed] [Google Scholar]
- 73.Safdar A, Bourgeois JM, Ogborn DI, et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci USA 2011;108:4135–4140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ono T, Isobe K, Nakada K, Hayashi JI. Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet 2001;28:272–275 [DOI] [PubMed] [Google Scholar]
- 75.Yu-Wai-Man P, Chinnery PF. Dysfunctional mitochondrial maintenance: what breaks the circle of life? Brain 2012;135:9–11 [DOI] [PubMed] [Google Scholar]
- 76.Payne BA, Wilson IJ, Hateley CA, et al. Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nat Genet 2011;43:806–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science 2012;337:1062–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vermulst M, Wanagat J, Loeb LA. On mitochondria, mutations, and methodology. Cell Metab 2009;10:437. [DOI] [PubMed] [Google Scholar]
- 79.Gentilini D, Mari D, Castaldi D, Remondini D, Ogliari G, Ostan R, et al. Role of epigenetics in human aging and longevity: genome-wide DNA methylation profile in centenarians and centenarians' offspring. Age (Dordr). 25 August 2012 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 80.Barrès R, Osler ME, Yan J, et al. Non-CpG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab 2009;10:189–198 [DOI] [PubMed] [Google Scholar]
- 81.Hoehn KL, Salmon AB, Hohnen-Behrens C, et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA 2009;106:17787–17792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scheepers A, Joost HG, Schürmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr 2004;28:364–371 [DOI] [PubMed] [Google Scholar]
- 83.Kuo LE, Kitlinska JB, Tilan JU, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med 2007;13:803–811 [DOI] [PubMed] [Google Scholar]
- 84.Picard M. Pathways to aging: the mitochondrion at the intersection of biological and psychosocial sciences. J Aging Res 2011;2011:814096. [DOI] [PMC free article] [PubMed] [Google Scholar]