Abstract
Purpose
Paclitaxel is an effective therapy for patients with solid tumors. While the albumin-bound formulation eliminates the hypersensitivity reaction caused by the Cremaphor solvent, significant peripheral neuropathy persists when given over the standard 30-minute infusion time. We sought to determine if the incidence and severity of peripheral neuropathy could be reduced when the infusion time is lengthened to 2-hours.
Methods
This was an open-label, single-arm, phase 2 study of albumin-bound paclitaxel given over 2-hours. Twenty-five patients with advanced non-small cell lung cancer were enrolled to determine whether the longer infusion reduced the severity of neuropathy compared to data from an earlier cohort of 40 similar patients treated over 30-minutes. Patients received 125 mg/m2 of albumin-bound paclitaxel IV over 2-hours without premedication on days 1, 8, and 15 of a 28-day cycle. Radiologic assessment was performed every 8 weeks.
Results
There was a significant 0.45 grade decrease in average peripheral neuropathy experienced by patients in the 2-hour group versus the 30-minute group (90% CI 0.03–0.87). There was, in addition, a significant decrease in grade ≥ 2 peripheral neuropathy in patients treated over 2-hours versus 30-minutes (28% vs. 55%, 2-sided P = .04). A decrease in grade ≥ 2 neutropenia (20% vs. 48%, 2-sided P = .07) was also observed. The median survival, 11 months, was the same for both groups.
Conclusion
Increasing the infusion time of albumin-bound paclitaxel from 30-minutes to 2-hours resulted in a significant reduction in both average and grade ≥ 2 peripheral neuropathy without affecting survival.
Keywords: albumin-bound paclitaxel, abraxane, neuropathy, non-small cell lung cancer
Introduction
Paclitaxel is used alone and in combination with other chemotherapeutics in the treatment of many solid tumors [12, 14]. The treatment-limiting adverse events associated with paclitaxel are neuropathy, neutropenia, and hypersensitivity reactions. While the predominant peripheral neuropathy associated with paclitaxel is sensory, a distinct motor neuropathy is also recognized [3]. In addition to the inherent neurotoxicity of the active agent [15], the lipid-based Cremophor solvent required to dissolve the compound appears to contribute independently to the development of neuropathy [9].
To reduce both the neurotoxicity and the severe hypersensitivity reactions caused by Cremophor or Tween 80 (the solvent used with docetaxel), an albumin-bound solvent-free version of paclitaxel was developed [6]. This formulation, albumin-bound paclitaxel (Abraxane®), eliminates solvent-associated toxicity. Moreover, because albumin-bound paclitaxel causes no severe hypersensitivity reactions, premedication with steroids or antihistamines is not required.
Albumin-bound paclitaxel is generally used in patients with metastatic breast cancer at 260 mg/m2 over 30-minutes every 3 weeks. When compared to paclitaxel in a randomized phase III trial in patients with metastatic breast cancer, albumin-bound paclitaxel improved time to progression, was associated with a decreased incidence of neutropenia, but appeared to cause an increased incidence of severe neuropathy [4]. Albumin-bound paclitaxel has also been studied in patients with NSCLC. When administered at 260 mg/m2 over 30-minutes once every3 weeks, grade 2 peripheral neuropathy developed in 12% of patients, grade 3 peripheral neuropathy in 5% of patients, grade 4 neutropenia in none [5].
In our phase I/II study of weekly albumin-bound paclitaxel in patients with NSCLC, the maximum tolerated dose (MTD) was determined to be 125 mg/m2 over 30-minutes on days 1, 8, and 15 of a 28-day cycle [13]. In the phase II portion of the study, there was a 30% objective response rate. However, 23% of patients developed grade 3 peripheral neuropathy (combined sensory and motor), which emerged as a treatment limiting toxicity. To date, all published studies with albumin-bound paclitaxel have used a 30-minute infusion [1, 4, 6–7, 10].
Prior clinical trials evaluating the relative contributions of dose and schedule to the development of paclitaxel-induced neuropathy produced mixed results. Comparison of a 24-hour infusion (24h) to a 3-hour infusion (3h) was addressed in three studies. In the NSABP B-26 trial, paclitaxel was given at 250 mg/m2 once every three weeks to 563 patients; neuropathy ≥ grade 3 arose in 13% in 24h compared to 22% in 3h (P = .005) [17]. Eisenhauer et al. evaluated 135 or 175 mg/m2 once every three weeks in 391 patients; neuropathy at any grade arose in 40% in 24h compared to 49% in 3h (P = .84) while grade 3 neuropathy arose in 0.6% in 24h compared to 0.7% in 3h (P = NS) [2]. Peretz et al. described paclitaxel given at 175 mg/m2 or larger doses (per study escalation) once every 3 weeks to 521 patients; neuropathy occurred in 65% in 24h compared to 78% in 3h (P = .001), however the 3h group had more dose escalations and thus received more paclitaxel [11]. Subsequently, two studies compared the 3-hour infusion to an even shorter 1-hour infusion. Mielke et al. treated 92 patients with paclitaxel at 100 mg/m2 weekly for 12 weeks [9]. They used a clinical scoring system (range 0–12) to evaluate peripheral neuropathy and describe the probability of developing a score > 3 at 12 weeks as 47% in 3h compared to 68% in 1h (P = .66). No patients treated with 3h had a score > 6 whereas 3 patients treated with 1h did. Lastly, CALGB 9840 evaluated 572 patients treated with two different regimens: paclitaxel at 175 mg/m2 over 3-hours every 3 weeks or 80 mg/m2 over 1-hour weekly [16]. Grade 2 neuropathy was identical at 21% in the 3h and 1h groups. Grade 3 neuropathy developed in 12% in 3h but in 24% in 1h (P = .0003). Grade 4 neuropathy occurred in no patient in 3h and 1 patient in 1h. Because the two groups differed in dose, in infusion time, and in schedule, it is difficult to know which variable was responsible for the differences in the adverse events reported in this study.
While some of these data demonstrate statistically significant differences and others trends, all five studies comparing longer infusions to shorter infusions agreed that shorter infusions of paclitaxel are associated with increased severity of neuropathy.
Based on these observations and on studies suggesting that peripheral neuropathy may develop from peak plasma levels during infusion [9], we hypothesized that lengthening the infusion time would reduce the incidence and/or severity of peripheral neuropathy. We therefore conducted a single-arm, phase 2 study of weekly albumin-bound paclitaxel at 125mg/m2 over 2-hours in order to assess the incidence and severity of peripheral neuropathy and antitumor efficacy when compared to a cohort treated over 30-minutes. This comparison group of 40 patients was treated on a protocol identical to that of the current study, with the length of albumin-bound paclitaxel infusion the only difference [13].
Patients and Methods
Study Design
The primary study endpoint was to determine whether a 2-hour infusion would reduce the severity of neuropathy compared to a 30-minute infusion. The study was designed so that treatment of 25 patients would allow us to detect an average one half grade difference in peripheral neuropathy with 80% power at a 2-sided P = .10 significance level when compared with patients in our earlier study treated with albumin-bound paclitaxel over 30-minutes, as described above [13].
In addition to the analysis planned by the protocol, we compared the two treatment groups using an adapted Mantel-Haenszel method [20]. This is used for 2-by-c tables when the response variable is ordinal and tests for the tendency for patients in one treatment group to have higher scores than patients in another treatment group. Further, we compared the two treatment groups with respect to the rate of clinically significant toxicities (grade ≥ 2) using Fisher’s exact test.
Patients
We expanded the phase 2 portion of our study to enroll an additional 25 patients who received albumin-bound paclitaxel over 2-hours. Patients with stage IV or recurrent NSCLC who had not received prior chemotherapy for advanced NSCLC were eligible. Patients may have received chemotherapy in the adjuvant or neoadjuvant setting. Patients with peripheral neuropathy grade more than 1 were excluded. All patients who had received any drug were evaluable for efficacy and toxicity. All patients underwent a medical history, physical examination, complete blood count with differential (CBC), and chemistry panel. The CBC and chemistry panel were repeated before each cycle and the CBC was performed before each dose. All patients had a CT scan of the chest every 2-cycles.
The protocol and informed consent documents were approved by the Institutional Review Board at Memorial Sloan-Kettering Cancer Center. All patients signed informed consent prior to enrollment onto this trial.
Data from patients in the comparison 30-minute infusion group, treated on an otherwise identical protocol, were obtained from our prior study of albumin-bound paclitaxel as reported previously.[13]
Drug and Treatment
Albumin-bound paclitaxel (Abraxane®, Abraxis BioScience Inc., Los Angeles, CA) was supplied in 50 mL vials containing 100 mg of paclitaxel and human albumin. Albumin-bound paclitaxel was reconstituted with normal saline and diluted in a PVC infusion bag. The drug was administered over 2-hours intravenously on days 1, 8, and 15 of a 28-day cycle. At the beginning of each cycle, laboratory values were checked with a requirement to meet baseline eligibility criteria. Prior to drug administration on days 8 and 15, an ANC ≥ 1 × 109 cells/L was required. Patients continued on treatment until progressive disease or treatment intolerance.
Dose reductions were allowed by 25 mg/m2 to a minimum dose of 100 mg/m2 for grade 3 or 4 thrombocytopenia or any grade 3 or 4 non-hematologic toxicity. The use of filgrastim or peg-filgrastim was encouraged in patients developing fever during drug-induced neutropenia and as prophylaxis in patients who had developed fever and neutropenia in previous cycles or neutropenia causing a delay in previous treatments.
Toxicity, including neuropathy, was evaluated at the start of each treatment cycle and graded according to NIH CTCAE v3.0.
Results
Patient Characteristics
Between March 2006 and September 2007, 25 patients were treated with 125 mg/m2 of albumin-bound paclitaxel over 2-hours on days 1, 8, and 15 of a 28-day cycle. Table 1 lists the patient characteristics of this group and the earlier 30-minute infusion cohort. There were no significant differences in median age, gender, performance status, smoking history, race, tumor histology, prior chemotherapy, or baseline neuropathy.
Table 1.
Baseline Characteristics of Patients Treated with 125 mg/m2 Albumin-bound Paclitaxel
| Characteristic | 2-Hour Infusion (N=25) | 30-Minute Infusion (N=40) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
|
| ||||
| Median | 66 | 70 | ||
|
| ||||
| Range | 49 – 83 | 43 – 84 | ||
|
| ||||
| Sex | ||||
|
| ||||
| Female | 14 | 56 | 21 | 53 |
|
| ||||
| Male | 11 | 44 | 19 | 48 |
|
| ||||
| Karnofsky Performance Status | ||||
|
| ||||
| 90% – 100% | 5 | 20 | 10 | 25 |
|
| ||||
| 70% – 80% | 20 | 80 | 30 | 75 |
|
| ||||
| Smoking history, pack-years | ||||
|
| ||||
| Never | 0 | 0 | 2 | 5 |
|
| ||||
| 0–15 | 5 | 20 | 7 | 18 |
|
| ||||
| > 15 | 20 | 80 | 31 | 78 |
|
| ||||
| Histology | ||||
|
| ||||
| Squamous | 7 | 28 | 8 | 20 |
|
| ||||
| Adenocarcinoma | 18 | 72 | 32 | 80 |
|
| ||||
| Baseline neuropathy | ||||
|
| ||||
| Grade 0 | 20 | 80 | 30 | 75 |
|
| ||||
| Grade 1 | 5 | 20 | 10 | 25 |
Drug Delivery
The median number of administered doses was 11 (range 2–30) for the 2-hour infusion and 11 (range 1–40) for the 30-minute infusion (Table 2). Six patients in each group required dose reduction for the following toxicities: hematologic (2 in 2-hour, 1 in 30-minute), grade 3 or 4 neuropathy (2 in 2-hour, 3 in 30-minute) and other grade 3 or 4 toxicity (2 in 2-hour, 2 in 30-minute). The outlier patient who received 40 treatments in the 30-minute infusion group required no dose modifications, having experienced only grade 1 sensory neuropathy and grade 1 anemia. The median cumulative dose was 1375 mg/m2 for the 2-hour infusion and 1225 mg/m2 for the 30-minute infusion. No hypersensitivity reactions were reported with either schedule. No premedications were administered.
Table 2.
Albumin-bound Paclitaxel Administration and Responses
| Doses and Responses | 2-Hour Infusion (N=25) | 30-Minute Infusion(N=40) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Number cycles given | ||||
|
| ||||
| Median | 4 | 4 | ||
|
| ||||
| Minimum, maximum | 1, 10 | 1, 14 | ||
|
| ||||
| Number doses given | ||||
|
| ||||
| Median | 11 | 11 | ||
|
| ||||
| Minimum, maximum | 2, 30 | 1, 40 | ||
|
| ||||
| Dose reductions | 6 | 24 | 6 | 15 |
|
| ||||
| Hematological toxicity | 2 | 8 | 1 | 3 |
|
| ||||
| Grade 3 neuropathy | 2 | 8 | 3 | 8 |
|
| ||||
| Number evaluable for response | 25 | 100 | 40 | 100 |
|
| ||||
| Responses | ||||
|
| ||||
| Complete | 0 | 0 | 1 | 3 |
|
| ||||
| Partial | 4 | 16 | 11 | 28 |
|
| ||||
| Median PFS, months | 5.3 | 5.3 | ||
|
| ||||
| 95% CI | 3.4 – 7.3 | 2.3 – 8.9 | ||
|
| ||||
| Median overall survival, months | 11 | 11 | ||
Abbreviations: PFS, progression-free survival; CI, confidence interval
Toxicity
Therapy was stopped in 5 patients (20%) for treatment-related neuropathy in the 2-hour group as compared to 14 patients (36%) in the 30-minute group. The average neuropathy grade (the primary endpoint of the study) was 1.2 for the 2-hour infusion and 1.65 for the 30-minute infusion. This 0.45 grade difference was not significantly different from the pre-specified 0.5 grade endpoint, and reflected a statistically significant decrease in the average grade neuropathy experienced by patients (90% CI: 0.03 to 0.85, P = 0.08).
An evaluation of distinct toxicity grades also revealed a significantly higher proportion of clinically-relevant toxicities (grade ≥ 2) in the 30-minute group as compared to the 2-hour group (Table 3). In the 2-hour group, grade 2 neuropathy occurred in 12% of patients (3/25) and grade 3 neuropathy in 16% of patients (4/25) (combined rate, 28%). In contrast, in the 30-minute group, grade 2 neuropathy occurred in 33% of patients (13/40) and grade 3 neuropathy in 23% of patients (9/40) (combined rate, 55%). One patient in the 30-minute group developed grade 3 motor neuropathy, and one patient in the 2-hour group developed grade 1 motor neuropathy. Both the ordinal test across the four toxicity scores and the exact test comparing the difference in clinically significant toxicities (grade ≥ 2) between the two treatment groups were statistically significant (two-sided P = 0.04). There was a similar, but not significant, trend towards a decrease in grade ≥ 3 neuropathy favoring the 2-hour infusion (16% vs. 23%, P = 0.75)
Table 3.
Toxicities of Albumin-bound paclitaxel by infusion duration and gradea
| Toxicity | 2-Hour Infusion (N=25) | 30-Minute Infusion (N=40) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number (%) | Number (%) | |||||||||
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | |
| Neuropathy | 6 (24) | 12 (48) | 3 (12) | 4 (16) | -- | 5 (13) | 13 (33) | 13 (33) | 9 (23) | -- |
|
| ||||||||||
| Neutropenia | 16 (64) | 3 (12) | 3 (12) | 1 (4) | 2 (8) | 16 (40) | 5 (13) | 11 (28) | 6 (15) | 2 (5) |
|
| ||||||||||
| Leukopenia | 11 (44) | 6 (24) | 5 (20) | 2 (8) | 1 (4) | 15 (38) | 9 (23) | 8 (20) | 8 (20) | -- |
|
| ||||||||||
| Fatigue | 7 (28) | 2 (8) | 8 (32) | 8 (32) | -- | 4 (10) | 14 (35) | 13 (33) | 9 (23) | -- |
|
| ||||||||||
| Diarrhea | 12 (48) | 9 (36) | 3 (12) | 1 (4) | -- | 20 (50) | 11 (28) | 4 (10) | 5 (13) | -- |
|
| ||||||||||
| Myalgia | 17 (68) | 4 (16) | 3 (12) | 1 (4) | -- | 28 (70) | 8 (20) | 4 (10) | -- | -- |
|
| ||||||||||
| Alopecia | 8 (32) | 5 (20) | 12 (48) | -- | -- | 8 (20) | 4 (10) | 28 (70) | -- | -- |
|
| ||||||||||
| Constipation | 15 (60) | 6 (24) | 4 (16) | -- | -- | 16 (40) | 16 (40) | 8 (20) | -- | -- |
|
| ||||||||||
| Anemia | 3 (12) | 18 (72) | 4 (16) | -- | -- | 3 (8) | 25 (63) | 9 (23) | 3 (8) | -- |
|
| ||||||||||
| Edema | 17 (68) | 5 (20) | 3 (12) | -- | -- | 26 (65) | 8 (20) | 6 (15) | -- | -- |
|
| ||||||||||
| Nausea | 11 (44) | 13 (52) | 1 (4) | -- | -- | 20 (50) | 16 (40) | 3 (8) | 1 (3) | -- |
|
| ||||||||||
| Rash | 15 (60) | 9 (36) | 1 (4) | -- | -- | 23 (58) | 15 (38) | 2 (5) | -- | -- |
|
| ||||||||||
| Anorexia | 19 (76) | 5 (20) | 1 (4) | -- | -- | 29 (73) | 7 (18) | 4 (10) | -- | -- |
|
| ||||||||||
| Hypersensitivity | 25 (100) | -- | -- | -- | -- | 40 (100) | -- | -- | -- | -- |
highest drug-related adverse events reported at any time on study
Finally, we noted a trend towards a reduction in neutropenia. Neutropenia grade 2 or greater occurred in 6 (24%) patients in the 2-hour group in contrast to 19 patients (48%) in the 30-minute group (P = .071). Grade ≥ 3 neutropenia showed a similar, though not significant, trend favoring the 2-hour infusion (12% vs. 20%, P = 0.51). Filgrastim or peg-filgrastim was administered to 2 patients in the 30-minute group and to 3 patients in the 2-hour group. In all cases, these agents were administered subsequent to the development of neutropenia. There were no other significant differences between adverse event groups.
Response/Survival
Of the 25 patients in the 2-hour cohort, 4 (16%) experienced a partial response (PR) and none a complete response (CR) (95% CI: 5–36%) (Table 2). This was not significantly different from the 30% response rate (PR+CR) seen in the 30-minute cohort (95% CI: 16–44%).
Concordant with these results, the median progression-free survival (PFS) was 5.3 months for both groups. The median survival was 11 months for the 2-hour infusion and 11 months for the 30-minute infusion (Table 2). The Kaplan-Meier survival curves for the two groups overlapped (Figure 1).
Figure 1.
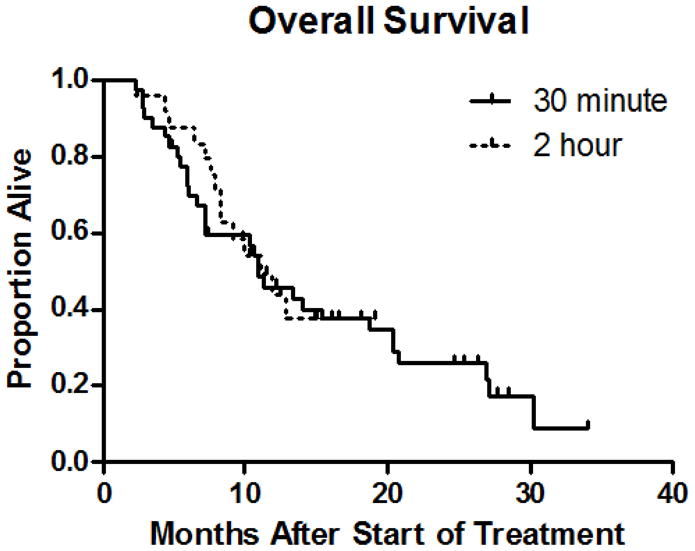
Kaplan-Meier survival curves of patients treated with weekly albumin-bound paclitaxel (Abraxane®) over 2-hours versus 30-minutes.
Discussion
Administering paclitaxel over shorter periods of time (1-hour versus 3-hours, 3-hours versus 24-hours) has generally led to increased neuropathy (see introduction as well as review by Lee and Swain) [8]. Studies of albumin-bound paclitaxel have thus far evaluated only 30-minute infusions, however.
In an attempt to reduce the severity of peripheral neuropathy, we studied a longer infusion schedule. Our findings demonstrate that by prolonging the infusion time from 30-minutes to 2-hours, study participants experienced a statistically significant reduction in the degree and incidence of peripheral neuropathy.
The trial was designed to use, as a primary endpoint, the difference in average grade of neuropathy between the two groups. The 2-hour infusion was associated with a significant reduction in average neuropathy of 0.45 grade points when compared to the 30-minute infusion, meeting the trial’s primary objective. We also noted a 51% decrease (from 56% to 28%) in the frequency of grade ≥ 2 neuropathy favoring the 2-hour group, which was statistically significant. This observation underscores the marked difference in neuropathy seen between the two groups, one that an unweighted average of all toxicities has a tendency to under-represent (e.g. four grade 1 toxicities, when averaged, is not equivalent to one grade 4 toxicity in a cohort of 4 patients).
In addition, we observed a 50% reduction in the frequency of grade ≥ 2 neutropenia when albumin-bound paclitaxel was administered over 2-hours that trended towards, but did not reach, statistical significance. Because filgrastim or peg-filgrastim was administered after the onset of neutropenia, it is unlikely that this impacted the incidence of neutropenia. The identical PFS and median overall survival, along with the overlapping survival curves (Figure 1), suggest that efficacy is unaffected by infusion duration.
The significant reduction in neuropathy coupled to a clinically important improvement in neutropenia is striking in light of previous studies of long versus short infusion times with paclitaxel, in which longer infusion times led to a reduction in peripheral neuropathy countered by an increase in neutropenia [2, 17].
Socinski et. al. recently reported results from a randomized, Phase III study of carboplatin (AUC=6) plus either paclitaxel or albumin-bound paclitaxel (100 mg/m2 over 30-minutes) as first-line therapy in patients with advanced NSCLC [18]. While the overall response rate (ORR) was higher with albumin-bound paclitaxel (33 vs. 25%, P = 0.005), the ORR difference was highest in patients with squamous-cell histology (41 vs. 24%, P < 0.001). Unfortunately, the smaller sample size of this study did not provide sufficient power to allow any meaningful histologic subgroup comparison, as only 15 patients (23%) held diagnoses of squamous-cell carcinoma (squamous vs. non-squamous RR 13% vs. 24%, P = 0.49).
The incidence of grade ≥ 3 neutropenia in the albumin-bound paclitaxel plus carboplatin arm was 44%, which was significantly lower than in the paclitaxel plus carboplatin arm (56%, P = 0.009). The incidence of grade ≥ 3 sensory neuropathy with albumin-bound paclitaxel plus carboplatin was 3%, which is considerably lower than that seen in this study. Part of this may be attributable to the lower dose of albumin-bound paclitaxel used in the Phase III study, as the incidence of grade ≥ 3 peripheral neuropathy in patients treated with carboplatin plus albumin-bound paclitaxel at 125 mg/m2 over 30-minutes in the preceding dose finding study was double that seen with the 100 mg/m2 dose level (16% vs. 8%) [19].
In summary, our data suggest that a change in the infusion time of albumin-bound paclitaxel from 30-minutes to 2-hours may lead to a significant reduction in the frequency and severity of peripheral neuropathy experienced by patients without affecting efficacy. Given the wide range of solid tumor malignancies for which albumin-bound paclitaxel is used, the resulting clinical impact that this alteration could have has the potential to be substantial, and is a maneuver that warrants consideration in patients who experience peripheral neuropathy while being treated with this drug.
Acknowledgments
Grant support: Abraxis BioScience, Los Angeles, CA
Footnotes
Financial disclosures: None
References
- 1.Blum JL, Savin MA, Edelman G, Pippen JE, Robert N, Sandbach J, Carrasco S, O’Shaughnessy JA. Long term disease control in taxane-refractory metastatic breast cancer treated with nab paclitaxel. J Clin Oncol (Meeting Abstracts) 2004;22:543. [Google Scholar]
- 2.Eisenhauer E, ten Bokkel Huinink W, Swenerton K, Gianni L, Myles J, van der Burg M, Kerr I, Vermorken J, Buser K, Colombo N. European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: high-dose versus low-dose and long versus short infusion. J Clin Oncol. 1994;12:2654–2666. doi: 10.1200/JCO.1994.12.12.2654. [DOI] [PubMed] [Google Scholar]
- 3.Freilich R, Balmaceda C, Seidman A. Motor neuropathy due to docetaxel and paclitaxel. Neurology. 1996;47:115–118. doi: 10.1212/wnl.47.1.115. [DOI] [PubMed] [Google Scholar]
- 4.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O’Shaughnessy J. Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared With Polyethylated Castor Oil-Based Paclitaxel in Women With Breast Cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 5.Green MR, Manikhas GM, Orlov S, Afanasyev B, Makhson AM, Bhar P, Hawkins MJ. Abraxane®, a novel Cremophor®-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Annals of Oncology. 2006;17:1263–1268. doi: 10.1093/annonc/mdl104. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim N, Samuels B, Page R, Guthrie G, Hortobagyi G. Nanoparticle paclitaxel (ABI–007) in metastatic breast cancer (MBC): Efficacy and evidence of dose-dependent activity in two multicenter phase II studies. Proc Am Soc Clin Oncol. 2002;21:abstr 209. [Google Scholar]
- 7.Ibrahim NK, Samuels B, Page R, Doval D, Patel KM, Rao SC, Nair MK, Bhar P, Desai N, Hortobagyi GN. Multicenter Phase II Trial of ABI–007, an Albumin-Bound Paclitaxel, in Women With Metastatic Breast Cancer. J Clin Oncol. 2005;23:6019–6026. doi: 10.1200/JCO.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Lee JJ, Swain SM. Peripheral Neuropathy Induced by Microtubule-Stabilizing Agents. J Clin Oncol. 2006;24:1633–1642. doi: 10.1200/JCO.2005.04.0543. [DOI] [PubMed] [Google Scholar]
- 9.Mielke S, Sparreboom A, Steinberg SM, Gelderblom H, Unger C, Behringer D, Mross K. Association of Paclitaxel Pharmacokinetics with the Development of Peripheral Neuropathy in Patients with Advanced Cancer. Clinical Cancer Research. 2005;11:4843–4850. doi: 10.1158/1078-0432.CCR-05-0298. [DOI] [PubMed] [Google Scholar]
- 10.O’Shaughnessy JBJ, Sandbach J. Weekly nanoparticle albumin paclitaxel (Abraxane) results in long-term disease control in patients with taxane-refractory metastatic breast cancer. 27th Annual San Antonio Breast Cancer Symposium; San Antonio, TX. 2004. p. abstr 1070. [Google Scholar]
- 11.Peretz T, Sulkes A, Chollet P, Gelmon K, Paridaens R, Gorbonuva V, Catimel G, Kuhnle H, Huinink WtB, Khayat D, Ditrich C, Klaassen U, Bergh J, Wilking N, Nabholtz JM, Calabresi F, Tubiana-Hulin M, Chazard M, Gallant G, Diergarten K, Westberg R, Bogaert J, Renard J, Weil C. A multicenter, randomized study of two schedules of paclitaxel (PTX) in patients with advanced breast cancer (ABC) European Journal of Cancer. 1995;31:75–75. [Google Scholar]
- 12.Ranson M, Davidson N, Nicolson M, Falk S, Carmichael J, Lopez P, Anderson H, Gustafson N, Jeynes A, Gallant G, Washington T, Thatcher N. Randomized Trial of Paclitaxel Plus Supportive Care Versus Supportive Care for Patients With Advanced Non-Small-Cell Lung Cancer. J Natl Cancer Inst. 2000;92:1074–1080. doi: 10.1093/jnci/92.13.1074. [DOI] [PubMed] [Google Scholar]
- 13.Rizvi NA, Riely GJ, Azzoli CG, Miller VA, Ng KK, Fiore J, Chia G, Brower M, Heelan R, Hawkins MJ, Kris MG. Phase I/II Trial of Weekly Intravenous 130-nm Albumin-Bound Paclitaxel As Initial Chemotherapy in Patients With Stage IV Non-Small-Cell Lung Cancer. J Clin Oncol. 2008;26:639–643. doi: 10.1200/JCO.2007.10.8605. [DOI] [PubMed] [Google Scholar]
- 14.Roszkowski K, Pluzanska A, Krzakowski M, Smith AP, Saigi E, Aasebo U, Parisi A, Pham Tran N, Olivares R, Berille J. A multicenter, randomized, phase III study of docetaxel plus best supportive care versus best supportive care in chemotherapy-naive patients with metastatic or non-resectable localized non-small cell lung cancer (NSCLC) Lung Cancer. 2000;27:145–157. doi: 10.1016/s0169-5002(00)00094-5. [DOI] [PubMed] [Google Scholar]
- 15.Roytta M, Raine S. Taxol induced neuropathy: chronic effects of local injection. J Neurocytol. 1986;15:483–496. doi: 10.1007/BF01611731. [DOI] [PubMed] [Google Scholar]
- 16.Seidman AD, Berry D, Cirrincione C, Harris L, Dressler L, Muss H, Norton L, Winer E, Hudis C. CALGB 9840: Phase III study of weekly (W) paclitaxel (P) via 1-hour(h) infusion versus standard (S) 3h infusion every third week in the treatment of metastatic breast cancer (MBC), with trastuzumab (T) for HER2 positive MBC and randomized for T in HER2 normal MBC. J Clin Oncol (Meeting Abstracts) 2004;22:512. [Google Scholar]
- 17.Smith RE, Brown AM, Mamounas EP, Anderson SJ, Lembersky BC, Atkins JH, Shibata HR, Baez L, DeFusco PA, Davila E, Tipping SJ, Bearden JD, Thirlwell MP. Randomized Trial of 3-Hour Versus 24-Hour Infusion of High-Dose Paclitaxel in Patients With Metastatic or Locally Advanced Breast Cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-26. J Clin Oncol. 1999;17:3403–3411. doi: 10.1200/JCO.1999.17.11.3403. [DOI] [PubMed] [Google Scholar]
- 18.Socinski M, Bondarenko I, Karaseva N, Makhson A, Vynnychenko I, Okamoto I, Hon J. Results of a randomized, phase III trial of nab-paclitaxel (nab-P) and carboplatin (C) compared with cremophor-based paclitaxel (P) and carboplatin as first-line therapy in advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2010;28:abstr LBA7511. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 19.Socinski MA, Manikhas GM, Stroyakovsky DL, Makhson AN, Cheporov SV, Orlov SV, Yablonsky PK, Bhar PH, Iglesias J. A Dose Finding Study of Weekly and Every-3-Week nab-Paclitaxel Followed by Carboplatin as First-Line Therapy in Patients with Advanced Non-small Cell Lung Cancer. Journal of Thoracic Oncology. 2010;5:852–861. doi: 10.1097/JTO.0b013e3181d5e39e. [DOI] [PubMed] [Google Scholar]
- 20.Stokes M, Davis C, Koch G. Categorical Data Analysis Using the SAS System. 2. SAS Institute and John Wiley & Sons, Inc; Cary, NC: 2000. [Google Scholar]


