Background: The serotonin transporter (SERT) relies exclusively on SEC24 paralog C for its ER export.
Results: A lysine to tyrosine mutation in the C terminus of SERT switches its preference from SEC24C to SEC24D.
Conclusion: The position +2 from the RI/RL/KL export motif determines SEC24 paralog requirement.
Significance: The role of SEC24C in ER export of neurotransmitter transporters is relevant to psychiatric disorders, e.g., bipolar disease and depression.
Keywords: Dopamine Transporters, Endoplasmic Reticulum (ER), Membrane Transport, Monoamine Transporters, Serotonin, Serotonin Transporters
Abstract
The serotonin transporter (SERT) maintains serotonergic neurotransmission via rapid reuptake of serotonin from the synaptic cleft. SERT relies exclusively on the coat protein complex II component SEC24C for endoplasmic reticulum (ER) export. The closely related transporters for noradrenaline and dopamine depend on SEC24D. Here, we show that discrimination between SEC24C and SEC24D is specified by the residue at position +2 downstream from the ER export motif (607RI608 in SERT). Substituting Lys610 with tyrosine, the corresponding residue found in the noradrenaline and dopamine transporters, switched the SEC24 isoform preference: SERT-K610Y relied solely on SEC24D to reach the cell surface. This analysis was extended to other SLC6 (solute carrier 6) transporter family members: siRNA-dependent depletion of SEC24C, but not of SEC24D, reduced surface levels of the glycine transporter-1a, the betaine/GABA transporter and the GABA transporter-4. Experiments with dominant negative versions of SEC24C and SEC24D recapitulated these findings. We also verified that the presence of two ER export motifs (in concatemers of SERT and GABA transporter-1) supported recruitment of both SEC24C and SEC24D. To the best of our knowledge, this is the first report to document a change in SEC24 specificity by mutation of a single residue in the client protein. Our observations allowed for deducing a rule for SLC6 family members: a hydrophobic residue (Tyr or Val) in the +2 position specifies interaction with SEC24D, and a hydrophilic residue (Lys, Asn, or Gln) recruits SEC24C. Variations in SEC24C are linked to neuropsychiatric disorders. The present findings provide a mechanistic explanation. Variations in SEC24C may translate into distinct surface levels of neurotransmitter transporters.
Introduction
Sodium- and chloride-dependent neurotransmitter symporters of the SLC6 (solute carrier 6) protein family are targeted to the presynaptic specialization, where they accomplish their eponymous action (1). Removal of neurotransmitters from the synaptic cleft not only terminates neurotransmission but also replenishes vesicular stores. Thus, changes in surface levels and, hence, in activity of the transporters are important in shaping the synaptic response. The biological relevance of changes in transporter levels is exemplified by the polymorphism in the promoter of the serotonin transporter (SERT)2 (SLC6A4): the short polymorphism results in lower expression of SERT and renders people more susceptible to depression and other depression-related disorders (2, 3). When compared with their bacterial homologs, e.g., TnaT (4) or LeuTAa (5), mammalian neurotransmitter sodium symporter members have long N and C termini. These additions are dispensable for the substrate translocation process, but they were presumably acquired during evolution in eukaryotic cells to support regulation and trafficking. In fact, the N and C termini harbor phosphorylation sites, and several proteins are known to bind to the N and C termini of neurotransmitter sodium symporter (1, 6). In addition, several of the naturally occurring human SERT variants occur in these regions; they affect the cycle of endocytosis and exocytosis of SERT that is regulated by phosphorylation via cGMP-dependent protein kinase, p38 MAPK, and protein kinase C isoforms (7).
Like all other integral membrane proteins, transporters of the SLC6 family are born in the endoplasmic reticulum (ER). Accordingly, they are subject to anterograde trafficking through the secretory pathway, and sorting decisions must be made to deliver them to specialized compartments of the plasma membrane, e.g., the apical membrane and the presynaptic terminal of the axon for epithelial transporters and neurotransmitter transporters, respectively. The early secretory pathway has been implicitly assumed to operate in a constitutive, housekeeping manner: chaperone-assisted folding allows the protein to adopt a stable conformation, and it is concentrated in ER exit sites, where it engages a SEC24 component of the COPII coat either directly or via the recruitment of an escort protein. It has recently been appreciated that this process is subject to regulation and integrates signals emanating from cell surface receptors at several levels (8). There are at least two kinases that impinge on the assembly of the COPII coat: p42 mitogen-activated protein kinase/ERK2 phosphorylates SEC16, and this promotes the activity of the protein, i.e., the nucleation of ERES sites (9). The isoforms SE24C and SEC24D are substrates for protein kinase B/AKT; phosphorylation is thought to affect the assembly of the SEC23/SEC24 dimer (10). Additional regulatory input has also been uncovered at later steps (8). This suggests that the cell surface levels of membrane proteins are also in part determined by their rate of anterograde trafficking. Accordingly, it is of interest to understand how neurotransmitter transporters are exported from the ER and how they are transferred through subsequent compartments to reach their destination. The individual steps are best understood for the GABA transporter-1: GAT-1 leaves the ER as an oligomer (11, 12) after recruiting SEC24D to a C-terminal RL/RI motif and the exocyst via its C-terminal PDZ domain (13, 14). Subsequently, it requires a trihydrophobic motif to exit the ER Golgi intermediate compartment (15). Recruitment of both SEC24D and ARF-GAP is required to supports delivery of GAT-1 into the axonal compartment (16). SERT (and other SLC6 family members) has an ER export motif on its C terminus (607RI608) similar to that of GAT-1 (17, 18). However, contrary to GAT-1 and the monoamine transporters most closely related to SERT (the norepinephrine transporter NET and the dopamine transporter DAT), ER export of SERT depends on SEC24C rather than SEC24D (18). In the present study, we addressed the basis for this selective interaction: we identified a lysine residue (Lys610) at position +2 from the ER export (607RI608), which specifies recruitment of SEC24C. Mutation to the corresponding tyrosine residue in NET and DAT suffices to switch the selectivity in favor of SEC24D. We extended our analysis to other SLC6 family members and confirmed that residues with hydrophilic side chains (i.e., Lys, Gln, and Asn) in this +2 position confer selectivity for SEC24C. To the best of our knowledge, this is the first instance where the source of the SEC24 isoform selectivity is understood.
EXPERIMENTAL PROCEDURES
Materials
[3H]GABA (76.2 Ci/mmol), [3H]glycine (56.4 Ci/mmol), and [3H]serotonin (28.1 Ci/mmol) were supplied by PerkinElmer Life Sciences. The QuikChange II site-directed mutagenesis kit was from Stratagene Cloning Systems (La Jolla, CA), and the oligonucleotides were from Operon Biotechnologies (Cologne, Germany). Predesigned stealth RNA duplex oligoribonucleotides (Invitrogen) were used to knock down SEC24A–D. The codes were as follows: SEC24AHSS145804, SEC24AHSS145805, and SEC24AHSS145806 (for SEC24A); SEC24DHSS114919, SEC24DHSS114920, and SEC24DHSS190682 (for SEC24D); SEC24CHSS114388, SEC24CHSS114389, and SEC24CHSS114390 (for SEC24C); and SEC24BHSS115967, SEC24BHSS115968, and SEC24BHSS173629 (for SEC24B). Negative controls used throughout the experiments were recommended by the manufacturer (Invitrogen). Cell culture media, supplements, and antibiotics were all from Invitrogen. All other chemicals were of analytical grade.
Mutagenesis and Transfections
The mutants SERT-K610Y and NET-Y590K were created using the QuikChange Lightning site-directed mutagenesis kit (Stratagene Cloning Systems) and the cDNA of human SERT or NET (both cloned into the pEYFP vector) as templates. The primer sequence of the sense strands (in the 5′ to 3′ direction, with mutations shown in bold underlined type) were GGGACATTTAAAGAGCGTATTATTTACAGTATTACCCCGG and GGGAGAGACTGGCCAAGGGCATCACGCC for SERT and NET mutants, respectively. The plasmids encoding SEC24C-796DD797-VN and SEC24D-733DD734-VN mutants, as well as alanine and truncation mutants of SERT, were generated as described previously (17, 18). The construction of the functionally active concatemers of SERT and GAT-1 (SEGA) and two SERT moieties has been described previously (19). HeLa or HEK293 cells were transfected using predesigned stealth RNA duplex oligoribonucleotides (Invitrogen) with appropriate negative controls (Invitrogen) and using Lipofectamine RNAiMAX (Invitrogen) according to the protocol provided by the manufacturer. Forty-eight hours following siRNA transfections, the cells were transfected with cDNAs encoding SERT, SERT-K610Y, GLYT-1a, BGT-1, or GAT-4, using Lipofectamine 2000 (Invitrogen). Uptake and confocal microscopy experiments were carried out 24 h later. Plasmid titration experiments using dominant negative mutants of SEC24C and SEC24D (i.e., SEC24C-796DD797-VN and SEC24D-733DD734-VN, respectively) were performed as described previously (18).
GST Protein Purification and GST Pulldown
The sequence corresponding to the C terminus of SERT was inserted into the pGEX5 vector, and the SERT-K610Y mutant was created in this background using the QuikChange Lightning site-directed mutagenesis kit (Stratagene Cloning Systems) and the appropriate primers (see above). Plasmids were used to transform XL10 gold Escherichia coli. Bacteria (0.6 liter of liquid culture) were grown to an optical density of <0.8 at 600 nm. Protein expression was induced by the addition of isopropyl 1-thio-β-d-galactopyranoside (0.7 mm) for 3 h. Thereafter, cells were harvested by centrifugation at 5,000 × g for 15 min. The cell pellet was resuspended in 20 ml of buffer (25 mm HEPES/NaOH, pH 8.0, 150 mm NaCl, 1 mm EDTA) containing 30 mg of lysozyme. After an incubation of 30 min at 4 °C under rotation, DNase (1 mg) and Triton X-100 (1%) were added, and the suspension was incubated for another 30 min, subsequently subjected to sonication, and left on ice for a further 15 min. The lysate was cleared by centrifugation at 50,000 × g for 1 h, and the resulting supernatant was loaded onto a GSH-Sepharose resin and rotated at 4 °C overnight. After removal of the supernatant, GSH-Sepharose was washed with buffer containing 1% Triton X-100 followed by buffer containing 1 mm ATP. Proteins were eluted with buffer containing glutathione at pH 8.0. Glutathione was removed, and the protein was concentrated by repeated cycles of concentration and dilution with pulldown buffer (130 mm KCl, 25 mm HEPES/NaOH, pH 7.2) in Amicon® Ultra-4 centrifugal filter units. The proteins were frozen in liquid nitrogen and stored at −80 °C. HEK293 cells were transfected with a plasmid encoding CFP-tagged Sec24C. After 48 h, the cells were harvested and lysed by sonication in 0.1 ml of pulldown buffer; the particulate fraction was removed by centrifugation (16,000 × g for 5 min). Cytosol (200 μg) was incubated with purified GST-tagged constructs (30 μg) for 1 h on ice. Pre-equilibrated GSH-Sepharose (corresponding to 50 μl of packed beads) was added, and samples were rotated at 4 °C overnight. The beads were collected by brief centrifugation and washed three times with pulldown buffer. The proteins were eluted by the addition of 50 μl of sample buffer (2% SDS, 100 mm β-mercaptoethanol) and shaking for 30 min at 65 °C. After centrifugation, 20 μl of the supernatant were loaded onto a SDS-polyacrylamide gel. The resolved proteins were electroblotted onto methanol-activated PVDF membranes. Nonspecific protein binding sites were saturated using 5% bovine serum albumin in 0.1% TBST for 1 h at room temperature. The blots were incubate at 4 °C overnight in 1:4000 rabbit anti-GFP antiserum in 0.1% TBST (20 mm Tris·HCl, pH 7.5, 150 mm NaCl, 0.1% Tween 20). The blots were washed four times using 0.1% TBST and incubated with 1:5000 horseradish peroxidase-conjugated anti-rabbit secondary antibody in 0.1% TBST. After a further four washes, the blots were incubated with substrate (SuperSignal West Pico chemiluminescent substrate or SuperSignal West Femto chemiluminescent substrate; Thermo Scientific). The resulting chemiluminescence was detected with photographic films. Cell lysates for use in Western blotting were prepared from cells transfected with the siRNAs against SEC24A–D, as described earlier (18).
Surface Biotinylation
Experiments were carried out according to the procedure described by Steinkellner et al. (20). In brief, the cells were treated twice for 15 min with sulfo-NHS-SS-biotin (1 mg/ml) in PBS supplemented with 1 mm MgCl2 and 0.1 mm CaCl2 (PBS2+) at 4 °C. The reactions were quenched with 100 mm glycine in PBS2+. After extensive washing in PBS2+, the cells were lysed in radioimmune precipitation assay buffer (10 mm Tris·HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% SDS) supplemented with protease inhibitor mixture (Roche Complete). The lysates were incubated with streptavidin beads (Thermo Fisher Scientific, Inc.) overnight at 4 °C. On the next day, the beads were washed thrice with radioimmune precipitation assay buffer, and the bound material was released in reducing sample buffer and resolved by SDS-polyacrylamide gel electrophoresis. Immunodetection was done with the antibody directed against GFP.
Cell Culture and Confocal Microscopy
HeLa and HEK293 cells were grown at 37 °C in a 5% CO2 humidified atmosphere in DMEM supplemented with 10% fetal calf serum and 1% kanamycin. In the case of siRNA transfections, antibiotics were excluded from the culture media to reduce cytotoxicity, as recommended by the supplier. The cells were seeded onto poly-d-lysine-coated 48-well culture plates (for uptake assays), 10-cm dishes for cell lysate preparations, or poly-d-lysine-coated 15-mm glass coverslips for confocal microscopy experiments. Confocal microscopy was performed as described earlier (18) using a Zeiss LSM510 confocal microscope (argon laser, 30 milliwatt; helium/neon laser, 1 milliwatt) equipped with an oil immersion objective (Zeiss Plan-Neofluar ×40/1.3). The images were analyzed with the Image Zeiss LSM Image Browser (version 4,2,2,121; Carl Zeiss Microimaging).
Radioligand Binding and Uptake Assays
For binding assays, the membranes were prepared from HEK293 cells transfected with the wild type or SERT-K610Y mutant and incubated with [3H]imipramine, in reactions of 250 μl. The buffer contained 20 mm Tris·HCl (pH 7.5), 1 mm EDTA, 2 mm MgCl2, 120 mm NaCl, and 3 mm KCl. Nonspecific binding was determined in the presence of 10 μm paroxetine. The reactions, lasting 20 min, were terminated by rapid washing with ice-cold buffer, collected onto glass fiber filters (Whatman GF/B), dissolved in scintillation medium, and counted for radioactivity. One day prior to performing uptake assays, the cells were seeded onto poly-d-lysine-coated 48-well culture plates. On the day of experiment, medium was aspirated, and the cells were carefully washed twice with Krebs-HEPES buffer at 25 °C. The cells were then incubated for 10 min at 25 °C with Krebs-HEPES buffer in the absence or presence of 10 μm paroxetine, 10 μm nisoxetine, 10 μm NNC 05-2090, 10 mm glycine or 600 μm GABA (to determine nonspecific uptake by SERT, NET, BGT-1, GLYT-1a, and GAT-4, respectively). Subsequently, solutions containing 0.2 μm [3H]5-HT (for SERT), 50 nm [3H]MPP+ (for NET), 50 nm [3H]GABA (for BGT-1 and GAT-4), or 50 nm [3H]glycine (for GLYT-1a) were added onto the cells for 1 min (SERT and mutants), 2 min (NET), or 5–15 min (GLYT-1a, BGT-1, and GAT-4). Uptake was terminated by rapid washing with ice-cold Krebs-HEPES buffer; the cells were lysed by the addition of 1% SDS, and the level of radioactivity in the lysates was determined by liquid scintillation counting. The values are shown as the arithmetic means ± S.E. Different conditions were compared using one-way analysis of variance (ANOVA), followed by Tukey's post hoc test or by t test with the appropriate Bonferroni correction.
RESULTS
SEC24C Interaction with SERT Occurs Upstream of E615 and Does Not Require a Flexible C Terminus
The wild type SERT interacts with SEC24C in an exclusive manner, unlike its close relatives NET and DAT that rely on SEC24D instead (18). An aspartate, nine residues C-terminal from the established RL/KL/RI export motif located on the transporter C terminus region (21), has been proposed to support the interaction of the glycine transporter GLYT-1 with SEC24. This acidic residue is conserved in most SLC6 family members: GAT-1, DAT, and NET have either Asp or Glu at this site (Table 1), but SERT differs by having a proline at the equivalent position. The C terminus of SERT can be truncated by up to 15 amino acid residues without impairing its cell surface expression in HEK293 cells (17, 22). The truncation eliminates this particular proline (Pro617). Accordingly, we examined whether the SEC24 selectivity was relaxed in the resulting SERT-ΔC15 mutant. As can be seen from Fig. 1A, this was not the case: when transiently expressed in HeLa cells, SERT-ΔC15 surface levels—and hence SERT-ΔC15-dependent cellular substrate uptake—was only reduced upon depletion of SEC24C by siRNA-mediated knockdown; sole depletion of SEC24D was as ineffective in the truncated SERT (right-hand set of bars in Fig. 1A) as in the wild type transporter (left-hand set of bars in Fig. 1A). We carried out these experiments and our previous work (18) in HeLa cells, because these cells expressed all four SEC24 isoforms (23). However, we noted that HEK293 cells also express all SEC24 isoforms, and their levels can be readily manipulated by transfection of siRNAs (Fig. 1B). Hence, subsequent experiments were conducted in HEK293 cells because SERT and concatemers are expressed to markedly higher levels in HEK293 cells than in HeLa cells.
TABLE 1.
Amino acid sequence alignment of SLC6 transporter C-terminal regions
The ER export motif (R/K-L/I/V) is indicated in bold type. Putative SEC24 isoform selectivity residues on cargo proteins are shown in bold underlined type.
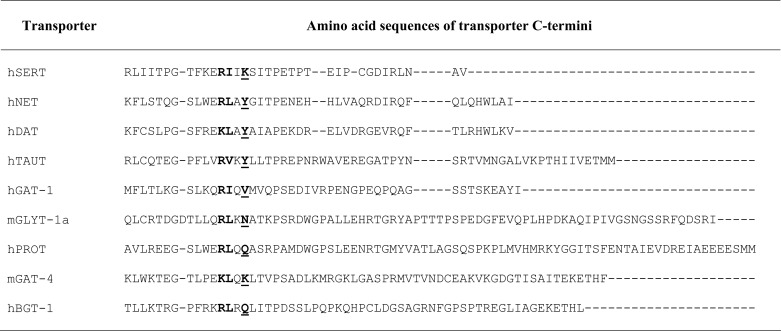
FIGURE 1.
SEC24 requirement of SERT C terminus truncation mutant (A) and of concatamer proteins comprised of SERT-SERT (C) or SERT-GAT-1 moieties (D) assessed by depleting SEC24 isoforms (B) using siRNA knockdown. A, HeLa cells were transfected with siRNAs directed against SEC24 isoforms C and D (10 nm, individually or combined SEC24A–D) or with the control siRNA. Forty-eight hours after siRNA transfection, the cells were transfected with plasmids encoding YFP-tagged wild type SERT or a mutant in which the last 15 residues were truncated (SERT-ΔC15). Transporter surface expression was assessed by determining [3H]5-HT uptake (n = 3–4 performed in triplicates; error bars indicate S.E.) as outlined under “Experimental Procedures.” B, HEK293 cells transfected with the indicated siRNAs were lysed to document isoform-specific down-regulation of each individual SEC24 paralog by immunoblotting. C and D, experimental conditions were as outlined for A, except that both HEK293 cells and HeLa cells were transfected with plasmids encoding a SERT-SERT concatemer (SESE, C) or a SERT-GAT-1 concatemer (SEGA, D). The data from the two cell lines were pooled. Because expression levels in HEK293 and HeLa cells differed, uptake values were normalized by setting the transport rate observed in the presence of control siRNA to 100%. These values were 58 ± 21 pmol/106 cells/min for SESE and 54 ± 20 pmol/106 cells/min for SEGA. The data were analyzed using one-way ANOVA. *, significantly different (p < 0.05) with respect to control.
Our siRNA-based approach relied on the assumption that relaxing the stringency of the interaction between the SEC24 isoform and SERT resulted in a reduction of transporters at the cell surface induced by both SEC24C- and SEC24D-directed siRNAs. We verified that this was amenable to detection by employing a concatamer comprising SERT and GAT-1 (referred to as SEGA in Fig. 1D). This concatemer contains two SEC24-binding sites, namely a SEC24C-specific one originating from the SERT moiety and a second, SEC24D-specific one provided by GAT-1. Surface levels of this construct were indeed reduced by depletion of either SEC24C or SEC24D (Fig. 1D). In contrast, the SERT-SERT concatemer (i.e., SESE) still relied exclusively on SEC24C (Fig. 1C).
Scanning SEC24 Isoforms Requirement of SERT C-terminal Mutants Sheds New Light onto SEC24 Isoform Selectivity
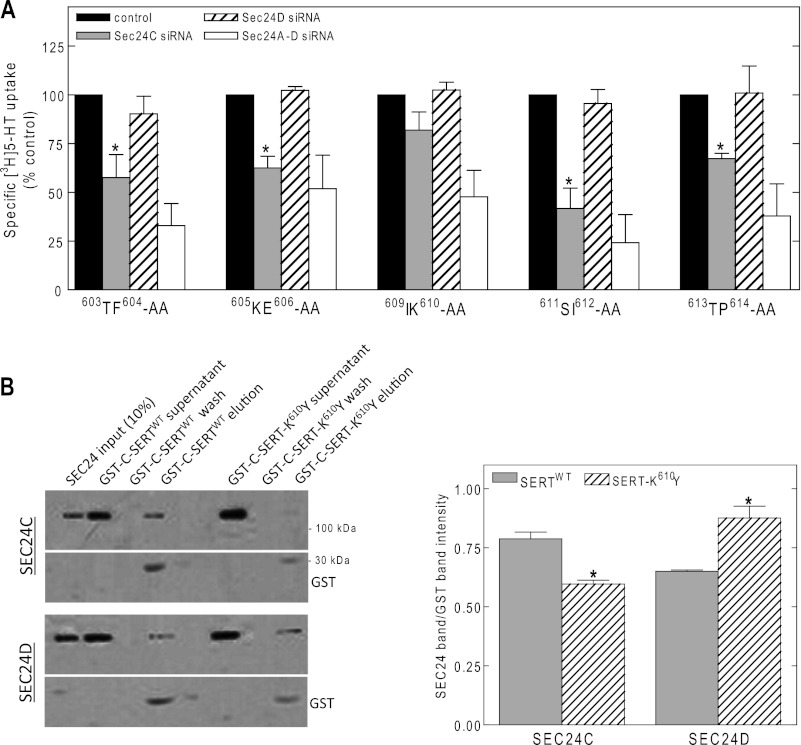
SEC24-binding motifs are thought to be comprised of short contiguous sequences (24). Truncation of the last 15 amino acids of SERT impaired cell surface expression of SERT in HeLa cells (but not in HEK293 cells; see Refs. 17 and 22), indicating that they are important for trafficking. However, in their absence, SERT still depends on SEC24C. We therefore surmised that one or several upstream residues were required to support discrimination between SEC24C and SEC24D. We therefore screened the next 12 amino acids by using double alanine substitutions. Our screen did not include 601PG602 and the core 607RI608 ER export motif: SERT-601PG602 fails to fold and is retained in the ER (17). SERT-R607A,I608A also incurs a folding problem, and the residual, low cell surface expression is independent of all SEC24 isoforms (17). We measured the effect of depleting SEC24C, SEC24D, or both on cell surface levels, i.e., cellular [3H]5-HT uptake by each mutant. Substrate uptake was found to be sensitive to SEC24C depletion for SERT mutants with alanines at the 603TF604, 605KE606, and 611SI612 positions (Fig. 2A). We noted two exceptions, namely SERT with alanines at positions 609IK610 (located adjacent to the 607RI608 motif) and 613TP614. These appeared to have a relaxed SEC24C requirement, i.e., depletion of either SEC24C or SEC24D did not suffice to cause a significant reduction in substrate reduce surface levels, but cell surface levels of the mutant transporters declined upon combined knockdown (Fig. 2A). Inspection of the other monoamine transporters revealed that the equivalent TP was also present in NET (Table 1). NET, however, requires SEC24D (18). Hence, the residues Thr and Pro did not account for the ability of SERT to discriminate between SEC24C and SEC24D. In fact, the corresponding proline residue is present in all SLC6 transporters (Table 1), which indicates that it plays a structural role. Inspection of the other monoamine transporters revealed that the lysine residue at position 610 was unique: DAT and NET have a bulky aromatic tyrosine at the equivalent position (Table 1). Based on these considerations, we created fusion proteins in which the C terminus of SERT was fused to GST and prepared a version in which Lys610 was substituted by Tyr. GST pulldown experiments indicated that the mutated C terminus indeed interacted to a lesser extent with SEC24C than the C terminus of wild type SERT (Fig. 2B, upper blots). Conversely, the mutated C terminus was more effective than the wild type C terminus in immobilizing SEC24D (Fig. 2B, lower blots).
FIGURE 2.
SEC24 requirement of alanine scanning mutants in the region flanking the 607RI608 motif of SERT (A) and pulldown of SEC24C by GST fusion proteins comprising the C terminus of wild type SERT or the K610Y mutant thereof (B). A, HeLa cells were transfected with siRNAs directed against SEC24 isoforms and plasmids encoding the indicated SERT mutants, which scan the region spanning from Thr603 to Pro614. Specific [3H]5-HT uptake (n = 3–4, performed in triplicates; error bars indicate S.E.) was measured to assess the impact of depleting either SEC24C, SEC24D, or all four SEC24 isoforms on cell surface expression of the mutated versions of SERT. The data are expressed as percentages of control for each of the mutants; mean control uptake values were: T603A,F604A (603TF604-AA), 17 ± 4 pmol/106 cells/min; K605A,E606A 605KE606-AA), 20 ± 6 pmol/106 cells/min; I609A,K610A (609IK610-AA), 20 ± 3 pmol/106 cells/min; S611A,I612A (611SI612-AA), 24 ± 5 pmol/106 cells/min; and T613A,P614A (613TP614-AA), 21 ± 4 pmol/106 cells/min. Asterisks indicate statistically significant differences (p < 0.05, t test for paired data) between down-regulation of SEC24C (gray bars) and SEC24D (hatched bars). The combined knockdown of all four SEC24 isoforms (white bar) differed in a statistically significant manner from control (full bar) (p < 0.01). B, GST pulldown experiments were performed as described under “Experimental Procedures” with purified fusion proteins comprising the C terminus of wild type SERT (GST-C-SERT) or of SERT-K610Y (GST-C-SERT-K610Y). The bands illustrate that wild type GST-C-SERT interacts with SEC24C to a visibly larger extent than the GST-C-SERT-K610Y mutant. Shown is the input (10%) and volume corrected aliquots of the supernatant, the last wash, and the eluate. The experiment was reproduced twice with similar results. The data were quantified (expressed as ratio of SEC24C/GST versus SEC24D/GST integrated band intensity), and the mutant was compared with the wild type SERT as shown in the rightmost bar diagram. *, significantly different (p < 0.05, t test) with respect to the wild type transporter.
The SERT-K610Y Mutant Fails to Recruit SEC24C
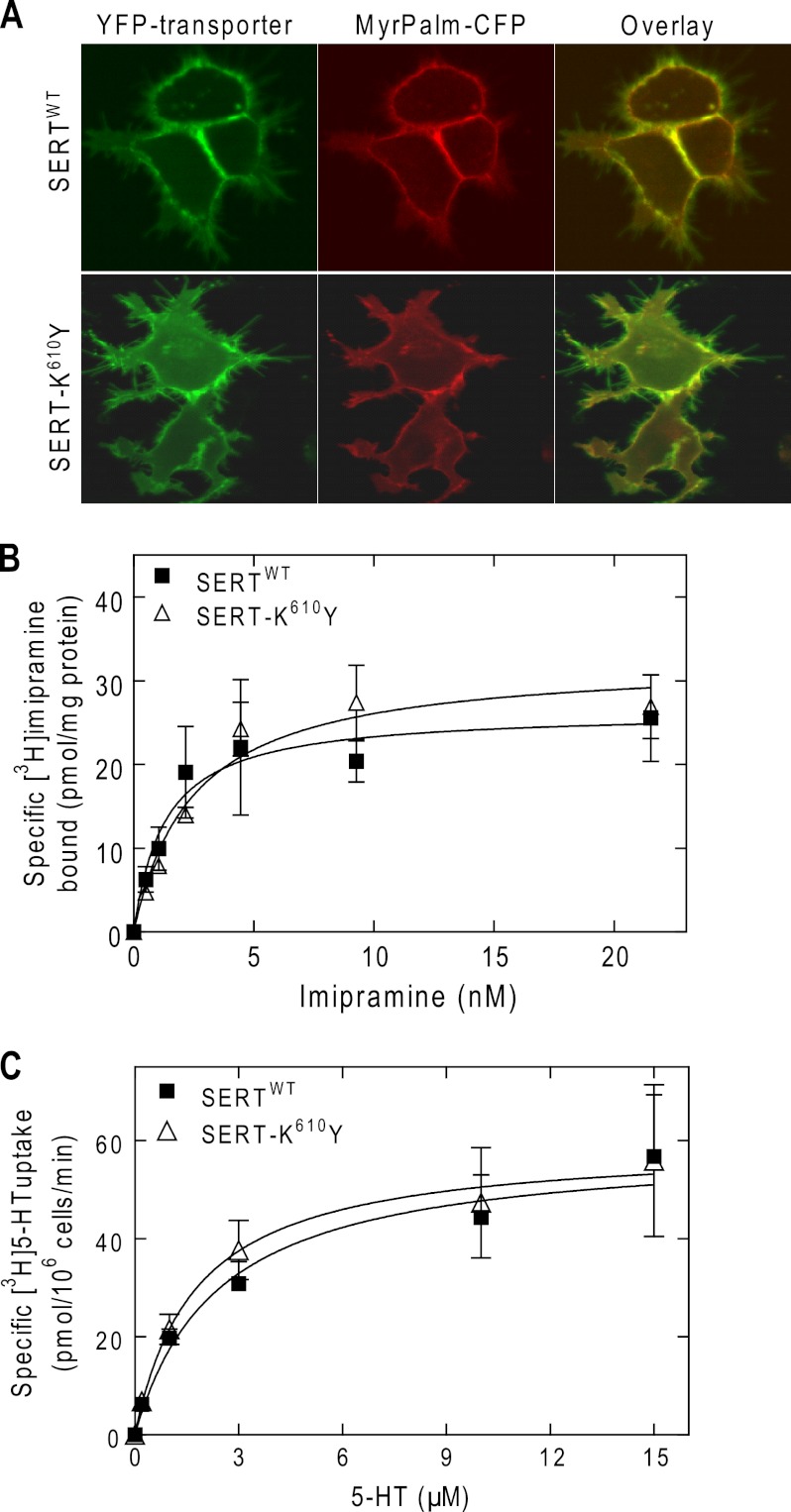
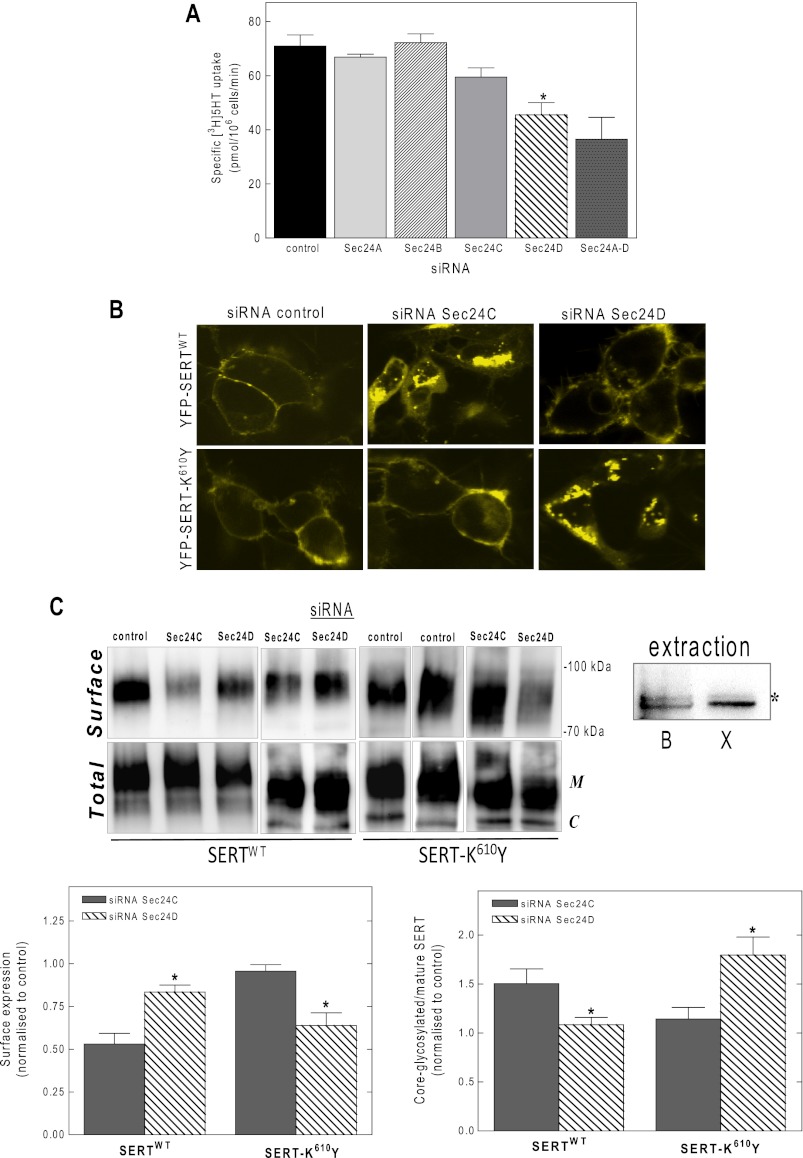
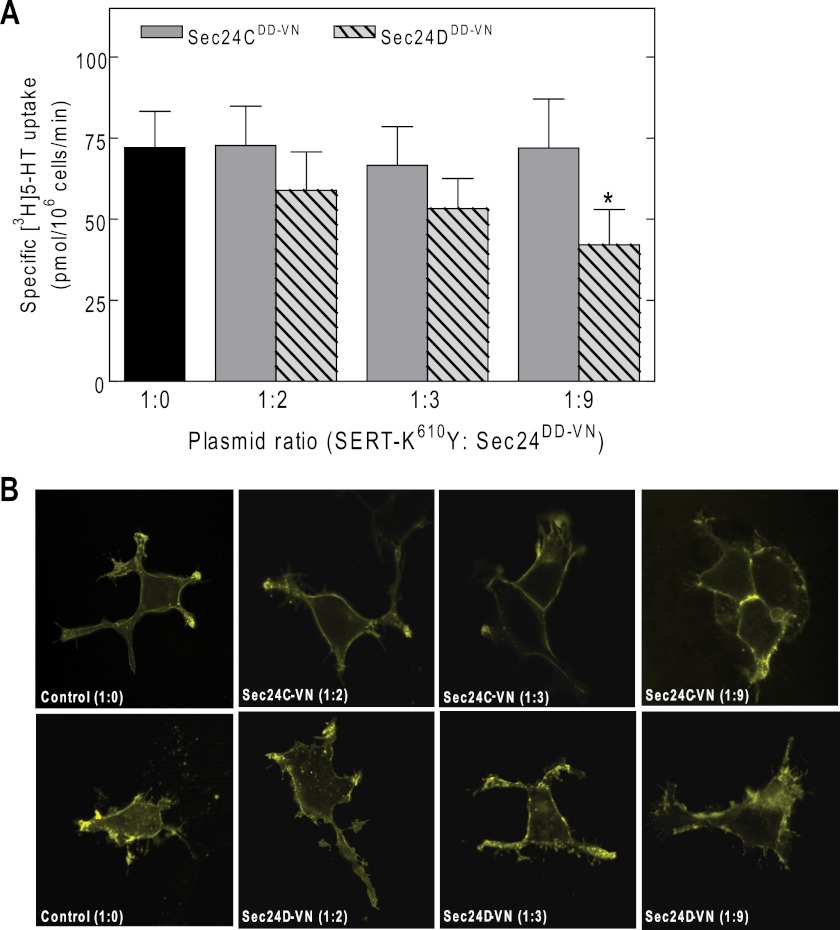
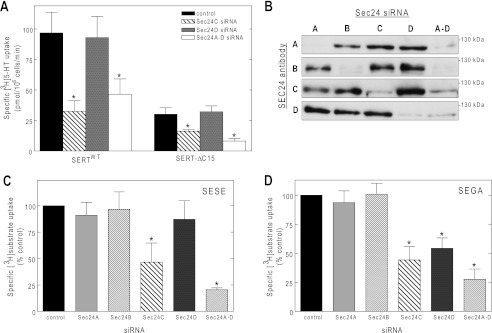
We created a mutated version of SERT, in which the residue Lys610 was replaced by tyrosine. Laser scanning microscopy showed that the YFP-tagged SERT-K610Y was targeted to the plasma membrane of transiently transfected HEK293 cells (Fig. 3A). Wild type SERT and SERT-K610Y had similar affinities for substrate (Fig. 3B) and for binding of the inhibitor [3H]imipramine (Fig. 3C). Because the Vmax (Fig. 3B) and Bmax parameters (Fig. 3C) were comparable, we conclude that substitution of Lys610 by Tyr did not affect the turnover number and hence the cycle between inward and outward facing conformations. Similar to wild type SERT, there were not any appreciable intracellular accumulations of SERT-K610Y (Fig. 3A). This suggested that SERT-K610Y was exported from the ER as efficiently as the wild type protein. Moreover, siRNA-induced knockdown of SEC24C did not reduce substrate uptake; this was only observed upon depletion of SEC24D (Fig. 4A). This observation was confirmed by visualizing the transporter by confocal microscopy: although siRNA-induced knockdown of SEC24C resulted in intracellular retention of the wild type SERT in HEK293 cells (Fig. 4B, top middle panel), accumulation of SERT-K610Y at the plasma membrane remained unimpaired (Fig. 4B, bottom middle panel). The contrary held true for SEC24D knockdown; unlike the wild type protein, the SERT-K610Y mutant became susceptible to depletion of SEC24 isoform D (Fig. 4B, top and bottom right panels, respectively). An identical outcome was obtained when the levels of transporter surface expression were examined by biotinylation (Fig. 4C). As can be seen from the representative immunoblot, lysates from SERT expressing cells contained two major bands: we previously verified that the lower band (labeled with C in Fig. 4C, lower blot) corresponds to the endoglycosidase H-sensitive, core-glycosylated (i.e., ER-retained) form of SERT, whereas the upper band was the mature, fully glycoslated protein (17). It is evident from the upper blot in Fig. 4C that the biotinylated material only contained a single species (consistent with surface labeling). It is also evident that there was some variability in expression levels. This is not surprising given that cells were subjected to two sequential transfections. We therefore quantified the effect of SEC24-directed siRNAs on biotinylation by relating it to the amount of total transporter in the detergent extract and comparing this ratio with that seen in cells transfected with control siRNA (bottom left panel in Fig. 4C). In addition, we also quantified the siRNA-induced change in the ER-resident form (bottom right panel in Fig. 4C). We stress that the detergent mixture employed in these experiments is optimized for recovering surface-expressed biotinylated SERT. It is less effective in extracting ER-retained SERT than the buffer used previously (17) (Fig. 4C, top right panel, lanes labeled extraction). This explains why the ER-retained bands in Fig. 4C appears to be less prominent than predicted from the confocal images showed in Fig. 4B. Because the ER-retained material is underrepresented in the extracts, the effect of the siRNA is underestimated. However, this systematic error does not affect the conclusion, namely that SEC24C is required for ER export of wild type SERT and that SEC24D supports ER export of SERT-K610Y. Moreover, co-expression of increasing amounts of dominant negative versions of SEC24C and SEC24D with SERT-K610Y in HEK293 cells showed a similar effect. Even at high plasmid ratios of the SEC24C-VN mutant, substrate uptake by SERT-K610Y was not decreased (gray bars in Fig. 5A), but a significant reduction in uptake was detected when the cells were co-transfected with the same amount of plasmid encoding the SEC24D-VN mutant (hatched bars in Fig. 5A). The findings were further confirmed by confocal microscopy (Fig. 5B).
FIGURE 3.
Cell surface expression (A), substrate transport (B), and inhibitor binding (C) of YFP-tagged SERT-K610Y compared with wild type SERT. A, HEK293 cells were transfected with plasmids driving the expression of YFP-tagged wild type SERT or SERT-K610Y mutant. The cell surface was visualized by co-expression of an N-terminally myristoylated and palmitoylated CFP (MyrPalm-CFP). The images were captured by confocal microscopy under settings that recorded the fluorescence of YFP (left panels) or CFP (middle panels). Overlay images (right panels) were generated to visualize co-localization of both wild type and mutant transporter and MyrPalm-CFP on the cell membrane. B, uptake of [3H]5-HT by cells expressing wild type SERT (filled symbols) or SERT-K610Y (open symbols) was measured in the absence or presence of 10 μm paroxetine (to determine nonspecific uptake, which was subtracted from total uptake). The Km values were 2.4 μm (0.5–4.3 μm) for the wild type and 1.8 μm (0.1–3.4 μm) for the SERT-K610Y mutant; the Vmax values were 59 ± 7 and 59 ± 8 pmol/106 cells/min for the wild type and mutant SERT, respectively. C, membranes (20 μg/assay) prepared from HEK293 cells expressing wild type SERT (filled symbols) or SERT-K610Y (open symbols) were incubated with the indicated concentrations of [3H]imipramine, as outlined under “Experimental Procedures.” Nonspecific binding was determined in the presence of 10 μm paroxetine. The data in B and C are from three independent experiments that were done in parallel and performed in triplicate; the error bars indicate S.E. The KD values were 1.3 nm (0.1–2.7 nm) for the wild type transporter and 2.5 nm (1.1–3.9 nm) for the mutant; the Bmax values were 26 ± 4 and 32 ± 3 pmol/mg protein for the wild type and SERT-K610Y mutant, respectively.
FIGURE 4.
Effect of siRNA-induced depletion of individual SEC24 isoforms on ER export of the SERT-K610Y mutant. siRNA-induced knockdown of SEC24A–D was done in HEK293 cells as outlined in the legend to Fig. 1 and under “Experimental Procedures.” Cell surface levels of SERT-K610Y were determined by measuring specific [3H]5-HT uptake (A), visualized by confocal microscopy (B), and quantified by cell surface biotinylation (C). The bars represent the means ± S.E. from three independent experiments performed in triplicate. *, significantly different (p < 0.05) from control, siRNA against SEC24A, SEC24B, and SEC24C (ANOVA). There was no statistically significant difference in uptake by cells transfected with siRNA for SEC24C and the combination of all siRNAs (siRNA SEC24A–D). Surface biotinylation experiments were carried out as described under “Experimental Procedures.” C, representative immunoblots showing the expression levels of wild type SERT (left panels) and SERT-K610Y (right panels). Top panels, surface expression; bottom panels, total lysate fractions. The integrated intensity of the biotinylated bands were quantified by Image J, related to the total amount of SERT immunoreactivity in the lysate (i.e., the bands with mature glycosylation M) and expressed as fraction of negative control (i.e., transfection with an irrelevant siRNA) for both wild type and mutant SERT (bottom left panel). In addition, the core-glycosylated (ER-resident) bands (C) and bands with mature glycosylation (M) were quantified in the lysate, and their ratio was calculated and expressed as a fraction of control (bottom right panel). The data are the means ± S.E. (n = 3); statistically significant differences were confirmed for both wild type and K610Y-SERT upon depletion of SEC24C versus SEC24D (*, p < 0.05) by a paired t test. In the lanes labeled extraction, two different buffers were compared for their ability to solubilize ER-resident SERT (buffer B optimized for biotinylation experiment: 10 mm Tris·HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate; buffer X optimized for retrieval of ER-resident SERT; see Ref. 17; 50 mm Tris·HCl, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 0.1% SDS, 0.5% sodium deoxycholate). HEK293 cells were transiently with a plasmid driving the expression of SERT and lysed 11 h after transfection, i.e., when the bulk of the transporters was still in the ER and identical amounts of membranes were extracted with these two buffers. Extracts (10 μg) were electrophoretically resolved, and the amount of SERT was visualized on nitrocellulose blots; buffer B was less effective in solubilizing the lower (core-glycosylated) band than buffer X. In contrast, there were comparable (albeit faint) amounts of the upper mature glycosylated band (indicated by an asterisk).
FIGURE 5.
A dominant negative mutant of SEC24D, but not of SEC24C, decreases substrate uptake by SERT-K610Y (A) by causing intracellular retention of the mutant transporter (B). HEK293 cells were transiently transfected with a plasmid driving the expression of SERT-K610Y, in the absence or presence of the increasing amounts of plasmids (plasmid ratio indicated in the figure caption) encoding dominant negative mutants of SEC24C (SEC24C-796DD797-VN) and SEC24D (SEC24D-733DD734-VN). Cell surface levels of SERT-K610Y were quantified by measuring specific [3H]5-HT uptake (A) or visualized by confocal microscopy (B). The bars represent the means ± S.E. from four independent experiments (means ± S.E.). *, significantly different (p < 0.01) from control and dominant negative SEC24C (one-way ANOVA, followed by Tukey's post hoc test). There was no statistically significant difference in uptake by cells transfected with control plasmid and a dominant negative version of SEC24C.
Specificity for SEC24C or SEC24D Is Determined by the +2 Position after the RL/RI Motif
Upon mutation of Lys610 to Tyr, ER export of SERT was dependent on SEC24D, indicating that this residue played an essential role in selecting the SEC24 isoform. We tested this interpretation by introducing the reverse mutation in the NET, substituting the wild type tyrosine residue by a lysine. We found that the resulting mutant (NET-Y590K) also depended on SEC24C for ER export (Fig. 6A) and thus phenocopied SERT. Furthermore, an inspection of the C termini of SLC6 family members reveals that murine GAT-4 (equivalent to rat GAT-3) also carries a lysine residue in the position corresponding to that of Lys610 of SERT (Table 1). Our observations predict that mGAT-4 ought to depend on SEC24C rather than SEC24D for its ER export. This prediction was confirmed by both siRNA-induced knockdown of SEC24A–D (Fig. 6B) and co-expression with dominant negative versions of SEC24C and SEC24D (Fig. 6C). The alignment shown in Table 1 highlights the fact that this position is either occupied by tyrosine (NET, DAT, and taurine transporter TAUT) and valine (GAT-1) or by lysine (SERT and mGAT-4) and glutamine and asparagine (BGT-1, GLYT-1a, and proline transporter PROT). GAT-1 (14), NET, and DAT (18) require SEC24D. These transporters carry the hydrophobic residues valine and tyrosine (hydrophobicity of 0.96 and 1.22, respectively) (25) in the +2 position. In contrast, asparagine and glutamine have a polar group, and lysine is positively charged at physiological pH such that their side chains are hydrophilic (hydrophobicity of −0.60, −0.22, and −0.90, respectively). We therefore surmised that asparagine and glutamine also specify an interaction with SEC24C. We verified this conjecture by selecting GLYT-1a and BGT-1, which have an asparagine and a glutamine, respectively, at the +2 position (Table 1): regardless of whether assessed by siRNA-induced knockdown (Fig. 7, A and C) or by co-expression with dominant negative versions of SEC24 (i.e., SEC24C-796DD797-VN or SEC24D-733DD734-VN; Fig. 7, B and D), surface levels of GLYT-1a and BGT-1 depended on SEC24C.
FIGURE 6.
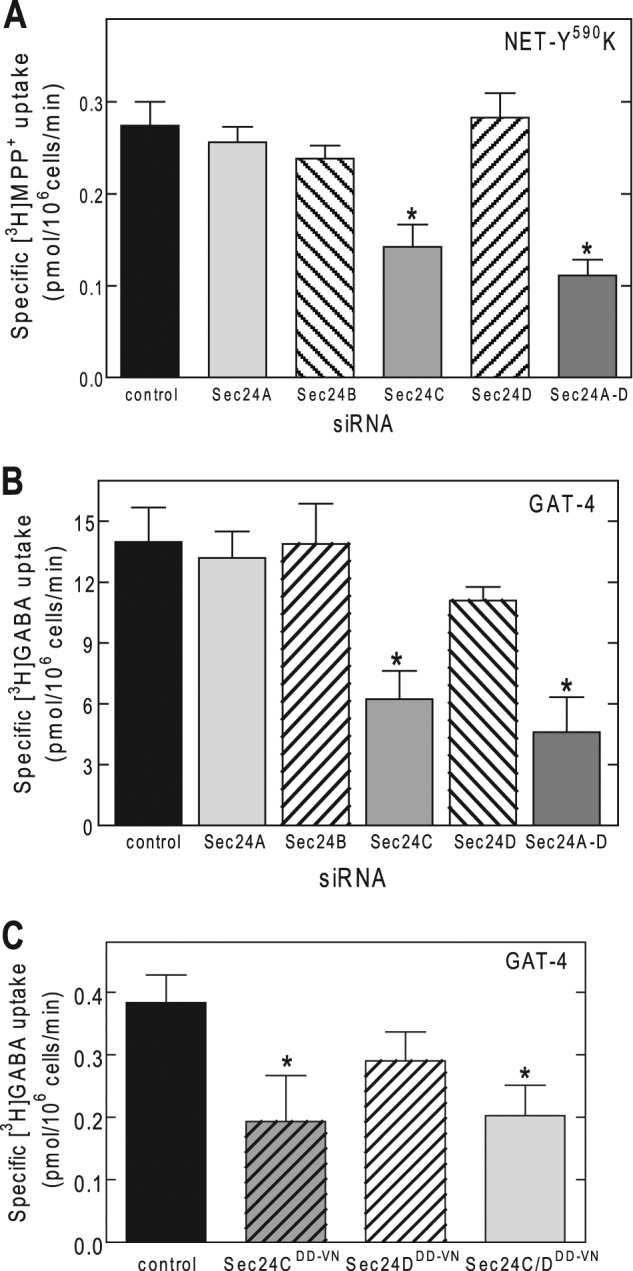
SEC24C is required for cell surface expression of NET-Y590K mutant and GAT-4. A and B, HEK293 cells were transfected with siRNAs directed against SEC24A–D and 48 h later with the cDNAs encoding NET-Y590K (A) or GAT-4 (B). Uptake of substrates (i.e., [3H]GABA for GAT-4 and [3H]MPP+ for NET-Y590K) was used to quantify cell surface expression of the transporters. Uptake was measured in the absence or presence of 10 μm nisoxetine or 600 μm GABA to determine nonspecific uptake by NET and GAT-4, respectively. C, HEK293 cells were transfected with a plasmid encoding GAT-4, in the absence or presence of a 6-fold excess of plasmids encoding dominant negative versions of SEC24C (SEC24C-796DD797-VN) and SEC24D (SEC24D-733DD734-VN) or the combination thereof. Co-expression with a dominant negative version of SEC24C, but not SEC24D, markedly reduces the expression of GAT-4. The data are from three or four independent experiments carried out in triplicate. *, significantly different (p < 0.01) from control (ANOVA followed by Tukey's post hoc test).
FIGURE 7.
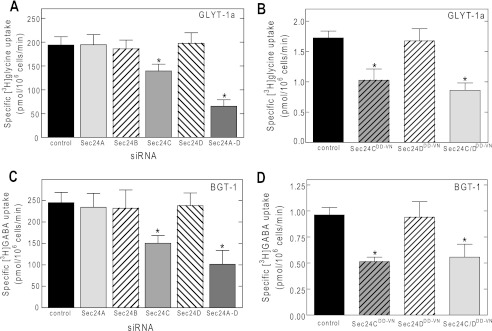
Other SLC6 transporter family members, GLYT-1a and BGT-1, also rely on SEC24C for their ER export. HEK293 cells were transfected with siRNAs or dominant negative versions of SEC24C or D with the plasmids encoding GLYT-1a (A and B) or BGT-1 (C and D) (as outlined under “Experimental Procedures” and above in the legend to Fig. 6). Uptake of substrates (i.e., [3H]GABA for BGT-1 and [3H]glycine for GLYT-1a) was used to quantify cell surface expression of the two transporters. The uptake was measured in the absence or presence of 10 μm NNC 05-2090 or 10 mm glycine to determine nonspecific uptake by BGT-1 or GLYT-1a, respectively. The data are from three to five independent experiments and carried out in triplicate. *, significantly different (p < 0.01) from control and dominant negative SEC24D (ANOVA followed by Tukey's post hoc test).
DISCUSSION
The mechanism of COPII assembly is understood in considerable detail (24, 26). It is less clear how cargo discriminates between different SEC23/SEC24 dimers. The human genome encodes about 6000 integral membrane proteins, the vast majority of which are subject to COPII-dependent ER export (27). There are four different SEC24 paralogs, and they differ in cargo specificity (23). To the best of our knowledge, our experiments are the first to examine the basis for discrimination by related cargo molecules among SEC24 isoforms. Although the RI/RL/KL motif is conserved in all SLC6 family members (and in species orthologs from worm to man), the flanking sequences are divergent. We observed a crucial contribution of the residue at the +2 position in determining the interaction with SEC24C or SEC24D. The findings allow for deducing the following rule: a hydrophobic residue at the +2 position specifies recruitment of SEC24D, whereas a polar side chain supports engagement of SEC24C. This conclusion is supported by the following observations: (i) Mutation of this position to alanine relaxed the specificity of SERT such that only a combined knockdown reduced cell surface levels (Fig. 2A). (ii) Replacing lysine by tyrosine produced a version of SERT that failed to reach the cell surface in cells depleted of SEC24D, but not of SEC24C (Fig. 4). This showed that it relied exclusively on SEC24D and thus phenocopied its closest relatives NET and DAT. This mutation also reduced the ability of the C terminus to bind SEC24C in vitro (Fig. 2B). (iii) Cell surface expression of those SLC6 family members, which carried a hydrophilic residue at the +2 position, was also only impaired upon siRNA-mediated depletion of SEC24C (Figs. 6 and 7). Between the invariant glycine (Gly602 in SERT) and proline (Pro614 in SERT), the amino acids flanking the core RI/RL/KL motif show a variable degree of conservation, but this does not allow discrimination a posteriori between SEC24C and SEC24D clients. Residues distal to the invariant proline are unlikely to account for any specificity because they can be truncated in SERT without relaxing the specificity for SEC24C. Similarly, the C terminus of GAT-1 can be truncated by 27 amino acids without impairing its ER export beyond that caused by elimination of the last three amino acids, which constitute the type II PDZ motif (13). (iv) The approach that relied on dominant negative versions of SEC24 recapitulated the observations that were obtained by siRNA-dependent depletion of SEC24C and SEC24D (Figs. 5A, 6C, and 7, B and D).
There are three known binding sites that have been visualized on SEC24C, with sites A and B recognizing a YXXXNPF motif, a diacidic motif (DXE and the related sequences LXXLE and LXXME), respectively (24). Site C is bipartite, is contributed in part by SEC23, and recognizes the inactive closed conformation of the SNARE protein SEC22 (28). Currently, we cannot rule out that additional portions of the SLC6 intracellular segments contribute to the ER export motif resulting in a bipartite—possibly conformation-sensitive—binding site. Conceptually, a bipartite, conformation-sensitive binding site is attractive, because it engineers proofreading into cargo selection. The transporter must adopt a stable conformation prior to COPII recruitment. This precludes premature ER export of partially folded transporters. There are three lines of circumstantial evidence to support the conjecture that additional segments of the transporter contribute to recruiting SEC24 paralogs: (i) We note that the GST fusion protein comprising the mutated C terminus (with the K610Y) mutation still bound SEC24C albeit less efficiently than the fusion protein with the wild type C terminus. In contrast, the results obtained with the siRNA knockdown experiments suggested that the mutation sufficed to completely switch isoform dependence of ER export of SERT from SEC24C to SEC24D. (ii) Substitution of the conserved residues 613TP614 by alanine relaxed the exclusive reliance on SEC24C. This may also point to the contribution of an additional segment of the transporter to the binding surface for SEC24C and/or the SEC23/SEC24C dimer. (iii) Finally, a fragment of SERT that comprises only transmembrane helices 11 and 12 and the C terminus fails to be exported from the ER; in fact, this domain, which can dimerize with the intact transporter, inhibits ER export of full-length SERT in a dominant negative manner (29). Regardless of whether the SLC6 family members engage a single or a bipartite binding site on the SEC24/SEC23 dimer, it appears justified to assume that the binding site resides in the vicinity of 796DD797 or 733DD734 on SEC24C and SEC24D, respectively. These two acidic residues are conserved in all SEC24 isoforms. Nevertheless, all SLC6 family members tested rely exclusively on either SEC24C or SEC24D. The sequence divergence in the SEC24 paralogs can be exploited to map the binding site. This is currently being explored.
Mutations in both genes encoding SEC23 and SEC24 isoforms have already been identified in a number of pathological conditions (26). Mutated SEC24B fails to recruit the planar cell polarity component Vangl2. The resulting defect in ER export of the transmembrane protein Vangl2 is thought to disrupt the polarity of the neuroepithelial sheet and to preclude closure of the neural tube leading to craniorachischisis (30, 31) and to impair development of the lung epithelium (32). Truncations in the medaka SEC24D impair ER export of collagen II and result in skeletal malformation (vertebra imperfecta) in the hatching fish (33). Mutations in sec23a and sec23b lead to cranio-lenticulo-sutural dysplasia (34) and congenital dysrythropoietic anemia type II (35), respectively. Cellular surveys with model cargo molecules suggest that, in some instances, the different isoforms of COPII components function in a redundant manner (23). The phenotypes of the individual mutations, however, prove that, at least for some cargos, the specificity is exquisite. In addition, these models establish a role for mutations in genes encoding SEC23 and SEC24 paralogs in monogenic diseases. It is also conceivable that many disease states are linked to COPII machinery deficiencies, i.e., inefficient export from ER compartments. The sec24c gene has repeatedly been identified in association studies of Alzheimer disease (36, 37). On statistical grounds, SEC24C was assigned the top rank in a list of transcripts that differed in levels in control samples of brain prefrontal cortex and patients with bipolar disease (38). Incidentally, SLC6A11 (human GAT-3 = murine GAT-4), which we identify as a client of SEC24C, was ranked fifth on this list of 103 differentially expressed genes (38). It is worth noting that this sample cannot assess SERT: although SERT containing axons project to the cortex, the cell bodies, and hence the mRNA, reside in the brain stem, i.e., the pontine raphe nuclei. Nevertheless, it is attractive to speculate that changes in SEC24C translate into distinct surface levels of neurotransmitter transporters and so alter synaptic transmission in the short term and neuronal wiring patterns in the long term. Thus, our findings do, in principle, have explanatory power and may bear some direct relation to human diseases.
Acknowledgments
We thank Nathan Nelson (Tel Aviv University) and Arne Schousboe and Bolette Christiansen (University of Copenhagen) for plasmids encoding mouse GAT-4 and human BGT-1, respectively. We are grateful to Jean-Pierre Paccaud (OM Pharma, Geneva, Switzerland) and Hans-Peter Hauri (Biozentrum, Universität Basel), Randy Schekman (University of California, Berkeley), and Roger Tsien (University of California, San Diego) for the plasmids encoding SEC24C, anti-SEC24A–D antibodies, and MyrPalm-CFP, respectively.
This work was supported by Project Program Grant SFB35 from the Austrian Science Fund/FWF (to M. F.).
- SERT
- serotonin transporter
- 5-HT
- 5-hydroxytryptamine (serotonin)
- BGT-1
- betaine/γ-aminobutyric acid transporter 1
- CFP
- cyan fluorescent protein
- COPII
- coat protein complex II
- DAT
- dopamine transporter
- ER
- endoplasmic reticulum
- GAT
- GABA transporter member
- GLYT-1a
- glycine transporter isoform 1a
- NET
- noradrenaline transporter
- YFP
- yellow fluorescent protein
- ANOVA
- analysis of variance.
REFERENCES
- 1. Kristensen A. S., Andersen J., Jørgensen T. N., Sørensen L., Eriksen J., Loland C. J., Strømgaard K., Gether U. (2011) SLC6 neurotransmitter transporters. Structure, function, and regulation. Pharmacol. Rev. 63, 585–640 [DOI] [PubMed] [Google Scholar]
- 2. Lesch K. P., Bengel D., Heils A., Sabol S. Z., Greenberg B. D., Petri S., Benjamin J., Müller C. R., Hamer D. H., Murphy D. L. (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531 [DOI] [PubMed] [Google Scholar]
- 3. Caspi A., Sugden K., Moffitt T. E., Taylor A., Craig I. W., Harrington H., McClay J., Mill J., Martin J., Braithwaite A., Poulton R. (2003) Influence of life stress on depression. Moderation by a polymorphism in the 5-HTT gene. Science 301, 386–389 [DOI] [PubMed] [Google Scholar]
- 4. Androutsellis-Theotokis A., Goldberg N. R., Ueda K., Beppu T., Beckman M. L., Das S., Javitch J. A., Rudnick G. (2003) Characterization of a functional bacterial homolog of sodium-dependent neurotransmitter transporters. J. Biol. Chem. 278, 12703–12709 [DOI] [PubMed] [Google Scholar]
- 5. Yamashita A., Singh S. K., Kawate T., Jin Y., Gouaux E. (2005) Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature 437, 215–223 [DOI] [PubMed] [Google Scholar]
- 6. Sager J. J., Torres G. E. (2011) Proteins interacting with monoamine transporters. Current state and future challenges. Biochemistry 50, 7295–7310 [DOI] [PubMed] [Google Scholar]
- 7. Prasad H. C., Zhu C. B., McCauley J. L., Samuvel D. J., Ramamoorthy S., Shelton R. C., Hewlett W. A., Sutcliffe J. S., Blakely R. D. (2005) Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc. Natl. Acad. Sci. U.S.A. 102, 11545–11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farhan H., Rabouille C. (2011) Signalling to and from the secretory pathway. J. Cell Sci. 124, 171–180 [DOI] [PubMed] [Google Scholar]
- 9. Farhan H., Wendeler M. W., Mitrovic S., Fava E., Silberberg Y., Sharan R., Zerial M., Hauri H. P. (2010) MAPK signaling to the early secretory pathway revealed by kinase/phosphatase functional screening. J. Cell Biol. 189, 997–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharpe L. J., Luu W., Brown A. J. (2011) Akt phosphorylates Sec24. New clues into the regulation of ER-to-Golgi trafficking. Traffic 12, 19–27 [DOI] [PubMed] [Google Scholar]
- 11. Scholze P., Freissmuth M., Sitte H. H. (2002) Mutations within an intramembrane leucine heptad repeat disrupt oligomer formation of the rat GABA transporter-1. J. Biol. Chem. 277, 43682–43690 [DOI] [PubMed] [Google Scholar]
- 12. Korkhov V. M., Farhan H., Freissmuth M., Sitte H. H. (2004) Oligomerization of the γ-aminobutyric acid transporter-1 is driven by an interplay of polar and hydrophobic interactions in transmembrane helix II. J. Biol. Chem. 279, 55728–55736 [DOI] [PubMed] [Google Scholar]
- 13. Farhan H., Korkhov V. M., Paulitschke V., Dorostkar M. M., Scholze P., Kudlacek O., Freissmuth M., Sitte H. H. (2004) Two discontinuous segments in the carboxyl terminus are required for membrane targeting of the rat γ-aminobutyric acid transporter-1 (GAT1). J. Biol. Chem. 279, 28553–28563 [DOI] [PubMed] [Google Scholar]
- 14. Farhan H., Reiterer V., Korkhov V. M., Schmid J. A., Freissmuth M., Sitte H. H. (2007) Concentrative export from the endoplasmic reticulum of the γ-aminobutyric acid transporter 1 requires binding to SEC24D. J. Biol. Chem. 282, 7679–7689 [DOI] [PubMed] [Google Scholar]
- 15. Farhan H., Reiterer V., Kriz A., Hauri H. P., Pavelka M., Sitte H. H., Freissmuth M. (2008) Signal-dependent export of GABA transporter 1 from the ER-Golgi intermediate compartment is specified by a C-terminal motif. J. Cell Sci. 121, 753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reiterer V., Maier S., Sitte H. H., Kriz A., Rüegg M. A., Hauri H. P., Freissmuth M., Farhan H. (2008) Sec24- and ARFGAP1-dependent trafficking of GABA transporter-1 is a prerequisite for correct axonal targeting. J. Neurosci. 28, 12453–12464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El-Kasaby A., Just H., Malle E., Stolt-Bergner P. C., Sitte H. H., Freissmuth M., Kudlacek O. (2010) Mutations in the carboxyl-terminal SEC24 binding motif of the serotonin transporter impair folding of the transporter. J. Biol. Chem. 285, 39201–39210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sucic S., El-Kasaby A., Kudlacek O., Sarker S., Sitte H. H., Marin P., Freissmuth M. (2011) The serotonin transporter is an exclusive client of the coat protein complex II (COPII) component SEC24C. J. Biol. Chem. 286, 16482–16490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seidel S., Singer E. A., Just H., Farhan H., Scholze P., Kudlacek O., Holy M., Koppatz K., Krivanek P., Freissmuth M., Sitte H. H. (2005) Amphetamines take two to tango. An oligomer-based counter-transport model of neurotransmitter transport explores the amphetamine action. Mol. Pharmacol. 67, 140–151 [DOI] [PubMed] [Google Scholar]
- 20. Steinkellner T., Yang J. W., Montgomery T. R., Chen W. Q., Winkler M. T., Sucic S., Lubec G., Freissmuth M., Elgersma Y., Sitte H. H., Kudlacek O. (2012) Ca2+/calmodulin-dependent protein kinase IIα (αCaMKII) controls the activity of the dopamine transporter. Implications for Angelman syndrome. J. Biol. Chem. 287, 29627–29635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fernández-Sánchez E., Díez-Guerra F. J., Cubelos B., Giménez C., Zafra F. (2008) Mechanisms of endoplasmic-reticulum export of glycine transporter-1 (GLYT-1). Biochem. J. 409, 669–681 [DOI] [PubMed] [Google Scholar]
- 22. Larsen M. B., Fjorback A. W., Wiborg O. (2006) The C-terminus is critical for the functional expression of the human serotonin transporter. Biochemistry 45, 1331–1337 [DOI] [PubMed] [Google Scholar]
- 23. Wendeler M. W., Paccaud J. P., Hauri H. P. (2007) Role of Sec24 isoforms in selective export of membrane proteins from the endoplasmic reticulum. EMBO Rep. 8, 258–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gillon A. D., Latham C. F., Miller E. A. (2012) Vesicle-mediated ER export of proteins and lipids. Biochim. Biophys. Acta 1821, 1040–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fauchère J., Pliska V. (1983) Hydrophobic parameters π of amino acid side chains from the partitioning of N-acetyl-amino-acid amides. Eur. J. Med. Chem. 18, 369–375 [Google Scholar]
- 26. Zanetti G., Pahuja K. B., Studer S., Shim S., Schekman R. (2012) COPII and the regulation of protein sorting in mammals. Nat. Cell Biol. 14, 20–28 [DOI] [PubMed] [Google Scholar]
- 27. Dancourt J., Barlowe C. (2010) Protein sorting receptors in the early secretory pathway. Annu. Rev. Biochem. 79, 777–802 [DOI] [PubMed] [Google Scholar]
- 28. Mancias J. D., Goldberg J. (2007) The transport signal on Sec22 for packaging into COPII-coated vesicles is a conformational epitope. Mol. Cell 26, 403–414 [DOI] [PubMed] [Google Scholar]
- 29. Just H., Sitte H. H., Schmid J. A., Freissmuth M., Kudlacek O. (2004) Identification of an additional interaction domain in transmembrane domains 11 and 12 that supports oligomer formation in the human serotonin transporter. J. Biol. Chem. 279, 6650–6657 [DOI] [PubMed] [Google Scholar]
- 30. Merte J., Jensen D., Wright K., Sarsfield S., Wang Y., Schekman R., Ginty D. D. (2010) Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat. Cell Biol. 12, 41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wansleeben C., Feitsma H., Montcouquiol M., Kroon C., Cuppen E., Meijlink F. (2010) Planar cell polarity defects and defective Vangl2 trafficking in mutants for the COPII gene Sec24b. Development 137, 1067–1073 [DOI] [PubMed] [Google Scholar]
- 32. Wansleeben C., van Gurp L., Feitsma H., Kroon C., Rieter E., Verberne M., Guryev V., Cuppen E., Meijlink F. (2011) An ENU-mutagenesis screen in the mouse. Identification of novel developmental gene functions. PLoS One 6, e19357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ohisa S., Inohaya K., Takano Y., Kudo A. (2010) sec24d encoding a component of COPII is essential for vertebra formation, revealed by the analysis of the medaka mutant, vbi. Dev. Biol. 342, 85–95 [DOI] [PubMed] [Google Scholar]
- 34. Boyadjiev S. A., Fromme J. C., Ben J., Chong S. S., Nauta C., Hur D. J., Zhang G., Hamamoto S., Schekman R., Ravazzola M., Orci L., Eyaid W. (2006) Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat. Genet. 38, 1192–1197 [DOI] [PubMed] [Google Scholar]
- 35. Schwarz K., Iolascon A., Verissimo F., Trede N. S., Horsley W., Chen W., Paw B. H., Hopfner K. P., Holzmann K., Russo R., Esposito M. R., Spano D., De Falco L., Heinrich K., Joggerst B., Rojewski M. T., Perrotta S., Denecke J., Pannicke U., Delaunay J., Pepperkok R., Heimpel H. (2009) Mutations affecting the secretory COPII coat component SEC23B cause congenital dyserythropoietic anemia type II. Nat. Genet. 41, 936–940 [DOI] [PubMed] [Google Scholar]
- 36. Morgan A. R., Turic D., Jehu L., Hamilton G., Hollingworth P., Moskvina V., Jones L., Lovestone S., Brayne C., Rubinsztein D. C., Lawlor B., Gill M., O'Donovan M. C., Owen M. J., Williams J. (2007) Association studies of 23 positional/functional candidate genes on chromosome 10 in late-onset Alzheimer's disease. Am. J. Med. Genet. B Neuropsychiatr. Genet. 144B, 762–770 [DOI] [PubMed] [Google Scholar]
- 37. Yu W., Clyne M., Khoury M. J., Gwinn M. (2010) Phenopedia and Genopedia. Disease-centered and gene-centered views of the evolving knowledge of human genetic associations. Bioinformatics 26, 145–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee S. A., Tsao T. T., Yang K. C., Lin H., Kuo Y. L., Hsu C. H., Lee W. K., Huang K. C., Kao C. Y. (2011) Construction and analysis of the protein-protein interaction networks for schizophrenia, bipolar disorder, and major depression. BMC Bioinformatics 12, (Suppl. 13) S20. [DOI] [PMC free article] [PubMed] [Google Scholar]