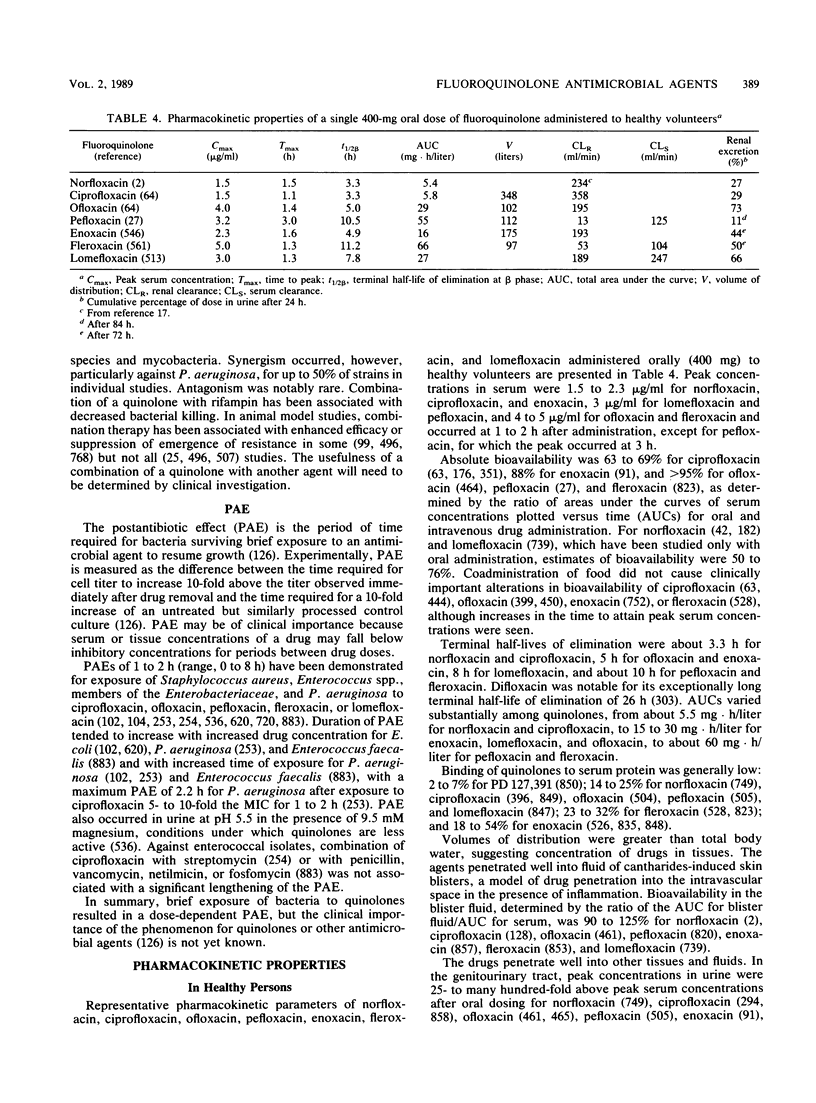
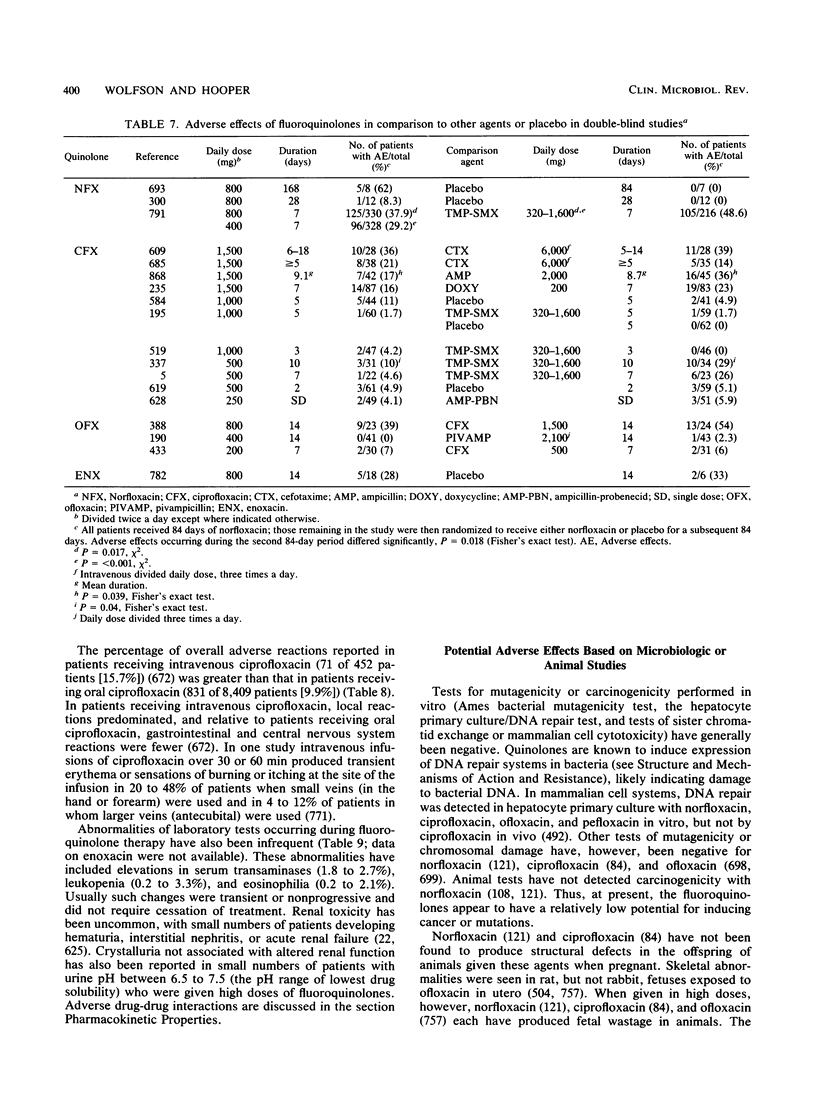
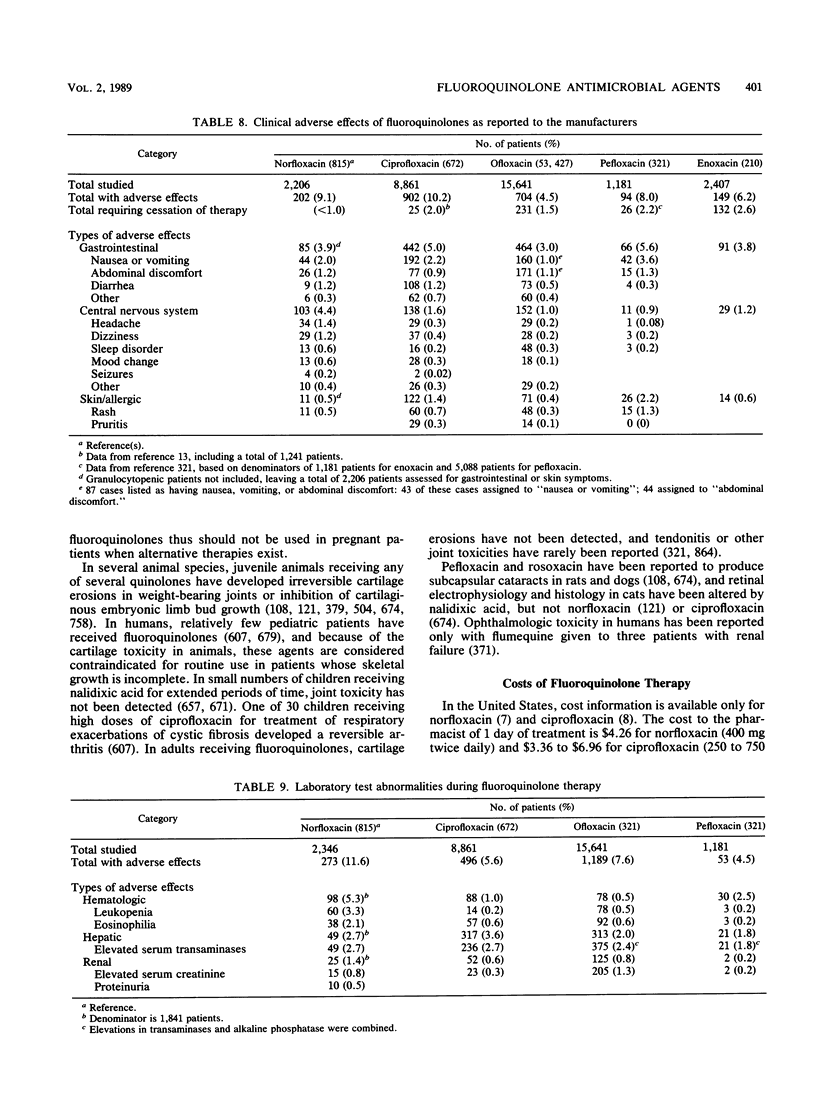
Abstract
The fluoroquinolones, a new class of potent orally absorbed antimicrobial agents, are reviewed, considering structure, mechanisms of action and resistance, spectrum, variables affecting activity in vitro, pharmacokinetic properties, clinical efficacy, emergence of resistance, and tolerability. The primary bacterial target is the enzyme deoxyribonucleic acid gyrase. Bacterial resistance occurs by chromosomal mutations altering deoxyribonucleic acid gyrase and decreasing drug permeation. The drugs are bactericidal and potent in vitro against members of the family Enterobacteriaceae, Haemophilus spp., and Neisseria spp., have good activity against Pseudomonas aeruginosa and staphylococci, and (with several exceptions) are less potent against streptococci and have fair to poor activity against anaerobic species. Potency in vitro decreases in the presence of low pH, magnesium ions, or urine but is little affected by different media, increased inoculum, or serum. The effects of the drugs in combination with a beta-lactam or aminoglycoside are often additive, occasionally synergistic, and rarely antagonistic. The agents are orally absorbed, require at most twice-daily dosing, and achieve high concentrations in urine, feces, and kidney and good concentrations in lung, bone, prostate, and other tissues. The drugs are efficacious in treatment of a variety of bacterial infections, including uncomplicated and complicated urinary tract infections, bacterial gastroenteritis, and gonorrhea, and show promise for therapy of prostatitis, respiratory tract infections, osteomyelitis, and cutaneous infections, particularly when caused by aerobic gram-negative bacilli. Fluoroquinolones have also proved to be efficacious for prophylaxis against travelers' diarrhea and infection with gram-negative bacilli in neutropenic patients. The drugs are effective in eliminating carriage of Neisseria meningitidis. Patient tolerability appears acceptable, with gastrointestinal or central nervous system toxicities occurring most commonly, but only rarely necessitating discontinuance of therapy. In 17 of 18 prospective, randomized, double-blind comparisons with another agent or placebo, fluoroquinolones were tolerated as well as or better than the comparison regimen. Bacterial resistance has been uncommonly documented but occurs, most notably with P. aeruginosa and Staphylococcus aureus and occasionally other species for which the therapeutic ratio is less favorable. Fluoroquinolones offer an efficacious, well-tolerated, and cost-effective alternative to parenteral therapies of selected infections.
Full text
PDF


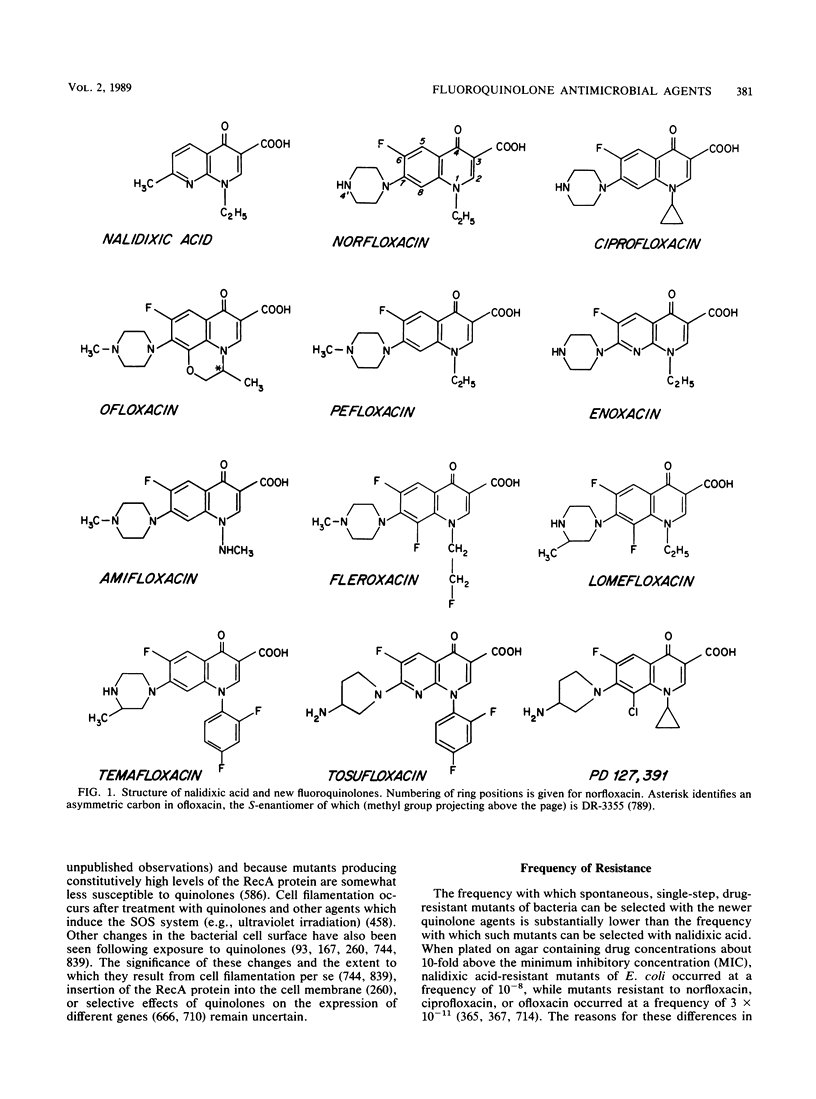


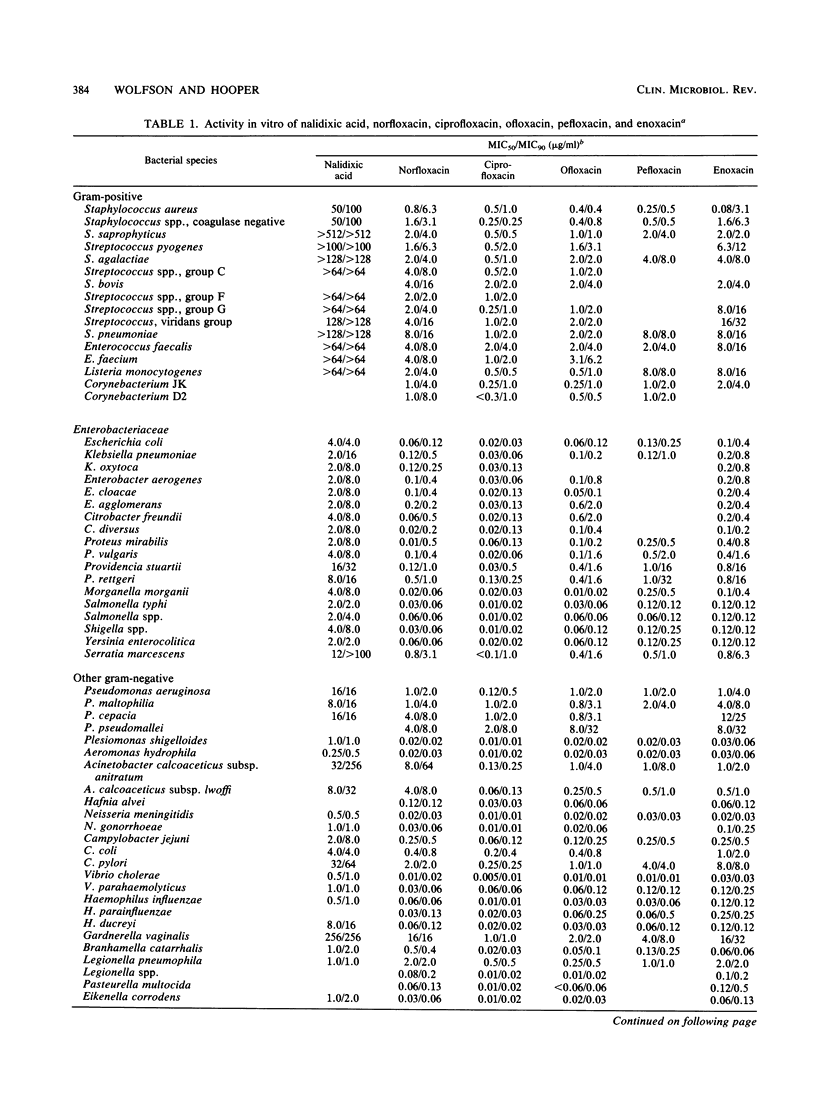
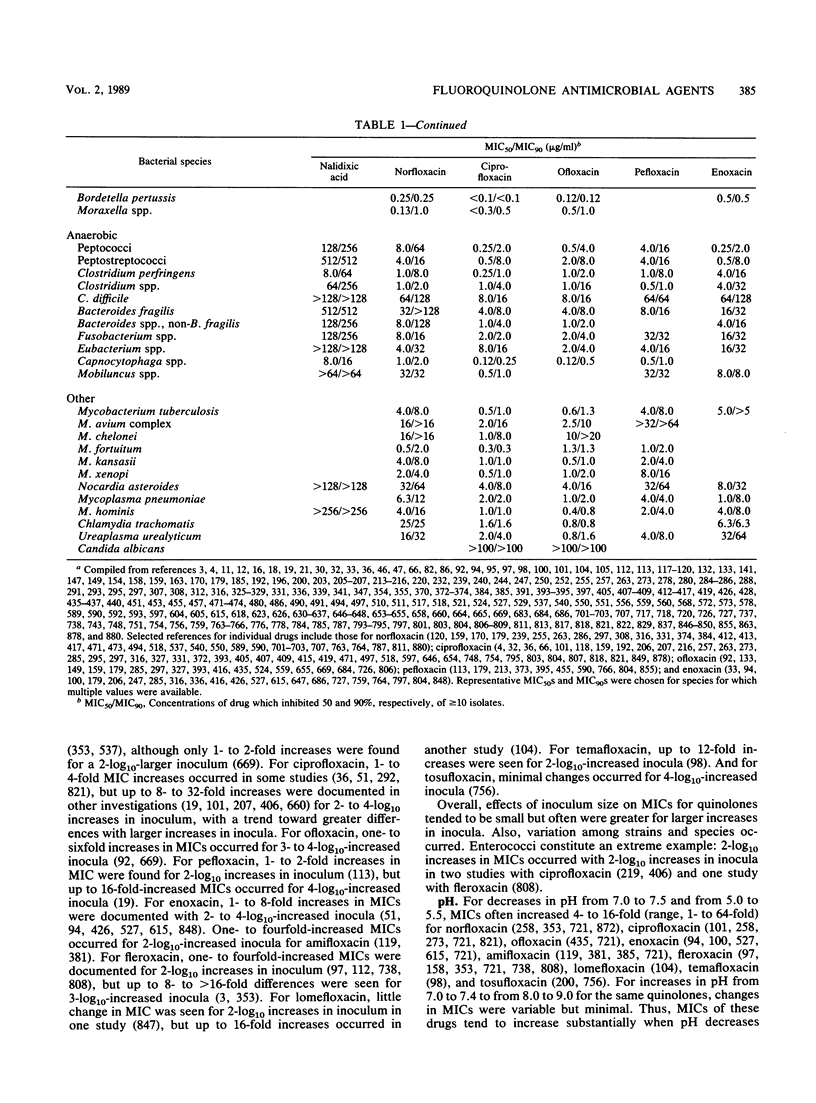
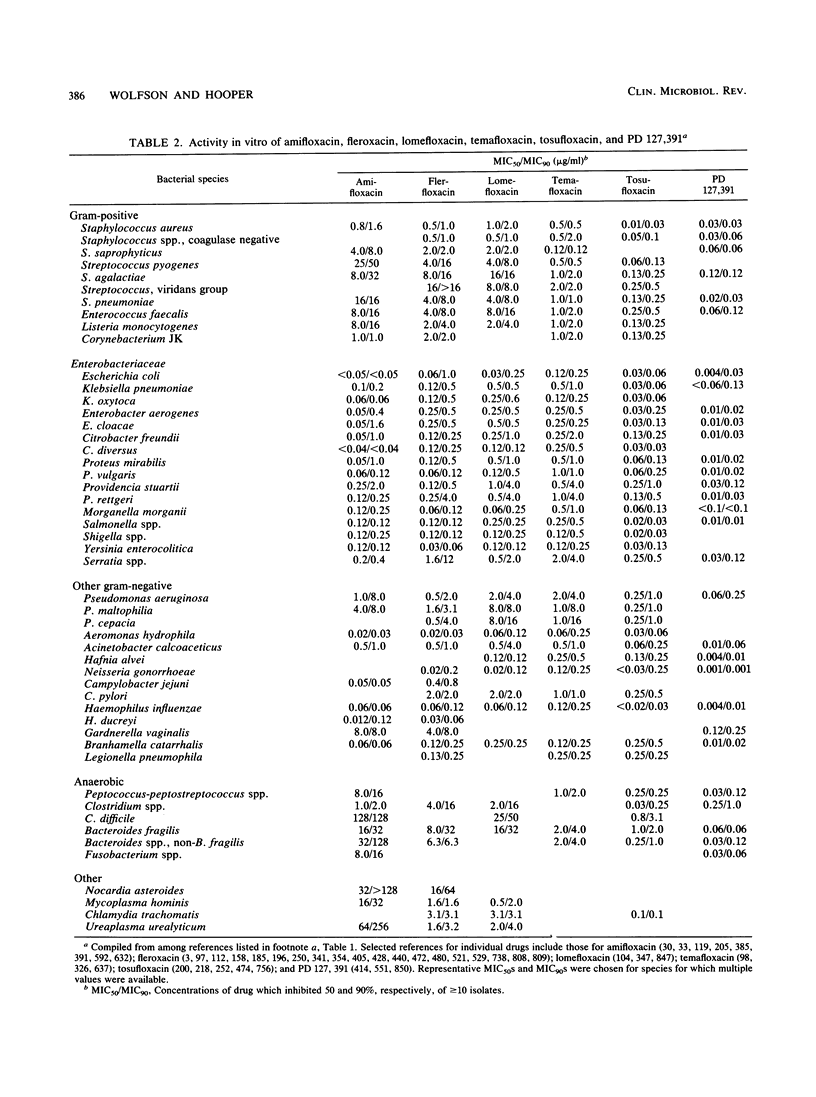



































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhami Z. N., Wise R., Weston D., Crump B. The pharmacokinetics and tissue penetration of norfloxacin. J Antimicrob Chemother. 1984 Jan;13(1):87–92. doi: 10.1093/jac/13.1.87. [DOI] [PubMed] [Google Scholar]
- Aldridge K. E., Schiro D. D., Sanders C. V. RO23-6240, a new orally absorbed quinolone: in vitro comparison with other broad-spectrum oral antimicrobial agents and imipenem. Diagn Microbiol Infect Dis. 1987 May;7(1):9–19. doi: 10.1016/0732-8893(87)90064-2. [DOI] [PubMed] [Google Scholar]
- Aldridge K. E., Valainis G. T., Sanders C. V. Comparison of the in vitro activity of ciprofloxacin and 24 other antimicrobial agents against clinical strains of Chromobacterium violaceum. Diagn Microbiol Infect Dis. 1988 May;10(1):31–39. doi: 10.1016/0732-8893(88)90124-1. [DOI] [PubMed] [Google Scholar]
- Allais J. M., Preheim L. C., Cuevas T. A., Roccaforte J. S., Mellencamp M. A., Bittner M. J. Randomized, double-blind comparison of ciprofloxacin and trimethoprim-sulfamethoxazole for complicated urinary tract infections. Antimicrob Agents Chemother. 1988 Sep;32(9):1327–1330. doi: 10.1128/aac.32.9.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama H., Inoue M., Mitsuhashi S. In-vitro and in-vivo antibacterial activity of fleroxacin, a new fluorinated quinolone. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):99–114. doi: 10.1093/jac/22.supplement_d.99. [DOI] [PubMed] [Google Scholar]
- Aoyama H., Sato K., Fujii T., Fujimaki K., Inoue M., Mitsuhashi S. Purification of Citrobacter freundii DNA gyrase and inhibition by quinolones. Antimicrob Agents Chemother. 1988 Jan;32(1):104–109. doi: 10.1128/aac.32.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum P. C., Spangler S. K., Tamarree T. Susceptibility of 310 nonfermentative gram-negative bacteria to aztreonam, carumonam, ciprofloxacin, ofloxacin and fleroxacin. Chemotherapy. 1988;34(1):40–45. doi: 10.1159/000238546. [DOI] [PubMed] [Google Scholar]
- Appleman M. E., Hadfield T. L., Gaines J. K., Winn R. E. Susceptibility of Bordetella pertussis to five quinolone antimicrobic drugs. Diagn Microbiol Infect Dis. 1987 Oct;8(2):131–133. doi: 10.1016/0732-8893(87)90162-3. [DOI] [PubMed] [Google Scholar]
- Arcieri G., Griffith E., Gruenwaldt G., Heyd A., O'Brien B., Screen P., Becker N., August R. A survey of clinical experience with ciprofloxacin, a new quinolone antimicrobial. J Clin Pharmacol. 1988 Feb;28(2):179–189. doi: 10.1002/j.1552-4604.1988.tb05741.x. [DOI] [PubMed] [Google Scholar]
- Arlet G., Sanson-Le Pors M. J., Casin I. M., Ortenberg M., Perol Y. In vitro susceptibility of 96 Capnocytophaga strains, including a beta-lactamase producer, to new beta-lactam antibiotics and six quinolones. Antimicrob Agents Chemother. 1987 Aug;31(8):1283–1284. doi: 10.1128/aac.31.8.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo G., Cavaliere G., D'Amico G., Passarella E., Broccali G. Pharmacokinetics of norfloxacin in chronic renal failure. Int J Clin Pharmacol Ther Toxicol. 1985 Sep;23(9):491–496. [PubMed] [Google Scholar]
- Ashdown L. R. In vitro activities of the newer beta-lactam and quinolone antimicrobial agents against Pseudomonas pseudomallei. Antimicrob Agents Chemother. 1988 Sep;32(9):1435–1436. doi: 10.1128/aac.32.9.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf S. J., Arya S. C., Parande C. M., Sahay R., Ageel A. R. Anti-delta antibody in primary hepatocellular carcinoma patients in the Gizan area of Saudi Arabia. Infection. 1986 Sep-Oct;14(5):250–251. doi: 10.1007/BF01644273. [DOI] [PubMed] [Google Scholar]
- Auckenthaler R., Michéa-Hamzehpour M., Pechère J. C. In-vitro activity of newer quinolones against aerobic bacteria. J Antimicrob Chemother. 1986 Apr;17 (Suppl B):29–39. doi: 10.1093/jac/17.suppl_b.29. [DOI] [PubMed] [Google Scholar]
- Avent C. K., Krinsky D., Kirklin J. K., Bourge R. C., Figg W. D. Synergistic nephrotoxicity due to ciprofloxacin and cyclosporine. Am J Med. 1988 Sep;85(3):452–453. doi: 10.1016/0002-9343(88)90613-4. [DOI] [PubMed] [Google Scholar]
- Aznar J., Caballero M. C., Lozano M. C., de Miguel C., Palomares J. C., Perea E. J. Activities of new quinoline derivatives against genital pathogens. Antimicrob Agents Chemother. 1985 Jan;27(1):76–78. doi: 10.1128/aac.27.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball A. P., Fox C., Ball M. E., Brown I. R., Willis J. V. Pharmacokinetics of oral ciprofloxacin, 100 mg single dose, in volunteers and elderly patients. J Antimicrob Chemother. 1986 May;17(5):629–635. doi: 10.1093/jac/17.5.629. [DOI] [PubMed] [Google Scholar]
- Ball P. Ciprofloxacin: an overview of adverse experiences. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):187–193. doi: 10.1093/jac/18.sd.187. [DOI] [PubMed] [Google Scholar]
- Ball P. Ciprofloxacin: an overview of adverse experiences. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):187–193. doi: 10.1093/jac/18.sd.187. [DOI] [PubMed] [Google Scholar]
- Baltch A. L., Bassey C., Fanciullo G., Smith R. P. In-vitro antimicrobial activity of enoxacin in combination with eight other antibiotics against Pseudomonas aeruginosa, Enterobacteriaceae and Staphylococcus aureus. J Antimicrob Chemother. 1987 Jan;19(1):45–48. doi: 10.1093/jac/19.1.45. [DOI] [PubMed] [Google Scholar]
- Bamberger D. M., Peterson L. R., Gerding D. N., Moody J. A., Fasching C. E. Ciprofloxacin, azlocillin, ceftizoxime and amikacin alone and in combination against gram-negative bacilli in an infected chamber model. J Antimicrob Chemother. 1986 Jul;18(1):51–63. doi: 10.1093/jac/18.1.51. [DOI] [PubMed] [Google Scholar]
- Barre J., Houin G., Tillement J. P. Dose-dependent pharmacokinetic study of pefloxacin, a new antibacterial agent, in humans. J Pharm Sci. 1984 Oct;73(10):1379–1382. doi: 10.1002/jps.2600731014. [DOI] [PubMed] [Google Scholar]
- Barriere S. L. Economic impact of oral ciprofloxacin. A pharmacist's perspective. Am J Med. 1987 Apr 27;82(4A):387–390. [PubMed] [Google Scholar]
- Barry A. L., Fass R. J., Anhalt J. P., Neu H. C., Thornsberry C., Tilton R. C., Painter B. G., Washington J. A., 2nd Ciprofloxacin disk susceptibility tests: interpretive zone size standards for 5-microgram disks. J Clin Microbiol. 1985 Jun;21(6):880–883. doi: 10.1128/jcm.21.6.880-883.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N. Comparative in vitro activity of amifloxacin and five other fluoroquinolone antimicrobial agents and preliminary criteria for the disk susceptibility test. Eur J Clin Microbiol. 1987 Apr;6(2):179–182. doi: 10.1007/BF02018204. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N. In vitro activity of ciprofloxacin against gram-positive cocci. Am J Med. 1987 Apr 27;82(4A):27–32. [PubMed] [Google Scholar]
- Barry A. L., Jones R. N., Thornsberry C., Ayers L. W., Gerlach E. H., Sommers H. M. Antibacterial activities of ciprofloxacin, norfloxacin, oxolinic acid, cinoxacin, and nalidixic acid. Antimicrob Agents Chemother. 1984 May;25(5):633–637. doi: 10.1128/aac.25.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassey C. M., Baltch A. L., Smith R. P. Comparative antimicrobial activity of enoxacin, ciprofloxacin, amifloxacin, norfloxacin and ofloxacin against 177 bacterial isolates. J Antimicrob Chemother. 1986 May;17(5):623–628. doi: 10.1093/jac/17.5.623. [DOI] [PubMed] [Google Scholar]
- Bates S. A., Elder M. G. An evaluation of pelvic tissue concentrations after oral administration of enoxacin. J Antimicrob Chemother. 1988 Feb;21 (Suppl B):79–85. doi: 10.1093/jac/21.suppl_b.79. [DOI] [PubMed] [Google Scholar]
- Bauernfeind A., Eberlein E., Hörl G. Bactericidal kinetics of various dosages of fleroxacin simulated in bacterial cultures. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):81–89. doi: 10.1093/jac/22.supplement_d.81. [DOI] [PubMed] [Google Scholar]
- Bauernfeind A., Petermüller C. In vitro activity of ciprofloxacin, norfloxacin and nalidixic acid. Eur J Clin Microbiol. 1983 Apr;2(2):111–115. doi: 10.1007/BF02001575. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Blomquist I. K., Kim K. S. Ciprofloxacin in experimental aortic valve endocarditis due to Pseudomonas aeruginosa. J Antimicrob Chemother. 1986 May;17(5):641–649. doi: 10.1093/jac/17.5.641. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Lindsay P., Yih J., Hirano L., Lee D., Blomquist I. K. Efficacy of ciprofloxacin in experimental aortic valve endocarditis caused by a multiply beta-lactam-resistant variant of Pseudomonas aeruginosa stably derepressed for beta-lactamase production. Antimicrob Agents Chemother. 1986 Oct;30(4):528–531. doi: 10.1128/aac.30.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayerdörffer E., Kasper G., Pirlet T., Sommer A., Ottenjann R. Ofloxacin in der Therapie Campylobacter-pylori-positiver Ulcera duodeni. Eine prospektive kontrollierte randomisierte Studie. Dtsch Med Wochenschr. 1987 Sep 11;112(37):1407–1411. doi: 10.1055/s-2008-1068260. [DOI] [PubMed] [Google Scholar]
- Bayerdörffer E., Simon T., Bästlein C., Ottenjann R., Kasper G. Bismuth/ofloxacin combination for duodenal ulcer. Lancet. 1987 Dec 19;2(8573):1467–1468. doi: 10.1016/s0140-6736(87)91169-x. [DOI] [PubMed] [Google Scholar]
- Bedard J., Wong S., Bryan L. E. Accumulation of enoxacin by Escherichia coli and Bacillus subtilis. Antimicrob Agents Chemother. 1987 Sep;31(9):1348–1354. doi: 10.1128/aac.31.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender S. W., Dalhoff A., Shah P. M., Strehl R., Posselt H. G. Ciprofloxacin pharmacokinetics in patients with cystic fibrosis. Infection. 1986 Jan-Feb;14(1):17–21. doi: 10.1007/BF01644804. [DOI] [PubMed] [Google Scholar]
- Bergan T., Dalhoff A., Rohwedder R. Pharmacokinetics of ciprofloxacin. Infection. 1988;16 (Suppl 1):S3–13. doi: 10.1007/BF01650500. [DOI] [PubMed] [Google Scholar]
- Bergeron M. G., Roy R., Lessard C., Foucault P. Enoxacin penetration into human prostatic tissue. Antimicrob Agents Chemother. 1988 Sep;32(9):1433–1434. doi: 10.1128/aac.32.9.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron M. G., Thabet M., Roy R., Lessard C., Foucault P. Norfloxacin penetration into human renal and prostatic tissues. Antimicrob Agents Chemother. 1985 Aug;28(2):349–350. doi: 10.1128/aac.28.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergogne-Bérézin E., Berthelot G., Even P., Stern M., Reynaud P. Penetration of ciprofloxacin into bronchial secretions. Eur J Clin Microbiol. 1986 Apr;5(2):197–200. doi: 10.1007/BF02013986. [DOI] [PubMed] [Google Scholar]
- Berkey P., Moore D., Rolston K. In vitro susceptibilities of Nocardia species to newer antimicrobial agents. Antimicrob Agents Chemother. 1988 Jul;32(7):1078–1079. doi: 10.1128/aac.32.7.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin O. G., Young L. S., Bruckner D. A. In-vitro activity of six fluorinated quinolones against Mycobacterium tuberculosis. J Antimicrob Chemother. 1987 May;19(5):611–615. doi: 10.1093/jac/19.5.611. [DOI] [PubMed] [Google Scholar]
- Berré J., Thys J. P., Husson M., Gangji D., Klastersky J. Penetration of ciprofloxacin in bronchial secretions after intravenous administration. J Antimicrob Chemother. 1988 Oct;22(4):499–504. doi: 10.1093/jac/22.4.499. [DOI] [PubMed] [Google Scholar]
- Blaser J., Dudley M. N., Gilbert D., Zinner S. H. Influence of medium and method on the in vitro susceptibility of Pseudomonas aeruginosa and other bacteria to ciprofloxacin and enoxacin. Antimicrob Agents Chemother. 1986 May;29(5):927–929. doi: 10.1128/aac.29.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Cozzarelli N. R. Use of site-specific recombination as a probe of DNA structure and metabolism in vivo. J Mol Biol. 1987 Mar 20;194(2):205–218. doi: 10.1016/0022-2836(87)90369-x. [DOI] [PubMed] [Google Scholar]
- Bodhidatta L., Taylor D. N., Chitwarakorn A., Kuvanont K., Echeverria P. Evaluation of 500- and 1,000-mg doses of ciprofloxacin for the treatment of chancroid. Antimicrob Agents Chemother. 1988 May;32(5):723–725. doi: 10.1128/aac.32.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerema J. B., Dalhoff A., Debruyne F. M. Ciprofloxacin distribution in prostatic tissue and fluid following oral administration. Chemotherapy. 1985;31(1):13–18. doi: 10.1159/000238308. [DOI] [PubMed] [Google Scholar]
- Boerema J. B., Moesker H. L., Notowicz, Van de Rhee H. J. Fleroxacin (Ro 23-6240) in the treatment of gonorrhoea. J Antimicrob Chemother. 1988 Jan;21(1):140–141. doi: 10.1093/jac/21.1.140. [DOI] [PubMed] [Google Scholar]
- Boerema J. B., Olthof B. J., van Saene H. K. Effects of norfloxacin on the faecal flora in patients with complicated urinary tract infections. Scand J Infect Dis Suppl. 1986;48:27–31. [PubMed] [Google Scholar]
- Boerema J. B., Pauwels R., Scheepers J., Crombach W. Efficacy and safety of pefloxacin in the treatment of patients with complicated urinary tract infections. J Antimicrob Chemother. 1986 Apr;17 (Suppl B):103–109. doi: 10.1093/jac/17.suppl_b.103. [DOI] [PubMed] [Google Scholar]
- Boerema J. B., van Saene H. K. Norfloxacin treatment in complicated urinary tract infection. Scand J Infect Dis Suppl. 1986;48:20–26. [PubMed] [Google Scholar]
- Boerema J., Boll B., Muytjens H., Branolte J. Efficacy and safety of ciprofloxacin (Bay 0 9867) in the treatment of patients with complicated urinary tract infections. J Antimicrob Chemother. 1985 Aug;16(2):211–217. doi: 10.1093/jac/16.2.211. [DOI] [PubMed] [Google Scholar]
- Bogaerts J., Martinez Tello W., Verbist L., Piot P., Vandepitte J. Norfloxacin versus thiamphenicol for treatment of uncomplicated gonorrhea in Rwanda. Antimicrob Agents Chemother. 1987 Mar;31(3):434–437. doi: 10.1128/aac.31.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologna M., Vaggi L., Flammini D., Carlucci G., Forchetti C. M. Norfloxacin in prostatitis: correlation between HPLC tissue concentrations and clinical results. Drugs Exp Clin Res. 1985;11(2):95–100. [PubMed] [Google Scholar]
- Borner K., Höffken G., Lode H., Koeppe P., Prinzing C., Glatzel P., Wiley R., Olschewski P., Sievers B., Reinitz D. Pharmacokinetics of ciprofloxacin in healthy volunteers after oral and intravenous administration. Eur J Clin Microbiol. 1986 Apr;5(2):179–186. doi: 10.1007/BF02013983. [DOI] [PubMed] [Google Scholar]
- Borobio M. V., Perea E. J. Effect of inoculum, pH, and medium on the activity of ciprofloxacin against anaerobic bacteria. Antimicrob Agents Chemother. 1984 Mar;25(3):342–343. doi: 10.1128/aac.25.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch J., Liñares J., López de Goicoechea M. J., Ariza J., Cisnal M. C., Martin R. In-vitro activity of ciprofloxacin, ceftriaxone and five other antimicrobial agents against 95 strains of Brucella melitensis. J Antimicrob Chemother. 1986 Apr;17(4):459–461. doi: 10.1093/jac/17.4.459. [DOI] [PubMed] [Google Scholar]
- Boscia J. A., Kobasa W. D., Kaye D. Comparison of difloxacin, enoxacin, and cefoperazone for treatment of experimental Enterobacter aerogenes endocarditis. Antimicrob Agents Chemother. 1987 Mar;31(3):458–460. doi: 10.1128/aac.31.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscia J. A., Kobasa W. D., Kaye D. Enoxacin compared with cefoperazone for the treatment of experimental Enterobacter aerogenes endocarditis. Antimicrob Agents Chemother. 1985 May;27(5):708–711. doi: 10.1128/aac.27.5.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosso J. A., Black P. G., Matsen J. M. Ciprofloxacin versus tobramycin plus azlocillin in pulmonary exacerbations in adult patients with cystic fibrosis. Am J Med. 1987 Apr 27;82(4A):180–184. [PubMed] [Google Scholar]
- Bourguignon G. J., Levitt M., Sternglanz R. Studies on the mechanism of action of nalidixic acid. Antimicrob Agents Chemother. 1973 Oct;4(4):479–486. doi: 10.1128/aac.4.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bow E. J., Louie T. J., Riben P. D., McNaughton R. D., Harding G. K., Ronald A. R. Randomized controlled trial comparing trimethoprim/sulfamethoxazole and trimethoprim for infection prophylaxis in hospitalized granulocytopenic patients. Am J Med. 1984 Feb;76(2):223–233. doi: 10.1016/0002-9343(84)90777-0. [DOI] [PubMed] [Google Scholar]
- Bow E. J., Rayner E., Louie T. J. Comparison of norfloxacin with cotrimoxazole for infection prophylaxis in acute leukemia. The trade-off for reduced gram-negative sepsis. Am J Med. 1988 May;84(5):847–854. doi: 10.1016/0002-9343(88)90062-9. [DOI] [PubMed] [Google Scholar]
- Bow E. J., Rayner E., Scott B. A., Louie T. J. Selective gut decontamination with nalidixic acid or trimethoprim-sulfamethoxazole for infection prophylaxis in neutropenic cancer patients: relationship of efficacy to antimicrobial spectrum and timing of administration. Antimicrob Agents Chemother. 1987 Apr;31(4):551–557. doi: 10.1128/aac.31.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie W. R., Willetts V., Sibau L. Failure of norfloxacin to eradicate Chlamydia trachomatis in nongonococcal urethritis. Antimicrob Agents Chemother. 1986 Oct;30(4):594–597. doi: 10.1128/aac.30.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles S. K., Popovski Z., Rybak M. J., Beckman H. B., Edwards D. J. Effect of norfloxacin on theophylline pharmacokinetics at steady state. Antimicrob Agents Chemother. 1988 Apr;32(4):510–512. doi: 10.1128/aac.32.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogard J. M., Jehl F., Monteil H., Adloff M., Blickle J. F., Levy P. Comparison of high-pressure liquid chromatography and microbiological assay for the determination of biliary elimination of ciprofloxacin in humans. Antimicrob Agents Chemother. 1985 Aug;28(2):311–314. doi: 10.1128/aac.28.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. M., Morris R., Stephenson T. P. The efficacy and safety of ciprofloxacin in the treatment of chronic Pseudomonas aeruginosa urinary tract infection. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):123–127. doi: 10.1093/jac/18.supplement_d.123. [DOI] [PubMed] [Google Scholar]
- Brumfitt W., Franklin I., Grady D., Hamilton-Miller J. M., Iliffe A. Changes in the pharmacokinetics of ciprofloxacin and fecal flora during administration of a 7-day course to human volunteers. Antimicrob Agents Chemother. 1984 Nov;26(5):757–761. doi: 10.1128/aac.26.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G. R-plasmid transfer and its response to nalidixic acid. J Bacteriol. 1977 Jul;131(1):76–81. doi: 10.1128/jb.131.1.76-81.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante C. I., Drusano G. L., Wharton R. C., Wade J. C. Synergism of the combinations of imipenem plus ciprofloxacin and imipenem plus amikacin against Pseudomonas aeruginosa and other bacterial pathogens. Antimicrob Agents Chemother. 1987 Apr;31(4):632–634. doi: 10.1128/aac.31.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne S. K., Crawford C. E., Geddes G. L., Black W. A. In vitro susceptibilities of Mycobacterium tuberculosis to 10 antimicrobial agents. Antimicrob Agents Chemother. 1988 Sep;32(9):1441–1442. doi: 10.1128/aac.32.9.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Juárez E., Setlow J. K. Gyrase activity and number of copies of the gyrase B subunit gene in Haemophilus influenzae. J Bacteriol. 1985 Nov;164(2):535–538. doi: 10.1128/jb.164.2.535-538.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoli-Richards D. M., Monk J. P., Price A., Benfield P., Todd P. A., Ward A. Ciprofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1988 Apr;35(4):373–447. doi: 10.2165/00003495-198835040-00003. [DOI] [PubMed] [Google Scholar]
- Carbon C., Weber P., Levy M., Boussougant Y., Cerf M. Short-term ciprofloxacin therapy for typhoid fever. J Infect Dis. 1987 Apr;155(4):833–833. doi: 10.1093/infdis/155.4.833. [DOI] [PubMed] [Google Scholar]
- Carlson J. R., Thornton S. A., DuPont H. L., West A. H., Mathewson J. J. Comparative in vitro activities of ten antimicrobial agents against bacterial enteropathogens. Antimicrob Agents Chemother. 1983 Oct;24(4):509–513. doi: 10.1128/aac.24.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter T. C., Hackbarth C. J., Chambers H. F., Sande M. A. Efficacy of ciprofloxacin for experimental endocarditis caused by methicillin-susceptible or -resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Sep;30(3):382–384. doi: 10.1128/aac.30.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkley L. J., Koornhof H. J. Antimicrobial activity of ciprofloxacin against Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus determined by the killing curve method: antibiotic comparisons and synergistic interactions. Antimicrob Agents Chemother. 1985 Aug;28(2):331–342. doi: 10.1128/aac.28.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. C., Oppenheim B. A., Anderson H., Swindell R., Scarffe J. H. Randomized trial comparing ciprofloxacin plus netilmicin versus piperacillin plus netilmicin for empiric treatment of fever in neutropenic patients. Antimicrob Agents Chemother. 1989 Jan;33(1):87–91. doi: 10.1128/aac.33.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T., Black A., Dunky A., Wolf R., Sedman A., Latts J., Welling P. G. Pharmacokinetics of intravenous and oral enoxacin in healthy volunteers. J Antimicrob Chemother. 1988 Feb;21 (Suppl B):49–56. doi: 10.1093/jac/21.suppl_b.49. [DOI] [PubMed] [Google Scholar]
- Chantot J. F., Bryskier A. Antibacterial activity of ofloxacin and other 4-quinolone derivatives: in-vitro and in-vivo comparison. J Antimicrob Chemother. 1985 Oct;16(4):475–484. doi: 10.1093/jac/16.4.475. [DOI] [PubMed] [Google Scholar]
- Chapman J. S., Georgopapadakou N. H. Routes of quinolone permeation in Escherichia coli. Antimicrob Agents Chemother. 1988 Apr;32(4):438–442. doi: 10.1128/aac.32.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrand S. A., Scribner R. K., Weber A. H., Welch D. F., Marks M. I. In vitro activity of CI-919 (AT-2266), an oral antipseudomonal compound. Antimicrob Agents Chemother. 1983 May;23(5):658–663. doi: 10.1128/aac.23.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau P. Y., Ng W. S., Leung Y. K., Lolekha S. In vitro susceptibility of strains of Pseudomonas pseudomallei isolated in Thailand and Hong Kong to some newer beta-lactam antibiotics and quinolone derivatives. J Infect Dis. 1986 Jan;153(1):167–170. doi: 10.1093/infdis/153.1.167. [DOI] [PubMed] [Google Scholar]
- Cherubin C., Stilwell S. Norfloxacin versus parenteral therapy in the treatment of complicated urinary tract infections and resistant organisms. Scand J Infect Dis Suppl. 1986;48:32–37. [PubMed] [Google Scholar]
- Chin N. X., Brittain D. C., Neu H. C. In vitro activity of Ro 23-6240, a new fluorinated 4-quinolone. Antimicrob Agents Chemother. 1986 Apr;29(4):675–680. doi: 10.1128/aac.29.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Figueredo V. M., Novelli A., Neu H. C. In vitro activity of temafloxacin, a new difluoro quinolone antimicrobial agent. Eur J Clin Microbiol Infect Dis. 1988 Feb;7(1):58–63. doi: 10.1007/BF01962176. [DOI] [PubMed] [Google Scholar]
- Chin N. X., Jules K., Neu H. C. Synergy of ciprofloxacin and azlocillin in vitro and in a neutropenic mouse model of infection. Eur J Clin Microbiol. 1986 Feb;5(1):23–28. doi: 10.1007/BF02013456. [DOI] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. Ciprofloxacin, a quinolone carboxylic acid compound active against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1984 Mar;25(3):319–326. doi: 10.1128/aac.25.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. In vitro activity of enoxacin, a quinolone carboxylic acid, compared with those of norfloxacin, new beta-lactams, aminoglycosides, and trimethoprim. Antimicrob Agents Chemother. 1983 Nov;24(5):754–763. doi: 10.1128/aac.24.5.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. Post-antibiotic suppressive effect of ciprofloxacin against gram-positive and gram-negative bacteria. Am J Med. 1987 Apr 27;82(4A):58–62. [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. Synergy of imipenem--a novel carbapenem, and rifampin and ciprofloxacin against Pseudomonas aeruginosa, Serratia marcescens and Enterobacter species. Chemotherapy. 1987;33(3):183–188. doi: 10.1159/000238493. [DOI] [PubMed] [Google Scholar]
- Chin N. X., Novelli A., Neu H. C. In vitro activity of lomefloxacin (SC-47111; NY-198), a difluoroquinolone 3-carboxylic acid, compared with those of other quinolones. Antimicrob Agents Chemother. 1988 May;32(5):656–662. doi: 10.1128/aac.32.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A. W., Cheng N., Bartlett K. H. In vitro susceptibility of Clostridium difficile to new beta-lactam and quinolone antibiotics. Antimicrob Agents Chemother. 1985 Dec;28(6):842–844. doi: 10.1128/aac.28.6.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A. W., Wong J., Bartlett K. H. Synergistic interactions of ciprofloxacin and extended-spectrum beta-lactams or aminoglycosides against multiply drug-resistant Pseudomonas maltophilia. Antimicrob Agents Chemother. 1988 May;32(5):782–784. doi: 10.1128/aac.32.5.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow R. T., Dougherty T. J., Fraimow H. S., Bellin E. Y., Miller M. H. Association between early inhibition of DNA synthesis and the MICs and MBCs of carboxyquinolone antimicrobial agents for wild-type and mutant [gyrA nfxB(ompF) acrA] Escherichia coli K-12. Antimicrob Agents Chemother. 1988 Aug;32(8):1113–1118. doi: 10.1128/aac.32.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysanthopoulos C. J., Skoutelis A. T., Starakis J. C., Arvaniti A., Bassaris H. P. Use of ciprofloxacin in biliary sepsis. Infection. 1988 Jul-Aug;16(4):249–249. doi: 10.1007/BF01650766. [DOI] [PubMed] [Google Scholar]
- Chu D. T., Fernandes P. B. Structure-activity relationships of the fluoroquinolones. Antimicrob Agents Chemother. 1989 Feb;33(2):131–135. doi: 10.1128/aac.33.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. M., Zemcov S. J., Campbell M. E. In-vitro activity of pefloxacin compared to enoxacin, norfloxacin, gentamicin and new beta-lactams. J Antimicrob Chemother. 1985 Jan;15(1):39–44. doi: 10.1093/jac/15.1.39. [DOI] [PubMed] [Google Scholar]
- Clarke A. M., Zemcov S. J. In vitro activity of the new 4-quinolone compound Ro 23-6240. Eur J Clin Microbiol. 1987 Apr;6(2):161–164. doi: 10.1007/BF02018199. [DOI] [PubMed] [Google Scholar]
- Cofsky R. D., duBouchet L., Landesman S. H. Recovery of norfloxacin in feces after administration of a single oral dose to human volunteers. Antimicrob Agents Chemother. 1984 Jul;26(1):110–111. doi: 10.1128/aac.26.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., Hooper D. C., Wolfson J. S., Souza K. S., McMurry L. M., Levy S. B. Endogenous active efflux of norfloxacin in susceptible Escherichia coli. Antimicrob Agents Chemother. 1988 Aug;32(8):1187–1191. doi: 10.1128/aac.32.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., McMurry L. M., Levy S. B. marA locus causes decreased expression of OmpF porin in multiple-antibiotic-resistant (Mar) mutants of Escherichia coli. J Bacteriol. 1988 Dec;170(12):5416–5422. doi: 10.1128/jb.170.12.5416-5422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. H., Uttley A. H. In-vitro susceptibility of mycobacteria to ciprofloxacin. J Antimicrob Chemother. 1985 Nov;16(5):575–580. doi: 10.1093/jac/16.5.575. [DOI] [PubMed] [Google Scholar]
- Cornaglia G., Pompei R., Dainelli B., Satta G. In vitro activity of ciprofloxacin against aerobic bacteria isolated in a southern European hospital. Antimicrob Agents Chemother. 1987 Oct;31(10):1651–1655. doi: 10.1128/aac.31.10.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett J. B., Wagner R. B., Dobson R. A., Wentland M. P., Bailey D. M. In vitro and in vivo antibacterial activities of the fluoroquinolone WIN 49375 (amifloxacin). Antimicrob Agents Chemother. 1985 Jan;27(1):4–10. doi: 10.1128/aac.27.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrado M. L., Cherubin C. E., Shulman M. The comparative activity of norfloxacin with other antimicrobial agents against Gram-positive and Gram-negative bacteria. J Antimicrob Chemother. 1983 Apr;11(4):369–376. doi: 10.1093/jac/11.4.369. [DOI] [PubMed] [Google Scholar]
- Corrado M. L., Struble W. E., Peter C., Hoagland V., Sabbaj J. Norfloxacin: review of safety studies. Am J Med. 1987 Jun 26;82(6B):22–26. doi: 10.1016/0002-9343(87)90614-0. [DOI] [PubMed] [Google Scholar]
- Cox C. E. A comparison of enoxacin and co-trimoxazole in the treatment of patients with complicated urinary tract infections. J Antimicrob Chemother. 1988 Feb;21 (Suppl B):113–118. doi: 10.1093/jac/21.suppl_b.113. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA gyrase and the supercoiling of DNA. Science. 1980 Feb 29;207(4434):953–960. doi: 10.1126/science.6243420. [DOI] [PubMed] [Google Scholar]
- Crawford J., Eye-Boland M. K., Cohen H. J. Clinical utility of erythrocyte sedimentation rate and plasma protein analysis in the elderly. Am J Med. 1987 Feb;82(2):239–246. doi: 10.1016/0002-9343(87)90063-5. [DOI] [PubMed] [Google Scholar]
- Crider S. R., Colby S. D., Miller L. K., Harrison W. O., Kerbs S. B., Berg S. W. Treatment of penicillin-resistant Neisseria gonorrhoeae with oral norfloxacin. N Engl J Med. 1984 Jul 19;311(3):137–140. doi: 10.1056/NEJM198407193110301. [DOI] [PubMed] [Google Scholar]
- Crump B., Wise R., Dent J. Pharmacokinetics and tissue penetration of ciprofloxacin. Antimicrob Agents Chemother. 1983 Nov;24(5):784–786. doi: 10.1128/aac.24.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumplin G. C., Kenwright M., Hirst T. Investigations into the mechanism of action of the antibacterial agent norfloxacin. J Antimicrob Chemother. 1984 May;13 (Suppl B):9–23. doi: 10.1093/jac/13.suppl_b.9. [DOI] [PubMed] [Google Scholar]
- Crumplin G. C., Smith J. T. Nalidixic acid: an antibacterial paradox. Antimicrob Agents Chemother. 1975 Sep;8(3):251–261. doi: 10.1128/aac.8.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullmann W., Stieglitz M., Baars B., Opferkuch W. Comparative evaluation of recently developed quinolone compounds--with a note on the frequency of resistant mutants. Chemotherapy. 1985;31(1):19–28. doi: 10.1159/000238309. [DOI] [PubMed] [Google Scholar]
- Dabernat H., Delmas C., Lareng M. B. Activité de l'ofloxacine sur Haemophilus influenzae, streptococcus pneumoniae et Neisseria meningitidis. Comparaison avec des molécules voisines. Pathol Biol (Paris) 1985 May;33(5):385–388. [PubMed] [Google Scholar]
- Daikos G. L., Kathpalia S. B., Sharifi R., Lolans V. T., Jackson G. G. Comparison of ciprofloxacin and beta-lactam antibiotics in the treatment of urinary tract infections and alteration of fecal flora. Am J Med. 1987 Apr 27;82(4A):290–294. [PubMed] [Google Scholar]
- Daikos G. L., Lolans V. T., Jackson G. G. Alterations in outer membrane proteins of Pseudomonas aeruginosa associated with selective resistance to quinolones. Antimicrob Agents Chemother. 1988 May;32(5):785–787. doi: 10.1128/aac.32.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan M., Golomb J., Gorea A., Braf Z., Berger S. A. Concentration of ciprofloxacin in human prostatic tissue after oral administration. Antimicrob Agents Chemother. 1986 Jul;30(1):88–89. doi: 10.1128/aac.30.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan M., Golomb J., Gorea A., Lindner A., Berger S. A. Penetration of norfloxacin into human prostatic tissue following single-dose oral administration. Chemotherapy. 1987;33(4):240–242. doi: 10.1159/000238501. [DOI] [PubMed] [Google Scholar]
- Dan M., Serour F., Gorea A., Levenberg A., Krispin M., Berger S. A. Concentration of norfloxacin in human gallbladder tissue and bile after single-dose oral administration. Antimicrob Agents Chemother. 1987 Feb;31(2):352–353. doi: 10.1128/aac.31.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan M., Verbin N., Gorea A., Nagar H., Berger S. A. Concentrations of ciprofloxacin in human liver, gallbladder, and bile after oral administration. Eur J Clin Pharmacol. 1987;32(2):217–218. doi: 10.1007/BF00542200. [DOI] [PubMed] [Google Scholar]
- Danan G., Montay G., Cunci R., Erlinger S. Pefloxacin kinetics in cirrhosis. Clin Pharmacol Ther. 1985 Oct;38(4):439–442. doi: 10.1038/clpt.1985.201. [DOI] [PubMed] [Google Scholar]
- Dangor Y., Miller S. D., Exposto F. da L., Koornhof H. J. Antimicrobial susceptibilities of southern African isolates of Haemophilus ducreyi. Antimicrob Agents Chemother. 1988 Sep;32(9):1458–1460. doi: 10.1128/aac.32.9.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daschner F. D., Westenfelder M., Dalhoff A. Penetration of ciprofloxacin into kidney, fat, muscle and skin tissue. Eur J Clin Microbiol. 1986 Apr;5(2):212–213. doi: 10.1007/BF02013992. [DOI] [PubMed] [Google Scholar]
- Davey P. G., Barza M., Stuart M. Dose response of experimental Pseudomonas endophthalmitis to ciprofloxacin, gentamicin, and imipenem: evidence for resistance to "late" treatment of infections. J Infect Dis. 1987 Mar;155(3):518–523. doi: 10.1093/infdis/155.3.518. [DOI] [PubMed] [Google Scholar]
- Davies B. I., Maesen F. P., Baur C. Ciprofloxacin in the treatment of acute exacerbations of chronic bronchitis. Eur J Clin Microbiol. 1986 Apr;5(2):226–231. doi: 10.1007/BF02013995. [DOI] [PubMed] [Google Scholar]
- Davies B. I., Maesen F. P., Teengs J. P. Serum and sputum concentrations of enoxacin after single oral dosing in a clinical and bacteriological study. J Antimicrob Chemother. 1984 Sep;14 (Suppl 100):83–89. doi: 10.1093/jac/14.suppl_c.83. [DOI] [PubMed] [Google Scholar]
- Davies G. S., Cohen J. In-vitro study of the activity of ciprofloxacin alone and in combination against strains of Pseudomonas aeruginosa with multiple antibiotic resistance. J Antimicrob Chemother. 1985 Dec;16(6):713–717. doi: 10.1093/jac/16.6.713. [DOI] [PubMed] [Google Scholar]
- Davies S., Sparham P. D., Spencer R. C. Comparative in-vitro activity of five fluoroquinolones against mycobacteria. J Antimicrob Chemother. 1987 May;19(5):605–609. doi: 10.1093/jac/19.5.605. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Koup J. R., Williams-Warren J., Weber A., Heggen L., Stempel D., Smith A. L. Pharmacokinetics of ciprofloxacin in cystic fibrosis. Antimicrob Agents Chemother. 1987 Jun;31(6):915–919. doi: 10.1128/aac.31.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gruttola V., Mayer K. H. Assessing and modeling heterosexual spread of the human immunodeficiency virus in the United States. Rev Infect Dis. 1988 Jan-Feb;10(1):138–150. doi: 10.1093/clinids/10.1.138. [DOI] [PubMed] [Google Scholar]
- De Lepeleire I., Van Hecken A., Verbesselt R., Tjandra-Maga T. B., De Schepper P. J. Comparative oral pharmacokinetics of fleroxacin and pefloxacin. J Antimicrob Chemother. 1988 Aug;22(2):197–202. doi: 10.1093/jac/22.2.197. [DOI] [PubMed] [Google Scholar]
- De Mol P., Mets T., Lagasse R., Vandepitte J., Mutwewingabo A., Butzler J. P. Treatment of bacillary dysentery: a comparison between enoxacin and nalidixic acid. J Antimicrob Chemother. 1987 May;19(5):695–698. doi: 10.1093/jac/19.5.695. [DOI] [PubMed] [Google Scholar]
- Debbia E., Mannelli S., Gianrossi G., Schito G. C. Susceptibility in vitro of gram-positive aerobe and anaerobe bacteria to ofloxacin. Drugs Exp Clin Res. 1987;13(4):213–217. [PubMed] [Google Scholar]
- Deitz W. H., Cook T. M., Goss W. A. Mechanism of action of nalidixic acid on Escherichia coli. 3. Conditions required for lethality. J Bacteriol. 1966 Feb;91(2):768–773. doi: 10.1128/jb.91.2.768-773.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker A. W., Rozenberg-Arska M., Sixma J. J., Verhoef J. Prevention of infection by trimethoprim-sulfamethoxazole plus amphotericin B in patients with acute nonlymphocytic leukaemia. Ann Intern Med. 1981 Nov;95(5):555–559. doi: 10.7326/0003-4819-95-5-555. [DOI] [PubMed] [Google Scholar]
- Dekker A. W., Rozenberg-Arska M., Verhoef J. Infection prophylaxis in acute leukemia: a comparison of ciprofloxacin with trimethoprim-sulfamethoxazole and colistin. Ann Intern Med. 1987 Jan;106(1):7–11. doi: 10.7326/0003-4819-106-1-7. [DOI] [PubMed] [Google Scholar]
- Dellamonica P., Bernard E., Etesse H., Garraffo R. The diffusion of pefloxacin into bone and the treatment of osteomyelitis. J Antimicrob Chemother. 1986 Apr;17 (Suppl B):93–102. doi: 10.1093/jac/17.suppl_b.93. [DOI] [PubMed] [Google Scholar]
- Delmee M., Avesani V. Comparative in vitro activity of seven quinolones against 100 clinical isolates of Clostridium difficile. Antimicrob Agents Chemother. 1986 Feb;29(2):374–375. doi: 10.1128/aac.29.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplaces N., Gutmann L., Carlet J., Guibert J., Acar J. F. The new quinolones and their combinations with other agents for therapy of severe infections. J Antimicrob Chemother. 1986 Mar;17 (Suppl A):25–39. doi: 10.1093/jac/17.suppl_a.25. [DOI] [PubMed] [Google Scholar]
- Digranes A., Benonisen E., Salveson A., Zahm F. In vitro studies of fleroxacin (Ro 23-6240), a new trifluorinated quinolone derivative. Chemotherapy. 1988;34(5):401–410. doi: 10.1159/000238599. [DOI] [PubMed] [Google Scholar]
- Digranes A., Dibb W. L., Benonisen E. In vitro activities of ciprofloxacin, ofloxacin, norfloxacin and rosoxacin compared with cinoxacin and trimethoprim. Chemotherapy. 1985;31(6):466–471. doi: 10.1159/000238375. [DOI] [PubMed] [Google Scholar]
- Diridl G., Pichler H., Wolf D. Treatment of chronic salmonella carriers with ciprofloxacin. Eur J Clin Microbiol. 1986 Apr;5(2):260–261. doi: 10.1007/BF02014006. [DOI] [PubMed] [Google Scholar]
- Divo A. A., Sartorelli A. C., Patton C. L., Bia F. J. Activity of fluoroquinolone antibiotics against Plasmodium falciparum in vitro. Antimicrob Agents Chemother. 1988 Aug;32(8):1182–1186. doi: 10.1128/aac.32.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs B. R., Gazeley L. R., Campbell A. J., Edwards I. R. The effect of age on the pharmacokinetics of enoxacin. Eur J Clin Pharmacol. 1987;33(1):101–104. doi: 10.1007/BF00610390. [DOI] [PubMed] [Google Scholar]
- Doern G. V., Tubert T. A. In vitro activities of 39 antimicrobial agents for Branhamella catarrhalis and comparison of results with different quantitative susceptibility test methods. Antimicrob Agents Chemother. 1988 Feb;32(2):259–261. doi: 10.1128/aac.32.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagala J. M., Hagen S. E., Heifetz C. L., Hutt M. P., Mich T. F., Sanchez J. P., Trehan A. K. 7-substituted 5-amino-1-cyclopropyl-6,8-difluoro-1,4-dihydro-4-oxo-3- quinolinecarboxylic acids: synthesis and biological activity of a new class of quinolone antibacterials. J Med Chem. 1988 Mar;31(3):503–506. doi: 10.1021/jm00398a003. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Hanna L. D., Heifetz C. L., Hutt M. P., Mich T. F., Sanchez J. P., Solomon M. New structure-activity relationships of the quinolone antibacterials using the target enzyme. The development and application of a DNA gyrase assay. J Med Chem. 1986 Mar;29(3):394–404. doi: 10.1021/jm00153a015. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Heifetz C. L., Hutt M. P., Mich T. F., Nichols J. B., Solomon M., Worth D. F. 1-Substituted 7-[3-[(ethylamino)methyl]-1-pyrrolidinyl]-6,8- difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acids. New quantitative structure-activity relationships at N1 for the quinolone antibacterials. J Med Chem. 1988 May;31(5):991–1001. doi: 10.1021/jm00400a017. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J., Saukkonen J. J. Membrane permeability changes associated with DNA gyrase inhibitors in Escherichia coli. Antimicrob Agents Chemother. 1985 Aug;28(2):200–206. doi: 10.1128/aac.28.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dournon E., Rajagopalan P., Vilde J. L., Pocidalo J. J. Efficacy of pefloxacin in comparison with erythromycin in the treatment of experimental guinea pig legionellosis. J Antimicrob Chemother. 1986 Apr;17 (Suppl B):41–48. doi: 10.1093/jac/17.suppl_b.41. [DOI] [PubMed] [Google Scholar]
- Downs J., Andriole V. T., Ryan J. L. In vitro activity of MK-0366 against clinical urinary pathogens including gentamicin-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Apr;21(4):670–672. doi: 10.1128/aac.21.4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drancourt M., Gallais H., Raoult D., Estrangin E., Mallet M. N., De Micco P. Ofloxacin penetration into cerebrospinal fluid. J Antimicrob Chemother. 1988 Aug;22(2):263–265. doi: 10.1093/jac/22.2.263. [DOI] [PubMed] [Google Scholar]
- Drew R. H., Gallis H. A. Ofloxacin: its pharmacology, pharmacokinetics, and potential for clinical application. Pharmacotherapy. 1988;8(1):35–46. doi: 10.1002/j.1875-9114.1988.tb04063.x. [DOI] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Engle E. C., Manes S. H. DNA gyrase on the bacterial chromosome: possibility of two levels of action. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6879–6883. doi: 10.1073/pnas.77.11.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L. An overview of the pharmacology of intravenously administered ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):339–345. [PubMed] [Google Scholar]
- Drusano G. L., Standiford H. C., Plaisance K., Forrest A., Leslie J., Caldwell J. Absolute oral bioavailability of ciprofloxacin. Antimicrob Agents Chemother. 1986 Sep;30(3):444–446. doi: 10.1128/aac.30.3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L., Weir M., Forrest A., Plaisance K., Emm T., Standiford H. C. Pharmacokinetics of intravenously administered ciprofloxacin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1987 Jun;31(6):860–864. doi: 10.1128/aac.31.6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil L., Devos J., Beerens H., Romond C. Activité in vitro de l'association ofloxacine-métronidazole sur les anaérobies stricts. Cinétique d'action du métronidazole sur Bacteroides fragilis. Pathol Biol (Paris) 1988 May;36(5):488–492. [PubMed] [Google Scholar]
- Dubreuil L., Devos J., Romond C., Bryskier A. Sensibilité des anaérobies stricts envers l'ofloxacine, la péfloxacine, l'enoxacine et la norfloxacine. Pathol Biol (Paris) 1985 May;33(5):421–425. [PubMed] [Google Scholar]
- Dupont H. L., Corrado M. L., Sabbaj J. Use of norfloxacin in the treatment of acute diarrheal disease. Am J Med. 1987 Jun 26;82(6B):79–83. doi: 10.1016/0002-9343(87)90624-3. [DOI] [PubMed] [Google Scholar]
- Dworzack D. L., Sanders C. C., Horowitz E. A., Allais J. M., Sookpranee M., Sanders W. E., Jr, Ferraro F. M. Evaluation of single-dose ciprofloxacin in the eradication of Neisseria meningitidis from nasopharyngeal carriers. Antimicrob Agents Chemother. 1988 Nov;32(11):1740–1741. doi: 10.1128/aac.32.11.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eandi M., Viano I., Di Nola F., Leone L., Genazzani E. Pharmacokinetics of norfloxacin in healthy volunteers and patients with renal and hepatic damage. Eur J Clin Microbiol. 1983 Jun;2(3):253–259. doi: 10.1007/BF02029528. [DOI] [PubMed] [Google Scholar]
- Easmon C. S., Crane J. P. Uptake of ciprofloxacin by human neutrophils. J Antimicrob Chemother. 1985 Jul;16(1):67–73. doi: 10.1093/jac/16.1.67. [DOI] [PubMed] [Google Scholar]
- Easmon C. S., Crane J. P. Uptake of ciprofloxacin by macrophages. J Clin Pathol. 1985 Apr;38(4):442–444. doi: 10.1136/jcp.38.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easmon C. S., Woodford N., Ison C. A. The activity of the 4 quinolone Ro 23 6240 and the cephalosporins Ro 15 8074 and Ro 19 5247 against penicillin sensitive and resistant gonococci. J Antimicrob Chemother. 1987 Jun;19(6):761–765. doi: 10.1093/jac/19.6.761. [DOI] [PubMed] [Google Scholar]
- Edlund C., Bergan T., Josefsson K., Solberg R., Nord C. E. Effect of norfloxacin on human oropharyngeal and colonic microflora and multiple-dose pharmacokinetics. Scand J Infect Dis. 1987;19(1):113–121. doi: 10.3109/00365548709032386. [DOI] [PubMed] [Google Scholar]
- Edlund C., Lidbeck A., Kager L., Nord C. E. Comparative effects of enoxacin and norfloxacin on human colonic microflora. Antimicrob Agents Chemother. 1987 Nov;31(11):1846–1848. doi: 10.1128/aac.31.11.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund C., Nord C. E. A review on the impact of 4-quinolones on the normal oropharyngeal and intestinal human microflora. Infection. 1988;16(1):8–12. doi: 10.1007/BF01646921. [DOI] [PubMed] [Google Scholar]
- Edwards D. J., Bowles S. K., Svensson C. K., Rybak M. J. Inhibition of drug metabolism by quinolone antibiotics. Clin Pharmacokinet. 1988 Sep;15(3):194–204. doi: 10.2165/00003088-198815030-00004. [DOI] [PubMed] [Google Scholar]
- Egede F., Kristensen I. A clinical comparative study of ofloxacin and pivampicillin in acute exacerbations of chronic bronchitis. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):139–142. doi: 10.1093/jac/22.supplement_c.139. [DOI] [PubMed] [Google Scholar]
- Eliopoulos G. M., Gardella A., Moellering R. C., Jr In vitro activity of ciprofloxacin, a new carboxyquinoline antimicrobial agent. Antimicrob Agents Chemother. 1984 Mar;25(3):331–335. doi: 10.1128/aac.25.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston R. A., Taylor J. Possible interaction of ciprofloxacin with cyclosporin A. J Antimicrob Chemother. 1988 May;21(5):679–680. doi: 10.1093/jac/21.5.679. [DOI] [PubMed] [Google Scholar]
- Engle E. C., Manes S. H., Drlica K. Differential effects of antibiotics inhibiting gyrase. J Bacteriol. 1982 Jan;149(1):92–98. doi: 10.1128/jb.149.1.92-98.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson C. D., Johnson P. C., Dupont H. L., Morgan D. R., Bitsura J. A., de la Cabada F. J. Ciprofloxacin or trimethoprim-sulfamethoxazole as initial therapy for travelers' diarrhea. A placebo-controlled, randomized trial. Ann Intern Med. 1987 Feb;106(2):216–220. doi: 10.7326/0003-4819-106-2-216. [DOI] [PubMed] [Google Scholar]
- Ernst F., van der Auwera P. In-vitro activity of fleroxacin (Ro 23-6240), a new fluoro-quinolone, and other agents, against Mycobacterium spp. J Antimicrob Chemother. 1988 Apr;21(4):501–504. doi: 10.1093/jac/21.4.501. [DOI] [PubMed] [Google Scholar]
- Ernst J. A., Sy E. R., Colon-Lucca H., Sandhu N., Rallos T., Lorian V. Ciprofloxacin in the treatment of pneumonia. Antimicrob Agents Chemother. 1986 Jun;29(6):1088–1089. doi: 10.1128/aac.29.6.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L. J., Harvey L., Hixon D. L., Poretz D. M. Ciprofloxacin therapy of infections caused by Pseudomonas aeruginosa and other resistant bacteria. Antimicrob Agents Chemother. 1985 Aug;28(2):308–310. doi: 10.1128/aac.28.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza A. M., Chin N. X., Novelli A., Neu H. C. Comparative in vitro activity of a new fluorinated 4-quinolone, T-3262 (A-60969). Antimicrob Agents Chemother. 1988 May;32(5):663–670. doi: 10.1128/aac.32.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S., Galante D., Bianchi W., Gagliardi R. Efficacy and safety of oral ciprofloxacin in the treatment of respiratory tract infections associated with chronic hepatitis. Am J Med. 1987 Apr 27;82(4A):211–214. [PubMed] [Google Scholar]
- Esposito S., Gupta A., Thadepalli H. In vitro synergy of ciprofloxacin and three other antibiotics against Bacteroides fragilis. Drugs Exp Clin Res. 1987;13(8):489–492. [PubMed] [Google Scholar]
- Etienne J., Coulet M., Brun Y., Blanchon J. F., Demoux F., Fleurette J. Susceptibilities of streptococcal strains associated with infective endocarditis to nine antibiotics. Chemotherapy. 1988;34(2):113–116. doi: 10.1159/000238557. [DOI] [PubMed] [Google Scholar]
- Fairweather N. F., Orr E., Holland I. B. Inhibition of deoxyribonucleic acid gyrase: effects on nucleic acid synthesis and cell division in Escherichia coli K-12. J Bacteriol. 1980 Apr;142(1):153–161. doi: 10.1128/jb.142.1.153-161.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon R. J., Brown W. M. In-vitro sensitivity of legionellas, meningococci and mycoplasmas to ciprofloxacin and enoxacin. J Antimicrob Chemother. 1985 Jun;15(6):787–789. doi: 10.1093/jac/15.6.787. [DOI] [PubMed] [Google Scholar]
- Fallon R. J. In-vitro sensitivity of legionellas and mycoplasmas to amifloxacin. J Antimicrob Chemother. 1988 Mar;21(3):381–382. doi: 10.1093/jac/21.3.381. [DOI] [PubMed] [Google Scholar]
- Fass R. J. Efficacy and safety of oral ciprofloxacin for treatment of serious urinary tract infections. Antimicrob Agents Chemother. 1987 Feb;31(2):148–150. doi: 10.1128/aac.31.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J. Efficacy and safety of oral ciprofloxacin in the treatment of serious respiratory infections. Am J Med. 1987 Apr 27;82(4A):202–207. [PubMed] [Google Scholar]
- Fass R. J., Helsel V. L. In vitro antistaphylococcal activity of pefloxacin alone and in combination with other antistaphylococcal drugs. Antimicrob Agents Chemother. 1987 Oct;31(10):1457–1460. doi: 10.1128/aac.31.10.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J. In vitro activity of ciprofloxacin (Bay o 9867). Antimicrob Agents Chemother. 1983 Oct;24(4):568–574. doi: 10.1128/aac.24.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J. The quinolones. Ann Intern Med. 1985 Mar;102(3):400–402. doi: 10.7326/0003-4819-102-3-400. [DOI] [PubMed] [Google Scholar]
- Fass R. J. Treatment of skin and soft tissue infections with oral ciprofloxacin. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):153–157. doi: 10.1093/jac/18.supplement_d.153. [DOI] [PubMed] [Google Scholar]
- Felmingham D., O'Hare M. D., Robbins M. J., Wall R. A., Williams A. H., Cremer A. W., Ridgway G. L., Grüneberg R. N. Comparative in vitro studies with 4-quinolone antimicrobials. Drugs Exp Clin Res. 1985;11(5):317–329. [PubMed] [Google Scholar]
- Felmingham D., Wall R. A. The comparative activity of twelve 4-quinolone antimicrobials and sulphadiazine against Neisseria meningitidis. Drugs Exp Clin Res. 1985;11(7):427–429. [PubMed] [Google Scholar]
- Fenlon C. H., Cynamon M. H. Comparative in vitro activities of ciprofloxacin and other 4-quinolones against Mycobacterium tuberculosis and Mycobacterium intracellulare. Antimicrob Agents Chemother. 1986 Mar;29(3):386–388. doi: 10.1128/aac.29.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P. B., Chu D. T., Swanson R. N., Ramer N. R., Hanson C. W., Bower R. R., Stamm J. M., Hardy D. J. A-61827 (A-60969), a new fluoronaphthyridine with activity against both aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1988 Jan;32(1):27–32. doi: 10.1128/aac.32.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P. B. Mode of action, and in vitro and in vivo activities of the fluoroquinolones. J Clin Pharmacol. 1988 Feb;28(2):156–168. doi: 10.1002/j.1552-4604.1988.tb05967.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guerrero M., Rouse M. S., Henry N. K., Geraci J. E., Wilson W. R. In vitro and in vivo activity of ciprofloxacin against enterococci isolated from patients with infective endocarditis. Antimicrob Agents Chemother. 1987 Mar;31(3):430–433. doi: 10.1128/aac.31.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Roblas R., Prieto S., Santamaría M., Ponte C., Soriano F. Activity of nine antimicrobial agents against Corynebacterium group D2 strains isolated from clinical specimens and skin. Antimicrob Agents Chemother. 1987 May;31(5):821–822. doi: 10.1128/aac.31.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreccio C., Morris J. G., Jr, Valdivieso C., Prenzel I., Sotomayor V., Drusano G. L., Levine M. M. Efficacy of ciprofloxacin in the treatment of chronic typhoid carriers. J Infect Dis. 1988 Jun;157(6):1235–1239. doi: 10.1093/infdis/157.6.1235. [DOI] [PubMed] [Google Scholar]
- Figueredo V. M., Neu H. C. Synergy of ciprofloxacin with fosfomycin in vitro against Pseudomonas isolates from patients with cystic fibrosis. J Antimicrob Chemother. 1988 Jul;22(1):41–50. doi: 10.1093/jac/22.1.41. [DOI] [PubMed] [Google Scholar]
- Fillastre J. P., Hannedouche T., Leroy A., Humbert G. Pharmacokinetics of norfloxacin in renal failure. J Antimicrob Chemother. 1984 Oct;14(4):439–439. doi: 10.1093/jac/14.4.439. [DOI] [PubMed] [Google Scholar]
- Fillastre J. P., Leroy A., Humbert G. Ofloxacin pharmacokinetics in renal failure. Antimicrob Agents Chemother. 1987 Feb;31(2):156–160. doi: 10.1128/aac.31.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filutowicz M., Jonczyk P. The gyrB gene product functions in both initiation and chain polymerization of Escherichia coli chromosome replication: suppression of the initiation deficiency in gyrB-ts mutants by a class of rpoB mutations. Mol Gen Genet. 1983;191(2):282–287. doi: 10.1007/BF00334827. [DOI] [PubMed] [Google Scholar]
- Filutowicz M. Requirement of DNA gyrase for the initiation of chromosome replication in Escherichia coli K-12. Mol Gen Genet. 1980 Jan;177(2):301–309. doi: 10.1007/BF00267443. [DOI] [PubMed] [Google Scholar]
- Finch R., Whitby M., Craddock C., Holliday A., Martin J., Pilkington R. Clinical evaluation of treatment with ciprofloxacin. Eur J Clin Microbiol. 1986 Apr;5(2):257–259. doi: 10.1007/BF02014005. [DOI] [PubMed] [Google Scholar]
- Fisher L. M., Barot H. A., Cullen M. E. DNA gyrase complex with DNA: determinants for site-specific DNA breakage. EMBO J. 1986 Jun;5(6):1411–1418. doi: 10.1002/j.1460-2075.1986.tb04375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L. M., Mizuuchi K., O'Dea M. H., Ohmori H., Gellert M. Site-specific interaction of DNA gyrase with DNA. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4165–4169. doi: 10.1073/pnas.78.7.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming L. W., Moreland T. A., Scott A. C., Stewart W. K., White L. O. Ciprofloxacin in plasma and peritoneal dialysate after oral therapy in patients on continuous ambulatory peritoneal dialysis. J Antimicrob Chemother. 1987 Apr;19(4):493–503. doi: 10.1093/jac/19.4.493. [DOI] [PubMed] [Google Scholar]
- Fleming L. W., Moreland T. A., Stewart W. K., Scott A. C. Ciprofloxacin and antacids. Lancet. 1986 Aug 2;2(8501):294–294. doi: 10.1016/s0140-6736(86)92120-3. [DOI] [PubMed] [Google Scholar]
- Fliegelman R. M., Petrak R. M., Goodman L. J., Segreti J., Trenholme G. M., Kaplan R. L. Comparative in vitro activities of twelve antimicrobial agents against Campylobacter species. Antimicrob Agents Chemother. 1985 Mar;27(3):429–430. doi: 10.1128/aac.27.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follath F., Bindschedler M., Wenk M., Frei R., Stalder H., Reber H. Use of ciprofloxacin in the treatment of Pseudomonas aeruginosa infections. Eur J Clin Microbiol. 1986 Apr;5(2):236–240. doi: 10.1007/BF02013997. [DOI] [PubMed] [Google Scholar]
- Fong I. W., Ledbetter W. H., Vandenbroucke A. C., Simbul M., Rahm V. Ciprofloxacin concentrations in bone and muscle after oral dosing. Antimicrob Agents Chemother. 1986 Mar;29(3):405–408. doi: 10.1128/aac.29.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong I. W., Linton W., Simbul M., Thorup R., McLaughlin B., Rahm V., Quinn P. A. Treatment of nongonococcal urethritis with ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):311–316. [PubMed] [Google Scholar]
- Fong I. W., Rittenhouse B. R., Simbul M., Vandenbroucke A. C. Bone penetration of enoxacin in patients with and without osteomyelitis. Antimicrob Agents Chemother. 1988 Jun;32(6):834–837. doi: 10.1128/aac.32.6.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong I. W., Vandenbroucke A., Simbul M. Penetration of enoxacin into bronchial secretions. Antimicrob Agents Chemother. 1987 May;31(5):748–751. doi: 10.1128/aac.31.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foot M., Williams G., Want S., Roe M., Quaghebeur G., Bates S. An open study of the safety and efficacy of enoxacin in complicated urinary tract infections. J Antimicrob Chemother. 1988 Feb;21 (Suppl B):97–103. doi: 10.1093/jac/21.suppl_b.97. [DOI] [PubMed] [Google Scholar]
- Forward K. R., Harding G. K., Gray G. J., Urias B. A., Ronald A. R. Comparative activities of norfloxacin and fifteen other antipseudomonal agents against gentamicin-susceptible and -resistant Pseudomonas aeruginosa strains. Antimicrob Agents Chemother. 1983 Oct;24(4):602–604. doi: 10.1128/aac.24.4.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. K., Lentino J. R., Strodtman R., DiVincenzo C. Comparison of in vitro activity of quinolone antibiotics and vancomycin against gentamicin- and methicillin-resistant Staphylococcus aureus by time-kill kinetic studies. Antimicrob Agents Chemother. 1986 Dec;30(6):823–827. doi: 10.1128/aac.30.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. TolF locus in Escherichia coli: chromosomal location and relationship to loci cmlB and tolD. J Bacteriol. 1976 Nov;128(2):604–608. doi: 10.1128/jb.128.2.604-608.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French G. L., Ling J., Ling T., Hui Y. W. Susceptibility of Hong Kong isolates of methicillin-resistant Staphylococcus aureus to antimicrobial agents. J Antimicrob Chemother. 1988 May;21(5):581–588. doi: 10.1093/jac/21.5.581. [DOI] [PubMed] [Google Scholar]
- Frydman A. M., Le Roux Y., Lefebvre M. A., Djebbar F., Fourtillan J. B., Gaillot J. Pharmacokinetics of pefloxacin after repeated intravenous and oral administration (400 mg bid) in young healthy volunteers. J Antimicrob Chemother. 1986 Apr;17 (Suppl B):65–79. doi: 10.1093/jac/17.suppl_b.65. [DOI] [PubMed] [Google Scholar]
- Fu K. P., Grace M. E., McCloud S. J., Gregory F. J., Hung P. P. Discrepancy between the antibacterial activities and the inhibitory effects on Micrococcus luteus DNA gyrase of 13 quinolones. Chemotherapy. 1986;32(6):494–498. doi: 10.1159/000238458. [DOI] [PubMed] [Google Scholar]
- Fuchs P. C., Barry A. L., Jones R. N., Thornsberry C. Evaluation of in vitro antibacterial activity of enoxacin: comparison with other orally absorbed antimicrobial agents, proposed disk diffusion test interpretive criteria, and quality control limits. Diagn Microbiol Infect Dis. 1985 May;3(3):213–221. doi: 10.1016/0732-8893(85)90033-1. [DOI] [PubMed] [Google Scholar]
- Fuchs P. C., Barry A. L., Jones R. N., Thornsberry C. Proposed disk diffusion susceptibility criteria for ofloxacin. J Clin Microbiol. 1985 Aug;22(2):310–311. doi: 10.1128/jcm.22.2.310-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. C., Barry A. L., Jones R. N., Thornsberry C. Tentative disk diffusion susceptibility interpretive criteria for pefloxacin. J Clin Microbiol. 1986 Sep;24(3):448–450. doi: 10.1128/jcm.24.3.448-450.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. C., Jones R. N., Barry A. L., Ayers L. W., Gavan T. L., Gerlach E. H., Thornsberry C. RO 23-6240 (AM-833), a new fluoroquinolone: in vitro antimicrobial activity and tentative disk diffusion interpretive criteria. Diagn Microbiol Infect Dis. 1987 May;7(1):29–35. doi: 10.1016/0732-8893(87)90066-6. [DOI] [PubMed] [Google Scholar]
- Fujimaki K., Noumi T., Saikawa I., Inoue M., Mitsuhashi S. In vitro and in vivo antibacterial activities of T-3262, a new fluoroquinolone. Antimicrob Agents Chemother. 1988 Jun;32(6):827–833. doi: 10.1128/aac.32.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuursted K. Comparative killing activity and postantibiotic effect of streptomycin combined with ampicillin, ciprofloxacin, imipenem, piperacillin or vancomycin against strains of Streptococcus faecalis and Streptococcus faecium. Chemotherapy. 1988;34(3):229–234. doi: 10.1159/000238574. [DOI] [PubMed] [Google Scholar]
- Fuursted K. Post-antibiotic effect of ciprofloxacin on Pseudomonas aeruginosa. Eur J Clin Microbiol. 1987 Jun;6(3):271–274. doi: 10.1007/BF02017611. [DOI] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadebusch H. H., Shungu D. L., Weinberg E., Chung S. K. Comparison of the antibacterial activity of norfloxacin (MK 0366, AM 715), a new organic acid, with that of other orally absorbed chemotherapeutic agents. Infection. 1982 Jan;10(1):41–44. doi: 10.1007/BF01640837. [DOI] [PubMed] [Google Scholar]
- Gahrn-Hansen B., Søgaard P., Arpi M. In vitro activity of ciprofloxacin against methicillin-susceptible and methicillin-resistant staphylococci. Eur J Clin Microbiol. 1987 Oct;6(5):581–584. doi: 10.1007/BF02014254. [DOI] [PubMed] [Google Scholar]
- Galante D., Pennucci C., Esposito S., Barba D. Comparative in vitro activity of ciprofloxacin and five other quinoline derivatives against gram-negative isolates. Drugs Exp Clin Res. 1985;11(5):331–334. [PubMed] [Google Scholar]
- Gargallo D., Moros M., Coll R., Esteve M., Parés J., Xicota M. A., Guinea J. Activity of E-3846, a new fluoroquinolone, in vitro and in experimental cystitis and pyelonephritis in rats. Antimicrob Agents Chemother. 1988 May;32(5):636–641. doi: 10.1128/aac.32.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlando F., Rietiker S., Täuber M. G., Flepp M., Meier B., Lüthy R. Single-dose ciprofloxacin at 100 versus 250 mg for treatment of uncomplicated urinary tract infections in women. Antimicrob Agents Chemother. 1987 Feb;31(2):354–356. doi: 10.1128/aac.31.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey N., St John A. C., Witkin E. M. Evidence for RecA protein association with the cell membrane and for changes in the levels of major outer membrane proteins in SOS-induced Escherichia coli cells. J Bacteriol. 1985 Sep;163(3):870–876. doi: 10.1128/jb.163.3.870-876.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt P. N., Lambert B. E. Single dose ciprofloxacin for the eradication of pharyngeal carriage of Neisseria meningitidis. J Antimicrob Chemother. 1988 Apr;21(4):489–496. doi: 10.1093/jac/21.4.489. [DOI] [PubMed] [Google Scholar]
- Gay J. D., DeYoung D. R., Roberts G. D. In vitro activities of norfloxacin and ciprofloxacin against Mycobacterium tuberculosis, M. avium complex, M. chelonei, M. fortuitum, and M. kansasii. Antimicrob Agents Chemother. 1984 Jul;26(1):94–96. doi: 10.1128/aac.26.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gellert M., Menzel R., Mizuuchi K., O'Dea M. H., Friedman D. I. Regulation of DNA supercoiling in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):763–767. doi: 10.1101/sqb.1983.047.01.087. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A. M., Levy S. B. Amplifiable resistance to tetracycline, chloramphenicol, and other antibiotics in Escherichia coli: involvement of a non-plasmid-determined efflux of tetracycline. J Bacteriol. 1983 Aug;155(2):531–540. doi: 10.1128/jb.155.2.531-540.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A. M., Levy S. B. Gene in the major cotransduction gap of the Escherichia coli K-12 linkage map required for the expression of chromosomal resistance to tetracycline and other antibiotics. J Bacteriol. 1983 Aug;155(2):541–548. doi: 10.1128/jb.155.2.541-548.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Dix B. A., Angehrn P., Wick A., Olson G. L. Monocyclic and tricyclic analogs of quinolones: mechanism of action. Antimicrob Agents Chemother. 1987 Apr;31(4):614–616. doi: 10.1128/aac.31.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos A., Breyer S., Georgopoulos M., Mailer H., Graninger W. In-vitro activity of fleroxacin. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):25–29. doi: 10.1093/jac/22.supplement_d.25. [DOI] [PubMed] [Google Scholar]
- Gevaudan M. J., Mallet M. N., Gulian C., Terriou P., Lagier P., de Micco P. Etude de la sensibilité de sept espèces de mycobactéries aux nouvelles quinolones. Pathol Biol (Paris) 1988 May;36(5):477–481. [PubMed] [Google Scholar]
- Giamarellou H., Daphnis E., Dendrinos C., Daikos G. K. Experience with ciprofloxacin in the treatment of various infections caused mainly by Pseudomonas aeruginosa. Drugs Exp Clin Res. 1985;11(5):351–356. [PubMed] [Google Scholar]
- Giamarellou H., Efstratiou A., Tsagarakis J., Petrikkos G., Daikos G. K. Experience with ciprofloxacin in vitro and in vivo. Arzneimittelforschung. 1984;34(12):1775–1778. [PubMed] [Google Scholar]
- Giamarellou H., Galanakis N., Dendrinos C., Stefanou J., Daphnis E., Daikos G. K. Evaluation of ciprofloxacin in the treatment of Pseudomonas aeruginosa infections. Eur J Clin Microbiol. 1986 Apr;5(2):232–235. doi: 10.1007/BF02013996. [DOI] [PubMed] [Google Scholar]
- Giamarellou H., Petrikkos G. Ciprofloxacin interactions with imipenem and amikacin against multiresistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Jun;31(6):959–961. doi: 10.1128/aac.31.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. N., Tice A. D., Marsh P. K., Craven P. C., Preheim L. C. Oral ciprofloxacin therapy for chronic contiguous osteomyelitis caused by aerobic gram-negative bacilli. Am J Med. 1987 Apr 27;82(4A):254–258. [PubMed] [Google Scholar]
- Gilbert M., Boscia J. A., Kobasa W. D., Kaye D. Enoxacin compared with vancomycin for the treatment of experimental methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1986 Mar;29(3):461–463. doi: 10.1128/aac.29.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie S. H., McEniry D. W., Felmingham D. In-vitro susceptibility of Pseudomonas pseudomallei to DNA gyrase inhibitors. J Antimicrob Chemother. 1987 Oct;20(4):612–614. doi: 10.1093/jac/20.4.612. [DOI] [PubMed] [Google Scholar]
- Gleadhill I. C., Ferguson W. P., Lowry R. C. Efficacy and safety of ciprofloxacin in patients with respiratory infections in comparison with amoxycillin. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):133–138. doi: 10.1093/jac/18.supplement_d.133. [DOI] [PubMed] [Google Scholar]
- Glupczynski Y., Hansen W., Freney J., Yourassowsky E. In vitro susceptibility of Alcaligenes denitrificans subsp. xylosoxidans to 24 antimicrobial agents. Antimicrob Agents Chemother. 1988 Feb;32(2):276–278. doi: 10.1128/aac.32.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glupczynski Y., Labbe M., Burette A., Delmee M., Avesani V., Bruck C. Treatment failure of ofloxacin in Campylobacter pylori infection. Lancet. 1987 May 9;1(8541):1096–1096. doi: 10.1016/s0140-6736(87)90527-7. [DOI] [PubMed] [Google Scholar]
- Goldfarb J., Stern R. C., Reed M. D., Yamashita T. S., Myers C. M., Blumer J. L. Ciprofloxacin monotherapy for acute pulmonary exacerbations of cystic fibrosis. Am J Med. 1987 Apr 27;82(4A):174–179. [PubMed] [Google Scholar]
- Goldstein E. J., Alpert M. L., Najem A., Eng R. H., Ginsburg B. P., Kahn R. M., Cherubin C. E. Norfloxacin in the treatment of complicated and uncomplicated urinary tract infections. A comparative multicenter trial. Am J Med. 1987 Jun 26;82(6B):65–69. doi: 10.1016/0002-9343(87)90621-8. [DOI] [PubMed] [Google Scholar]
- Goldstein E. J., Citron D. M., Bendon L., Vagvolgyi A. E., Trousdale M. D., Appleman M. D. Potential of topical norfloxacin therapy. Comparative in vitro activity against clinical ocular bacterial isolates. Arch Ophthalmol. 1987 Jul;105(7):991–994. doi: 10.1001/archopht.1987.01060070135043. [DOI] [PubMed] [Google Scholar]
- Goldstein E. J., Citron D. M. Comparative activities of cefuroxime, amoxicillin-clavulanic acid, ciprofloxacin, enoxacin, and ofloxacin against aerobic and anaerobic bacteria isolated from bite wounds. Antimicrob Agents Chemother. 1988 Aug;32(8):1143–1148. doi: 10.1128/aac.32.8.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E. J., Citron D. M. Comparative activity of the quinolones against anaerobic bacteria isolated at community hospitals. Antimicrob Agents Chemother. 1985 Apr;27(4):657–659. doi: 10.1128/aac.27.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E. J., Citron D. M., Corrado M. L. Effect of inoculum size on in vitro activity of norfloxacin against fecal anaerobic bacteria. Rationale for selective decontamination of the digestive tract. Am J Med. 1987 Jun 26;82(6B):84–87. doi: 10.1016/0002-9343(87)90625-5. [DOI] [PubMed] [Google Scholar]
- Goldstein E. J., Citron D. M., Vagvolgyi A. E., Gombert M. E. Susceptibility of Eikenella corrodens to newer and older quinolones. Antimicrob Agents Chemother. 1986 Jul;30(1):172–173. doi: 10.1128/aac.30.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E. J., Kahn R. M., Alpert M. L., Ginsberg B. P., Greenway F. L., Citron D. M. Ciprofloxacin versus cinoxacin in therapy of urinary tract infections. A randomized, double-blind trial. Am J Med. 1987 Apr 27;82(4A):284–287. [PubMed] [Google Scholar]
- Golper T. A., Hartstein A. I., Morthland V. H., Christensen J. M. Effects of antacids and dialysate dwell times on multiple-dose pharmacokinetics of oral ciprofloxacin in patients on continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother. 1987 Nov;31(11):1787–1790. doi: 10.1128/aac.31.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombert M. E., Aulicino T. M. Comparison of agar dilution, microtitre broth dilution and tube macrodilution susceptibility testing of ciprofloxacin against several pathogens at two different inocula. J Antimicrob Chemother. 1985 Dec;16(6):709–712. doi: 10.1093/jac/16.6.709. [DOI] [PubMed] [Google Scholar]
- Gombert M. E., Aulicino T. M. Susceptibility of multiply antibiotic-resistant pneumococci to the new quinoline antibiotics, nalidixic acid, coumermycin, and novobiocin. Antimicrob Agents Chemother. 1984 Dec;26(6):933–934. doi: 10.1128/aac.26.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombert M. E., Aulicino T. M., duBouchet L., Berkowitz L. R. Susceptibility of Nocardia asteroides to new quinolones and beta-lactams. Antimicrob Agents Chemother. 1987 Dec;31(12):2013–2014. doi: 10.1128/aac.31.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. A., Uribe F., Moisen S. D., Fuster A. P., Selen A., Welling P. G., Painter B. Multiple-dose pharmacokinetics and safety of ciprofloxacin in normal volunteers. Antimicrob Agents Chemother. 1984 Nov;26(5):741–744. doi: 10.1128/aac.26.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman L. J., Fliegelman R. M., Trenholme G. M., Kaplan R. L. Comparative in vitro activity of ciprofloxacin against Campylobacter spp. and other bacterial enteric pathogens. Antimicrob Agents Chemother. 1984 Apr;25(4):504–506. doi: 10.1128/aac.25.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goormans E., Dalhoff A., Kazzaz B., Branolte J. Penetration of ciprofloxacin into gynecological tissues following oral and intravenous administration. Chemotherapy. 1986;32(1):7–17. doi: 10.1159/000238383. [DOI] [PubMed] [Google Scholar]
- Goossens H., De Mol P., Coignau H., Levy J., Grados O., Ghysels G., Innocent H., Butzler J. P. Comparative in vitro activities of aztreonam, ciprofloxacin, norfloxacin, ofloxacin, HR 810 (a new cephalosporin), RU28965 (a new macrolide), and other agents against enteropathogens. Antimicrob Agents Chemother. 1985 Mar;27(3):388–392. doi: 10.1128/aac.27.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotuzzo E., Guerra J. G., Benavente L., Palomino J. C., Carrillo C., Lopera J., Delgado F., Nalin D. R., Sabbaj J. Use of norfloxacin to treat chronic typhoid carriers. J Infect Dis. 1988 Jun;157(6):1221–1225. doi: 10.1093/infdis/157.6.1221. [DOI] [PubMed] [Google Scholar]
- Grabe M., Forsgren A., Björk T., Hellsten S. Controlled trial of a short and a prolonged course with ciprofloxacin in patients undergoing transurethral prostatic surgery. Eur J Clin Microbiol. 1987 Feb;6(1):11–17. doi: 10.1007/BF02097183. [DOI] [PubMed] [Google Scholar]
- Granneman G. R., Snyder K. M., Shu V. S. Difloxacin metabolism and pharmacokinetics in humans after single oral doses. Antimicrob Agents Chemother. 1986 Nov;30(5):689–693. doi: 10.1128/aac.30.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R. N., Kennedy D. J., Reilly P. M., Luppen K. L., Weinandt W. J., Bollinger M. R., Aguirre F., Kodesch F., Saeed A. M. Treatment of bone, joint, and soft-tissue infections with oral ciprofloxacin. Antimicrob Agents Chemother. 1987 Feb;31(2):151–155. doi: 10.1128/aac.31.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R. N., Tice A. D., Marsh P. K., Craven P. C., Reilly P. M., Bollinger M., Weinandt W. J. Randomized trial of ciprofloxacin compared with other antimicrobial therapy in the treatment of osteomyelitis. Am J Med. 1987 Apr 27;82(4A):266–269. [PubMed] [Google Scholar]
- Greenwood D., Laverick A. Activities of newer quinolones against Legionella group organisms. Lancet. 1983 Jul 30;2(8344):279–280. doi: 10.1016/s0140-6736(83)90257-x. [DOI] [PubMed] [Google Scholar]
- Greenwood D., Osman M., Goodwin J., Cowlishaw W. A., Slack R. Norfloxacin: activity against urinary tract pathogens and factors influencing the emergence of resistance. J Antimicrob Chemother. 1984 Apr;13(4):315–323. doi: 10.1093/jac/13.4.315. [DOI] [PubMed] [Google Scholar]
- Gregoire S. L., Grasela T. H., Jr, Freer J. P., Tack K. J., Schentag J. J. Inhibition of theophylline clearance by coadministered ofloxacin without alteration of theophylline effects. Antimicrob Agents Chemother. 1987 Mar;31(3):375–378. doi: 10.1128/aac.31.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs D. J., Wise R., Kirkpatrick B., Ashby J. P. The metabolism and pharmacokinetics of fleroxacin in healthy subjects. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):191–194. doi: 10.1093/jac/22.supplement_d.191. [DOI] [PubMed] [Google Scholar]
- Grimm H. In vitro study with ciprofloxacin: interpretive criteria of agar diffusion test according to standards of the NCCLS and DIN. Am J Med. 1987 Apr 27;82(4A):376–380. [PubMed] [Google Scholar]
- Grimm H. In-vitro-Aktivität von sieben Gyrase-Hemmern aus der Gruppe heterozyklischer Carbonsäuren gegen nichtfermentative gramnegative Stäbchen (Nonfermenter). Arzneimittelforschung. 1985;35(3):570–572. [PubMed] [Google Scholar]
- Guay D. R., Awni W. M., Peterson P. K., Obaid S., Breitenbucher R., Matzke G. R. Pharmacokinetics of ciprofloxacin in acutely ill and convalescent elderly patients. Am J Med. 1987 Apr 27;82(4A):124–129. [PubMed] [Google Scholar]
- Guelpa-Lauras C. C., Perani E. G., Giroir A. M., Grosset J. H. Activities of pefloxacin and ciprofloxacin against Mycobacterium leprae in the mouse. Int J Lepr Other Mycobact Dis. 1987 Mar;55(1):70–77. [PubMed] [Google Scholar]
- Guibert J., Destrée D., Konopka C., Acar J. Ciprofloxacin in the treatment of urinary tract infection due to enterobacteria. Eur J Clin Microbiol. 1986 Apr;5(2):247–248. doi: 10.1007/BF02014000. [DOI] [PubMed] [Google Scholar]
- Guimaraes M. A., Noone P. The comparative in-vitro activity of norfloxacin, ciprofloxacin, enoxacin and nalidixic acid against 423 strains of gram-negative rods and staphylococci isolated from infected hospitalised patients. J Antimicrob Chemother. 1986 Jan;17(1):63–67. doi: 10.1093/jac/17.1.63. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Billot-Klein D., Williamson R., Goldstein F. W., Mounier J., Acar J. F., Collatz E. Mutation of Salmonella paratyphi A conferring cross-resistance to several groups of antibiotics by decreased permeability and loss of invasiveness. Antimicrob Agents Chemother. 1988 Feb;32(2):195–201. doi: 10.1128/aac.32.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Moreau N., Kitzis M. D., Collatz E., Acar J. F., Goldstein F. W. Cross-resistance to nalidixic acid, trimethoprim, and chloramphenicol associated with alterations in outer membrane proteins of Klebsiella, Enterobacter, and Serratia. J Infect Dis. 1985 Mar;151(3):501–507. doi: 10.1093/infdis/151.3.501. [DOI] [PubMed] [Google Scholar]
- Hackbarth C. J., Chambers H. F., Sande M. A. Serum bactericidal activity of rifampin in combination with other antimicrobial agents against Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Apr;29(4):611–613. doi: 10.1128/aac.29.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajji M., el Mdaghri N., Benbachir M., el Filali K. M., Himmich H. Prospective randomized comparative trial of pefloxacin versus cotrimoxazole in the treatment of typhoid fever in adults. Eur J Clin Microbiol Infect Dis. 1988 Jun;7(3):361–363. doi: 10.1007/BF01962337. [DOI] [PubMed] [Google Scholar]
- Haller I. Comprehensive evaluation of ciprofloxacin-aminoglycoside combinations against Enterobacteriaceae and Pseudomonas aeruginosa strains. Antimicrob Agents Chemother. 1985 Nov;28(5):663–666. doi: 10.1128/aac.28.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handsfield H. H., Judson F. N., Holmes K. K. Treatment of uncomplicated gonorrhea with rosoxacin. Antimicrob Agents Chemother. 1981 Nov;20(5):625–629. doi: 10.1128/aac.20.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D. J., Hanson C. W., Hensey D. M., Beyer J. M., Fernandes P. B. Susceptibility of Campylobacter pylori to macrolides and fluoroquinolones. J Antimicrob Chemother. 1988 Nov;22(5):631–636. doi: 10.1093/jac/22.5.631. [DOI] [PubMed] [Google Scholar]
- Hardy D. J., Swanson R. N., Hensey D. M., Ramer N. R., Bower R. R., Hanson C. W., Chu D. T., Fernandes P. B. Comparative antibacterial activities of temafloxacin hydrochloride (A-62254) and two reference fluoroquinolones. Antimicrob Agents Chemother. 1987 Nov;31(11):1768–1774. doi: 10.1128/aac.31.11.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartinger A., Korting H. C., Neubert U., Blaufuss H. In-Vitro-Wirksamkeit von Ofloxacin und Ciprofloxacin bei genitalen Chlamydia trachomatis-Isolaten gemessen an minimaler Hemmkonzentration (MHK) und minimaler bakterizider Konzentration (MBK). Z Hautkr. 1988 Jan 18;63(1):29–32. [PubMed] [Google Scholar]
- Hawkey P. M., Hawkey C. A. Comparative in-vitro activity of quinolone carboxylic acids against Proteeae. J Antimicrob Chemother. 1984 Nov;14(5):485–489. doi: 10.1093/jac/14.5.485. [DOI] [PubMed] [Google Scholar]
- Hawkey P. M., Smith S. D., Haynes J., Malnick H., Forlenza S. W. In vitro susceptibility of Capnocytophaga species to antimicrobial agents. Antimicrob Agents Chemother. 1987 Feb;31(2):331–332. doi: 10.1128/aac.31.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa I., Atarashi S., Yokohama S., Imamura M., Sakano K., Furukawa M. Synthesis and antibacterial activities of optically active ofloxacin. Antimicrob Agents Chemother. 1986 Jan;29(1):163–164. doi: 10.1128/aac.29.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heessen F. W., Muytjens H. L. In vitro activities of ciprofloxacin, norfloxacin, pipemidic acid, cinoxacin, and nalidixic acid against Chlamydia trachomatis. Antimicrob Agents Chemother. 1984 Jan;25(1):123–124. doi: 10.1128/aac.25.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets L. B., Iseman M. D., Lindholm-Levy P. J. Determination of MICs of conventional and experimental drugs in liquid medium by the radiometric method against Mycobacterium avium complex. Drugs Exp Clin Res. 1987;13(9):529–538. [PubMed] [Google Scholar]
- Heifets L. B., Lindholm-Levy P. J. Bacteriostatic and bactericidal activity of ciprofloxacin and ofloxacin against Mycobacterium tuberculosis and Mycobacterium avium complex. Tubercle. 1987 Dec;68(4):267–276. doi: 10.1016/0041-3879(87)90067-5. [DOI] [PubMed] [Google Scholar]
- Heifets L. Qualitative and quantitative drug-susceptibility tests in mycobacteriology. Am Rev Respir Dis. 1988 May;137(5):1217–1222. doi: 10.1164/ajrccm/137.5.1217. [DOI] [PubMed] [Google Scholar]
- Heifetz C. L., Bien P. A., Cohen M. A., Dombrowski M. E., Griffin T. J., Malta T. E., Sesnie J. C., Shapiro M. A., Wold S. A. Enoxacin: in-vitro and animal evaluation as a parenteral and oral agent against hospital bacterial isolates. J Antimicrob Chemother. 1988 Feb;21 (Suppl B):29–42. doi: 10.1093/jac/21.suppl_b.29. [DOI] [PubMed] [Google Scholar]
- Henry D., Skidmore A. G., Ngui-Yen J., Smith A., Smith J. A. In vitro activities of enoxacin, ticarcillin plus clavulanic acid, aztreonam, piperacillin, and imipenem and comparison with commonly used antimicrobial agents. Antimicrob Agents Chemother. 1985 Aug;28(2):259–264. doi: 10.1128/aac.28.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry N. K., Schultz H. J., Grubbs N. C., Muller S. M., Ilstrup D. M., Wilson W. R. Comparison of ciprofloxacin and co-trimoxazole in the treatment of uncomplicated urinary tract infection in women. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):103–106. doi: 10.1093/jac/18.supplement_d.103. [DOI] [PubMed] [Google Scholar]
- Henwood J. M., Monk J. P. Enoxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1988 Jul;36(1):32–66. doi: 10.2165/00003495-198836010-00004. [DOI] [PubMed] [Google Scholar]
- Heppleston C., Richmond S., Bailey J. Antichlamydial activity of quinolone carboxylic acids. J Antimicrob Chemother. 1985 May;15(5):645–647. doi: 10.1093/jac/15.5.645. [DOI] [PubMed] [Google Scholar]
- Hessen M. T., Ingerman M. J., Kaufman D. H., Weiner P., Santoro J., Korzeniowski O. M., Boscia J., Topiel M., Bush L. M., Kaye D. Clinical efficacy of ciprofloxacin therapy for gram-negative bacillary osteomyelitis. Am J Med. 1987 Apr 27;82(4A):262–265. [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Hosaka M., Oomori Y., Niwata Y., Suzue S., Irikura T. In vitro and in vivo antibacterial activity of AM-833, a new quinolone derivative. Antimicrob Agents Chemother. 1986 Jun;29(6):1059–1066. doi: 10.1128/aac.29.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Isolation and characterization of norfloxacin-resistant mutants of Escherichia coli K-12. Antimicrob Agents Chemother. 1986 Aug;30(2):248–253. doi: 10.1128/aac.30.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Apr;31(4):582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T., Okezaki E., Kato H., Ito Y., Inoue M., Mitsuhashi S. In vitro and in vivo activity of NY-198, a new difluorinated quinolone. Antimicrob Agents Chemother. 1987 Jun;31(6):854–859. doi: 10.1128/aac.31.6.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho G., Tierney M. G., Dales R. E. Evaluation of the effect of norfloxacin on the pharmacokinetics of theophylline. Clin Pharmacol Ther. 1988 Jul;44(1):35–38. doi: 10.1038/clpt.1988.109. [DOI] [PubMed] [Google Scholar]
- Hodson M. E., Roberts C. M., Butland R. J., Smith M. J., Batten J. C. Oral ciprofloxacin compared with conventional intravenous treatment for Pseudomonas aeruginosa infection in adults with cystic fibrosis. Lancet. 1987 Jan 31;1(8527):235–237. doi: 10.1016/s0140-6736(87)90062-6. [DOI] [PubMed] [Google Scholar]
- Hohl P., Felber A. M. Effect of method, medium, pH and inoculum on the in-vitro antibacterial activities of fleroxacin and norfloxacin. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):71–80. doi: 10.1093/jac/22.supplement_d.71. [DOI] [PubMed] [Google Scholar]
- Hohl P., Salfinger M., Kafader F. M. In vitro activity of the new quinolone RO 23-6240 (AM-833) and the new cephalosporins RO 15-8074 and RO 19-5247 (T-2525) against Mycobacterium fortuitum and Mycobacterium chelonae. Eur J Clin Microbiol. 1987 Aug;6(4):487–488. doi: 10.1007/BF02013118. [DOI] [PubMed] [Google Scholar]
- Hohl P., von Graevenitz A., Zollinger-Iten J. Fleroxacin (Ro 23-6240): activity in vitro against 355 enteropathogenic and non-fermentative gram-negative bacilli and Legionella pneumophila. J Antimicrob Chemother. 1987 Sep;20(3):373–378. doi: 10.1093/jac/20.3.373. [DOI] [PubMed] [Google Scholar]
- Holmes B., Brogden R. N., Richards D. M. Norfloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1985 Dec;30(6):482–513. doi: 10.2165/00003495-198530060-00003. [DOI] [PubMed] [Google Scholar]
- Hoogkamp-Korstanje J. A. Antibiotics in Yersinia enterocolitica infections. J Antimicrob Chemother. 1987 Jul;20(1):123–131. doi: 10.1093/jac/20.1.123. [DOI] [PubMed] [Google Scholar]
- Hoogkamp-Korstanje J. A., Klein S. J. Ciprofloxacin in acute exacerbations of chronic bronchitis. J Antimicrob Chemother. 1986 Sep;18(3):407–413. doi: 10.1093/jac/18.3.407. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., McHugh G. L., Swartz M. D., Tung C., Swartz M. N. Elimination of plasmid pMG110 from Escherichia coli by novobiocin and other inhibitors of DNA gyrase. Antimicrob Agents Chemother. 1984 May;25(5):586–590. doi: 10.1128/aac.25.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Ng E. Y., Swartz M. N. Mechanisms of action of and resistance to ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):12–20. [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Ng E. Y., McHugh G. L., Swartz M. N. Mechanisms of quinolone resistance in Escherichia coli: characterization of nfxB and cfxB, two mutant resistance loci decreasing norfloxacin accumulation. Antimicrob Agents Chemother. 1989 Mar;33(3):283–290. doi: 10.1128/aac.33.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. The fluoroquinolones: pharmacology, clinical uses, and toxicities in humans. Antimicrob Agents Chemother. 1985 Nov;28(5):716–721. doi: 10.1128/aac.28.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf G., Böcker R., Estler C. J., Radtke H. J., Floh W. Concentration of ciprofloxacin in human serum, lung and pleural tissues and fluids during and after lung surgery. Infection. 1988;16(1):29–30. doi: 10.1007/BF01646928. [DOI] [PubMed] [Google Scholar]
- Hopf G., Böcker R., Estler C. J., Radtke H. J., Floh W. Concentration of ciprofloxacin in human serum, lung and pleural tissues and fluids during and after lung surgery. Infection. 1988;16(1):29–30. doi: 10.1007/BF01646928. [DOI] [PubMed] [Google Scholar]
- Horowitz D. S., Wang J. C. Mapping the active site tyrosine of Escherichia coli DNA gyrase. J Biol Chem. 1987 Apr 15;262(11):5339–5344. [PubMed] [Google Scholar]
- Hughes W. T. A tribute to toilet paper. Rev Infect Dis. 1988 Jan-Feb;10(1):218–222. doi: 10.1093/clinids/10.1.218. [DOI] [PubMed] [Google Scholar]
- Hupertz V., Carr H., Czinn S. J. Susceptibility of Campylobacter pylori isolated from pediatric and adult patients to seven new quinolone antibiotics and nalidixic acid. Chemotherapy. 1988;34(4):341–344. doi: 10.1159/000238589. [DOI] [PubMed] [Google Scholar]
- Hurault de Ligny B., Sirbat D., Kessler M., Trechot P., Chanliau J. Effets secondaires oculaires de la fluméquine. Trois observations d'atteintes maculaires. Therapie. 1984 Sep-Oct;39(5):595–600. [PubMed] [Google Scholar]
- Husson M. O., Izard D., Bouillet L., Leclerc H. Comparative in-vitro activity of ciprofloxacin against non-fermenters. J Antimicrob Chemother. 1985 Apr;15(4):457–462. doi: 10.1093/jac/15.4.457. [DOI] [PubMed] [Google Scholar]
- Husson M. O., Izard D., Bouillet L., Leclerc H. In vitro activity of pefloxacin. Drugs Exp Clin Res. 1986;12(4):313–317. [PubMed] [Google Scholar]
- Hussy P., Maass G., Tümmler B., Grosse F., Schomburg U. Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase alpha primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts. Antimicrob Agents Chemother. 1986 Jun;29(6):1073–1078. doi: 10.1128/aac.29.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen M., Kunnas K., Saloranta P. Enoxacin treatment of urinary tract infections in elderly patients. J Antimicrob Chemother. 1988 Feb;21 (Suppl B):105–111. doi: 10.1093/jac/21.suppl_b.105. [DOI] [PubMed] [Google Scholar]
- Höffken G., Borner K., Glatzel P. D., Koeppe P., Lode H. Reduced enteral absorption of ciprofloxacin in the presence of antacids. Eur J Clin Microbiol. 1985 Jun;4(3):345–345. doi: 10.1007/BF02013667. [DOI] [PubMed] [Google Scholar]
- Höffken G., Lode H., Prinzing C., Borner K., Koeppe P. Pharmacokinetics of ciprofloxacin after oral and parenteral administration. Antimicrob Agents Chemother. 1985 Mar;27(3):375–379. doi: 10.1128/aac.27.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høiby N. Clinical uses of nalidixic acid analogues: the fluoroquinolones. Eur J Clin Microbiol. 1986 Apr;5(2):138–140. doi: 10.1007/BF02013968. [DOI] [PubMed] [Google Scholar]
- Ichiyama S., Tsukamura M. Ofloxacin and the treatment of pulmonary disease due to Mycobacterium fortuitum. Chest. 1987 Dec;92(6):1110–1112. doi: 10.1378/chest.92.6.1110. [DOI] [PubMed] [Google Scholar]
- Imamura M., Shibamura S., Hayakawa I., Osada Y. Inhibition of DNA gyrase by optically active ofloxacin. Antimicrob Agents Chemother. 1987 Feb;31(2):325–327. doi: 10.1128/aac.31.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Sato K., Fujii T., Hirai K., Inoue M., Iyobe S., Mitsuhashi S. Some properties of subunits of DNA gyrase from Pseudomonas aeruginosa PAO1 and its nalidixic acid-resistant mutant. J Bacteriol. 1987 May;169(5):2322–2325. doi: 10.1128/jb.169.5.2322-2325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani A., Welty G. S., Newton B. R., Richard G. A. Effects of changes in pH, medium, and inoculum size on the in vitro activity of amifloxacin against urinary isolates of Staphylococcus saprophyticus and Escherichia coli. Antimicrob Agents Chemother. 1985 Apr;27(4):449–451. doi: 10.1128/aac.27.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs D., Slack M. P., Wilkinson A. R., Westwood A. W. Successful treatment of Pseudomonas ventriculitis with ciprofloxacin. J Antimicrob Chemother. 1986 Apr;17(4):535–538. doi: 10.1093/jac/17.4.535. [DOI] [PubMed] [Google Scholar]
- Isaacs R. D., Ellis-Pegler R. B. Successful treatment of Morganella morganii meningitis with pefloxacin mesylate. J Antimicrob Chemother. 1987 Nov;20(5):769–770. doi: 10.1093/jac/20.5.769. [DOI] [PubMed] [Google Scholar]
- Ito A., Hirai K., Inoue M., Koga H., Suzue S., Irikura T., Mitsuhashi S. In vitro antibacterial activity of AM-715, a new nalidixic acid analog. Antimicrob Agents Chemother. 1980 Feb;17(2):103–108. doi: 10.1128/aac.17.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus N. V., Tally F. P., Barza M. Antimicrobial spectrum of Win 49375. Antimicrob Agents Chemother. 1984 Jul;26(1):104–107. doi: 10.1128/aac.26.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janin N., Meugnier H., Desnottes J. F., Woehrle R., Fleurette J. Recovery of pefloxacin in saliva and feces and its action on oral and fecal floras of healthy volunteers. Antimicrob Agents Chemother. 1987 Nov;31(11):1665–1668. doi: 10.1128/aac.31.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T., Pedersen S. S., Høiby N., Koch C. Efficacy of oral fluoroquinolones versus conventional intravenous antipseudomonal chemotherapy in treatment of cystic fibrosis. Eur J Clin Microbiol. 1987 Dec;6(6):618–622. doi: 10.1007/BF02013055. [DOI] [PubMed] [Google Scholar]
- Jensen T., Pedersen S. S., Nielsen C. H., Høiby N., Koch C. The efficacy and safety of ciprofloxacin and ofloxacin in chronic Pseudomonas aeruginosa infection in cystic fibrosis. J Antimicrob Chemother. 1987 Oct;20(4):585–594. doi: 10.1093/jac/20.4.585. [DOI] [PubMed] [Google Scholar]
- Joet F. P., Nourrit J., Frances Y., Arnaud A. Enoxacin in the treatment of bronchopulmonary infections. Eur J Clin Microbiol. 1985 Oct;4(5):512–513. doi: 10.1007/BF02014438. [DOI] [PubMed] [Google Scholar]
- John J. F., Jr, Twitty J. A. Amifloxacin activity against well-defined gentamicin-resistant, gram-negative bacteria. Antimicrob Agents Chemother. 1984 Nov;26(5):781–784. doi: 10.1128/aac.26.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. C., Ericsson C. D., Morgan D. R., Dupont H. L., Cabada F. J. Lack of emergence of resistant fecal flora during successful prophylaxis of traveler's diarrhea with norfloxacin. Antimicrob Agents Chemother. 1986 Nov;30(5):671–674. doi: 10.1128/aac.30.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M., Roberts G. D. In vitro activity of ciprofloxacin and ofloxacin against the Mycobacterium avium-intracellulare complex. Diagn Microbiol Infect Dis. 1987 May;7(1):89–91. doi: 10.1016/0732-8893(87)90077-0. [DOI] [PubMed] [Google Scholar]
- Joly-Guillou M. L., Bergogne-Berezin E. Etude comparative in vitro de l'activité de cinq quinolones vis-à-vis d'Acinetobacter calcoaceticus. Pathol Biol (Paris) 1985 May;33(5):416–420. [PubMed] [Google Scholar]
- Jones B. M., Geary I., Lee M. E., Duerden B. I. Activity of pefloxacin and thirteen other antimicrobial agents in vitro against isolates from hospital and genitourinary infections. J Antimicrob Chemother. 1986 Jun;17(6):739–746. doi: 10.1093/jac/17.6.739. [DOI] [PubMed] [Google Scholar]
- Joos B., Ledergerber B., Flepp M., Bettex J. D., Lüthy R., Siegenthaler W. Comparison of high-pressure liquid chromatography and bioassay for determination of ciprofloxacin in serum and urine. Antimicrob Agents Chemother. 1985 Mar;27(3):353–356. doi: 10.1128/aac.27.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Doern G. V., Thornsberry C., Preston D. A., Redding J. S., Maher L. A., Tubert T. Susceptibility of multiply resistant Haemophilus influenzae to newer antimicrobial agents. Diagn Microbiol Infect Dis. 1988 Jan;9(1):27–32. doi: 10.1016/0732-8893(88)90057-0. [DOI] [PubMed] [Google Scholar]
- Kaatz G. W., Barriere S. L., Schaberg D. R., Fekety R. Ciprofloxacin versus vancomycin in the therapy of experimental methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1987 Apr;31(4):527–530. doi: 10.1128/aac.31.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalager T., Digranes A., Bergan T., Rolstad T. Ofloxacin: serum and skin blister fluid pharmacokinetics in the fasting and non-fasting state. J Antimicrob Chemother. 1986 Jun;17(6):795–800. doi: 10.1093/jac/17.6.795. [DOI] [PubMed] [Google Scholar]
- Kaplowitz L. G., Vishniavsky N., Evans T., Vartivarian S., Dalton H., Simpson M., Gruninger R. P. Norfloxacin in the treatment of uncomplicated gonococcal infections. Am J Med. 1987 Jun 26;82(6B):35–39. doi: 10.1016/0002-9343(87)90616-4. [DOI] [PubMed] [Google Scholar]
- Karp J. E., Merz W. G., Hendricksen C., Laughon B., Redden T., Bamberger B. J., Bartlett J. G., Saral R., Burke P. J. Infection management during antileukemia treatment-induced granulocytopenia: the role for oral norfloxacin prophylaxis against infections arising from the gastrointestinal tract. Scand J Infect Dis Suppl. 1986;48:66–78. [PubMed] [Google Scholar]
- Kaukoranta-Tolvanen S. S., Renkonen O. V. In vitro susceptibility of Neisseria gonorrhoeae to RO 23-6240 and ciprofloxacin. Eur J Clin Microbiol. 1987 Jun;6(3):315–317. doi: 10.1007/BF02017623. [DOI] [PubMed] [Google Scholar]
- Kayser F. H., Novak J. In vitro activity of ciprofloxacin against gram-positive bacteria. An overview. Am J Med. 1987 Apr 27;82(4A):33–39. [PubMed] [Google Scholar]
- Kelley S. G., Bertram M. A., Young L. S. Activity of ciprofloxacin against resistant clinical isolates. J Antimicrob Chemother. 1986 Mar;17(3):281–286. doi: 10.1093/jac/17.3.281. [DOI] [PubMed] [Google Scholar]
- Kenny G. E., Hooton T. M., Roberts M. C., Cartwright F. D., Hoyt J. Susceptibilities of genital mycoplasmas to the newer quinolones as determined by the agar dilution method. Antimicrob Agents Chemother. 1989 Jan;33(1):103–107. doi: 10.1128/aac.33.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent A. F., Speller D. C. Sensitivity of urinary staphylococci to ciprofloxacin and acrosoxacin. Eur J Clin Microbiol. 1985 Apr;4(2):139–140. doi: 10.1007/BF02013582. [DOI] [PubMed] [Google Scholar]
- Kern W., Kurrle E., Vanek E. Ofloxacin for prevention of bacterial infections in granulocytopenic patients. Infection. 1987 Nov-Dec;15(6):427–433. doi: 10.1007/BF01647222. [DOI] [PubMed] [Google Scholar]
- Ketterl R., Beckurts T., Stübinger B., Claudi B. Use of ofloxacin in open fractures and in the treatment of post-traumatic osteomyelitis. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):159–166. doi: 10.1093/jac/22.supplement_c.159. [DOI] [PubMed] [Google Scholar]
- Khan M. Y., Gruninger R. P., Nelson S. M., Klicker R. E. Comparative in vitro activity of norfloxacin (MK-0366) and ten other oral antimicrobial agents against urinary bacterial isolates. Antimicrob Agents Chemother. 1982 May;21(5):848–851. doi: 10.1128/aac.21.5.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. Y., Siddiqui Y., Gruninger R. P. Comparative in vitro activity of Mk-0366 and other selected oral antimicrobial agents against Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1981 Aug;20(2):265–266. doi: 10.1128/aac.20.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid-Smith P. S. Alpha blockade. An overview of efficacy data. Am J Med. 1987 Jan 5;82(1A):21–25. doi: 10.1016/0002-9343(87)90139-2. [DOI] [PubMed] [Google Scholar]
- King A., Boothman C., Phillips I. The in-vitro activity of PD127,391, a new quinolone. J Antimicrob Chemother. 1988 Aug;22(2):135–141. doi: 10.1093/jac/22.2.135. [DOI] [PubMed] [Google Scholar]
- King A., Shannon K., Phillips I. The in-vitro activities of enoxacin and ofloxacin compared with that of ciprofloxacin. J Antimicrob Chemother. 1985 May;15(5):551–558. doi: 10.1093/jac/15.5.551. [DOI] [PubMed] [Google Scholar]
- King A., Shannon K., Phillips I. The in-vitro activity of ciprofloxacin compared with that of norfloxacin and nalidixic acid. J Antimicrob Chemother. 1984 Apr;13(4):325–331. doi: 10.1093/jac/13.4.325. [DOI] [PubMed] [Google Scholar]
- King A., Warren C., Shannon K., Phillips I. In vitro antibacterial activity of norfloxacin (MK-0366). Antimicrob Agents Chemother. 1982 Apr;21(4):604–607. doi: 10.1128/aac.21.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevan L., Wang J. C. Deoxyribonucleic acid gyrase-deoxyribonucleic acid complex containing 140 base pairs of deoxyribonucleic acid and an alpha 2 beta 2 protein core. Biochemistry. 1980 Nov 11;19(23):5229–5234. doi: 10.1021/bi00564a012. [DOI] [PubMed] [Google Scholar]
- Klinger J. D., Aronoff S. C. In-vitro activity of ciprofloxacin and other antibacterial agents against Pseudomonas aeruginosa and Pseudomonas cepacia from cystic fibrosis patients. J Antimicrob Chemother. 1985 Jun;15(6):679–684. doi: 10.1093/jac/15.6.679. [DOI] [PubMed] [Google Scholar]
- Klopman G., Macina O. T., Levinson M. E., Rosenkranz H. S. Computer automated structure evaluation of quinolone antibacterial agents. Antimicrob Agents Chemother. 1987 Nov;31(11):1831–1840. doi: 10.1128/aac.31.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H. Clinical efficacy of ciprofloxacin in the treatment of patients with respiratory tract infections in Japan. Am J Med. 1987 Apr 27;82(4A):169–173. [PubMed] [Google Scholar]
- Koga H. High-performance liquid chromatography measurement of antimicrobial concentrations in polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1987 Dec;31(12):1904–1908. doi: 10.1128/aac.31.12.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korvick J., Yu V. L., Sharp J. A. Interaction of rifampicin or rifapentine with other agents against Pseudomonas aeruginosa. J Antimicrob Chemother. 1987 Jun;19(6):847–848. doi: 10.1093/jac/19.6.847. [DOI] [PubMed] [Google Scholar]
- Kosmidis J., Gargalianos P., Adamis G., Petropoulou D., Makris D. Fleroxacin in single dose oral therapy of uncomplicated lower urinary tract infection. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):219–221. doi: 10.1093/jac/22.supplement_d.219. [DOI] [PubMed] [Google Scholar]
- Kouno K., Inoue M., Mitsuhashi S. In vitro and in vivo antibacterial activity of AT-2266. Antimicrob Agents Chemother. 1983 Jul;24(1):78–84. doi: 10.1128/aac.24.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausse R., Ullmann U. Comparative in vitro activity of fleroxacin (RO 23-6240) against Ureaplasma urealyticum and Mycoplasma hominis. Eur J Clin Microbiol Infect Dis. 1988 Feb;7(1):67–69. doi: 10.1007/BF01962178. [DOI] [PubMed] [Google Scholar]
- Kresken M., Wiedemann B. Development of resistance to nalidixic acid and the fluoroquinolones after the introduction of norfloxacin and ofloxacin. Antimicrob Agents Chemother. 1988 Aug;32(8):1285–1288. doi: 10.1128/aac.32.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer K. N., Cozzarelli N. R. Escherichia coli mutants thermosensitive for deoxyribonucleic acid gyrase subunit A: effects on deoxyribonucleic acid replication, transcription, and bacteriophage growth. J Bacteriol. 1979 Nov;140(2):424–435. doi: 10.1128/jb.140.2.424-435.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer K. N., Cozzarelli N. R. Formation and resolution of DNA catenanes by DNA gyrase. Cell. 1980 May;20(1):245–254. doi: 10.1016/0092-8674(80)90252-4. [DOI] [PubMed] [Google Scholar]
- Kromann-Andersen B., Sommer P., Pers C., Larsen V., Rasmussen F. Ofloxacin compared with ciprofloxacin in the treatment of complicated lower urinary tract infections. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):143–147. doi: 10.1093/jac/22.supplement_c.143. [DOI] [PubMed] [Google Scholar]
- Krueger J. H., Walker G. C. groEL and dnaK genes of Escherichia coli are induced by UV irradiation and nalidixic acid in an htpR+-dependent fashion. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1499–1503. doi: 10.1073/pnas.81.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada T., Neu H. C. In-vitro activity of ofloxacin, a quinolone carboxylic acid compared to other quinolones and other antimicrobial agents. J Antimicrob Chemother. 1985 Nov;16(5):563–574. doi: 10.1093/jac/16.5.563. [DOI] [PubMed] [Google Scholar]
- Kurrle E., Dekker A. W., Gaus W., Haralambie E., Krieger D., Rozenberg-Arska M., de Vries-Hospers H. G., van der Waaij D., Wendt F. Prevention of infection in acute leukemia: a prospective randomized study on the efficacy of two different drug regimens for antimicrobial prophylaxis. Infection. 1986 Sep-Oct;14(5):226–232. doi: 10.1007/BF01644268. [DOI] [PubMed] [Google Scholar]
- Kurzynski T. A., Boehm D. M., Rott-Petri J. A., Schell R. F., Allison P. E. Antimicrobial susceptibilities of Bordetella species isolated in a Multicenter Pertussis Surveillance Project. Antimicrob Agents Chemother. 1988 Jan;32(1):137–140. doi: 10.1128/aac.32.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Mégraud F., Gerbaud G., Courvalin P. Susceptibility of Campylobacter pyloridis to 20 antimicrobial agents. Antimicrob Agents Chemother. 1986 Sep;30(3):510–511. doi: 10.1128/aac.30.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassus A., Renkonen O. V., Ellmén J. Fleroxacin versus standard therapy in gonococcal urethritis. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):223–225. doi: 10.1093/jac/22.supplement_d.223. [DOI] [PubMed] [Google Scholar]
- Lauwers S., Vincken W., Naessens A., Pierard D. Efficacy and safety of pefloxacin in the treatment of severe infections in patients hospitalized in intensive care units. J Antimicrob Chemother. 1986 Apr;17 (Suppl B):111–115. doi: 10.1093/jac/17.suppl_b.111. [DOI] [PubMed] [Google Scholar]
- Le Saux N. M., Slaney L. A., Plummer F. A., Ronald A. R., Brunham R. C. In vitro activity of ceftriaxone, cefetamet (Ro 15-8074), ceftetrame (Ro 19-5247; T-2588), and fleroxacin (Ro 23-6240; AM-833) versus Neisseria gonorrhoeae and Haemophilus ducreyi. Antimicrob Agents Chemother. 1987 Jul;31(7):1153–1154. doi: 10.1128/aac.31.7.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBel M., Barbeau G., Bergeron M. G., Roy D., Vallée F. Pharmacokinetics of ciprofloxacin in elderly subjects. Pharmacotherapy. 1986 Mar-Apr;6(2):87–91. doi: 10.1002/j.1875-9114.1986.tb03458.x. [DOI] [PubMed] [Google Scholar]
- LeBel M., Bergeron M. G., Vallée F., Fiset C., Chassé G., Bigonesse P., Rivard G. Pharmacokinetics and pharmacodynamics of ciprofloxacin in cystic fibrosis patients. Antimicrob Agents Chemother. 1986 Aug;30(2):260–266. doi: 10.1128/aac.30.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel M., Bergeron M. G. Pharmacokinetics in the elderly. Studies on ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):108–114. [PubMed] [Google Scholar]
- Ledergerber B., Bettex J. D., Joos B., Flepp M., Lüthy R. Effect of standard breakfast on drug absorption and multiple-dose pharmacokinetics of ciprofloxacin. Antimicrob Agents Chemother. 1985 Mar;27(3):350–352. doi: 10.1128/aac.27.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh D. A., Emmanuel F. X., Petch V. J. Ciprofloxacin therapy in complicated urinary tract infections caused by Pseudomonas aeruginosa and other resistant bacteria. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):117–121. doi: 10.1093/jac/18.supplement_d.117. [DOI] [PubMed] [Google Scholar]
- Leigh D. A., Smith E. C., Marriner J. Comparative study using norfloxacin and amoxycillin in the treatment of complicated urinary tract infections in geriatric patients. J Antimicrob Chemother. 1984 May;13 (Suppl B):79–83. doi: 10.1093/jac/13.suppl_b.79. [DOI] [PubMed] [Google Scholar]
- Leigh D. A., Walsh B., Harris K., Hancock P., Travers G. Pharmacokinetics of ofloxacin and the effect on the faecal flora of healthy volunteers. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):115–125. doi: 10.1093/jac/22.supplement_c.115. [DOI] [PubMed] [Google Scholar]
- Leor J., Matetzki S. Ofloxacin and warfarin. Ann Intern Med. 1988 Nov 1;109(9):761–761. doi: 10.7326/0003-4819-109-9-761_1. [DOI] [PubMed] [Google Scholar]
- Leroy A., Borsa F., Humbert G., Bernadet P., Fillastre J. P. The pharmacokinetics of ofloxacin in healthy adult male volunteers. Eur J Clin Pharmacol. 1987;31(5):629–630. doi: 10.1007/BF00606645. [DOI] [PubMed] [Google Scholar]
- Lesage D., Delisle-Mizon F., Vergez P., Daguet G. L. Comparaison de l'activité in vitro de six quinolones sur Pseudomonas sp. Pathol Biol (Paris) 1985 May;33(5):412–415. [PubMed] [Google Scholar]
- Lesse A. J., Freer C., Salata R. A., Francis J. B., Scheld W. M. Oral ciprofloxacin therapy for gram-negative bacillary osteomyelitis. Am J Med. 1987 Apr 27;82(4A):247–253. [PubMed] [Google Scholar]
- Lewis A. M., Chattopadhyay B. Comparative in-vitro activity of thirteen antibiotics against faecal isolates of Yersinia spp. J Antimicrob Chemother. 1987 Mar;19(3):406–408. doi: 10.1093/jac/19.3.406. [DOI] [PubMed] [Google Scholar]
- Licitra C. M., Brooks R. G., Sieger B. E. Clinical efficacy and levels of ciprofloxacin in tissue in patients with soft tissue infection. Antimicrob Agents Chemother. 1987 May;31(5):805–807. doi: 10.1128/aac.31.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligtvoet E. E., Wickerhoff-Minoggio T. In-vitro activity of pefloxacin compared with six other quinolones. J Antimicrob Chemother. 1985 Oct;16(4):485–490. doi: 10.1093/jac/16.4.485. [DOI] [PubMed] [Google Scholar]
- Limb D. I., Dabbs D. J., Spencer R. C. In-vitro selection of bacteria resistant to the 4-quinolone agents. J Antimicrob Chemother. 1987 Jan;19(1):65–71. doi: 10.1093/jac/19.1.65. [DOI] [PubMed] [Google Scholar]
- Ling J., Kam K. M., Lam A. W., French G. L. Susceptibilities of Hong Kong isolates of multiply resistant Shigella spp. to 25 antimicrobial agents, including ampicillin plus sulbactam and new 4-quinolones. Antimicrob Agents Chemother. 1988 Jan;32(1):20–23. doi: 10.1128/aac.32.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Micrococcus luteus DNA gyrase: active components and a model for its supercoiling of DNA. Proc Natl Acad Sci U S A. 1978 May;75(5):2098–2102. doi: 10.1073/pnas.75.5.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo W. L. Ofloxacin in treatment of typhoid fever: a preliminary study. J Antimicrob Chemother. 1988 May;21(5):681–682. doi: 10.1093/jac/21.5.681. [DOI] [PubMed] [Google Scholar]
- Lockley M. R., Wise R., Dent J. The pharmacokinetics and tissue penetration of ofloxacin. J Antimicrob Chemother. 1984 Dec;14(6):647–652. doi: 10.1093/jac/14.6.647. [DOI] [PubMed] [Google Scholar]
- Lockshon D., Morris D. R. Sites of reaction of Escherichia coli DNA gyrase on pBR322 in vivo as revealed by oxolinic acid-induced plasmid linearization. J Mol Biol. 1985 Jan 5;181(1):63–74. doi: 10.1016/0022-2836(85)90324-9. [DOI] [PubMed] [Google Scholar]
- Lode H., Höffken G., Olschewski P., Sievers B., Kirch A., Borner K., Koeppe P. Pharmacokinetics of ofloxacin after parenteral and oral administration. Antimicrob Agents Chemother. 1987 Sep;31(9):1338–1342. doi: 10.1128/aac.31.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lode H., Wiley R., Höffken G., Wagner J., Borner K. Prospective randomized controlled study of ciprofloxacin versus imipenem-cilastatin in severe clinical infections. Antimicrob Agents Chemother. 1987 Oct;31(10):1491–1496. doi: 10.1128/aac.31.10.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo P. S., Ridgway G. L., Oriel J. D. Single dose ciprofloxacin for treating gonococcal infections in men. Genitourin Med. 1985 Oct;61(5):302–305. doi: 10.1136/sti.61.5.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopitaux R., Hermet R., Sirot J., Terver S., Levai J. P. Notre expérience de l'ofloxacine dans le traitement des infections ostéo-articulaires. Pathol Biol (Paris) 1988 May;36(5):557–561. [PubMed] [Google Scholar]
- Lynn R., Giaever G., Swanberg S. L., Wang J. C. Tandem regions of yeast DNA topoisomerase II share homology with different subunits of bacterial gyrase. Science. 1986 Aug 8;233(4764):647–649. doi: 10.1126/science.3014661. [DOI] [PubMed] [Google Scholar]
- MacGowan A. P., Greig M. A., Clarke E. A., White L. O., Reeves D. S. The pharmacokinetics of norfloxacin in the aged. J Antimicrob Chemother. 1988 Nov;22(5):721–727. doi: 10.1093/jac/22.5.721. [DOI] [PubMed] [Google Scholar]
- Machka K., Braveny I. Comparative in vitro activity of RO 23-6240 (fleroxacin), a new 4-quinolone derivative. Eur J Clin Microbiol. 1987 Aug;6(4):482–485. doi: 10.1007/BF02013116. [DOI] [PubMed] [Google Scholar]
- Machka K. In vitro activity of ciprofloxacin and norfloxacin against Gardnerella vaginalis. Eur J Clin Microbiol. 1984 Aug;3(4):374–374. doi: 10.1007/BF01977503. [DOI] [PubMed] [Google Scholar]
- Machka K., Vogelsang G. In vitro activity of norfloxacin against multi-resistant bacteria. Eur J Clin Microbiol. 1983 Jun;2(3):270–271. doi: 10.1007/BF02029531. [DOI] [PubMed] [Google Scholar]
- Maeda H., Fujii A., Nakata K., Arakawa S., Kamidono S. In vitro activities of T-3262, NY-198, fleroxacin (AM-833; RO 23-6240), and other new quinolone agents against clinically isolated Chlamydia trachomatis strains. Antimicrob Agents Chemother. 1988 Jul;32(7):1080–1081. doi: 10.1128/aac.32.7.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maesen F. P., Davies B. I., Geraedts W. H., Sumajow C. A. Ofloxacin and antacids. J Antimicrob Chemother. 1987 Jun;19(6):848–850. doi: 10.1093/jac/19.6.848. [DOI] [PubMed] [Google Scholar]
- Maesen F. P., Davies B. I., Teengs J. P. Pefloxacin in acute exacerbations of chronic bronchitis. J Antimicrob Chemother. 1985 Sep;16(3):379–388. doi: 10.1093/jac/16.3.379. [DOI] [PubMed] [Google Scholar]
- Maesen F. P., Teengs J. P., Baur C., Davies B. I. Quinolones and raised plasma concentrations of theophylline. Lancet. 1984 Sep 1;2(8401):530–530. doi: 10.1016/s0140-6736(84)92617-5. [DOI] [PubMed] [Google Scholar]
- Malek R. S., Omess P. J., Benson R. C., Jr, Zincke H. Renal cell carcinoma in von Hippel-Lindau syndrome. Am J Med. 1987 Feb;82(2):236–238. doi: 10.1016/0002-9343(87)90062-3. [DOI] [PubMed] [Google Scholar]
- Malmborg A. S., Rannikko S. Enoxacin distribution in human tissues after multiple oral administration. J Antimicrob Chemother. 1988 Feb;21 (Suppl B):57–60. doi: 10.1093/jac/21.suppl_b.57. [DOI] [PubMed] [Google Scholar]
- Mandell W., Neu H. C. Disk susceptibility of ofloxacin, a new carboxyquinolone. J Clin Microbiol. 1985 Nov;22(5):786–788. doi: 10.1128/jcm.22.5.786-788.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manek N., Andrews J. M., Wise R. In vitro activity of Ro 23-6240, a new difluoroquinolone derivative, compared with that of other antimicrobial agents. Antimicrob Agents Chemother. 1986 Aug;30(2):330–332. doi: 10.1128/aac.30.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manek N., Wise R., Andrews J. In-vitro activity of pefloxacin in combination with other antimicrobial agents. J Antimicrob Chemother. 1986 Jun;17(6):827–828. doi: 10.1093/jac/17.6.827. [DOI] [PubMed] [Google Scholar]
- Marians K. J. DNA gyrase-catalyzed decatenation of multiply linked DNA dimers. J Biol Chem. 1987 Jul 25;262(21):10362–10368. [PubMed] [Google Scholar]
- Martin C., Gouin F., Fourrier F., Junginger W., Prieur B. L. Pefloxacin in the treatment of nosocomial lower respiratory tract infections in intensive care patients. J Antimicrob Chemother. 1988 Jun;21(6):795–799. doi: 10.1093/jac/21.6.795. [DOI] [PubMed] [Google Scholar]
- Martino P., Venditti M., Valente B., Brandimarte C., Serra P. N-formimidoyl-thienamycin and norfloxacin against multiple-resistant Pseudomonas aeruginosa strains. Combined in vitro activity and comparison with 14 other antibiotics. Drugs Exp Clin Res. 1985;11(4):247–251. [PubMed] [Google Scholar]
- Marty N., Clave D., Cancet B., Henry-Ferry S., Didier J. Corynebacterium groupe D2. Etude clinique, identification biochimique et sensibilité aux antibiotiques. Pathol Biol (Paris) 1988 May;36(5):460–464. [PubMed] [Google Scholar]
- Mascellino M. T., Lorenzi A., Bonanni M., Iegri F. Antimicrobial activity of norfloxacin in enteric and urinary tract infections: combined effect of norfloxacin with aminoglycosides, tetracycline and chloramphenicol. Drugs Exp Clin Res. 1986;12(4):319–323. [PubMed] [Google Scholar]
- Mayrer A. R., Andriole V. T. Urinary tract antiseptics. Med Clin North Am. 1982 Jan;66(1):199–208. doi: 10.1016/s0025-7125(16)31453-5. [DOI] [PubMed] [Google Scholar]
- McDaniel L. S., Rogers L. H., Hill W. E. Survival of recombination-deficient mutants of Escherichia coli during incubation with nalidixic acid. J Bacteriol. 1978 Jun;134(3):1195–1198. doi: 10.1128/jb.134.3.1195-1198.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty C. A., Dent J. C. Susceptibility of clinical isolates of Campylobacter pylori to twenty-one antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1988 Aug;7(4):566–569. doi: 10.1007/BF01962617. [DOI] [PubMed] [Google Scholar]
- McNulty C. A., Dent J., Wise R. Susceptibility of clinical isolates of Campylobacter pyloridis to 11 antimicrobial agents. Antimicrob Agents Chemother. 1985 Dec;28(6):837–838. doi: 10.1128/aac.28.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehtar S., Drabu Y., Blakemore P. Ciprofloxacin in the treatment of infections caused by gentamicin-resistant gram-negative bacteria. Eur J Clin Microbiol. 1986 Apr;5(2):248–251. doi: 10.1007/BF02014001. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert H., Weil G., Millott G. In vitro activity of norfloxacin against Chlamydia trachomatis. Eur J Clin Microbiol. 1983 Jun;2(3):271–272. doi: 10.1007/BF02029532. [DOI] [PubMed] [Google Scholar]
- Mensing H. Treatment of chancroid with enoxacin. Acta Derm Venereol. 1985;65(5):455–457. [PubMed] [Google Scholar]
- Michéa-Hamzehpour M., Pechère J. C., Marchou B., Auckenthaler R. Combination therapy: a way to limit emergence of resistance? Am J Med. 1986 Jun 30;80(6B):138–142. doi: 10.1016/0002-9343(86)90491-2. [DOI] [PubMed] [Google Scholar]
- Mikhail I. A., Bourgeois A. L., Hyams K. C., Podgore J. K., Lissner C. R., Walz S. In vitro activity of ciprofloxacin compared to trimethoprim-sulfamethoxazole against Campylobacter spp., Shigella spp. and Enterotoxigenic Escherichia coli causing travellers' diarrhea in Egypt. Scand J Infect Dis. 1987;19(4):479–481. doi: 10.3109/00365548709021682. [DOI] [PubMed] [Google Scholar]
- Miller R. V., Scurlock T. R. DNA gyrase (Topoisomerase II) from Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1983 Jan 27;110(2):694–700. doi: 10.1016/0006-291x(83)91205-6. [DOI] [PubMed] [Google Scholar]
- Mitscher L. A., Sharma P. N., Chu D. T., Shen L. L., Pernet A. G. Chiral DNA gyrase inhibitors. 1. Synthesis and antimicrobial activity of the enantiomers of 6-fluoro-7-(1-piperazinyl)-1-(2'-trans-phenyl-1'-cyclopropyl)-1, 4-dihydro-4-oxoquinoline-3-carboxylic acid. J Med Chem. 1986 Oct;29(10):2044–2047. doi: 10.1021/jm00160a042. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., Fisher L. M., O'Dea M. H., Gellert M. DNA gyrase action involves the introduction of transient double-strand breaks into DNA. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1847–1851. doi: 10.1073/pnas.77.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondorf A. W., Buch A., Steinbacher G., Rüdel C., Falkenberg F. W. Renal tolerance of fleroxacin in healthy volunteers. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):179–189. doi: 10.1093/jac/22.supplement_d.179. [DOI] [PubMed] [Google Scholar]
- Monk J. P., Campoli-Richards D. M. Ofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1987 Apr;33(4):346–391. doi: 10.2165/00003495-198733040-00003. [DOI] [PubMed] [Google Scholar]
- Montay G., Goueffon Y., Roquet F. Absorption, distribution, metabolic fate, and elimination of pefloxacin mesylate in mice, rats, dogs, monkeys, and humans. Antimicrob Agents Chemother. 1984 Apr;25(4):463–472. doi: 10.1128/aac.25.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montay G., Jacquot C., Bariety J., Cunci R. Pharmacokinetics of pefloxacin in renal insufficiency. Eur J Clin Pharmacol. 1985;29(3):345–349. doi: 10.1007/BF00544092. [DOI] [PubMed] [Google Scholar]
- Moody J. A., Gerding D. N., Peterson L. R. Evaluation of ciprofloxacin's synergism with other agents by multiple in vitro methods. Am J Med. 1987 Apr 27;82(4A):44–54. [PubMed] [Google Scholar]
- Moody J. A., Peterson L. R., Gerding D. N. In vitro activity of ciprofloxacin combined with azlocillin. Antimicrob Agents Chemother. 1985 Dec;28(6):849–850. doi: 10.1128/aac.28.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhouse E. C., Clarke P. C. Efficacy of ofloxacin in the treatment of lower respiratory tract infections in general practice. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):135–138. doi: 10.1093/jac/22.supplement_c.135. [DOI] [PubMed] [Google Scholar]
- Moorhouse E. C., Mulvihill T. E., Jones L., Mooney D., Falkiner F. R., Keane C. T. The in-vitro activity of some antimicrobial agents against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1985 Mar;15(3):291–295. doi: 10.1093/jac/15.3.291. [DOI] [PubMed] [Google Scholar]
- Morelli G. An unusual course for hepatitis A. Infection. 1986 Nov-Dec;14(6):300–300. doi: 10.1007/BF01643968. [DOI] [PubMed] [Google Scholar]
- Morris J. G., Jr, Tenney J. H., Drusano G. L. In vitro susceptibility of pathogenic Vibrio species to norfloxacin and six other antimicrobial agents. Antimicrob Agents Chemother. 1985 Sep;28(3):442–445. doi: 10.1128/aac.28.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A., Cozzarelli N. R. Site-specific cleavage of DNA by E. coli DNA gyrase. Cell. 1979 May;17(1):175–184. doi: 10.1016/0092-8674(79)90305-2. [DOI] [PubMed] [Google Scholar]
- Morrison P. J., Mant T. G., Norman G. T., Robinson J., Kunka R. L. Pharmacokinetics and tolerance of lomefloxacin after sequentially increasing oral doses. Antimicrob Agents Chemother. 1988 Oct;32(10):1503–1507. doi: 10.1128/aac.32.10.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. E., Ruane P. J., Johnston L., Wong P., Wheelock J. P., MacDonald K., Reinhardt J. F., Johnson C. C., Statner B., Blomquist I. Ciprofloxacin for eradication of methicillin-resistant Staphylococcus aureus colonization. Am J Med. 1987 Apr 27;82(4A):215–219. [PubMed] [Google Scholar]
- Munshi M. H., Sack D. A., Haider K., Ahmed Z. U., Rahaman M. M., Morshed M. G. Plasmid-mediated resistance to nalidixic acid in Shigella dysenteriae type 1. Lancet. 1987 Aug 22;2(8556):419–421. doi: 10.1016/s0140-6736(87)90957-3. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Rensimer E. R., DuPont H. L. Emergence of high-level trimethoprim resistance in fecal Escherichia coli during oral administration of trimethoprim or trimethoprim--sulfamethoxazole. N Engl J Med. 1982 Jan 21;306(3):130–135. doi: 10.1056/NEJM198201213060302. [DOI] [PubMed] [Google Scholar]
- Muytjens H. L., van der Ros-van de Repe J. Comparative in vitro susceptibilities of eight Enterobacter species, with special reference to Enterobacter sakazakii. Antimicrob Agents Chemother. 1986 Feb;29(2):367–370. doi: 10.1128/aac.29.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muytjens H. L., van der Ros-van de Repe J., van Veldhuizen G. Comparative activities of ciprofloxacin (Bay o 9867), norfloxacin, pipemidic acid, and nalidixic acid. Antimicrob Agents Chemother. 1983 Aug;24(2):302–304. doi: 10.1128/aac.24.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller B. R., Kaspersen P., Mamsen A., Korsager B., Quitzau K. Fleroxacin in the treatment of uncomplicated acute cystitis in women. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):215–218. doi: 10.1093/jac/22.supplement_d.215. [DOI] [PubMed] [Google Scholar]
- Naamara W., Plummer F. A., Greenblatt R. M., D'Costa L. J., Ndinya-Achola J. O., Ronald A. R. Treatment of chancroid with ciprofloxacin. A prospective, randomized clinical trial. Am J Med. 1987 Apr 27;82(4A):317–320. [PubMed] [Google Scholar]
- Naber K. G., Sörgel F., Gutzler F., Bartosik-Wich B. In vitro activity, pharmacokinetics, clinical safety and therapeutic efficacy of enoxacin in the treatment of patients with complicated urinary tract infections. Infection. 1985 Sep-Oct;13(5):219–224. doi: 10.1007/BF01667215. [DOI] [PubMed] [Google Scholar]
- Naber K. G., Sörgel F., Kees F., Schumacher H., Metz R., Grobecker H. In-vitro activity of fleroxacin against isolates causing complicated urinary tract infections and concentrations in seminal and prostatic fluid and in prostatic adenoma tissue. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):199–207. doi: 10.1093/jac/22.supplement_d.199. [DOI] [PubMed] [Google Scholar]
- Nagayama A., Nakao T., Taen H. In vitro activities of ofloxacin and four other new quinoline-carboxylic acids against Chlamydia trachomatis. Antimicrob Agents Chemother. 1988 Nov;32(11):1735–1737. doi: 10.1128/aac.32.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Inoue S., Shimizu M., Iyobe S., Mitsuhashi S. Inhibition of conjugal transfer of R plasmids by pipemidic acid and related compounds. Antimicrob Agents Chemother. 1976 Nov;10(5):779–785. doi: 10.1128/aac.10.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Kurobe N., Kashimoto S., Ohue T., Takase Y., Shimizu M. Pharmacokinetics of AT-2266 administered orally to mice, rats, dogs, and monkeys. Antimicrob Agents Chemother. 1983 Jul;24(1):54–60. doi: 10.1128/aac.24.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Minami A., Katae H., Inoue S., Yamagishi J., Takase Y., Shimizu M. In vitro antibacterial properties of AT-2266, a new pyridonecarboxylic acid. Antimicrob Agents Chemother. 1983 May;23(5):641–648. doi: 10.1128/aac.23.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M., Kanamaru M., Uematsu T., Takiguchi A., Mizuno A., Itaya T., Kawahara F., Ooie T., Saito S., Uchida H. Clinical pharmacokinetics and tolerance of fleroxacin in healthy male volunteers. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):133–144. doi: 10.1093/jac/22.supplement_d.133. [DOI] [PubMed] [Google Scholar]
- Nayagam A. T., Ridgway G. L., Oriel J. D. Efficacy of ofloxacin in the treatment of non-gonococcal urethritis in men and genital infections caused by Chlamydia trachomatis in men and women. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):155–158. doi: 10.1093/jac/22.supplement_c.155. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Chin N. X. In-vitro activity of two new quinolone antimicrobial agents, S-25930 and S-25932 compared with that of other agents. J Antimicrob Chemother. 1987 Feb;19(2):175–185. doi: 10.1093/jac/19.2.175. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Ciprofloxacin: an overview and prospective appraisal. Am J Med. 1987 Apr 27;82(4A):395–404. [PubMed] [Google Scholar]
- Neu H. C. Clinical use of the quinolones. Lancet. 1987 Dec 5;2(8571):1319–1322. doi: 10.1016/s0140-6736(87)91205-0. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Kumada T., Chin N. X., Mandell W. The post-antimicrobial suppressive effect of quinolone agents. Drugs Exp Clin Res. 1987;13(2):63–67. [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. In vitro activity of norfloxacin, a quinolinecarboxylic acid, compared with that of beta-lactams, aminoglycosides, and trimethoprim. Antimicrob Agents Chemother. 1982 Jul;22(1):23–27. doi: 10.1128/aac.22.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman M., Esanu A. Gaps and perspectives of new fluoroquinolones. Drugs Exp Clin Res. 1988;14(6):385–391. [PubMed] [Google Scholar]
- Newsom S. W., Matthews J., Amphlett M., Warren R. E. Norfloxacin and the antibacterial gamma pyridone beta carboxylic acids. J Antimicrob Chemother. 1982 Jul;10(1):25–30. doi: 10.1093/jac/10.1.25. [DOI] [PubMed] [Google Scholar]
- Newsom S. W., Murphy P., Matthews J. A comparative study of ciprofloxacin and trimethoprim in the treatment of urinary tract infections in geriatric patients. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):111–115. doi: 10.1093/jac/18.supplement_d.111. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki Y., Soejima R., Kawane H., Sumi M., Umeki S. New synthetic quinolone antibacterial agents and serum concentration of theophylline. Chest. 1987 Oct;92(4):663–669. doi: 10.1378/chest.92.4.663. [DOI] [PubMed] [Google Scholar]
- Nix D. E., DeVito J. M., Whitbread M. A., Schentag J. J. Effect of multiple dose oral ciprofloxacin on the pharmacokinetics of theophylline and indocyanine green. J Antimicrob Chemother. 1987 Feb;19(2):263–269. doi: 10.1093/jac/19.2.263. [DOI] [PubMed] [Google Scholar]
- Nix D. E., Schentag J. J. The quinolones: an overview and comparative appraisal of their pharmacokinetics and pharmacodynamics. J Clin Pharmacol. 1988 Feb;28(2):169–178. doi: 10.1002/j.1552-4604.1988.tb05740.x. [DOI] [PubMed] [Google Scholar]
- Nix D. E., Schultz R. W., Frost R. W., Sedman A. J., Thomas D. J., Kinkel A. W., Schentag J. J. The effect of renal impairment and haemodialysis on single dose pharmacokinetics of oral enoxacin. J Antimicrob Chemother. 1988 Feb;21 (Suppl B):87–95. doi: 10.1093/jac/21.suppl_b.87. [DOI] [PubMed] [Google Scholar]
- Nord C. E., Edlund C., Lahnborg G. The efficacy of pefloxacin in comparison to gentamicin in the treatment of experimentally induced peritonitis in rats. J Antimicrob Chemother. 1986 Apr;17 (Suppl B):59–63. doi: 10.1093/jac/17.suppl_b.59. [DOI] [PubMed] [Google Scholar]
- Norrby S. R., Jonsson M. Antibacterial activity of norfloxacin. Antimicrob Agents Chemother. 1983 Jan;23(1):15–18. doi: 10.1128/aac.23.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby S. R., Jonsson M. Comparative in vitro activity of PD 127,391, a new fluorinated 4-quinolone derivative. Antimicrob Agents Chemother. 1988 Aug;32(8):1278–1281. doi: 10.1128/aac.32.8.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notowicz A., Stolz E., van Klingeren B. A double blind study comparing two dosages of enoxacin for the treatment of uncomplicated urogenital gonorrhoea. J Antimicrob Chemother. 1984 Sep;14 (Suppl 100):91–94. doi: 10.1093/jac/14.suppl_c.91. [DOI] [PubMed] [Google Scholar]
- Noyes M., Polk R. E. Norfloxacin and absorption of magnesium-aluminum. Ann Intern Med. 1988 Jul 15;109(2):168–169. doi: 10.7326/0003-4819-109-2-168_2. [DOI] [PubMed] [Google Scholar]
- O'Connor M. B., Malamy M. H. Mapping of DNA gyrase cleavage sites in vivo oxolinic acid induced cleavages in plasmid pBR322. J Mol Biol. 1985 Feb 20;181(4):545–550. doi: 10.1016/0022-2836(85)90426-7. [DOI] [PubMed] [Google Scholar]
- O'Hare M. D., Felmingham D., Ridgway G. L., Grüneberg R. N. The comparative in vitro activity of twelve 4-quinolone antimicrobials against enteric pathogens. Drugs Exp Clin Res. 1985;11(4):253–257. [PubMed] [Google Scholar]
- Ode B., Walder M., Forsgren A. Failure of a single dose of 100 mg ofloxacin in lower urinary tract infections in females. Scand J Infect Dis. 1987;19(6):677–679. doi: 10.3109/00365548709117203. [DOI] [PubMed] [Google Scholar]
- Okazaki O., Miyazaki K., Tachizawa H. Lack of effect of ofloxacin on theophylline pharmacokinetics in rats. Chemotherapy. 1987;33(6):402–411. doi: 10.1159/000238528. [DOI] [PubMed] [Google Scholar]
- Orr E., Staudenbauer W. L. Bacillus subtilis DNA gyrase: purification of subunits and reconstitution of supercoiling activity. J Bacteriol. 1982 Jul;151(1):524–527. doi: 10.1128/jb.151.1.524-527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada Y., Ogawa H. Antimycoplasmal activity of ofloxacin (DL-8280). Antimicrob Agents Chemother. 1983 Mar;23(3):509–511. doi: 10.1128/aac.23.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek B. P., Rozenberg-Arska M., Verhoef J. Do quinolones really augment the antifungal effect of amphotericin B in vitro? Drugs Exp Clin Res. 1985;11(11):745–746. [PubMed] [Google Scholar]
- Panneton A. C., Bergeron M. G., LeBel M. Pharmacokinetics and tissue penetration of fleroxacin after single and multiple 400- and 800-mg-dosage regimens. Antimicrob Agents Chemother. 1988 Oct;32(10):1515–1520. doi: 10.1128/aac.32.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish L. C., Asper R. Systemic treatment of cutaneous infections. A comparative study of ciprofloxacin and cefotaxime. Am J Med. 1987 Apr 27;82(4A):227–229. [PubMed] [Google Scholar]
- Parpia S. H., Nix D. E., Hejmanowski L. G., Goldstein H. R., Wilton J. H., Schentag J. J. Sucralfate reduces the gastrointestinal absorption of norfloxacin. Antimicrob Agents Chemother. 1989 Jan;33(1):99–102. doi: 10.1128/aac.33.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry M. F., Smego D. A., Digiovanni M. A. Hepatobiliary kinetics and excretion of ciprofloxacin. Antimicrob Agents Chemother. 1988 Jul;32(7):982–985. doi: 10.1128/aac.32.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. H., Williams E. W. Interaction between ciprofloxacin and vancomycin against staphylococci. J Antimicrob Chemother. 1987 Aug;20(2):251–254. doi: 10.1093/jac/20.2.251. [DOI] [PubMed] [Google Scholar]
- Patton W. N., Smith G. M., Leyland M. J., Geddes A. M. Multiply resistant Salmonella typhimurium septicaemia in an immunocompromised patient successfully treated with ciprofloxacin. J Antimicrob Chemother. 1985 Nov;16(5):667–669. doi: 10.1093/jac/16.5.667. [DOI] [PubMed] [Google Scholar]
- Pattyn S. R. Activity of ofloxacin and pefloxacin against Mycobacterium leprae in mice. Antimicrob Agents Chemother. 1987 Apr;31(4):671–672. doi: 10.1128/aac.31.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn S. R., Van Caekenberghe D. L., Verhoeven J. R. In vitro activity of five new quinolones against cultivable mycobacteria. Eur J Clin Microbiol. 1987 Oct;6(5):572–573. doi: 10.1007/BF02014249. [DOI] [PubMed] [Google Scholar]
- Pecquet S., Andremont A., Tancrède C. Effect of oral ofloxacin on fecal bacteria in human volunteers. Antimicrob Agents Chemother. 1987 Jan;31(1):124–125. doi: 10.1128/aac.31.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecquet S., Andremont A., Tancrède C. Selective antimicrobial modulation of the intestinal tract by norfloxacin in human volunteers and in gnotobiotic mice associated with a human fecal flora. Antimicrob Agents Chemother. 1986 Jun;29(6):1047–1052. doi: 10.1128/aac.29.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S. S., Jensen T., Hvidberg E. F. Comparative pharmacokinetics of ciprofloxacin and ofloxacin in cystic fibrosis patients. J Antimicrob Chemother. 1987 Oct;20(4):575–583. doi: 10.1093/jac/20.4.575. [DOI] [PubMed] [Google Scholar]
- Perronne C. M., Malinverni R., Glauser M. P. Treatment of Staphylococcus aureus endocarditis in rats with coumermycin A1 and ciprofloxacin, alone or in combination. Antimicrob Agents Chemother. 1987 Apr;31(4):539–543. doi: 10.1128/aac.31.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H. J. Comparison of intravenous ciprofloxacin and mezlocillin in treatment of complicated urinary tract infection. Eur J Clin Microbiol. 1986 Apr;5(2):253–255. doi: 10.1007/BF02014003. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Stein D., Guay D. R., Logan G., Obaid S., Gruninger R., Davies S., Breitenbucher R. Prospective study of lower respiratory tract infections in an extended-care nursing home program: potential role of oral ciprofloxacin. Am J Med. 1988 Aug;85(2):164–171. doi: 10.1016/s0002-9343(88)80336-x. [DOI] [PubMed] [Google Scholar]
- Petrou M. A., Rogers T. R. In-vitro activity of antifungal agents in combination with four quinolones. Drugs Exp Clin Res. 1988;14(1):9–18. [PubMed] [Google Scholar]
- Philip-Joet F., Nourrit J., Frances Y., Arnaud A. Enoxacin in the treatment of lower respiratory tract infections. J Antimicrob Chemother. 1988 Feb;21 (Suppl B):125–129. doi: 10.1093/jac/21.suppl_b.125. [DOI] [PubMed] [Google Scholar]
- Phillips I., Culebras E., Moreno F., Baquero F. Induction of the SOS response by new 4-quinolones. J Antimicrob Chemother. 1987 Nov;20(5):631–638. doi: 10.1093/jac/20.5.631. [DOI] [PubMed] [Google Scholar]
- Pichler H. E., Diridl G., Stickler K., Wolf D. Clinical efficacy of ciprofloxacin compared with placebo in bacterial diarrhea. Am J Med. 1987 Apr 27;82(4A):329–332. [PubMed] [Google Scholar]
- Pichler H., Diridl G., Wolf D. Ciprofloxacin in the treatment of acute bacterial diarrhea: a double blind study. Eur J Clin Microbiol. 1986 Apr;5(2):241–243. doi: 10.1007/BF02013998. [DOI] [PubMed] [Google Scholar]
- Piddock L. J., Wise R. The effect of altered porin expression in Escherichia coli upon susceptibility to 4-quinolones. J Antimicrob Chemother. 1986 Oct;18(4):547–549. doi: 10.1093/jac/18.4.547. [DOI] [PubMed] [Google Scholar]
- Piercy E. A., Barbaro D., Luby J. P., Mackowiak P. A. Ciprofloxacin for methicillin-resistant Staphylococcus aureus infections. Antimicrob Agents Chemother. 1989 Jan;33(1):128–130. doi: 10.1128/aac.33.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piffaretti J. C., Arini A., Peduzzi R. In vitro activity of norfloxacin against Neisseria gonorrhoeae. Chemotherapy. 1983;29(6):415–418. doi: 10.1159/000238229. [DOI] [PubMed] [Google Scholar]
- Pizzo P. A., Robichaud K. J., Edwards B. K., Schumaker C., Kramer B. S., Johnson A. Oral antibiotic prophylaxis in patients with cancer: a double-blind randomized placebo-controlled trial. J Pediatr. 1983 Jan;102(1):125–133. doi: 10.1016/s0022-3476(83)80310-2. [DOI] [PubMed] [Google Scholar]
- Pohlod D. J., Saravolatz L. D. Activity of quinolones against Legionellaceae. J Antimicrob Chemother. 1986 Apr;17(4):540–541. doi: 10.1093/jac/17.4.540. [DOI] [PubMed] [Google Scholar]
- Pohlod D. J., Saravolatz L. D. In vitro susceptibilities of 393 recent clinical isolates to WIN 49375, cefotaxime, tobramycin, and piperacillin. Antimicrob Agents Chemother. 1984 Mar;25(3):377–379. doi: 10.1128/aac.25.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlod D. J., Saravolatz L. D., Somerville M. M. In-vitro susceptibility of staphylococci to fleroxacin in comparison with six other quinolones. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):35–41. doi: 10.1093/jac/22.supplement_d.35. [DOI] [PubMed] [Google Scholar]
- Poitevin M., Ly M., Daubras M., Ichou F. Etude in vivo de la sensibilité de Treponema pallidum à l'ofloxacine. Pathol Biol (Paris) 1988 May;36(5):482–487. [PubMed] [Google Scholar]
- Prabhala R. H., Rao B., Marshall R., Bansal M. B., Thadepalli H. In vitro susceptibility of anaerobic bacteria to ciprofloxacin (Bay o 9867). Antimicrob Agents Chemother. 1984 Nov;26(5):785–786. doi: 10.1128/aac.26.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preheim L. C., Cuevas T. A., Roccaforte J. S., Mellencamp M. A., Bittner M. J. Ciprofloxacin and antacids. Lancet. 1986 Jul 5;2(8497):48–48. doi: 10.1016/s0140-6736(86)92596-1. [DOI] [PubMed] [Google Scholar]
- Preheim L. C., Cuevas T. A., Roccaforte J. S., Mellencamp M. A., Bittner M. J. Oral ciprofloxacin in the treatment of elderly patients with complicated urinary tract infections due to trimethoprim/sulfamethoxazole-resistant bacteria. Am J Med. 1987 Apr 27;82(4A):295–300. [PubMed] [Google Scholar]
- Preiksaitis J. K., Thompson L., Harding G. K., Marrie T. J., Hoban S., Ronald A. R. A comparison of the efficacy of nalidixic acid and cephalexin in bacteriuric women and their effect on fecal and periurethral carriage of enterobacteriaceae. J Infect Dis. 1981 Apr;143(4):603–608. doi: 10.1093/infdis/143.4.603. [DOI] [PubMed] [Google Scholar]
- Prigogine T., Glupczynski Y., Carpiaux J. P., Blogie M., Yourassowsky E., Schmerber J. S. Enoxacin in acute exacerbations of chronic bronchitis: a comparison with amoxycillin. J Antimicrob Chemother. 1988 Feb;21 (Suppl B):131–136. doi: 10.1093/jac/21.suppl_b.131. [DOI] [PubMed] [Google Scholar]
- Pugsley M. P., Dworzack D. L., Horowitz E. A., Cuevas T. A., Sanders W. E., Jr, Sanders C. C. Efficacy of ciprofloxacin in the treatment of nasopharyngeal carriers of Neisseria meningitidis. J Infect Dis. 1987 Jul;156(1):211–213. doi: 10.1093/infdis/156.1.211. [DOI] [PubMed] [Google Scholar]
- Pust R. A., Ackenheil-Köppe H. R., Weidner W., Meier-Ewert H. Clinical efficacy and tolerance of fleroxacin in patients with urethritis caused by Chlamydia trachomatis. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):227–230. doi: 10.1093/jac/22.supplement_d.227. [DOI] [PubMed] [Google Scholar]
- Puthucheary S. D., Parasakthi N. Antimicrobial susceptibility of Pseudomonas pseudomallei. J Antimicrob Chemother. 1987 Dec;20(6):921–922. doi: 10.1093/jac/20.6.921. [DOI] [PubMed] [Google Scholar]
- Pérez-Ruvalcaba J. A., Quintero-Pérez N. P., Morales-Reyes J. J., Huitrón-Ramírez J. A., Rodríguez-Chagollán J. J., Rodríguez-Noriega E. Double-blind comparison of ciprofloxacin with cefotaxime in the treatment of skin and skin structure infections. Am J Med. 1987 Apr 27;82(4A):242–246. [PubMed] [Google Scholar]
- Pérez J. L., Riera L., Valls F., Berrocal C. I., Berrocal L. A comparison of the in-vitro activity of seventeen antibiotics against Streptococcus faecalis. J Antimicrob Chemother. 1987 Sep;20(3):357–362. doi: 10.1093/jac/20.3.357. [DOI] [PubMed] [Google Scholar]
- Quentin R., Koubaa N., Cattier B., Gavignet M., Goudeau A. In vitro activities of five new quinolones against 88 genital and neonatal Haemophilus isolates. Antimicrob Agents Chemother. 1988 Jan;32(1):147–149. doi: 10.1128/aac.32.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintiliani R., Cooper B. W., Briceland L. L., Nightingale C. H. Economic impact of streamlining antibiotic administration. Am J Med. 1987 Apr 27;82(4A):391–394. [PubMed] [Google Scholar]
- Raeburn J. A., Govan J. R., McCrae W. M., Greening A. P., Collier P. S., Hodson M. E., Goodchild M. C. Ciprofloxacin therapy in cystic fibrosis. J Antimicrob Chemother. 1987 Sep;20(3):295–296. doi: 10.1093/jac/20.3.295. [DOI] [PubMed] [Google Scholar]
- Ramirez-Ronda C. H., Saavedra S., Rivera-Vázquez C. R. Comparative, double-blind study of oral ciprofloxacin and intravenous cefotaxime in skin and skin structure infections. Am J Med. 1987 Apr 27;82(4A):220–223. [PubMed] [Google Scholar]
- Ramirez C. A., Bran J. L., Mejia C. R., Garcia J. F. Open, prospective study of the clinical efficacy of ciprofloxacin. Antimicrob Agents Chemother. 1985 Jul;28(1):128–132. doi: 10.1128/aac.28.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D. S., Parfitt A. M., Villanueva A. R., Dorman P. J., Kleerekoper M. Hypophosphatemic osteomalacia and adult Fanconi syndrome due to light-chain nephropathy. Another form of oncogenous osteomalacia. Am J Med. 1987 Feb;82(2):333–338. doi: 10.1016/0002-9343(87)90081-7. [DOI] [PubMed] [Google Scholar]
- Raoof S., Wollschlager C., Khan F. A. Ciprofloxacin increases serum levels of theophylline. Am J Med. 1987 Apr 27;82(4A):115–118. [PubMed] [Google Scholar]
- Raoof S., Wollschlager C., Khan F. Treatment of respiratory tract infections with ciprofloxacin. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):139–145. doi: 10.1093/jac/18.supplement_d.139. [DOI] [PubMed] [Google Scholar]
- Raoult D., Roussellier P., Galicher V., Perez R., Tamalet J. In vitro susceptibility of Rickettsia conorii to ciprofloxacin as determined by suppressing lethality in chicken embryos and by plaque assay. Antimicrob Agents Chemother. 1986 Mar;29(3):424–425. doi: 10.1128/aac.29.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoult D., Roussellier P., Vestris G., Galicher V., Perez R., Tamalet J. Susceptibility of Rickettsia conorii and R. rickettsii to pefloxacin, in vitro and in ovo. J Antimicrob Chemother. 1987 Mar;19(3):303–305. doi: 10.1093/jac/19.3.303. [DOI] [PubMed] [Google Scholar]
- Rau D. C., Gellert M., Thoma F., Maxwell A. Structure of the DNA gyrase-DNA complex as revealed by transient electric dichroism. J Mol Biol. 1987 Feb 5;193(3):555–569. doi: 10.1016/0022-2836(87)90266-x. [DOI] [PubMed] [Google Scholar]
- Reeves D. S., Bywater M. J., Holt H. A. The activity of enoxacin against clinical bacterial isolates in comparison with that of five other agents, and factors affecting that activity. J Antimicrob Chemother. 1984 Sep;14 (Suppl 100):7–17. doi: 10.1093/jac/14.suppl_c.7. [DOI] [PubMed] [Google Scholar]
- Rella M., Haas D. Resistance of Pseudomonas aeruginosa PAO to nalidixic acid and low levels of beta-lactam antibiotics: mapping of chromosomal genes. Antimicrob Agents Chemother. 1982 Aug;22(2):242–249. doi: 10.1128/aac.22.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudin H., Quentin C., de Barbeyrac B., Bebear C. Activité in vitro de nouvelles quinolones sur les mycoplasmes pathogènes pour l'homme. Pathol Biol (Paris) 1988 May;36(5):496–499. [PubMed] [Google Scholar]
- Renkonen O. V., Sivonen A., Visakorpi R. Effect of ciprofloxacin on carrier rate of Neisseria meningitidis in army recruits in Finland. Antimicrob Agents Chemother. 1987 Jun;31(6):962–963. doi: 10.1128/aac.31.6.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescott D. L., Nix D. E., Holden P., Schentag J. J. Comparison of two methods for determining in vitro postantibiotic effects of three antibiotics on Escherichia coli. Antimicrob Agents Chemother. 1988 Apr;32(4):450–453. doi: 10.1128/aac.32.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S. M., Higgins C. F., Lilley D. M. The genetic control of DNA supercoiling in Salmonella typhimurium. EMBO J. 1984 Aug;3(8):1745–1752. doi: 10.1002/j.1460-2075.1984.tb02041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond S. J., Bhattacharyya M. N., Maiti H., Chowdhury F. H., Stirland R. M., Tooth J. A. The efficacy of ofloxacin against infection caused by Neisseria gonorrhoeae and Chlamydia trachomatis. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):149–153. doi: 10.1093/jac/22.supplement_c.149. [DOI] [PubMed] [Google Scholar]
- Ridgway G. L., O'Hare M. D., Felmingham D., Grüneberg R. N. The comparative activity of twelve 4-quinolone antimicrobials against Haemophilus influenzae and Streptococcus pneumoniae. Drugs Exp Clin Res. 1985;11(4):259–262. [PubMed] [Google Scholar]
- Righter J. Ciprofloxacin treatment of Staphylococcus aureus infections. J Antimicrob Chemother. 1987 Oct;20(4):595–597. doi: 10.1093/jac/20.4.595. [DOI] [PubMed] [Google Scholar]
- Rippelmeyer D. J., Synhavsky A. Ciprofloxacin and allergic interstitial nephritis. Ann Intern Med. 1988 Jul 15;109(2):170–170. doi: 10.7326/0003-4819-109-2-170_1. [DOI] [PubMed] [Google Scholar]
- Robbins M. J., O'Hare M. D., Felmingham D., Ridgway G. L., Grüneberg R. N. The comparative activity of twelve 4-quinolone antimicrobials against gram-positive and gram-negative anaerobes. Drugs Exp Clin Res. 1985;11(7):431–434. [PubMed] [Google Scholar]
- Robillard N. J., Scarpa A. L. Genetic and physiological characterization of ciprofloxacin resistance in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1988 Apr;32(4):535–539. doi: 10.1128/aac.32.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy R. E., Handsfield H. H., Hook E. W., 3rd Comparative trial of single-dose ciprofloxacin and ampicillin plus probenecid for treatment of gonococcal urethritis in men. Antimicrob Agents Chemother. 1986 Aug;30(2):267–269. doi: 10.1128/aac.30.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerie F., Ott D., Vandepitte J., Verbist L., Lemmens P., Habiyaremye I. Comparison of norfloxacin and nalidixic acid for treatment of dysentery caused by Shigella dysenteriae type 1 in adults. Antimicrob Agents Chemother. 1986 May;29(5):883–886. doi: 10.1128/aac.29.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing S. R., Landmesser J. E., Gerster J. F., Pecore S. E., Stern R. M. Differentiation of fluorinated quinolone antibacterials with Neisseria gonorrhoeae isolates. J Antimicrob Chemother. 1985 May;15(5):539–544. doi: 10.1093/jac/15.5.539. [DOI] [PubMed] [Google Scholar]
- Rolston K. V., Alvarez M. E., Hsu K. C., Bodey G. P. In-vitro activity of cefpirome (HR-810), WIN-49375, BMY-28142 and other antibiotics against nosocomially important isolates from cancer patients. J Antimicrob Chemother. 1986 Apr;17(4):453–457. doi: 10.1093/jac/17.4.453. [DOI] [PubMed] [Google Scholar]
- Rolston K. V., Anaissie E. A., Bodey G. P. In-vitro susceptibility of Pseudomonas species to fifteen antimicrobial agents. J Antimicrob Chemother. 1987 Feb;19(2):193–196. doi: 10.1093/jac/19.2.193. [DOI] [PubMed] [Google Scholar]
- Rolston K. V., Bodey G. P. In vitro activity of various new antimicrobial agents against group JK corynebacteria. Eur J Clin Microbiol. 1987 Aug;6(4):491–492. doi: 10.1007/BF02013120. [DOI] [PubMed] [Google Scholar]
- Rolston K. V., Bodey G. P. In vitro susceptibility of Acinetobacter species to various antimicrobial agents. Antimicrob Agents Chemother. 1986 Nov;30(5):769–770. doi: 10.1128/aac.30.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolston K. V., Ho D. H., LeBlanc B., Bodey G. P. Activity of newer antimicrobial agents against Aeromonas hydrophila. Eur J Clin Microbiol. 1986 Aug;5(4):454–456. doi: 10.1007/BF02075707. [DOI] [PubMed] [Google Scholar]
- Rolston K. V., Ho D. H., LeBlanc B., Gooch G., Bodey G. P. Comparative in vitro activity of the new difluoro-quinolone temafloxacin (A-62254) against bacterial isolates from cancer patients. Eur J Clin Microbiol Infect Dis. 1988 Oct;7(5):684–686. doi: 10.1007/BF01964255. [DOI] [PubMed] [Google Scholar]
- Rolston K. V. Susceptibility of group B and group G streptococci to newer antimicrobial agents. Eur J Clin Microbiol. 1986 Oct;5(5):534–536. doi: 10.1007/BF02017697. [DOI] [PubMed] [Google Scholar]
- Romanowski B., Wood H., Draker J., Tsianco M. C. Norfloxacin in the therapy of uncomplicated gonorrhea. Antimicrob Agents Chemother. 1986 Sep;30(3):514–515. doi: 10.1128/aac.30.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A. R., Turck M., Petersdorf R. G. A critical evaluation of nalidixic acid in urinary-tract infections. N Engl J Med. 1966 Nov 17;275(20):1081–1089. doi: 10.1056/NEJM196611172752001. [DOI] [PubMed] [Google Scholar]
- Ronco E., Pilliot J. Activité antistaphylococcique comparée in vitro de la norfloxacine et de la péfloxacine sur 312 souches hospitaliéres. Pathol Biol (Paris) 1985 May;33(5):381–384. [PubMed] [Google Scholar]
- Rosen T., Chu D. T., Lico I. M., Fernandes P. B., Marsh K., Shen L., Cepa V. G., Pernet A. G. Design, synthesis, and properties of (4S)-7-(4-amino-2-substituted-pyrrolidin-1-yl)quinolone-3-carboxylic acids. J Med Chem. 1988 Aug;31(8):1598–1611. doi: 10.1021/jm00403a020. [DOI] [PubMed] [Google Scholar]
- Rosen T., Chu D. T., Lico I. M., Fernandes P. B., Shen L., Borodkin S., Pernet A. G. Asymmetric synthesis and properties of the enantiomers of the antibacterial agent 7-(3-aminopyrrolidin-1-yl)-1-(2,4-difluorophenyl)-1,4-dihydro-6- fluoro-4-oxo-1,8-naphthyridine-3-carboxylic acid hydrochloride. J Med Chem. 1988 Aug;31(8):1586–1590. doi: 10.1021/jm00403a017. [DOI] [PubMed] [Google Scholar]
- Rubinstein E., Segev S. Drug interactions of ciprofloxacin with other non-antibiotic agents. Am J Med. 1987 Apr 27;82(4A):119–123. [PubMed] [Google Scholar]
- Rubio T. T. Ciprofloxacin: comparative data in cystic fibrosis. Am J Med. 1987 Apr 27;82(4A):185–188. [PubMed] [Google Scholar]
- Rubio T. T., Shapiro C. Ciprofloxacin in the treatment of Pseudomonas infection in cystic fibrosis patients. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):147–152. doi: 10.1093/jac/18.supplement_d.147. [DOI] [PubMed] [Google Scholar]
- Rudin J. E., Norden C. W., Shinners E. M. In vitro activity of ciprofloxacin against aerobic gram-negative bacteria. Antimicrob Agents Chemother. 1984 Oct;26(4):597–598. doi: 10.1128/aac.26.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudrik J. T., Cavalieri S. J., Britt E. M. In vitro activities of enoxacin and 17 other antimicrobial agents against multiply resistant, gram-negative bacteria. Antimicrob Agents Chemother. 1984 Jul;26(1):97–100. doi: 10.1128/aac.26.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummens J. L., Gordts B., Van Landuyt H. W. In vitro susceptibility of Capnocytophaga species to 29 antimicrobial agents. Antimicrob Agents Chemother. 1986 Nov;30(5):739–742. doi: 10.1128/aac.30.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. L., Berenson C. S., Greco T. P., Mangi R. J., Sims M., Thornton G. F., Andriole V. T. Oral ciprofloxacin in resistant urinary tract infections. Am J Med. 1987 Apr 27;82(4A):303–306. [PubMed] [Google Scholar]
- Saavedra S., Ramírez-Ronda C. H., Nevárez M. Ciprofloxacin in the treatment of urinary tract infections caused by Pseudomonas aeruginosa and multiresistant bacteria. Eur J Clin Microbiol. 1986 Apr;5(2):255–257. doi: 10.1007/BF02014004. [DOI] [PubMed] [Google Scholar]
- Sabbaj J., Hoagland V. L., Cook T. Norfloxacin versus co-trimoxazole in the treatment of recurring urinary tract infections in men. Scand J Infect Dis Suppl. 1986;48:48–53. [PubMed] [Google Scholar]
- Sabbaj J., Hoagland V. L., Shih W. J. Multiclinic comparative study of norfloxacin and trimethoprim-sulfamethoxazole for treatment of urinary tract infections. Antimicrob Agents Chemother. 1985 Mar;27(3):297–301. doi: 10.1128/aac.27.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A., Koga H., Shigeno H., Watanabe K., Mori K., Kohno S., Shigeno Y., Suzuyama Y., Yamaguchi K., Hirota M. The antimicrobial activity of ciprofloxacin against Legionella species and the treatment of experimental Legionella pneumonia in guinea pigs. J Antimicrob Chemother. 1986 Aug;18(2):251–260. doi: 10.1093/jac/18.2.251. [DOI] [PubMed] [Google Scholar]
- Saito A., Sawatari K., Fukuda Y., Nagasawa M., Koga H., Tomonaga A., Nakazato H., Fujita K., Shigeno Y., Suzuyama Y. Susceptibility of Legionella pneumophila to ofloxacin in vitro and in experimental Legionella pneumonia in guinea pigs. Antimicrob Agents Chemother. 1985 Jul;28(1):15–20. doi: 10.1128/aac.28.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Tomioka H., Nagashima K. In vitro and in vivo activities of ofloxacin against Mycobacterium leprae infection induced in mice. Int J Lepr Other Mycobact Dis. 1986 Dec;54(4):560–562. [PubMed] [Google Scholar]
- Salam M. A., Bennish M. L. Therapy for shigellosis. I. Randomized, double-blind trial of nalidixic acid in childhood shigellosis. J Pediatr. 1988 Nov;113(5):901–907. doi: 10.1016/s0022-3476(88)80029-5. [DOI] [PubMed] [Google Scholar]
- Samonis G., Ho D. H., Gooch G. F., Rolston K. V., Bodey G. P. In vitro susceptibility of Citrobacter species to various antimicrobial agents. Antimicrob Agents Chemother. 1987 May;31(5):829–830. doi: 10.1128/aac.31.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Joaquin V. H., Scribner R. K., Pickett D. A., Welch D. F. Antimicrobial susceptibility of Aeromonas species isolated from patients with diarrhea. Antimicrob Agents Chemother. 1986 Nov;30(5):794–795. doi: 10.1128/aac.30.5.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J. P., Domagala J. M., Hagen S. E., Heifetz C. L., Hutt M. P., Nichols J. B., Trehan A. K. Quinolone antibacterial agents. Synthesis and structure-activity relationships of 8-substituted quinoline-3-carboxylic acids and 1,8-naphthyridine-3-carboxylic acids. J Med Chem. 1988 May;31(5):983–991. doi: 10.1021/jm00400a016. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V. Overview of preclinical studies with ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):2–11. [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V., Werner V. Selection of multiple antibiotic resistance by quinolones, beta-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob Agents Chemother. 1984 Dec;26(6):797–801. doi: 10.1128/aac.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Watanakunakorn C. Emergence of resistance to beta-lactams, aminoglycosides, and quinolones during combination therapy for infection due to Serratia marcescens. J Infect Dis. 1986 Mar;153(3):617–619. doi: 10.1093/infdis/153.3.617. [DOI] [PubMed] [Google Scholar]
- Sanders W. E., Jr Efficacy, safety, and potential economic benefits of oral ciprofloxacin in the treatment of infections. Rev Infect Dis. 1988 May-Jun;10(3):528–543. doi: 10.1093/clinids/10.3.528. [DOI] [PubMed] [Google Scholar]
- Sanson-Le Pors M. J., Casin I. M., Thebault M. C., Arlet G., Perol Y. In vitro activities of U-63366, a spectinomycin analog; roxithromycin (RU 28965), a new macrolide antibiotic; and five quinolone derivatives against Haemophilus ducreyi. Antimicrob Agents Chemother. 1986 Sep;30(3):512–513. doi: 10.1128/aac.30.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanzey B. Modulation of gene expression by drugs affecting deoxyribonucleic acid gyrase. J Bacteriol. 1979 Apr;138(1):40–47. doi: 10.1128/jb.138.1.40-47.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Inoue Y., Fujii T., Aoyama H., Inoue M., Mitsuhashi S. Purification and properties of DNA gyrase from a fluoroquinolone-resistant strain of Escherichia coli. Antimicrob Agents Chemother. 1986 Nov;30(5):777–780. doi: 10.1128/aac.30.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Matsuura Y., Inoue M., Une T., Osada Y., Ogawa H., Mitsuhashi S. In vitro and in vivo activity of DL-8280, a new oxazine derivative. Antimicrob Agents Chemother. 1982 Oct;22(4):548–553. doi: 10.1128/aac.22.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaglione F., Ferrara F., Braga P. C., Zanollo A., Esposti D., Fraschini F. Differentiated distribution of norfloxacin in the medullary-cortical part of the kidney in man. Int J Clin Pharmacol Res. 1986;6(5):351–354. [PubMed] [Google Scholar]
- Schaad U. B., Wedgwood-Krucko J. Nalidixic acid in children: retrospective matched controlled study for cartilage toxicity. Infection. 1987 May-Jun;15(3):165–168. doi: 10.1007/BF01646040. [DOI] [PubMed] [Google Scholar]
- Schlenkhoff D., Dalhoff A., Knopf J., Opferkuch W. Penetration of ciprofloxacin into human lung tissue following intravenous injection. Infection. 1986 Nov-Dec;14(6):299–300. doi: 10.1007/BF01643967. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Jauregui L., Lettieri J., Bachmann K. Impact of ciprofloxacin on theophylline clearance and steady-state concentrations in serum. Antimicrob Agents Chemother. 1988 Jan;32(1):75–77. doi: 10.1128/aac.32.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman M. S., Franck W. A. Vitamin D toxicity complicating the treatment of senile, postmenopausal, and glucocorticoid-induced osteoporosis. Four case reports and a critical commentary on the use of vitamin D in these disorders. Am J Med. 1987 Feb;82(2):224–230. doi: 10.1016/0002-9343(87)90060-x. [DOI] [PubMed] [Google Scholar]
- Scott A. C., Fleming L. W., Stewart W. K. Efficacy of ciprofloxacin in treatment of CAPD peritonitis. Eur J Clin Microbiol. 1987 Oct;6(5):599–599. doi: 10.1007/BF02014261. [DOI] [PubMed] [Google Scholar]
- Scott G. R., McMillan A., Young H. Ciprofloxacin versus ampicillin and probenecid in the treatment of uncomplicated gonorrhoea in men. J Antimicrob Chemother. 1987 Jul;20(1):117–121. doi: 10.1093/jac/20.1.117. [DOI] [PubMed] [Google Scholar]
- Scully B. E., Jules K., Chin N. X., Neu H. C. Effect of ciprofloxacin on fecal flora of patients with cystic fibrosis and other patients treated with oral ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):336–338. [PubMed] [Google Scholar]
- Scully B. E., Nakatomi M., Ores C., Davidson S., Neu H. C. Ciprofloxacin therapy in cystic fibrosis. Am J Med. 1987 Apr 27;82(4A):196–201. [PubMed] [Google Scholar]
- Scully B. E., Neu H. C., Parry M. F., Mandell W. Oral ciprofloxacin therapy of infections due to Pseudomonas aeruginosa. Lancet. 1986 Apr 12;1(8485):819–822. doi: 10.1016/s0140-6736(86)90937-2. [DOI] [PubMed] [Google Scholar]
- Segev S., Rehavi M., Rubinstein E. Quinolones, theophylline, and diclofenac interactions with the gamma-aminobutyric acid receptor. Antimicrob Agents Chemother. 1988 Nov;32(11):1624–1626. doi: 10.1128/aac.32.11.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev S., Rubinstein E., Shick J., Rabinovitch O., Dolitsky M. Penetration of ciprofloxacin into female pelvic tissues. Eur J Clin Microbiol. 1986 Apr;5(2):207–209. doi: 10.1007/BF02013989. [DOI] [PubMed] [Google Scholar]
- Segreti J., Kessler H. A., Kapell K. S., Trenholme G. M. In vitro activity of A-56268 (TE-031) and four other antimicrobial agents against Chlamydia trachomatis. Antimicrob Agents Chemother. 1987 Jan;31(1):100–101. doi: 10.1128/aac.31.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert G., Limbert M., Klesel N. Comparison of the antibacterial in vitro and in vivo activity of ofloxacin (HOE 280 DL 8280) and nalidixic acid analogues. Eur J Clin Microbiol. 1983 Dec;2(6):548–553. doi: 10.1007/BF02016563. [DOI] [PubMed] [Google Scholar]
- Shah P. M., Ottrad M., Stille W. In vitro activity of norfloxacin in urine compared to that of cinnoxacin, nalidixic acid and pipemidic acid. Eur J Clin Microbiol. 1983 Jun;2(3):272–274. doi: 10.1007/BF02029533. [DOI] [PubMed] [Google Scholar]
- Shah P. M., Sammann A., Schäfer V., Seczendi M., Knothe H. Fleroxacin: safety, tolerance and effect on the faecal flora of healthy volunteers. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):209–213. doi: 10.1093/jac/22.supplement_d.209. [DOI] [PubMed] [Google Scholar]
- Shahmanesh M., Shukla S. R., Phillips I., Westwood A., Thin R. N. Ciprofloxacin for treating urethral gonorrhoea in men. Genitourin Med. 1986 Apr;62(2):86–87. doi: 10.1136/sti.62.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalit I., Greenwood R. B., Marks M. I., Pederson J. A., Frederick D. L. Pharmacokinetics of single-dose oral ciprofloxacin in patients undergoing chronic ambulatory peritoneal dialysis. Antimicrob Agents Chemother. 1986 Jul;30(1):152–156. doi: 10.1128/aac.30.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalit I., Stutman H. R., Marks M. I., Chartrand S. A., Hilman B. C. Randomized study of two dosage regimens of ciprofloxacin for treating chronic bronchopulmonary infection in patients with cystic fibrosis. Am J Med. 1987 Apr 27;82(4A):189–195. [PubMed] [Google Scholar]
- Shea M. J., Deanfield J. E., Wilson R. A., Delandsheere C. M., Selwyn A. P. Silent myocardial ischemia during mastication. Am J Med. 1987 Feb;82(2):357–360. doi: 10.1016/0002-9343(87)90087-8. [DOI] [PubMed] [Google Scholar]
- Sheehan G. J., Harding G. K., Haase D. A., Thomson M. J., Urias B., Kennedy J. K., Hoban D. J., Ronald A. R. Double-blind, randomized comparison of 24 weeks of norfloxacin and 12 weeks of norfloxacin followed by 12 weeks of placebo in the therapy of complicated urinary tract infection. Antimicrob Agents Chemother. 1988 Aug;32(8):1292–1293. doi: 10.1128/aac.32.8.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley E. D., Shelley W. B. The subcorneal pustular drug eruption: an example induced by norfloxacin. Cutis. 1988 Jul;42(1):24–27. [PubMed] [Google Scholar]
- Shen L. L., Kohlbrenner W. E., Weigl D., Baranowski J. Mechanism of quinolone inhibition of DNA gyrase. Appearance of unique norfloxacin binding sites in enzyme-DNA complexes. J Biol Chem. 1989 Feb 15;264(5):2973–2978. [PubMed] [Google Scholar]
- Shen L. L., Pernet A. G. Mechanism of inhibition of DNA gyrase by analogues of nalidixic acid: the target of the drugs is DNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):307–311. doi: 10.1073/pnas.82.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibl A. M., Hackbarth C. J., Sande M. A. Evaluation of pefloxacin in experimental Escherichia coli meningitis. Antimicrob Agents Chemother. 1986 Mar;29(3):409–411. doi: 10.1128/aac.29.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada H., Ebine Y., Sato T., Kurosawa Y., Arauchi T. Dominant lethal study in male mice treated with ofloxacin, a new antimicrobial drug. Mutat Res. 1985 Sep;144(1):51–55. doi: 10.1016/0165-7992(85)90123-x. [DOI] [PubMed] [Google Scholar]
- Shimada J., Yamaji T., Ueda Y., Uchida H., Kusajima H., Irikura T. Mechanism of renal excretion of AM-715, a new quinolonecarboxylic acid derivative, in rabbits, dogs, and humans. Antimicrob Agents Chemother. 1983 Jan;23(1):1–7. doi: 10.1128/aac.23.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungu D. L., Nalin D. R., Gilman R. H., Gadebusch H. H., Cerami A. T., Gill C., Weissberger B. Comparative susceptibilities of Campylobacter pylori to norfloxacin and other agents. Antimicrob Agents Chemother. 1987 Jun;31(6):949–950. doi: 10.1128/aac.31.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungu D. L., Tutlane V. K., Weinberg E., Gadebusch H. H. In vitro antibacterial activity of norfloxacin and other agents against ocular pathogens. Chemotherapy. 1985;31(2):112–118. doi: 10.1159/000238322. [DOI] [PubMed] [Google Scholar]
- Shungu D. L., Weinberg E., Gadebusch H. H. In vitro antibacterial activity of norfloxacin (MK-0366, AM-715) and other agents against gastrointestinal tract pathogens. Antimicrob Agents Chemother. 1983 Jan;23(1):86–90. doi: 10.1128/aac.23.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungu D. L., Weinberg E., Gadebusch H. H. Tentative interpretive standards for disk diffusion susceptibility testing with norfloxacin (MK-0366, AM-715). Antimicrob Agents Chemother. 1983 Feb;23(2):256–260. doi: 10.1128/aac.23.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siboulet A., Bohbot J. M., Catalan F. Enoxacin in the treatment of sexually transmitted diseases. J Antimicrob Chemother. 1988 Feb;21 (Suppl B):119–124. doi: 10.1093/jac/21.suppl_b.119. [DOI] [PubMed] [Google Scholar]
- Simberkoff M. S., Rahal J. J., Jr Bactericidal activity of ciprofloxacin against amikacin- and cefotaxime-resistant gram-negative bacilli and methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1986 Jun;29(6):1098–1100. doi: 10.1128/aac.29.6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C., Lindner U. In vitro activity of norfloxacin against Mycoplasma hominis and Ureaplasma urealyticum. Eur J Clin Microbiol. 1983 Oct;2(5):479–480. doi: 10.1007/BF02013911. [DOI] [PubMed] [Google Scholar]
- Singlas E., Taburet A. M., Landru I., Albin H., Ryckelinck J. P. Pharmacokinetics of ciprofloxacin tablets in renal failure; influence of haemodialysis. Eur J Clin Pharmacol. 1987;31(5):589–593. doi: 10.1007/BF00606636. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Kubo M., Imamoto F. Promoter-specific inhibition of transcription by antibiotics which act on DNA gyrase. Nature. 1978 Oct 5;275(5679):420–423. doi: 10.1038/275420a0. [DOI] [PubMed] [Google Scholar]
- Smith G. M., Leyland M. J., Farrell I. D., Geddes A. M. A clinical, microbiological and pharmacokinetic study of ciprofloxacin plus vancomycin as initial therapy of febrile episodes in neutropenic patients. J Antimicrob Chemother. 1988 May;21(5):647–655. doi: 10.1093/jac/21.5.647. [DOI] [PubMed] [Google Scholar]
- Smith G. M., Leyland M. J., Farrell I. D., Geddes A. M. Preliminary evaluation of ciprofloxacin, a new 4-quinolone antibiotic, in the treatment of febrile neutropenic patients. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):165–174. doi: 10.1093/jac/18.supplement_d.165. [DOI] [PubMed] [Google Scholar]
- Smith J. T. Frequency and expression of mutational resistance to the 4-quinolone antibacterials. Scand J Infect Dis Suppl. 1986;49:115–123. [PubMed] [Google Scholar]
- Smith M. J., Hodson M. E., Batten J. C. Ciprofloxacin in cystic fibrosis. Lancet. 1986 May 10;1(8489):1103–1103. doi: 10.1016/s0140-6736(86)91374-7. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H. Activity of ciprofloxacin against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 May;27(5):688–691. doi: 10.1128/aac.27.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H., Berman E. The effect of ciprofloxacin on methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1986 Mar;17(3):287–295. doi: 10.1093/jac/17.3.287. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H., Cherubin C. E. Conditions affecting the results of susceptibility testing for the quinolone compounds. Chemotherapy. 1988;34(4):308–314. doi: 10.1159/000238584. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H. Interaction of ciprofloxacin with ampicillin and vancomycin for Streptococcus faecalis. Diagn Microbiol Infect Dis. 1988 Apr;9(4):239–243. doi: 10.1016/0732-8893(88)90115-0. [DOI] [PubMed] [Google Scholar]
- Smith S. M. In vitro comparison of A-56619, A-56620, amifloxacin, ciprofloxacin, enoxacin, norfloxacin, and ofloxacin against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Feb;29(2):325–326. doi: 10.1128/aac.29.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneller M., Wellborne F., Barile M. F., Plotz P. Prosthetic joint infection with Mycoplasma hominis. J Infect Dis. 1986 Jan;153(1):174–175. doi: 10.1093/infdis/153.1.174. [DOI] [PubMed] [Google Scholar]
- Snyder M., Drlica K. DNA gyrase on the bacterial chromosome: DNA cleavage induced by oxolinic acid. J Mol Biol. 1979 Jun 25;131(2):287–302. doi: 10.1016/0022-2836(79)90077-9. [DOI] [PubMed] [Google Scholar]
- Somogyi A. A., Bochner F., Keal J. A., Rolan P. E., Smith M. Effect of food on enoxacin absorption. Antimicrob Agents Chemother. 1987 Apr;31(4):638–639. doi: 10.1128/aac.31.4.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgel F., Metz R., Naber K., Seelmann R., Muth P. Pharmacokinetics and body fluid penetration of fleroxacin in healthy volunteers. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):155–167. doi: 10.1093/jac/22.supplement_d.155. [DOI] [PubMed] [Google Scholar]
- Speciale A., Stefani S., Caccamo F., Nicolosi V. M., Nicoletti G. The sensitivity of gram-negative and gram-positive bacteria to ofloxacin. Drugs Exp Clin Res. 1987;13(9):555–561. [PubMed] [Google Scholar]
- Staib A. H., Harder S., Mieke S., Beer C., Stille W., Shah P. M., Frech K. Gyrase-Hemmer können die Coffein-Elimination verzögern. Dtsch Med Wochenschr. 1986 Sep 26;111(39):1500–1500. [PubMed] [Google Scholar]
- Staib A. H., Harder S., Mieke S., Beer C., Stille W., Shah P. Gyrase-inhibitors impair caffeine elimination in man. Methods Find Exp Clin Pharmacol. 1987 Mar;9(3):193–198. [PubMed] [Google Scholar]
- Staib A. H., Harder S., Papenburg A., Stille W. Gyrasehemmer beeinflussen Metabolisierungsprozesse in der Leber. Dtsch Med Wochenschr. 1987 Oct 30;112(44):1720–1721. [PubMed] [Google Scholar]
- Stamey T. A., Bragonje J. Resistance to nalidixic acid. A misconception due to underdosage. JAMA. 1976 Oct 18;236(16):1857–1860. [PubMed] [Google Scholar]
- Steck T. R., Drlica K. Bacterial chromosome segregation: evidence for DNA gyrase involvement in decatenation. Cell. 1984 Apr;36(4):1081–1088. doi: 10.1016/0092-8674(84)90058-8. [DOI] [PubMed] [Google Scholar]
- Steck T. R., Pruss G. J., Manes S. H., Burg L., Drlica K. DNA supercoiling in gyrase mutants. J Bacteriol. 1984 May;158(2):397–403. doi: 10.1128/jb.158.2.397-403.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. E. Review of the bioavailability and pharmacokinetics of oral norfloxacin. Am J Med. 1987 Jun 26;82(6B):18–21. doi: 10.1016/0002-9343(87)90613-9. [DOI] [PubMed] [Google Scholar]
- Stille W., Harder S., Mieke S., Beer C., Shah P. M., Frech K., Staib A. H. Decrease of caffeine elimination in man during co-administration of 4-quinolones. J Antimicrob Chemother. 1987 Nov;20(5):729–734. doi: 10.1093/jac/20.5.729. [DOI] [PubMed] [Google Scholar]
- Stiver H. G., Bartlett K. H., Chow A. W. Comparison of susceptibility of gentamicin-resistant and -susceptible "Acinetobacter anitratus" to 15 alternative antibiotics. Antimicrob Agents Chemother. 1986 Oct;30(4):624–625. doi: 10.1128/aac.30.4.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobberingh E. E., Houben A. W., van Boven C. P. In vitro evaluation of Ro 23-6240, a new fluorinated 4-quinolone. Chemotherapy. 1987;33(3):197–203. doi: 10.1159/000238495. [DOI] [PubMed] [Google Scholar]
- Stone J. W., Andrews J. M., Ashby J. P., Griggs D., Wise R. Pharmacokinetics and tissue penetration of orally administered lomefloxacin. Antimicrob Agents Chemother. 1988 Oct;32(10):1508–1510. doi: 10.1128/aac.32.10.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J. W., Wise R., Donovan I. A., Gearty J. Failure of ciprofloxacin to eradicate Campylobacter pylori from the stomach. J Antimicrob Chemother. 1988 Jul;22(1):92–93. doi: 10.1093/jac/22.1.92. [DOI] [PubMed] [Google Scholar]
- Strunk R. W., Gratz J. C., Maserati R., Scheld W. M. Comparison of ciprofloxacin with azlocillin plus tobramycin in the therapy of experimental Pseudomonas aeruginosa endocarditis. Antimicrob Agents Chemother. 1985 Sep;28(3):428–432. doi: 10.1128/aac.28.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A. W. Comparison of antimicrobial susceptibility patterns of fifty-seven strains of Haemophilus ducreyi isolated in Amsterdam from 1978 to 1985. J Antimicrob Chemother. 1987 Feb;19(2):187–191. doi: 10.1093/jac/19.2.187. [DOI] [PubMed] [Google Scholar]
- Suerbaum S., Leying H., Kroll H. P., Gmeiner J., Opferkuch W. Influence of beta-lactam antibiotics and ciprofloxacin on cell envelope of Escherichia coli. Antimicrob Agents Chemother. 1987 Jul;31(7):1106–1110. doi: 10.1128/aac.31.7.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Bott K. F. Bacillus subtilis deoxyribonucleic acid gyrase. J Bacteriol. 1980 Mar;141(3):1331–1339. doi: 10.1128/jb.141.3.1331-1339.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullam P. M., Täuber M. G., Hackbarth C. J., Chambers H. F., Scott K. G., Sande M. A. Pefloxacin therapy for experimental endocarditis caused by methicillin-susceptible or methicillin-resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1985 May;27(5):685–687. doi: 10.1128/aac.27.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Kwok Y. Y., Bulkacz J. Comparative activity of ciprofloxacin against anaerobic bacteria. Antimicrob Agents Chemother. 1985 Mar;27(3):427–428. doi: 10.1128/aac.27.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson B. N., Boppana V. K., Vlasses P. H., Rotmensch H. H., Ferguson R. K. Norfloxacin disposition after sequentially increasing oral doses. Antimicrob Agents Chemother. 1983 Feb;23(2):284–288. doi: 10.1128/aac.23.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Wallace R. J., Jr, Silcox V. A., Thornsberry C. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother. 1985 Dec;28(6):807–811. doi: 10.1128/aac.28.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds J., Javaid A., Bone M., Turner A. The penetration of ofloxacin into bronchial secretions. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):91–95. doi: 10.1093/jac/22.supplement_c.91. [DOI] [PubMed] [Google Scholar]
- Sziegoleit A. Antibacterial activity of ciprofloxacin, in particular against polyresistant strains. Drugs Exp Clin Res. 1987;13(4):205–207. [PubMed] [Google Scholar]
- Sörgel F., Seelmann R., Naber K., Metz R., Muth P. Metabolism of fleroxacin in man. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):169–178. doi: 10.1093/jac/22.supplement_d.169. [DOI] [PubMed] [Google Scholar]
- Tabary X., Moreau N., Dureuil C., Le Goffic F. Effect of DNA gyrase inhibitors pefloxacin, five other quinolones, novobiocin, and clorobiocin on Escherichia coli topoisomerase I. Antimicrob Agents Chemother. 1987 Dec;31(12):1925–1928. doi: 10.1128/aac.31.12.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata M., Otsuki M., Nishino T. In-vitro and in-vivo activities of T-3262, a new pyridone carboxylic acid. J Antimicrob Chemother. 1988 Aug;22(2):143–154. doi: 10.1093/jac/22.2.143. [DOI] [PubMed] [Google Scholar]
- Takayama S., Watanabe T., Akiyama Y., Ohura K., Harada S., Matsuhashi K., Mochida K., Yamashita N. Reproductive toxicity of ofloxacin. Arzneimittelforschung. 1986 Aug;36(8):1244–1248. [PubMed] [Google Scholar]
- Tatsumi H., Senda H., Yatera S., Takemoto Y., Yamayoshi M., Ohnishi K. Toxicological studies on pipemidic acid. V. Effect on diarthrodial joints of experimental animals. J Toxicol Sci. 1978 Nov;3(4):357–367. doi: 10.2131/jts.3.357. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Ng L. K., Lior H. Susceptibility of Campylobacter species to nalidixic acid, enoxacin, and other DNA gyrase inhibitors. Antimicrob Agents Chemother. 1985 Nov;28(5):708–710. doi: 10.1128/aac.28.5.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegelberg-Stassen M. J., van der Hoek J. C., Mooi L., Wagenvoort J. H., van Joost T., Michel M. F., Stolz E. Treatment of uncomplicated gonococcal urethritis in men with two dosages of ciprofloxacin. Eur J Clin Microbiol. 1986 Apr;5(2):244–246. doi: 10.1007/BF02013999. [DOI] [PubMed] [Google Scholar]
- Tegelberg-Stassen M. J., van der Willigen A. H., van der Hoek J. C., Wagenvoort J. H., van Vliet H. J., van Klingeren B., van Joost T., Michel M. F., Stolz E. Treatment of uncomplicated urogenital gonorrhoea in women with a single dose of enoxacin. Eur J Clin Microbiol. 1986 Aug;5(4):395–398. doi: 10.1007/BF02075693. [DOI] [PubMed] [Google Scholar]
- Tenney J. H., Maack R. W., Chippendale G. R. Rapid selection of organisms with increasing resistance on subinhibitory concentrations of norfloxacin in agar. Antimicrob Agents Chemother. 1983 Jan;23(1):188–189. doi: 10.1128/aac.23.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney J. H., Warren J. W. Bactericidal activity of norfloxacin and nine other urinary tract antibiotics against Gram-negative bacilli causing bacteriuria in chronically catheterized patients. J Antimicrob Chemother. 1983 Mar;11(3):287–290. doi: 10.1093/jac/11.3.287. [DOI] [PubMed] [Google Scholar]
- Teoh-Chan C. H., Cowlishaw A., Eley A., Slater G., Greenwood D. Laboratory evaluation of enoxacin: comparison with norfloxacin and nalidixic acid. J Antimicrob Chemother. 1985 Jan;15(1):45–52. doi: 10.1093/jac/15.1.45. [DOI] [PubMed] [Google Scholar]
- Texier-Maugein J., Mormède M., Fourche J., Bébéar C. In vitro activity of four fluoroquinolones against eighty-six isolates of mycobacteria. Eur J Clin Microbiol. 1987 Oct;6(5):584–586. doi: 10.1007/BF02014255. [DOI] [PubMed] [Google Scholar]
- Thabaut A., Durosoir J. L. Activité antibactérienne comparée in vitro de la péfloxacine (1589 RB), de l'acide nalidixique, de l'acide pipémidique et de la fluméquine. Pathol Biol (Paris) 1982 Jun;30(6):394–397. [PubMed] [Google Scholar]
- Thadepalli H., Gollapudi S. V., Chuah S. K. Therapeutic evaluation of difloxacin (A-56619) and A-56620 for experimentally induced Bacteroides fragilis-associated intra-abdominal abscess. Antimicrob Agents Chemother. 1986 Oct;30(4):574–576. doi: 10.1128/aac.30.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauvin C., Lemeland J. F., Humbert G., Fillastre J. P. Efficacy of pefloxacin-fosfomycin in experimental endocarditis caused by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1988 Jun;32(6):919–921. doi: 10.1128/aac.32.6.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Then R. L., Angehrn P. Multiply resistant mutants of Enterobacter cloacae selected by beta-lactam antibiotics. Antimicrob Agents Chemother. 1986 Nov;30(5):684–688. doi: 10.1128/aac.30.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. G., Ellis-Pegler R. B. Enoxacin treatment of urinary tract infections. J Antimicrob Chemother. 1985 Jun;15(6):759–763. doi: 10.1093/jac/15.6.759. [DOI] [PubMed] [Google Scholar]
- Thorsteinsson S. B., Bergan T., Johannesson G., Thorsteinsson H. S., Rohwedder R. Tolerance of ciprofloxacin at injection site, systemic safety and effect on electroencephalogram. Chemotherapy. 1987;33(6):448–451. doi: 10.1159/000238534. [DOI] [PubMed] [Google Scholar]
- Toon S., Hopkins K. J., Garstang F. M., Aarons L., Sedman A., Rowland M. Enoxacin-warfarin interaction: pharmacokinetic and stereochemical aspects. Clin Pharmacol Ther. 1987 Jul;42(1):33–41. doi: 10.1038/clpt.1987.104. [DOI] [PubMed] [Google Scholar]
- Torre D. Influence of charcoal on ciprofloxacin activity. Rev Infect Dis. 1988 Nov-Dec;10(6):1231–1231. doi: 10.1093/clinids/10.6.1231. [DOI] [PubMed] [Google Scholar]
- Traub W. H. Incomplete cross-resistance of nalidixic and pipemidic acid-resistant variants of Serratia marcescens against ciprofloxacin, enoxacin, and norfloxacin. Chemotherapy. 1985;31(1):34–39. doi: 10.1159/000238311. [DOI] [PubMed] [Google Scholar]
- Traub W. H., Kohl K. H., Spohr M. In vitro activity of three fluoroquinolones against enterococci and Pseudomonas aeruginosa: modified categorization system for discrepant broth dilution and agar disk diffusion test results. Chemotherapy. 1988;34(2):101–106. doi: 10.1159/000238555. [DOI] [PubMed] [Google Scholar]
- Traub W. H. Multiple drug-resistant Corynebacteriaceae: in vitro and in vivo (murine) studies. Chemotherapy. 1985;31(5):372–382. doi: 10.1159/000238362. [DOI] [PubMed] [Google Scholar]
- Traub W. H., Spohr M., Bauer D. Pseudomonas aeruginosa: in vitro susceptibility to antimicrobial drugs, single and combined, with and without defibrinated human blood. Chemotherapy. 1988;34(4):284–297. doi: 10.1159/000238582. [DOI] [PubMed] [Google Scholar]
- Traub W. H., Spohr M. In vitro antibiotic susceptibility of Legionellaceae: search for alternative antimicrobial drugs. Chemotherapy. 1984;30(3):182–187. doi: 10.1159/000238266. [DOI] [PubMed] [Google Scholar]
- Trautmann M., Krause B., Birnbaum D., Wagner J., Lenk V. Serum bactericidal activity of two newer quinolones against Salmonella typhi compared with standard therapeutic regimens. Eur J Clin Microbiol. 1986 Jun;5(3):297–302. doi: 10.1007/BF02017785. [DOI] [PubMed] [Google Scholar]
- Trimble K. A., Clark R. B., Sanders W. E., Jr, Frankel J. W., Cacciatore R., Valdez H. Activity of ciprofloxacin against Mycobacteria in vitro: comparison of BACTEC and macrobroth dilution methods. J Antimicrob Chemother. 1987 May;19(5):617–622. doi: 10.1093/jac/19.5.617. [DOI] [PubMed] [Google Scholar]
- Tsuei S. E., Darragh A. S., Brick I. Pharmacokinetics and tolerance of enoxacin in healthy volunteers administered at a dosage of 400 mg twice daily for 14 days. J Antimicrob Chemother. 1984 Sep;14 (Suppl 100):71–74. doi: 10.1093/jac/14.suppl_c.71. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Sato H., Kume Y., Tamai I., Okezaki E., Nagata O., Kato H. Inhibitory effects of quinolone antibacterial agents on gamma-aminobutyric acid binding to receptor sites in rat brain membranes. Antimicrob Agents Chemother. 1988 Feb;32(2):190–194. doi: 10.1128/aac.32.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamura M. In vitro antimycobacterial activity of a new antibacterial substance DL-8280--differentiation between some species of mycobacteria and related organisms by the DL-8280 susceptibility test. Microbiol Immunol. 1983;27(12):1129–1132. doi: 10.1111/j.1348-0421.1983.tb02933.x. [DOI] [PubMed] [Google Scholar]
- Tsukamura M. In vitro antituberculosis activity of a new antibacterial substance ofloxacin (DL8280). Am Rev Respir Dis. 1985 Mar;131(3):348–351. doi: 10.1164/arrd.1985.131.3.348. [DOI] [PubMed] [Google Scholar]
- Tsukamura M., Nakamura E., Yoshii S., Amano H. Therapeutic effect of a new antibacterial substance ofloxacin (DL8280) on pulmonary tuberculosis. Am Rev Respir Dis. 1985 Mar;131(3):352–356. doi: 10.1164/arrd.1985.131.3.352. [DOI] [PubMed] [Google Scholar]
- Uemura T., Morikawa K., Yanagida M. The nucleotide sequence of the fission yeast DNA topoisomerase II gene: structural and functional relationships to other DNA topoisomerases. EMBO J. 1986 Sep;5(9):2355–2361. doi: 10.1002/j.1460-2075.1986.tb04504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T., Fujimoto T., Sato K., Osada Y. In vitro activity of DR-3355, an optically active ofloxacin. Antimicrob Agents Chemother. 1988 Sep;32(9):1336–1340. doi: 10.1128/aac.32.9.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T., Nakajima R., Otani T., Katami K., Osada Y., Otani M. Lack of effectiveness of ofloxacin against experimental syphilis in rabbits. Arzneimittelforschung. 1987 Sep;37(9):1048–1051. [PubMed] [Google Scholar]
- Valero-Guillén P. L., Martín-Luengo F., Quintanilla I. In-vitro activity of some quinoline derivatives against Mycobacterium fortuitum. J Antimicrob Chemother. 1985 Feb;15(2):254–255. doi: 10.1093/jac/15.2.254. [DOI] [PubMed] [Google Scholar]
- Van Caekenberghe D. L., Breyssens J. In vitro synergistic activity between bismuth subcitrate and various antimicrobial agents against Campylobacter pyloridis (C. pylori). Antimicrob Agents Chemother. 1987 Sep;31(9):1429–1430. doi: 10.1128/aac.31.9.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Caekenberghe D. L., Pattyn S. R. In vitro activity of ciprofloxacin compared with those of other new fluorinated piperazinyl-substituted quinoline derivatives. Antimicrob Agents Chemother. 1984 Apr;25(4):518–521. doi: 10.1128/aac.25.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Poppel H., Wegge M., Dammekens H., Chysky V. Ciprofloxacin in the treatment of urinary tract infection in patients with multiple sclerosis. Eur J Clin Microbiol. 1986 Apr;5(2):251–253. doi: 10.1007/BF02014002. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P. Interaction of gentamicin, dibekacin, netilmicin and amikacin with various penicillins, cephalosporins, minocycline and new fluoro-quinolones against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother. 1985 Nov;16(5):581–587. doi: 10.1093/jac/16.5.581. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P., Joly P. Comparative in-vitro activities of teicoplanin, vancomycin, coumermycin and ciprofloxacin, alone and in combination with rifampicin or LM 427, against Staphylococcus aureus. J Antimicrob Chemother. 1987 Mar;19(3):313–320. doi: 10.1093/jac/19.3.313. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P., Klastersky J., Lieppe S., Husson M., Lauzon D., Lopez A. P. Bactericidal activity and killing rate of serum from volunteers receiving pefloxacin alone or in combination with amikacin. Antimicrob Agents Chemother. 1986 Feb;29(2):230–234. doi: 10.1128/aac.29.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera P., Matsumoto T., Husson M. Intraphagocytic penetration of antibiotics. J Antimicrob Chemother. 1988 Aug;22(2):185–192. doi: 10.1093/jac/22.2.185. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P., Scorneaux B. In vitro susceptibility of Campylobacter jejuni to 27 antimicrobial agents and various combinations of beta-lactams with clavulanic acid or sulbactam. Antimicrob Agents Chemother. 1985 Jul;28(1):37–40. doi: 10.1128/aac.28.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoof R., Hubrechts J. M., Roebben E., Leger J., De Schepper N. L'activité bactériostatique et bactéricide in vitro de la ciprofloxacine ainsi que d'autres dérivés quinoloniques contre les Campylobacters jejuni. Pathol Biol (Paris) 1988 Feb;36(2):163–166. [PubMed] [Google Scholar]
- Vanhoof R., Hubrechts J. M., Roebben E., Nyssen H. J., Nulens E., Leger J., De Schepper N. The comparative activity of pefloxacin, enoxacin, ciprofloxacin and 13 other antimicrobial agents against enteropathogenic microorganisms. Infection. 1986 Nov-Dec;14(6):294–298. doi: 10.1007/BF01643966. [DOI] [PubMed] [Google Scholar]
- Varoli O., Ferri M. In vitro study of the antibacterial activity of ofloxacin against recent clinical isolates. Drugs Exp Clin Res. 1987;13(4):209–212. [PubMed] [Google Scholar]
- Venezio F. R., Tatarowicz W., DiVincenzo C. A., O'Keefe J. P. Activity of ciprofloxacin against multiply resistant strains of Pseudomonas aeruginosa, Staphylococcus epidermidis, and group JK corynebacteria. Antimicrob Agents Chemother. 1986 Dec;30(6):940–941. doi: 10.1128/aac.30.6.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist L. Comparative in-vitro activity of Ro 23-6240, a new trifluorinated quinolone. J Antimicrob Chemother. 1987 Sep;20(3):363–372. doi: 10.1093/jac/20.3.363. [DOI] [PubMed] [Google Scholar]
- Verschraegen G., Claeys G., Van den Abeele A. M. Comparative in vitro activity of the new quinolone fleroxacin (RO 23-6240). Eur J Clin Microbiol Infect Dis. 1988 Feb;7(1):63–66. doi: 10.1007/BF01962177. [DOI] [PubMed] [Google Scholar]
- Vuye A. In vitro comparison of norfloxacin with nalidixic acid, cinoxacin and oxolinic acid. Arzneimittelforschung. 1983;33(12):1623–1627. [PubMed] [Google Scholar]
- Wagenvoort J. H., van der Willigen A. H., van Vliet H. J., Michel M. F., Van Klingeren B. Resistance of Neisseria gonorrhoeae to enoxacin. J Antimicrob Chemother. 1986 Sep;18(3):429–429. doi: 10.1093/jac/18.3.429. [DOI] [PubMed] [Google Scholar]
- Wall R. A., Mabey D. C., Bello C. S., Felmingham D. The comparative in-vitro activity of twelve 4-quinolone antimicrobials against Haemophilus ducreyi. J Antimicrob Chemother. 1985 Aug;16(2):165–168. doi: 10.1093/jac/16.2.165. [DOI] [PubMed] [Google Scholar]
- Walton L., Elwell L. P. In vitro cleavable-complex assay to monitor antimicrobial potency of quinolones. Antimicrob Agents Chemother. 1988 Jul;32(7):1086–1089. doi: 10.1128/aac.32.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Sabbaj J., Corrado M., Hoagland V. World-wide clinical experience with norfloxacin: efficacy and safety. Scand J Infect Dis Suppl. 1986;48:81–89. [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wang W. L., Reller L. B., Blaser M. J. Comparison of antimicrobial susceptibility patterns of Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1984 Sep;26(3):351–353. doi: 10.1128/aac.26.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt B., Brown F. V. Is ciprofloxacin active against clinically important anaerobes? J Antimicrob Chemother. 1986 May;17(5):605–613. doi: 10.1093/jac/17.5.605. [DOI] [PubMed] [Google Scholar]
- Watt B., Brown F. V. The in-vitro activity of ciprofloxacin in combination with other agents against anaerobes of clinical interest. J Antimicrob Chemother. 1986 May;17(5):679–680. doi: 10.1093/jac/17.5.679-a. [DOI] [PubMed] [Google Scholar]
- Webberley J. M., Andrews J. M., Ashby J. P., McLeod A., Wise R. Pharmacokinetics and tissue penetration of orally administered pefloxacin. Eur J Clin Microbiol. 1987 Oct;6(5):521–524. doi: 10.1007/BF02014239. [DOI] [PubMed] [Google Scholar]
- Weber A. H., Scribner R. K., Marks M. I. In vitro activity of ciprofloxacin against pediatric pathogens. Chemotherapy. 1985;31(6):456–465. doi: 10.1159/000238374. [DOI] [PubMed] [Google Scholar]
- Weber D. J., Saviteer S. M., Rutala W. A., Thomann C. A. In vitro susceptibility of Bacillus spp. to selected antimicrobial agents. Antimicrob Agents Chemother. 1988 May;32(5):642–645. doi: 10.1128/aac.32.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidekamm E., Portmann R., Partos C., Dell D. Single and multiple dose pharmacokinetics of fleroxacin. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):145–154. doi: 10.1093/jac/22.supplement_d.145. [DOI] [PubMed] [Google Scholar]
- Weidner W., Schiefer H. G., Dalhoff A. Treatment of chronic bacterial prostatitis with ciprofloxacin. Results of a one-year follow-up study. Am J Med. 1987 Apr 27;82(4A):280–283. [PubMed] [Google Scholar]
- Weinberg E., Shungu D. L., Gadebusch H. H. Effectiveness of the antimicrobial removal device, BACTEC 16B medium, and thiol broth in neutralizing antibacterial activities of imipenem, norfloxacin, and related agents. J Clin Microbiol. 1984 Feb;19(2):207–209. doi: 10.1128/jcm.19.2.207-209.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisser J., Wiedemann B. Elimination of plasmids by new 4-quinolones. Antimicrob Agents Chemother. 1985 Nov;28(5):700–702. doi: 10.1128/aac.28.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisser J., Wiedemann B. Inhibition of R-plasmid transfer in Escherichia coli by 4-quinolones. Antimicrob Agents Chemother. 1987 Apr;31(4):531–534. doi: 10.1128/aac.31.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N. J., Dance D. A. Clinical and laboratory studies of malaria and melioidosis. Trans R Soc Trop Med Hyg. 1988;82(1):15–20. doi: 10.1016/0035-9203(88)90249-0. [DOI] [PubMed] [Google Scholar]
- Whiting J. L., Cheng N., Chow A. W. Interactions of ciprofloxacin with clindamycin, metronidazole, cefoxitin, cefotaxime, and mezlocillin against gram-positive and gram-negative anaerobic bacteria. Antimicrob Agents Chemother. 1987 Sep;31(9):1379–1382. doi: 10.1128/aac.31.9.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnands W. J., Vree T. B., Baars A. M., Hafkenscheid J. C., Kohler B. E., van Herwaarden C. L. The penetration of ofloxacin into lung tissue. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):85–89. doi: 10.1093/jac/22.supplement_c.85. [DOI] [PubMed] [Google Scholar]
- Wijnands W. J., Vree T. B., Baars A. M., van Herwaarden C. L. Pharmacokinetics of enoxacin and its penetration into bronchial secretions and lung tissue. J Antimicrob Chemother. 1988 Feb;21 (Suppl B):67–77. doi: 10.1093/jac/21.suppl_b.67. [DOI] [PubMed] [Google Scholar]
- Wijnands W. J., Vree T. B. Interaction between the fluoroquinolones and the bronchodilator theophylline. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):109–114. doi: 10.1093/jac/22.supplement_c.109. [DOI] [PubMed] [Google Scholar]
- Wijnands W. J., Vree T. B., van Herwaarden C. L. The influence of quinolone derivatives on theophylline clearance. Br J Clin Pharmacol. 1986 Dec;22(6):677–683. doi: 10.1111/j.1365-2125.1986.tb02957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnands W. J., van Griethuysen A. J., Vree T. B., Van Klingeren B., van Herwaarden C. L. Enoxacin in lower respiratory tract infections. J Antimicrob Chemother. 1986 Dec;18(6):719–727. doi: 10.1093/jac/18.6.719. [DOI] [PubMed] [Google Scholar]
- Wijnands W. J., van Herwaarden C. L., Vree T. B. Enoxacin raises plasma theophylline concentrations. Lancet. 1984 Jul 14;2(8394):108–109. doi: 10.1016/s0140-6736(84)90283-6. [DOI] [PubMed] [Google Scholar]
- Willems F. T., Boerema J. B., von Summeren T. R. The in-vitro comparative activity of quinolones against bacteria from urinary tract infections in general practice. J Antimicrob Chemother. 1986 Jan;17(1):69–73. doi: 10.1093/jac/17.1.69. [DOI] [PubMed] [Google Scholar]
- Williams A. H., Grüneberg R. N. Ciprofloxacin and co-trimoxazole in urinary tract infection. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):107–110. doi: 10.1093/jac/18.supplement_d.107. [DOI] [PubMed] [Google Scholar]
- Williams P. Sub-MICs of cefuroxime and ciprofloxacin influence interaction of complement and immunoglobulins with Klebsiella pneumoniae. Antimicrob Agents Chemother. 1987 May;31(5):758–762. doi: 10.1128/aac.31.5.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Guiney D. G. Failure of oral trimethoprim-sulfamethoxazole prophylaxis in acute leukemia: isolation of resistant plasmids from strains of Enterobacteriaceae causing bacteremia. N Engl J Med. 1982 Jan 7;306(1):16–20. doi: 10.1056/NEJM198201073060105. [DOI] [PubMed] [Google Scholar]
- Winston D. J., Ho W. G., Champlin R. E., Karp J., Bartlett J., Finley R. S., Joshi J. H., Talbot G., Levitt L., Deresinski S. Norfloxacin for prevention of bacterial infections in granulocytopenic patients. Am J Med. 1987 Jun 26;82(6B):40–46. doi: 10.1016/0002-9343(87)90617-6. [DOI] [PubMed] [Google Scholar]
- Winston D. J., Ho W. G., Nakao S. L., Gale R. P., Champlin R. E. Norfloxacin versus vancomycin/polymyxin for prevention of infections in granulocytopenic patients. Am J Med. 1986 May;80(5):884–890. doi: 10.1016/0002-9343(86)90633-9. [DOI] [PubMed] [Google Scholar]
- Winton M. D., Everett E. D., Dolan S. A. Activities of five new fluoroquinolones against Pseudomonas pseudomallei. Antimicrob Agents Chemother. 1988 Jun;32(6):928–929. doi: 10.1128/aac.32.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Ashby J. P., Matthews R. S. In vitro activity of lomefloxacin, a new quinolone antimicrobial agent, in comparison with those of other agents. Antimicrob Agents Chemother. 1988 May;32(5):617–622. doi: 10.1128/aac.32.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Danks G. In-vitro activity of enoxacin (CL-919), a new quinoline derivative, compared with that of other antimicrobial agents. J Antimicrob Chemother. 1984 Mar;13(3):237–244. doi: 10.1093/jac/13.3.237. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Edwards L. J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob Agents Chemother. 1983 Apr;23(4):559–564. doi: 10.1128/aac.23.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Ashby J. P., Andrews J. M. In vitro activity of PD 127,391, an enhanced-spectrum quinolone. Antimicrob Agents Chemother. 1988 Aug;32(8):1251–1256. doi: 10.1128/aac.32.8.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Baker S. L., Misra M., Griggs D. The pharmacokinetics of enoxacin in elderly patients. J Antimicrob Chemother. 1987 Mar;19(3):343–350. doi: 10.1093/jac/19.3.343. [DOI] [PubMed] [Google Scholar]
- Wise R., Honeybourne D., Andrews J. M., Ashby J. P. The penetration of fleroxacin into bronchial mucosa. J Antimicrob Chemother. 1988 Aug;22(2):203–206. doi: 10.1093/jac/22.2.203. [DOI] [PubMed] [Google Scholar]
- Wise R., Kirkpatrick B., Ashby J., Griggs D. J. Pharmacokinetics and tissue penetration of Ro 23-6240, a new trifluoroquinolone. Antimicrob Agents Chemother. 1987 Feb;31(2):161–163. doi: 10.1128/aac.31.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Lockley M. R. The pharmacokinetics of ofloxacin and a review of its tissue penetration. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):59–64. doi: 10.1093/jac/22.supplement_c.59. [DOI] [PubMed] [Google Scholar]
- Wise R., Lockley R. M., Webberly M., Dent J. Pharmacokinetics of intravenously administered ciprofloxacin. Antimicrob Agents Chemother. 1984 Aug;26(2):208–210. doi: 10.1128/aac.26.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Lockley R., Webberly M., Adhami Z. N. The pharmacokinetics and tissue penetration of enoxacin and norfloxacin. J Antimicrob Chemother. 1984 Sep;14 (Suppl 100):75–81. doi: 10.1093/jac/14.suppl_c.75. [DOI] [PubMed] [Google Scholar]
- Wiström J., Norrby S. R., Burman L. G., Lundholm R., Jellheden B., Englund G. Norfloxacin versus placebo for prophylaxis against travellers' diarrhoea. J Antimicrob Chemother. 1987 Oct;20(4):563–574. doi: 10.1093/jac/20.4.563. [DOI] [PubMed] [Google Scholar]
- Wolff M., Boutron L., Singlas E., Clair B., Decazes J. M., Regnier B. Penetration of ciprofloxacin into cerebrospinal fluid of patients with bacterial meningitis. Antimicrob Agents Chemother. 1987 Jun;31(6):899–902. doi: 10.1128/aac.31.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M., Regnier B., Daldoss C., Nkam M., Vachon F. Penetration of pefloxacin into cerebrospinal fluid of patients with meningitis. Antimicrob Agents Chemother. 1984 Sep;26(3):289–291. doi: 10.1128/aac.26.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C., Ng E. Y., Souza K. S., McHugh G. L., Swartz M. N. Antagonism of wild-type and resistant Escherichia coli and its DNA gyrase by the tricyclic 4-quinolone analogs ofloxacin and S-25930 stereoisomers. Antimicrob Agents Chemother. 1987 Nov;31(11):1861–1863. doi: 10.1128/aac.31.11.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollschlager C. M., Raoof S., Khan F. A., Guarneri J. J., LaBombardi V., Afzal Q. Controlled, comparative study of ciprofloxacin versus ampicillin in treatment of bacterial respiratory tract infections. Am J Med. 1987 Apr 27;82(4A):164–168. [PubMed] [Google Scholar]
- Wood L. V., Morgan D. R., DuPont H. L. Antimicrobial resistance of gram-negative bacteria isolated from foods in Mexico. J Infect Dis. 1983 Oct;148(4):766–766. doi: 10.1093/infdis/148.4.766. [DOI] [PubMed] [Google Scholar]
- Wood M. E., Newland A. C. Intravenous ciprofloxacin in the treatment of infection in immunocompromised patients. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):175–178. doi: 10.1093/jac/18.supplement_d.175. [DOI] [PubMed] [Google Scholar]
- Wood M. J., Logan M. N. Ciprofloxacin for soft tissue infections. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):159–164. doi: 10.1093/jac/18.supplement_d.159. [DOI] [PubMed] [Google Scholar]
- Xicota M. A., Coll R., Esteve M., Moros M., Parés J. In vitro antibacterial activity of E-3604, a new 6-fluoroquinolone, on clinical isolates. Drugs Exp Clin Res. 1987;13(3):133–136. [PubMed] [Google Scholar]
- Yajko D. M., Kirihara J., Sanders C., Nassos P., Hadley W. K. Antimicrobial synergism against Mycobacterium avium complex strains isolated from patients with acquired immune deficiency syndrome. Antimicrob Agents Chemother. 1988 Sep;32(9):1392–1395. doi: 10.1128/aac.32.9.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi J., Furutani Y., Inoue S., Ohue T., Nakamura S., Shimizu M. New nalidixic acid resistance mutations related to deoxyribonucleic acid gyrase activity. J Bacteriol. 1981 Nov;148(2):450–458. doi: 10.1128/jb.148.2.450-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi J., Yoshida H., Yamayoshi M., Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986 Sep;204(3):367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]
- Yeaman M. R., Mitscher L. A., Baca O. G. In vitro susceptibility of Coxiella burnetii to antibiotics, including several quinolones. Antimicrob Agents Chemother. 1987 Jul;31(7):1079–1084. doi: 10.1128/aac.31.7.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Kojima T., Yamagishi J., Nakamura S. Quinolone-resistant mutations of the gyrA gene of Escherichia coli. Mol Gen Genet. 1988 Jan;211(1):1–7. doi: 10.1007/BF00338386. [DOI] [PubMed] [Google Scholar]
- Young L. S., Berlin O. G., Inderlied C. B. Activity of ciprofloxacin and other fluorinated quinolones against mycobacteria. Am J Med. 1987 Apr 27;82(4A):23–26. [PubMed] [Google Scholar]
- Yu V. L., Goetz A., Wagener M., Smith P. B., Rihs J. D., Hanchett J., Zuravleff J. J. Staphylococcus aureus nasal carriage and infection in patients on hemodialysis. Efficacy of antibiotic prophylaxis. N Engl J Med. 1986 Jul 10;315(2):91–96. doi: 10.1056/NEJM198607103150204. [DOI] [PubMed] [Google Scholar]
- Zackrisson G., Brorson J. E., Björnegård B., Trollfors B. Susceptibility of Bordetella pertussis to doxycycline, cinoxacin, nalidixic acid, norfloxacin, imipenem, mecillinam and rifampicin. J Antimicrob Chemother. 1985 May;15(5):629–632. doi: 10.1093/jac/15.5.629. [DOI] [PubMed] [Google Scholar]
- Zeiler H. J. Evaluation of the in vitro bactericidal action of ciprofloxacin on cells of Escherichia coli in the logarithmic and stationary phases of growth. Antimicrob Agents Chemother. 1985 Oct;28(4):524–527. doi: 10.1128/aac.28.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccarelli M., Simeon de Buochberg M., Maillols H., Dusart G., Attisso M. A. Effet post-antibiotique de la ciprofloxacine seule et en association sur Streptococcus faecalis. Pathol Biol (Paris) 1988 May;36(5):410–413. [PubMed] [Google Scholar]
- Zuccarelli M., Simeon de Buochberg M., Maillols M., Armynot du Chatelet A. M., Attisso M. A. Cinétiques de bactéricidie comparées de la ciprofloxacine, de l'ofloxacine et de la péfloxacine seules et en association sur des souches de streptocoques du groupe D. Pathol Biol (Paris) 1988 May;36(5):403–409. [PubMed] [Google Scholar]
- Zweerink M. M., Edison A. M. The uptake of 3H-norfloxacin by human polymorphonuclear leucocytes. J Antimicrob Chemother. 1988 Feb;21(2):266–267. doi: 10.1093/jac/21.2.266. [DOI] [PubMed] [Google Scholar]
- Zweerink M. M., Edison A. Inhibition of Micrococcus luteus DNA gyrase by norfloxacin and 10 other quinolone carboxylic acids. Antimicrob Agents Chemother. 1986 Apr;29(4):598–601. doi: 10.1128/aac.29.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Auwera P., de Moor G., Lacroix G., Mambour A., Rossion N., Schuyteneer F. In vitro activity of enoxacin compared with norfloxacin and amikacin. Eur J Clin Microbiol. 1985 Feb;4(1):55–58. doi: 10.1007/BF02148662. [DOI] [PubMed] [Google Scholar]
- van der Graaf M., Diepersloot R. J. Transmission of human immunodeficiency virus (HIV/HTLV-III/LAV): a review. Infection. 1986 Sep-Oct;14(5):203–211. doi: 10.1007/BF01644263. [DOI] [PubMed] [Google Scholar]
- van der Willigen A. H., van der Hoek J. C., Wagenvoort J. H., van Vliet H. J., van Klingeren B., Schalla W. O., Knapp J. S., van Joost T., Michel M. F., Stolz E. Comparative double-blind study of 200- and 400-mg enoxacin given orally in the treatment of acute uncomplicated urethral gonorrhea in males. Antimicrob Agents Chemother. 1987 Apr;31(4):535–538. doi: 10.1128/aac.31.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]



