Background: Receptors activate channels of sensory nerves to cause inflammation and pain by unknown mechanisms.
Results: Protease-activated receptor 2 (PAR2) stimulated transient receptor potential vanilloid 4 (TRPV4) by generation of channel agonists. This required a key TRPV4 tyrosine and induced inflammation.
Conclusion: PAR2 opens TRPV4 by functional coupling.
Significance: Antagonism of PAR2-TRPV4 coupling could alleviate inflammation and pain.
Keywords: G Protein-coupled Receptors (GPCR), Inflammation, Pain, Signal Transduction, TRP Channels
Abstract
G protein-coupled receptors of nociceptive neurons can sensitize transient receptor potential (TRP) ion channels, which amplify neurogenic inflammation and pain. Protease-activated receptor 2 (PAR2), a receptor for inflammatory proteases, is a major mediator of neurogenic inflammation and pain. We investigated the signaling mechanisms by which PAR2 regulates TRPV4 and determined the importance of tyrosine phosphorylation in this process. Human TRPV4 was expressed in HEK293 cells under control of a tetracycline-inducible promoter, allowing controlled and graded channel expression. In cells lacking TRPV4, the PAR2 agonist stimulated a transient increase in [Ca2+]i. TRPV4 expression led to a markedly sustained increase in [Ca2+]i. Removal of extracellular Ca2+ and treatment with the TRPV4 antagonists Ruthenium Red or HC067047 prevented the sustained response. Inhibitors of phospholipase A2 and cytochrome P450 epoxygenase attenuated the sustained response, suggesting that PAR2 generates arachidonic acid-derived lipid mediators, such as 5′,6′-EET, that activate TRPV4. Src inhibitor 1 suppressed PAR2-induced activation of TRPV4, indicating the importance of tyrosine phosphorylation. The TRPV4 tyrosine mutants Y110F, Y805F, and Y110F/Y805F were expressed normally at the cell surface. However, PAR2 was unable to activate TRPV4 with the Y110F mutation. TRPV4 antagonism suppressed PAR2 signaling to primary nociceptive neurons, and TRPV4 deletion attenuated PAR2-stimulated neurogenic inflammation. Thus, PAR2 activation generates a signal that induces sustained activation of TRPV4, which requires a key tyrosine residue (TRPV4-Tyr-110). This mechanism partly mediates the proinflammatory actions of PAR2.
Introduction
Injury and inflammation trigger the activation of proteases from the circulation, immune cells, and epithelial tissues that regulate cells by cleaving protease-activated receptors (PARs)3, members of a family of four G protein-coupled receptors (GPCRs) (1, 2). By cleaving PARs at specific sites within the extracellular N-terminal domains, proteases reveal tethered ligands that bind to and activate the cleaved receptors. Synthetic peptides that mimic the tethered ligand domains (PAR-activating peptides, APs) can directly activate PARs and are useful tools to probe receptor functions. Once activated, PARs regulate multiple pathophysiological processes, including inflammation, pain, hemostasis, and healing.
PAR2 is coexpressed with substance P and calcitonin gene-related peptide by a subpopulation of primary spinal-afferent neurons that control neurogenic inflammation and pain transmission (3, 4). Activation of PAR2 on sensory nerve endings evokes the local release of these neuropeptides, which stimulate extravasation of plasma proteins, infiltration of neutrophils, and vasodilation (neurogenic inflammation). PAR2 activation also promotes the central release of neuropeptides that activate second-order spinal neurons that transmit pain. These mechanisms contribute to painful inflammation of the intestine (5, 6), pancreas (7, 8), and joints (9). Therefore, it is of considerable interest to understand the mechanisms by which PARs regulate the activity of nociceptive neurons.
Members of the transient receptor potential (TRP) family of ion channels, including TRP vanilloids 1 and 4 (TRPV1 and TRPV4) and TRP ankyrin 1 (TRPA1), mediate neurogenic inflammation and pain (10, 11) and are major downstream targets of PAR2 (12–19). Activation of these non-selective cation channels stimulates the influx of extracellular Ca2+ ions and the release of neuropeptides in peripheral tissues and the spinal cord, which induces neurogenic inflammation and pain. During injury and inflammation, several factors are generated that can directly activate these channels (10, 11). Elevated temperatures, protons, and lipid mediators activate TRPV1 (20–22); mechanical shear stress, osmotic stimuli, and lipid mediators activate TRPV4 (23–25); and products of reactive oxygen species and reactive prostaglandin metabolites activate TRPA1 (26, 27). However, indirect mechanisms, particularly those triggered by GPCRs, play a prominent role in TRP channel activation. Many GPCRs that induce neurogenic inflammation and pain indirectly regulate TRP channels, which mediate their proinflammatory and pronociceptive actions. Sensitization, whereby pretreatment with a GPCR agonist amplifies responses to a TRP channel agonist, is a well recognized mechanism of indirect regulation. For example, agonists of PAR2 (12–19), bradykinin-B2 (22, 28), histamine H2 (16, 29), neurokinin NK1 (30) and NK2 (31), prostaglandin E2 (32, 33), prokineticin PKR1 and PKR2 (34), and purinergic P2Y1 and P2Y2 receptors (35, 36) sensitize TRP channels. The mechanisms by which GPCRs sensitize TRPs are not fully understood. However, in a manner that is reminiscent of the process by which rhodopsin sensitizes ancestral TRP channels in the Drosophila eye (37), a major mechanism depends on phospholipase C-mediated cleavage of phosphatidyl inositol 4,5 bisphosphate in the plasma membrane (17, 38, 39). This mechanism relieves tonic TRP inhibition and activates protein kinases A and C, which phosphorylate TRPs and modify channel gating (12, 22, 40, 41).
In addition to sensitization, emerging evidence suggests that GPCR signaling can directly activate TRP channels. Responses of dorsal root ganglion (DRG) neurons to bradykinin and histamine are largely dependent on Ca2+ influx through TRPV1 (28, 42). Products of phospholipase A2 (PLA2) and lipoxygenase can directly activate TRPV1, including N-arachidonoylethanolamide, which is derived from membrane phospholipids, and hydroperoxy or hydroxy eicosatetraenoic acids and leukotriene B4 products of the lipoxygenase-dependent metabolism of arachidonic acid (43). Diacylglycerol directly activates TRPV1 through a kinase-independent mechanism that underlies cellular responses to M3 muscarinic and glutamate mGluR5 agonists (44, 45). 5-Hydroxytryptamine and acetylcholine activate TRPV4 by a mechanism that is dependent on generation of epoxyeicosatrienoic acid (5′,6′-EET and 8′,9′-EET) products of cytochrome P450 epoxygenase-dependent catalysis of arachidonic acid (46–48).
We have identified a new mechanism by which PAR2 activates TRPV4 channels. Although TRPV1, TRPV4, and TRPA1 mediate the pronociceptive actions of PAR2 (12–19), the mechanisms underlying the functional interactions between TRPs and PAR2 are not fully understood. HEK293 cells endogenously express PAR2 (49) and respond robustly to PAR2 stimuli, including trypsin or the tethered ligand mimetic peptide, SLIGRL-NH2, with a rapid, transient increase in intracellular calcium ([Ca2+]i). By modulating the expression of TRP channels, we report the novel finding that TRPV4, but not TRPA1, contributes to the cellular response to PAR2 agonists in HEK cells. This mechanism involves PLA2- and cytochrome P450 epoxygenase-dependent catalysis of arachidonic acid and requires the phosphorylation of a key TRPV4 tyrosine residue (Tyr-110) that has been implicated in sensitization of TRPV4 (50). We propose that the activation of TRPV4 serves to prolong PAR2 signaling and to amplify its proinflammatory and pronociceptive actions. These findings have implications for the consequences of protease-dependent PAR2 activation during injury and inflammation.
EXPERIMENTAL PROCEDURES
Reagents
The following reagents were used: mouse/rat PAR2-AP (SLIGRL-NH2) (CPC Scientific, San Jose, CA); 4α-phorbol didecanoate (4α-PDD) and bisindolylmaleimide I (BIM-1) (Calbiochem, La Jolla, CA); 17-octadecynoic acid (17-ODYA) and methyl arachidonyl fluorophosphonate (MAFP) (Enzo Life Sciences, Waterloo, Australia); HC067047 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); and capsaicin, GSK1016790A, indomethacin, Ruthenium Red, trypsin, Src inhibitor 1, and ATP (Sigma-Aldrich, St. Louis, MO).
Site-directed Mutagenesis
Tyrosines 110, 805, and 110/805 of human TRPV4 were mutated to phenylalanine using the QuikChangeTM site-directed mutagenesis kit (Stratagene, Santa Clara, CA) according to the directions of the manufacturer. The following primer sequences were used: hTRPV4 Y110F, ATGGACTCACTGTTTGACTTTGGCACCTATCGTCACCACT (forward) and AGTGGTGACGATAGGTGCCAAAGTCAAACAGTGAGTCCAT (reverse); hTRPV4 Y805F, GGGCAAGAATGAGACCTACCAGTATTATGGCTTC (forward) and GAAGCCATAATACTGGTAGGTCTCATTCTTGCCC (reverse). Sequences were confirmed by automated DNA sequencing.
Cell Lines
HEK293 cell lines stably expressing human TRPV4 or rat TRPA1 were generated using a tetracycline-inducible system as described (18, 26, 51). Briefly, Flp-InTM T-RexTM HEK293 cells were transfected with pcDNA5/FRT/TO containing human TRPV4 (HEK-TRPV4 cells, TRPV4 untagged or with C-terminal intracellular HA.11 epitope) or rat TRPA1 (HEK-TRPA1 cells) using Lipofectamine 2000 (Invitrogen). Cells were grown in DMEM containing 10% tetracycline-free FBS, hygromycin (50 μg/ml), and blasticidin (5 μg/ml). To induce TRP channel expression, tetracycline (0.001–0.1 μg/ml) was added to the medium 16 h before use. For controls, HEK cells were transfected with an empty vector control, or non-transfected cells were used (HEK control).
Animals
Rats (Sprague-Dawley, 200–250 g, male) and mice (trpv4−/− and trpv4+/+ littermates, 20–30 g, male) (52) were studied. Procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco or the University of Melbourne Animal Experimentation Ethics Committee.
Immunofluorescence
HEK-TRPV4-HA cells (2.5 × 105/35-mm dish) were plated onto poly-D-lysine-coated coverslips (100 μg/ml). Cells were incubated with graded concentrations of tetracycline (0–0.1 μg/ml, 16 h). Cells were washed with PBS (pH 7.4) and fixed (4% paraformaldehyde, 100 mm PBS (pH 7.4), 20 min on ice). Cells were incubated in blocking buffer (1% normal horse serum in PBS with 0.1% saponin, 3 × 10 min) and then incubated with rat anti-hemagglutinin (1:1,000, overnight, 4 °C; clone 3F10, Roche). Cells were washed and incubated with donkey anti-rat IgG coupled to Rhodamine Red-X (1:200, 2 h, room temperature; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Slides were examined using a Zeiss LSM510 Meta confocal microscope (Carl Zeiss Inc., Thornwood, NY). Images were acquired using Zeiss Neofluar ×40 (numerical aperture 1.3) or Fluar Plan Apochromat ×63 (numerical aperture 1.4) objectives.
Measurement of [Ca2+]i in HEK Cells
For population studies, HEK cells were seeded onto 96-well plates (25,000 cells/well) coated with poly-D-lysine (100 μg/ml) and cultured for 48 h. Cells were loaded with Fura2-AM ester (2.5 μm, 30 min, 37 °C) in Hanks' balanced salt solution (HBSS) containing 20 mm HEPES and 0.1% BSA. Fluorescence was measured (4-s intervals) at 340 nm and 380 nm excitation and 510 nm emission wavelengths using a FlexStation 3 plate reader (Molecular Devices, Sunnyvale, CA) as described (53). In some experiments, cells were incubated in Ca2+-free HBSS containing EDTA (20 mm). Results were expressed as the 340/380-nm fluorescence emission ratio, which is proportional to the [Ca2+]i. For measurement of [Ca2+]i in individual cells, HEK cells were studied at 37 °C using a Leica DMI-6000B imaging system (Leica, Germany) with ×10 or ×20 objectives. Images were collected (5 s intervals) at 340 nm and 380 nm excitation and 510 nm emission wavelengths. Images were processed using ImageJ software with McMaster Biophotonics Facility plug-ins (v1.46b) as described (53). Cells were challenged with PAR2-AP (50 μm) or trypsin (300 units/ml). In some experiments, cells were preincubated for 30 min with MAFP (1μm, 10 μm, PLA2 inhibitor), 17-ODYA (50 μm, cytochrome P450 epoxygenase inhibitor), indomethacin (50 μm, cyclooxygenase inhibitor), BIM-1 (100 nm, PKC inhibitor), or Src inhibitor 1 (Src1, 10 μm, Src family kinase inhibitor) 30 min before stimulation. Cells were also assayed in Ca2+-free HBSS containing EDTA (20 mm) or after treatment with Ruthenium Red (10 μm, TRPV antagonist).
Measurement of [Ca2+]i in DRG Neurons
Rats were anesthetized with isoflurane and killed by bilateral thoracotomy. DRG were collected from all spinal levels and cultured as described (13, 53). Neurons were plated onto glass coverslips coated with poly-l-lysine and laminin (100 μg/ml) and cultured for 48–72 h. Neurons were loaded with Fura2-AM ester (2 μm, 30 min, 37 °C) in HBSS, washed, and incubated in HBSS for 30 min before study. Responses of individual neurons to agonists were measured by microscopy as described for HEK cells. Neurons were challenged sequentially with trypsin (300 units/ml), GSK1016790A (10 nm, TRPV4 agonist), capsaicin (100 nm, TRPV1 agonist), and KCl (50 mm). In some experiments cells were pretreated with HC067047 (10 μm, TRPV4 antagonist) 30 min prior to addition of agonists or were assayed in Ca2+-free HBSS.
Analysis of Ca2+ Signals
The transient Ca2+ response induced by PAR2-AP challenge of HEK cells was calculated as the difference between the basal 340/380-nm fluorescence emission ratio (average of four readings immediately prior to application of PAR2-AP) and the maximal fluorescence that was measured 10–20 s after PAR2-AP application. The sustained Ca2+ response was calculated as the difference between the basal fluorescence and the fluorescence measured at 50–60 s after PAR2-AP application. The results were expressed as a ratio of the transient to the sustained response. A diagram illustrating these measurements is presented in Fig. 2B. Representative traces were obtained by averaging values recorded from three different wells with paired vehicle and treated groups. At least three technical repeats were performed for each experiment.
FIGURE 2.
TRPV4 expression amplifies the sustained phase of PAR2 Ca2+ signaling. A, induction of TRPV4 (HEK-TRPV4, 0.1 μg/ml tetracycline) increased the magnitude and duration of the sustained phase of the Ca2+ response to PAR2-AP relative to non-transfected HEK control cells. B, diagram delineating the transient and sustained components of the Ca2+ response to PAR2 activation. C, quantitative analysis demonstrated that the magnitude of the sustained phase (50–60 s post-PAR2-AP) relative to the transient phase (10–20 s post-PAR2-AP) (sustained:transient ratio) of the response was dependent on the tetracycline concentration used for induction (0.001–0.1 μg/ml) and, thus, the level of TRPV4 expression. Tetracycline had no effect on control cells. *, p < 0.05; **, p < 0.01 compared with HEK control cells for each tetracycline condition (Student's t test). n ≥ 3 experiments. D, trypsin induced a sustained phase only in tetracycline (0.1 μg/ml)-induced HEK-TRPV4 cells. E, expression of TRPV4 did not affect the magnitude or duration of the Ca2+ response to ATP compared with HEK control cells. F, TRPA1 expression (HEK-TRPA1, 0.1 μg tetracycline) had no effect on the magnitude or duration of the PAR2-AP response compared with HEK control cells.
For analysis of DRG, Ca2+ responses were included of cells that responded to K+ stimulation with an increase in 340/380-nm fluorescence emission ratio of > 0.1 units above the initial base line. The trypsin-responsive population was subdivided further on the basis of the magnitude of responses to GSK1016790A, with lower and upper quartiles designated as “low” and “high” responders, respectively. At least 640 neurons were analyzed (n = 5 independent cultures) per treatment group.
Cell Surface Biotinylation
Cell surface labeling was performed with EZLink sulfo-NHS-LC-biotin (Pierce) as described in detail (54).
Western Blotting
Proteins were resolved in Criterion 4–15% Tris-glycine gels (Bio-Rad), electroblotted onto nitrocellulose membrane (Protran, Whatman, Rydalmere, Australia), and blocked in 5% skim milk/Tris-buffered saline + 0.05% Tween 20 (TBS-T). Membranes were probed with rabbit anti-TRPV4 antibody (1:1,000 in 5% skim milk/TBS-T overnight, 4 °C; Abcam, Waterloo, Australia) and then washed and incubated with IRDye 800 donkey anti-mouse IgG (1:5,000, 1 h, room temperature; Li-Cor Biosciences, Lincoln, NE). Membranes were washed and analyzed using an Odyssey infrared imager (Li-Cor Biosciences). Signal density was quantified using ImageJ software.
Assessment of Inflammation
Mice were anesthetized with isoflurane (2%), and baseline paw thickness was measured using a digital caliper (Mitutoyo, Aurora, IL). PAR2-AP (50 μg/paw) or 0.9% NaCl (50 μl) were administered by intraplantar injection. The paw thickness was measured from 30–180 min after injection. In some experiments, mice were treated with 17-ODYA (5 mg/kg, 150 μl, intraperitoneal) or vehicle (25% DMSO, 0.9% NaCl, 150 μl, intraperitoneal) 30 min before the intraplantar injections. The paw thickness was normalized to base line (0 min). Mice were killed 6 h after the injection. Paws were collected, snap-frozen in liquid nitrogen, and assessed for tissue myeloperoxidase (MPO) activity as described (55). MPO was solubilized with hexadecyltrimethylammonium bromide, and MPO activity was measured with a dianisidine-H2O2 assay. Changes in absorbance at 450 nm over a 15-min period were determined using a microplate reader (Molecular Devices). Data were expressed as MPO activity relative to total protein (units/mg) and normalized to controls.
Statistical Analysis
Results were expressed as the mean ± S.E. and were compared by Student's or one-sample t test (one-tailed) or one-way analysis of variance and Newman-Keuls test, as indicated, using GraphPad Prism (v5.0). Differences were considered significant when p < 0.05.
RESULTS
PAR2 Couples to TRPV4, Which Mediates Influx of Extracellular Ca2+ Ions
We confirmed that Flp-InTM T-RexTM HEK293 cells express endogenous PAR2 by examining the effects of graded concentrations of PAR2-AP (SLIGRL-NH2) on [Ca2+]i. PAR2-AP caused a transient and concentration-dependent increase in [Ca2+]i with a pEC50 (negative logarithm of the EC50) of 4.67 ± 0.15 and a maximal response to 100 μm (Fig. 1, A and B).
FIGURE 1.
Expression and function of PAR2 and TRPV4 in HEK cell lines. A, PAR2-AP stimulated a concentration-dependent increase in [Ca2+]i in HEK-TRPV4 cells that were not treated with tetracycline. B, the peak increase in [Ca2+]i to PAR2-AP was concentration-dependent. C, the level of expression of functional TRPV4 was dependent on the tetracycline concentration (0–0.1 μg/ml) used for induction, as demonstrated by elevated [Ca2+]i in response to stimulation with 4α-PDD (100 nm). A–C, n = 3 experiments. D, TRPV4-HA expression at the plasma membrane of HEK-TRPV4 cells was dependent on the concentration of tetracycline (0.001–0.1 μg/ml) that was used for induction, as demonstrated by immunofluorescence using HA.11 antibody (red). Nuclei are indicated by DAPI staining (cyan). Scale bar = 5 μm.
To examine the effect of TRPV4 expression on PAR2-evoked Ca2+ signaling, we generated HEK cell lines expressing TRPV4 with or without an HA tag. TRPV4 was expressed under control of a tetracycline-inducible promoter, which enabled controlled expression of the channel. The expression of TRPV4 was examined by measuring changes in [Ca2+]i in response to the TRPV4 activator 4α-PDD and by immunofluorescence and confocal microscopy using an antibody to the HA.11 epitope. In HEK-TRPV4 cells not exposed to tetracycline, 4α-PDD had no effect on [Ca2+]i (Fig. 1C), and immunoreactive TRPV4 was undetectable (D). Incubation of HEK-TRPV4 cells with graded concentrations of tetracycline (0.0001–0.1 μg/ml, 16 h) induced graded expression of functional and immunoreactive TRPV4. In tetracycline-treated cells, 4α-PDD elicited a gradual increase in [Ca2+]i that was sustained for the period of observation (200 s) and was graded with the concentration of tetracycline (Fig. 1C). Responses to 4α-PDD were detected after incubation with 0.001 μg/ml tetracycline and were maximal after 0.1 μg/ml tetracycline. The basal [Ca2+]i was also elevated by the highest expression levels of TRPV4 (0.01, 0.1 μg/ml), although the cells were microscopically normal and remained responsive to TRPV4 and PAR2 agonists. Immunoreactive TRPV4 was detected in some cells after incubation with 0.001 μg/ml tetracycline and present at the plasma membrane of all cells after incubation with 0.1 μg/ml tetracycline (Fig. 1D).
In HEK control cells (expressing the empty vector without a TRPV4 insert), PAR2-AP (50 μm) evoked a transient increase in [Ca2+]i that was maximal after 10–20 s and that declined to base line after ∼75 s of stimulation (Fig. 2A). In tetracycline-treated (0.1 μg/ml, 16 h) HEK-TRPV4 cells, PAR2-AP evoked a similar rapid increase in [Ca2+]i that was markedly sustained (Fig. 2A). The sustained response was quantified by determining the ratio of the [Ca2+]i at the maximal point of the transient phase (10–20 s) and during the sustained phase (50–60 s) (Fig. 2B). This analysis revealed that the magnitude of the sustained phase was proportional to the concentration of tetracycline and, thus, the level of TRPV4 expression (Fig. 2C). TRPV4 expression similarly enhanced the sustained increase in [Ca2+]i to trypsin (300 units/ml), a physiologically relevant PAR2 agonist (Fig. 2D).
To determine whether TRPV4 similarly affects signaling by other GPCRs, we examined the consequences of TRPV4 expression on responses to ATP, which mobilizes Ca2+ in HEK293 cells by activating P2Y receptors (56). ATP (1 μm) evoked a rapid and transient increase in [Ca2+]i in HEK control cells (Fig. 2E). The magnitude and duration of the ATP-evoked Ca2+ response were unaffected by TRPV4 expression (p = 0.6 and p = 0.7 to control cells, respectively). Thus, not all GPCRs can activate TRPV4.
To determine whether PAR2 can activate other TRP channels, which would represent a more general mechanism of TRP regulation, we examined the effect of expression of TRPA1 on the responses to PAR2-AP. TRPA1 was selected because it is coexpressed with PAR2 by nociceptive neurons and because pretreatment with PAR2 agonists amplifies responses to TRPA1 agonists, which is indicative of channel sensitization. TRPA1 was expressed in HEK cells using a tetracycline-inducible promoter to allow for regulated expression. Tetracycline-induced expression of TRPA1 was confirmed by responsiveness to allyl-isothiocyanate or cinnamaldehyde (data not shown). In contrast to TRPV4, TRPA1 expression did not affect the amplitude or duration of the response to PAR2-AP relative to identically treated control cells (Fig. 2F). Thus, TRPA1 does not contribute to PAR2-evoked Ca2+ signaling. Addition of the HA epitope tag did not affect PAR2 or TRPV4 responses to activating stimuli (data not shown). However, all subsequent experiments used untagged TRPV4.
To assess the cell-to-cell variability of PAR2-induced activation of TRPV4, we examined responses of individual HEK-TRPV4 cells using microscopy. In HEK-control cells, PAR2-AP caused a transient increase in [Ca2+]i, with no sustained phase (Fig. 3, A, C, and E). In HEK-TRPV4 cells incubated with tetracycline (0.1 μg/ml, 16 h), most cells exhibited a sustained plateau of [Ca2+]i after treatment with PAR2-AP (Fig. 3, B, D, and E). These results indicate that activation of PAR2 results in a transient increase in [Ca2+]i and that a more sustained response requires coexpression of TRPV4.
FIGURE 3.
TRPV4 expression amplifies the sustained phase of PAR2 Ca2+ signaling in a subset of cells. A and B, ratiometric images showing non-transfected cells (HEK control, A) and TRPV4-expressing cells (HEK-TRPV4, 0.1 μg tetracycline, B) before (15 s) and after (30 s, 75 s) application of PAR2-AP (50 μm). Images taken at 30 s represent the maximal transient phase of the response to PAR2-AP and at 75 s represent the sustained phase. A major subset of cells remains activated at 75 s in the HEK-TRPV4 population. C and D, individual traces from 50 randomly selected cells show responses from HEK-control (C) and HEK-TRPV4 (D) cells when stimulated with PAR2-AP. E, mean data showing the augmentation of the sustained phase of the PAR2 response in TRPV4-expressing cells.
Our results suggest that PAR2 can activate TRPV4, possibly by generating endogenous TRPV4 agonists or by activating signaling pathways that alter channel gating or localization. We refer to this activation as PAR2 “coupling” to TRPV4. The mechanism of this coupling is not related to the rapid mobilization of intracellular Ca2+ per se because ATP stimulation of P2Y receptors did not activate TRPV4 despite eliciting a substantial release of intracellular Ca2+ ions.
PAR2 Stimulates an Influx of Extracellular Ca2+ through TRPV4
To determine the source of the sustained TRPV4-dependent Ca2+ response and to further assess the involvement of TRPV4, we either removed extracellular Ca2+ ions or treated cells with the non-selective TRPV inhibitor Ruthenium Red. Omission of extracellular Ca2+ abolished the sustained phase of the PAR2-AP response of tetracycline-induced HEK-TRPV4 cells (Fig. 4, A and B; p = 0.002). Ruthenium Red (10 μm) also abolished the sustained phase of the PAR2-AP response (Fig. 4, C and D). However, removal of extracellular Ca2+ or addition of Ruthenium Red had no effect on the transient phase of the response to PAR2-AP. These results suggest that PAR2 couples to TRPV4, which mediates an influx of extracellular Ca2+ ions that comprise the sustained phase of the PAR2 response.
FIGURE 4.
The duration of the PAR2 [Ca2+]i response depends on influx of extracellular Ca2+ ions through TRPV4. A, in HEK-TRPV4 cells (0.1 μg/ml tetracycline), removal of extracellular Ca2+ attenuated the sustained phase of the Ca2+ response to PAR2-AP compared with the control with extracellular Ca2+ (representative trace). B, quantitative analysis demonstrated that removal of extracellular Ca2+ significantly inhibited the sustained phase of the response for all levels of TRPV4 expression. **, p < 0.01; ***, p < 0.001 compared with the vehicle control for each tetracycline condition (Student's t test), n ≥ 3 experiments. C, in HEK-TRPV4 cells (0.1 μg/ml tetracycline), the non-selective TRPV antagonist Ruthenium Red (RR) attenuated the sustained phase of the Ca2+ response to PAR2-AP compared with the vehicle control (representative trace). D, quantitative analysis demonstrated that Ruthenium Red significantly inhibited the sustained phase of the response for all levels of TRPV4 expression. *, p < 0.05 compared with vehicle control for each tetracycline condition (Student's t test), n ≥ 3 experiments.
PLA2 and Cytochrome P450 Epoxygenase Contribute to PAR2-induced Activation of TRPV4
PAR2 couples to PLA2, which generates arachidonic acid (57), a substrate for synthesis of endogenous TRPV4 activators including 5′,6′-EET (25). We used a pharmacological approach to determine the contribution of PLA2 and downstream enzymes to PAR2-dependent activation of TRPV4. The irreversible PLA2 inhibitor MAFP (1, 10 μm) inhibited both the transient and sustained phases of the response to PAR2-AP (Fig. 5, A and F), but lower concentrations of MFAP had no effect (data not shown). Thus, we could not ascribe a selective effect to inhibition of PLA2 by this compound. MAFP also slightly inhibited TRPV4 activation by the synthetic agonist GSK1016790A (100 nm, Fig. 5A, p = 0.045). The cytochrome P450 epoxygenase inhibitor 17-ODYA (50 μm) inhibited the sustained phase of the response to PAR2-AP without reducing the transient phase of the response (Fig. 5, B and F; p = 0.0001). 17-ODYA had a minor inhibitory effect on the response to the TRPV4 agonist 4αPDD (100 nm), indicating that TRPV4 activity was mostly retained (Fig. 5B). Inhibition of cyclooxygenase by indomethacin (50 μm) did not alter the responses to PAR2-AP (p = 0.27; Fig. 5, C and F). Thus, PAR2 coupling to TRPV4 appears to involve activation of cytochrome P450 epoxygenase, which can generate arachidonic acid-derived TRPV4 activators such as 5′,6′-EET.
FIGURE 5.
PLA2, cytochrome P450 epoxygenase, and Src kinase-dependent mechanisms mediate PAR2 activation of TRPV4. The effects of inhibitors on PAR2-AP-evoked changes in [Ca2+]i in HEK-TRPV4 cells (0.1 μg/ml tetracycline). A, the PLA2 inhibitor MAFP inhibited the transient and sustained phases of the Ca2+ response to PAR2-AP and also had a small inhibitory effect on the response to GSK1016790A. B, the cytochrome P450 epoxygenase inhibitor 17-ODYA inhibited the sustained phase of the Ca2+ response to PAR2-AP and also had a small inhibitory effect on the response to 4α-PDD. C and D, the cyclooxygenase 1/2 inhibitor indomethacin (C) and PKC inhibitor BIM-1 (D) did not reduce the sustained phase of the Ca2+ response to PAR2-AP. E, the Src family inhibitor Src 1 did not affect the transient response to PAR2-AP but reduced the sustained phase in TRPV4 cells compared with vehicle. F, the summarized data demonstrate the effects of inhibitors on the sustained phase by comparing the sustained:transient ratio for inhibitors and matching vehicle treatments. “Control” sustained:transient data compare HEK control and TRPV4-HEK cells (0.1 μg/ml tetracycline) from Fig. 2A. *, p < 0.05; **, p < 0.01; ***, p < 0.001 relative to vehicle; Student's t test; n ≥ 3 experiments.
Certain GPCRs, including PAR2, can sensitize TRP channels through activation of second messenger kinases, including PKCϵ, which can phosphorylate TRPs and regulate channel gating (12, 13, 16–18). Therefore, we examined the effects of kinase inhibitors on PAR2 coupling to TRPV4. The PKC inhibitor BIM-1 (100 nm) had no effect on the PAR2-AP responses (Fig. 5, D and F). However, the inhibitor of Src-family kinases, Src inhibitor 1 (Src1, 10 μm), significantly reduced the sustained phase of the PAR2-AP [Ca2+]i response without affecting the transient phase of the response (Fig. 5, E and F; p = 0.0028). These data suggest the involvement of tyrosine kinase activity in the TRPV4-dependent sustained phase of the PAR2 response.
Tyrosine 110 Is Required for TRPV4 Activation
To identify the putative sites of tyrosine phosphorylation, we mutated tyrosine residues within the N and C termini of TRPV4 (Y110F, Y805F, and Y110/805F). Cell surface biotinylation and Western blotting for TRPV4 indicated that wild-type TRPV4 and all TRPV4 mutants were expressed at similar levels at the cell surface (Fig. 6A). PAR2-AP induced a rapid and transient increase in [Ca2+]i that was similar in cells expressing wild-type and mutant TRPV4 channels (Fig. 6B). However, the sustained phase of the response was markedly attenuated in cells expressing TRPV4-Y110F (Fig. 6, B and C; p < 0.0001 compared with the wild type). The sustained response to PAR2-AP of cells expressing TRPV4-Y805F was only slightly reduced (p = 0.03 compared with the wild type), whereas the response of cells expressing the double mutant TRPV4-Y110/805F resembled that of cells expressing the single mutant TRPV4-Y110F (Fig. 6, B and C; p = 0.001 compared with the wild type). Responses to the TRPV4 agonist GSK1016790A were unaffected by TRPV4 mutations (data not shown).
FIGURE 6.

PAR2-dependent activation of TRPV4 requires key tyrosine residues. A, TRPV4 WT and the TRPV4 Y110F, Y805F, and Y110F/Y805F mutants were expressed at similar levels at the cell surface of HEK cells and detected by cell surface biotinylation and TRPV4 Western blotting. B and C, the sustained phase of the Ca2+ response to PAR2-AP was significantly reduced by mutation of the phosphorylation sites Tyr-110 and Tyr-805. A marked reduction in [Ca2+]i for Y110F and the double mutant (Y110F and Y805F) was evident at 60 s post-PAR2-AP treatment (p < 0.0001) compared with the Y805F single TRPV4 mutant (expressed as percentage relative to the wild-type control, p = 0.03 (C)). *, p < 0.05; ***, p < 0.0001 relative to the wild type; one sampled t test; n = 9 experiments.
These data suggest an involvement of tyrosine kinases in the generation of the sustained phase of the PAR2 response, presumably through modulation of TRPV4 by phosphorylation of Tyr-110, which we have identified as a key residue required for the TRPV4-dependent response to PAR2 activation.
TRP Channels Regulate PAR2-dependent Ca2+ Signaling in DRG Neurons
To determine whether PAR2 couples to TRPV4 in cells that naturally express these proteins, we examined responses of DRG neurons from rats to agonists of PAR2 and TRP channels. DRG were first challenged with trypsin (300 units/ml), which was selected as a physiological agonist of PAR2 that gave more robust responses than PAR2-AP (data not shown). DRG cultures were then challenged sequentially with the TRPV4 agonist GSK1016790A (10 nm) and the TRPV1 agonist capsaicin (100 nm). Cells were finally exposed to K+ (50 mm), which depolarizes neurons. Only those cells that responded to K+ and that were neurons were analyzed. Trypsin stimulated a rapid increase in [Ca2+]i in neurons, with a peak within 5–15 s that gradually returned to prestimulation levels after 120 s (Fig. 7A). Approximately half of all PAR2-responsive neurons also responded to GSK1016790A (52.78 ± 12.69%, 649 neurons, n = 5 experiments) with a prompt and sustained elevation in [Ca2+]i, although the magnitude of those responses was variable. Capsaicin elicited further responses in many of the neurons, which were generally sustained. Not every neuron that responded to GSK1016790A also responded to capsaicin and vice versa. These results indicate that PAR2 and TRPV4 are coexpressed by > 50% of DRG neurons.
FIGURE 7.
PAR2 activates TRPV4 in DRG neurons. A, Ca2+ responses of 50 randomly selected rat DRG neurons (gray traces, individual neurons; black trace, mean response of 50 neurons) to sequential stimulation with trypsin (25 s, PAR2 agonist) (i), GSK1016790A (150 s, TRPV4 agonist) (ii), capsaicin (270 s, TRPV1 agonist) (iii), and KCl (50 mm, 380 s, positive selection for neurons) (iv). B, omission of extracellular Ca2+ ions reduced the baseline [Ca2+]i and lowered the transient and sustained phases of the response to PAR2-AP. The TRPV4 antagonist HC067047 significantly reduced the magnitude of the transient phase (0.440 ± 0.012 to 0.391 ± 0.017; p = 0.047) but not the sustained phase (0.379 ± 0.020 to 0.357 ± 0.012; p = 0.355) of the PAR2-AP response. N ≥ 3 experiments. C and D, the magnitude of the response to GSK1016790A was used to assess TRPV4 expression and activity. Neurons were segregated into upper and lower quartiles of GSK1016790A responsiveness. Analysis of the area under the curve, measured up to 125 s post-PAR2-AP, indicated that neurons in the upper quartile exhibited larger transient and sustained responses to PAR2-AP compared with neurons in the lower quartile (maximal transient responses at 30 s post-PAR2-AP: upper quartile, 0.137 ± 0.036; lower quartile, 0.067 ± 0.014; p = 0.024; Student's t test). Neurons were similarly segregated into upper and lower quartiles of capsaicin (100 nm) responsiveness to assess TRPV1 expression and activity (D). There was no difference in the PAR2-AP response between neurons in the upper and lower quartiles of capsaicin responsiveness (p = 0.346). *, p < 0.05; Student's t test; n = 5 experiments.
The omission of extracellular Ca2+ ions reduced the basal [Ca2+]i and blunted the peak and sustained phase of the Ca2+ response to trypsin (Fig. 7B). The TRPV4 antagonist HC067047 (10 μm) did not affect the basal [Ca2+]i but markedly attenuated the peak and sustained Ca2+ response to trypsin (Fig. 7B).
The variability in responsiveness to GSK1016790A probably reflects variable levels of TRPV4 expression in different neurons. Therefore, we sought to determine whether the magnitude of the PAR2 responses correlated with the magnitude of the responses to GSK1016790A. To do so, we compared the Ca2+ response to trypsin, which was quantified as the area under the curve measured up to 125 s after challenge with trypsin, for those neurons in the upper and lower quartiles of the GSK1016790A responses (Fig. 7C). The PAR2 responses of the neurons in the upper quartile of GSK1016790A responsiveness were ∼2-fold greater than the responses of the lower quartile (Fig. 7D, p = 0.002). This finding is consistent with a role for TRPV4 in mediating the Ca2+ responses of the neurons to PAR2 activation. In contrast, when neuronal populations were similarly subdivided on the basis of their responsiveness to TRPV1 activation with capsaicin, there was no difference between the responsiveness of the upper and lower quartiles (Fig. 7D, p = 0.06). Our results indicate that TRPV4 largely mediates the PAR2-induced Ca2+ signals in DRG neurons.
TRPV4 Mediates PAR2-evoked Inflammation
PAR2 agonists evoke neurogenic inflammation that depends on the local release of neuropeptides from primary spinal afferent neurons (3, 5). To investigate the functional relevance of PAR2 coupling to TRPV4 in the intact animal, we assessed the effects of PAR2-AP on peripheral inflammation in trpv4+/+ and trpv4−/− mice. Intraplantar injection of PAR2-AP into trpv4+/+ mice resulted in an increase in paw thickness that was maximal within 30 min and maintained for 180 min, indicative of tissue edema and consistent with previous reports (Fig. 8A). The magnitude of PAR2-induced paw edema at early time points was reduced in trpv4−/− mice compared with trpv4+/+ mice (p = 0.006 at 30 min, p = 0.052 at 60 min, n = 10 mice). Paw thickness was similar in both groups at 120 and 180 min after PAR2-AP (Fig. 8A, p < 0.05 relative to NaCl-treated mice). No difference in paw thickness was detected between trpv4+/+ and trpv4−/− mice after intraplantar injection of 0.9% NaCl. PAR2-AP also increased MPO activity in the paws of trpv4+/+ and trpv4−/− mice (Fig. 8B, p = 0.0028 and p = 0.0088, respectively, compared with NaCl; n = 8–10). However, MPO activity was similar in trpv4+/+ and trpv4−/− mice (p = 0.8340).
FIGURE 8.
PAR2-AP induced paw edema and granulocyte infiltration. A, intraplantar injection of PAR2-AP induced sustained paw edema (*, p < 0.05 compared with NaCl-treated mice; n = 8–10 mice/group). This effect was significantly attenuated in trpv4−/− mice compared with trpv4+/+ mice at an early time point (#, p = 0.006 at 30 min; n = 10 mice/group). NaCl injection had no effect. B, intraplantar PAR2-AP also increased MPO activity in both trpv4+/+ and trpv4−/− mice (p = 0.0028 and p = 0.0088 compared with NaCl-treated animals, n ≥ 8 experiments), but there was no difference between genotypes (p = 0.8340). C and D, pretreatment with 17-ODYA reduced PAR2-AP-induced paw edema at 30 min (**, p = 0.0305 compared with PAR2-AP/vehicle-treated mice; #, p < 0.05 compared with NaCl-treated mice; n = 5) without affecting MPO activity (p = 0.019 compared with NaCl-treated mice; n = 5) Analysis of variance and Newman-Keuls test were used.
Pretreatment with 17-ODYA (5 mg/kg, intraperitoneal, 30 min before PAR2-AP) reduced the paw edema response to PAR2-AP at an early time point (Fig. 8C, p = 0.0305 at 30 min compared with vehicle, n = 5 mice). Paw thickness was similar in both groups at other time points after PAR2-AP (Fig. 8C, p < 0.05 relative to NaCl-treated mice). 17-ODYA did not affect the PAR2-induced MPO activity (Fig. 8D).
These results indicate that the release of arachidonic acid metabolites and the activation of TRPV4 contributes to the initial phases of PAR2-evoked edema. However, TRPV4 is not involved in PAR2-mediated recruitment of inflammatory cells.
DISCUSSION
Our results reveal an unexpected functional coupling between PAR2 and TRP channels, which involves the generation of an arachidonic acid-derived TRPV4 activator and a tyrosine kinase signaling pathway (Fig. 9). We identified this coupling in a model HEK cell line and in primary nociceptive neurons. The coupling gives rise to long-lasting TRPV4-dependent Ca2+ signals and demonstrates a direct involvement of TRPV4 in the cellular response to PAR2 activation, which contributes to the proinflammatory effects of this receptor. We propose that this coupling represents a mechanism of TRP channel regulation that is distinct from the process of sensitization. This convergence of GPCR and TRP signaling may provide a mechanism through which the specificity and magnitude of cellular responses is conferred and controlled. This proposal is supported by other studies demonstrating functional coupling between GPCRs and TRP channels in native cells (28, 29, 37, 44–48). However, the process of PAR2 coupling to TRPV4 shows specificity because ATP acting through P2Y receptors did not activate TRPV4, and PAR2 did not activate TRPA1. These observations suggest that the mechanism of PAR2 coupling to TRPV4 is not simply due to elevated [Ca2+]i, which was similarly stimulated by ATP. Two different modes of PAR2 activation (PAR2-AP and trypsin) induced coupling between PAR2 and TRPV4. Thus, coupling is not an artifact of using the synthetic tethered ligand-based activating peptide and can also occur in response to an endogenous activator.
FIGURE 9.
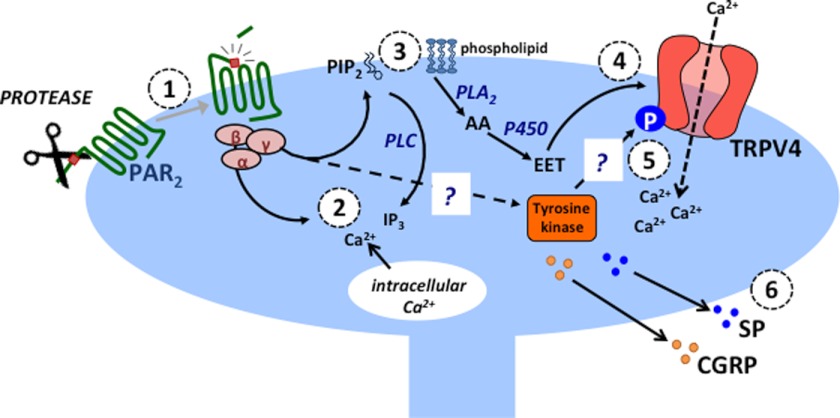
Hypothesized mechanism of PAR2 and TRVP4-dependent neurogenic inflammation. Proteases released during inflammation and injury cleave and activate PAR2 on the terminals of nociceptive spinal afferent neurons (1). PAR2 activates heterotrimeric G proteins, which leads to release of intracellular Ca2+ (2) via the phospholipase C (PLC), phosphatidylinositol 4,5-bisphosphate (PIP2), and inositol 1,4,5-trisphosphate (IP3) pathways. PLA2 hydrolyzes membrane phospholipids to form arachidonic acid (AA), and cytochrome P450 epoxygenase (P450) generates EETs (3). As endogenous TRPV4 agonists, EETs (most likely 5′,6′-EET) promote TRPV4 channel opening and influx of extracellular Ca2+ (4). This requires tyrosine 110, a known target for phosphorylation (P) by tyrosine kinases (5). PAR2 activation triggers calcium-dependent release of calcitonin gene-related peptide (CGRP) and substance P (SP), which mediate neurogenic inflammation (6). Question marks indicate that the tyrosine kinase remains to be identified and that PAR2-induced phosphorylation of TRPV4 has not been examined directly.
Significance of PAR2-TRPV4 Coupling for Neurogenic Inflammation and Pain
PAR2 agonists promote neurogenic inflammation and pain in multiple tissues (1, 3, 4). TRPV4 is coexpressed with PAR2 in nociceptive spinal-afferent neurons, and sensitization of these channels following PAR2 activation is a major mechanism through which both neurogenic pain and inflammation develop (13, 16–18). In our proposed model, the activity of TRPV4 influences the duration of PAR2-dependent signaling, thereby augmenting the effects of acute PAR2 activation. Thus, both coupling and sensitization of TRP channels may contribute to pathophysiological changes associated with PAR2 activation.
Our experiments with cultured DRG neurons demonstrate that both the magnitude and duration of PAR2 signaling are modulated by TRPV4 activity, whereas in HEK293 cells only the sustained phase of the PAR2-evoked Ca2+ response was affected by TRPV4 expression. The PAR2 response was greatly reduced in DRG neurons treated with the TRPV4 antagonist HC067047 or after omission of extracellular Ca2+ ions. These findings are consistent with preliminary studies showing that Ruthenium Red also suppresses PAR2 signaling in DRG neurons (data not shown). We conclude that PAR2-induced Ca2+ signaling in DRG neurons depends in large part upon activation of TRPV4 and a TRPV4-mediated influx of extracellular Ca2+. However, a substantial component of the increase in [Ca2+]i also derives from intracellular Ca2+ stores.
In this study, we have demonstrated a role for PAR2-TRPV4 coupling in the regulation of PAR2-mediated paw edema but not in granulocyte infiltration. These findings are consistent with a role for TRPV4 in PAR2-dependent signaling in DRG neurons, as the development of paw edema is neurogenic in origin, whereas granulocyte recruitment occurs independently of sensory innervation (3).
PAR2-TRP Channel Coupling in Other Systems
Our observations may have important functional implications for other cell types in which PAR2 and TRPV4 are coexpressed, such as bladder urothelium, colonic epithelium, and bronchial epithelial cells (18, 57–60). Whether physiological and pathophysiological responses to PAR2 activation are augmented by TRPV4 in these tissues has yet to be determined. PAR2 activation in these cell types leads to increased barrier permeability and is associated with inflammation (61). Similarly, TRPV4 activation results in inflammation and altered cellular permeability of colonic epithelial cells (58). Thus, as with DRG neurons, TRP channels may also regulate PAR2-dependent signaling in these cells.
Signaling Pathways Involved in TRP Activation
PARs signal through phospholipase C and PLA2 (57, 62, 63). Arachidonic acid derivatives are endogenous activators of TRPV1, TRPV4, and TRPA1 (24, 25, 43, 64). PAR2 coupling with TRPV4 involves production of arachidonic acid derivatives, as demonstrated using MAFP, an irreversible inhibitor of PLA2 (65). This observation is consistent with evidence that PAR2 activation leads to increased arachidonic acid release and prostaglandin production in enterocytes (57) and with other studies examining GPCR interaction with TRPV4 channels (47). Whether this is a common mechanism in cells that coexpress GPCRs and TRPV4 channels remains to be determined. PLA2-derived TRP channel activators contribute to both the transient and sustained phases of the PAR2 response because MAFP inhibited both components of the response. The inability of indomethacin to block TRPV4 activation excludes involvement of cyclooxygenase-derived prostaglandin in signaling to TRPV4. Lipoxygenase-derived arachidonic acid derivatives, such as hydroxyeicosatetraenoic acids, are endogenous TRPV1 agonists and are not reported to activate TRPV4 (28, 43, 66, 67). In our experiments, lipoxygenase inhibition reduced cell viability and was not examined further (data not shown). We did not observe coupling between PAR2 and TRPA1. This result is at variance with a recent study in which 5′,6′-EET dependent neuronal activation of TRPA1 was reported (68). The reason for this discrepancy is presently unknown but may relate to the differences in the studied receptors and the experimental systems.
TRPV4 is modulated by a range of serine/threonine and tyrosine kinases and contains target residues, including those for tyrosine kinases (50, 69) and PKC (40). In addition, PKC-activating phorbol esters directly activate TRPV4 (41). We found that PKC-specific inhibition (BIM-1) had no effect on PAR2 signaling, which excludes a role for PKC in coupling with TRPV4.
Two observations implicate tyrosine phosphorylation of TRPV4 in PAR2-TRPV4 coupling. First, the Src family kinase inhibitor, Src1, attenuated TRPV4-dependent PAR2 signaling. Second, the magnitude of coupling was markedly attenuated by mutation of Tyr-110, a target residue for Src family kinases (50). A limitation of our work is that we have not identified the particular tyrosine kinase involved in PAR2-TRPV4 coupling. Although Src1 inhibited coupling, preliminary studies indicated that PP2 was less effective (data not shown). Thus, it is possible that other kinases are involved because nanomolar concentrations of PP2 block certain Src kinases (70). Another caveat of our study is that we did not directly demonstrate phosphorylation of TRPV4-Y110 in response to PAR2 activation. Further studies using pharmacological and genetic approaches for manipulating kinase activity are required to identify the kinases that mediate tyrosine phosphorylation. Proteomic analyses are necessary to study PAR2-induced tyrosine phosphorylation of TRPV4. Once the specific kinase is identified, it will be possible to examine its contribution to protease-evoked inflammation and pain in mice.
Summary
In summary, we have demonstrated coupling between PAR2 and TRPV4. This activation of TRP channels is mediated by production of endogenous activators and is dependent on key tyrosine residues of TRPV4. Our study has identified critical components of the intracellular signaling pathways underlying the activation of TRP channels. These may represent novel targets for therapeutics aimed at reducing augmented signaling under pathophysiological conditions while leaving normal responses intact. This selective inhibition of aberrant signaling is a more attractive target compared with global inhibition of TRP channels, which have important physiological roles in thermoregulation, osmoregulation, and nociception.
This work was supported, in whole or in part, by National Institutes of Health Grants DK57840 and DK43207 (to N. W. B.). This work was also supported by National Health and Medical Research Council Grants 1049682 and 633033 (to N. W. B.), 1024683 and 1046860 (to P. M. and N. W. B.), and 454858 (to D. P. P.).
- PAR
- protease-activated receptor
- GPCR
- G protein-coupled receptor
- AP
- activating peptide
- TRPA1
- transient receptor potential ankyrin 1
- TRPV
- transient receptor potential vanilloid
- DRG
- dorsal root ganglia
- PLA2
- phospholipase A2
- 4α-PDD
- 4α-phorbol didecanoate
- BIM-1
- bisindolylmaleimide I
- EET
- epoxyeicosatrienoic acid
- 17-ODYA
- 17-octadecynoic acid
- MAFP
- methyl arachidonyl fluorophosphonate
- PKC
- protein kinase C
- HBSS
- Hanks' balanced salt solution
- MPO
- myeloperoxidase.
REFERENCES
- 1. Ossovskaya V. S., Bunnett N. W. (2004) Protease-activated receptors. Contribution to physiology and disease. Physiol. Rev. 84, 579–621 [DOI] [PubMed] [Google Scholar]
- 2. Ramachandran R., Noorbakhsh F., Defea K., Hollenberg M. D. (2012) Targeting proteinase-activated receptors. Therapeutic potential and challenges. Nat. Rev. Drug. Discov. 11, 69–86 [DOI] [PubMed] [Google Scholar]
- 3. Steinhoff M., Vergnolle N., Young S. H., Tognetto M., Amadesi S., Ennes H. S., Trevisani M., Hollenberg M. D., Wallace J. L., Caughey G. H., Mitchell S. E., Williams L. M., Geppetti P., Mayer E. A., Bunnett N. W. (2000) Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 6, 151–158 [DOI] [PubMed] [Google Scholar]
- 4. Vergnolle N., Bunnett N. W., Sharkey K. A., Brussee V., Compton S. J., Grady E. F., Cirino G., Gerard N., Basbaum A. I., Andrade-Gordon P., Hollenberg M. D., Wallace J. L. (2001) Proteinase-activated receptor-2 and hyperalgesia. A novel pain pathway. Nat. Med. 7, 821–826 [DOI] [PubMed] [Google Scholar]
- 5. Cenac N., Coelho A. M., Nguyen C., Compton S., Andrade-Gordon P., MacNaughton W. K., Wallace J. L., Hollenberg M. D., Bunnett N. W., Garcia-Villar R., Bueno L., Vergnolle N. (2002) Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am. J. Pathol. 161, 1903–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cenac N., Andrews C. N., Holzhausen M., Chapman K., Cottrell G., Andrade-Gordon P., Steinhoff M., Barbara G., Beck P., Bunnett N. W., Sharkey K. A., Ferraz J. G., Shaffer E., Vergnolle N. (2007) Role for protease activity in visceral pain in irritable bowel syndrome. J. Clin. Invest. 117, 636–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoogerwerf W. A., Zou L., Shenoy M., Sun D., Micci M. A., Lee-Hellmich H., Xiao S. Y., Winston J. H., Pasricha P. J. (2001) The proteinase-activated receptor 2 is involved in nociception. J. Neurosci. 21, 9036–9042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ceppa E. P., Lyo V., Grady E. F., Knecht W., Grahn S., Peterson A., Bunnett N. W., Kirkwood K. S., Cattaruzza F. (2011) Serine proteases mediate inflammatory pain in acute pancreatitis. Am. J. Physiol. Gastrointest Liver Physiol. 300, G1033–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lohman R. J., Cotterell A. J., Barry G. D., Liu L., Suen J. Y., Vesey D. A., Fairlie D. P. (2012) An antagonist of human protease activated receptor-2 attenuates PAR2 signaling, macrophage activation, mast cell degranulation, and collagen-induced arthritis in rats. FASEB J. 26, 2877–2887 [DOI] [PubMed] [Google Scholar]
- 10. Basbaum A. I., Bautista D. M., Scherrer G., Julius D. (2009) Cellular and molecular mechanisms of pain. Cell 139, 267–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nilius B. (2007) TRP channels in disease. Biochim. Biophys. Acta 1772, 805–812 [DOI] [PubMed] [Google Scholar]
- 12. Amadesi S., Cottrell G. S., Divino L., Chapman K., Grady E. F., Bautista F., Karanjia R., Barajas-Lopez C., Vanner S., Vergnolle N., Bunnett N. W. (2006) Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cϵ- and A-dependent mechanisms in rats and mice. J. Physiol. 575, 555–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amadesi S., Nie J., Vergnolle N., Cottrell G. S., Grady E. F., Trevisani M., Manni C., Geppetti P., McRoberts J. A., Ennes H., Davis J. B., Mayer E. A., Bunnett N. W. (2004) Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J. Neurosci. 24, 4300–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cattaruzza F., Spreadbury I., Miranda-Morales M., Grady E. F., Vanner S., Bunnett N. W. (2010) Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am. J. Physiol. Gastrointest Liver Physiol. 298, G81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cenac N., Altier C., Chapman K., Liedtke W., Zamponi G., Vergnolle N. (2008) Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology 135, 937–946 [DOI] [PubMed] [Google Scholar]
- 16. Dai Y., Moriyama T., Higashi T., Togashi K., Kobayashi K., Yamanaka H., Tominaga M., Noguchi K. (2004) Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J. Neurosci. 24, 4293–4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dai Y., Wang S., Tominaga M., Yamamoto S., Fukuoka T., Higashi T., Kobayashi K., Obata K., Yamanaka H., Noguchi K. (2007) Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J. Clin. Invest. 117, 1979–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grant A. D., Cottrell G. S., Amadesi S., Trevisani M., Nicoletti P., Materazzi S., Altier C., Cenac N., Zamponi G. W., Bautista-Cruz F., Lopez C. B., Joseph E. K., Levine J. D., Liedtke W., Vanner S., Vergnolle N., Geppetti P., Bunnett N. W. (2007) Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J. Physiol. 578, 715–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sipe W. E., Brierley S. M., Martin C. M., Phillis B. D., Cruz F. B., Grady E. F., Liedtke W., Cohen D. M., Vanner S., Blackshaw L. A., Bunnett N. W. (2008) Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am. J. Physiol. Gastrointest Liver Physiol. 294, G1288–1298 [DOI] [PubMed] [Google Scholar]
- 20. Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. (1997) The capsaicin receptor. A heat-activated ion channel in the pain pathway. Nature 389, 816–824 [DOI] [PubMed] [Google Scholar]
- 21. Tominaga M., Caterina M. J., Malmberg A. B., Rosen T. A., Gilbert H., Skinner K., Raumann B. E., Basbaum A. I., Julius D. (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531–543 [DOI] [PubMed] [Google Scholar]
- 22. Vellani V., Mapplebeck S., Moriondo A., Davis J. B., McNaughton P. A. (2001) Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J. Physiol. 534, 813–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alessandri-Haber N., Yeh J. J., Boyd A. E., Parada C. A., Chen X., Reichling D. B., Levine J. D. (2003) Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 39, 497–511 [DOI] [PubMed] [Google Scholar]
- 24. Vriens J., Watanabe H., Janssens A., Droogmans G., Voets T., Nilius B. (2004) Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. U.S.A. 101, 396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watanabe H., Vriens J., Prenen J., Droogmans G., Voets T., Nilius B. (2003) Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424, 434–438 [DOI] [PubMed] [Google Scholar]
- 26. Andrè E., Campi B., Materazzi S., Trevisani M., Amadesi S., Massi D., Creminon C., Vaksman N., Nassini R., Civelli M., Baraldi P. G., Poole D. P., Bunnett N. W., Geppetti P., Patacchini R. (2008) Cigarette smoke-induced neurogenic inflammation is mediated by α,β-unsaturated aldehydes and the TRPA1 receptor in rodents. J. Clin. Invest. 118, 2574–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trevisani M., Siemens J., Materazzi S., Bautista D. M., Nassini R., Campi B., Imamachi N., Andrè E., Patacchini R., Cottrell G. S., Gatti R., Basbaum A. I., Bunnett N. W., Julius D., Geppetti P. (2007) 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. U.S.A. 104, 13519–13524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shin J., Cho H., Hwang S. W., Jung J., Shin C. Y., Lee S. Y., Kim S. H., Lee M. G., Choi Y. H., Kim J., Haber N. A., Reichling D. B., Khasar S., Levine J. D., Oh U. (2002) Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc. Natl. Acad. Sci. U.S.A. 99, 10150–10155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shim W. S., Tak M. H., Lee M. H., Kim M., Kim M., Koo J. Y., Lee C. H., Kim M., Oh U. (2007) TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J. Neurosci. 27, 2331–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang H., Cang C. L., Kawasaki Y., Liang L. L., Zhang Y. Q., Ji R. R., Zhao Z. Q. (2007) Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCϵ. A novel pathway for heat hyperalgesia. J. Neurosci. 27, 12067–12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sculptoreanu A., Aura Kullmann F., de Groat W. C. (2008) Neurokinin 2 receptor-mediated activation of protein kinase C modulates capsaicin responses in DRG neurons from adult rats. Eur. J. Neurosci. 27, 3171–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aley K. O., Levine J. D. (1999) Role of protein kinase A in the maintenance of inflammatory pain. J. Neurosci. 19, 2181–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moriyama T., Higashi T., Togashi K., Iida T., Segi E., Sugimoto Y., Tominaga T., Narumiya S., Tominaga M. (2005) Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol. Pain 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vellani V., Colucci M., Lattanzi R., Giannini E., Negri L., Melchiorri P., McNaughton P. A. (2006) Sensitization of transient receptor potential vanilloid 1 by the prokineticin receptor agonist Bv8. J. Neurosci. 26, 5109–5116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moriyama T., Iida T., Kobayashi K., Higashi T., Fukuoka T., Tsumura H., Leon C., Suzuki N., Inoue K., Gachet C., Noguchi K., Tominaga M. (2003) Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J. Neurosci. 23, 6058–6062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tominaga M., Wada M., Masu M. (2001) Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc. Natl. Acad. Sci. U.S.A. 98, 6951–6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chevesich J., Kreuz A. J., Montell C. (1997) Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron 18, 95–105 [DOI] [PubMed] [Google Scholar]
- 38. Chuang H. H., Prescott E. D., Kong H., Shields S., Jordt S. E., Basbaum A. I., Chao M. V., Julius D. (2001) Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411, 957–962 [DOI] [PubMed] [Google Scholar]
- 39. Prescott E. D., Julius D. (2003) A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science 300, 1284–1288 [DOI] [PubMed] [Google Scholar]
- 40. Peng H., Lewandrowski U., Müller B., Sickmann A., Walz G., Wegierski T. (2010) Identification of a protein kinase C-dependent phosphorylation site involved in sensitization of TRPV4 channel. Biochem. Biophys. Res. Commun. 391, 1721–1725 [DOI] [PubMed] [Google Scholar]
- 41. Xu F., Satoh E., Iijima T. (2003) Protein kinase C-mediated Ca2+ entry in HEK 293 cells transiently expressing human TRPV4. Br. J. Pharmacol. 140, 413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim B. M., Lee S. H., Shim W. S., Oh U. (2004) Histamine-induced Ca(2+) influx via the PLA(2)/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci. Lett. 361, 159–162 [DOI] [PubMed] [Google Scholar]
- 43. Hwang S. W., Cho H., Kwak J., Lee S. Y., Kang C. J., Jung J., Cho S., Min K. H., Suh Y. G., Kim D., Oh U. (2000) Direct activation of capsaicin receptors by products of lipoxygenases. Endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 97, 6155–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim Y. H., Park C. K., Back S. K., Lee C. J., Hwang S. J., Bae Y. C., Na H. S., Kim J. S., Jung S. J., Oh S. B. (2009) Membrane-delimited coupling of TRPV1 and mGluR5 on presynaptic terminals of nociceptive neurons. J. Neurosci. 29, 10000–10009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Woo D. H., Jung S. J., Zhu M. H., Park C. K., Kim Y. H., Oh S. B., Lee C. J. (2008) Direct activation of transient receptor potential vanilloid 1(TRPV1) by diacylglycerol (DAG). Mol. Pain 4, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Adapala R. K., Talasila P. K., Bratz I. N., Zhang D. X., Suzuki M., Meszaros J. G., Thodeti C. K. (2011) PKCα mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 301, H757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ducret T., Guibert C., Marthan R., Savineau J. P. (2008) Serotonin-induced activation of TRPV4-like current in rat intrapulmonary arterial smooth muscle cells. Cell Calcium 43, 315–323 [DOI] [PubMed] [Google Scholar]
- 48. Zhang D. X., Mendoza S. A., Bubolz A. H., Mizuno A., Ge Z. D., Li R., Warltier D. C., Suzuki M., Gutterman D. D. (2009) Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension 53, 532–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McGuire J. J., Saifeddine M., Triggle C. R., Sun K., Hollenberg M. D. (2004) 2-furoyl-LIGRLO-amide. A potent and selective proteinase-activated receptor 2 agonist. J. Pharmacol. Exp. Ther. 309, 1124–1131 [DOI] [PubMed] [Google Scholar]
- 50. Wegierski T., Lewandrowski U., Müller B., Sickmann A., Walz G. (2009) Tyrosine phosphorylation modulates the activity of TRPV4 in response to defined stimuli. J. Biol. Chem. 284, 2923–2933 [DOI] [PubMed] [Google Scholar]
- 51. Story G. M., Peier A. M., Reeve A. J., Eid S. R., Mosbacher J., Hricik T. R., Earley T. J., Hergarden A. C., Andersson D. A., Hwang S. W., McIntyre P., Jegla T., Bevan S., Patapoutian A. (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829 [DOI] [PubMed] [Google Scholar]
- 52. Liedtke W., Friedman J. M. (2003) Abnormal osmotic regulation in trpv4−/− mice. Proc. Natl. Acad. Sci. U.S.A. 100, 13698–13703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Veldhuis N. A., Lew M. J., Abogadie F. C., Poole D. P., Jennings E. A., Ivanusic J. J., Eilers H., Bunnett N. W., McIntyre P. (2012) N-glycosylation determines ionic permeability and desensitization of the TRPV1 capsaicin receptor. J. Biol. Chem. 287, 21765–21772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dragoni I., Guida E., McIntyre P. (2006) The cold and menthol receptor TRPM8 contains a functionally important double cysteine motif. J. Biol. Chem. 281, 37353–37360 [DOI] [PubMed] [Google Scholar]
- 55. Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. (1982) Measurement of cutaneous inflammation. Estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 78, 206–209 [DOI] [PubMed] [Google Scholar]
- 56. Schachter J. B., Sromek S. M., Nicholas R. A., Harden T. K. (1997) HEK293 human embryonic kidney cells endogenously express the P2Y1 and P2Y2 receptors. Neuropharmacology 36, 1181–1187 [DOI] [PubMed] [Google Scholar]
- 57. Kong W., McConalogue K., Khitin L. M., Hollenberg M. D., Payan D. G., Böhm S. K., Bunnett N. W. (1997) Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc. Natl. Acad. Sci. U.S.A. 94, 8884–8889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. D'Aldebert E., Cenac N., Rousset P., Martin L., Rolland C., Chapman K., Selves J., Alric L., Vinel J. P., Vergnolle N. (2011) Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology 140, 275–285 [DOI] [PubMed] [Google Scholar]
- 59. D'Andrea M. R., Saban M. R., Nguyen N. B., Andrade-Gordon P., Saban R. (2003) Expression of protease-activated receptor-1, -2, -3, and -4 in control and experimentally inflamed mouse bladder. Am. J. Pathol. 162, 907–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Birder L., Kullmann F. A., Lee H., Barrick S., de Groat W., Kanai A., Caterina M. (2007) Activation of urothelial transient receptor potential vanilloid 4 by 4α-phorbol 12,13-didecanoate contributes to altered bladder reflexes in the rat. J. Pharmacol. Exp. Ther. 323, 227–235 [DOI] [PubMed] [Google Scholar]
- 61. Jacob C., Yang P. C., Darmoul D., Amadesi S., Saito T., Cottrell G. S., Coelho A. M., Singh P., Grady E. F., Perdue M., Bunnett N. W. (2005) Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and β-arrestins. J. Biol. Chem. 280, 31936–31948 [DOI] [PubMed] [Google Scholar]
- 62. Macfarlane S. R., Seatter M. J., Kanke T., Hunter G. D., Plevin R. (2001) Proteinase-activated receptors. Pharmacol. Rev. 53, 245–282 [PubMed] [Google Scholar]
- 63. Nagataki M., Moriyuki K., Sekiguchi F., Kawabata A. (2008) Evidence that PAR2-triggered prostaglandin E2 (PGE2) formation involves the ERK-cytosolic phospholipase A2-COX-1-microsomal PGE synthase-1 cascade in human lung epithelial cells. Cell Biochem. Funct. 26, 279–282 [DOI] [PubMed] [Google Scholar]
- 64. Materazzi S., Nassini R., Andrè E., Campi B., Amadesi S., Trevisani M., Bunnett N. W., Patacchini R., Geppetti P. (2008) Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. U.S.A. 105, 12045–12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Balsinde J., Dennis E. A. (1996) Distinct roles in signal transduction for each of the phospholipase A2 enzymes present in P388D1 macrophages. J. Biol. Chem. 271, 6758–6765 [DOI] [PubMed] [Google Scholar]
- 66. Ferreira J., da Silva G. L., Calixto J. B. (2004) Contribution of vanilloid receptors to the overt nociception induced by B2 kinin receptor activation in mice. Br. J. Pharmacol. 141, 787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McLean P. G., Aston D., Sarkar D., Ahluwalia A. (2002) Protease-activated receptor-2 activation causes EDHF-like coronary vasodilation. Selective preservation in ischemia/reperfusion injury. Involvement of lipoxygenase products, VR1 receptors, and C-fibers. Circ. Res. 90, 465–472 [DOI] [PubMed] [Google Scholar]
- 68. Sisignano M., Park C. K., Angioni C., Zhang D. D., von Hehn C., Cobos E. J., Ghasemlou N., Xu Z. Z., Kumaran V., Lu R., Grant A., Fischer M. J., Schmidtko A., Reeh P., Ji R. R., Woolf C. J., Geisslinger G., Scholich K., Brenneis C. (2012) 5,6-EET is released upon neuronal activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals. J. Neurosci. 32, 6364–6372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xu H., Zhao H., Tian W., Yoshida K., Roullet J. B., Cohen D. M. (2003) Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation. SRC family kinase-dependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress. J. Biol. Chem. 278, 11520–11527 [DOI] [PubMed] [Google Scholar]
- 70. Bain J., Plater L., Elliott M., Shpiro N., Hastie C. J., McLauchlan H., Klevernic I., Arthur J. S., Alessi D. R., Cohen P. (2007) The selectivity of protein kinase inhibitors. A further update. Biochem. J. 408, 297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]