Abstract
Basic helix–loop–helix (bHLH) transcription factors control developmental decisions for a wide range of embryonic cell types. Hand1 and Hand2 are closely related bHLH proteins that control cardiac, craniofacial, and limb development. Within the developing heart, Hand1 expression becomes restricted predominantly to the left ventricle, whereas Hand2 becomes restricted predominantly to the left ventricle, for which findings have shown each Hand factor to be necessary for normal chamber formation. Forced overexpression of Hand1 throughout the early developing heart induces abnormal interventricular septal development, with resulting pathogenesis of congenital heart defects. To investigate the potential transcriptional mechanisms involved in heart morphogenesis by Hand2, this study used a replacement targeting approach to knock Hand2 into the Hand1 locus and ectopically express one copy of Hand2 within the endogenous Hand1 expression domain in the developing hearts of transgenic mice. The findings show that high-percentage Hand1Hand2 chimeras die at birth and exhibit a range of congenital heart defects. These findings suggest that Hand factors may act via unique transcriptional mechanisms mediated by bHLH factor partner choice, supporting the notion that alterations of Hand factor stoichiometry may be as deleterious to normal heart morphogenesis as Hand factor loss of function.
Keywords: bHLH transcription factors, Chimeras, Congenital heart defects
Proper development of all multicellular organisms requires the spatial and temporal coordination of numerous transcriptional pathways and integrated extracellular signaling cues. Developing embryos comprise a complex and dynamic environment in which cells are typically exposed to a great deal of overlapping and transient transcriptional information, growth factors, and signaling pathways that may serve synergistic, parallel, and/or antagonistic roles. A key question is how cells within such an environment respond to these competing influences to enact appropriate cell fate specification and differentiation programs.
Congenital heart defects affect 1% of live births [21] and frequently require intervention for the treatment of childhood heart failure in an attempt to prevent neonatal death [6, 13]. Although the deleterious consequences of such cardiac malformations usually are evident only after birth, the underlying causes of these defects frequently involve deregulation of events within the transcriptional programs that control cardiac specification, differentiation, and morphogenesis in utero.
Hand1 and Hand2 are evolutionarily conserved basic helix–loop–helix (bHLH) transcription factors. The first class B genes of the bHLH superfamily identified to play a role in cardiogenesis were the Hand transcription factors Hand1 (previously termed eHAND, Hxt, Thing1) and Hand2 (previously termed dHAND, Hed, Thing2).
Hand factors show high amino acid identity between species, suggesting conserved biologic function [6, 8, 9, 14, 15, 39], whereas functional studies have shown that Hand1 and Hand2 exhibit broad dimerization profiles [17]. Hand1 and Hand2 are evolutionarily conserved basic transcription factors that exhibit dynamic and partially overlapping spatiotemporal expression patterns during cardiovascular development. Cardiac expression of Hand1 and Hand2 is initiated after cell specification (E7.0 in mice; HH stage 8 in chick; day 21 in human). Although mammalian Hand1 and Hand2 are initially coexpressed, during morphogenesis and asymmetric looping of the early embryonic heart tube, Hand1 becomes predominantly restricted to the left ventricle, whereas Hand2 becomes predominantly restricted to the right ventricle [16, 39]. However, both genes remain coexpressed in the embryonic aortic sac, the great vessels that exit the heart, and the nascent interventricular septum that eventually septates the left and right ventricular chambers [6, 15].
As cardiogenesis progresses, Hand gene expression is progressively downregulated [9, 39]. A study in human patients showed that HAND genes may be expressed at very low levels in the adult human heart but can be reexpressed during heart disease [30, 35] and significantly upregulated in response to cardiac hypertrophy [43]. Recently, a functional genetic study identified HAND1 mutations in septation defects within tissue samples from human heart patient samples [31].
Transgenic analysis of Hand factors shows that both are required for normal cardiovascular development. Both systemic Hand1- and Hand2-targeted knock-out mice exhibit heart morphologic abnormalities [16, 33, 40]. Hand2 nulls are embryonically lethal and die between E9.5 and E10.5 due to cardiac and vascular defects [40, 48]. Morphologic analysis of Hand2 nulls shows that the region of the looping heart tube destined to become the right ventricle is missing. Although the morphologic phenotype could be the result of a looping defect, it likely is not based on the finding that Hand2 expression tracks with alterations in sidedness putting it downstream of right-left polarity signals [44].
Molecular analysis in mice shows that cardiac specification occurs because cardiac-restricted molecular markers are normally expressed [40]. However, expression of the chamber-restricted marker natriuretic peptide precursor type A (Nppa) [20] is downregulated in Hand2 nulls, and this reduction is via a direct transcriptional effect [42].
In contrast, Hand1 does not transcriptionally influence Nppa expression, suggesting that Hand1 and Hand2 may not be functionally redundant during cardiogenesis. Although the Hand1 null cardiovascular phenotype is difficult to analyze due to the extraembryonic defects and early E8.5 to E9.5 in utero lethality [16, 33], the cardiac lineage is specified, and cell differentiation (assessed via expression of cardiac-specific structural genes Mlc2a and Mlc2v) occurs in Hand1 nulls [16]. Tetraploid rescue of Hand1 null embryos has suggested a looping defect [33] Combined, these data suggest that like Hand2, the function of Hand1 during cardiogenesis most likely is to regulate heart morphogenesis and is not required during early cardiac lineage commitment or initial cell differentiation. In support of this, when a conditional Hand1-null allele was deleted in only the cardiomyocytes lineage, perinatal Hand1 conditional mutants displayed defects in the left ventricle and endocardial cushions and exhibited dysregulated ventricular gene expression [28]. Moreover, creation of Hand1/2 double-mutant mice showed gene dose-sensitive functions of Hand transcription factors in the control of cardiac morphogenesis and ventricular gene expression [28].
Although these results must be interpreted in light of the technical limitations of Cre-mediated gene deletion, these data do suggest that mammalian Hand genes may play both overlapping and unique cardiac functions during evolution [28]. Additionally, when the Hand1 cDNA was knocked into the Mlc2v locus and Hand1 was robustly overexpressed ectopically throughout the developing left and right ventricles, septation defects resulted [46].
To understand further the mechanisms whereby HAND factors regulate heart formation during cardiovascular morphogenesis and to test the functional redundancy of Hand1 and Hand2 directly, we substituted Hand2 for Hand1 by knocking Hand2 into the Hand1 locus (Hd1Hd2). Remarkably, our results show that high-percentage Hd1Hd2 chimeras die at birth and exhibit a range of congenital heart defects (CHDs). The observed phenotypes occur specifically where endogenous Hand1 is expressed during normal heart development. These findings show that Hand1 and Hand2 convey unique transcriptional regulation during cardiogenesis and suggest that Hand factor partner choice is critical for normal cardiac morphogenesis.
Materials and Methods
Gene Targeting
We used a replacement targeting approach to knock a Hand2 genomic NotI-BamHI fragment containing both exons and lacking the transcriptional start site into the Hand1 locus using our previously published targeting strategy [16]. The Hand2 fragment was cloned downstream of the Hand1 transcriptional initiation codon contained in the 3.0-kb BstEII Hand1 5′ targeting arm, and an additional EcoRI site was engineered to create a unique restriction fragment length polymorphism (RFLP) site, enabling us to detect a 3.9-kb EcoRI fragment when a 3′ external HindIII-BssHII probe is used, as previously described [16]. All cloning junctions within the Hand1Hand2 (Hd1Hd2) targeting vector were confirmed by DNA sequencing, and the vector was linearized with NotI, before electroporation into 129SvJ mouse embryonic stem (ES) cells by the Indiana University PUI (IUPUI) ES Cell and Transgenic Core Facility. Genomic DNA was isolated from ES clones that survived positive-negative selection using previously established protocols [16, 34]. Southern hybridization on EcoRI-digested DNA confirmed homologous recombination at a frequency of 1:30 in the 60 ES clones analyzed via both the internal and the 3′ external HindIII-BssHII probes previously described [16].
F0 Chimera Production and Analysis
Two correctly targeted ES clones were identified, and both independent clones were subsequently implanted into pseudopregnant wild-type C57BL/6 host blastocysts and into foster female mice to obtain F0 chimeric embryos sacrificed at E10.5 and newborn stages using standard protocols [16, 34]. To obtain primarily high-percentage early embryonic chimeras, 10 to 14 targeted Hd1Hd2 ES cells were injected per blastocyst. Southern analysis of genomic DNA extracted from embryo yolk sacs and newborn tails was used to estimate the percentage of chimerism by comparing the molar ratio of the wild-type and knock-in Hd1Hd2 mutant bands via a personal FX phosphoimager (BioRad, Hercules, CA). The animal use protocols were approved by the Institutional Animal Care and Use Committee at IUPUI.
Histologic, Immunohistochemical, and Gene Expression Analysis
Tissue isolation, 4% paraformaldehyde fixation, processing, paraffin embedding, hematoxylin and eosin (H&E) staining, and immunohistochemical detection of α-smooth muscle actin (αSMA) (1:5,000 dilution αSMA; Sigma, St. Louis, MO) were performed as described [34, 38]. Sections (3 individual embryos/newborns of each genotype) were cut at 10-μm thickness and counterstained. Immunologic reactions were visualized by use of a Vector ABC kit (Sigma, St. Louis, MO) and a peroxidase-diaminobenzidine reaction. The sections were counterstained with hematoxylin and mounted on glass slides. Negative controls were obtained by substituting the primary antibody with serum. In situ hybridization using published Hand1 and Hand2 [47], Tbx20 and Nppa [20], and Ncx1 [24] cDNA probes was performed as previously described [5, 38]. Both sense and antisense S35-uridine triphosphate (UTP)-labeled probes were used, and specific signal was observed only with hybridization of the antisense probe in serial sections within at least three independent embryos/newborns of each genotype.
Results
Forced Expression of a Hand2 Within a Hand1 Locus Can Result in Neonatal Lethality
We used a replacement targeting approach to knock Hand2 into the Hand1 locus, generating a Hd1Hd2 knock-in allele (Fig. 1). The generation of heterozygous Hd1Hd2 ES cells allowed us to test whether Hand1 and Hand2 are interchangeable in regions of endogenous Hand1 expression. After injection of two separately targeted ES cell lines, approximately 55% of the chimeras (n = 14) were stillborn. Genotyping showed that only the high-percentage chimeras were nonviable. Significantly, approximately 60% of the dead chimeras showed more than 95% chimerism based on the equal molar ratio of the wild-type and mutant bands observed via Southern analysis (Fig. 1), and all stillborn chimeras contained more than 70% chimerism (6 of the 6 high-percentage chimeras were stillborn).
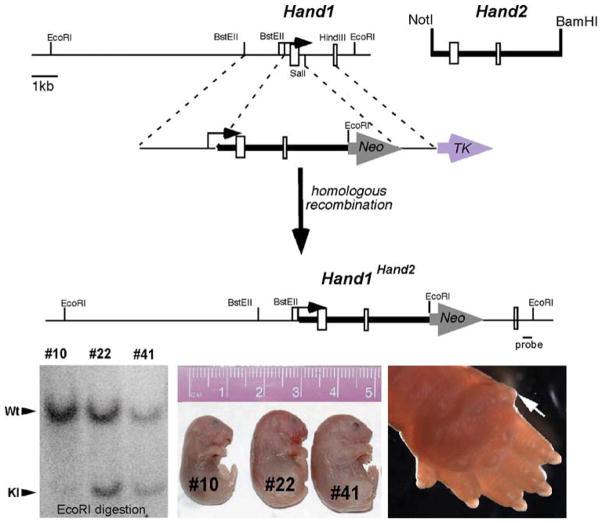
Fig. 1.
Analysis of Hand1Hand2 chimeric mice. (upper panel). The schematic depicts the targeting strategy used. A Hand2 genomic NotI-BamHI fragment containing both exons and lacking the transcriptional start site was cloned downstream of the Hand1 transcriptional start site contained in the Hand1 5′ targeting arm. An additional EcoRI site creates an RFLP, enabling detection of a 3.9-kb EcoRI fragment when a 3′ external probe (indicated) is used (lower right panel). Southern analysis demonstrates chimerism via comparison of the molar ratio of the wild-type (Wt) and knock-in (KI) mutant bands in three newborn F0 age-matched mutant littermate chimeras. Chimera #10 contains less than 15% chimerism control (#10) whereas chimeras #22 and #41 show greater than 95% chimerism (lower middle panel). Stillborn high-percentage Hand1Hand2 (Hd1Hd2) chimeras (#22 and #41) exhibit wholesale edema compared with viable low-percentage unaffected littermates (#10) (lower left panel). Closer examination shows polydactyly in high-percentage Hd1Hd2 chimeras (ectopic posterior digit indicated by the white arrow)
Although we cannot be certain that the percentage contribution of the Hd1Hd2 ES cells is similar between the hearts and the tails, the consistency of the observed phenotypes is highly suggestive that this is the case. Phenotypic examination of the chimeric pups showed generalized whole-body edema, most severe in stillborn high-percentage chimeric pups #22 and #41, whereas viable low-percentage pup #10 appeared grossly unaffected (Fig. 1). Closer examination also showed a high incidence of polydactyly in the stillborn high-percentage chimeras (5 of the 6 high-percentage chimeras showing an extra digit on all four limbs were stillborn). An ectopic digit was present on the posterior side of the limb (Fig. 1), in a position usually occupied by the zone of polarizing activity (ZPA; [45]) and opposite the thumb/big toe. Of the surviving low- and medium-percentage chimeras, one was small and frail (~50 to 60% by coat color), but the remaining ones (~15 to 50% by coat color) were phenotypically normal and fertile but had yet to transmit the Hd1Hd2 allele germ line (26 litters).
High-Percentage Hand1Hand2 Chimerism Results in Congenital Heart Defects
Pathologic examination of the stillborn high-percentage chimeric neonatal Hd1Hd2 pups showed severe dilated cardiomyopathy (both ventricles and atria, Fig. 2a), suggesting that cardiac failure is most likely the cause of postnatal death. Significantly, the high-percentage chimeric pups showed signs of congenital double-outlet right ventricle (DORV) heart defect, in which the aorta and pulmonary trunk are located side by side and exit the right ventricle, compared with the normal arrangement of out-flow tract (OFT) vessels in which the aorta exits the left ventricle and the pulmonary trunk exits the right ventricle (Fig. 2b, c).
Fig. 2.
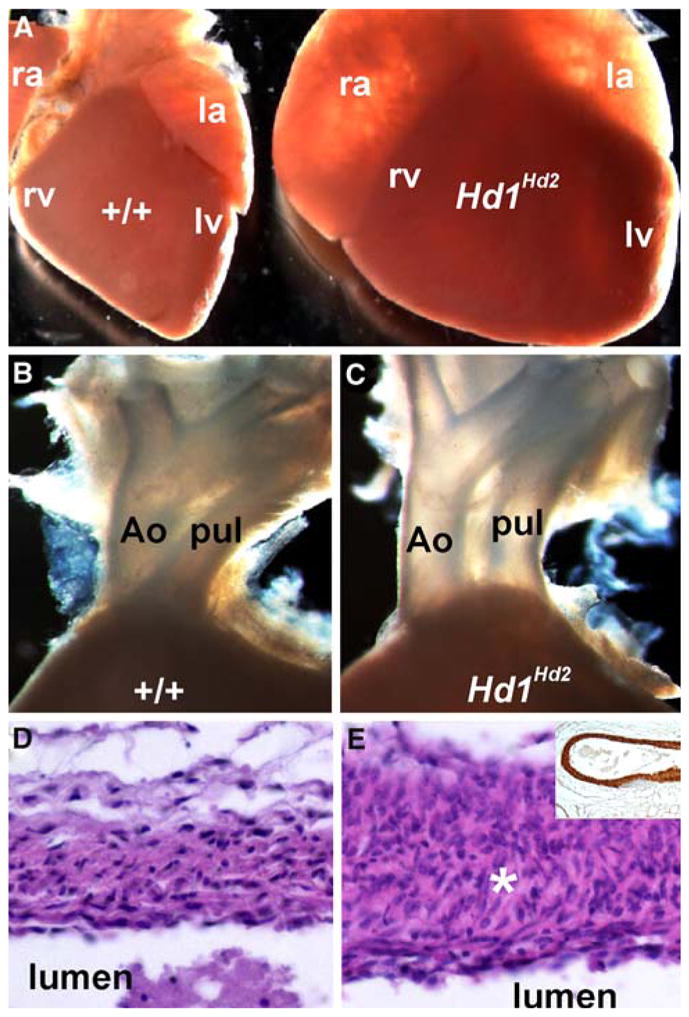
Cardiac defects in the high-percentage Hand1Hand2 chimeric newborns. a–c Wild-type (+/+) and stillborn high-percentage Hd1Hd2 chimeric age-matched littermate hearts. Note the dilation of both atria and ventricles and the rounded apex at the base of the heart compared with controls. Closer examination shows that high-percentage chimeras exhibit double-outlet right ventricle (DORV) as both the aorta (Ao) and pulmonary trunk (pul) exit the right ventricle (c) compared with wild-type littermates (b). d, e Hematoxylin and eosin (H&E) sections through the wild-type (d) and Hd1Hd2 chimeric aortic arch show hyperplasia of the smooth muscle layer (asterisk) around the outflow tract (OFT) vessels. Note however, that there is appropriate α-smooth muscle actin (αSMA) staining of the Hd1Hd2 chimeric vasculature (shown in the inset in e). ra right atria, rv right ventricle, la left atria, lv left ventricle
Histologic examination showed that although αSMA expression was unperturbed, the Hd1Hd2 chimeric OFT vessel walls were hyperplastic, and the lamella organization of the smooth muscle surrounding the aorta, pulmonary artery, and ductus arteriosus was disrupted (Fig. 2e). Although the high-percentage Hd1Hd2 placenta appeared unaffected, their newborn livers were enlarged and engorged with fetal blood, supporting the implication of hemodynamic overload and heart failure [7].
Detailed histologic analysis of a stillborn high-percentage chimeric neonatal Hd1Hd2 cardiovascular system confirmed severe cardiac malformations (Fig. 3), specifically DORV with concomitant interventricular septal defects (VSDs) and patent ductus arteriosus (PDA) in all the stillborns (6 of the 6 high-percentage chimeras were stillborn). Sections showed that all the dead Hd1Hd2 neonates show VSDs, that their hearts had a thinned myocardium, and that the myocardial architecture was extensively disorganized (Fig. 3b–f). In high-percentage chimeric hearts, both multiple muscular and obligatory large perimembraneous VSDs were present (Fig. 3b–e), but in the lower-percentage Hd1Hd2 hearts, only an isolated muscular VSD was observed at the base of the heart (Fig. 3f). The most severely affected chimera (#41) showed that the entire septum was hypoplastic with multiple VSDs (Fig. 3c). Furthermore, the high-percentage chimeric Hd1Hd2 coronary vascular system was affected as the coronaries were dilated and lacked complete supporting muscle layer (because αSMA expression is irregular; Fig. 3d), further suggesting heart failure as the cause of neonatal Hd1Hd2 lethality.
Fig. 3.
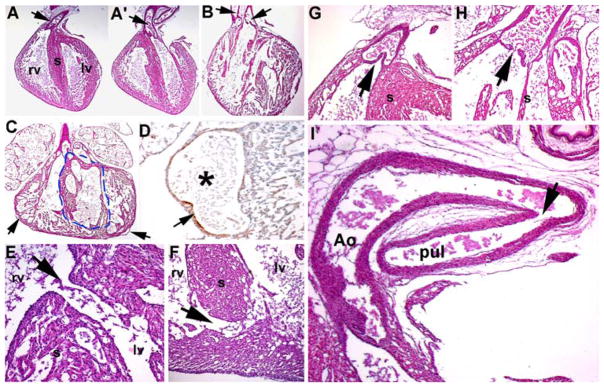
Histologic examination of cardiovascular defects in Hand1Hand2 newborns. a, a′ Wild-type heart depicting the exit of the pulmonary trunk from the right ventricle (arrow in a) and exit of the aorta from the left ventricle (arrow in a′). b Stillborn high-percentage Hd1Hd2 chimeric heart exhibiting double-outlet right ventricle (DORV) as both outflow tract (OFT) vessels exit the right ventricle (arrows), as well as mild hypoplasia of the ventricles, thin myocardial walls, and abnormal perforated septum resulting in both membranous and muscular interventricular septal defects (VSDs). c, d Additional example of high-percentage Hd1Hd2 chimeric heart illustrating ventricular septal hypoplasia (indicated via the dotted line) and dilated coronaries (arrows in c). High-power view of fluid-filled mutant coronary artery (asterisk in d) and incomplete α-smooth muscle actin (αSMA) expression in the supporting muscle layer of the dilated coronaries (arrow in d). e, f Histologic sectioning showing associated perimembranous VSDs (high-percentage chimera shown in panel b) and muscular VSDs (low-percentage chimera). g, h Hd1Hd2 chimeric valve leaflets are hypoplastic and misshapen (chimera in panel b) compared with wild-type littermates. i Stillborn high-percentage Hd1Hd2 chimeras (n = 6) all exhibiting patent ductus arteriosus as the ductus remains open (arrow in i) between the descending aorta (Ao) and the pulmonary trunk (pul), resulting in a high-volume burden on the neonatal lungs and respiratory failure. rv right ventricle, lv left ventricle
Significantly, all the dead chimeras also exhibited hypoplastic/misshapen OFT valve leaflets and PDA ductus arteriosus (Fig. 3). Collectively, in the absence of any other obvious defects (apart from polydactyly), the replacement of one allele of Hand1 for one expressing Hand2 in regions expressing Hand1 was sufficient to cause the DORV, VSD, and PDA congenital defects which undoubtedly would have caused hemodynamic distress that resulted in neonatal respiratory failure [7].
Ectopic Hand2 Expression Within the Hand1 Locus Results in Upregulation of Nppa
Analysis of the cardiac gene expression via in situ hybridization in newborn offspring showed upregulation of Nppa expression in Hd1Hd2 hearts (Fig. 4). Nppa is secreted by cardiac myocytes [10], is one of the first hallmarks of chamber formation [20], has been implicated in the control of extracellular fluid volume and electrolyte homeostasis, and is one of the most commonly used molecular markers of cardiac failure [2, 22, 49].
Fig. 4.
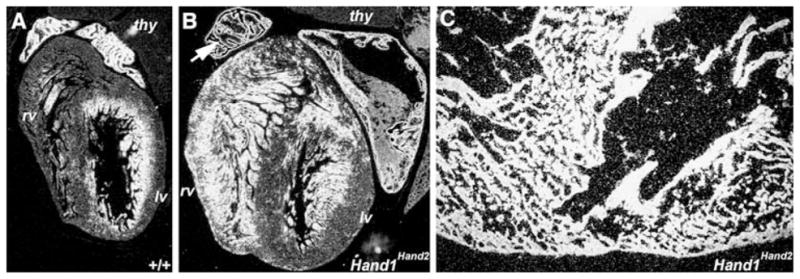
Natriuretic peptide precursor type A (Nppa) is upregulated in Hand1Hand2 newborn hearts. a, b According to S35 in situ hybridization analysis, Nppa mRNA expression is significantly and ectopically upregulated in F0 medium-percentage (~40 to 60%) chimeric newborn Hd1Hd2 hearts compared with wild-type (+/+) age-matched littermate hearts. Note that in mutant hearts (b), upregulation of Nppa is clearly detectable in the atria and ventricles (but not in the thymus [thy]), whereas in normal hearts (a), expression usually is confined to the ventricular trabecular region and the atria. High-level Nppa expression in the chimeric right atria has a burnt-in signal and at this point appears black in a dark field image (arrow in b). c In a high-power view of stillborn high-percentage Hd1Hd2 chimeras, Nppa is abnormally ubiquitously expressed in all cardiomyocytes in the dilated mutant ventricles. All in situs were probed with the same S35 probe and exposed for an equivalent exposure time. rv right ventricle, lv left ventricle
Significantly, Nppa was upregulated in both viable medium-percentage (~40 to 60%) and stillborn high-percentage (~60%+) chimeric pup hearts compared with age-matched littermate control subjects. Whereas Nppa was significantly upregulated in medium-percentage Hd1Hd2 chimeric atrial and ventricular cardiomyocytes (Fig. 4b), it was expressed in all the high-percentage chimeric cardiomyocytes, indicating complete heart failure (Fig. 4c). As expected, expression of sodium calcium exchanger-1 (Ncx1) also was upregulated in high-percentage chimeric hearts (data not shown), as Ncx1 is known to be upregulated during heart failure [29]. However, expression of Hand1, Hand2, and another chamber-restricted gene T-box20 [20] was unaffected in newborns (data not shown).
Although Hand1 and Hand2 are normally downregulated during embryogenesis by E13.5 and undetectable in newborns [6, 15, 16, 47], we did not detect any prolonged ectopic Hand2 expression in stillborn knock-in mutant hearts. Similarly, chamber-restricted gene Tbx20 also was not ectopically misexpressed, suggesting that Nppa and Ncx1 upregulation are indicative of heart failure rather than chamber identity/morphologic abnormalities.
Replacement of Hand1 with Hand2 Disrupts Embryonic Cardiac Morphogenesis and Heart Looping
Given that Hand1 cardiac expression is downregulated between E11.5 and E13.5 [15, 16] and that it is uncertain whether early morphologic and molecular events mediated by the Hd1Hd2 allele can result in defects that became deleterious at later developmental stages in our high-percentage chimeras, we reinjected our Hd1Hd2 targeted ES cells. This enabled us to collect F0 chimeric embryos at E10.5 when both Hand1 and Hand2 were both robustly expressed in the heart [6, 15, 47].
After identifying their genotypes via Southern analysis of yolk sacs, we recovered several high-percentage E10.5 Hd1Hd2 chimeras (n = 4) that exhibited cardiac looping anomalies (Fig. 5). The Hd1Hd2 cardiac tube appears angular and loops in an anterior-to-posterior manner rather than right to left (Fig. 5b, c), as is seen in age-matched control littermates. Clearly visible in these high-percentage chimeras were regular heartbeats indicating that these mice likely would survive to later stages of development. Based on phenotypic observations from newborn high-percentage chimeras (Figs. 1, 2 and 3), in which severe VSD and OFT abnormalities were observed, these morphologic abnormalities observed in Hd1Hd2 high-percentage chimeras at earlier time points were consistent with the VSD and OFT defects observed in the nonviable Hd1Hd2 neonates. Moreover, if the known temporal-spatial expression of Hand1 in cardiac development (E7.0–E13.5) is taken into account, the severe neonatal phenotypes observed are likely due to molecular events occurring in the early embryonic period during initial heart remodeling and formation of a four-chambered heart.
Fig. 5.
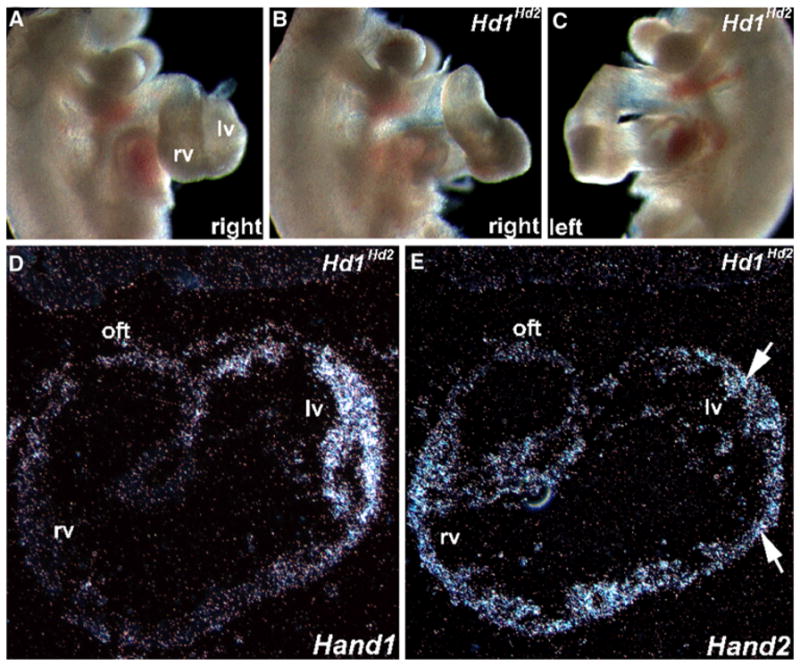
In utero analysis of E10.5 high-percentage Hand1Hand2 chimeric embryos. a–c Whole-embryo right-sided view of a normal E10.5 (a) and both right- (b) and left- (c) sided views of age-matched littermate F0 Hd1Hd2 high-percentage chimeric embryos. Note that the chimeric heart is “unlooped” and farther away from the body of the normal littermate (i.e., both inflow and outflow tract [OFT] are elongated). Otherwise, the chimeric embryos are comparable in size, shape, and structure with their nonchimeric littermates. Also, the chimeric hearts beat at rates comparable with that of control siblings. d, e Using S35 in situ hybridization analysis, sections of the chimeric embryo heart (shown in b and c) were probed with Hand1 (d) and Hand2 (e) cDNA probes. Note that Hand1 mRNA is appropriately expressed in the left ventricle but that Hand2 mRNA is both appropriately expressed in the right ventricle and ectopically expressed in the left ventricle (arrows in e). rv right ventricle, lv left ventricle, oft outflow tract
To confirm ectopic expression of Hand2 within regions of the developing heart that exclusively express Hand1, we used in situ hybridization analysis (Fig. 5d, e). Hand1 is normally expressed within the developing left embryonic ventricle and OFT, whereas Hand2 expression is normally localized largely to the developing right embryonic ventricle and OFT [15, 16]. As expected, endogenous Hand1 mRNA expression is restricted to the E10.5 left ventricle of mutant Hd1Hd2 high-percentage chimeras (Fig. 5d) and indistinguishable from that of nonchimeric control litter-mates (data not shown). As predicted, endogenous Hand2 mRNA expression is detected in the right ventricle and OFT, but knock-in Hand2 mRNA is also ectopically expressed at this point in the E10.5 mutant Hd1Hd2 high-percentage chimera left ventricle (Fig. 5e), where Hand1 is now aberrantly coexpressed within these knock-in mutants.
Importantly, it should be noted that the expression levels of endogenous Hand2 in the right ventricle and transgenic Hand2 in the left ventricle were similar because only a single copy of Hand2 was knocked in using our replacement targeting approach (Fig. 1). The findings of both morphologic defects and ectopic pharmacologically relevant Hand2 expression within the Hd1Hd2 left ventricle suggest that the observed neonatal high-percentage Hd1Hd2 lethal phenotypes are the result of substituting one allele of Hand1 for one transiently coexpressed Hand2 allele during early chamber morphogenesis.
Discussion
We and others previously demonstrated a critical role for Hand factors in the development of limb and heart, and in the regulation of chamber morphogenesis [16, 28, 33, 40, 46]. The results of this study extend those findings and lead to the unexpected conclusion that the activity of Hand factors is mediated by partner choice, regulated stoichiometry, and restricted expression patterns. Hand2, which shares high amino acid identity with Hand1, is incapable of replacing Hand1 during limb bud patterning and cardiac chamber morphogenesis. Moreover, alterations of Hand factor stoichiometry may be as deleterious to normal heart morphogenesis (as well as limb formation) as Hand factor loss of function. Our results suggest that the molecular mechanisms by which Hand factors function during development are more complicated than traditional models of bHLH protein function predict.
To test the functional redundancy of Hand1 and Hand2 directly, we substituted Hand2 for Hand1 by knocking Hand2 into the Hand1 locus (Hd1Hd2). High-percentage chimeric Hd1Hd2 pups die at birth and exhibit polydactyly, OFT misalignment defects, and VSDs, indicating that Hand1 and Hand2 are not functionally redundant, and more importantly, that 50% substitution of ectopic Hand2 for Hand1 expression in Hand1-expressing cells is sufficient to alter cardiomyocyte morphology and limb patterning. The cardiovascular VSD and DORV defects undoubtedly cause hemodynamic distress and resultant neonatal respiratory failure, and in the absence of other obvious defects (apart from polydactyly), we suggest that the replacement of one allele of Hand1 for one expressing Hand2 in regions expressing Hand1 is sufficient to cause lethal CHDs. The presence of polydactyly and the cardiovascular Hd1Hd2 phenotypes in tissues in which Hand1 is expressed strongly argues that ectopic expression of Hand2 affects only tissues that normally express Hand1.
Moreover, because this mouse model expresses Hand2 at physiologically relevant levels (50% of total Hand1 expression) from endogenous transcriptional regulatory elements in the Hand1 locus, the total Hand expression should be equal to endogenous Hand1. Thus, any resultant phenotypes should be only in tissues that express Hand1. In fact, this is what was observed. Phenotypes are evident in the developing limbs [12]. Furthermore, the dilated ventricles, VSDs, and OFT defects all are associated with sites of Hand1 heart expression [14]. Given that Hand1 heterozygous mice are viable, a 50% reduction in expression of Hand1 is obviously not a lethal condition. Mechanistically, this could result from Hand1 and Hand2 not being redundant or from ectopic Hand2 affecting another bHLH factors function.
Hand factors, like other tissue-specific (class B) bHLH proteins, are thought to act primarily as heterodimers with widely expressed E-proteins [26]. However, Hand factors also can form homodimers themselves as well as hetero-dimers with all members of the Twist class of bHLH factors and with bHLH proteins in the HES-related transcription factor family [1, 17–19]. Mice null for Hey2 display membranous VSDs that result in the majority of null mice succumbing to heart failure as neonates [36]. We have shown that Hand dimerization can be regulated in part by phosphorylation of key residues found in helix-I of the bHLH domain and that hypophosphorylation and phosphorylation mimic mutations, resulting in distinct phenotypic limb outcomes when expressed in vivo [18]. Thus an alteration of dimerization affinities can modulate Hand factor function, and the chimeric phenotypes observed may represent the combined effects of diminished expression levels and a shift of the phosphorylation state of helix-I due to distorted dimer partner choice.
The ectopic digit is present on the posterior side of the Hd1Hd2 high-percentage chimeric limbs in a region adjacent to the zone of polarizing activity (ZPA [45]) and opposite the thumb/big toe. The ZPA is a group of mesenchymal cells producing a gradient of Sonic hedgehog (Shh) at the posterior limb margin that controls digit identities along the anteroposterior (thumb to little finger) limb axis [32, 45, 50]. This is significant because the developing limb is a site of Hand1 expression, and Hand2 (along with 5′-HoxD) expression within the ZPA itself controls Shh activation [4]. Furthermore, high-level transgenic misexpression of Hand2 throughout the anterior compartment of the limb bud induces ectopic Shh expression, with resulting preaxial polydactyly and mirror image duplications of posterior digits [27]. Given that conditional inactivation of Shh at specific time points during limb morphogenesis has shown that Shh functions early and transiently in the specification of digit identities [51], these data demonstrate that Hand2 is unable to substitute for Hand1 expression during limb organogenesis. Further analysis is required to determine whether Shh, 5′-HoxD, or both are ectopically expressed in the early embryonic Hd1Hd2 high-percentage chimeric limbs or whether ectopic Hand2 expression within the endogenous Hand1 expression domain alters the temporal exposure of the developing limb to Shh activity.
The DORV heart defect is associated with both separate and combined deficiencies in the cardiac neural crest, left-right specification, abnormalities in looping during cardiac remodeling [3, 23, 25], and cardiomyocyte morphogenesis. Pathogenesis of VSDs can exhibit different etiologies that result in similar structural anomalies. Because Hand1 and Hand2 are both expressed in the early OFT and because DORV/looping defects are observed in Hd1Hd2 chimeras, we must consider that the underlying cardiomyocytes may influence the adjacent endocardial cells and that this region of the embryonic heart is considered to be a contiguous signaling center in which each lineage relies on its neighbors for normal morphogenesis.
We hypothesize that Hd1Hd2 DORV and associated cardiovascular anomalies are not due to neural crest abnormalities because we observe comparable αSMA–positive neural crest-derived cells surrounding the OFT vessels as well as normal development of other Hand1-expressing neural crest–derived structures, such as the cranial ganglia and thymus (data not shown). Analysis of Hand2 nulls shows that the neural crest-derived components of the branchial arch are present, suggesting that normal migration of the neural crest occurs even in the absence of Hand2 [15]. Thymic gland aplasia usually results when neural crest deficiencies are present, as observed in DiGeorge/CATCH-22 [37, 41]. We suggest heart-looping abnormalities (secondary to poor cardiac function, morphogenesis, or both) as the likely cause of the cardiac alignment defects and of the DORV defects seen in Hd1Hd2 chimeras (the presence of muscular VSDs underscores a cardiomyocyte origin). If the processes of heart looping and remodeling are compromised, the apposition of the great vessels and ventricles is disturbed, resulting in DORV and VSDs. Indeed, VSDs are the most prevalent CHDs in humans [7, 21].
The ventricular septum forms when the trabeculae condense at the interventricular groove and when the medial walls of the expanding ventricles fuse together and grow inward. The poor development of the muscular septum in the Hd1Hd2 chimeras could be accounted for by the lack of ventricular wall expansion. The septum is the thickest portion of the ventricle in normal newborns. In Hd1Hd2 mutants, the septum is thin-walled with poor contribution from the compact zone of the ventricle. Regions of the atrioventricular (AV) cushion contribute to both the atrial and ventricular septa, and alterations in AV cushion remodeling result in septal defects involving the membranous ventricular septum [11]. It is likely that defects in AV cushion remodeling, a lack of fusion, or both also could contribute to the observed membranous VSDs observed in Hd1Hd2 chimeras.
In addition to AV cushion abnormalities, mutant mice exhibit a disorganized muscular septum and muscular VSDs, suggesting that growth of septal myocytes, elevated cell death, or positioning of the interventricular septum could underlie the Hd1Hd2 knock-in defects. In situ data show that Hand1 expression is excluded from all but a small subpopulation of septal myocytes, signifying that it plays a non-cell autonomous role in definition of the septal boundary or that septal defects are secondary to abnormal growth and morphogenesis of the left ventricle. Evidence that Hand1 is indeed an important regulator of the inter-ventricular boundary is observed in Mlc2vHand1 mice that die at midgestation lacking a septum [46].
Summary and Future Directions
The results of the aforementioned Hd1Hd2 knock-in chimeric experiments suggest that Hand factors are not functionally redundant and may have regulated dimerization. The survival of patients with CHDs, treated or untreated, is expected to increase, requiring the training of more cardiologists to manage moderate and complex congenital lesions [21]. Further basic developmental biologic studies are needed to help define the pathogenesis of VSDs, one of the most frequent CHDs. Hopefully, these studies will someday identify common underlying pathologic pathways and lineages that enable researchers and clinicians to design more focused interventions. Gaining insight into the early molecular mechanisms regulating cardiogenesis will undoubtedly provide a greater understanding of cardiac development as well as the genetic and cellular insults that result in CHDs affecting human newborns.
Acknowledgments
This study was supported in part by the Riley Children’s Foundation, the Indiana University Department of Pediatrics (Cardiology), and NIH P01 HL085098 (to SJC and ABF).
References
- 1.Barnes RM, Firulli AB. A Twist of insight, the role of Twist family bHLH factors in development. Int J Dev Biol. 2009;53:909–924. doi: 10.1387/ijdb.082747rb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bettencourt PM. Clinical usefulness of B-type natriuretic peptide measurement: present and future perspectives. Heart. 2005;91:1489–1494. doi: 10.1136/hrt.2005.063784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouman HG, Broekhuizen ML, Baasten AM, Gittenberger-De Groot AC, Wenink AC. Spectrum of looping disturbances in stage 34 chicken hearts after retinoic acid treatment. Anatomical Record. 1995;243:101–108. doi: 10.1002/ar.1092430112. [DOI] [PubMed] [Google Scholar]
- 4.Capellini TD, Di Giacomo G, Salsi V, Brendolan A, Ferretti E, Srivastava D, Zappavigna V, Selleri L. Pbx1/Pbx2 requirement for distal limb patterning is mediated by the hierarchical control of Hox gene spatial distribution and Shh expression. Development. 2006;133:2263–2273. doi: 10.1242/dev.02395. [DOI] [PubMed] [Google Scholar]
- 5.Conway SJ. In situ hybridization of cell and tissue sections. Humana Press Inc; Totowa: 1996. [DOI] [PubMed] [Google Scholar]
- 6.Conway SJ, Firulli AB. A bHLH code for cardiac morphogenesis. Pediatr Cardiol. 2009 doi: 10.1007/s00246-009-9608-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conway SJ, Kruzynska-Frejtag A, Kneer PL, Machnicki M, Koushik SV. What cardiovascular defect does my prenatal mouse mutant have, and why? Genesis. 2003;35:1–21. doi: 10.1002/gene.10152. [DOI] [PubMed] [Google Scholar]
- 8.Cross JC, Flannery ML, Blanar MA, Steingrimsson E, Jenkins NA, Copeland NG, Rutter WJ, Werb Z. Hxt encodes a basic helix–loop–helix transcription factor that regulates trophoblast cell development. Development. 1995;121:2513–2523. doi: 10.1242/dev.121.8.2513. [DOI] [PubMed] [Google Scholar]
- 9.Cserjesi P, Brown D, Lyons GE, Olson EN. Expression of the novel basic helix–loop–helix gene eHAND in neural crest derivatives and extraembryonic membranes during mouse development. Dev Biol. 1995;170:664–678. doi: 10.1006/dbio.1995.1245. [DOI] [PubMed] [Google Scholar]
- 10.de Bold A. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg LM, Markwald RR. Cellular recruitment and the development of the myocardium. Dev Biol. 2004;274:225–232. doi: 10.1016/j.ydbio.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Teran M, Piedra ME, Rodriguez-Rey JC, Talamillo A, Ros MA. Expression and regulation of eHAND during limb development. Dev Dyn. 2003;226:690–701. doi: 10.1002/dvdy.10271. [DOI] [PubMed] [Google Scholar]
- 13.Field LJ, Shou W, Caldwell RL. 2008 Riley Heart Center symposium on cardiac development: growth and morphogenesis of the ventricular wall. Pediatr Cardiol. 2009;30:577–579. doi: 10.1007/s00246-009-9407-4. [DOI] [PubMed] [Google Scholar]
- 14.Firulli AB. A HANDful of questions: the molecular biology of the HAND subclass of basic helix–loop–helix transcription factors. Gene. 2003;312C:27–40. doi: 10.1016/s0378-1119(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 15.Firulli AB, Conway SJ. Combinatorial transcriptional interaction within the cardiac neural crest: a pair of HANDs in heart formation. Birth Defects Research, Part C. Embryo Today. 2004;72:151–161. doi: 10.1002/bdrc.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Firulli AB, McFadden DG, Lin Q, Srivastava D, Olson EN. Heart and extra-embryonic mesodermal defects in mouse embryos lacking the bHLH transcription factor Hand1. Nat Genet. 1998;18:266–270. doi: 10.1038/ng0398-266. [DOI] [PubMed] [Google Scholar]
- 17.Firulli BA, Hadzic DB, McDaid JR, Firulli AB. The basic helix–loop–helix transcription factors dHAND and eHAND exhibit dimerization characteristics that suggest complex regulation of function. J Biol Chem. 2000;275:33567–33573. doi: 10.1074/jbc.M005888200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Firulli B, Howard MJ, McDaid JR, McIlreavey L, Dionne KM, Centonze V, Cserjesi P, Virshup DMa, Firulli AB. PKA, PKC, and the protein phosphatase 2A influence HAND factor function: a mechanism for tissue-specific transcriptional regulation. Mol Cell. 2003;12:1225–1237. doi: 10.1016/s1097-2765(03)00425-8. [DOI] [PubMed] [Google Scholar]
- 19.Firulli BA, Krawchuk D, Centonze VE, Virshup DE, Conway SJ, Cserjesi P, Laufer E, Firulli AB. Altered Twist1 and Hand2 dimerization is associated with Saethre-Chotzen syndrome and limb abnormalities. Nat Genet. 2005;37:373–381. doi: 10.1038/ng1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habets PEMH, Moorman AFM, Clout DEW, van Roon MA, Lingbeek M, van Lohuizen M, Campione M, Christoffels VM. Cooperative action of Tbx2 and Nkx2.5 inhibits ANF expression in the atrioventricular canal: implications for cardiac chamber formation. Genes Dev. 2002;16:1234–1246. doi: 10.1101/gad.222902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congential heart disease. Am Heart J. 2004;147:425–439. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Horsthuis T, Houweling AC, Habets PEMH, de Lange FJ, el Azzouzi H, Clout DEW, Moorman AFM, Christoffels VM. Distinct regulation of developmental and heart disease-induced atrial natriuretic factor expression by two separate distal sequences. Circ Res. 2008;102:849–859. doi: 10.1161/CIRCRESAHA.107.170571. [DOI] [PubMed] [Google Scholar]
- 23.Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220:1059–1061. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- 24.Koushik SV, Wang J, Rogers R, Moskophidis D, Lambert NA, Creazzo TL, Conway SJ. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J. 2001;15:1209–1211. doi: 10.1096/fj.00-0696fje. [DOI] [PubMed] [Google Scholar]
- 25.Lin CR, Kioussi C, O’Connell S, Briata P, Szeto D, Liu F, Izpisua-Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning, and pituitary and tooth morphogenesis. Nature. 1999;401:279–282. doi: 10.1038/45803. [DOI] [PubMed] [Google Scholar]
- 26.Massari ME, Murre C. Helix–loop–helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McFadden DG, McAnally J, Richardson JA, Charite’ J, Olson EN. Misexpression of dHAND induces ectopic digits in the developing limb bud in the absence of direct DNA binding. Development. 2002;129:3077–3088. doi: 10.1242/dev.129.13.3077. [DOI] [PubMed] [Google Scholar]
- 28.McFadden DG, Barbosa AC, Richardson JA, Schneider MD, Srivastava D, Olson EN. The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development. 2005;132:189–201. doi: 10.1242/dev.01562. [DOI] [PubMed] [Google Scholar]
- 29.Menick DR, Renaud L, Buchholz A, Muller JG, Zhou H, Kappler CS, Kubalak SW, Conway SJ, Xu L. Regulation of Ncx1 gene expression in the normal and hypertrophic heart. Ann N Y Acad Sci. 2007;1099:195–203. doi: 10.1196/annals.1387.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natarajan A, Yamagishi H, Ahmad F, Li D, Roberts R, Matsuoka R, Hill S, Srivastava D. Human eHAND, but not dHAND, is downregulated in cardiomyopathies. J Mol Cellular Cardiol. 2001;33:1607–1614. doi: 10.1006/jmcc.2001.1434. [DOI] [PubMed] [Google Scholar]
- 31.Reamon-Buettner SM, Ciribilli Y, Traverso I, Kuhls B, Inga A, Borlak J. A functional genetic study identifies HAND1 mutations in septation defects of the human heart. Hum Mol Genet. 2009;18:3567–3578. doi: 10.1093/hmg/ddp305. [DOI] [PubMed] [Google Scholar]
- 32.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 33.Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet. 1998;18:271–275. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- 34.Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, Feng JQ, Conway SJ. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cellular Biol. 2005;25:11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritter O, Haase H, Schulte HD, Lange PE, Morano I. Remodeling of the hyperophied human myocardium by cardiac bHLH transcription factors. J Cellular Biochem. 1999;74:551–561. doi: 10.1002/(sici)1097-4644(19990915)74:4<551::aid-jcb5>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Sakata Y, Kamei CN, Nakagami H, Bronson R, Liao JK, Chin MT. Ventricular septal defect and cardiomyopathy in mice lacking the transcription factor CHF1/Hey2. Proc Natl Acad Sci USA. 2002;99:16197–16202. doi: 10.1073/pnas.252648999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snider P, Olaopa M, Firulli AB, Conway SJ. Cardiovascular development and the colonizing cardiac neural crest lineage. Sci World J. 2007;7:1090–1113. doi: 10.1100/tsw.2007.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, Firulli AB, Molkentin JD, Markwald R, Conway SJ. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008;102:752–760. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srivastava D, Cserjesi P, Olson EN. A subclass of bHLH proteins required for cardiac morphogenesis. Science. 1995;270:1995–1999. doi: 10.1126/science.270.5244.1995. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, Olson EN. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet. 1997;16:154–160. doi: 10.1038/ng0697-154. [DOI] [PubMed] [Google Scholar]
- 41.Stalmans I, Lambrechts D, De Smet F, Jansen S, Wang J, Maity S, Kneer P, von der Ohe M, Swillen A, Maes C, Gewillig M, Molin DGM, Hellings P, Boetel T, Haardt M, Compernolle V, Dewerchin M, Plaisance S, Vlietinck R, Emanuel B, Gittenberger-de Groot AC, Scambler P, Morrow B, Driscol DA, Moons L, Esguerra CV, Carmeliet G, Behn-Krappa A, Devriendt K, Collen D, Conway SJ, Carmeliet P. VEGF: a modifier of the del22q11 (DiGeorge) syndrome? Nat Med. 2003;9:173–182. doi: 10.1038/nm819. [DOI] [PubMed] [Google Scholar]
- 42.Thattaliyath BD, Firulli BA, Firulli AB. The basic-helix–loop–helix transcription factor HAND2 directly regulates transcription of the atrial naturetic peptide gene. J Mol Cell Cardiol. 2002;34:1325–1344. doi: 10.1006/jmcc.2002.2085. [DOI] [PubMed] [Google Scholar]
- 43.Thattaliyath BD, Livi CB, Steinhelper ME, Toney GM, Firulli AB. HAND1 and HAND2 are expressed in the adult rodent heart and are modulated during cardiac hypertrophy. Biochem Biophys Res Com. 2002;297:870–875. doi: 10.1016/s0006-291x(02)02297-0. [DOI] [PubMed] [Google Scholar]
- 44.Thomas T, Yamagishi H, Overbeek PA, Olson EN, Srivastava D. The bHLH factors, dHAND and eHAND, specify pulmonary and systemic cardiac ventricles independent of left-right sidedness. Dev Biol. 1998;196:228–236. doi: 10.1006/dbio.1998.8849. [DOI] [PubMed] [Google Scholar]
- 45.Tickle C. The number of polarizing region cells required to specify additional digits in the developing chick wing. Nature. 1981;289:295–298. doi: 10.1038/289295a0. [DOI] [PubMed] [Google Scholar]
- 46.Togi K, Kawamoto T, Yamauchi R, Yoshida Y, Kita T, Tanaka M. Role of Hand1/eHAND in the dorsoventral patterning and interventricular septum formation in the embryonic heart. Mol Cell Biol. 2004;24:4627–4635. doi: 10.1128/MCB.24.11.4627-4635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vincentz JW, Barnes RM, Rodgers R, Firulli BA, Conway SJ, Firulli AB. An absence of Twist1 results in aberrant cardiac neural crest morphogenesis. Dev Biol. 2008;320:131–139. doi: 10.1016/j.ydbio.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamagishi H, Olson EN, Srivastava D. The basic helix–loop–helix transcription factor, dHAND, is required for vascular development. J Clin Invest. 2000;105:261–270. doi: 10.1172/JCI8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, Ogawa H, Okumura K, Mukoyama M, Nakao K. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/01.cir.90.1.195. [DOI] [PubMed] [Google Scholar]
- 50.Zeller R, Lopez-Rios J, Zuniga A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet. 2009;10:845–858. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- 51.Zhu J, Nakamura E, Nguyen M-T, Bao X, Akiyama H, Mackem S. Uncoupling sonic hedgehog control of pattern and expansion of the developing limb bud. Dev Cell. 2008;14:624–632. doi: 10.1016/j.devcel.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]