Summary
IFN-γ and IL-17 producing T cells autoreactive across myelin components are central to the pathogenesis of multiple sclerosis (MS). Using direct in vivo, adoptive transfer, and in vitro systems, here we show that the generation of these effectors in MOG35–55 induced EAE depends on interactions of locally produced C3a/C5a with APC and T cell C3aR/C5aR. In the absence of the cell surface C3/C5 convertase inhibitor decay accelerating factor (DAF) but not the combined absence of DAF and C5aR and/or C3aR on APC and T cells, a heightened local autoimmune response occurs in which myelin destruction is markedly augmented in concert with markedly more IFN-γ+ and IL-17+ T cell generation. The augmented T cell response is due to increased IL-12 and IL-23 elaboration by APCs together with increased T cell expression of the receptors for each cytokine. The results apply to initial generation of the IL-17 phenotype since naïve CD62Lhi Daf1−/− T cells produce 3-fold more IL-17 in response to TGF-β and IL-6 while CD62Lhi Daf1−/−C5aR−/−C3aR−/− T cells produce 4-fold less.
Keywords: Decay Accelerating Factor, EAE, T cell, Autoimmunity, Epitope spreading
Introduction
Experimental autoimmune encephalomyelitis [EAE (1)] is a rodent model that has been valuable for characterizing immunopathogenic processes that underlie multiple sclerosis (MS). One way that disease is induced is adjuvant and pertussis toxin boosted immunization of C57BL/6 mice with the encephalitogenic peptide (p35–55) of myelin oligodendrocyte glycoprotein (MOG), an external myelin component. Following this immunization, myelin sheaths of oligodendrocytes are attacked (2).
According to current concepts (3), myelin destruction in EAE (and MS) is caused by T cells autoreactive with myelin which are peripherally activated, and upon encountering microglia bearing myelin peptides in the brain become further activated (4). A feature of EAE pathogenesis that closely relates to MS pathogenesis is that autoreactivity induced against the myelin immunogen, e.g. MOG, broadens to other myelin components, e.g. proteolipid protein (PLP) and myelin basic protein (MBP). This process termed “epitope spreading” is thought to be due to endogenous priming to new self determinants during initial and subsequent inflammatory interactions (5). For reproducing it, rather than the C57BL/6 H-2b strain, the autoreactive-prone SJL H-2s strain generally is used.
In previous studies (6), we found that decay accelerating factor (DAF or CD55), a regulator originally characterized as a plasma membrane shield that circumvents C3b deposition on self cell surfaces and prevents C5 activation (7), modulates T cell responses (see Discussion). In its absence on APCs or on cognate CD4+ and CD8+ T cells interacting with them, proliferative and IFN-γ responses are 5–22 fold augmented. Findings that double deficiency of DAF and factor D (fD), an alternative pathway component, abolished the enhancement in vitro as well in vivo linked the effect to complement locally produced by APC•T cell partners. Prompted by this result, we analyzed flow sorted WT APCs and T cells following mixing with specific peptide and found that early during cognate interactions both partners locally synthesize all three alternative pathway components i.e. C3, factor B (fB), and fD together with C5 as well as C5a and C3a receptors (C5aR and C3aR). We found that concomitant with this local complement synthesis, both APCs and T cells downregulate surface DAF expression lifting restraint on junctional activation. We further found that C5a and C3a are locally generated by both partners and that the interaction of these activation fragments with C5aR and C3aR on both partners promotes both APC and T cell cytokine production (8). While other investigators have reported that autoimmune diseases are diminished in the absence of C5aR or C3aR and attributed the findings to effects of serum complement (9–11), our findings that a) APC and T cells locally synthesize complement b) DAF restraint on junctional activation is lifted in concert with the local complement synthesis and c) that interactions of locally generated C5a and C3a with C5aR and C3aR promotes cytokine production, prompted us to investigate whether interaction of locally produced C5a and C3a with C5aR and C3aR on APCs and T cells impacts autoimmunity and, if so, how.
In the present investigation, we exploited Daf deficient mice in the MOG 35–55-induced EAE model to study a) the extent to which DAF influences T cell autoreactivity to self antigens, b) whether its presence is necessary to protect against broadening of the anti-myelin T cell response, c) the nature of the T cell response elicited in its absence, and d) the mechanism of the any observed differences.
Materials and Methods
Animals
Eight to 12 week old female Daf1−/− mice (12) and their Daf1+/+ female littermates backcrossed 5 generations on the C57BL/6 background were used. Although mice possess a second Daf gene [Daf2 (13)], which encodes transmembrane-anchored Daf expressed almost exclusively in the testes. Studies were carried out using an approved Institutional Animal Care and Use Center (IACUC) protocol.
Antigens
Rat MOG35–55 MEVGWYRSPFSRVVHLYRNGK, the immunodominant encephalitogen for C57BL/6 mice (14) was synthesized using standard solid phase methodology and 9-fluorenylmethoxycarbonyl (FMOC) side chain protected amino acids, purified >90% by reverse phase HPLC, and confirmed by mass spectrometry. PLP was purified from a washed total lipid extract of human white matter and converted to aqueous form as described (15). Mouse MBP was purchased from Sigma (St. Louis, MO). Mouse IL-4 and GM-CSF were purchased from Peprotech (Rockyhill, NJ). Anti-CD3, anti-CD28, anti-IFN-γ and anti-IL-4 monoclonal antibodies (mAbs) were purchased from BD Pharmingen (San Diego, CA).
Induction and Evaluation of EAE
Animals were injected sc with 100 µg of MOG33–35 in CFA containing 400 µg of mycobacterium tuberculosis H37RA (Difco, Detroit, MI). Upon immunization and two days later, 200 ng of pertussis toxin (List Biological Labs Inc., Campbell, CA) was injected ip. Mice were weighed and scored for neurological deficits daily: 0=no disease; 1=decreased tail tone or slightly clumsy gait; 2=tail atony; 3=limb weakness; 4=limb paralysis; 5=moribund state.
Adoptive transfer of T cells
18 days after priming 8–12 wk old female Daf1−/− or Daf1+/+ mice with MOG35–55 in CFA containing H37RA, splenocytes were cultured for 72 hr with 20 µg/ml of MOG35–55 and 10 ng/ml of IL-12 or IL-23. In initial studies (3 × 107) washed cells from Daf1−/− mice or Daf1+/+ mice were administered ip into Daf1+/+ recipients that received 400 rads of γ irradiation. Guided by 4 or 3-fold higher numbers of IFN-γ or IL-17 producing cells, in subsequent studies, washed spleen cells (40 or 30 × 106) from Daf1+/+ mice vs 10 × 106 from Daf1−/− mice were transferred to obtain equal numbers of IFN-γ or IL-17 producing cells. Clinical scores and weights were monitored as above.
Histology and Myelin Staining
Spinal cords were collected at days 18 and 56 from 4–8 mice in each group, fixed after infusion with 100 ml of 4% paraformaldehyde for 5 min, embedded in OCT, quick frozen in liquid N2 and stored at −70°C. Cryostat sections (10–20 µm) were evaluated at ten levels. H&E staining was done by standard methods. Alcohol-dehydrated sections were stained for myelin in Luxol Fast Blue (Sigma Inc., St. Louis MO) overnight at 37°C and agitated for 10 sec in 0.0005% lithium carbonate and 60 sec in 70% alcohol.
Electron Microscopy (EM)
Spinal cords were fixed in formaldehyde/glutaraldehyde (3%)/0.1 M sodium cacodylate, post-fixed in 1% osmium tetroxide and embedded in LX112 resin (Polysciences Inc., PA). Ultrathin uranyl acetate/lead citrate-stained sections were cut and examined with a JEOL 100cx microscope.
Proliferation and ELISPOT Assays
Splenocytes (2×105 cells in 200 µl) were cultured for 96 hr in triplicate in 96-well microtiter flat bottom plates with 0.01–100 µg/ml of MOG35–55, pulsed for 16 hr with [methyl-3H] thymidine (1 µCi/well; sp. act., 6.7 Ci/mmol; New England Biolabs, Boston, MA) and incorporated radioactivity expressed as the mean cpm of triplicate Ag containing wells. In ELISPOT assays, splenocytes (2–6 ×105) were incubated for 24 hr in anti-IFN-γ or IL-17 mAb (R&D System, Minneapolis, MN) coated and PBS-1% BSA blocked 96 well ELISPOT plates (Whatman, Clifton, NJ) with 0.01–20 µg/ml of MOG 35–55, 0.01–50 µg/ml of PLP or 100 µg/ml of MBP and spots were developed and quantitated on an ELISPOT analyzer (Cellular Technology Ltd., Cleveland, OH).
Murine DCs
Bone marrow cells from mice were grown in RPMI 1640 (containing 10% FBS, 10 µg/ml IL-4 and 10 µg/ml GM-CSF). The medium was changed on day 3 and day 5. Cells were used on Day 6.
RNA purification, cDNA synthesis and qPCR
Total RNA was purified by Qiagen RNeasy mini kit (Qiagen Inc, La Jolla, CA). cDNA was synthesized from 20 µl of mRNA in Sprint PowerScript Single Shots (Clontech, Mountain View, CA). Diluted cDNA (10 µl) was mixed with 2 µl of primer and 10 µl SYBR green master mix (Applied Biosystems, Foster City, CA). Each sample was assayed in duplicate on an ABI prism 7000 cycler. In all assays, fold-increases are relative to basal levels and standardized to Actin.
Western Blotting
All blots were performed as previously described (16) using HRP-conjugated secondary antibody, and an ECL enhancer (GE Healthcare, Buckinghamshire, UK).
Induction of IL-17 with IL-6 and TGF-β
Lymph node cells were harvested from 5 Daf1+/+, 5 Daf1−/−, and 5 Daf1−/−C5aR−/−C3aR−/− mice and naïve CD4 +CD62Lhi cells were sorted. The sorted (CD4+CD62Lhi) T cells were stimulated with anti-CD3 (5 µg/ml), anti-CD28 (10 µg/ml), anti-IFN-γ (10 µg/ml), anti-IL-4 (10 µg/ml) antibodies together with TGF-β (20 ng/ml) or TGF-β plus IL-6 (20 ng/ml). IL-17 production in culture supernatants was measured by the Beadlyte Mouse 21-plex Cytokine Detection System (Upstate, CA) after 72 hr of activation.
Statistics
A repeated measures cumulative logit model (17), using the equation, log it [P (Y ≤ j | x)] = αj + β1genotype + β2time + β3genotype * time, was used to test for the differences in groups across time points, with αj corresponding to the P (Y ≤ j) for each threshold or score and β corresponding to the coefficients to be estimated for the main effects of genotype and time as well as for the interaction between genotype and time. Calculations were performed on SAS for Windows 9.1. Statistics for the in vitro studies were done by Student’s t-test.
Results
Severity of EAE is increased in Daf1−/− mice
To determine how DAF deficiency influences CNS neuronal injury in EAE, we immunized 12 Daf1−/− mice and 12 Daf1+/+ littermates with MOG35–55 and monitored them daily. While all mice in both groups developed EAE, neurologic dysfunction in Daf1−/− mice was markedly more severe. Fig. 1A shows clinical scores over a 62 day period from the above mice combined with 12 more Daf1−/− and Daf1+/+ mice from an identical repeat experiment (total of 24 mice in each group). In the Daf1−/− mice, the mean clinical score was 3.5 ± 1.1 vs. 1.7 ± 1.3 in Daf1+/+ littermates (p < 0.001), and the mean weight decrease (over days 10 to 62) was greater (91.2±2.8% of original weight vs 97.8±3.4%, p < 0.05) (not shown). While by day 40 onward most Daf1+/+ mice exhibited only minimal physical changes, some Daf1−/− mice had to be euthanized because they barely moved.
Figure 1. Clinical courses in MOG35–55 immunized Daf1−/− and Daf1+/+ mice.
A) Clinical severity was scored daily in Daf1−/− mice (n=24) and Daf1+/+ littermates (n=24). B) Clinical severity was scored daily in Daf1+/+ recipients of adoptively transferred with spleen cells from Daf1+/+ mice (n=5) and in Daf1+/+ recipients of spleen cells from Daf1−/− mice (n=5). Data are an average from two independent experiments. Daf1−/− vs. Daf1+/+ p<0.001.
Heightened disease severity in Daf1−/− mice is due to heightened cellular autoreactivity
To document that the heightened disease severity in Daf1−/− mice was mediated by increased cellular immunity (since DAF also inhibits cytotoxicity conferred by systemic complement), we performed adoptive transfer experiments in which we injected equal numbers of splenocytes from MOG35–55-immunized Daf1−/− or Daf1+/+ mice into naïve Daf1+/+ recipients. As seen in Fig 1B, compared to Daf1+/+ recipients that received Daf1+/+ splenocytes, Daf1+/+ recipients that received Daf1−/− splenocytes showed ≥ 2 fold higher clinical scores and more profound weight decreases (data not shown). The difference in disease severity was comparable to that following direct immunization with MOG35–55.
In contrast to the enhancement of T cell responses in Daf1−/− mice, assays of day 56 sera showed that both groups elaborated similar amounts of anti-MOG antibodies. Moreover, Daf1−/− mice did not generate more complement fixing IgG2a, IgG2b, or IgM (not shown). Staining of [perfused (see Methods)] spinal cords (day 56) for deposited C3 and C9 showed that while some deposition of both components was detectable in Daf1−/− mice and Daf1+/+ littermates, pixel counts showed no significant differences [650 ± 123 vs 530 ± 120 for C3 (p > 0.05) and 630 ± 194 vs 570 ± 121 for C9 (p > 0.05) (data not shown)].
Daf1 −/− mice suffer greater CNS leukocyte infiltration and demyelination and myelin does not recover
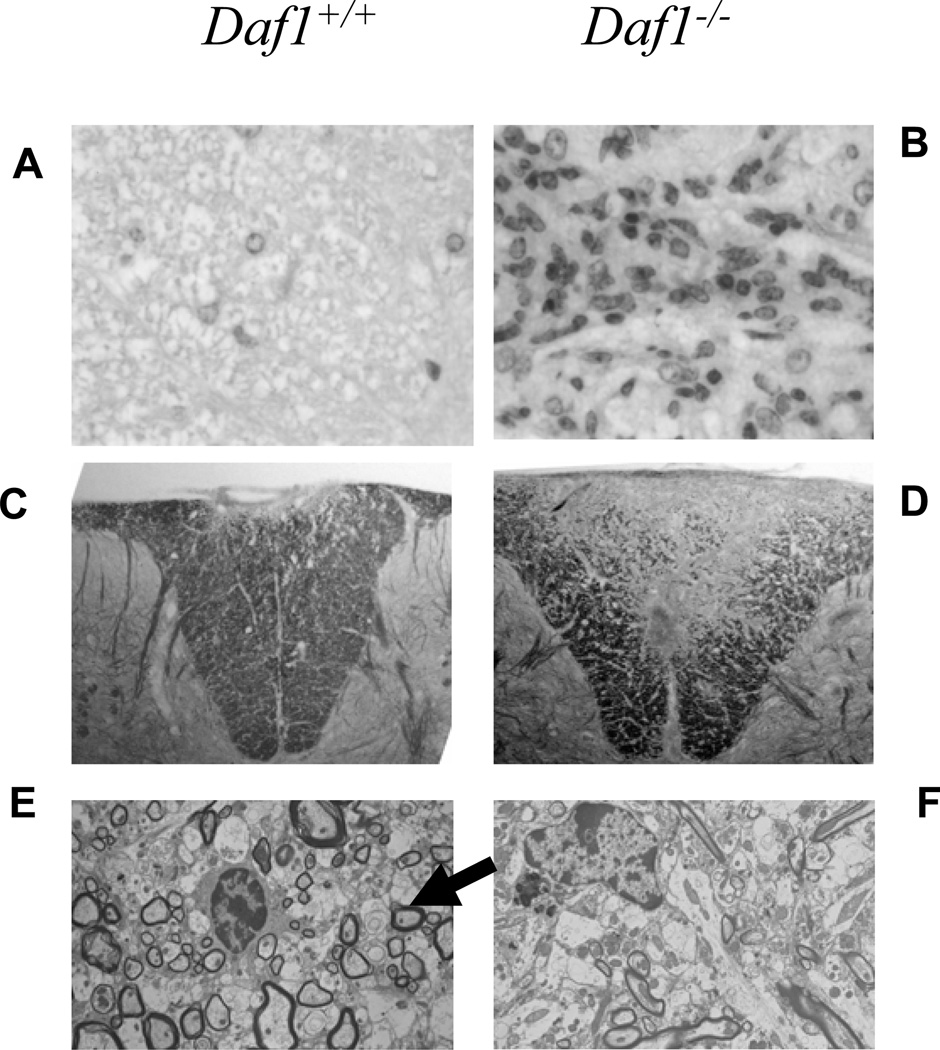
To compare pathology, we examined dorsal columns of Daf1−/− and Daf1+/+ mice at early (day 18) and late (day 56) disease stages. At the day 18 time point, H&E staining showed markedly increased leukocyte infiltration in Daf1−/− mice which on high power examination were >90% lymphocytes and macrophages (Fig. 2 A,B). Luxol Fast Blue staining at both day 18 and 56 showed markedly more myelin loss in Daf1−/− mice (Fig. 2 C,D). Ultrastructural analyses at both the early and late time points confirmed markedly increased destruction of myelin sheaths (not shown). Moreover, while at late stages of disease (day 84), Daf1+/+ mice demonstrated remyelination [as indicated by small thinly myelinated axons in lesional areas (Fig. 2 E)], little, if any, remyelination was detectable in the Daf1−/− mice (Fig. 2F).
Figure 2. Neuropathology of spinal cords in MOG35–55 immunized Daf1−/− and Daf1+/+ mice.
A and B) H&E staining of spinal dorsal column (magnification X100). C and D) Luxol fast Blue stains of dorsal column sections (magnification X100). E and F) Electron microscopy of lesions in dorsal columns at day 84 post immunization. E) The arrow shows thinly remyelinated axons in Daf1+/+ mice.
Greater disease severity in Daf1−/− mice is due to the augmented generation of IFN-γ and IL-17 producing cells
To determine the immunological basis for the heightened disease in Daf1−/− mice, we measured T cell responses to MOG35–55 peptide. In [3H]-thymidine incorporation assays (not shown) at day 28 postimmunization, Daf1−/− splenic T cells expanded 5-fold more than Daf1+/+ spleen cells (2000 vs 400 cpm/105 spleen cells at 1µg/ml MOG35–55). IFN-γ and IL-17 ELISPOT assays at 30 days showed 5–6 fold more IFN-γ producing cells and 2-fold more IL-17 producing cells than in Daf1+/+ spleens (Fig. 3). Later in disease (60 days), these assays showed 4–5-fold more IFN-γ producing cells and 5–7-fold more IL-17 producing cells than in Daf1+/+ spleens. At an even later time point (75 days) the ELISPOT assays showed that the ratio of IL-17 producing cells to IFN-γ producing cells in Daf1+/+ mice declined to 2:1, while the ratio of IL-17 producing cells to IFN-γ producing cells in Daf1−/− mice remained more than 6:1 indicating that the IL-17 response in Daf1−/− mice is more sustained. ELISAs of supernatants of spleen cells from Daf1−/− and Daf1+/+ mice at day 56 showed increases in IL-2, IFN-γ, and IL-17, and decreases in IL-5 and IL-10 in the Daf1−/− mice, consistent with augmented Th1 and Th17 responses (data not shown). IFN-γ and IL-17 ELISAs of cultured spleen supernatants at day 56 verified 3–4 fold elevated levels of IFN-γ and 5–6 fold elevated levels of IL-17 (not shown). Computer analyses of mean ELISPOT sizes showed similar IL-17 spot sizes but larger IFN-γ spot sizes (Fig 3C). Repeat adoptive transfer experiments this time adjusting spleen cells for equal numbers of IFN-γ or IL-17 producing cells showed that, even under these conditions, Daf1−/− spleen cells in each case conferred greater pathology (Fig 3D).
Figure 3.
T cell responses in MOG35–55 immunized Daf1−/− and Daf1+/+ mice to MOG35–55 peptide and adoptive transfer of spleen cells from MOG immunized mice to naïve recipients
A) IFN-γ and IL-17 producing cells as assessed by ELISPOT assays at day 30 postimmunization. B) IFN-γ and IL-17 producing cells at day 60 post immunization. C) IFN-γ and IL-17 spot sizes in the presence of MOG35–55. The data represent studies with 5 mice. The IFN-γ and IL-17 assays were done in the same spleens. The larger IFN-γ spot sizes of Daf1−/− T cells could be due to more IFN-γ or earlier onset of IFN-γ production (representative of 3 independent experiments at low spot density). D) Clinical scores of Daf1+/+ recipients of the same numbers of adoptively transferred IFN-γ or IL-17 producing spleen cells from MOG35–55 immunized Daf1−/− and Daf1+/+ mice. For these studies with equal numbers of IFN-γ or IL-17 producing cells, a portion of spleen cells was cultured with MOG35–55 and 10 ng/ml IL-12 or 10 ng/ml of IL-23 for 72 hr, while another portion from the same spleen was incubated for 72 hr on ELISPOT plates to quantitate IFN-γ or IL-17 producing cells. Based on the IFN-γ ELISPOT counts following removal of adherent cells, 40 × 106 IL-12 treated spleen cells from Daf1+/+ mice and 10 × 106 of the IL-12 treated spleen cells from Daf1−/− mice were transferred to Daf1+/+ recipients. Based on the IL-17 ELISPOT, following removal of adherent cells 30×106 of IL-23 treated spleen cells from Daf1+/+ mice and 10×106 of the IL-23 treated spleen cells from Daf1−/− mice were transferred to Daf1+/+ recipients. The data represent 2 independent experiments.
The T cell response in Daf1−/− mice shows intermolecular epitope spreading
To determine whether the enhanced immunoreactivity in Daf1−/− mice was sufficient to overcome the normal resistance in H-2b mice to epitope spreading (5), we performed IFN-γ ELISPOT recall assays at days 13–64 with PLP and MBP. Consistent with past literature (18), no PLP reactivity was observed with spleen cells from Daf1+/+ controls (Fig. 4A). In contrast, dose-dependent PLP reactivity (Fig 4A) developed between day 56 and 64 (Fig. 4B) in Daf1−/− mice. As with PLP, Daf1−/− spleen cells also showed reactivity to MBP (Fig. 4C). Reactivity to neither PLP nor MBP was detectable in spleens of unprimed Daf1−/− mice, verifying that the elicited autoreactives were dependent upon MOG immunization.
Figure 4. Epitope spreading to PLP and MBP in MOG35–55 immunized Daf1−/− and Daf1+/+ mice.
A) IFN-γ responses to PLP protein of MOG35–55 immunized Daf1−/− and Daf1+/+ mice at day 56. In one Daf1−/− mouse, low reactivity with splenocytes (detectable in the absence of PLP probably representing spontaneous blastogenesis) did not differ in the presence of Ova323–339 control. B) Kinetics of T cell responses of MOG35–55 immunized mice spreading to PLP. Ratio of Daf−/− IFN-γ producing cells to Daf1+/+ IFN-γ producing cells in response to the MOG35–55 immunogen are shown on the left and spreading to PLP is shown on the right. C) IFN-γ recall responses in MOG35–55 immunized mice at day 56 to PLP (50 µg/ml) and MBP (100 µg/ml).
The heightened IFN-γ and IL-17 production by Daf1−/− T cells is dependent on amplified interactions of C3a and C5a with APC C3aR and C5aR resulting in increased IL-12 and IL-23 production
Our previous studies (6) showed that when APCs and cognate T cells interact, both partners turn on the local synthesis of complement and concomitantly downregulate DAF. Because a) DAF regulates C3/C5 convertases (7), b) prior reports have suggested that the anaphylatoxin receptors C3aR and C5aR can be involved in T cell mediated disease models (9, 10), and c) we showed that exogenous C5a augments alloreactive T cell immunity (6), we tested whether increased local C5a/C3a•C5aR/C3aR interaction by Daf1−/− APCs and T cells can account for the greater anti-MOG autoreactive response in Daf1−/− mice as well as explain previous findings by others with C5aR−/− and C3aR−/− mice attributed to “crosstalk” from serum complement.
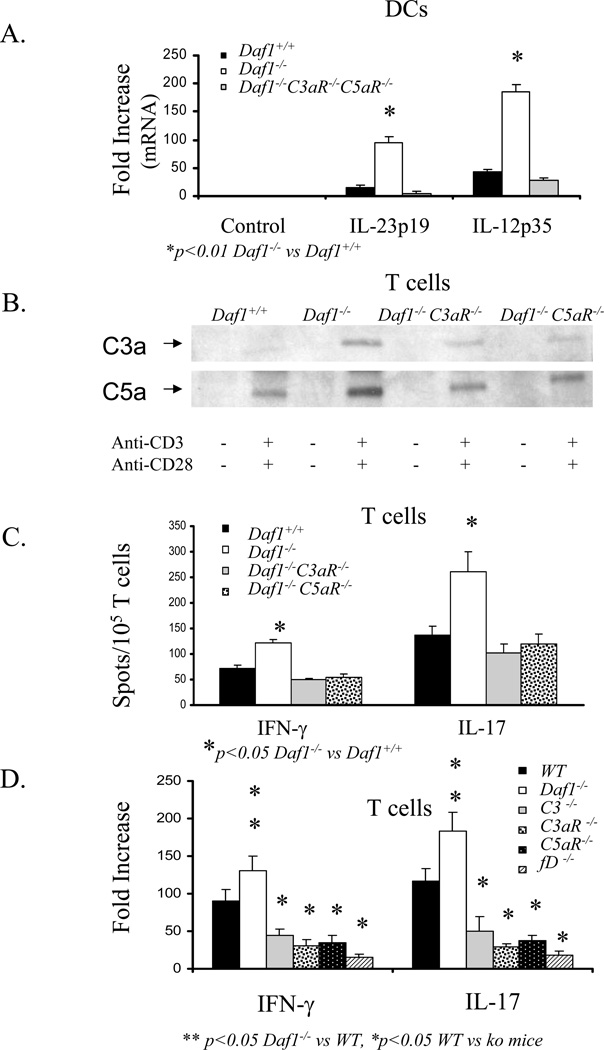
To determine whether increased local C5a/C3a•C5aR/C3aR interaction by Daf1−/− APCs and T cells is involved in the increased IFN-γ and IL-17 production in Daf1−/− mice and, if so, how the two processes are mechanistically linked. We performed two sets of in vitro studies. In one, we stimulated transgenic OT-II T cells with Daf1+/+, Daf1−/−, and Daf1−/− C3aR−/− C5aR−/− dendritic cells (DCs) in the presence of specific ova323–339 peptide and examined the production of IL-12 and IL-23 by the DCs. In the other, we stimulated Daf1+/+, Daf1−/−, Daf1−/− C3aR−/−, and Daf1−/− C5aR−/− T cells with anti-CD3 and anti-CD28 mAbs and assessed the effects of IL-12 or IL-23 addition on T cell IFN-γ and IL-17 production. The two sets of studies showed that a) Daf1−/− DCs produced 4-fold more IL-12 and 5-fold more IL-23 than Daf1+/+ DCs (Fig 5A), b) the addition of IL-12 to anti-CD3/CD28 stimulated Daf1−/− T cells produced 4-fold more IFN-γ producing cells and the addition of IL-23 produced 5-fold more IL-17 producing cells compared to Daf1+/+ controls (Fig 5B), c) the heightened responses of Daf1−/− T cells were associated with upregulation of their IL-12Rβ2 and IL-23R levels (Fig 5C), and d) the augmented IFN-γ and IL-17 production by Daf1−/− T cells depended on T cell C3aR and C5aR (Figs 5B and 6C).
Figure 5. Effects of Daf deficiency and combined Daf•C5aR/C3aR deficiency on cytokine expression by APCs and cytokine receptor expression by T cells.
A) IL-12p35 and IL-23p19 mRNA expression in flow separated DCs from Daf1+/+ and Daf1−/− mice following 3 hr incubation with OT-II cells and ova323–339. B) IFN-γ and IL-17 ELISPOT assays: T cells isolated by CD3+ T cell enrichment columns (R&D Systems) from Daf1−/− and Daf1+/+ littermates were stimulated with anti-CD3 and anti-CD28 antibodies in the presence of increasing concentrations of IL-23 or IL-12. C) IL-23R and IL-12Rβ2 mRNA expression levels on T cells from Daf1+/+, Daf1−/−, and Daf1−/−C3aR−/−C5aR−/− mice stimulated with anti-CD3 and anti-CD28 antibodies.
Figure 6. Augmented T cell responses by Daf deficient APCs and T cells depend on C5aR and C3aR.
A) IL-23p19 and IL-12p35 mRNA expression in sorted Daf1+/+, Daf1−/−, and Daf1−/−C3aR−/−C5aR−/− DCs after incubation with ova323–339 and OT-II cells. B). Western Blots of C3a and C5a in supernatants of Daf1+/+, Daf−/−, Daf1−/−C3aR−/− and Daf1−/−C5aR−/− T cells following anti-CD3 and anti-CD28 antibody stimulation. C) IFN-γ and IL-17 ELISPOT assays of Daf1+/+, Daf1−/−, Daf1−/− C5aR−/−, and Daf1−/−C3aR−/− T cells following anti-CD3 and anti-CD28 antibody stimulation. D) IFN-γ and IL-17 protein levels in supernatants of anti-CD3 and anti-CD28 antibody stimulated T cells from WT and relevant knockout mice. T cell were isolated by CD3+ T cell enrichment columns (R&D Systems).
In support of the dependence of the augmented IFN-γ and IL-17 responses on C5aR and C3aR on both APCs and T cells, a) the increased production of IL-12 and IL-23 by Daf1−/− APCs was abrogated in Daf1−/− C3aR−/− C5aR−/− triple knockout APCs (Fig 6A), b) western blots of supernatants of anti-CD3/CD28 stimulated Daf1+/+, Daf1−/−, Daf1−/− C3aR−/−, and Daf1−/− C5aR−/− T cells showed that Daf1−/− cells but not Daf1−/− C3aR−/− or Daf1−/− C5aR−/− cells produced more C3a and C5a (the ligands for C3aR and C5aR) than Daf1+/+ cells (Fig 6B), c) the increased IFN-γ and IL-17 responsiveness of Daf1−/− T cells to IL-12 and IL-23 was abrogated in Daf1−/−C5aR−/− and Daf1−/−C3aR−/− mice (Fig 5B), d) ELISPOT assays showed that the increased numbers of IFN-γ and IL-17 producing cells generated by anti-CD3/CD28 stimulated Daf1−/− T cells were abolished with Daf1−/−C5aR−/− and Daf1−/−C3aR−/− T cells (Fig 6C), and e) ELISAs showed that the augmented IFN-γ and IL-17 also depended C3 and fD (Fig 6D).
Daf1−/− T cells produce more IL-17 in response to TGF-β and IL-6
Recent studies (19) of sorted naïve CD4+ CD62Lhi cells have shown that IL-17 producing cells are not generated directly from IL-23 stimulation but rather are initially induced by IL-6 and TGF-β. Once differentiated by these cytokines, IL-23 augments and sustains IL-17 production. To ascertain how the results in this study relate to this finding, we flow sorted Daf1+/+ and Daf1−/− CD4+ CD62Lhi cells and incubated the sorted cells with anti-CD3+anti-CD28 mAbs for 72 hr in the presence of IL-6 and TGF-β together with anti-IFN-γ and anti-IL-4 mAbs, exactly as described in the above published study (19). An ELISA of supernatants (Fig 7A) of the treated cells showed three times more IL-17 production by naïve Daf1−/− T cells than by naïve Daf1+/+ cells. Compared to WT T cells, IL-17 production was reduced 4-fold when Daf1−/−C3aR−/−C5aR−/− cells were used.
Figure 7. Differentiation of naïve CD4+CD62Lhi T cells into IL-17 producing cells is augmented in the absence of Daf and markedly diminished in the absence of C5aR/C3aR and clinical scores are correspondingly decreased.
A) Generation of IL-17 producing cells. Sorted naïve (CD4+ CD62Lhi) T cells were stimulated with anti-CD3 (5µg/ml), anti-CD28 (10 µg/ml), anti-IFN-γ (10 µg/ml), and anti-IL-4 (10 µg/ml) together with TGF-β or TGF-β plus IL-6 for 72 hr. Supernatants were assayed for IL-17 protein by the Beadlyte mouse multicytokine detection system. Naïve T cells treated with mAbs only served as a control. B) More severe EAE in Daf1−/− mice depends on C5aR and C3aR. Clinical scores of Daf1−/− (n=5) and Daf1+/+ (n=5), Daf1−/−C3aR−/− (n=3), and Daf1−/−C5aR−/− (n=3) mice measured daily after immunization with MOG35–55.
The dependence of T cell IFN-γ and IL-17 production on C5a/C3a•C5aR/C3aR interactions applies in vivo
To document that the above in vitro linkage of C5a/C3a•C5aR/C3aR interactions with T cell IFN-γ and IL-17 production applies in vivo and correlates with EAE disease severity, we compared clinical scores in Daf1−/−, Daf1−/− C5aR−/−, Daf1−/− C3aR−/−, and Daf1+/+ mice following immunization with MOG35–55. Consistent with the in vitro data in Fig 6A–D, the increased disease severity in Daf1−/− mice over that in Daf1+/+ mice was not only abrogated in Daf1−/− C5aR−/− and Daf1−/− C3aR−/− mice, but clinical scores went below those of Daf1+/+ mice (Fig 7B).
Taken together, the data indicate that heightened disease activity in Daf1−/− mice is due to 1) heightened production of IL-12/IL-23 by Daf1−/− APCs and 2) heightened IFN-γ and IL-17 responses by Daf1−/− T cells to the two APC “signal 3” cytokines resulting (at least in part) from higher induced levels of IL-12β2R and IL-23R. The studies with Daf1−/− cells doubly deficient in C5aR or C3aR show that a) the augmented cytokine production by both partners depends on APC•T cell C5a/C3a•C5aR/C3aR interactions and b) the dependence on these interactions applies in vivo in that they control EAE disease severity.
Discussion
The results of this study document an important role for DAF function in shielding against T cell autoreactivity. T cell autoimmunity in Daf1−/− mice immunized with MOG35–55 is stronger; EAE is more severe; more T cells are present in the CNS; myelin loss is more extensive; and remyelination is impaired. That the heightened CNS injury is, in fact, mediated by augmented T cell reactivity was formally established by adoptive transfer. The autoreactive response is both quantitatively and qualitatively different in that it is not only associated with more IFN-γ producing cells but even more IL-17 producing cells. As assessed by both proliferation and by IFN-γ and IL-17 ELISPOT assays, the increased lymphocyte infiltration in Daf1−/− mice is due to up to a 5-fold more robust T cell IFN-γ response and 7-fold more robust T cell IL-17 response to the priming by MOG immunogen. When equalized for IFN-γ producing cells or IL-17 producing cell numbers, adoptively transferred spleen cells induce greater disease severity. As a result, despite the inherent resistance of the H-2b background to epitope spreading, the enhanced autoreactivity allows intermolecular spreading to PLP and MBP. Taken together, the findings argue that DAF serves as a barrier to the induction of T cell autoreactivity in EAE and that its protection is conferred via inhibition of the induced T cell autoreactivity to myelin and spreading of the autoreactivity to other myelin components.
Until this report, significant epitope spreading has been observed primarily in SJL/J or SWXJ mice and has not been extensively described in C57BL/6 H-2b mice. Our findings of markedly stronger T cell responses in C57BL/6 H-2b Daf1−/− mice and spreading of autoreactivity to PLP and MBP are consistent with the observed augmented myelin destruction. To our knowledge, the finding that T cell reactivity to PLP and MBP is induced by MOG35–55 in the absence of DAF, i.e. that the barrier to extension of T cell reactivity to myelin components other than the MOG35–55 immunogen is overcome, constitutes the first definitive demonstration of H-2b-restricted intermolecular epitope spreading.
The adoptive transfer experiments (Fig 1C) showed that the enhanced disease was directly related to Daf1−/− donor cells. Thus, priming to self in a DAF-deficient milieu produces an uncontrolled hyper-autoreactivity that leads to severe disease sequelae. Although it is clear that DAF serves as a global negative regulator of T cell activation, the overall permissive impact of DAF deficiency in EAE severity is the overrepresentation of the Th17 as well as the Th1 lineage. While the association is not causally proven in this study, the linkage of severe EAE outcome to increased frequencies of IL-17 producing T cells is consistent with many recent studies showing a) greater autoimmune severity following transfer of such Th17 T cells, b) amelioration of disease following IL-17 neutralization in WT mice, and c) reduced EAE susceptibility in IL17−/− mice (20–23). It is possible that DAF expression may tip this fine balance away from IFN-γ and Th-17 mediated enhanced disease and that unregulated local complement production by APCs, T cells, or both may shape the autoimmune repertoire by allowing excessive selection of the IFN-γ and Th17 lineages. In support of this, in addition to the number of anti-MOG35–55 IL-17 producing cells being greater in the Daf1−/− mice, the rate of decline in the number of these cells was slower than in Daf1+/+ mice possibly accounting for the absence in these mice of myelin repair. Thus, DAF may serve overall to prevent or limit severe autoimmune outcomes due to IFN-γ and particularly to Th17 mediated events.
Our studies with Daf1−/− DCs and T cells doubly deficient in C5aR−/−C3aR−/− or C3−/− relate the mechanism underlying DAF’s T cell immunomodulatory activity to our newly uncovered findings (6) that an early event (<1 hr) during APC•T cell cognate interactions is that both partners turn on local synthesis of C3, fB, fD (the three alternative pathway components) and downregulate DAF. More recent work1 has shown that both cognate partners additionally synthesize C5, upregulate C5aR and C3aR, and in the context of diminished junctional DAF, generate C5a and C3a from the locally synthesized components. This recent work has shown that interactions of these locally generated anaphylatoxins with upregulated C5aR and C3aR in both partners augments costimulation. The in vitro experiments in this study with Daf1−/−, Daf1+/+ and doubly deficient Daf1−/−C5aR−/− or doubly deficient DCs and T cells shows that the augmented IFN-γ and IL-17 production in Daf1−/− mice is due both to a) more IL-12 and IL-23 production by Daf1−/− DCs than WT DCs, b) upregulated expression of IL-12Rβ2 and IL-23R on Daf1−/− T cells, and c) increased production of IFN-γ and IL-17 of Daf1−/− T cells in response to IL-12 and IL-23, respectively. The western blot analyses of culture supernatants showing increased C5a and C3a production by Daf1−/− T cells and the ELISPOT assays and ELISAs showing reversal of the heightened IFN-γ and IL-17 production when Daf1−/−C5aR−/− or Daf1−/−C3aR−/− T cells were used document that the T cell autoreactivity is dependent on local C5a/C3a generation and consequent C5aR/C3aR signaling. While the work in this study was done with Daf deficient cells, our previous findings in WT cells (6) that in concert with local complement/C5aR/C3aR synthesis, DAF downregulates on both APCs and T cells establish that Daf1−/− T cells simulate activation events in normal cells. Since the heightened reactivity of T cells occurs in the absence of DAF on APCs (6) and T cells2, this newly uncovered process is not related to older studies (24, 25) putatively linking DAF to direct signaling per se.
Our studies with naïve CD4+ CD62Lhi cells relate our results to recent findings (26) that differentiation of naïve T cells into the Th17 lineage is dependent on TGF-β and IL-6. It has been shown that TGF-β mediates upregulation of IL-23 receptors on T cells (19, 27–31). Our incubations of sorted CD4+ CD62Lhi naïve Daf1−/− and Daf1+/+ T cells with TGF-β and IL-6 showed that Daf1−/− T cells produce 3-fold more IL-17. Our studies with Daf1−/−C5aR−/−C3aR−/− cells showed that the increases depended on C5aR/C3aR function. More work is needed to determine the source of these cytokines and whether local complement synthesis is involved in their production.
Humoral immunity against myelin has been implicated in EAE, but its involvement generally relates to induction of disease with whole protein rather than peptide (32). When immunized with whole MOG protein, B cell-deficient mice develop less severe EAE (32). In contrast, if MOG35–55 is used, demyelination in the B cell knockouts is similar to that in WTs (33). The experiments in this study showing no significant difference in complement-fixing Igs or C3/C9 deposition, document that DAF’s activity on T cells is distinct from DAF’s traditional self cell-shielding role against systemic complement-mediated injury. Lower clinical scores in C3−/− mice (34) and in C3aR−/− mice (11) are consistent with the results in this study concerning locally generated complement activation fragments enhancing activation during T cell priming (6). Whether the findings in this study that DAF participates in protecting myelin from autoreactive T cells in EAE is a general phenomenon for autoimmunity and T cell reactivity in other contexts must await further work. Results available so far with corneal transplantation (35) would support this possibility.
In MS, like EAE, MOG is a target autoantigen (36). As in EAE, MHC class II restricted T cells reactive with MOG are present in MS patients' cerebrospinal fluid (37). Currently, acute attacks are treated with intravenous steroids, IFN-β, Copaxone, and immunosuppressives, but overall, available treatments are unsatisfactory (38). Our findings that markedly enhanced EAE occurs in Daf1−/− mice due to lowering the threshold to T cell autoreactivity raise the possibility that appropriately targeted recombinant DAF could have therapeutic relevance for MS.
Acknowledgments
The authors thank Dr. BP Morgan, Cardiff, UK, for rabbit anti-rat C9, Dr. Mireya Diaz-Insua (Dept of Statistics, CWRU) for statistical analyses, and Denny Hatala for preparing tissue sections.
Abbreviations used in this paper
- MS
multiple sclerosis
- EAE
experimental autoimmune encephalomyelitis
- BBB
blood brain barrier
- MOG
myelin oligodendrocyte glycoprotein
- CNS
central nervous system;
- MBP
myelin basic protein
- PLP
proteolipid protein
- DAF
decay accelerating factor
- daf1
mouse homolog of human DAF protein
- C5aR and C3aR
C5a receptor and C3a receptor
Footnotes
The work was supported by National Institute of Health, MEM (AI23598 and EY-11288), VKT (AI-51837 and DC-006422), FL (RG3664-A-1 and NS052471), RHM (N536674), and E. Pearlman (core facility EY015476).
Strainic et. al., submitted and Lalli, et. al., submitted)
Strainic et. al., submitted and Lalli, et. al., submitted)
References
- 1.Gold R, Hartung HP, Toyka K. Animal models for autoimmune demyelinating disorders of the nervous system. Mol Med Today. 2000;6:88–88. doi: 10.1016/s1357-4310(99)01639-1. [DOI] [PubMed] [Google Scholar]
- 2.Merkler D, Oertle T, Buss A, Pinschewer DD, Schnell L, Bareyre FM, Kerschensteiner M, Buddeberg BS, Schwab M. Rapid induction of autoantibodies against Nogo-A and MOG in the absence of an encephalitogenic T cell response: implication for immunotherapeutic approaches in neurological diseases. FASEB Journal. 2003;17:2275–2275. doi: 10.1096/fj.02-1203fje. [DOI] [PubMed] [Google Scholar]
- 3.Kohm AP, Miller SD. Role of ICAM-1 and P-selectin expression in the development and effector function of CD4+CD25+regulatory T cells. J Autoimmun. 2003;21:261–271. doi: 10.1016/s0896-8411(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 4.Linnington C, Berger T, Perry L, Weerth S, Hinze-Selch D, Zhang Y, Lu HC, Lassmann H, Wekerle H. T cells specific for the myelin oligodendrocyte glycoprotein mediate an unusual autoimmune inflammatory response in the central nervous system. Eur J Immunol. 1993;23:1364–1364. doi: 10.1002/eji.1830230627. [DOI] [PubMed] [Google Scholar]
- 5.Tuohy VK, Yu M, Yin L, Kawczak JA, Johnson JM, Mathisen PM, Weinstock-Guttman B, Kinkel RP. The epitope spreading cascade during progression of experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol Rev. 1998;164:93–100. doi: 10.1111/j.1600-065x.1998.tb01211.x. [DOI] [PubMed] [Google Scholar]
- 6.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, Xu Y, Medof ME. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201:1523–1530. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160:1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenderfer SE, Ke B, Hollmann TJ, Wetsel RA, Lan HY, Braun MC. C5a receptor deficiency attenuates T cell function and renal disease in MRLlpr mice. J Am Soc Nephrol. 2005;16:3572–3582. doi: 10.1681/ASN.2005040373. [DOI] [PubMed] [Google Scholar]
- 9.Kumar V, Ali SR, Konrad S, Zwirner J, Verbeek JS, Schmidt RE, Gessner JE. Cell-derived anaphylatoxins as key mediators of antibody-dependent type II autoimmunity in mice. J Clin Invest. 2006;116:512–520. doi: 10.1172/JCI25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nataf S, Davoust N, Barnum SR. Kinetics of anaphylatoxin C5a receptor expression during experimental allergic encephalomyelitis. J Neuroimmunol. 1998;91:147–155. doi: 10.1016/s0165-5728(98)00169-6. [DOI] [PubMed] [Google Scholar]
- 11.Boos L, Campbell IL, Ames R, Wetsel RA, Barnum SR. Deletion of the complement anaphylatoxin C3a receptor attenuates, whereas ectopic expression of C3a in the brain exacerbates, experimental autoimmune encephalomyelitis. J Immunol. 2004;173:4708–4714. doi: 10.4049/jimmunol.173.7.4708. [DOI] [PubMed] [Google Scholar]
- 12.Lin F, Fukuoka Y, Spicer A, Ohta R, Okada N, Harris CL, Emancipator SN, Medof ME. Tissue distribution of products of the mouse decay-accelerating factor (DAF) genes. Exploitation of a Daf1 knock-out mouse and site-specific monoclonal antibodies. Immunology. 2001;104:215–225. doi: 10.1046/j.0019-2805.2001.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spicer AP, Seldin MF, Gendler SJ. Molecular cloning and chromosomal localization of the mouse decay-accelerating factor genes. Duplicated genes encode glycosylphosphatidylinositol-anchored and transmembrane forms. Journal of Immunology. 1995;155:3079–3091. [PubMed] [Google Scholar]
- 14.Kerlero de Rosbo N, Mendel I, Ben-Nun A. Chronic relapsing experimental autoimmune encephalomyelitis with a delayed onset and an atypical clinical course, induced in PL/J mice by myelin oligodendrocyte glycoprotein (MOG)-derived peptide: preliminary analysis of MOG T cell epitopes. Eur J Immunol. 1995;25:985–993. doi: 10.1002/eji.1830250419. [DOI] [PubMed] [Google Scholar]
- 15.Tuohy VK, Lu ZJ, Sobel RA, Laursen RA, Lees MB. A synthetic peptide from myelin proteolipid protein induces experimental allergic encephalomyelitis. J Immunol. 1988;141:1126–1130. [PubMed] [Google Scholar]
- 16.Lin F, Fukuoka Y, Spicer A, Ohta R, Okada N, Harris CL, Emancipator SN, Medof ME. Tissue distribution of products of the mouse decay-accelerating factor (DAF) genes. Exploitation of a Daf1 knock-out mouse and site-specific monoclonal antibodies. Immunology. 2001;104:215–225. doi: 10.1046/j.0019-2805.2001.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agresti A, Min Y. Unconditional small-sample confidence intervals for the odds ratio. Biostatistics. 2002;3:379–386. doi: 10.1093/biostatistics/3.3.379. [DOI] [PubMed] [Google Scholar]
- 18.Jabs C, Greve B, Chang TT, Sobel RA, Sharpe AH, Kuchroo VK. Genetic background determines the requirement for B7 costimulation in induction of autoimmunity. Eur J Immunol. 2002;32:2687–2697. doi: 10.1002/1521-4141(200209)32:9<2687::AID-IMMU2687>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 20.Furuzawa-Carballeda J, Vargas-Rojas MI, Cabral AR. Autoimmune inflammation from the Th17 perspective. Autoimmun Rev. 2007;6:169–175. doi: 10.1016/j.autrev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 23.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 24.Shenoy-Scaria AM, Timson Gauen LK, Kwong J, Shaw AS, Lublin DM. Palmitoylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Molecular and Cellular Biology. 1993;13:6385–6392. doi: 10.1128/mcb.13.10.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shenoy-Scaria AM, Kwong J, Fujita T, Olszowy MW, Shaw AS, Lublin DM. Signal transduction through decay-accelerating factor. Interaction of glycosylphophatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn. Journal of Immunology. 1992;149:3535–3541. [PubMed] [Google Scholar]
- 26.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 27.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 28.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 29.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17- producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Lyons JA, S M, Happ MP, Cross AH. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur J Immunol. 1999;29:3432–3439. doi: 10.1002/(SICI)1521-4141(199911)29:11<3432::AID-IMMU3432>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Hjelmstrom P, J A, Fjell J, Ruddle NH. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J Immunol. 1998;161:4480–4483. [PubMed] [Google Scholar]
- 34.Nataf S, Carroll SL, Wetsel RA, Szalai AJ, Barnum SR. Attenuation of experimental autoimmune demyelination in complement-deficient mice. J Immunol. 2000;165:5867–5873. doi: 10.4049/jimmunol.165.10.5867. [DOI] [PubMed] [Google Scholar]
- 35.Esposito A, Suedekum B, Lin F, Lass JH, Medof ME. Essential Roles of DAF and CD59 in Sustaining Successful Corneal Engraftment. Immunology 2004, International Proceedings, 12th International Congress of Immunology and 4th Annual Conference of FOCIS; Canada. 2004. pp. 375–379. Publisher MEDIMOND S.r.l. Ed.Monduzzi, ISBN 88-7587-069-1. [Google Scholar]
- 36.Gardinier MV, A P, Linington C, Matthieu JM. Myelin/oligodendrocyte glycoprotein is a unique member of the immunoglobulin superfamily. J Neurosci Res. 1992;33:177–187. doi: 10.1002/jnr.490330123. [DOI] [PubMed] [Google Scholar]
- 37.Sun J, L H, Olsson T, Xiao BG, Andersson G, Ekre HP, Linington C, Diener P. T and B cell responses to myelin oligodendrocyte glycoprotein in multiple sclerosis. J Immunol. 1991;146:1490–1495. [PubMed] [Google Scholar]
- 38.Killestein J, Polman CH. Current trials in multiple sclerosis: established evidence and future hopes. Curr Opin Neurol. 2005;18:253–260. doi: 10.1097/01.wco.0000169741.29535.cc. [DOI] [PubMed] [Google Scholar]