Abstract
Mutations in GUCY2D are associated with recessive Leber congenital amaurosis-1 (LCA1). GUCY2D encodes photoreceptor-specific, retinal guanylate cyclase-1 (RetGC1). Reports of retinal degeneration in LCA1 are conflicting; some describe no obvious degeneration and others report loss of both rods and cones. Proof of concept studies in models representing the spectrum of phenotypes is warranted. We have previously demonstrated adeno-associated virus (AAV)-mediated RetGC1 is therapeutic in GC1ko mice, a model exhibiting loss of cones only. The purpose of this study was to characterize AAV-mediated gene therapy in the RetGC1/RetGC2 double knockout (GCdko) mouse, a model lacking rod and cone function and exhibiting progressive loss of both photoreceptor subclasses. Use of this model also allowed for the evaluation of the functional efficiency of transgenic RetGC1 isozyme. Subretinal delivery of AAV8(Y733F) vector containing the human rhodopsin kinase (hGRK1) promoter driving murine Gucy2e was performed in GCdko mice at various postnatal time points. Treatment resulted in restoration of rod and cone function at all treatment ages and preservation of retinal structure in GCdko mice treated as late as 7 weeks of age. Functional gains and structural preservation were stable for at least 1 year. Treatment also conferred cortical- and subcortical-based visually-guided behavior. Functional efficiency of transgenic RetGC1 was indistinguishable from that of endogenous isozyme in congenic wild-type (WT) mice. This study clearly demonstrates AAV-mediated RetGC1 expression restores function to and preserves structure of rod and cone photoreceptors in a degenerative model of retinal guanylate cyclase deficiency, further supporting development of an AAV-based vector for treatment of LCA1.
Boye and colleagues report that subretinal injection of AAV8 vector carrying the murine retinal guanylate cyclase-1 gene results in restoration of rod and cone function in a mouse model of Leber congenital amaurosis (LCA). Correction was seen at all treatment ages, with functional gains and structural preservation stable for at least 1 year.
Introduction
Ongoing clinical trials for RPE65 Leber congenital amaurosis (LCA2) have established that adeno-associated virus (AAV) can be used to safely deliver therapeutic transgene to the retinal pigment epithelium thereby restoring useful vision to patients (Bainbridge et al., 2008; Cideciyan et al., 2008; Hauswirth et al., 2008; Maguire et al., 2008; Ashtari et al., 2011; Bennett et al., 2012; Jacobson et al., 2012). However, most retinal degenerations are caused by mutations in genes expressed in photoreceptors (Wright et al., 2010), a cell type yet to be targeted in a clinical setting. Retinal guanylate/guanylyl cyclase-1 (GUCY2D) encodes RetGC1 isozyme of the retinal membrane guanylate cyclase (GC), a protein expressed predominantly in photoreceptor outer segments (Dizhoor et al., 1994; Liu et al., 1994). The physiological link between RetGC1, guanylate cyclase-activating proteins (GCAPs), and intracellular Ca2+ affects the polarization state of the photoreceptor (Burns and Arshavsky, 2005; Karan et al., 2010). Upon light stimulation, cyclic guanosine monophosphate (cGMP) hydrolysis by cGMP phosphodiesterase leads to closure of cGMP-gated channels, a reduction in intracellular Ca2+, and hyperpolarization of photoreceptors. In response to the lowered free Ca2+ in the outer segment, Ca2+-sensitive guanylate cyclase-activating proteins—GCAP1 (Palczewski et al., 1994) and GCAP2 (Dizhoor et al., 1995)—activate cGMP production by RetGC1 and RetGC2 (Makino et al., 2008; Olshevskaya et al., 2012) thus accelerating the recovery phase of the photoresponse. This restoration of cGMP levels reopens cGMP-gated channels and, via increase in intracellular Ca2+, deactivates RetGC by converting their GCAP sensors back into their inhibitory state.
Mutations in GUCY2D are associated with recessive Leber congenital amaurosis-1 (LCA1) (Perrault et al., 1996) as well as dominant and recessive forms of cone–rod dystrophy, CORD6 (Kelsell et al., 1998; Perrault et al., 1998; Weigell-Weber et al., 2000), and CORD (Ugur Iseri et al., 2010), respectively. LCA is a clinically and genetically heterogeneous group of severe, early-onset retinal dystrophies characterized by a reduced or absent electroretinogram (ERG), nystagmus, digito-ocular signs, and apparently normal fundus appearance (Perrault et al., 1999). Among the specific LCA1 studies, there are those that describe retinal degeneration (Milam et al., 2003; Porto et al., 2003) and others suggesting no obvious retinal degeneration (Simonelli et al., 2007; Pasadhika et al., 2010). Until a more thorough analysis of patients with GUCY2D mutations is performed, it is unclear to what extent progressive retinal degeneration is part of the human LCA1 disease phenotype and whether there exists a spectrum of disease expression.
With the goal of developing a gene replacement strategy for treatment of this disease, it is important that proof-of-concept studies are performed in animal models that represent the spectrum of LCA1 phenotypes. Based on the patient reports to date, treatment should thus be evaluated in models of guanylate cyclase deficiency that exhibit reduced photoreceptor-based function and varying degrees of retinal degeneration. More specifically, models should exhibit either a progressive loss of cones only or both rod and cone photoreceptor subclasses. The RetGC1 gene knockout (ko) mouse (GC1ko or GC-E null, after Gucy2e, the murine homologue of GUCY2D) exhibits loss of cone function and cone degeneration (Yang et al., 1999). However, rods retain 30 to 50% of their function and do not degenerate due to the presence of RetGC2 (encoded by Gucy2f), another functional guanylate cyclase isozyme found in mammalian rod photoreceptors (Lowe et al., 1995; Yang et al., 1995, 1999; Yang and Garbers, 1997). We have previously shown that AAV-mediated delivery of Gucy2e results in restoration of cone function detected by electroretinography (ERG), visually-guided behavior, and preservation of cone photoreceptors over the lifetime of the GC1ko mouse (Boye et al., 2010). These findings were confirmed, and it was also demonstrated that AAV-mediated delivery of GUCY2D resulted in improvements in the rod-driven ERG (Mihelec et al., 2011). There is complexity to the latter observation because untreated GC1ko mice maintain 30 to 50% of rod function and exhibit normal visually-guided behavior under scotopic conditions (Yang et al., 1999; Boye et al., 2010). To clarify whether AAV-mediated Gucy2e gene replacement will restore otherwise absent function to rod photoreceptors, treatment would best be applied to a model that lacks intrinsic rod function. The RetGC1/RetGC2 double knockout (GCdko) mouse that carries disruptions in both Gucy2e and Gucy2f genes exhibits loss of both rod and cone structure and function (Baehr et al., 2007) and thus serves as a model in which therapeutic effects on rod function can be addressed. In addition, it is only within the context of the GCdko mouse, which lacks all retinal guanylate cyclase activity, that we can accurately measure the functional efficiency of AAV-vectored RetGC1. The purpose of this study was to test whether Gucy2e gene replacement would restore retinal function, visually-guided behavior, and preserve rod and cone photoreceptors in the GCdko mouse, to establish a window of therapy and evaluate the functional efficiency of vector-mediated RetGC1 in an LCA1 model with dysfunction and degeneration in both photoreceptor types.
Materials and Methods
Animals
Guanylate cyclase 1/2 double knockout (GCdko) mice (Baehr et al., 2007) and C57BL/6J wild-type mice were bred and maintained at the University of Florida Health Science Center Animal Care Services Facility under a 12-hour/12-hour light/dark cycle. Food and water were available ad libitum. All experiments were approved by the University of Florida's Institutional Animal Care and Use Committee and were conducted in accordance with the Association for Research in Vision and Ophthalmology's (ARVO's) Statement for the Use of Animals in Ophthalmic and Vision Research and with National Institutes of Health regulations.
Construction of AAV vectors
Vector plasmid containing the photoreceptor-specific human rhodopsin kinase, −112 to +180 (numbers represent nucleotide positions relative to transcriptional start site) promoter (hGRK1) (Khani et al., 2007) driving murine RetGC1 cDNA was generated, packaged into AAV8(Y733F) capsids, purified, and titered according to previously described methods (Zolotukhin et al., 2002; Jacobson et al., 2006). Therapeutic RetGC1 cDNA was replaced with green fluorescent protein (GFP) cDNA for generation of control vector AAV8(Y733F)-hGRK1-GFP, which was packaged and purified with identical methods. Resulting titers for AAV8(Y733F)-hGRK1-mGC1 and AAV8(Y733F)-hGRK1-GFP vectors were 1.08×1013 vector genomes/milliliter (vg/mL) and 1.95×1013vg/mL, respectively.
Subretinal injections
One microliter of AAV8(Y733F)-hGRK1-mGC1 was delivered subretinally to one eye of GCdko mice. Contralateral eyes were either injected subretinally with 1 microliter of control vector, AAV8(Y733F)-hGRK1-GFP, or remained uninjected. Injections were performed in four separate cohorts of GCdko mice that varied in their respective treatment age; cohort 1, postnatal day 18 (P18); cohort 2, P21–P25; cohort 3, P37–P49; and cohort 4, P108. A separate group of animals designated for guanylate cyclase activity assays were subretinally injected between P30–P60 with identical vector. Subretinal injections were performed as previously described (Timmers et al., 2001). Further analysis was carried out only on animals that received comparable, successful injections (≥60% retinal detachment and minimal complications). It is well established that the area of vector transduction corresponds to at least the area of retinal detachment (Timmers et al., 2001; Cideciyan et al., 2008).
Electroretinographic analysis
ERG of GCdko mice treated with either AAV-GC1 or AAV-GFP, untreated GCdko mice, and age-matched C57BL/6J control mice were recorded using a UTAS Visual Diagnostic System equipped with Big Shot Ganzfeld (LKC Technologies, Gaithersburg, MD) according to methods previously described (Pang et al., 2010). ERG recordings of treated GCdko mice began at 1 month postinjection and were repeated, along with age-matched C57BL/6J controls, bimonthly thereafter until the animals reached at least 1 year of age. Following overnight dark adaptation, scotopic ERGs were elicited at intensities ranging from −20 to 0 dB with interstimulus intervals of 30 sec, averaged from five measurements at each intensity. Mice were then light-adapted to a 30 cds/m2 white background for 2 min. Photopic responses were elicited, with intensities ranging from −3 dB to 10 dB. Fifty responses with interstimulus intervals of 0.4 sec were recorded in the presence of a 20 cds/m2 white background and averaged at each intensity. The b-wave amplitudes were defined as the difference between the a-wave troughs to the positive peaks of each waveform. At each time point, maximum scotopic and photopic b-wave amplitudes (those generated at 0 dB and 10 dB, respectively) from all treated, untreated, and control mice within each cohort were averaged as mean±standard error. Values were imported into SigmaPlot for final graphical presentation.
Optical coherence tomography
Outer nuclear layer (ONL) thickness was quantified by optical coherence tomography (OCT) (Bioptigen Inc, Durham, NC) to assess the natural history of photoreceptor nuclear layer change in GCdko mice and treatment efficacy. OCT was performed at 59 timepoints in the GCdko animals, spanning 3 to 15 months of age after treatment. Outer nuclear layer (ONL) segmentation could not be reliably performed in nine treated and two untreated eyes due to reduced quality of these scans. Horizontal scans across the optic nerve head (ONH), extending ∼0.6 mm to either side, were obtained. Postacquisition processing of OCT data was performed with custom programs written in MATLAB (MATLAB6.5; MathWorks, Natick, MA) as described previously (Aleman et al., 2011; Huang et al., 2012). The longitudinal reflectivity profiles (LRPs) of the OCT images were aligned manually by straightening the retinal pigment epithelium (RPE) reflection, which was defined as the second hyperreflective peak from the sclerad side (Ruggeri et al., 2007). The ONL is the hyporeflective band sclerad to the outer plexiform layer (OPL) and corresponds to the signal trough delimited by the signal peaks defining the OPL and outer limiting membrane (OLM). ONL thickness was measured in a semiautomated fashion using the LRP sample having maximum slope on both sides of the signal trough (Jacobson et al., 2005; Huang et al., 2012).
Optomotor testing
Using the two-alternative forced choice paradigm as previously described (Umino et al., 2008; Boye et al., 2010), light-adapted spatial frequency and contrast sensitivity measurements were made in 1-year-old C57BL/6J mice (M1-M5), and AAV-GC1-treated (M6-M9) and untreated (M10-M14) GCdko mice with a virtual optokinetic instrument (Optomotry, Cerebral Mechanics). The single eye-treated GCdko mice analyzed were chosen from cohort 1 (P18-treated, “M6”) and cohort 3 (P37–P49-treated,“M7,” “M8,” and “M9”). As previously shown (Douglas et al., 2005), visual signals originating from right or left eyes were determined separately and simultaneously via stepwise functions for correct responses in both the clockwise and counterclockwise directions. The spatial frequency at threshold was determined using targets of 100% contrast. Contrast sensitivity was defined as 100 divided by the lowest percent contrast yielding a threshold response. For photopic acuity, the initial stimulus was a 0.200 cycles/degree sinusoidal pattern with a fixed 100% contrast. For photopic contrast sensitivity measurements, the initial pattern was presented at 100% contrast, with a fixed spatial frequency of 0.128 cycles/degree. Photopic vision was measured at a mean luminance of 70 cds/m2. Spatial frequencies and contrast sensitivities were measured for both eyes of each mouse over 4 to 5 trials for a period of 3 days. Student's t-tests were performed to determine significance of results. Significance was defined as a P-value <0.05.
Morris Water Maze testing
Rod- and cone-mediated behavior was assessed using Morris Water Maze as previously described (Pang et al., 2006, 2008). Testing consisted of four blocks of three trials per day for four consecutive days under each respective lighting condition. Cone-mediated testing in a well-lit room preceded dark-adapted, rod-mediated testing (light levels not detectable with Extech Datalogging Light Meter, Model 401036; Extech Instruments, Waltham, MA). Escape latencies were timed using a stopwatch and recorded in a notebook. On the fourth day of both cone- and rod-mediated testing, trials were recorded with a Sony Handycam camcorder (rod-mediated behavior was filmed in “nightshot plus” mode). The single-eye, AAV-GC1-treated GCdko mice used in this assay were taken from cohort 1 (P18-treated, n=2) and cohort 3 (P37–P49-treated, n=3) and were 1 year of age at the time of testing. These mice were the same mice tested in the optomotor task described above. Age-matched, untreated GCdko mice (n=5) and C57BL/6J mice (n=5) were also tested. Release and platform locations were randomized across each mouse and each session. Three days after completion of rod-mediated behavior testing, treated GCdko mice were anesthetized and their treated eyes sutured shut. Following a 24-hour recovery period, the cone-mediated behavior test was repeated once (1 day consisting of four blocks and three trials). Student's t-tests were used to compare all escape latencies recorded throughout the testing procedure between cohorts. Significant difference was defined as a P-value <0.05.
Guanylate cyclase activity assays
A separate cohort of GCdko mice was injected with AAV-GC1 between P30–P60. Scotopic and photopic ERGs were performed 1 month post-treatment to confirm rescue. Successfully injected mice (at least 60% retinal detachment and concomitant rod/cone rescue, n=21) were then used for RetGC1 activity assays. Mice were dark-adapted overnight, sacrificed under infrared illumination, and the retinas were extracted and assayed for guanylate cyclase activity in the dark using [α-32P]GTP as a substrate according to a previously described protocol (Olshevskaya et al., 2004; Peshenko et al., 2011). The assay mixture (25 μL) contained 30 mM MOPS–KOH (pH 7.2), 60 mM KCl, 4 mM NaCl, 1 mM DTT, 2 mM Ca2+/EGTA buffer, 1 mM free Mg2+, 0.3 mM ATP, 4 mM cGMP, 1 mM GTP, 1 μCi of [α-32P]GTP, 0.1 μCi of [8-3H]cGMP (Perkin Elmer, Waltham, MA), phosphodiesterase inhibitors zaprinast and dipyridamole, 10 mM creatine phosphate, and 0.5 unit of creatine phosphokinase. The resultant [32P]cGMP product, together with the internal standard of [8-3H]cGMP, was purified using fluorescently backed polyethyleneimine cellulose thin-layer chromatography plate (Merck, Whitehouse Station, NJ) as described previously (Olshevskaya et al., 1997), and the radioactivity of both tracers was counted after elution in 1 M LiCl using ScintiSafe scintillation cocktail (Fisher/Thermo Scientific, Waltham, MA) containing 20% ethanol. Ca2+/EGTA buffers containing calibrated free Ca2+ and Mg2+ concentrations were prepared using published methods (Tsien and Pozzan, 1989) and verified by fluorescent Ca2+ indicator dyes as previously described (Peshenko and Dizhoor, 2006).
Tissue preparation, immunohistochemistry, and microscopy
Single eye-treated GCdko mice were sacrificed between 12 and 17 months of age. All mice analyzed were from cohort 2 (P21–P25-treated) or cohort 3 (P37–P49-treated). A limited number of age-matched wild-type (WT) mice served as controls. At sacrifice, tissue was prepared according to published methods (Boye et al., 2011). Eyes designated for cryosectioning were processed and immunostained as described (Haire et al., 2006; Pang et al., 2010). Retinal cross sections from treated and untreated eyes of GCdko mice were immunostained with antibodies specific for RetGC1 (rabbit polyclonal sc50512, 1:200; Santa Cruz Biotechnology), mouse cone arrestin (rabbit polyclonal “LUMIj,” 1:1000; generously provided by Dr. Cheryl Craft, Univ. of Southern California), GCAP1 (rabbit polyclonal “UW101,” 1:5000), GCAP2 (rabbit polyclonal “UW50,” 1:4000), M opsin (goat polyclonal sc-22117, 1:200; Santa Cruz Biotechnology) or S opsin (rabbit polyclonal, AB5407, 1:300, Millipore). Following an overnight incubation in primary antibodies, IgG secondary antibodies Alexafluor-488 or Alexafluor-594 were applied for 1 hr at room temperature. Sections were counterstained with 4’, 6’-diaminio-2-phenylindole (DAPI) for 5 min at room temperature. Retinal sections were imaged as previously described (Boye et al., 2011).
Immunoblotting
At 12 months of age, a single eye-treated GCdko mouse from cohort 3 (P37-P49-treated), and an age-matched WT control mouse were sacrificed, enucleated, and processed according to previously described methods (Molday et al., 2007; Boye et al., 2011). The tissue was lysed by sonication (3×30 sec) in 100 microliters of sucrose buffer (0.23 M sucrose, 2 mM EDTA, 5 mM Tris-HCl, pH 7.5). Cell debris was removed by centrifugation at 14,000 rpm for 10 min at room temperature. The protein concentration of the supernatant was determined with bicinchoninic acid (BCA) (Thermo Fisher Scientific), and equal amounts of protein were separated on 12% polyacrylamide gels (BioRad, Hercules, CA) and subsequently transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA). Blots were labeled with antibodies specific for RetGC1 (mouse monoclonal “IS4,” 1:3000; generously provided by Dr. Kris Palczewski), RetGC2 (rabbit polyclonal “L670,” 1:5000), GCAP1 (rabbit polyclonal “UW101,” 1:30,000), GCAP2 (rabbit polyclonal “UW50,” 1:2000) and β-actin (1:5000; Abcam, Cambridge, MA).
Results
Effects of treatment on rod-and-cone photoreceptor function in GCdko mice
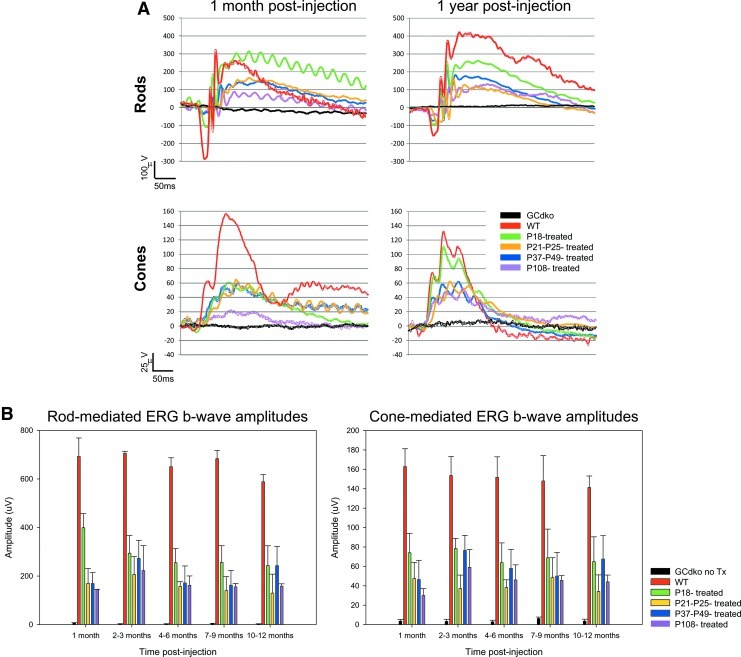
While mouse cones rely exclusively on RetGC1 for guanylate cyclase activity, mouse rods utilize both RetGC1 and RetGC2 (Yang et al., 1999; Baehr et al., 2007). Thus, both rods and cones in the GCdko mouse have no GCAP-regulated cyclase activity (Peshenko et al., 2011) and lack photoresponses (Baehr et al., 2007). As such, the GCdko mouse affords us the opportunity to test whether AAV-delivered RetGC1 can restore function to both photoreceptor subclasses. We therefore subretinally delivered AAV vector–expressing Gucy2e to GCdko mice at ages ranging from P18–P108. Bimonthly ERG analysis beginning at 1 month post-treatment revealed that treated eyes of all cohorts displayed robust rod- and cone-driven b-waves that were stable and of significantly higher amplitudes than responses from untreated eyes at all time points (P<0.001) (Fig. 1). Representative traces from all treated cohorts were compared to age-matched WT traces at 1 month and 1 year post-treatment (Fig. 1A). Treated-eye average rod responses ranged from 26% of WT following late treatment (P108) to 42% of WT following early treatment (P18). Treated-eye average cone responses ranged from 29% of WT (P108-treatment) to 44% of WT (P18-treatment). The level of cone rescue achieved in AAV8(Y733F)-hGRK1-mGC1-treated GCdko mice was comparable to that we reported earlier in GC1ko mice treated with the same vector (Boye et al., 2011).
FIG. 1.
Electroretinogram (ERG) analysis of AAV-GC1-treated GCdko mice and age-matched GCdko and WT controls. (A) Representative scotopic (top) and photopic (bottom) ERG traces from each cohort of AAV-treated GCdko mice and age matched untreated and WT controls are shown at 1 month post (left) or 1 year post (right) treatment. (B) Temporal analysis of average rod (left) and cone (right) b-wave amplitudes in 4 cohorts of AAV-treated GCdko mice relative to untreated and WT controls. GC1, guanylate cyclase-1; WT, wild-type; GCdko, retGC1/retGC2 double knockout.
In vivo retinal structure of GCdko mice and treatment efficacy
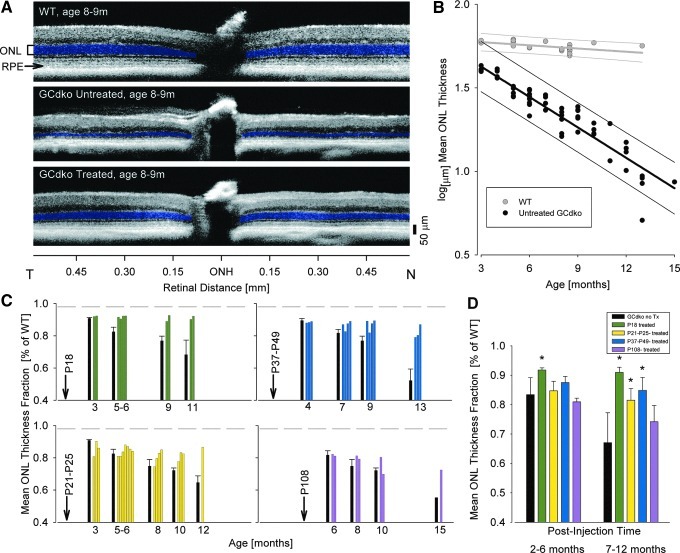
To better understand the progress of retinal degeneration and the effect of treatment with AAV-GC1 on GCdko retina morphology, OCT retinal scanning was performed and analyzed in treated and untreated GCdko eyes (ages 3–15 months) and compared with WT (C57BL/6) mice of similar ages (Fig. 2). Twenty-two WT mice and 12 GCdko mice that received uniocular treatment at either postnatal day 18 (cohort 1), 21–25 (cohort 2), 37–49 (cohort 3), or 108 (cohort 4) were analyzed. OCT imaging in these animals occurred ∼2 months postinjection, and follow-up OCTs were carried out at ∼2- to 3-month intervals and extended to 12 months after treatment.
FIG. 2.
OCT abnormalities in GCdko retinas and the effect of treatment. (A) Representative OCT horizontal scans ∼0.6mm to either side of the ONH to illustrate ONL thickness reduction in an untreated GCdko eye compared to an age-matched WT eye; ONL is retained in the treated eye of the same GCdko animal. ONL and RPE laminae are marked at the edge of the WT scan and ONL is highlighted on all scans (blue). (B) ONL thickness as a function of age in GCdko retinas and WT retina. Nasal-temporal OCT sections were quantified for ONL thickness in GCdko mice (black circles) and age-related WT mice (gray circles). Regression lines describe log-linear change of the parameters over time with 95% prediction intervals shown. The two data points for older WT mice in the analyses were derived from published data (Xu et al., 2009). (C) Effect of treatment delivered to one eye of GCdko mice at different ages postnatal (P, days). ONL thickness fraction change (expressed as percent of WT) in treated and untreated GCdko eyes as a function of time. Arrow and labels indicate the time of treatment; color bars, GCdko-treated eyes at various timepoints; black bars, mean (error bar, +SD) of untreated GCdko eyes at the corresponding age groups; gray dashed line at top of each graph, lower limits of WT data. (D) GCdko-treated eyes (color bars as in C) were separated into two groups (2–6 and 7–12 months post-treatment) and compared to age-related untreated eyes (black bar). Asterisk indicates significant difference between treated and untreated eyes (p<0.001). OCT, optical coherence tomography; OCT, optical coherence tomography; ONL, outer nuclear layer.
Representative OCT scans across the ONH show the retinal laminar architecture in a WT mouse, and in untreated and treated eyes from a GCdko mouse at 8–9 months of age (Fig. 2A). The ONL thickness was markedly reduced compared to WT in the untreated GCdko eye but retained in the treated eye. Thickness measurements along the horizontal meridian of the GCdko eyes (53 timepoints) and in 22 WT eyes (16 timepoints) spanning 3–15 months of age are shown (Fig. 2B). ONL thickness declined with age in the GCdko eyes in a manner that can be described with a log-linear function having a negative slope (log10(ONL)=1.8096 –0.0607*age [mos]; r2=0.88, p<0.001). There is little or no reduction of WT ONL with age that can also be described by a log-linear function (log10(ONL)=1.7931−0.0064*age[mos]; r2=0.31, p<0.05). The slope of ONL thickness decline in GCdko eyes is significantly different from that of C57BL/6 WT (P<0.001).
ONL thickness in treated GCdko eyes was quantified (Fig. 2C and D). In Figure 2C, arrows indicate the ages (in postnatal days) when the animals received treatment; colored bars represent ONL thickness fraction of individual treated eyes; black bars to the left of each of the follow-up age groups represent the mean (+/− standard deviation, SD) of the untreated eyes at the corresponding ages. In summary, the ONL thickness was either retained or its loss over time was slowed significantly in the treated eyes. To further examine the treatment effect, treated GCdko eyes were separated into two groups (2–6 and 7–12 months after injection) and compared to their untreated partner eyes at the corresponding age window (Fig. 2D). Significant differences (P<0.001) were detected in the P18 treatment cohort as early as 2–6 months after treatment. P18, P21–P25, and P37–P49 treatment cohorts also showed significant differences compared to untreated controls at these postinjection times. The P108 treatment cohort, however, was not significantly different from untreated eyes at the post-treatment ages studied.
Effects of treatment on cortically- and subcortically-driven visual behavior
In order to determine whether cortically- and/or subcortically-driven visual behavior could be restored to an animal model that lacked retinal function prior to AAV-GC1 treatment, we employed the Morris Water Maze and virtual optokinetic tests, respectively.
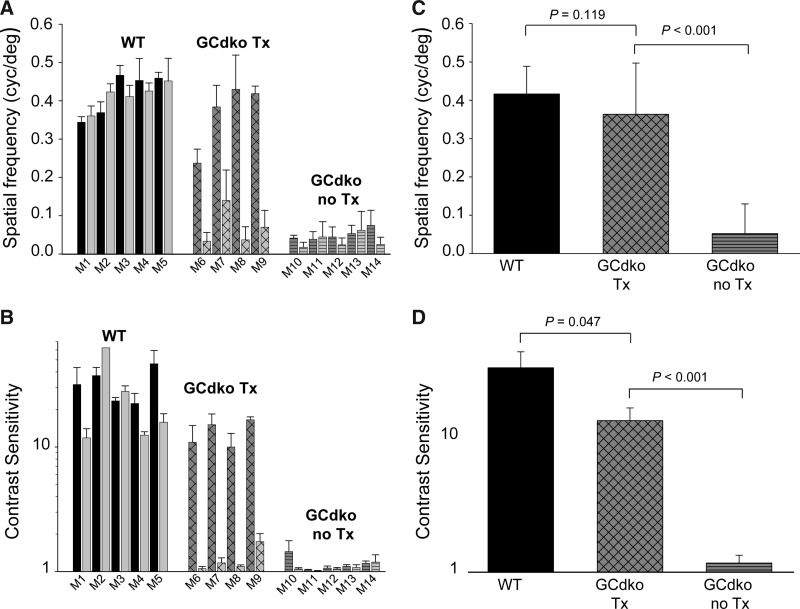
A virtual optokinetic system provided a measure of the capability of subcortical retinal efferents since lesions of the visual cortex have no effect on the rodent's ability to perform this task (Douglas et al., 2005). We found that AAV-GC1 treatment led to significant improvements in light-adapted behavior, as assessed by spatial frequency and contrast sensitivity measurements (Fig. 3). Responses from the right and left eyes of individual mice are plotted in Figure 3A and B (AAV-GC1 was delivered subretinally to the right eyes only of treated GCdko mice). Figure 3C and D depict the averages and standard deviations within each group. Spatial frequency at threshold in 1-year-old GCdko mice treated between P18–P49 was significantly improved relative to results in age-matched, untreated GCdko controls (P<0.001) and was not significantly different from age-matched, WT controls (P=0.119) (Fig. 3C). Contrast sensitivities were also significantly improved in AAV-GC1-treated GCdko mice relative to untreated controls (P<0.001) (Fig. 3D).
FIG. 3.
Optomotry-based analysis of subcortical function in single eye, AAV-treated GCdko mice (red) and age-matched untreated GCdko (green) and WT (black) controls. M1–M14 corresponds to the 14 mice used for testing. Right eyes, solid bars; left eyes, hatched bars. GCdko mice were treated in their right eyes only. (A) Photopic spatial frequencies and (B) contrast sensitivities of WT controls (M1–M5), AAV-treated GCdko (M6-M9), and untreated GCdko controls (M10–M14) reveal significant improvements in the cone-mediated vision of treated mice. Values within each group were averaged (C) and (D) and compared with student's t-test. P values were calculated between WT and treated GCdko mice and between treated and untreated GCdko mice (C) and (D).
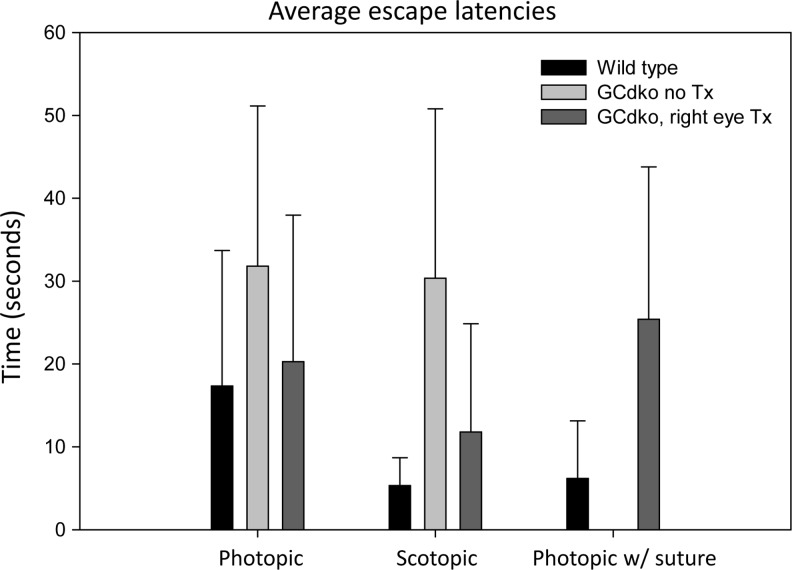
The Morris Water Maze, a test to measure cortically-driven visual behavior, was used to evaluate treatment effect on both cone and rod photoreceptors. The average amount of time required for single-eye-treated GCdko mice to find the escape platform under both dark- and light-adapted conditions (11.8 sec and 20.3 sec, respectively) was significantly less than that required by untreated GCdko (30.4 sec and 31.8 sec, respectively) (P<0.001) (Fig. 4). Light-adapted behavior of AAV-treated GCdko mice was very similar to WT (escape latencies of 20.3 sec and 17.3 sec, respectively). This difference was not significant (P=0.060). While large improvements were seen in dark-adapted behavior following treatment, a significant difference in escape times remained between AAV-treated GCdko (11.8 seconds) and WT mice (5.3 seconds) (P<0.001) (Fig. 4). To confirm that behavioral improvements in AAV-treated GCdko mice were dependent on use of the treated eye, it was sutured shut, light-adapted behavior tests were repeated (Fig. 4), and no significant difference was found between sutured, AAV-treated GCdko mice (25.4 sec) and untreated GCdko controls (31.8 sec) (P=0.063). Due to the learning component of the Morris Water Maze task (D'Hooge and De Deyn, 2001), escape latency times of sutured GCdko would be expected to be marginally lower than untreated GCdko controls as they performed the task more than any other cohort.
FIG. 4.
Morris Water Maze-based analysis of cortical function in AAV-treated GCdko mice and age-matched untreated GCdko and WT controls. Average escape latencies of WT (black bars, n=5), untreated GCdko (red bars, n=5), and AAV-treated GCdko mice (green bars, n=5) were calculated under photopic and scotopic conditions. A final comparison was made between WT mice and AAV-treated GCdko mice that had their treated eyes sutured, followed by testing under photopic conditions (“photopic w/suture”). Error bars represent one standard deviation.
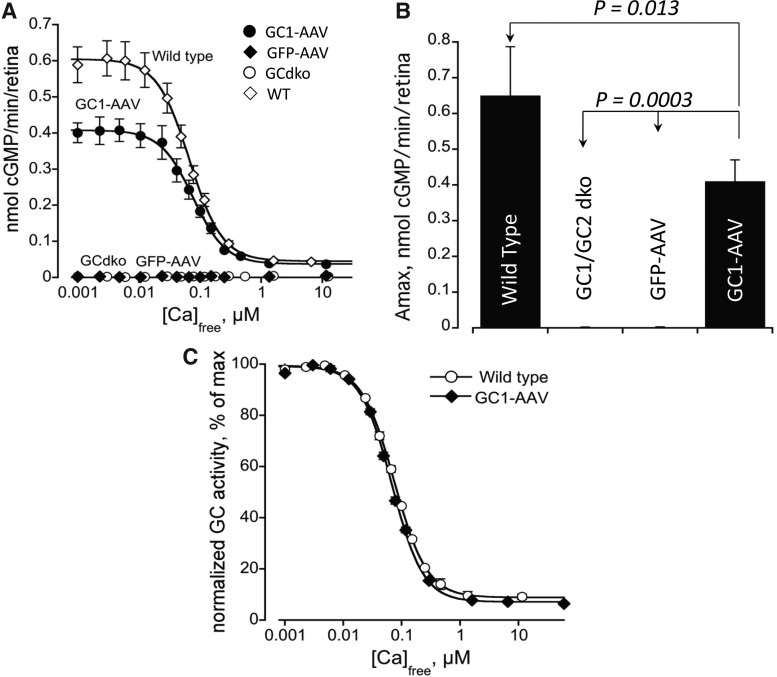
Functional efficiency of AAV-delivered RetGC1
In order to determine the functional efficiency of RetGC1 enzyme encoded by AAV-delivered Gucy2e, we performed guanylate cyclase activity assays in treated GCdko mice. Results were compared to RetGC activity in untreated GCdko mice, GCdko mice injected with control vector (AAV-GFP), and WT controls (Fig. 5A). RetGC activity in our assay measures only that of GCAP-regulated RetGC isozymes present in the retina, lacking in GCdko (Peshenko et al., 2011). Thus, total guanylate cyclase activity in the AAV-treated GCdko mice represents solely the activity of the AAV-mediated RetGC1 expression whereas total cyclase activity in WT mice is produced by both endogenous isozymes, RetGC1 and RetGC2 (Pehsenko et al., 2011). Maximal GC activity (at [Ca2+] free<20 nM) in treated mice was restored to ∼63% of WT (Fig. 5B). As expected, there was no detectable guanylate cyclase activity in untreated GCdko mice or in GCdko mice treated with AAV-GFP. Studies have shown that RetGC2 contributes 20% to 28% of total GC activity in mouse retina in whole mouse retina preparations and mouse rod outer segments, respectively (Peshenko et al., 2011). If the contribution of RetGC2 is subtracted from the total guanylate cyclase activity in WT mice, the levels of RetGC1 activity restored in AAV-treated GCdko mice approach 83–93% of the GC activity of WT mice. Since the area of retinal detachment corresponds to the area of the retina transduced by the vector and expresses the transgene (Timmers et al., 2001; Cideciyan et al., 2008), this level of RetGC1 activity suggests its near-complete restoration for the area exposed to AAV vector during the subretinal injection procedure. The calcium sensitivity of the delivered enzyme was evaluated by measuring RetGC1 activity in the presence of different free Ca2+ concentrations and normalization to the maximal RetGC1 activity at low free Ca2+. The sensitivity of RetGC1 in AAV-GC1-treated GCdko mice remains the same as in WT controls (Fig. 5C); there was no significant difference between the two groups (P=0.18). Taken together with the maximal activity measurements, this contends that the functional efficiency of the AAV-delivered RetGC1 was normal.
FIG. 5.
Functional efficiency of AAV-mediated RetGC1 activity in vivo. (A) RetGC activity in AAV-GC1–treated GCdko mice (closed circles), control vector AAV-GFP-treated GCdko mice (closed diamonds), age matched GCdko (open circles), and WT (open diamonds) controls at different free Ca2+. Values are the average of three measurements, mean±SE. In both AAV-GFP treated and untreated GCdko mice, the activity was undetectable. (B) Maximal activity of RetGC (at [Ca2+] free<20 nM). On the average, AAV-GC1 restored ∼63% of WT RetGC activity (0.41±0.06 SD, N=3; vs. 0.65±0.137 SD nmol cGMP/min/retina, N=8, respectively). ANOVA/Bonferroni data processing at 99% CL. (C) Ca2+ sensitivity of RetGC in AAV-GC1-treated GCdko mice remains the same as in WT retinas. RetGC activity was normalized by the maximal activity in each series and then averaged from all series for each genotype. The data are fitted by the Hill function, a=(amax − amin)/(1+([Ca]f/Ca1/2)h)+amin; the Ca1/2=68 nM±6 SD, N=8 (wild type) and 74 nM±6 SD, N=3 (GC1-AAV), not a significant difference by t-test (p=0.18); h=1.6±0.056 (WT) and 1.52±0.053 (GC1AAV), not a significant difference by t-test (p=0.1).
AAV-mediated RetGC1 expression and cone photoreceptor densities
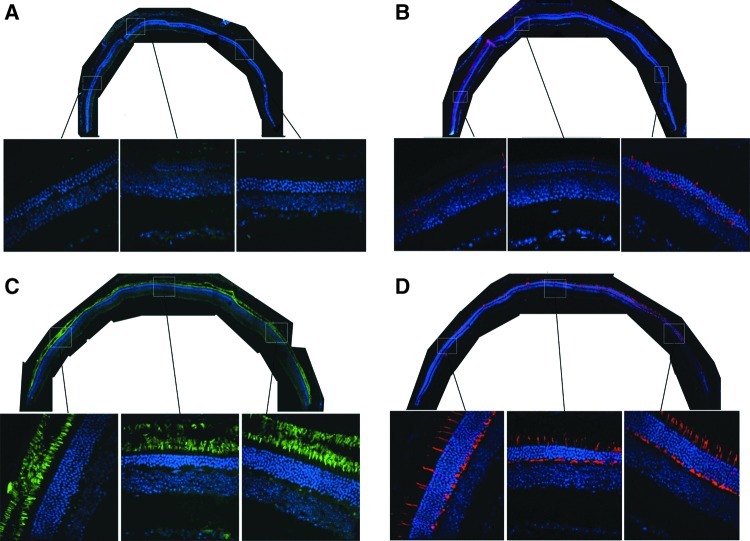
Microscopic analysis of representative retinal cross sections taken from 1-year-old GCdko mice that received single eye injections at P40 of AAV-GC1 revealed several key treatment-related changes (Fig. 6). Consistent with OCT findings, DAPI nuclear counterstain revealed pronounced thinning of the ONL in untreated GCdko mice (∼4 nuclei rows remaining) (Fig. 6A and B), whereas AAV-GC1-treated mice retained more ONL nuclei (∼10 nuclei rows remained throughout most of the treated retina) (Fig. 6C and D). AAV-mediated RetGC1 presence was detected exclusively in photoreceptor outer segments throughout the treated eyes (Fig. 6C) but, as expected, was absent from untreated contralateral control eyes of the same mice (Fig. 6A). Immunostaining with an antibody specific for cone arrestin revealed that cone photoreceptors had degenerated significantly in 1-year-old, untreated GCdko mice. Consistent with previous findings (Baehr et al., 2007), the remaining GCdko cones had an altered morphology and their outer segments were not detectable (Fig. 6B). In contrast, cones were abundant and their morphology remained intact in GCdko eyes treated with AAV-GC1 (Fig. 6D).
FIG. 6.
Expression of RetGC1 and cone arrestin in treated GCdko and untreated contralateral controls. Retinas from a 1-year-old GCdko mouse that received AAV-GC1 treatment at P40 in one eye only were stained with antibodies raised against RetGC1 and cone arrestin. Samples were counterstained with DAPI. Untreated GCdko retina (top) lacked RetGC1 expression (A), exhibited reduced cone photoreceptor densities abnormal morphology of remaining cones (B), and outer nuclear layer (ONL) thinning (4–5 nuclei rows remaining). AAV-GC1 treated retina contained pan-retinal RetGC1 expression that was exclusive to photoreceptor outer segments (C), increased cone densities with apparently normal morphology (D), and relatively preserved ONL throughout most of the retina. Entire eyecups (top, 4×) and magnified portions of each (bottom, 40×) are shown.
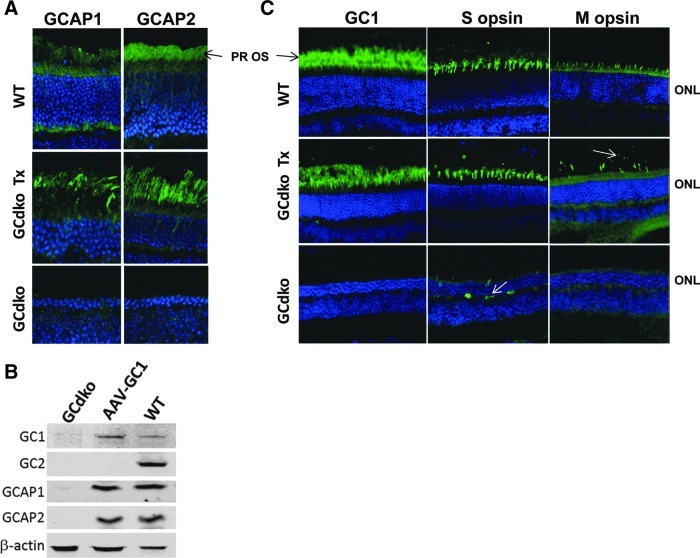
Effects of treatment on expression of GCAP1, GCAP2, and cone opsins
Guanylate cyclase-activating proteins 1 and 2, GCAP1 and GCAP2, the Ca2+-binding proteins that function as regulators of GC1 and GC2, are downregulated in GCdko mice (Baehr et al., 2007). Additionally, S-opsin and M-opsin are mislocalized and their expression reduced in GCdko (Baehr et al., 2007). To gain a better understanding of how AAV-GC1 treatment affects expression of GCAPs and cone opsins, we stained representative retinal sections with antibodies directed against each. Treated retina from a P40-injected GCdko mouse sacrificed at 17 months of age exhibited presence of both GCAP1 and GCAP2 predominantly in photoreceptor outer segments, whereas the contralateral untreated eye lacked detectable expression of either GCAP (Fig. 7A). Immunoblotting of another P40-treated retina, sacrificed at 12 months of age, revealed that GCAP1 and GCAP2 expression levels were identical to those in the age-matched WT controls, while only faint traces of GCAP1 and none of the GCAP2 were detectable in the untreated GCdko retina (Fig. 7B). Our ability to detect GCAP1 expression only by immunoblotting may be due to the younger age of the retina used for this assay compared to that used for the immunofluorescence analysis (12 months vs. 17 months, respectively).
FIG. 7.
Expression of GCAPs and cone opsins in AAV-treated (P40) GCdko retinas and age-matched untreated and WT controls. (A) Retinas from 17-month-old mice were immunostained for GCAP1 and GCAP2 (green), and the nuclei were counterstained with DAPI (blue); note that GCAP1 and GCAP2 are both detectable in WT or treated GCdko and undetectable in untreated GCdko. PR OS, photoreceptor outer segments, ONL, outer nuclear layer. (B) Immunoblots of 12-month-old, AAV-treated (P40) GCdko retinas and age-matched GCdko and WT controls revealed the presence of RetGC1, GCAP1, and GCAP2 in AAV-GC1-treated eyes. β-actin was used as a loading control. (C) Immunofluorescence (green) of RetGC1, S-opsin and M-opsin, and DAPI nuclear counterstain in 17-month-old mouse retinas. Note that RetGC1 and M-opsin were absent while S-opsin signal was reduced and mislocalized (white arrow) in untreated GCdko retinas. In treated retinas, GCAP1 and GCAP2 were upregulated. S-opsin was present in the inferior retina of treated GCdko mice at levels similar to WT and was found exclusively in photoreceptor outer segments. M-opsin was present at reduced levels in the superior retina of treated GCdko mice and its expression appeared restricted to sparse cone outer segments (white arrow).
S-opsin was abundant and properly localized in cones of the treated GCdko mice but was significantly downregulated and mislocalized in the untreated contralateral retinas (Fig. 7C). M-opsin expression was present in sparse outer segments of treated GCdko retinas, whereas it was undetectable in the untreated contralateral retinas (Fig. 7C). Care was taken to image sections from identical retinal areas across samples such that results were not distorted by the natural dorsal-ventral gradient of mouse cone opsin expression (sections from the superior and inferior hemispheres were chosen for analysis of M-opsin and S-opsin expression, respectively).
Discussion
Currently, 17 genes are known to be associated with LCA, accounting for ∼70% of cases classified as such by ophthalmic exam. The proteins these genes encode are involved in a wide variety of cellular functions within the retina (den Hollander et al., 2008; Wang et al., 2009; Estrada-Cuzcano et al., 2011; Falk et al., 2012; Koenekoop et al., 2012), and there can be clinical differences among the various genotypes (Hanein et al., 2004; den Hollander et al., 2008). Whereas there is severely impaired vision in all LCA1, there is no consensus about whether there are underlying retinal structure abnormalities in such patients. Some reports describe retinal degeneration (Milam et al., 2003; Porto et al., 2003) and others show normal-appearing, noninvasive, cross-sectional retinal images (Simonelli et al., 2007; Pasadhika et al., 2010). Such studies suggest that there may indeed be loss of rod and cone structure as well as function in some LCA1 patients, and it is important to evaluate gene therapies in an animal model exhibiting degeneration as well as dysfunction. The value of performing AAV-mediated Gucy2e gene replacement in the GCdko mouse lies in the ability to ask questions not addressable in other animal models of RetGC1 deficiency. These questions are: (1) In the absence of functional retinal GC, does vector-mediated RetGC1 supplementation confer functionality to rod photoreceptors? (2) In a model of RetGC deficiency in which rods degenerate, does AAV-GC1 preserve rods? (3) What is the functional efficiency of the vector-mediated enzyme?
To date, all reports of gene replacement in a mammalian model of RetGC1 deficiency have utilized the GC1ko mouse (Haire et al., 2006; Boye et al., 2010; Boye et al., 2011; Mihelec et al., 2011). While this mouse appropriately models the loss of cone function and structure seen in LCA1, it maintains variable levels of rod function (30–50%) and exhibits no rod degeneration (Yang et al., 1999; Karan et al., 2010; Olshevskaya et al., 2012). We have shown that AAV-mediated GC1 expression in photoreceptors restores cone function and preserves cones over the lifetime of the treated GC1ko mouse (Boye et al., 2010; Boye et al., 2011). The ability to increase rod function in this model has also been demonstrated (Mihelec et al., 2011). The current study extends these findings by demonstrating that restoration of rod function is achievable in a model with rods that are physiologically “silent” prior to AAV-Gucy2e treatment. Based on ERG responses, rod function in treated GCdko mice was restored up to 42% of WT. Consistent with our findings in the GC1ko mouse, cone function in treated mice was also restored up to 44% of WT (Fig. 1). Importantly, functional restoration (rods and cones) was stable for at least 1 year post-treatment. Recovery of retinal function was associated with improvements in visual behavior. Through the use of two visually-guided behavioral tasks, we show that treated GCdko mice with only partial recovery of rod and cone ERG amplitudes exhibited near WT-like behavior.
OCT and histology revealed that improvements in ERG and visually-guided behavior correlated with the long-term preservation of retinal structure. By 7 to 12 months post treatment, significant structural preservation was seen in all treatment groups, with the exception of the P108 treatment cohort (the oldest treatment age in our study). However, mean ONL thickness in this group was greater than that in untreated, age-matched eyes; the difference, however, did not achieve statistical significance. Our data show the rate of photoreceptor cell loss in GCdko mice treated as late as P49 is comparable to the natural rate of age-related loss in WT mice. What does this mean in terms of the window for therapy? Late stage therapy with AAV-RPE65 in Rpe65-/- mice retaining some outer nuclear layer was shown to be successful (Jacobson et al., 2005). Consistent with this, LCA2 patients in their late 3rd decade that retained residual islands of photoreceptors demonstrated clear improvements in visual function upon subretinal delivery of AAV-RPE65 to those areas (Jacobson et al., 2012). These animal model and human clinical trial results suggest that the success of gene therapy is related to the state of photoreceptor preservation at the time of treatment rather than simply the patient's age. Some studies indicate that LCA1 represents biochemical dysfunction in the absence of retinal degeneration (Simonelli et al., 2007; Pasadhika et al., 2010) suggesting that the window of therapy in these patients may be protracted. Predicting the true window of therapy for LCA1 patients is therefore likely to depend on results of detailed analyses of the retinal structure and function in large cohorts of these patients.
In addition to providing a means to evaluate the effects of Gucy2e gene replacement on rod photoreceptors, the GCdko mouse affords us the unique opportunity to measure the functional efficiency of vector-mediated RetGC1 enzyme. The guanylate cyclase activity assays used here specifically measure the activity of both RetGC isozymes in photoreceptors, but not other potential guanylate cyclase activites in the retinal tissue (Peshenko et al., 2011). Yet, the endogenous RetGC2 activity would skew the accuracy of the restored RetGC1 in treated GC1ko mice. It is only within the context of a model lacking both isozymes that AAV-mediated RetGC1 enzyme activity can be studied. Here, we find that the functional efficiency of the AAV-delivered enzyme is normal. The calcium sensitivity of exogenously delivered GC1 was identical to that seen in WT mice. Total GC1 activity was approximately 83–93% of WT, when corrected for contribution of RetGC2 to the total RetGC activity in WT retinas (Peshenko et al., 2011). This number roughly corresponds to the average percentage of retinal detachment/exposure to the vector achieved during subretinal injection and, if corrected for the photoreceptors' exposure area, amounts to a near-complete RetGC1 activity restoration in the transduced photoreceptors.
This study is the first to evaluate the functional efficiency of transgenic RetGC1 in a therapeutic setting. The ability to measure functional efficiency of the AAV-mediated RetGC1 delivery will be of use in establishing a dose-response curve. Additionally, such an assay could be developed into a bioassay of vector potency as has been done for AAV-RPE65 (Roman et al., 2007). This would be highly advantageous for comparing individual vector preparations and qualifying clinical grade vector. It should be noted that our study focused on murine RetGC1, (encoded by Gucy2e) whereas clinical grade vector would incorporate the human transgene (GUCY2D). Given a previous report that AAV-mediated GUCY2D expression was therapeutic in the GC1ko mouse (Mihelec et al., 2011), and our findings that it also conferred rod and cone rescue to the GCdko mouse (data not shown), it is expected that human RetGC1 will be amenable to the activity assays described herein.
We have demonstrated that AAV-delivered RetGC1 is fully functional, that it expresses in a large fraction of photoreceptors (Fig. 6) following subretinal injection, and that RetGC1 traffics properly to the photoreceptor outer segments (Boye et al., 2010; Boye et al., 2011; Haire et al., 2006; Mihelec et al., 2011). In spite of these findings, cone-mediated ERGs in both treated GC1KO and GCdko mice did not measure more than between 45–65% of WT (Boye et al., 2010, 2011; Mihelec et al., 2011). We restrict our focus to mouse cones because this photoreceptor subclass does not express GC2, and thus their function is not impaired by its absence (Lowe et al., 1995). Our findings of significant but incomplete cone rescue is in contrast to reports of other cone-targeted gene therapies in mouse models of retinal disease (Alexander et al., 2007; Carvalho et al., 2011). In two different models of achromatopsia, CNGB3-/- and Gnat2Cpfl3 mice, AAV-mediated delivery of the therapeutic transgene restored cone-mediated responses over the long term to 90%–100% of WT, respectively (Alexander et al., 2007; Carvalho et al., 2011). Why we do not see more robust ERG recovery following AAV-mediated Gucy2e expression is unknown. Whether this functional deficit relative to WT is significant in terms of limiting gains in useful vision is yet to be determined, but in behavioral tests, the mice with less than 100% ERG restoration did show a significant improvement (Fig. 3).
Key considerations for applying proof of principle experiments in mice to the clinic include confirming the activity and specificity of the promoter and vector serotype used in as closely related a species to man as possible. Multiple studies have shown that the human rhodopsin kinase (hGRK1) promoter drives efficient and rod/cone-specific transgene expression in mice (Khani et al., 2007; Boye et al., 2010, 2011; Pawlyk et al., 2010; Sun et al., 2010; Mihelec et al., 2011) and most recently in nonhuman primates (Boye et al., 2012). Notably, within the NHP retina, the hGRK1 promoter was active in foveal cones (Boye et al., 2012). Viral serotype choice is dependent upon the route of administration as transduction profiles differ greatly depending on where the vector is deposited (Vandenberghe and Auricchio, 2012). Most photoreceptor-targeted studies have focused on AAV5 and AAV8-based vectors. AAV5 has proven utility following subretinal injection in mouse and dog models of photoreceptor-mediated disease (Min et al., 2005; Alexander et al., 2007; Boye et al., 2010; Gorbatyuk et al., 2010; Komaromy et al., 2010; Pang et al., 2010; Li et al., 2011; Mao et al., 2011; Yao et al., 2011; Beltran et al., 2012; Pang et al., 2012). In addition, AAV5 is the only serotype tested thus far to confer a gain of visual function to photoreceptors of nonhuman primates (Mancuso et al., 2009). Multiple reports also highlight the ability of AAV8-based vectors to provide therapy to rodent models of retinal degeneration (Auricchio, 2011).
We used the AAV8(Y733F) serotype in this study because it was superior to AAV5 (treated ERG responses were higher) in the context of the GC1ko mouse (Boye et al., 2011). Despite this advantage over AAV5 within rodents, a recent study performed in PDE6β mutant dogs indicated that AAV5 and AAV8 were equally suitable for conferring therapy (Petit et al., 2012). Ultimately, the choice of serotype for clinical treatment of LCA1 (and perhaps other photoreceptor-mediated diseases) will depend on its ability to transduce photoreceptors in the primate (and the relative toxicities of the vectors, once tested). To date, very little data exist to compare AAV8- and AAV5-based vector performance in NHP retina. The only reports of AAV8-mediated photoreceptor expression in NHP indicated that AAV8 at (1×1010 vg) did not efficiently transduce foveal, parafoveal, or perifoveal cones following subretinal injection (Vandenberghe et al., 2011a 2011b). In contrast, AAV5 efficiently transduces cones in all these areas of NHP retina at comparable vector concentration (Boye et al., 2012). Hence, assuming a subretinal delivery route, the evidence to date would support the use of AAV5 in conjunction with the GRK1 promoter for driving GC1 expression in a clinical setting.
Acknowledgments
The authors would like to thank James Peterson and Vince Chiodo for their excellent technical assistance. This work was supported by NIH grants EY13729, EY11123, EY08571, RR025777, P30-EY021721, and EY11522, and grants from the CURE Formula Grant from Pennsylvania Department of Health, Macular Vision Research Foundation, Foundation Fighting Blindness, Creed's Cause Foundation, Eldon Family Foundation, Vision for Children, and Research to Prevent Blindness, Inc. Unrestricted grants from Research to Prevent Blindness to State University of New York Upstate Medical University and the Lions of Central New York.
Author Disclosure Statement
W.W.H. and the University of Florida have a financial interest in the use of AAV therapies and own equity in a company (AGTC, Inc.) that might, in the future, commercialize some aspects of this work. The other coauthors have no financial interests to disclose.
References
- Aleman T.S., et al. Human CRB1-associated retinal degeneration: comparison with the rd8 Crb1-mutant mouse model. Invest Ophthalmol.Vis. Sci. 2011;52:6898–6910. doi: 10.1167/iovs.11-7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J.J., et al. Restoration of cone vision in a mouse model of achromatopsia. Nat. Med. 2007;13:685–687. doi: 10.1038/nm1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M., et al. The human visual cortex responds to gene therapy-mediated recovery of retinal function. J. Clin. Invest. 2011;121:2160–2168. doi: 10.1172/JCI57377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auricchio A. Fighting blindness with adeno-associated virus serotype 8. Hum. Gene Ther. 2011;22:1169–1170. doi: 10.1089/hum.2011.2521. [DOI] [PubMed] [Google Scholar]
- Baehr W., et al. The function of guanylate cyclase 1 (GC1) and guanylate cyclase 2 (GC2) in rod and cone photoreceptors. J. Biol. Chem. 2007;282:8837–47. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge J.W., et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Beltran W.A., et al. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc. Natl. Acad. Sci. U.S.A. 2012;109:2132–2137. doi: 10.1073/pnas.1118847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J., et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci. Transl. Med. 2012;4:120ra15. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye S.E., et al. Functional and behavioral restoration of vision by gene therapy in the guanylate cyclase-1 (GC1) knockout mouse. PLoS. One. 2010;5:e11306. doi: 10.1371/journal.pone.0011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye S.L., et al. Long-term preservation of cone photoreceptors and restoration of cone function by gene therapy in the guanylate cyclase-1 knockout (GC1KO) mouse. Invest Ophthalmol. Vis. Sci. 2011;52:7098–7108. doi: 10.1167/iovs.11-7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye S.E., et al. The human rhodopsin kinase promoter in an aav5 vector confers rod- and cone-specific expression in the primate retina. Hum. Gene Ther. 2012;23:1101–15. doi: 10.1089/hum.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns M.E. Arshavsky V.Y. Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron. 2005;48:387–401. doi: 10.1016/j.neuron.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Carvalho L.S. Xu J. Pearson R.A. Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy. Hum. Mol. Genet. 2011;20:3161–3175. doi: 10.1093/hmg/ddr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan A.V., et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. U.S.A. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander A.I. Roepman R. Koenekoop R.K. Cremers F.P. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- D'Hooge R. De Deyn P.P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Dizhoor A.M. Lowe D.G. Olshevskaya E.V., et al. The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron. 1994;12:1345–1352. doi: 10.1016/0896-6273(94)90449-9. [DOI] [PubMed] [Google Scholar]
- Dizhoor A.M. Olshevskaya E.V. Henzel W. J. Cloning, sequencing, and expression of a 24-kDa Ca(2+)-binding protein activating photoreceptor guanylyl cyclase. J. Biol. Chem. 1995;270:25200–25206. doi: 10.1074/jbc.270.42.25200. [DOI] [PubMed] [Google Scholar]
- Douglas R.M. Alam N.M. Silver B.D., et al. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis. Neurosci. 2005;22:677–684. doi: 10.1017/S0952523805225166. [DOI] [PubMed] [Google Scholar]
- Estrada-Cuzcano A., et al. IQCB1 mutations in patients with leber congenital amaurosis. Invest Ophthalmol. Vis. Sci. 2011;52:834–839. doi: 10.1167/iovs.10-5221. [DOI] [PubMed] [Google Scholar]
- Falk M.J., et al. NMNAT1 mutations cause Leber congenital amaurosis. Nat. Genet. 2012;44:1040–1045. doi: 10.1038/ng.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk M.S. Knox T. LaVail M.M., et al. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc. Natl. Acad. Sci. U.S.A. 2010;107:5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haire S.E. Pang J. Boye S.L., et al. Light-driven cone arrestin translocation in cones of postnatal guanylate cyclase-1 knockout mouse retina treated with AAV-GC1. Invest Ophthalmol. Vis. Sci. 2006;47:3745–3753. doi: 10.1167/iovs.06-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanein S., et al. Leber congenital amaurosis: comprehensive survey of the genetic heterogeneity, refinement of the clinical definition, and genotype-phenotype correlations as a strategy for molecular diagnosis. Hum. Mutat. 2004;23:306–317. doi: 10.1002/humu.20010. [DOI] [PubMed] [Google Scholar]
- Hauswirth W.W., et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum. Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.C., et al. RPGR-associated retinal degeneration in human x-linked rp and a murine model. Invest Ophthalmol. Vis. Sci. 2012;53:5594–608. doi: 10.1167/iovs.12-10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S.G., et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: Prerequisite for human gene therapy success. Proc. Natl. Acad. Sci. U.S.A. 2005;102:6177–6182. doi: 10.1073/pnas.0500646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S.G., et al. Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection. Mol. Ther. 2006;13:1074–1084. doi: 10.1016/j.ymthe.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Jacobson S.G., et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch. Ophthalmol. 2012;130:9–24. doi: 10.1001/archophthalmol.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan S. Frederick J.M. Baehr W. Novel functions of photoreceptor guanylate cyclases revealed by targeted deletion. Mol. Cell Biochem. 2010;334:141–155. doi: 10.1007/s11010-009-0322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsell R.E., et al. Mutations in the retinal guanylate cyclase (RETGC-1) gene in dominant cone-rod dystrophy. Hum. Mol. Genet. 1998;7:1179–1184. doi: 10.1093/hmg/7.7.1179. [DOI] [PubMed] [Google Scholar]
- Khani S.C., et al. AAV-mediated expression targeting of rod and cone photoreceptors with a human rhodopsin kinase promoter. Invest Ophthalmol. Vis. Sci. 2007;48:3954–3961. doi: 10.1167/iovs.07-0257. [DOI] [PubMed] [Google Scholar]
- Koenekoop R.K., et al. Mutations in NMNAT1 cause Leber congenital amaurosis and identify a new disease pathway for retinal degeneration. Nat. Genet. 2012;44:1035–1039. doi: 10.1038/ng.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaromy A.M., et al. Gene therapy rescues cone function in congenital achromatopsia. Hum. Mol. Genet. 2010;19:2581–2593. doi: 10.1093/hmg/ddq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., et al. Gene therapy rescues cone structure and function in the 3-month-old rd12 mouse: a model for midcourse RPE65 leber congenital amaurosis. Invest Ophthalmol. Vis. Sci. 2011;52:7–15. doi: 10.1167/iovs.10-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Seno K. Nishizawa Y., et al. Ultrastructural localization of retinal guanylate cyclase in human and monkey retinas. Exp. Eye Res. 1994;59:761–768. doi: 10.1006/exer.1994.1162. [DOI] [PubMed] [Google Scholar]
- Lowe D.G. Dizhoor A.M. Liu K., et al. Cloning and expression of a second photoreceptor-specific membrane retina guanylyl cyclase (RetGC), RetGC-2. Proc. Natl. Acad. Sci. U.S.A. 1995;92:5535–5539. doi: 10.1073/pnas.92.12.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M., et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino C.L. Peshenko I.V. Wen X.H., et al. A role for GCAP2 in regulating the photoresponse. Guanylyl cyclase activation and rod electrophysiology in GUCA1B knock-out mice. J. Biol. Chem. 2008;283:29135–29143. doi: 10.1074/jbc.M804445200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso K. Hauswirth W.W. Li Q., et al. Gene therapy for red-green colour blindness in adult primates. Nature. 2009;461:784–787. doi: 10.1038/nature08401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H. James T., Jr. Schwein A., et al. AAV delivery of wild-type rhodopsin preserves retinal function in a mouse model of autosomal dominant retinitis pigmentosa. Hum. Gene Ther. 2011;22:567–575. doi: 10.1089/hum.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihelec M. Pearson R.A. Robbie S.J., et al. Long-term preservation of cones and improvement in visual function following gene therapy in a mouse model of leber congenital amaurosis caused by guanylate cyclase-1 deficiency. Hum. Gene Ther. 2011;22:1179–1190. doi: 10.1089/hum.2011.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam A.H., et al. Clinicopathologic effects of mutant GUCY2D in Leber congenital amaurosis. Ophthalmology. 2003;110:549–558. doi: 10.1016/S0161-6420(02)01757-8. [DOI] [PubMed] [Google Scholar]
- Min S.H., et al. Prolonged recovery of retinal structure/function after gene therapy in an Rs1h-deficient mouse model of x-linked juvenile retinoschisis. Mol. Ther. 2005;12:644–651. doi: 10.1016/j.ymthe.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Molday L.L. Wu W.W. Molday R.S. Retinoschisin (RS1), the protein encoded by the X-linked retinoschisis gene, is anchored to the surface of retinal photoreceptor and bipolar cells through its interactions with a Na/K ATPase-SARM1 complex. J. Biol. Chem. 2007;282:32792–32801. doi: 10.1074/jbc.M706321200. [DOI] [PubMed] [Google Scholar]
- Olshevskaya E.V. Hughes R.E. Hurley J.B. Dizhoor A.M. Calcium binding, but not a calcium-myristoyl switch, controls the ability of guanylyl cyclase-activating protein GCAP-2 to regulate photoreceptor guanylyl cyclase. J. Biol. Chem. 1997;272:14327–14333. doi: 10.1074/jbc.272.22.14327. [DOI] [PubMed] [Google Scholar]
- Olshevskaya E.V. Calvert P.D. Woodruff M. L., et al. The Y99C mutation in guanylyl cyclase-activating protein 1 increases intracellular Ca2+ and causes photoreceptor degeneration in transgenic mice. J. Neurosci. 2004;24:6078–6085. doi: 10.1523/JNEUROSCI.0963-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshevskaya E.V. Peshenko I.V. Savchenko A.B. Dizhoor A.M. Retinal guanylyl cyclase isozyme 1 is the preferential in vivo target for constitutively active GCAP1 mutants causing congenital degeneration of photoreceptors. J. Neurosci. 2012;32:7208–7217. doi: 10.1523/JNEUROSCI.0976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K., et al. Molecular cloning and characterization of retinal photoreceptor guanylyl cyclase-activating protein. Neuron. 1994;13:395–404. doi: 10.1016/0896-6273(94)90355-7. [DOI] [PubMed] [Google Scholar]
- Pang J.J., et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol. Ther. 2006;13:565–572. doi: 10.1016/j.ymthe.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Pang J.J., et al. AAV-mediated gene therapy for retinal degeneration in the rd10 mouse containing a recessive PDEbeta mutation. Invest Ophthalmol. Vis. Sci. 2008;49:4278–4283. doi: 10.1167/iovs.07-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J., et al. Self-complementary AAV-mediated gene therapy restores cone function and prevents cone degeneration in two models of Rpe65 deficiency. Gene Ther. 2010;17:815–826. doi: 10.1038/gt.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J.J., et al. AAV-mediated cone rescue in a naturally occurring mouse model of CNGA3-achromatopsia. PLoS. One. 2012;7:e35250. doi: 10.1371/journal.pone.0035250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasadhika S. Fishman G.A. Stone E. M., et al. Differential macular morphology in patients with RPE65-, CEP290-, GUCY2D-, and AIPL1-related Leber congenital amaurosis. Invest Ophthalmol. Vis. Sci. 2010;51:2608–2614. doi: 10.1167/iovs.09-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlyk B.S., et al. Replacement gene therapy with a human RPGRIP1 sequence slows photoreceptor degeneration in a murine model of Leber congenital amaurosis. Hum. Gene Ther. 2010;21:993–1004. doi: 10.1089/hum.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault I., et al. Retinal-specific guanylate cyclase gene mutations in Leber's congenital amaurosis. Nat.Genet. 1996;14:461–464. doi: 10.1038/ng1296-461. [DOI] [PubMed] [Google Scholar]
- Perrault I. Rozet J.M. Gerber S., et al. A retGC-1 mutation in autosomal dominant cone-rod dystrophy. Am. J. Hum. Genet. 1998;63:651–654. doi: 10.1086/301985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault I., et al. Leber congenital amaurosis. Mol. Genet. Metab. 1999;68:200–208. doi: 10.1006/mgme.1999.2906. [DOI] [PubMed] [Google Scholar]
- Peshenko I.V. Dizhoor A.M. Ca2+ and Mg2+ binding properties of GCAP-1. Evidence that Mg2+-bound form is the physiological activator of photoreceptor guanylyl cyclase. J. Biol. Chem. 2006;281:23830–23841. doi: 10.1074/jbc.M600257200. [DOI] [PubMed] [Google Scholar]
- Peshenko I.V. Olshevskaya E.V. Savchenko A. B., et al. Enzymatic properties and regulation of the native isozymes of retinal membrane guanylyl cyclase (RetGC) from mouse photoreceptors. Biochemistry. 2011;25:5590–5600. doi: 10.1021/bi200491b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L., et al. Restoration of vision in the pde6beta-deficient dog, a large animal model of rod-cone dystrophy. Mol. Ther. 2012;20:2019–2030. doi: 10.1038/mt.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto F.B. Perrault I. Hicks D., et al. Prenatal human ocular degeneration occurs in Leber's Congenital Amaurosis (LCA1 and 2) Adv. Exp. Med. Biol. 2003;533:59–68. [PubMed] [Google Scholar]
- Roman A.J. Boye S.L. Aleman T.S., et al. Electroretinographic analyses of Rpe65-mutant rd12 mice: developing an in vivo bioassay for human gene therapy trials of Leber congenital amaurosis. Mol. Vis. 2007;13:1701–1710. [PubMed] [Google Scholar]
- Ruggeri M. Wehbe H. Jiao S., et al. In vivo three-dimensional high-resolution imaging of rodent retina with spectral-domain optical coherence tomography. Invest Ophthalmol. Vis. Sci. 2007;48:1808–1814. doi: 10.1167/iovs.06-0815. [DOI] [PubMed] [Google Scholar]
- Simonelli F., et al. Clinical and molecular genetics of Leber's congenital amaurosis: a multicenter study of Italian patients. Invest Ophthalmol. Vis. Sci. 2007;48:4284–4290. doi: 10.1167/iovs.07-0068. [DOI] [PubMed] [Google Scholar]
- Sun X., et al. Gene therapy with a promoter targeting both rods and cones rescues retinal degeneration caused by AIPL1 mutations. Gene Ther. 2010;17:117–131. doi: 10.1038/gt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Timmers A.M. Zhang H. Squitieri A. Gonzalez-Pola C. Subretinal injections in rodent eyes: effects on electrophysiology and histology of rat retina. Mol. Vis. 2001;7:131–137. [PubMed] [Google Scholar]
- Tsien R. Pozzan T. Measurement of cytosolic free Ca2+ with quin2. Methods Enzymol. 1989;172:230–262. doi: 10.1016/s0076-6879(89)72017-6. [DOI] [PubMed] [Google Scholar]
- Ugur Iseri S.A. Durlu Y.K. Tolun A. A novel recessive GUCY2D mutation causing cone-rod dystrophy and not Leber's congenital amaurosis. Eur. J. Hum. Genet. 2010;18:1121–1126. doi: 10.1038/ejhg.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umino Y. Solessio E. Barlow R.B. Speed, spatial, and temporal tuning of rod and cone vision in mouse. J. Neurosci. 2008;28:189–198. doi: 10.1523/JNEUROSCI.3551-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe L.H. Auricchio A. Novel adeno-associated viral vectors for retinal gene therapy. Gene Ther. 2012;19:162–168. doi: 10.1038/gt.2011.151. [DOI] [PubMed] [Google Scholar]
- Vandenberghe L.H., et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci. Transl. Med. 2011a;3:88ra54. doi: 10.1126/scitranslmed.3002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe L.H. Bell P. Maguire A.M., et al. Cone and rod transduction with alternative AAV serotypes in the macula of non-human primates. ARVO; Ft. Lauderdale, FL: 2011b. [Google Scholar]
- Wang H., et al. Mutations in SPATA7 cause Leber congenital amaurosis and juvenile retinitis pigmentosa. Am. J. Hum. Genet. 2009;84:380–387. doi: 10.1016/j.ajhg.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigell-Weber M. Fokstuen S. Torok B., et al. Codons 837 and 838 in the retinal guanylate cyclase gene on chromosome 17p: hot spots for mutations in autosomal dominant cone-rod dystrophy? Arch. Ophthalmol. 2000;118:300. doi: 10.1001/archopht.118.2.300. [DOI] [PubMed] [Google Scholar]
- Wright A.F. Chakarova C.F. Abd El-Aziz M.M. Bhattacharya S.S. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat. Rev. Genet. 2010;11:273–284. doi: 10.1038/nrg2717. [DOI] [PubMed] [Google Scholar]
- Xu J. Molday L.L. Molday R.S. Sarunic M.V. In vivo imaging of the mouse model of X-linked juvenile retinoschisis with fourier domain optical coherence tomography: Invest Ophthalmol.Vis. Sci. 2009;50:2989–2993. doi: 10.1167/iovs.08-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R.B. Foster D.C. Garbers D.L. Fulle H.J. Two membrane forms of guanylyl cyclase found in the eye. Proc. Natl. Acad. Sci. U.S.A. 1995;92:602–606. doi: 10.1073/pnas.92.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R.B. Garbers D.L. Two eye guanylyl cyclases are expressed in the same photoreceptor cells and form homomers in preference to heteromers. J. Biol. Chem. 1997;272:13738–13742. doi: 10.1074/jbc.272.21.13738. [DOI] [PubMed] [Google Scholar]
- Yang R.B. Robinson S.W. Xiong W.H., et al. Disruption of a retinal guanylyl cyclase gene leads to cone-specific dystrophy and paradoxical rod behavior. J. Neurosci. 1999;19:5889–5897. doi: 10.1523/JNEUROSCI.19-14-05889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J. Feathers K.L. Khanna H., et al. XIAP therapy increases survival of transplanted rod precursors in a degenerating host retina. Invest Ophthalmol. Vis. Sci. 2011;52:1567–1572. doi: 10.1167/iovs.10-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin S., et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]