Abstract
Migraine is a debilitating neurological disorder characterized by mild to severe headache that is often accompanied by aura and other neurological symptoms. Among proposed mechanisms, dilation of the dural vasculature especially the middle meningeal artery (MMA) has been implicated as one component underlying this disorder. Several regulatory peptides from trigeminal sensory and sphenopalatine postganglionic parasympathetic fibers innervating these vessels have been implicated in the process including pituitary adenylate cyclase-activating polypeptide (PACAP). Although PACAP has been well described as a potent dilator in many vascular beds, the effects of PACAP on the dural vasculature are unclear. In the current study, we examined the ability of PACAP to dilate MMAs that were isolated from rats and pressurized ex vivo. PACAP38 potently dilated pressurized MMAs with an EC50 of 1 pM. The PAC1 receptor antagonist, PACAP(6-38), abolished MMA dilation caused by picomolar concentrations of PACAP. In contrast, cerebellar arteries isolated from the brain surface were ~1,000-fold less sensitive to PACAP than MMAs. Although cerebellar arteries expressed transcripts for all three PACAP receptor subtypes (PAC1, VPAC1, and VPAC2 receptors) by RT-PCR analyses, MMA demonstrated only PAC1 and VPAC2 receptor expression. Further, multiple variants of the PAC1 receptor were identified in the MMA. The expression of PAC1 receptors and the high potency of PACAP to induce MMA vasodilation are consistent with their potential roles in the etiology of migraine.
Keywords: Migraine, Vascular smooth muscle, Neurotransmitter, Pituitary adenylate cyclase-activating polypeptide (PACAP), Calcitonin gene-related peptide (CGRP)
Introduction
Migraine is one of the most prevalent contributors to the global burden of mental disorders (Collins et al. 2011). It is a complex episodic neurological disorder characterized by intense unilateral pulsating headaches that may last hours to days, which may be accompanied by aura, nausea, photophobia, phonophobia, and other neurological symptoms (Asghar et al. 2011). Although the causes and mechanisms underlying migraine are multifactorial and remain poorly understood, one likely contributing factor, first proposed decades ago (Ray et al. 1940), involves dilation of the dural vasculature. Subsequent investigations have implicated trigeminal sensory and/or sphenopalatine parasympathetic postganglionic neuronal signaling as a contributor to the experience of migraine pain (Mayberg et al. 1981; Brain et al. 1985; Lassen et al. 2002; Olesen et al. 2009; Schytz et al. 2009, 2010; Asghar et al. 2011; Amin et al. 2012). In one current model, enhanced neurotransmitter/neuropeptide release causes middle meningeal artery (MMA) dilation leading to sensitization of the trigeminal sensory neuronal fibers innervating the MMA that contributes to the perception of pain (Goadsby et al. 1993). Bioactive neuropeptides may participate in all of these events and one prototypic neuroregulator in migraine is calcitonin gene-related peptide (CGRP). CGRP is a potent vasodilator (Brain et al. 1985), is highly expressed in dorsal root/trigeminal sensory and sphenopalatine autonomic ganglia neurons, has been localized to perivascular nerve terminals (McCulloch et al. 1986), and has been shown to induce migraine-like headaches indistinguishable from spontaneous migraine attacks (Lassen et al. 2002). Notably, magnetic resonance angiography (MRA) imaging studies have correlated MMA vasodilation following exogenous CGRP administration with headache onset to further implicate roles for neurally mediated vasodilation in migraine (Asghar et al. 2011).
Similar to CGRP, more recent studies have also implicated pituitary adenylate cyclase-activating polypeptide (PACAP) signaling in migraine. PACAP is a neurotransmitter and neurotrophic peptide in the secretin, glucagon, and vasoactive intestinal peptide (VIP) superfamily and shares some G protein-coupled receptor selectivity with VIP (Vaudry et al. 2009). PACAP binds potently and specifically to the PAC1 receptor which may be coupled to multiple intracellular signaling cascades; by contrast, both PACAP and VIP bind with near-equal affinity to VPAC1 and VPAC2 receptors coupled principally to adenylyl cyclase. Among its many functions, PACAP has prominent roles in nociception and like CGRP causes relaxation of vascular and respiratory smooth muscle (Seki et al. 1995). The peptide is highly expressed in diverse central and peripheral nervous system tissues, including sensory and autonomic ganglia, and appears co-localized with CGRP for concomitant release upon neuronal stimulation (Csati et al. 2012). Hence, PACAP shares expression and regulatory parallels with CGRP, and significantly, recent clinical studies have shown that intravenous infusion of PACAP can produce MMA vasodilation and induce headache in both migraineurs and healthy subjects (Schytz et al. 2009; Amin et al. 2012). Further, the administration of the serotonin 5-HT1B and 5-HT1D receptor agonist sumatriptan, frequently used to treat migraine headaches, attenuated both PACAP-induced MMA vasodilation and headache pain. These studies indicate that PACAP signaling causes MMA dilation that may contribute to the etiology of migraine (Amin et al. 2012) and represent an important therapeutic target to aid migraineurs.
Despite the immediate relevance to migraine, PACAP signaling in the MMA has not been well characterized. In the current study, we examined the effects of PACAP on isolated segments of MMA using pressurized artery myography. Compared to other intracranial vessels, the high potency of PACAP and the expression of PACAP receptors in the MMA appear unique, suggesting that the MMA vasodilatory responses to PACAP/PAC1 receptor signaling may be an important component of the complexities of migraine.
Methods
Isolation of Middle Meningeal and Cerebellar Arteries
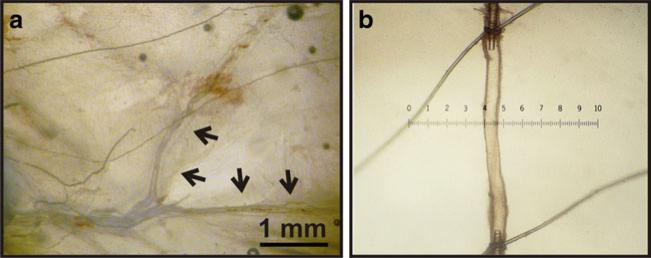
All experiments and protocols were conducted in accordance with the Guide for the Care and Use of Laboratory animals (NIH Pub. No. 85-23, revised 1996) and approved by the Institutional Animal Use and Care Committee of the University of Vermont. Sprague–Dawley rats (males, 300–350 g; Charles River Laboratories, Saint Constant, QC, Canada) were euthanized by decapitation under deep isoflurane anesthesia. The brain and calvaria were carefully removed and placed in ice-cold 3-(N-morpholino)propanesulfonic acid (MOPS)-buffered saline solution containing (in millimolar): 145 NaCl, 5 KCl, 1 MgSO4, 2.5 CaCl2, 1 KH2PO4, 0.02 EDTA, 3 MOPS, 2 pyruvate, 5 glucose, and 1 % bovine serum albumin (pH 7.4). For MMA isolation, the dura mater was gently removed from the calvaria under a dissection microscope (Fig. 1a). To enable a comparison of the MMA to pial arteries, cerebellar arteries were dissected from the brain using iris scissors. The MMA and cerebellar artery are both innervated by trigeminal sensory nerves (Simons et al. 1988; Goadsby et al. 1993).
Fig. 1.
Preparation of rat middle meningeal artery (MMA) for arteriograph studies. a Rat MMA embedded in the dura matter prior to dissection. Arrows indicate the MMA region typically used for ex vivo studies. b Isolated rat MMA cannulated on glass micropipettes mounted in an arteriograph chamber. Both ends of MMA were tied by suture thread, and the artery pressurized to 40 mmHg. Arterial diameter was measured using video edge detection and recorded using data acquisition software (details in “Methods”). One graduation of scale (e.g., 0 to 1) represents 100 μm
Pressurized Artery Myography
Middle meningeal and cerebellar arteries were cannulated onto glass micropipettes mounted in a 5-mL myograph chamber (Fig. 1b) and intravascular pressure was adjusted using a pressure servo-controller and a peristaltic pump (Living Systems Instruments, St. Albans, VT) as previously described (Ishiguro et al. 2002; Nystoriak et al. 2011). Arteries were continuously superfused with warmed artificial cerebral spinal fluid (aCSF; 37 °C, in millimolar: 125 NaCl, 3 KCl, 18 NaHCO3, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, and 5 glucose, aerated with 5 % CO2, 20 % O2, 75 %N2, pH 7.35–7.40). Changes in vessel diameter were measured by video edge detection (Living Systems Instrumentation, St Albans, VT) using WinDaq data acquisition software (Dataq Instruments; Akron, OH). Following equilibration (1 h), arterial viability was evaluated by brief exposure to 60 mM KCl (<2 min; isosmotic replacement of NaCl with KCl in aCSF). Only arteries that demonstrated a constriction representing a decrease in diameter of 50 % or greater were used in subsequent studies. Pressure-induced constriction is expressed as a percent decrease of the fully dilated diameter of individual arteries at the same intravascular pressure. These values were obtained by using the following equation: % constriction0[(DP−DA)/DP]×100, where DP=the (passive) diameter of the artery in Ca2+-free aCSF containing the vasodilators diltiazem (100 μM) and forskolin (1 μM), and DA=the (active) diameter of the artery in Ca2+-containing aCSF. Dilations in response to PACAP38 and VIP are expressed as a percent reduction in the level of arterial constriction. The values were obtained using the following equation: % dilation=[(DV–DA)/(DP–DA)]×100, where DV=arterial diameter in the presence of vasodilator, DA=the active diameter of the artery prior to addition of vasodilator, and DP=the passive diameter of the artery. The 38 amino acid form of PACAP, referred to as PACAP38 (Miyata et al. 1989), was used in all experimental series. The concentrations of PACAP38 or VIP that caused half maximal vasodilation (EC50) were calculated from individual concentration response curves using Graphpad Prism 5 software (Graphpad software, Inc. La Jolla, CA). PACAP38, VIP, and related peptides (American Peptide Sunnyvale, CA) were prepared as 100 μM stock in deionized water; aliquots were stored at –80 °C. All aliquots were diluted to their final concentrations immediately before use and were administered to arteries by bath superfusion (5 mL/min flow rate) for a minimum of 20 min.
MMA and Cerebellar Artery PACAP Receptor Transcript Expression
Total RNA extracted from isolated MMAs and cerebellar arteries using RNA STAT-60 total RNA isolation reagent (Tel-test, Friendswood, TX) was reverse-transcribed to cDNA using SuperScript First-Strand synthesis system (Invitrogen, Carlsbad, CA) as previously described (Nystoriak et al. 2009). To detect mRNA expression of PACAP receptor subtypes, semiquantitative RT-PCR was performed using primer sets for PAC1, VPAC1, and VPAC2 receptor transcripts; amplification of the same templates for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal control (Table 1). To examine vessel expression of PAC1 receptor splice variants, amplification using primers flanking the alternative splice site within the third cytoplasmic loop of the PAC1 receptor was performed as previously described (Braas et al. 1999). Amplification of single-stranded cDNA was performed in a 13-μL reaction volume consisting of 15 mM Tris–HCl, pH 8.0, containing 50 mM KCl, 2.5 mM MgCl2, 200 μM deoxynucleotide triphosphates, 0.5 μM primers, 0.5 μL of cDNA template, and 0.3 U AmpliTaq Gold DNA polymerase (Applied Biosystems, Norwalk, CT) with the following cycling parameters: (1) initial denaturation and enzyme activation 95 °C, 10 min; (2) denaturation and enzyme activation 94 °C, 1 min; annealing, primer specific (Table 1), 1 min; extension 72 °C, 1 min (32–40 cycles); and (3) final extension, 72 °C; 10 min. For PAC1 receptor variant identification, the amplified cDNA product was subjected to diagnostic restriction enzyme digestion as previously described (Braas et al. 1999). The amplified products were resolved on 2 % agarose gel and visualized by UV illumination following ethidium bromide staining.
Table 1.
Primer information for PCR studies examining PACAP receptor transcript expression
| Target | Sequences | GenBank accession number | Amplification region | Annealing temp °C | Product size |
|---|---|---|---|---|---|
| PACAPR1 | 5′-CTTGTACAGAAGCTGCAGTCCCCAGACATG-3′ | Z23272 | 1078–1107 | 60 | 303–387 |
| PACAPR2 | 5′-CGGTGCTTGAAGTCCATAGTGAAGTAACGGTTCACCTT-3′ | 1510–1548 | |||
| PACAPR3 | 5′-AACGACCTGATGGGACTAAAC-3′ | NM_133511 | 211–231 | 56 | 413 |
| PACAPR4 | 5′-CGGAAGCGGCACAAGATGACC-3′ | 603–623 | |||
| VPAC1R3 | 5′-CGGCCACCCGACATTGGGAAG-3′ | M86835 | 1034–1054 | 61 | 323 |
| VPAC1R4 | 5′-CTGCATGTGGCGCCGTTGCTG-3′ | 1336–1356 | |||
| VPAC2R1 | 5′-AAGCTAACTTCTCCAGATGTT-3′ | Z25885 | 990–1010 | 55 | 396 |
| VPAC2R2 | 5′-CAGCTAAATGACTGAGGTCTC-3′ | 1365–1385 | |||
| GAPDHF | 5′-GACAGCCGCATCTTCTTGTG-3′ | NM_017008 | 30–49 | 62 | 210 |
| GAPDHR | 5′-TGAACTTGCCGTGGGTAGAG-3′ | 220–239 |
Statistics
Data are expressed as mean ± SEM and analyzed by Student's unpaired t test. Statistical significance was considered at p<0.05.
Results
Isolated Middle Meningeal Arteries Exhibit Pressure-Induced Myogenic Tone
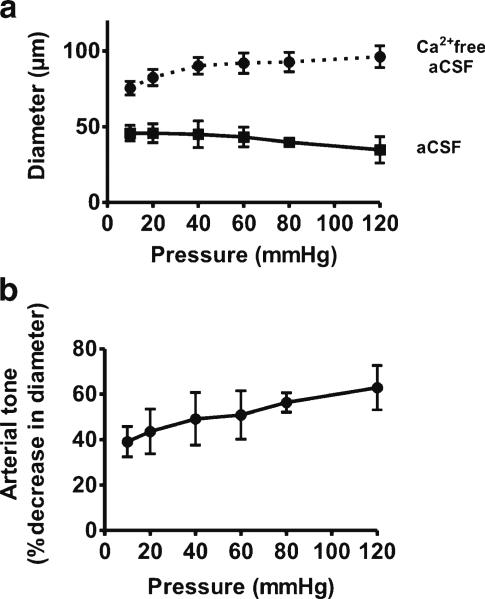
Small diameter arteries and arterioles isolated from a variety of vascular beds, including those supplying blood to the heart and brain, constrict in response to increases in intravascular pressure (Bayliss 1902; Johnson 1980). In the cerebral circulation, pressure-induced constriction, also termed myogenic tone, plays an important role in the regulation of cerebral artery diameter (Nelson et al. 1995; Ishiguro et al. 2002; Nystoriak et al. 2011). To evaluate myogenic tone in freshly isolated MMA segments, changes in arterial diameter were examined in response to stepwise increases in intravascular pressure. At 10 mmHg, MMA diameter in aCSF was 45.8±5.2 μm, representing a constriction of 39.1±5.8 % decrease from the maximally dilated diameter of 75.5±4.6 μm obtained in Ca2+-free aCSF containing the vasodilators diltiazem (100 μM) and forskolin (1 μM). The level of myogenic tone increased as intravascular pressure was elevated, reaching a constriction of 62.9±8.4 % at 120 mmHg (Fig. 2). At the physiologically relevant pressure of 40 mmHg, myogenic tone represented a constriction of 49.1±10 %. These data demonstrate that isolated MMA exhibit pressure-induced myogenic tone. Subsequent experimental series were performed using isolated MMAs held at 40 mmHg in the absence of exogenous vasoconstrictors.
Fig. 2.
Pressure-induced myogenic tone in isolated rat middle meningeal arteries (MMAs). a Diameter measurements of isolated rat MMA obtained in response to stepwise increases in intravascular pressure from 10 to 120 mmHg. Squares with solid line represent active diameter measurements obtained in artificial cerebral spinal fluid (aCSF) (n=4). Circles with dashed line represent maximally dilated (passive) diameter measurements obtained from the same arteries in Ca2+-free aCSF containing the vasodilators diltiazem (100 μM) and forskolin (1 μM). b Pressure-induced constriction (myogenic tone) in rat MMAs superfused with aCSF ex vivo. Constriction is expressed as a percent decrease from maximally dilated diameter obtained at the same intravascular pressure (n=4)
Picomolar Concentrations of PACAP38 Dilate Pressurized MMAs via Activation of PAC1 Receptors
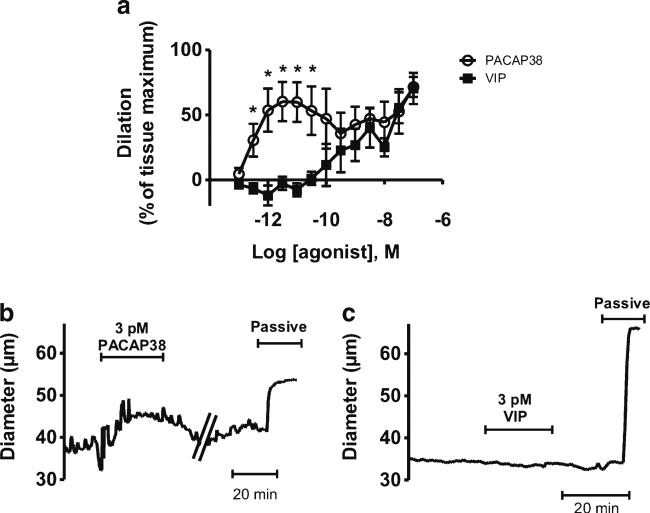
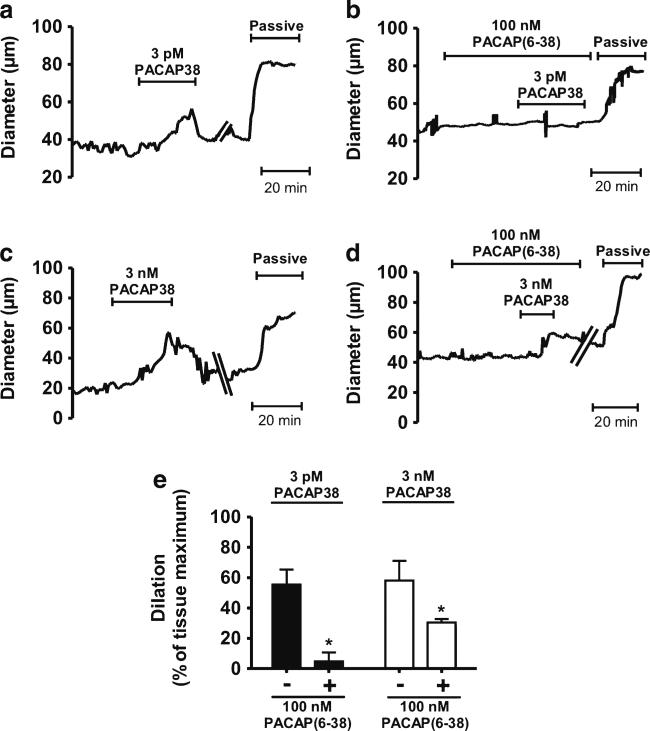
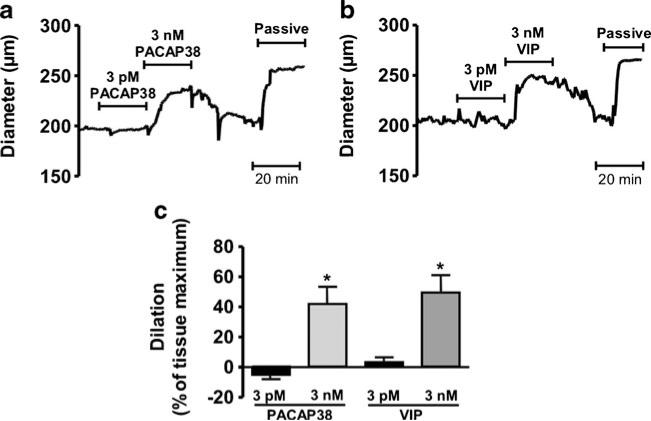
The ability of PACAP to induce MMA dilation ex vivo is unclear. To date, studies of PACAP-induced responses in isolated MMA segments have examined changes in isometric force generation in unpressurized arteries pre-contracted with exogenous vasoconstrictors (Baun et al. 2011). To examine the impact of PACAP on ex vivo pressurized MMAs exhibiting myogenic tone, increasing concentrations of PACAP38 or VIP (0.1 pM to 100 nM) were added to the superfusate bathing MMAs held at an intravascular pressure of 40 mmHg (Fig. 3a). PACAP38 potently dilated MMA with an EC50 of 1 pM (n=4). In marked contrast, VIP was approximately 1,000-fold less potent dilator of MMA (EC50, 1.4 nM, n=4). As an example, 3 pM PACAP38 caused MMA dilation of 60.3±15.1 % of tissue maximum, whereas 3 pM VIP did not significantly alter arteriolar diameter (Fig. 3b, c). Based on the differential potency of PACAP38 and VIP binding to PACAP receptor subtypes (Harmar et al. 2012), we hypothesized that MMA dilation to picomolar concentrations of PACAP38 are mediated via activation of PAC1 receptors. Consistent with this concept, MMA dilation to 3 pM PACAP38 was abolished by the PAC1 receptor antagonist PACAP (6-38) (Harmar et al. 2012) (Fig. 4a, b). Interestingly, MMA dilation to 1,000-fold higher concentrations of PACAP38 (i.e., 3 nM) were not abolished but were reduced by approximately 50 % (Fig. 4c–e), consistent with higher concentrations of PACAP38 causing activation of PAC1 receptors as well as VPAC1 and/or VPAC2 receptors. These data demonstrate that PACAP38 potently dilates pressurized MMAs via activation of PAC1 receptors.
Fig. 3.
Low picomolar concentrations of PACAP, but not VIP, dilate isolated pressurized rat middle meningeal arteries (MMAs). a Cumulative concentration response curves of PACAP and VIP obtained from rat MMAs pressurized to 40 mmHg ex vivo. Arteries were exposed to aCSF containing each concentration of PACAP38 or VIP for 20 min. Dilation to PACAP38 or VIP are expressed as percentage of maximum dilation obtained in the presence of Ca2+-free aCSF containing 100 μM diltiazem and 1 μM forskolin. p<0.05 by unpaired t test, n=4. b, c Diameter recordings of an isolated MMA treated with aCSF containing a single concentration (3 pM) of PACAP38 (b) and VIP (c). At the end of the experiment, maximally dilated diameter was obtained in the presence of Ca2+-free aCSF containing 100 μM diltiazem and 1 μM forskolin (shown as passive)
Fig. 4.
Block of picomolar PACAP-induced dilation of isolated pressurized middle meningeal artery (MMA) by the PAC1 receptor antagonist PACAP(6-38). a–d Diameter recordings from isolated MMAs obtained in the absence (a, c) and presence (b, d) of the PAC1 antagonist, PACAP(6-38). Arteries, pressurized to 40 mmHg, were treated with either 3 pM PACAP38 (a, b) or 3 nM PACAP38 (c, d). Maximum dilation (passive) was obtained by treatment with 100 μM diltiazem and 1 μM forskolin in Ca2+-free aCSF. e Summary data of PACAP38-induced dilation in the absence or presence of PACAP(6-38). PACAP(6-38) abolished 3 pM PACAP38-induced vasodilation but caused only partial inhibition of 3 nM PACAP38-induced vasodilation in rat MMAs (n=4 each). Diameter changes by PACAP38 treatment were expressed as percentage of maximum dilation. *p<0.05 vs PACAP-induced vasodilation in the absence of PACAP(6-38)
Nanomolar, but Not Picomolar, Concentrations of PACAP38 Dilate Pressurized Cerebellar Arteries
Intracranial cerebral arteries are innervated by sensory trigeminal nerves containing PACAP but appear to contribute little to migraine symptoms (Asghar et al. 2011; Amin et al. 2012). To examine whether PACAP dilates pressurized cerebral arteries with an efficacy similar to that observed in MMA, cerebellar arteries were cannulated and studied ex vivo. In contrast to MMAs, 3 pM PACAP38 did not alter cerebellar artery diameter (n=4, Fig. 5a, c). However, 1,000-fold higher concentrations of PACAP38 (3 nM) did significantly dilate cerebellar arteries (34±12 %, n=4). The EC50 value of PACAP-induced cerebellar dilation was 11 nM (n=6). Similarly, VIP caused cerebellar artery dilation at nanomolar, but not picomolar, concentrations (Fig. 5b, c; EC50=6 nM, n=3). These data demonstrate that PACAP is considerably more potent vasodilator of MMA compared to cerebellar arteries, suggesting that the PACAP- and VIP-induced vasodilation in cerebellar arteries are likely mediated by a VPAC receptor subtype.
Fig. 5.
Nanomolar, but not picomolar, concentrations of PACAP38 and VIP dilate pressurized cerebellar arteries. a Diameter recording obtained from pressurized rat cerebellar artery treated with PACAP38. PACAP38 induced dilation at 3 nM but not 3 pM. Maximum dilation was obtained using a combination of 100 μM diltiazem and 1 μM forskolin in Ca2+-free aCSF (passive). b Diameter recording obtained from pressurized rat cerebellar artery treated with VIP. VIP-induced dilation at 3 nM but not 3 pM. Maximum dilation was obtained using a combination of 100 μM diltiazem and 1 μM forskolin in Ca2+-free aCSF (passive). c Summarized data of PACAP38 and VIP treatment in rat cerebellar arteries. PACAP38-induced diameter changes are expressed as percentage of maximum dilation (n=4 each). *p<0.05 by paired t test
MMA and Cerebellar Arteries Express Different PAC1 and VPAC Receptor Subtypes
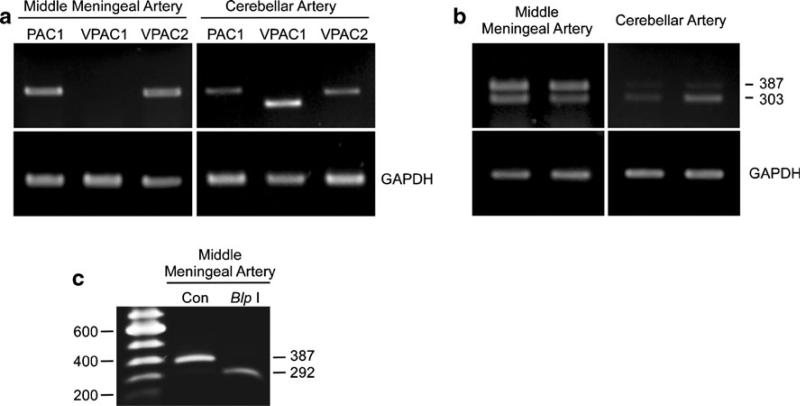
PACAP and VIP share receptor subtypes; the PAC1 receptor is specific for PACAP peptides while VPAC1 and VPAC2 receptors bind VIP and PACAP with similar affinities. Differential PACAP receptor expression may contribute to the disparity in the sensitivity of MMA and cerebellar arteries to PACAP. To establish PACAP/VIP receptor expression in MMA and cerebellar arteries, semiquantitative PCR studies were performed using primers unique to each receptor transcript, as described in previous work (Braas et al. 1999). Interestingly, only PAC1 and VPAC2 receptor transcripts were detected in MMA; there was little to no VPAC1 receptor expression in MMA. By contrast, all three PACAP/VIP receptor subtypes (PAC1, VPAC1, and VPAC2 receptors) were identified in cerebellar arteries (Fig. 6a).
Fig. 6.
PACAP receptor transcripts are differentially expressed in rat middle meningeal and cerebellar arteries. Total RNA from rat MMA and cerebellar arteries were reverse-transcribed for semiquantitative PCR analyses of PAC1 (413 bp), VPAC1 (323 bp), and VPAC2 (396 bp) receptor transcript expression. a Unlike cerebellar artery preparations which demonstrated mRNA for all three receptor subtypes (right panel, n=5), the MMA appeared to express only PAC1 and VPAC2 receptor transcripts (left panel, n=5). b To examine whether there were differences in PAC1 receptor isoform expression levels between the two arteries, primers flanking the third cytoplasmic loop were employed in the PCR studies. While cerebellar arteries appeared to possess predominantly the PAC1null receptor isoform (right panel, neither Hip nor Hop; 303 bp), the MMA expressed near-equal levels of the PAC1null (303 bp) and the one-cassette PAC1 receptor variant (387 bp; left panel). Each lane represents an independent sample replicate. Amplification of the same templates for GAPDH demonstrates approximate equal sample input. c The specific one-cassette PAC1 receptor variant could be identified by diagnostic restriction digest; isolation of the MMA 387-bp PAC1 receptor band followed by Blp I restriction enzyme incubation resulted in amplicon digestion to a smaller 292-bp product diagnostic of the Hop receptor variant
There are multiple PAC1 receptor isoforms from alternative splicing of Hip and/or Hop cassettes in the receptor gene corresponding to the G protein interactive sites in the third cytoplasmic loop, which are important for downstream activation of signaling cascades (Spengler et al. 1993). When PAC1 receptor variant transcript analyses were performed using primers flanking the third cytoplasmic loop, MMA and cerebellar arteries not only expressed the PAC1-null receptor (neither Hip nor Hop, Fig. 6b, 303-bp band) but also the one-cassette variant (Hip or Hop, Fig. 6b, 387-bp band). Furthermore, the relative abundance of the one-cassette variant was much greater in the MMA than the cerebellar artery. As the Hip and Hop cassettes are both 84 bp, the one-cassette 387-bp amplified product could not distinguish the specific PAC1 receptor splice variant expressed in MMA. However, restriction enzyme digestion can be diagnostic (Braas et al. 1999) and digestion of the 387-bp band with Blp I generated the 292-bp products consistent with the expression of the PAC1Hop receptor variant in MMA (Fig. 6c). While the PAC1null receptor is predominantly coupled to adenylyl cyclase, the PAC1Hop receptor variant can be potently coupled to multiple intracellular signaling cascades including adenylyl cyclase, phospholipase C, MAPK, and Akt (Braas et al. 1999). The expression and coordinate signaling downstream of both PAC1 receptor variants in MMA may have engendered the potent PACAP vasodilatory responses; maladaptions of the same PACAP/PAC1 receptor signaling pathways in MMA, by contrast, may be a contributing factor to migraine.
Discussion
Migraine is one of the leading contributors to the global burden of mental and neurological disorders. The few therapeutic options are often unsatisfactory and hence an understanding of the underlying mechanisms and the identification of new clinical targets remain important goals for effective treatments. From the many proposed causes, the neurovascular component in migraine has remained a viable mediator especially in light of the recent data using high-resolution MRA techniques (Asghar et al. 2011). In humans infused with the well-studied sensory/autonomic vasodilator CGRP peptide, the resulting migraine attacks correlated temporally and spatially with middle meningeal and middle cerebral artery dilation. The arterial dilations were bilateral when the headaches were bilateral and unilateral on the corresponding side when uni-hemispheric headaches were presented. Further, treatments with sumatripans to ease the peptide-induced migraines always resulted in vessel constriction. These studies appear to supplant earlier Doppler studies which failed to correlate vascular changes with migraine (Zwetsloot et al. 1993). Although these studies do not address definitive mechanisms, the results do implicate cranial vascular dynamics in the presentation of migraine. Among intracranial and extra-cranial arteries, the meningeal artery vasodilatory responses were better correlated with headache onset and treatments, and considered to be particularly significant as dural afferent nociceptor sensitization appears central to pain generation in migraine attacks (Burstein et al. 2005).
PACAP peptides behave as neurotransmitters and neurotrophic peptides in the central and peripheral nervous systems with diverse activities in neuroendocrine development and function. PACAP peptides are highly expressed in nociception sensory dorsal root and trigeminal ganglion neurons and in autonomic systems regulating vasodilatory control; hence the effects of PACAP parallel those for CGRP in facilitating migraines. PACAP38 infusions in in vivo and ex vivo preparations cause intra- and extracranial artery vasodilation and can induce migraine-like attacks in normal subjects and migraineurs (Schytz et al. 2009; Amin et al. 2012). As in other physiological systems, the PACAP-elicited responses can be long lasting which may be one of the distinguishing characteristics for PACAP-induced headache pain (Amin et al. 2012). Interestingly, while the related VIP peptide can also produce marked dilation in many arterial vessels, it induces only modest headache and no migraine-like attacks (Hansen et al. 2006; Rahmann et al. 2008) suggesting the selective expression and activation of the shared PAC1/VPAC receptors in the different cranial arterial systems.
Yet despite the unequivocal abilities for PACAP to instill migraine, the relative roles of PACAP and VIP in migraine, and whether specific PAC1/VPAC receptor subtype expression and function in the different cranial arteries can underlie the distinct PACAP/VIP vasodilatory and migraine responses, are still very much unclear. Some of the variability observed in the in vivo infusion studies may have stemmed from PACAP/VIP-mediated endothelial cell activation of nitric oxide pathways, rapid peptide degradation in the circulation, the poor ability for peptides in general to cross the blood–brain barrier, and in the case of rodents, the presence of anesthetics during the course of the studies. The putative selectivity of the available receptor agonists and antagonists may have contributed to the uncertainties, and the ex vivo isometric force studies may have been complicated by the use of high concentrations of reagents including prostaglandin F2α and serotonin to precontract the unpressurized wire-mounted vessels.
From these considerations, the use of a pressurized arteriograph system offers a number of advantages in characterizing PACAP and VIP vasodilatory responses. The pressurized arterial vessels develop myogenic tone in the absence of exogenous vasoconstrictors, are extremely sensitive and responsive to regulatory dynamics (Dunn et al. 1994; Nelson et al. 1995), and obviate the need for drug-induced arterial precontraction, which can potentially dampen the peptide vasodilatory effects. The MMA possesses the ability to autoregulate its blood flow and pressure intrinsically (Michalicek et al. 1996); hence, the pressurized MMA myography preparation presents a physiologically relevant means to examine PACAP-induced effects.
Using these preparations, the current studies demonstrated potent PACAP38 effects on MMA diameter. In contrast to previous isometric force myograph studies which suggested little or no PACAP effects on MMA contractility (Baun et al. 2011), subpicomolar concentrations of PACAP38 reproducibly dilated pressurized MMAs ex vivo. Picomolar PACAP concentrations maximally dilated the MMA to 50–60 % of passive diameter; high nanomolar PACAP concentrations had little additional vasodilatory effects. VIP also dilated MMA but only at nanomolar peptide concentrations. The picomolar potency of PACAP has been well described for several physiological systems including protection from ischemia in the brain and potentiation of glucose-induced insulin release in pancreatic beta cells (Yamada et al. 2004; Dejda et al. 2011). These differential PACAP/VIP response profiles in the MMA differed from those observed for cerebellar arteries in which PACAP38 and VIP appeared equipotent in inducing vasodilations at nanomolar concentrations.
The higher potency of PACAP compared to VIP in the MMA suggest PAC1 receptor activation in the vasodilatory responses. Both PAC1 and VPAC2 receptor transcripts were identified in the MMA; interestingly, VPAC1 receptor expression in the MMA was not detected, which varied with a previous work (Boni et al. 2009). Diagnostic PCR and restriction digests identified PAC1null and PAC1Hop1 receptor isoforms in the MMA which may have allowed second messenger coupling not only to adenylyl cyclase but to other intracellular cascades to potently stimulate MMA dilation. PACAP(6-38) pretreatment blocked the PACAP38 responses in the MMA, and although PACAP (6-38) may antagonize both PAC1 and VPAC2 receptors (Dickinson et al. 1997) to obfuscate clear identification of the relevant receptor subtype in the PACAP-induced responses, the apparent differential potencies observed for PACAP38 and VIP were consistent with PAC1 receptor-mediated vasodilation in the MMA. These results appear consistent with previous interpretations implicating PAC1 receptors in migraine (Olesen et al. 2009; Schytz et al. 2009, 2010). Unlike the MMA, however, the cerebellar arteries exhibited expression for all three PACAP/VPAC receptor subtypes. The apparent equipotency of PACAP38 and VIP in eliciting cerebellar artery vasodilation (Fig. 5) implicate VPAC receptor signaling. These results were in good agreement with previous observations implicating VPAC1 receptors in cerebellar artery vasodilation (Fahrenkrug et al. 2000).
In summary, the pressurized myograph preparations in the current study demonstrated differences between PACAP and VIP in eliciting MMA and cerebellar artery vasodilation which may be notable with respect to migraine physiology. The very high potency for PACAP38 to elicit and sustain MMA vasodilation suggested that even very modest levels of neuropeptide release and receptor activation may be sufficient to mediate migraine pain. As suggested in previous studies, the physiological and molecular analyses demonstrated differential PAC1/VPAC receptor expression profiles among the cranial vessels examined. The high potency of PACAP38, the expression of PAC1 receptor subtype and isoforms, and the abilities for the antagonist PACAP(6-38) to block PACAP-induced MMA dilation all implicated PAC1 receptor signaling in MMA vasodilation. As MMA vasodilatory dynamics well correlated with migraine onset and abatement in humans, PACAP/PAC1 receptor regulation of the MMA may be a crucial component to the development of migraine attacks. This action of PACAP on MMA contrasts with PACAP/VIP dilation of cerebellar arteries which appeared to be mediated via VPAC1 receptor activation. Together with the human PACAP infusion studies, these observations in sum suggest that PACAP and PAC1 receptor signaling may be a potential therapeutic target for the treatment of migraine.
Acknowledgments
The authors wish to thank Mr. Kevin O'Connor for his assistance and acknowledge the University of Vermont Neuroscience COBRE molecular biology core facility. This work was supported by the Totman Medical Research Trust and National Institutes of Health Grants P01-HL-2095488, P20-RR-16435, and R01-HL-078983.
Footnotes
Disclosures No conflicts of interest, financial or otherwise, are declared by the authors.
Contributor Information
Arsalan U. Syed, Department of Pharmacology, University of Vermont, 89 Beaumont Avenue, Burlington, VT 05405-0068, USA
Masayo Koide, Department of Pharmacology, University of Vermont, 89 Beaumont Avenue, Burlington, VT 05405-0068, USA.
Karen M. Braas, Department of Neurological Sciences, University of Vermont College of Medicine, 149 Beaumont Avenue, Burlington, VT 05405-0068, USA
Victor May, Department of Pharmacology, University of Vermont, 89 Beaumont Avenue, Burlington, VT 05405-0068, USA; Department of Neurological Sciences, University of Vermont College of Medicine, 149 Beaumont Avenue, Burlington, VT 05405-0068, USA.
George C. Wellman, Department of Pharmacology, University of Vermont, 89 Beaumont Avenue, Burlington, VT 05405-0068, USA george.wellman@uvm.edu
References
- Amin FM, Asghar MS, Guo S, et al. Headache and prolonged dilatation of the middle meningeal artery by PACAP38 in healthy volunteers. Cephalalgia. 2012;32:140–149. doi: 10.1177/0333102411431333. [DOI] [PubMed] [Google Scholar]
- Asghar MS, Hansen AE, Amin FM, et al. Evidence for a vascular factor in migraine. Ann Neurol. 2011;69:635–645. doi: 10.1002/ana.22292. [DOI] [PubMed] [Google Scholar]
- Baun M, Hay-Schmidt A, Edvinsson L, Olesen J, Jansen-Olesen I. Pharmacological characterization and expression of VIP and PACAP receptors in isolated cranial arteries of the rat. Eur J Pharmacol. 2011;670:186–194. doi: 10.1016/j.ejphar.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol (Lond) 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni LJ, Ploug KB, Olesen J, Jansen-Olesen I, Gupta S. The in vivo effect of VIP, PACAP-38 and PACAP-27 and mRNA expression of their receptors in rat middle meningeal artery. Cephalalgia. 2009;29:837–847. doi: 10.1111/j.1468-2982.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways. J Biol Chem. 1999;274:27702–27710. doi: 10.1074/jbc.274.39.27702. [DOI] [PubMed] [Google Scholar]
- Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M. Implications of multimechanism therapy: when to treat? Neurology. 2005;64:16–20. doi: 10.1212/wnl.64.10_suppl_2.s16. [DOI] [PubMed] [Google Scholar]
- Collins PY, Patel V, Joestl SS, et al. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csati A, Tajti J, Kuris A, et al. Distribution of vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, nitric oxide synthase, and their receptors in human and rat sphenopalatine ganglion. Neuroscience. 2012;202:158–168. doi: 10.1016/j.neuroscience.2011.10.055. [DOI] [PubMed] [Google Scholar]
- Dejda A, Seaborn T, Bourgault S, et al. PACAP and a novel stable analog protect rat brain from ischemia: insight into the mechanisms of action. Peptides. 2011;32:1207–1216. doi: 10.1016/j.peptides.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Dickinson T, Fleetwood-Walker SM, Mitchell R, Lutz EM. Evidence for roles of vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) receptors in modulating the responses of rat dorsal horn neurons to sensory inputs. Neuropeptides. 1997;31:175–185. doi: 10.1016/s0143-4179(97)90087-1. [DOI] [PubMed] [Google Scholar]
- Dunn WR, Wellman GC, Bevan JA. Enhanced resistance artery sensitivity to agonists under isobaric compared with isometric conditions. Am J Physiol Heart Circ Physiol. 1994;266:H147–H155. doi: 10.1152/ajpheart.1994.266.1.H147. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Hannibal J, Tams J, Georg B. Immunohistochemical localization of the VIP1 receptor (VPAC1R) in rat cerebral blood vessels: relation to PACAP and VIP containing nerves. J Cereb Blood Flow Metab. 2000;20:1205–1214. doi: 10.1097/00004647-200008000-00006. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- Hansen JM, Sitarz J, Birk S, et al. Vasoactive intestinal polypeptide evokes only a minimal headache in healthy volunteers. Cephalalgia. 2006;26:992–1003. doi: 10.1111/j.1468-2982.2006.01149.x. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Fahrenkrug J, Gozes I, et al. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR Review 1. Br J Pharmacol. 2012;166:4–17. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro M, Puryear CB, Bisson E, et al. Enhanced myogenic tone in cerebral arteries from a rabbit model of subarachnoid hemorrhage. Am J Physiol Heart Circ Physiol. 2002;283:H2217–H2225. doi: 10.1152/ajpheart.00629.2002. [DOI] [PubMed] [Google Scholar]
- Johnson PC. The myogenic response, vascular smooth muscle II. In: Bohr DF, Somlyo AP, Sparks HV Jr, editors. Handbook of physiology; Section 2: The cardiovascular system. American Physiological Society; Bethesda: 1980. pp. 409–44. [Google Scholar]
- Lassen LH, Haderslev PA, Jacobsen VB, et al. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- Mayberg M, Langer RS, Zervas NT, Moskowitz MA. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science. 1981;213:228–230. doi: 10.1126/science.6166046. [DOI] [PubMed] [Google Scholar]
- McCulloch J, Uddman R, Kingman TA, Edvinsson L. Calcitonin gene-related peptide: functional role in cerebrovascular regulation. Proc Natl Acad Sci U S A. 1986;83:5731–5735. doi: 10.1073/pnas.83.15.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalicek J, Gordon V, Lambert G. Autoregulation in the middle meningeal artery. J Cereb Blood Flow Metab. 1996;16:507–516. doi: 10.1097/00004647-199605000-00018. [DOI] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Nystoriak MA, Murakami K, Penar PL, Wellman GC. CaV1.2 splice variant with exon 9* is critical for regulation of cerebral artery diameter. Am J Physiol Heart Circ Physiol. 2009;297:H1820–H1828. doi: 10.1152/ajpheart.00326.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystoriak MA, O'Connor KP, Sonkusare SK, et al. Fundamental increase in pressure-dependent constriction of brain parenchymal arterioles from subarachnoid hemorrhage model rats due to membrane depolarization. Am J Physiol Heart Circ Physiol. 2011;300:H803–H812. doi: 10.1152/ajpheart.00760.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- Rahmann A, Wienecke T, Hansen JM, et al. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28:226–236. doi: 10.1111/j.1468-2982.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- Ray BS, Wolff HG. Experimental studies on headache: pain-sensitive structures of the head and their significance in headache. Arch Surg. 1940;41:813–856. [Google Scholar]
- Schytz HW, Birk S, Wienecke T, et al. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain. 2009;132:1–25. doi: 10.1093/brain/awn307. [DOI] [PubMed] [Google Scholar]
- Schytz HW, Olesen J, Ashina M. The PACAP receptor: a novel target for migraine treatment. Neurotherapeutics. 2010;7:191–196. doi: 10.1016/j.nurt.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki Y, Suzuki Y, Baskaya MK, et al. The effects of pituitary adenylate cyclase-activating polypeptide on cerebral arteries and vertebral artery blood flow in anesthetized dogs. Eur J Pharmacol. 1995;275:259–266. doi: 10.1016/0014-2999(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Simons T, Ruskell GL. Distribution and termination of trigeminal nerves to the cerebral arteries in monkeys. J Anat. 1988;159:57–71. [PMC free article] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, et al. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Yamada H, Watanabe M, Yada T. Cytosolic Ca2+ responses to sub-picomolar and nanomolar PACAP in pancreatic beta-cells are mediated by VPAC2 and PAC1 receptors. Regul Pept. 2004;123:147–153. doi: 10.1016/j.regpep.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Zwetsloot CP, Caekebeke JF, Ferrari MD. Lack of asymmetry of middle cerebral artery blood velocity in unilateral migraine. Stroke. 1993;24:1335–1338. doi: 10.1161/01.str.24.9.1335. [DOI] [PubMed] [Google Scholar]