Abstract
An association between autism and large head size has been previously described. Historically a subset of these cases have been correlated with mutations in the gene phosphatase and tensin homolog (PTEN). However, for the majority of cases the etiology is not known. We have studied 33 patients with autism and large head size. Within this group, we confirm the association of PTEN mutations and extreme head size and identify mutations in 22% of cases, including three novel PTEN mutations. In addition we define three novel phenotypic subgroups: (1) cases associated with somatic overgrowth (2) those with disproportionate macrocephaly and (3) those with relative macrocephaly. Members of these subgroups lack changes in the PTEN gene and furthermore we report two novel copy number changes in these patients.
An association between autism and macrocephaly has been previously described. A subset of cases with extreme macrocephaly (>3SD, 99.7th %ile) have been correlated to mutations in the gene phosphatase and tensin homolog (PTEN). However, the phenotypic and genetic characterization of the remaining cases remains unclear. We report the phenotypic classification and genetic testing evaluation of a cohort of 33 patients with autism and macrocephaly. Within our cohort, we confirm the association of PTEN mutations and extreme macrocephaly (> 3SD, 99.7th %ile) and identify mutations in 22% of cases, including three novel PTEN mutations. In addition we define three phenotypic subgroups: (1) those cases associated with somatic overgrowth, (2) those with disproportionate macrocephaly, and (3) those will relative macrocephaly. We have devised a novel way to segregate patients into these subgroups that will aide in the stratification of autism macrocephaly cases. Within these subgroups, we further expand the genetic etiologies for autism cases with macrocephaly by describing two novel suspected pathogenic Copy Number Variants (CNVs) located at 6q23.2 and 10q24.32. These findings demonstrate the phenotypic heterogeneity of autism cases associated with macrocephaly and their genetic etiologies. The clinical yield from PTEN mutation analysis is 22% and 9% from Chromosomal Microarray (CMA) testing within this cohort. The identification of three distinct phenotypic subgroups within macrocephaly autism patients may allow for the identification of their respective distinct genetic etiologies which to date have remained elusive.
Keywords: Autism, Macrocephaly, PTEN, Overgrowth, Hypotonia
INTRODUCTION
Autism was first reported by Kanner, in 1943 [Kanner 1943], as a developmental disorder in which patients present with severely affected expressive and receptive language skills, stereotypical behaviors, and diminished social interactive skills [Kanner 1943] [Kanner 1943] The incidence of this condition appears to be as high as 1:100 children. . Its etiology is unclear but a number of studies have demonstrated strong support for a genetic basis for the pathogenesis of autism [Bruining and others 2010; Iurov and others 2010], both from twin and familial studies [Muhle and others 2004; Spence 2004]. In addition, there are numerous single gene disorders (Fragile X, OMIM: 300624; Bannayan–Riley–Ruvalcaba, OMIM: 153480; and Rett Syndrome, OMIN: 312750) and chromosome abnormalities (for example, 15q and 1q21.1 duplications) that are associated with autistic behaviors. A few single genes have been identified that cause autism, including CNTNAP2 [Whitehouse and others 2011] and Neuroligins [Etherton and others 2011]. Cumulatively these genetic etiologies account for less than 10% of cases [Brunetti-Pierri and others 2008], and the exact cause of the remaining 90% remains elusive.
There has been a long-standing association between macrocephaly and autism that was first reported by Kanner [Kanner 1943][Kanner 1943; Kanner 1968], and this has been confirmed by many studies (Courchesne, 2004;Courchesne and others 2003]). Interestingly, cases of extreme macrocephaly (>3SD) have been correlated to mutations in the gene phosphatase and tensin homolog (PTEN) [Brunetti-Pierri and others 2008; Buxbaum and others 2007; Herman and others 2007a; McBride and others 2010]. This has made PTEN testing the standard of care for patients who present with autism and macrocephaly [Herman and others 2007b]. A number of recent studies have demonstrated the association of copy number variations (CNVs) with autism [Rosenfeld and others 2010]. The high diagnostic yield of chromosomal microarray analysis (CMA) testing has prompted standard practice guidelines from the American College of Medical Genetics (ACMG), which recommends this testing modality as part of an autism evaluation [Manning and Hudgins 2010].
In this study we report the phenotypic classification, PTEN status and genetic testing results from a cohort of 33 cases of autism and macrocephaly and describe novel phenotypic subgroups.
MATERIALS AND METHODS
This study was approved by the David Geffen School of Medicine Institutional Review Board. A retrospective chart review was carried out for all patients evaluated at the UCLA Medical Genetics clinic from 2008-2011 who presented with autism and macrocephaly, defined as a head circumference measurement at least at the 90th %ile (~1.5 standard deviations) above the mean for age or head circumference above 85th%ile with height below the 50th%ile. Somatic overgrowth was defined as a height and weight above the 95th%ile (around 1.8 standard deviations above the mean for age). Disproportionate macrocephaly refers to a height %ile/head circumfernce %ile ratio of <0.7. Relative macrocephaly refers to a height %ile/head circumfernce %ile >0.7. All growth parameter percentiles were calculated using the 2010 CDC growth charts [Rollins and others 2010]. Patients were referred to the clinic by a neurologist or a phychiatrist who evaluated the patients based on the DSM IV criteria for the diagnosis, including instruments such as ADOS, PL-ADOS, CHAT and STAT. Head circumference was obtained by two independent measurements and plotted on standard growth charts by two dysmorphologists. The chromosomal microarray platform was developed by Affymetrix and its performance characteristics determined by UCLA clinical laboratory as required by the CLIA ‘88 regulations. The assay compared the patient’s DNA to 270 HapMap normal controls, using the genome-wide SNP array 6.0. This array platform contains 1.8 million markers for copy number variant detection chosen at ~ 696 bp spacing throughout the human genome. Oligonucleotide probe information is based on the 36 build of the Human Genome (UCSC Genome Browser, hg18, March 2006). Non-diagnostic copy number changes are referenced to the Database of Genomic Variants (http://projects.tcag.ca/variation).
RESULTS
We report on a group of 33 patients evaluated in a Medical Genetics Clinic for autism and macrocephaly, their phenotypic findings, and sub-groupings (Table 1)
Table One.
Phenotypic features of individuals with Autism and Macrocephaly
| Individuals with confirmed PTEN Mutations | ADM (Autism with disproportionate Macrocephaly) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| Gender | M | M | M | M | M | M | M | M | M | F | M | M | F | M | M |
| Age | 15y | 3y | 4y | 2 y 6m |
4y 4m |
18y 11m |
17y | 5y 4m |
3y | 3y 2m |
6y 11m |
17y 6m |
7y | 2y 8m |
2y 9m |
| Height cm (%ile) |
169 (50) |
102 (>95) |
125.6 (>95) |
99 (>95) |
106.8 (75) |
158 (<5) |
168.3 (15) |
106.5 (20) |
92.5 (25) |
91.8 (25) |
117.5 (25) |
174.5 (48) |
123 (53) |
92 (50) |
95 (60) |
| Weight kg (%ile) |
62.6 (74) |
17.4 (95) |
24.8 (>95) |
13.2 (50) |
18.7 (80) |
91.8 (93) |
73.6 (76) |
17.3 (25) |
14.3 (50) |
14.2 (50) |
35.8 (>95) |
61.9 (30) |
26.5 (80) |
14.5 (73) |
16.6 (95) |
| HC cm (%ile) |
58.5 (>99.7) |
56 (>99.7) |
56 (>99.7) |
55.5 (>99.7) |
53 (92) |
62.5 (>99.7) |
57.3 (85) |
53 (87) |
53 (>97) |
51 (92) |
53.5 (85) |
58 (91) |
54 (>97) |
51 (85) |
53 (>97) |
| HC SD | > 3 | > 3 | > 3 | > 3 | 1-3 | >3 | 1-3 | 1-3 | 1-3 | 1-3 | 1-3 | 1-3 | 1-3 | 1-3 | 1-3 |
| HT/HC %ile | .5 | .98 | .98 | .96 | .82 | .05 | .18 | .23 | .25 | .27 | .29 | .53 | .55 | .59 | .62 |
| Hypotonia | − | + | − | − | − | + | + | + | + | + | + | − | + | − | + |
| Flat Nasal Bridge |
− | − | − | − | − | + | + | + | − | − | + | − | + | − | − |
| Clinodactyly | − | − | − | − | − | − | − | − | − | + | − | + | − | − | − |
| CMA | ? | ? | ? | ? | − | ? | dup6q23.2 | − | − | − | − | − | − | − | ? |
| PTEN | P38H | R130X | Y68N | R130L | V255A | − | − | − | − | − | − | − | − | − | ? |
| ARM (Autism with Relative Macrocephaly) | AMSO (Autism Macrocephaly with Somatic Overgrowth) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 |
| Gender | F | F | M | F | M | F | M | M | M | M | M | M | M | F | M | M | M | M |
| Age | 7y 10m |
2y 7m |
3y 1m |
4y 7m |
3y 10m |
2y 7m |
7y | 3y 8m |
4y 8m |
6y | 1y 6m |
3y 2m |
3y | 3y 6m |
14y 4m |
3y 1m |
4y 1m |
6y 11m |
| Height cm (%ile) |
129 (74) |
94 (75) |
98 (73) |
107.5 (74) |
104 (76) |
94.6 (85) |
126.7 (80) |
104.5 (89) |
112 (89) |
120 (80) |
87.5 (95) |
102 (93) |
113 (>95) |
109.8 (>95) |
181.3 (>95) |
104.8 (>95) |
116.7 (>95) |
140.3 (>95) |
| Weight kg (%ile) |
32.3 (91) |
17.6 (>95) |
16.8 (91) |
17.5 (50) |
17.2 (80) |
17.5 (>95) |
24.9 (74) |
18.4 (92) |
21 (90) |
23.3 (80) |
13.1 (80) |
17.1 (90) |
35 (>95) |
20.3 (>95) |
70.3 (92) |
19 (>95) |
22.2 (>95) |
56.7 (>95) |
| HC cm (%ile) |
55.5 (>97) |
53 (>97) |
52 (93) |
52.5 (95) |
53 (96) |
55.5 (>99.7) |
54 (90) |
54 (>97) |
54 (>97) |
53.5 (90) |
52.5 (>99.7) |
52.5 (96) |
55 (>99.7) |
52 (>97) |
57.5 (96) |
53 (>97) |
54.5 (>97) |
56 (>97) |
| HC 1-3SD | + | + | + | + | + | >3SD | + | + | + | + | >3SD | + | >3SD | + | + | + | + | + |
| HT/HC %ile | .76 | .77 | .79 | .79 | .79 | .85 | .89 | .91 | .91 | .89 | .95 | .97 | .98 | .98 | .98 | .98 | .98 | .98 |
| Hypotonia | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | + | − | − |
| Flat Nasal Bridge |
+ | − | − | + | − | − | − | − | − | − | − | − | + | − | − | − | + | − |
| Clinodactyly | − | − | − | − | + | − | − | + | − | + | + | + | + | − | − | − | − | − |
| CMA | ? | − | ? | − | − | ? | − | − | − | ? | del10q24.32 | − | ? | − | − | − | − | − |
| PTEN | − | ? | ? | ? | − | ? | − | − | ? | ? | ? | ? | ? | − | ? | − | − | − |
CMA: Chromosomal Microarray; HC: Head Circumference; HT: Height; %ile: percentile; ?: unknown; +: feature present; − : normal or not present; SD: standard deviation
Confirmed PTEN Mutations
Within our cohort five patients had confirmed PTEN mutations. Careful phenotypic analysis revealed that (4/5) 80% of those positive for mutations in PTEN presented with “extreme macrocephaly” or head circumference that was greater than or equal to 3SD (99.7th %ile) above the mean. This extent of macrocephaly was present in a range of ages, from 31 months to 15 years old. The one patient (Patient 5) whose head circumference was 1-2 SD ( 92nd %ile) above the mean also carries a novel missense mutation in PTEN, V255A. This mutation is in a well-conserved residue in many species, and is reported to be “possibly damaging” by Polyphen software (PP1). Additionally, patients 1 and 3 presented with novel PTEN mutations, P38H and Y68N. The clinical findings for patient 1 were recurrent otitis media, lipomas, and pigmented glans of the penis. This mutation (P38H) is predicted to be “probably damaging” by Polyphen software (PP1) and also the amino acid shows conservation across numerous species. . Patient 3 presented with a flattened nasal bridge and plagiocephaly. The mutation (Y68N) is predicted to be “possibly damaging” by Poly phen software (PP1) and the amino acid is well conserved . Additional clinical findings of these patients can be found in Figure 1.
Figure 1.
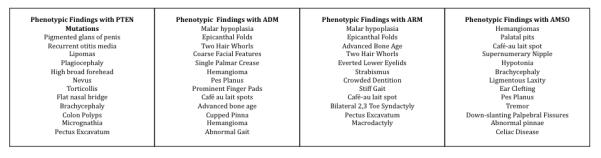
Phenotypic features of individuals with Autism Macrocephaly subtypes
Autism with Disproportionate Macrocephaly (ADM)
Among patients with macrocephaly, we identified a novel phenotype consisting of macrocephaly that was disproportionate to height. There were 10 patients with this phenotype; 9/10 (90%) of these patients had normal PTEN testing. Interestingly 8/10 (80%) of the patients in this group presented with hypotonia and 5/10 (50%) of these patients presented with flattened nasal bridges. A complete list of the physical findings of this group can be found in Figure 1. Chromosomal microarray analysis (CMA) revealed a copy number variation (CNV) in one case. Patient 7 had a 767 kb duplication of an interstitial region at the cytogenetic position 6q23.2 and also presented with the clinical findings of bilateral epicanthal folds and a flattened nasal bridge.
Autism with Relative Macrocephaly
In our cohort we identified a group (n=12) with “relative macrocephaly”. Of these, 5/12 (42%) presented with bilateral clinodactyly. In comparison to the ADM subgroup only 2/12 (17%) had de novo subgroup only 2/12 (17%) had hypotonia and 1/12 (8%) had a flattened nasal bridge. CMA testing revealed a de novo 324 kb deletion of 10q24.32 in Patient 26. Additional clinical findings in this patient included flattened nasal bridge and bilateral clinodactyly.
Autism Macrocephaly with Somatic Overgrowth (AMSO)
An additional subgroup of patients presented with somatic overgrowth (n=6) in addition to macrocephaly. The additional physical findings of this group can be found in Figure 1, including normal bone ages.
DISSCUSSION
Autism is a complex disease, which has highly variable severity and associated physical findings. The evidence for a genetic basis of the disease is compelling, although the large number of genome-wide association studies (GWAS) performed points to many genetic alterations in non-overlapping associated regions of the genome [Hu and others 2011] . While this is suggestive of a multifactorial etiology, it is also likely that lumping of all autism cases confounds what may be a heterogeneous group of phenotypes and clouds the ability to demonstrate true causality. Approaches at identifying “endophenotypes” offer the ability to parse out possible distinct genetic etiologies and enhance genotype-phenotype correlations.
We have described a cohort of patients with autism and macrocephalyassociated with PTEN mutations, some previously unreported which add to those already described in the literature. All but one of our cases with documented PTEN mutations exhibited “Extreme Macrocephaly,” defined as 3SD (>99.7 %ile) above the mean. Interestingly, we identified a patient with a PTEN mutation and a head circumference that is between 1-2SD (92nd %ile) above the mean (Patient 5). This extent of macrocephaly is less than what has been reported previously in cases of PTEN-associated macrocephaly autism syndrome [Butler and others 2005; McBride and others 2010; Mester and others 2011]. In that case, the mutation identified, V255A, is novel and may reflect a hypomorphic allele, which manifests with a lesser degree of macrocephaly. However further causative studies are needed to confirm that this mutation is in fact pathogenic. We identified two additional novel PTEN mutations, P38H (Patient 1) and Y68N (Patient 3) that presented with unique clinical findings including brachycephaly, nevi, and plagiocephaly (Figure 1). The pigmented macules on the glans of the penis along with reported lipomas suggest that patient 1 has Bannayan-Riley-Ruvalcaba-Syndrome (OMIM 15348) [Hobert and Eng 2009].
We report a clinical yield of 22% (5/23) for PTEN testing in this cohort, which is larger than the previously reported value of 10% reported by Herman et al in 2007, perhaps due to our small sample size. We, suggest that disproptionate macrocephaly may very well have a genetic etiology different from PTEN, which is evidenced by the lack of PTEN mutations in that cohort. Future genome analysis of this group may uncover a common genetics etiology which would allow for a clinical determinant of the need for PTEN testing.
In our analysis we found a group (N=6) with not only macrocephaly but also generalized somatic overgrowth. We contend that this represents a distinct subgroup of macrocephaly autism cases. This subgroup will be a cohort amenable for future genomic studies that may contribute to the understanding of autism as well as the etiology of somatic overgrowth. Autistic findings have been described in overgrowth syndromes [Cohen 2003; Kent and others 2008], and this subgroup of macrocephaly autism cases with somatic overgrowth may share genetic defects in a common signaling pathway related to PTEN.
In our remaining patients without somatic overgrowth we have herein used a novel clinical calculation to separate disproportionate from relative macrocephaly. We contest that the Autism Dispropotionate Macrocephaly (ADM) Subgroup is a distinct cohort whose genetic etiology for autism and/or macrocephaly may be different from the PTEN-positive and somatic overgrowth subtypes. In particular, hypotonia (90%) and a flattened nasal bridge (50%) are the most common clinical findings that distinguish this group and may, in the future, help to further subdivide these cases into subphenotypes. Recent reports have identified gene mutations that can be associated with autism and disproportionate macrocephaly, including the small GTPase RAB39B [Giannandrea and others 2010], and GlialCAM [Lopez-Hernandez and others 2011]; mild mutations in these genes may be associated with this subset of patients who present with autism-macrocephaly in the absence of somatic overgrowth and mutations in PTEN.
We stress the importance of CMA testing in all cases of macrocepahly autism. A significant number of chromosomal CNVs, both duplications and deletions, have been associated with autistic manifestations [Vorstman and others 2006]. Two separate reports have previously demonstrated that both duplications and deletions can result in autism and macrocephaly [Brunetti-Pierri and others 2008; Moreno-De-Luca and others 2010; Naqvi and others 2000]. We have observed two CNVs in our cohort: a novel duplication (767kB) at 6q23, and an additional de novo variant deletion located at 10q24.32 similar to a previously reported overlapping deletion at 104251636-104668797(genome assembly hg18). This patient presented with severe mental retardation, autistic behavior, hypotonia, macrocephaly, cerebellar vermis agenesis and partial sight [Jaillard and others 2010]. The duplicated genomic region at 6q23 (132,777,055-133,543,604) was reported as a “copy number change of unknown significance” however it contains over 10 genes some of which have roles in neurological development. A search of copy number variants did not identify any report of this duplication in the general population, indicating that it is clinically relevant.
We report a 22% yield for PTEN testing, a 7% yield with CMA, and 0% with fragile X in cases with macrocephaly. These yields are significantly higher than previously reported which may be due to the small sample size of this study, [Herman and others 2007b; Shen and others 2010] suggesting that the use of head circumference as a clinical indicator will increase overall yield in identifying a genetic etiology.
We contend that there is a subgroup of patients with autism and macrocephaly who do not have PTEN mutations and that represent a distinct subgroup of phenotypes amenable for gene association studies. Furthermore, there is a cohort of these patients who present with somatic overgrowth (AMSO), which may represent a distinct genetic etiology. Clinical features, such as macrocephaly, that are associated with autism, allow for subgroups to be established and facilitate genetic association studies, significantly enhancing testing yield.
In conclusion, we report on a group of patients with autism and macrocephaly. We recommend that two distinct subgroups be recognized: (1) autism with disproportionate macrocephaly (ADM), and (2) autism macrocephaly with somatic overgrowth (AMSO), both distinct from Autism with relative macropcephaly (ARM) and can be segregated based on clinical measurements. These will be amenable for genetic characterization in future studies.
Table 2.
CNVs in patients with macrocephaly autism
| Study | CNVs | Subgroup | Clinical Findings | Parental Origin |
|---|---|---|---|---|
| Klein, 2012 | Duplication of 6q23.2 (132777055- 133543604) |
Autism Disproportionate Macrocephaly (ADM) |
Bilateral epicanthal folds Flattened nasal bridge Strabismus Hyperpigmented lesion |
Unknown |
| Klein, 2012 | Deletion of 10q24.32 (104345460- 104670581) |
Autism Relative Macrocephaly (ARM) |
Flattened nasal bridge Clinodactyly of 4th and 5th finger and 5th toe bilaterally Plagiocephaly |
De Novo |
Acknowledgements
We would like to thank the patients and their families for their participation in this work. SK performed the retrospective chart review, compiled and analyzed the data, and wrote the manuscript. JAMA performed the clinical evaluation and genetic testing of all cases, performed the analysis and wrote the manuscript. SK was supported by a Summer Scholars Grant from the American College of Medical Genetics and NHGRI T32HG002536. Supported in Part by Research Grant No. 6-324 from the March of Dimes Foundation and theToday’s and Tomorrow’s Children Fund (TTCF).
REFERENCES
- Bruining H, de Sonneville L, Swaab H, de Jonge M, Kas M, van Engeland H, Vorstman J. Dissecting the clinical heterogeneity of autism spectrum disorders through defined genotypes. PLoS One. 2010;5(5):e10887. doi: 10.1371/journal.pone.0010887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Berg JS, Scaglia F, Belmont J, Bacino CA, Sahoo T, Lalani SR, Graham B, Lee B, Shinawi M, Shen J, Kang SH, Pursley A, Lotze T, Kennedy G, Lansky-Shafer S, Weaver C, Roeder ER, Grebe TA, Arnold GL, Hutchison T, Reimschisel T, Amato S, Geragthy MT, Innis JW, Obersztyn E, Nowakowska B, Rosengren SS, Bader PI, Grange DK, Naqvi S, Garnica AD, Bernes SM, Fong CT, Summers A, Walters WD, Lupski JR, Stankiewicz P, Cheung SW, Patel A. Recurrent reciprocal 1q21.1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nat Genet. 2008;40(12):1466–71. doi: 10.1038/ng.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, Miles JH, Wang CH, Stratton R, Pilarski R, Eng C. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. Journal of Medical Genetics. 2005;42(4):318–21. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Cai G, Chaste P, Nygren G, Goldsmith J, Reichert J, Anckarsater H, Rastam M, Smith CJ, Silverman JM, Hollander E, Leboyer M, Gillberg C, Verloes A, Betancur C. Mutation screening of the PTEN gene in patients with autism spectrum disorders and macrocephaly. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(4):484–91. doi: 10.1002/ajmg.b.30493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM., Jr. Mental deficiency, alterations in performance, and CNS abnormalities in overgrowth syndromes. Am J Med Genet C Semin Med Genet. 2003;117C(1):49–56. doi: 10.1002/ajmg.c.10013. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Brain development in autism: early overgrowth followed by premature arrest of growth. Ment Retard Dev Disabil Res Rev. 2004;10(2):106–11. doi: 10.1002/mrdd.20020. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290(3):337–44. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Etherton MR, Tabuchi K, Sharma M, Ko J, Sudhof TC. An autism-associated point mutation in the neuroligin cytoplasmic tail selectively impairs AMPA receptor-mediated synaptic transmission in hippocampus. EMBO J. 2011;30(14):2908–19. doi: 10.1038/emboj.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannandrea M, Bianchi V, Mignogna ML, Sirri A, Carrabino S, D’Elia E, Vecellio M, Russo S, Cogliati F, Larizza L, Ropers HH, Tzschach A, Kalscheuer V, Oehl-Jaschkowitz B, Skinner C, Schwartz CE, Gecz J, Van Esch H, Raynaud M, Chelly J, de Brouwer AP, Toniolo D, D’Adamo P. Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly. Am J Hum Genet. 2010;86(2):185–95. doi: 10.1016/j.ajhg.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman GE, Butter E, Enrile B, Pastore M, Prior TW, Sommer A. Increasing knowledge of PTEN germline mutations: Two additional patients with autism and macrocephaly. American Journal of Medical Genetics Part A. 2007a;143(6):589–93. doi: 10.1002/ajmg.a.31619. [DOI] [PubMed] [Google Scholar]
- Herman GE, Henninger N, Ratliff-Schaub K, Pastore M, Fitzgerald S, McBride KL. Genetic testing in autism: how much is enough? Genet Med. 2007b;9(5):268–74. doi: 10.1097/gim.0b013e31804d683b. [DOI] [PubMed] [Google Scholar]
- Hobert JA, Eng C. PTEN hamartoma tumor syndrome: an overview. Genet Med. 2009;11(10):687–94. doi: 10.1097/GIM.0b013e3181ac9aea. [DOI] [PubMed] [Google Scholar]
- Hu VW, Addington A, Hyman A. Novel autism subtype-dependent genetic variants are revealed by quantitative trait and subphenotype association analyses of published GWAS data. PLoS One. 2011;6(4):e19067. doi: 10.1371/journal.pone.0019067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurov I, Vorsanova SG, Saprina EA, Iurov Iu B. Identification of candidate genes of autism on the basis of molecular cytogenetic and in silico studies of the genome organization of chromosomal regions involved in unbalanced rearrangements. Genetika. 2010;46(10):1348–51. [PubMed] [Google Scholar]
- Jaillard S, Drunat S, Bendavid C, Aboura A, Etcheverry A, Journel H, Delahaye A, Pasquier L, Bonneau D, Toutain A, Burglen L, Guichet A, Pipiras E, Gilbert-Dussardier B, Benzacken B, Martin-Coignard D, Henry C, David A, Lucas J, Mosser J, David V, Odent S, Verloes A, Dubourg C. Identification of gene copy number variations in patients with mental retardation using array-CGH: Novel syndromes in a large French series. Eur J Med Genet. 2010;53(2):66–75. doi: 10.1016/j.ejmg.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217–50. [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Acta Paedopsychiatr. 1968;35(4):100–36. [PubMed] [Google Scholar]
- Kent L, Bowdin S, Kirby GA, Cooper WN, Maher ER. Beckwith Weidemann syndrome: a behavioral phenotype-genotype study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(7):1295–7. doi: 10.1002/ajmg.b.30729. [DOI] [PubMed] [Google Scholar]
- Lopez-Hernandez T, Ridder MC, Montolio M, Capdevila-Nortes X, Polder E, Sirisi S, Duarri A, Schulte U, Fakler B, Nunes V, Scheper GC, Martinez A, Estevez R, van der Knaap MS. Mutant GlialCAM causes megalencephalic leukoencephalopathy with subcortical cysts, benign familial macrocephaly, and macrocephaly with retardation and autism. Am J Hum Genet. 2011;88(4):422–32. doi: 10.1016/j.ajhg.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Hudgins L. Array-based technology and recommendations for utilization in medical genetics practice for detection of chromosomal abnormalities. Genet Med. 2010;12(11):742–5. doi: 10.1097/GIM.0b013e3181f8baad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride KL, Varga EA, Pastore MT, Prior TW, Manickam K, Atkin JF, Herman GE. Confirmation study of PTEN mutations among individuals with autism or developmental delays/mental retardation and macrocephaly. Autism Res. 2010;3(3):137–41. doi: 10.1002/aur.132. [DOI] [PubMed] [Google Scholar]
- Mester JL, Tilot AK, Rybicki LA, Frazier TW, 2nd, Eng C. Analysis of prevalence and degree of macrocephaly in patients with germline PTEN mutations and of brain weight in Pten knock-in murine model. European Journal of Human Genetics. 2011;19(7):763–8. doi: 10.1038/ejhg.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-De-Luca D, Mulle JG, Kaminsky EB, Sanders SJ, Myers SM, Adam MP, Pakula AT, Eisenhauer NJ, Uhas K, Weik L, Guy L, Care ME, Morel CF, Boni C, Salbert BA, Chandrareddy A, Demmer LA, Chow EWC, Surti U, Aradhya S, Pickering DL, Golden DM, Sanger WG, Aston E, Brothman AR, Gliem TJ, Thorland EC, Ackley T, Iyer R, Huang SW, Barber JC, Crolla JA, Warren ST, Martin CL, Ledbetter DH, Consortium S, Genetics SSC, GeneSTAR Deletion 17q12 Is a Recurrent Copy Number Variant that Confers High Risk of Autism and Schizophrenia. American Journal of Human Genetics. 2010;87(5):618–630. doi: 10.1016/j.ajhg.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113(5):e472–86. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- Naqvi S, Cole T, Graham JM., Jr Cole-Hughes macrocephaly syndrome and associated autistic manifestations. Am J Med Genet. 2000;94(2):149–52. doi: 10.1002/1096-8628(20000911)94:2<149::aid-ajmg7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Rollins JD, Collins JS, Holden KR. United States head circumference growth reference charts: birth to 21 years. J Pediatr. 2010;156(6):907–13. 913, e1–2. doi: 10.1016/j.jpeds.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JA, Ballif BC, Torchia BS, Sahoo T, Ravnan JB, Schultz R, Lamb A, Bejjani BA, Shaffer LG. Copy number variations associated with autism spectrum disorders contribute to a spectrum of neurodevelopmental disorders. Genet Med. 2010;12(11):694–702. doi: 10.1097/GIM.0b013e3181f0c5f3. [DOI] [PubMed] [Google Scholar]
- Shen Y, Dies KA, Holm IA, Bridgemohan C, Sobeih MM, Caronna EB, Miller KJ, Frazier JA, Silverstein I, Picker J, Weissman L, Raffalli P, Jeste S, Demmer LA, Peters HK, Brewster SJ, Kowalczyk SJ, Rosen-Sheidley B, McGowan C, Duda AW, 3rd, Lincoln SA, Lowe KR, Schonwald A, Robbins M, Hisama F, Wolff R, Becker R, Nasir R, Urion DK, Milunsky JM, Rappaport L, Gusella JF, Walsh CA, Wu BL, Miller DT. Clinical genetic testing for patients with autism spectrum disorders. Pediatrics. 2010;125(4):e727–35. doi: 10.1542/peds.2009-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SJ. The genetics of autism. Semin Pediatr Neurol. 2004;11(3):196–204. doi: 10.1016/j.spen.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Staal WG, van Daalen E, van Engeland H, Hochstenbach PF, Franke L. Identification of novel autism candidate regions through analysis of reported cytogenetic abnormalities associated with autism. Mol Psychiatry. 2006;11(1):1, 18–28. doi: 10.1038/sj.mp.4001781. [DOI] [PubMed] [Google Scholar]
- Whitehouse AJ, Bishop DV, Ang QW, Pennell CE, Fisher SE. CNTNAP2 variants affect early language development in the general population. Genes Brain Behav. 2011;10(4):451–6. doi: 10.1111/j.1601-183X.2011.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


