Abstract
Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis are the three leading bacteria species associated with otitis media. Defining the molecular epidemiology of bacteria known to cause otitis media is of great importance, in both clinical and research settings. PFGE and MLST provide data for the characterization of isolates’ genetic relatedness, yet they differ in the types of studies for which they are most useful. Consequently, knowledge of both techniques is important for laboratories intending to study the molecular epidemiology of otitis media–associated bacterial pathogens.
Keywords: Molecular epidemiology, multilocus sequence typing, pulsed field gel electrophoresis, eBURST, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, otitis media
1. Introduction
The ability to establish the genetic relatedness of bacteria is important in the study of species known to cause otitis media, including Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis. This is crucial in determining the population structure and transmission patterns of each species, as well as for tracking the spread of antibiotic-resistant clones or those particularly adapted to disease. The two most commonly used techniques used to distinguish between isolates of otitis media–associated pathogens are pulsed field gel electrophoresis (PFGE) and multilocus sequence typing (MLST).
MLST involves the sequencing of internal fragments of, on average, seven housekeeping genes distributed around the bacterial chromosome. The first MLST scheme was proposed for the bacterium Neisseria meningitidis by Maiden et al. (1), while a scheme for S. pneumoniae soon followed (2). MLST schemes for H. influenzae (3) and M. catarrhalis (available at http://web.mpiib-berlin.mpg.de/mlst/dbs/Mcatarrhalis) have also been developed. MLST has since become the most frequently used tool for genotypically characterizing a number of bacterial pathogens (4).
Once established, the sequence of each locus is queried against a central, curated database in which each allele is assigned an allelic number. In the event of a sequence not being present in the central database, it can be added by the database’s curator. Once an allelic number has been assigned to each locus, an allelic profile will be determined and a sequence type (ST) allocated. The relationship between STs is ascertained by the number of alleles shared between strains.
PFGE involves the comparison of patterns of bacterial genomic DNA digested with a rare cutting restriction enzyme, such as SmaI (5). Genomic DNA is embedded in agarose plugs to prevent shearing of large DNA molecules. The embedded DNA is then digested with a restriction enzyme that cuts the genomic DNA into a variable number of fragments, depending on the polymorphisms within potential cleavage sites throughout the genome. The digested DNA is then subjected to pulsed field electrophoresis on an agarose gel. Standard gel electrophoresis can separate fragments of DNA up to 30–50 kb in size. Fragments larger than 30–50 kb in size are not effectively separated, because they do not tend to experience a difference in mobility (6). In contrast, PFGE is capable of resolving large DNA molecules of up to 5 Mb in size. Rather than the continuous field used in a standard electrophoresis process, the orientation of the field is repeatedly changed, or pulsed, thus causing the separation of larger fragments of DNA. The more often the direction of the field is altered, the greater the separation between fragments and hence the greater resolution of subtypes (7).
PFGE is often regarded as the gold standard in epidemiology due to its ability to discriminate between very closely related isolates. The technique may be used to compare middle ear and nasopharyngeal isolates from a single patient (8), or to distinguish between isolates obtained from children in the same daycare facility (9). PFGE has been shown to be the most discriminatory method for the comparison of nontypeable H. influenzae isolates (10). However, there are distinct advantages and drawbacks to both MLST and PFGE. MLST offers discrete, unambiguous data that may be easily shared and compared between laboratories across the globe, in contrast to the banding patterns of PFGE that often limit such data to the laboratory in which it was generated. However, PFGE is far cheaper to perform than MLST and provides more discriminatory results, although the cost of sequencing is continually decreasing. Both methods are hampered by the time taken to perform the protocols each requires (11).
MLST and PFGE protocols exist for S. pneumoniae, H. influenzae, and M. catarrhalis, but for the purposes of this chapter the methodology will concentrate on S. pneumoniae (2, 3, 9). The methodology for S. pneumoniae can be adapted for the other two bacteria species. The main differences in protocol involve (1) the media used to grow H. influenzae and M. catarrhalis for DNA extraction or the preparation of PFGE plugs, (2) the specific housekeeping genes that are amplified for MLST, and (3) the specific PCR conditions for MLST.
2. Materials
2.1. Crude DNA Extraction for PCR Amplification of MLST Loci
Trypticase soy agar (TSA) plates, supplemented with 5% (v/v) sheep’s blood.
TE buffer: 10 mM Tris-HCl, pH 7.5, and 1 mM EDTA, pH 7.5. Store at room temperature.
48-well PCR plate and adhesive PCR film.
2.2. PCR Amplification of MLST Housekeeping Loci
48-well PCR plate and adhesive PCR film.
PCR Master Mix.
Seven pairs of PCR primers (Table 11.1), designed by Enright and Spratt (2) to amplify internal fragments of the seven housekeeping genes aroE, gdh, gki, recP, spi, xpt, and ddl, although the primers described here have been adapted to include tails that correlate to the M13F and M13R sequencing primers, as described by Pettigrew et al. (12) (see Note 1).
10X Tris-Borate-EDTA (TBE) buffer, pH 8.3: 0.9 M Trisbase, 0.9 M boric acid, 30 mM EDTA.
Ethidium bromide (10 mg/mL) dissolved in distilled water. Store in a darkened environment.
1 kb DNA ladder.
Table 11.1.
Primers utilized in the multilocus sequence typing of S. pneumoniae
| Locus | Primer | Sequence (5′-3′) |
|---|---|---|
| aroE | aroM13F aroM13R |
TGTAAAACGACGGCCAGTGCCTTTGAGGCGACAGC AGGAAACAGCTATGACCATTGCAGTTCARAAACATWTTCTAA |
| gdh | gdhM13F gdhM13R |
TGTAAAACGACGGCCAGTATGGACAAACCAGCNAGYTT AGGAAACAGCTATGACCATGCTTGAGGTCCCATRCTNCC |
| gki | gkiM13F gkiM13R |
TGTAAAACGACGGCCAGTGGCATTGGAATGGGATCACC AGGAAACAGCTATGACCATTCTCCCGCAGCTGACAC |
| recP | recPM13F recPM13R |
TGTAAAACGACGGCCAGTGCCAACTCAGGTCATCCAGG AGGAAACAGCTATGACCATTGCAACCGTAGCATTGTAAC |
| spi | spiM13F spiM13R |
TGTAAAACGACGGCCAGTTTATTCCTCCTGATTCTGTC AGGAAACAGCTATGACCATGTGATTGGCCAGAAGCGGAA |
| xpt | xptM13F xptM13R |
TGTAAAACGACGGCCAGTTTATTAGAAGAGCGCATCCT AGGAAACAGCTATGACCATAGATCTGCCTCCTTAAATAC |
| ddl | ddlM13F ddlM13R |
TGTAAAACGACGGCCAGTTGCYCAAGTTCCTTATGTGG AGGAAACAGCTATGACCATCACTGGGTRAAACCWGGCAT |
| M13 | M13F M13R |
TGTAAAACGACGGCCAGT AGGAAAGACGTATGACCAT |
Underlined sections represent M13 gene specific primer sequences (see Note 1).
2.3. Purification of PCR Amplicons and DNA Sequence Reactions
Polyethylene glycol (PEG) solution: 20% PEG, 2.5 M NaCl mixture (w/v). Store at room temperature.
70% and 95% ethanol (v/v), diluted with distilled water. Store at 4 °C.
3 M sodium acetate, pH 5.2.
15 mL tubes.
Saran wrap.
2.4. DNA Sequencing Reaction
48-well PCR plate and adhesive PCR film.
BigDye (version 3; PE Applied Biosystems, UK) fluorescent terminators. The BigDye version required may differ depending on the setup of individual sequencing facilities.
Sequencing primers M13F and M13R (Table 11.1).
2.5. PFGE
PET IV buffer for cell suspension: 1 M NaCl, 10 mM Tris, pH 7.5. Store at room temperature.
InCert agarose (Cambrex, Bio Science Rockland Inc., ME, USA).
EC lysis buffer: 6 mM Tris-HCl, pH 7.5, 1 M NaCl, 100 mM EDTA, pH 8.0, 0.5% polyoxyethylene 20 cetylether (Brij 58™), 0.2% deoxycholate, 0.5% N-laurylsarcosyl. Store in aliquots at −20 °C.
ESP buffer: 30 mL 0.5 M EDTA (pH 8.5), 1.5 mL 10% sarkosyl, and 60 mg proteinase K. Store in aliquots at −20 °C.
TE buffer: 10 mM Tris-HCl, pH 7.5, 1 mM EDTA, pH 7.5. Store at room temperature.
SmaI restriction enzyme and appropriate reaction buffers. Store at −20 °C.
10X TBE buffer, pH 8.3: 0.9 M Tris-base, 0.9 M boric acid, and 30 mM EDTA.
Ethidium bromide (10 mg/mL) dissolved in distilled water. Store in a darkened environment.
SeaKem HGT agarose (BioWhittaker Molecular Applications, ME, USA).
Midrange PFG Marker I.
3. Methods
3.1. Multilocus Sequence Typing
3.1.1. Crude DNA Extraction for PCR Amplification of MLST Loci
For many species, a crude lysis of bacterial cells is sufficient for the extraction of DNA for MLST.
Streak the isolate to be studied for single colonies on half of a TSA plate, supplemented with 5% sheep’s blood (v/v). Incubate overnight at 37 °C with 5% carbon dioxide.
Pipette 50 µL of TE into each well of a PCR plate.
Inoculate each well with 1–2 bacterial colonies and seal the plate with adhesive PCR film.
Heat each sample to 100 °C for 10 min in a thermal cycler.
3.1.2. PCR Amplification of MLST Housekeeping Loci
Amplify internal fragments of the housekeeping genes aroE, gdh, gki, recP, spi, xpt, and ddl in separate polymerase chain reactions (PCR). The PCR mixture consists of 2 µL of chromosomal DNA, 1 µL of forward and reverse PCR primers (10 pM; Table 11.1), and 25 µL of PCR Master Mix. Prepare reactions on ice in 48-well PCR plates and seal with adhesive PCR film. Thermally cycle the reactions at 95 °C for 3 min, 35 cycles of 95 °C for 30 sec, 53 °C for 30 sec, and 72 °C for 1 min, followed by a final elongation step of 72 °C for 10 min. The reactions are held in the thermal cycler at 4 °C until their removal.
Prepare a 1% (w/v) agarose gel by mixing 1 g of agarose powder in 100 mL of 0.5X TBE and heat until completely dissolved. Allow the gel to cool until safe to touch and add 5 µL of 10 mg/mL ethidium bromide. Pour the gel, which should set in approximately 20 min.
Dilute 100 mL of 10X TBE with 1.9 L of distilled water for use as running buffer. Fill the gel tank with running buffer.
Once the gel has set, carefully remove the comb(s) and position in the gel tank. Replenish with running buffer, in order to ensure that the gel is submerged.
Load 6 µL of each PCR mixture in a well. Include one well of molecular marker for each comb used in the gel. Secure the gel tank cover in place and connect to a power supply, ensuring that the gel runs from the negative to the positive electrode. Run at 150 V for 30 min. Visualize the gel under UV light using a UVIdoc and UVIPhotoMW software (UVItec Ltd., Cambridge, UK), to ascertain whether the reaction was successful and that the amplicon is of the correct size.
3.1.3. Purification of PCR Amplicons
Add 60 µL of PEG/NaCl mixture to each sample well and reseal (see Note 2). Vortex thoroughly and centrifuge at 200 g for 20 sec. Incubate at room temperature for 30 min.
Centrifuge at 2,465 g for 30 min at 4 °C in order to pellet any DNA precipitate. Remove the excess PEG/NaCl mixture by removing the adhesive PCR seal and inverting the PCR plate onto tissue and centrifuging at 200 g for 20 sec followed by a separate spin for 1 min on fresh tissue (see Note 3).
Add 150 µL of 70% ethanol (v/v) to each well and reseal with the same adhesive PCR seal. Wash the pellet by centrifuging at 2,465 g for 30 min at 4 °C. Remove excess ethanol by removing the adhesive PCR seal and inverting onto tissue for 30 sec. Place on fresh tissue and centrifuge for 1 min at 200 g.
Incubate the open PCR plate at 37 °C for 2 min on a thermal cycler in order to dry the DNA pellet. Pipette 12 µL of sterile distilled water into each well. Reseal the plate with adhesive PCR film and vortex vigorously, before centrifuging for 20 sec at 200 g. Repeat the vortex/centrifuge processes for a total of three times each. Resuspended, purified PCR product may be stored stably at 4 °C for several days, provided that the plate is well sealed. For long-term storage, place the PCR product at −20 °C (see Note 4).
3.1.4. DNA Sequencing Reaction
It may not be necessary to perform the following steps of the MLST protocol, but they have been included for the sake of completeness. The stages to which the protocol needs to be completed will depend on the requirements of the facility or company employed for DNA sequencing. Some companies only require the unpurified amplicon from the PCR reaction mix, while some companies ask for a purified PCR product.
Pipette 2 µL of resuspended, purified PCR amplicon into a new PCR plate. Add 1 µL of either the forward or the reverse (1 pM) sequencing primer and 2 µL of BigDye fluorescent terminators. Prepare a second DNA sequencing reaction for each housekeeping gene of each isolate, utilizing the alternative sequencing primer. Seal the PCR plate with adhesive PCR film and centrifuge for 20 sec at 200 g.
Thermally cycle the reactions for 24 cycles of 95 °C for 10 sec, 50 °C for 5 sec, and 60 °C for 2 min. The DNA sequencing reactions are held in the thermal cycler at 4 °C until their removal.
3.1.5. Purification of DNA Sequencing Reactions
Add 12 µL of sterile distilled water to dilute each sequencing reaction (see Note 5).
Pipette 6 mL of 95% ethanol (v/v) and 240 µL of 3 M sodium acetate into a 15 mL falcon tube. Vortex to mix.
Add 52 µL of the ethanol/sodium acetate mix to each reaction, reseal, and vortex. Centrifuge for 20 sec at 200 g before incubating the plate for 30 min at 4 °C.
Centrifuge the PCR plate(s) for 30 min at 2,465 g in order to precipitate DNA. Remove excess ethanol/sodium acetate mix by inverting the plate onto tissue. Place on fresh tissue and centrifuge at 200 g for 1 min.
Add 150 µL of 70% ethanol (v/v) to each well in order to wash the DNA pellet. Reseal the plate and centrifuge at 2,465 g for 30 min. Remove excess ethanol by inverting the plate onto tissue for 1 min. Transfer to fresh tissue and centrifuge for 1 min at 200 g.
Air dry each PCR plate for 15 min at room temperature, before resealing and storing at 4 °C until ready to be run on an automated DNA sequencer.
3.1.6. DNA Sequence Analysis
Assemble the forward and reverse trace files for each housekeeping gene into individual contigs using a sequence viewing program such as SeqMan II from the DNAStar package (Lasergene, WI, USA). An example of an allele of each housekeeping gene may be obtained from the MLST site, www.mlst.net, and used as a reference to trim each contig to the appropriate length.
Following the generation of sequences, determine the allele numbers by querying the central database at www.mlst.net. Once an allelic number has been obtained for each locus, determine the allelic profile of the isolate and query this against the database at www.mlst.net in order to acquire the ST of the isolate (see Note 6).
Generate a graphical representation of the relatedness of isolates using the program enhanced Based Upon Related Sequence Types (eBURST) (13), available at http://eBURST.mlst.net. For highly recombinogenic species, such as S. pneumoniae, the most stringent settings are often used to define the number of loci isolates required to share in order to belong to the same clonal group.
3.2. Pulsed Field Gel Electrophoresis
3.2.1. Preparation of PFGE Plugs
Streak half of a TSA plate, supplemented with 5% sheep’s blood (v/v), with the isolate to be studied. Incubate overnight at 37 °C with 5% carbon dioxide.
Transfer the entire bacterial growth to 1 mL of PET IV buffer in a 1.5 mL microcentrifuge tube, using a sterile swab (see Note 7).
Centrifuge the bacterial suspension at 18,000 g for 3 min. Remove the supernatant by pipetting. Be careful not to disturb the bacterial pellet.
Add 100 µL of PET IV buffer to each sample and vortex to resuspend the bacterial pellet.
Dissolve 0.4 g of InCert agarose in 50 mL of distilled water by gently mixing and heating for 20 sec intervals until completely dissolved. Allow to cool for 2 min.
Add 110 µL of 0.8% (w/v) InCert agarose to each sample and mix by gently pipetting up and down. Pipette 24 µL of the resulting mixture onto a weighboat and repeat until the entire bacterial agar suspension has been transferred to plugs in the weighboat. Cool at 4 °C for 15 min.
Transfer plugs for each isolate to a 1.5 mL microcentrifuge tube.
Add 0.5 mL of EC buffer to each tube and incubate at 37 °C for at least 2 h.
Remove the EC buffer by pipetting (see Note 8).
Add 0.5 mL of ESP buffer to each sample and incubate overnight at 50 °C.
Remove the ESP buffer by pipetting. Add 1 mL of TE buffer and incubate at 37 °C for 30 min to wash the plugs.
Remove the TE buffer and replace with a fresh 1 mL of TE buffer. Incubate at 37 °C for 30 min. Repeat the process once more. The plugs may be stored at 4°C in this final TE buffer wash.
3.2.2. Restriction Enzyme Digest of Agarose Plug
Transfer one plug to a new 1.5 mL microcentrifuge tube (see Note 9).
Add 100 µL of 1X reaction buffer, specific to the restriction enzyme to be used, SmaI. Incubate at room temperature for 30 min.
Remove the 1X reaction buffer by pipette. Add 63 µL of sterile distilled water, 7 µL of 10X reaction buffer, and 1 µL of SmaI. A master mix for this step may be prepared during the previous 30 min incubation.
Incubate at room temperature for 2 h.
Remove the enzyme and reaction buffer mixture by pipette. Add 100 µL of 0.5X TBE buffer and incubate at room temperature for 15 min.
3.2.3. Preparation of the Pulsed Field Gel
Prepare 1.3% agarose gel (w/v) by mixing 1.3 g of SeaKem HGT agarose in 100 mL of 0.5X TBE and heating until completely dissolved. Allow the gel to cool until the bottle is safe to touch and add 5 µL of 10 mg/mL ethidium bromide. Pour the agarose into a gel tray with a comb in place. The gel should set in ~30 min. This step may be conducted while the plugs are digesting.
Add 2 L of 0.5X TBE to the PFGE chamber. Turn on the pump and cooling module, setting the cooling module to 14 °C. The pump may not immediately function and often requires some attention (see Note 10).
Once the gel has set, carefully remove the comb.
Remove the 0.5X TBE buffer from each sample and melt the plugs one at a time by placing in a heat block, set to 90 °C, for 20 sec.
Pipette the melted plug into one of the empty wells. This step must be achieved promptly to ensure that the plug does not begin to set again. Two wells of the gel must be reserved for loading with a molecular marker, although this does not require melting. Cut a thin slice of molecular marker and transfer to the appropriate well.
Transfer the gel to the centre of the electrophoresis chamber and ensure that the gel is submerged. Replenish the 0.5X TBE buffer if required.
- Set the required parameters in the control panel of the electrophoresis chamber.
- Voltage: 6V/cm
- Included angle: 120°
- Initial switch time: 4 sec
- Final switch time: 16 sec
- Run time: 18 h
Visualize the gel under UV light using UVIdoc and UVIPhotoMW software (UVItec Ltd., Cambridge, UK).
3.2.4. Banding Pattern Comparison
The criteria proposed by Tenover et al. (14) are those most commonly used in the analysis of PFGE banding patterns. Briefly, these criteria classify banding patterns into four separate categories – indistinguishable, closely related, possibly related, and different – depending on the number of differences between banding patterns. Isolates that have identical banding patterns are categorized as indistinguishable, while isolates that differ by 2–3 bands or 4–6 bands are classified as closely and possibly related, respectively. Isolates differing by seven or more bands are classified as different (see Note 11).
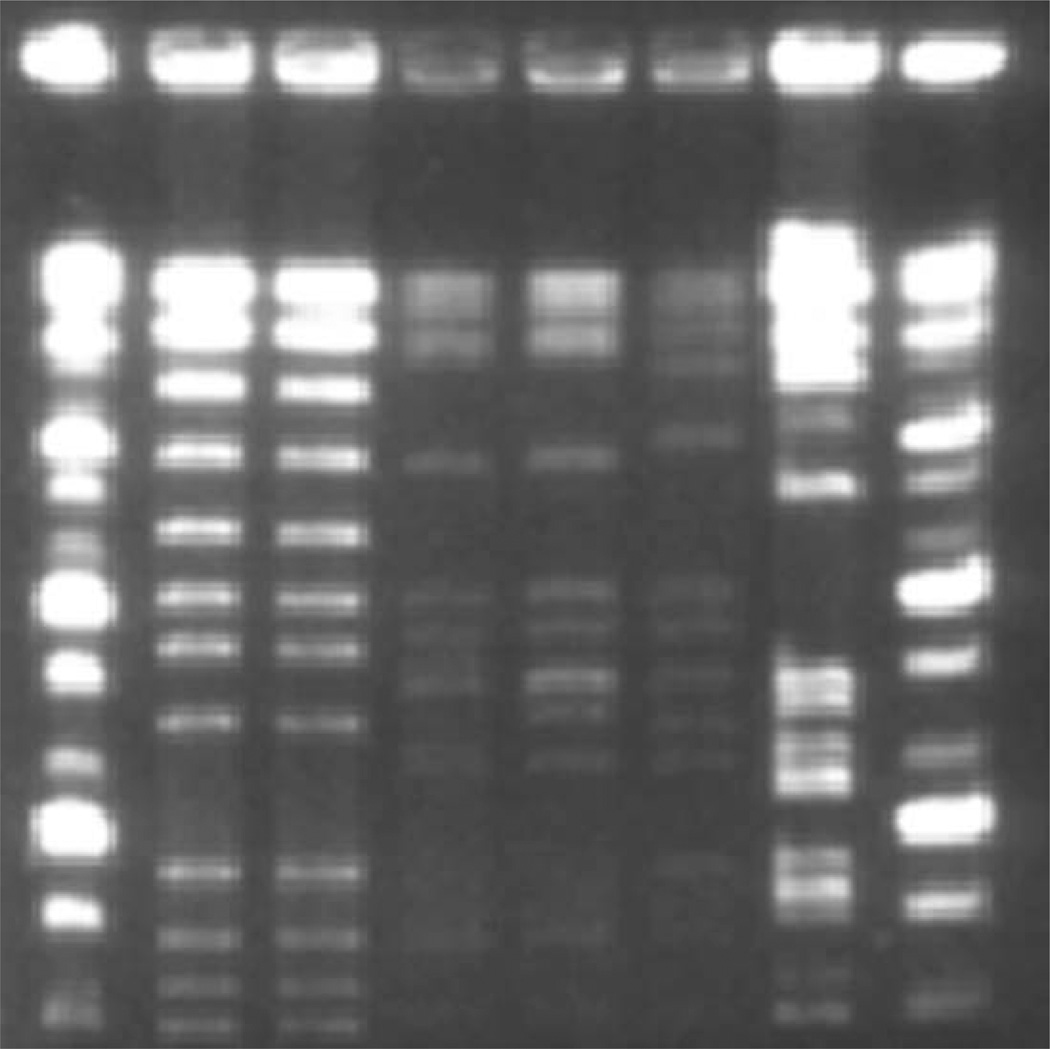
An example of PFGE analysis of S. pneumoniae is shown in Fig. 11.1. Using the Tenover criteria, isolates MP1-MEF and MP1-NP are classified as identical, as are isolates MP2-MEF and MP2-NP. Isolate MP3 is classified as possibly related to isolates MP2-MEF and MP2-NP, while MP4 is classified as different from the other five isolates.
Fig. 11.1.
Pulsed field gel electrophoresis image. Lane 1: Ladder; Lane 2: MP1-MEF; Lane 3: MP1-NP; Lane 4: MP2-MEF; Lane 5: MP2-NP; Lane 6: MP3; Lane 7: MP4; Lane 8: Ladder.
During the course of a large epidemiological or clinical study, the banding patterns of a large number of isolates may need to be compared. However, due to the limitations enforced by the number of lanes available in a PFGE gel, it is not always possible to run all samples on the same gel. Thus, several gels may have to be compared. In order to normalize any differences that may be introduced between gels, software such as Bionumerics Gel-Compare (Applied Maths, Austin, TX) has been developed. Such software utilizes the ladder as a marker, adjusting the banding patterns of each gel to a standard setting so that they might be compared directly.
Acknowledgments
This work was supported by funding awarded to MMP by the National Institute of Allergy and Infectious Diseases (R01 AI068043).
Footnotes
M13F and M13R tails are added to each of the gene-specific primers to increase the ease of sequencing in a 96-well format. Each of the alleles can be sequenced with the same forward and reverse primer regardless of the housekeeping gene being sequenced, without compromising the accuracy of the sequence data.
During the PCR product purification stage and DNA sequencing reaction purification, the same PCR adhesive films may be used after each centrifugation and removal of excess fluid.
The sequencing plates and tissue should be wrapped in saran wrap when being centrifuged to remove the excess PEG/NaCl, in order to prevent the PEG/sodium chloride solution from leaking into the centrifuge, as this may impair the functioning of this equipment.
While it is possible to purify the PCR products/DNA sequencing reactions of four plates at once, the authors have noted a decrease in quality of the sequences obtained when more than three plates are processed at once, while the number of repeats required also tends to increase. For PCRs, a plate is considered to be of 48-wells, while for DNA sequencing reactions a plate is considered to be of 96-wells (or the two 48-well plates for forward and reverse reactions of a given PCR plate). The quality of the purified PCR product may be ascertained by running 3 µL on a 1% agarose gel (w/v) at this stage.
The addition of sterile distilled water at this point dilutes the excess BigDye fluorescent terminators not utilized during the DNA sequencing reaction, thus reducing the number and size of dye blots obtained when the reaction is run on the automated sequencer.
The program Phineus (available at http://www.phineus.org/) has been designed to largely automate this process and has been extensively tested for the MLST schemes of N. meningitidis and S. pneumoniae, but can also be utilized for the analysis of MLST data of other species.
EC and ESP buffers may be removed from the −20 °C freezer at this point, due to the prolonged period of time required for these buffers to thaw.
The agarose plugs will be virtually transparent at this stage and during all subsequent washes. Therefore, a 100 µL pipette should be used, to avoid accidentally damaging any of the agarose plugs in the process. Positioning of the pipette at the very bottom of the tube should improve the chance of the pipette tip avoiding an agarose plug.
A 10 µL inoculating loop is effective at obtaining an agarose plug from the TE buffer.
In order to remove all air bubbles from the system, it is often necessary to reduce the pump speed to nearly zero, before detaching the tubing connecting the pump and chamber from the chamber (ensure that a container is placed in position to collect the TBE buffer that will escape from the chamber at this point). Replenish the TBE buffer level in the pump apparatus by pouring TBE directly into the tubing until nearly full and then reattach to the chamber. Increase the pump speed incrementally. Each step should result in another air bubble being forced into the chamber, and hence being eradicated from the system, until all air bubbles have been removed. The electrophoresis chamber and pump apparatus should be rinsed/flushed regularly with distilled water in order to prevent the build up of salts and excess agarose pieces that may block the pump.
The Tenover criteria were established for use with a small number of isolates (≤30) in an outbreak setting. PFGE data should be used in conjunction with epidemiological and/or clinical data to draw appropriate conclusions regarding the relationship between strains. When typing a much larger number of strains, digital normalization methods should be used.
References
- 1.Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant DA, Feavers IM, Achtman M, Spratt BG. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enright M, Spratt B. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144:3049–3060. doi: 10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- 3.Meats E, Feil EJ, Stringer S, Cody AJ, Goldstein R, Kroll JS, Popovic T, Spratt BG. Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J. Clin. Microbiol. 2003;41:1623–1636. doi: 10.1128/JCM.41.4.1623-1636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feil EJ, Enright MC. Analyses of clonality and the evolution of bacterial pathogens. Curr. Opinion Microbiol. 2004;7:308–313. doi: 10.1016/j.mib.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Lee M, Lee Y, Chiou C. The suitable restriction enzymes for pulsed-field gel electrophoresis analysis of Bordetella pertussis. Diagn. Microbiol. Infect. Dis. 2006;56:217–219. doi: 10.1016/j.diagmicrobio.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz DC, Cantor CR. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984;37:67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- 7.Birren B, Lai E. Rapid pulsed field separation of DNA molecules up to 250 kb. Nucleic Acids Res. 1994;22:5366–5370. doi: 10.1093/nar/22.24.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagan R, Leibovitz G, Cheletz G, Leiberman A, Porat N. Antibiotic treatment in acute otitis media promotes superinfection with resistant Streptococcus pneumoniae carried before initiation of treatment. J. Infect. Dis. 2001;183:800–806. doi: 10.1086/319250. [DOI] [PubMed] [Google Scholar]
- 9.Yano H, Suetake M, Kuga A, Irinoda K, Okamoto R, Kobayashi T, Inoue M. Pulsed-field gel electrophoresis analysis of nasopharyngeal flora in children attending a day care center. J. Clin. Microbiol. 2000;38:625–629. doi: 10.1128/jcm.38.2.625-629.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettigrew MM, Foxman B, Ecevit Z, Marrs CF, Gilsdorf J. Use of pulsed-field gel electrophoresis, enterobacterial repetitive intergenic consensus typing, and automated ribotyping to assess genomic variability among strains of nontypeable Haemophilus influenzae. J. Clin. Microbiol. 2002;40:660–662. doi: 10.1128/JCM.40.2.660-662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malchowa N, Sabat A, Gniadkowski M, Krzyszton-Russjan J, Empel J, Miedzobrodzki J, Kosowska-Shick K, Appelbaum PC, Hryniewicz W. Comparison of multiple-locus variable-number tandem repeat analysis with pulsed-field gel electophoresis, spa typing, and multilocus sequence typing for clonal characterization of Staphylococcus aureus isolates. J. Clin. Microbiol. 2005;43:3095–3100. doi: 10.1128/JCM.43.7.3095-3100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettigrew MM, Fennie KP, York MP, Daniels J, Ghaffar F. Variation in the presence of neuraminidase genes among Streptococcus pneumoniae isolates with identical sequence types. Infect. Immun. 2006;74:3360–3365. doi: 10.1128/IAI.01442-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. eBURST: Inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 2004;186:1518–1530. doi: 10.1128/JB.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]