Abstract
Imaging studies indicate smaller orbitofrontal cortex (OFC) volume in mood disorder patients compared with healthy subjects. We sought to determine whether child and adolescent patients with bipolar disorder have smaller OFC volumes than healthy controls. Fourteen children and adolescents meeting DSM-IV criteria for bipolar disorder (six males and eight females with a mean age ± S.D. = 15.5 ± 3.2 years) and 20 healthy controls (11 males and nine females with mean age ± S.D. = 16.9 ± 3.8 years) were studied. Orbitofrontal cortex volume was measured using magnetic resonance imaging. Male bipolar patients had smaller gray matter volumes in medial (p = 0.044), right medial (0.037) and right (p = 0.032) lateral OFC subdivisions compared to male controls. In contrast, female patients had larger gray matter volumes in left (p = 0.03), lateral (p = 0.012), left lateral (p = 0.007), and trends for larger volumes in right lateral and left medial OFC subdivisions compared with female controls. Male patients exhibit smaller gray matter volumes, while female patients exhibit larger volumes in some OFC sub-regions. Gender differences in OFC abnormalities may be involved in illness pathophysiology among young bipolar patients.
Keywords: Orbitofrontal cortex, Adolescents, Bipolar disorder, MRI, Mood disorders
Orbitofrontal cortex (OFC) consists of the ventral-most regions of the prefrontal cortex, extending from the anterior perforated substance, posteriorly, to the frontal pole, anteriorly. The OFC is implicated in such neuropsychological functions as impulse control, stimulus-reward association, mood regulation, and reward-guided behavior [16]. Structural and functional OFC abnormalities have been reported in patients with major depressive disorder (MDD), and these abnormalities could participate in the pathophysiology of mood disorders [7,19,20]. Magnetic resonance imaging studies have shown smaller OFC volumes in patients with MDD compared with healthy controls [4,11,12]. MDD patients have smaller gray matter volumes in right medial and left lateral OFC subdivisions; whereas male, but not female patients exhibit smaller left and right medial OFC volumes compared with healthy controls [11]. Bilateral reductions of OFC volumes are also reported in patients with MDD compared with healthy subjects [4,12]. Also, increased lesion density in OFC white matter was reported in elderly MDD patients [12]. Moreover, functional neuroimaging studies showed abnormally increased OFC regional cerebral blood flow in medicated MDD patients [3,6]. With respect to bipolar disorder, a voxel-based morphometry study [21] demonstrated smaller gray matter OFC volumes in bipolar adolescents compared to healthy controls. Taken together these studies provide several lines of evidence linking OFC abnormalities to the pathophysiology of mood disorders.
No neuroimaging studies utilizing a region-of-interest approach have examined OFC volumes in patients with bipolar disorder. The purpose of this study was to investigate possible structural abnormalities of the OFC and its subdivisions in child and adolescent bipolar disorder patients compared to healthy controls. Based on the previous findings in mood disorder patients, we hypothesized smaller OFC volumes in child and adolescent bipolar individuals.
Fourteen children and adolescents (mean age ± S.D. = 15.5 ± 3.2 years, age range = 10–21 years; six males and eight females; 14 Caucasian; 11 bipolar type I, 2 bipolar type II, 1 bipolar NOS; 12 right handed) diagnosed with DSM-IV bipolar disorder were studied. Twelve patients were euthymic and two were depressed at the time of the study. The patients had mean illness duration of 3.6 years, and the mean age at onset was 11.9 years. Ten patients were receiving treatment for bipolar disorder (six on lithium, one on valproate, and three on lithium and valproate). Twenty healthy controls (mean age ± S.D. = 17 ± 3.9 years, range: 10–21 years, 11 males and nine females; 19 right handed) were recruited. Healthy controls were excluded if they had any DSM-IV axis I disorders, any first degree relatives with a history of psychiatric disorders, or any current serious medical problem. Psychiatric diagnoses were determined through the schedule for affective disorders and schizophrenia for school-age children- present and life version [9], for Children up to 17-years-old, or the structural clinical interview for DSM-IV (American Psychiatric Association, 1994), for subjects of 18-year-old or older. The pubertal development of all subjects was measured using the Petersen pubertal development scale [15]. Written informed consent was obtained from all study participants and their guardians for the subjects younger than 18 years, following complete study description. The study protocol was approved by the University of Pittsburgh Institutional Review Board and conforms with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
MRI scans were acquired using a 1.5T GE Signa Imaging System running version Signa 5.4.3 (General Electric Medical Systems, Milwaukee, WI) software. One hundred and twenty-four images of the whole brain were acquired with a 3D-gradient echo imaging by spoiled gradient recalled acquisition in the coronal plane, with 1.5 mm thick slices. A single trained rater, who achieved intraclass correlation coefficients >0.95, did all anatomical measurements, with subjects’ identity and diagnosis masked, with BRAINS2 software, developed at the University of Iowa Hospitals and Clinics [2]. The OFC and its subdivisions were traced manually in the coronal plane. The tip of the genu of the corpus callosum, as determined in the sagittal plane, was used as the posterior boundary. The OFC tracing ended at the most anterior coronal slice where brain could be identified. The superior border was divided into two parts: for the subgenual regions, the superior boundary was represented by a midpoint on the interhempispheric fissure 5 mm below the intercommissural line; a midpoint on the intercommissural line served as the superior boundary for all slices anterior to the corpus callosum. The geometric method and validation are described elsewhere [10]. Intra-cranial volume was manually traced in the coronal plane and measured by a well trained rater who achieved high inter-rater reliability (intraclass correlation coefficients >0.97). Intra-cranial volume measurements included total cerebral gray and white matter, cerebrospinal fluid, dura matter and sinuses.
Data were analyzed using analysis of covariance (SPSS, Inc., Chicago, IL) with gender and diagnostic group as factors and age and intra-cranial volume as covariates. Groups were compared with respect to demographic (age, ethnicity and handedness) and clinical variables (listed in Table 1), using the Fisher exact test and Mann–Whitney U-test. Association between sub-regional volumes and selected demographic and clinical variables were assessed using Pearson and Spearman rank order correlation methods. Two-sided p-values less than 0.05 were considered statistically significant.
Table 1.
Demographics and clinical features of BP subjects
| BP males (n = 6) | BP females (n = 8) | All BP (n = 14) | |
|---|---|---|---|
| Clinical factors | |||
| Age at onset (years) | 10.7 (5.1) | 12.7 (2.9) | 11.8 (4) |
| Illness duration (years) | 4 (1.6) | 3.4 (2.9) | 3.6 (2.4) |
| Number of episodes (Mean ± S.D.) | 3.2 (2) | 5.1 (2.3) | 4.3 (2.3) |
| BP type I, n (%) | 5 (83.3) | 6 (75) | 11 (78.5) |
| Family psychiatric history | 6 | 7 | 13 |
| Petersen pubertal scale scores, post-pubertal, (Mean ± S.D.) | 3 ± 1 | 4.1 ± .8a | 3.6 ± 1.4 |
Females were significantly more mature than males (p < 0.05).
Bipolar patients and healthy controls did not differ significantly on age, gender or race. Regarding clinical characteristics, no significant differences were observed between males and females, except for pubertal development, where females were more advanced (see Table 1). We first analyzed only the data collected from the subjects less than 19-year-old (bipolars n = 12; controls n = 11) and then repeated the analysis using the entire sample. These two analyses yielded uniformly similar results, and so we report here only the results for the entire sample. The main effect for diagnostic group was not statistically significant for any OFC subregion; however, the group by gender interaction effect was significant for right, left, medial, right medial, lateral and left lateral. Simple effects analysis was used to examine males and females separately following significant group by gender interactions. Female patients showed larger gray matter volumes in left, lateral and left lateral regions compared with female controls, and female patients showed trends for larger volumes in right lateral and medial left regions (see Table 2 and Fig. 1). In contrast, male patients exhibited smaller volumes in medial, right medial and right lateral OFC subdivisions compared to male controls (see Table 2 and Fig. 1). No significant association was found between OFC measurements and treatment status, length of illness, age at onset, current mood state, or family psychiatric history in either male or female patients.
Table 2.
Orbitofrontal cortex gray matter volumes in bipolar disorder patients and healthy subjects
| Gray matter volumes (cm3) (Mean ± S.D.)
| ||||
|---|---|---|---|---|
| Males
|
F | p* | ||
| Patients (n = 6) | Controls (n = 11) | |||
| Region | ||||
| Total OFC | 18.35 ± 4.09 | 19.76 ± 3.14 | 1.44 | 0.24 |
| Right | 9.48 ± 2.62 | 10.46 ± 1.82 | 2.1 | 0.16 |
| Left | 8.99 ± 1.65 | 9.44 ± 1.73 | 0.56 | 0.46 |
| Mediala | 6.62 ± 2.03 | 7.97 ± 0.78 | 4.46 | 0.04 |
| Right medial | 3.43 ± 1.14 | 4.17 ± 0.47 | 4.79 | 0.04 |
| Left medial | 3.65 ± 0.98 | 3.89 ± 0.42 | 0.55 | 0.46 |
| Lateralb | 5.41 ± 0.54 | 6.46 ± 1.52 | 2.89 | 0.10 |
| Right lateral | 2.79 ± 0.55 | 3.42 ± 0.54 | 5.11 | 0.03 |
| Left lateral | 2.62 ± 0.43 | 3.04 ± 1.05 | 0.99 | 0.33 |
| Females
|
||||
| Patients (n = 8) | Controls (n = 9) | |||
|
| ||||
| Region | ||||
| Total OFC | 18.35 ± 3.36 | 14.18 ± 4.33 | 2.84 | 0.10 |
| Right | 9.70 ± 2.01 | 7.76 ± 2.66 | 1.14 | 0.30 |
| Left | 8.82 ± 1.47 | 6.54 ± 1.81 | 5.22 | 0.03 |
| Mediala | 7.73 ± 1.55 | 6.13 ± 1.56 | 3.13 | 0.09 |
| Right medial | 4.18 ± 0.81 | 3.32 ± 0.90 | 2.5 | 0.13 |
| Left medial | 3.56 ± 0.78 | 2.82 ± 0.72 | 3.45 | 0.07 |
| Lateralb | 6.3 ± 1.38 | 4.33 ± 1.24 | 7.2 | 0.01 |
| Right lateral | 3.16 ± 0.69 | 2.4 ± 0.75 | 3.45 | 0.07 |
| Left lateral | 3.13 ± 0.79 | 1.92 ± 0.53 | 8.51 | 0.007 |
The p-values shown are uncorrected for multiple comparisons. Only the difference for left lateral OFC volume in females is significant at the p < 0.05 level after Bonferroni correction. All F-tests were based on 1 and 28 degrees of freedom.
Medial = right medial + left medial.
Lateral = right lateral + left lateral.
Fig. 1.
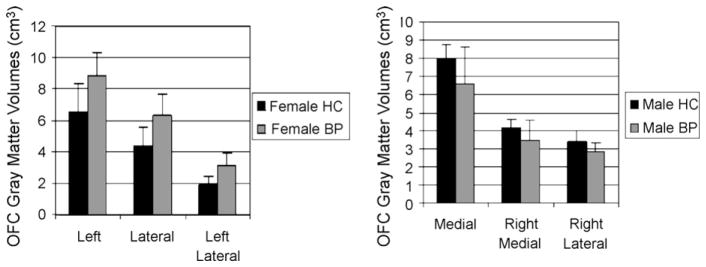
Gray matter volumes (cm3) of selected OFC regions as a function of diagnosis and gender.
Our findings of smaller medial, right medial and right lateral OFC gray matter volumes in male patients compared to male controls, and larger volumes in left, lateral, and left lateral sub-regions of female patients compared to female controls are consistent with previous studies showing different neuroanatomic abnormalities in men and women with different psychiatric diagnoses [5,8,17]. Smaller left and right medial OFC gray matter volumes were reported in adult male MDD patients compared with controls, but no differences were found for female patients [7].
In a previous study using a voxel-based approach [21], adolescent bipolar patients had smaller OFC volumes compared to healthy controls, but the authors did not analyze gender separately; this may explain the discrepancies with our findings. Our results could also be related to human and animal studies indicating potential neuroprotective effects from estrogens [1,13,18], and increased vulnerability to neurotoxic processes caused by testosterone [14]. The larger gray matter volumes we found in female patients may be the result of neuroprotective effects from estrogens, and the smaller gray matter volumes found in our male patients may be caused by brain mechanisms involved in increased vulnerability to bipolar disorder. Nevertheless the neuroprotective effect of estrogens may not explain why the lateral OFC in females with bipolar disorder is larger than that of controls. Perhaps alternative explanations such as pathological hyperactivity in these brain regions leading to volume increases may be worth considering. In summary, our results suggest that gender differences in OFC abnormalities could be involved in illness pathophysiology among young bipolar patients.
Acknowledgments
This work was partly supported by grants MH 01736, MH 069774, MH 55123, MH 30915, MH 59929 and RR02057 from the National Institutes of Health, NARSAD, the Krus Endowed Chair in Psychiatry (The University of Texas Health Science Center at San Antonio) and CAPES Foundation (Brazil).
References
- 1.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen NC, Cohen G, Harris G, Cizadlo T, Parkkinen J, Rezai K, Swayze VW., 2nd Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci. 1992;4:125–133. doi: 10.1176/jnp.4.2.125. [DOI] [PubMed] [Google Scholar]
- 3.Biver F, Goldman S, Delvenne V, Luxen A, De Maertelaer V, Hubain P, Mendlewicz J, Lotstra F. Frontal and parietal metabolic disturbances in unipolar depression. Biol Psychiatry. 1994;36:381–388. doi: 10.1016/0006-3223(94)91213-0. [DOI] [PubMed] [Google Scholar]
- 4.Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Staib LH, Charney DS. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 5.Briellmann RS, Berkovic SF, Jackson GD. Men may be more vulnerable to seizure-associated brain damage. Neurology. 2000;55:1479–1485. doi: 10.1212/wnl.55.10.1479. [DOI] [PubMed] [Google Scholar]
- 6.Cohen RM, Gross M, Nordahl TE, Semple WE, Oren DA, Rosenthal N. Preliminary data on the metabolic brain pattern of patients with winter seasonal affective disorder. Arch Gen Psychiatry. 1992;49:545–552. doi: 10.1001/archpsyc.1992.01820070039006. [DOI] [PubMed] [Google Scholar]
- 7.Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol Psychiatry. 1998;3:220–226. 190–1. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- 8.Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, Leinsinger G, Bottlender R, Hahn K, Moller HJ. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Lacerda AL, Hardan AY, Yorbik O, Keshavan MS. Measurement of the orbitofrontal cortex: a validation study of a new method. NeuroImage. 2003;19:665–673. doi: 10.1016/s1053-8119(03)00137-x. [DOI] [PubMed] [Google Scholar]
- 11.Lacerda AL, Keshavan MS, Hardan AY, Yorbik O, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Soares JC. Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol Psychiatry. 2004;55:353–358. doi: 10.1016/j.biopsych.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Lai T, Payne ME, Byrum CE, Steffens DC, Krishnan KR. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry. 2000;48:971–975. doi: 10.1016/s0006-3223(00)01042-8. [DOI] [PubMed] [Google Scholar]
- 13.Miller DB, Ali SF, O’Callaghan JP, Laws SC. The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann N Y Acad Sci. 1998;844:153–165. [PubMed] [Google Scholar]
- 14.Nishino H, Nakajima K, Kumazaki M, Fukuda A, Muramatsu K, Deshpande SB, Inubushi T, Morikawa S, Borlongan CV, Sanberg PR. Estrogen protects against while testosterone exacerbates vulnerability of the lateral striatal artery to chemical hypoxia by 3-nitropropionic acid. Neurosci Res. 1998;30:303–312. doi: 10.1016/s0168-0102(98)00010-8. [DOI] [PubMed] [Google Scholar]
- 15.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 16.Price JL. Prefrontal cortical networks related to visceral function and mood. Ann N Y Acad Sci. 1999;877:383–396. doi: 10.1111/j.1749-6632.1999.tb09278.x. [DOI] [PubMed] [Google Scholar]
- 17.Roy MA, Maziade M, Labbe A, Merette C. Male gender is associated with deficit schizophrenia: a meta-analysis. Schizophr Res. 2001;47:141–147. doi: 10.1016/s0920-9964(99)00231-5. [DOI] [PubMed] [Google Scholar]
- 18.Singer CA, Rogers KL, Dorsa DM. Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons. Neuroreport. 1998;9:2565–2568. doi: 10.1097/00001756-199808030-00025. [DOI] [PubMed] [Google Scholar]
- 19.Soares JC, Mann JJ. The anatomy of mood disorders—review of structural neuroimaging studies. Biol Psychiatry. 1997;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 20.Steffens DC, Krishnan KR. Structural neuroimaging and mood disorders: recent findings, implications for classification, and future directions. Biol Psychiatry. 1998;43:705–712. doi: 10.1016/s0006-3223(98)00084-5. [DOI] [PubMed] [Google Scholar]
- 21.Wilke M, Kowatch RA, DelBello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Res. 2004;131:57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]


