Noncoding genetic variation in the locus encoding for the Wnt signaling effector TCF7L2 remains the strongest genetic determinant of type 2 diabetes (T2D) risk in humans. This association raises the hypothesis that disease variants alter the quantitative, spatial, and/or temporal expression patterns of this gene. Understanding the mechanisms by which TCF7L2 and Wnt signaling regulate glucose metabolism may reveal novel insights into the pathogenesis of T2D, as well as highlight cellular and genetic pathways amenable to becoming novel therapeutic targets. Therefore, a large body of work has emerged over the past 4 years describing previously unknown glucose metabolism roles of TCF7L2.
Curiously, most of this work has focused on TCF7L2 actions in pancreatic β-cells, despite evidence that canonical Wnt signaling is not active in adult β-cells (1). The extensive evaluation of TCF7L2 actions or Wnt signaling effects in β-cells have resulted in contradictory findings at the molecular (2,3), cellular (1,4), and whole-animal physiology levels (5–7). Particularly difficult to reconcile is the incongruence of data showing that the presumed diabetogenic effects of TCF7L2 in β-cells, such as a blunted glucose-stimulated insulin secretion response, arises from reducing TCF7L2 expression (3,8). In contrast, evidence from human genetics and genomics studies suggests the risk alleles associated with T2D lead to increased expression of TCF7L2 (2,9–11), a notion recently confirmed by mouse models harboring germline null alleles or overexpressing TCF7L2 (6,7).
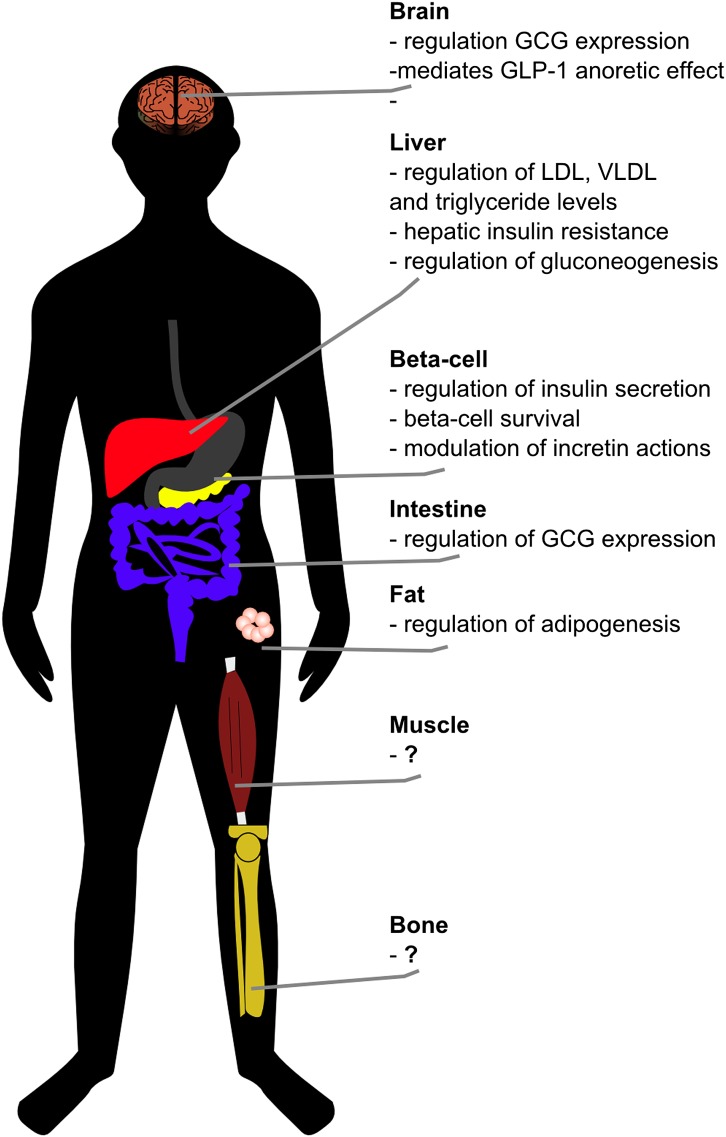
Not surprisingly, the lack of agreement among these reports has resulted in examination of the appropriateness of techniques, animal models, or developmental stages used in those studies. Some of these considerations are interesting and remain unexplored, such as the possibility that disease alleles may promote an imbalance of alternative splice forms of TCF7L2, thereby resulting in protein isoforms with opposing physiological effects (12). Critically lacking in the discussion, however, is the appreciation that TCF7L2 is expressed in a broad spatial domain pattern, including tissues with important roles in glucose metabolism such as brain, liver, skeletal muscle, fat, and bones (Fig. 1). This raises the possibility that, in vivo, TCF7L2 may not regulate glucose metabolism primarily through actions in β-cells, but rather in tissues outside the pancreas that remain poorly characterized. Importantly, the physiological roles of TCF7L2 in these tissues may underlie the mechanisms linking genetic variation and T2D risk.
FIG. 1.
Tissue-specific metabolic actions of TCF7L2. While significant attention has been directed toward TCF7L2’s effects in pancreatic β-cells, this transcription factor is highly expressed in multiple organs and tissues involved in glucose metabolism. The physiological roles of TCF7L2 in these tissues remain incompletely defined and may uncover additional mechanisms by which genetic variants in the TCF7L2 locus are associated with increased risk to T2D. In this issue of Diabetes, Shao et al. (13) describe how TCF7L2 expression in the brain intersects with aspects of the central regulation of glucose metabolism. GCG, glucagon gene.
In this issue of Diabetes, Shao et al. (13) illustrate one such example. They show a TCF7L2-mediated pathway regulating glucose metabolism in the brain (13) that uncovers cross-talk between Wnt and glucagon-like peptide 1 (GLP-1)/cAMP signaling that controls aspects of glucose metabolism in mice (Fig. 1). GLP-1, a transcription product of the glucagon gene (Gcg) in intestinal L cells and certain brainstem neurons, exerts powerful actions that promote glucose disposal in the body, including stimulation of insulin secretion in β-cells and a central anoretic effect in the brain. However, the mechanisms by which brain GLP-1 controls glucose metabolism remain incompletely defined, as well as the demonstration of a possible intersection of incretin effects with Wnt signaling in the brain.
Extending similar observations made by this laboratory in the gut (14), Shao et al. now demonstrate that TCF7L2 regulates expression of Gcg in the brain, and they also show colocalization of TCF7L2 and Gcg transcripts in brainstem neurons. To determine the functional consequences of alterations in TCF7L2 levels on GLP-1 biology, the authors engineered transgenic mice carrying a dominant negative form of TCF7L2, effectively creating a functional knockdown of TCF7L2 exclusively in Gcg-expressing cells in the brain and gut. This led to a significant decrease in Gcg transcription in intestinal enteroendocrine cells and brainstem neurons, but not pancreatic α-cells. Transgenic mice expressing the dominant negative TCF7L2 isoform showed impaired glucose tolerance and insulin sensitivity when fed a high-fat diet. These results indicate that TCF7L2 may be an important mediator of the GLP-1 signaling pathway in the brain, and indicate a central action of TCF7L2 in control of glucose homeostasis. While brain GLP-1 production is restricted to a neuronal subpopulation in the brainstem, GLP-1 receptors are expressed in a broader pattern, including hypothalamic nuclei, suggesting they are a site of action for GLP-1–mediated control of glucose metabolism in the brain. Mechanistically, injections of forskolin, which result in increased cAMP levels, led to increased Gcg transcription both in gut and brainstem, as well as phosphorylation of cAMP-responsive element-binding and β-catenin in hypothalamic neurons. These data suggest a model whereby brain TCF7L2 activates GLP-1 expression in the brainstem, and GLP-1 acts in hypothalamic nuclei promoting Wnt signaling activation via cAMP/cAMP-dependent protein kinase. The ensuing repression of AMP-activated protein kinase mediates the anorectic effect of GLP-1.
Yet unanswered is how this novel cross-talk between Wnt and GLP-1 signaling controls peripheral glucose disposal and insulin sensitivity. Moreover, it remains unclear whether this mechanism is altered in humans harboring alleles associated with increased T2D risk. Also, it is interesting to note that TCF7L2 has a broad expression pattern in the brain, particularly high in the hypothalamus, where TCF7L2 regulates processes directing the differentiation and patterning of neurons. TCF7L2 is dynamically expressed in the mediobasal hypothalamus during development, a region of the brain that plays a critical role in regulating glucose homeostasis. It will be interesting to specifically target the hypothalamic actions of TCF7L2 and their possible interface with glucose metabolism.
Showing how TCF7L2 controls a pathway that regulates glucose homeostasis in the brain, Shao et al. illustrate the likely roles of this transcription factor as an important regulator of glucose metabolism in multiple tissues (Fig. 1). Recent studies have also reported on significant glucose metabolism phenotypes following TCF7L2 knockdown in rat hepatocytes (15), coupled with data showing that TCF7L2 directly binds to cis-regulatory elements and promoters of a large number of genes involved in hepatic glucose metabolism. Little is known about the possible metabolic roles of TCF7L2 in tissues such as muscle, fat, and bones. The realization of these effects and the dissection of the molecular and physiological pathways regulated by TCF7L2 beyond pancreatic β-cells may uncover the basis for its association with T2D and result in novel targets for therapeutic development.
ACKNOWLEDGMENTS
M.A.N. is supported by the National Institutes of Health (Grant DK093972).
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 789.
REFERENCES
- 1.Krützfeldt J, Stoffel M. Regulation of wingless-type MMTV integration site family (WNT) signalling in pancreatic islets from wild-type and obese mice. Diabetologia 2010;53:123–127 [DOI] [PubMed] [Google Scholar]
- 2.Lyssenko V, Lupi R, Marchetti P, et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 2007;117:2155–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shu L, Sauter NS, Schulthess FT, Matveyenko AV, Oberholzer J, Maedler K. Transcription factor 7-like 2 regulates beta-cell survival and function in human pancreatic islets. Diabetes 2008;57:645–653 [DOI] [PubMed] [Google Scholar]
- 4.Murtaugh LC, Law AC, Dor Y, Melton DA. Beta-catenin is essential for pancreatic acinar but not islet development. Development 2005;132:4663–4674 [DOI] [PubMed] [Google Scholar]
- 5.da Silva Xavier G, Mondragon A, Sun G, et al. Abnormal glucose tolerance and insulin secretion in pancreas-specific Tcf7l2-null mice. Diabetologia 2012;55:2667–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savic D, Ye H, Aneas I, Park SY, Bell GI, Nobrega MA. Alterations in TCF7L2 expression define its role as a key regulator of glucose metabolism. Genome Res 2011;21:1417–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Li Q, Lee JH, Shu Y. Reduction in Tcf7l2 expression decreases diabetic susceptibility in mice. Int J Biol Sci 2012;8:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shu L, Matveyenko AV, Kerr-Conte J, Cho JH, McIntosh CH, Maedler K. Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired beta-cell function. Hum Mol Genet 2009;18:2388–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaulton KJ, Nammo T, Pasquali L, et al. A map of open chromatin in human pancreatic islets. Nat Genet 2010;42:255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savic D, Park SY, Bailey KA, Bell GI, Nobrega MA. In vitro scan for enhancers at the TCF7L2 locus. Diabetologia 2013;56:121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stitzel ML, Sethupathy P, Pearson DS, et al. NISC Comparative Sequencing Program Global epigenomic analysis of primary human pancreatic islets provides insights into type 2 diabetes susceptibility loci. Cell Metab 2010;12:443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansson O, Zhou Y, Renström E, Osmark P. Molecular function of TCF7L2: Consequences of TCF7L2 splicing for molecular function and risk for type 2 diabetes. Curr Diab Rep 2010;10:444–451 [DOI] [PubMed] [Google Scholar]
- 13.Shao W, Wang D, Chiang Y-T, et al. The Wnt signaling pathway effector TCF7L2 controls gut and brain proglucagon gene expression and glucose homeostasis. Diabetes 2013;62:789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi F, Brubaker PL, Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J Biol Chem 2005;280:1457–1464 [DOI] [PubMed] [Google Scholar]
- 15.Norton L, Fourcaudot M, Abdul-Ghani MA, et al. Chromatin occupancy of transcription factor 7-like 2 (TCF7L2) and its role in hepatic glucose metabolism. Diabetologia 2011;54:3132–3142 [DOI] [PubMed] [Google Scholar]