Background: A putative association between the trafficking protein TMED1 and the IL-33 receptor ST2L has previously been reported; however, the functional consequence of this remained unknown.
Results: TMED1 binds ST2L, and TMED1 knockdown impairs IL-33-induced IL-8 and IL-6 production.
Conclusion: TMED1 is required for optimal IL-33-induced signal activation.
Significance: These results further implicate the TMED family as an important family in immune signaling pathways.
Keywords: Immunology, Interleukin, Intracellular Trafficking, Signal Transduction, Toll IL-1 Receptor (TIR) Domain, IL-33
Abstract
The proinflammatory danger signal IL-33, which is released from damaged or dying cells, achieves its effects via the IL-1R family member ST2L. The detection of IL-33 by ST2L initiates downstream signaling pathways that result in the activation of MAPKs and NF-κB. Here, we show that TMED1 associates with ST2L. Using a series of mutation and deletion constructs, we demonstrate that this interaction is mediated by the GOLD domain of TMED1 and the TIR domain of ST2L. Our findings also demonstrate that TMED1 is required for optimal IL-33-induced IL-8 and IL-6 production. This discovery provides additional support to the concept that the TMED family members are important players in innate immune signaling.
Introduction
ST2 is a member of the IL-1 receptor (IL-1R)2/Toll-like receptor (TLR) superfamily. The ST2 gene encodes three known isoforms: ST2L, a transmembrane receptor; sST2, a secreted soluble form that can act as a decoy receptor; and ST2V, which is present mainly in gastrointestinal organs (1, 2). ST2L (also known as T1, IL-1RL1, and DER4) is a classic type I membrane receptor that contains three extracellular Ig-like domains, a transmembrane domain, and an intracellular TIR (Toll/IL-1 receptor) domain and is the primary receptor for the IL-1 family member IL-33 (3). It is expressed on several immune cell types, including Th2 lymphocytes, mast cells, natural killer cells, natural killer T-cells, monocytes, dendritic cells, and granulocytes as well as epithelial and endothelial cells (4).
Following IL-33 association with ST2L, the IL-1R accessory protein is in turn recruited to the complex in a ligand-dependent manner, allowing the initiation of downstream signaling pathways (5, 6). MyD88 (myeloid differentiation factor 88), IRAK-4 (interleukin-1 receptor-associated kinase 4), IRAK-1, and TRAF6 (TNF-α receptor-associated factor 6) are also recruited to ST2L, similar to other members of the IL-1R/TLR superfamily (3, 7). The activation of these pathways results in the phosphorylation of the MAPKs ERK1/2, p38, and JNK. In addition, the phosphorylation and degradation of IκBα (inhibitor of κBα) allows the NF-κB protein subunits p50 and p65 to enter the nucleus and induce gene expression (8). Both MyD88 and TRAF6 are required for the activation of NF-κB, p38, and JNK, whereas ERK activation can occur in the absence of TRAF6 (7). IL-33 is an endogenous proinflammatory danger signal that is released from damaged or dying cells (9, 10), and the IL-33/ST2L signaling axis has been implicated in numerous conditions, notably certain allergic and cardiovascular diseases (11, 12). Accordingly, a number of negative regulators of this pathway have been identified, including sST2 and SIGIRR (8, 13). In addition, IL-33 induces the internalization, phosphorylation, and ubiquitination of ST2L, targeting it for proteasomal degradation, thereby limiting IL-33-induced signaling (14).
The TMED (transmembrane emp24 domain-containing protein)/p24 family is involved in the vesicular trafficking of proteins. These proteins are highly conserved in eukaryotes, and 10 TMED genes have been identified in mammals. They are separated into four subfamilies, α, β, δ, and γ (15), and have been reported to exist as dimers, oligomers, and hetero-oligomers (16, 17). TMED proteins localize to membranes of the early secretory pathway, such as the endoplasmic reticulum (ER) and Golgi (15), and possess a GOLD (Golgi dynamics) domain, which is a β-strand-rich domain found in several proteins involved in Golgi dynamics and intracellular protein trafficking (18). In recent years, several GOLD domain-containing proteins have been implicated in the regulation of innate immune signaling pathways. A TMED homolog in Drosophila, LOGJAM, has been implicated in the negative regulation of a large number of immune-related genes, including targets of the Toll and Imd signaling pathways (19). In addition, the GOLD domain-containing proteins TAG (TRAM adaptor with GOLD domain) and the TMED family member TMED7 have been shown to limit TLR4 signaling to the transcription factor IRF3 (interferon regulatory factor 3). They act by displacing the adaptor TRIF (TIR domain-containing adaptor-inducing interferon-β) from TRAM (TRIF-related adaptor molecule) in the endosomal TLR4 signaling complex (20, 21).
For many years, ST2L was known as an orphan receptor with no known ligand. Prior to the discovery of IL-33, studies were undertaken to identify the ligand for ST2L. In one such study, although unsuccessful in discovering the ST2L ligand, Gayle et al. (22) identified and cloned a putative ST2L-binding protein. Sequence analysis revealed that this putative ST2L-binding protein is a 227-amino acid type I transmembrane protein known as tp24, p24γ1, or TMED1 (15).
In this study, we sought to investigate the putative association between TMED1 and ST2L and to determine whether TMED1 is involved in IL-33 signaling. We demonstrate that TMED1 and ST2L interact. Using a series of mutation and deletion constructs, we demonstrate that the GOLD domain of TMED1 and the TIR domain of ST2L are required for this interaction. Protease protection assays revealed that the GOLD domain of TMED1 protrudes into the cytosolic compartment, facilitating the interaction. Furthermore, we illustrate that TMED1 positively modulates IL-33-induced IL-8 and IL-6 production. These data therefore further implicate the TMED family in innate immunity by implicating TMED1 in IL-33 signaling.
EXPERIMENTAL PROCEDURES
Plasmids and Reagents
TMED1-GFP and TMED7-GFP were purchased from GeneCopoeia. Antibody HA.11 was from Cambridge Bioscience. Anti-TMED1 antibody (HPA018507), anti-rabbit IgG, puromycin, and α-thioglycerol were purchased from Sigma-Aldrich. Anti-ST2L antibody (AF523) and IL-8 and IL-6 ELISA kits were purchased from R&D Systems. Anti-GFP antibody and Protein A/G Plus-agarose beads were purchased from Santa Cruz Biotechnology. Horseradish peroxidase-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, and Alexa Fluor 647-labeled anti-mouse antibody was purchased from Invitrogen. Primers were obtained from MWG. AllStars negative control siRNA and siRNA oligonucleotides targeting TMED1 (SI00092568 and SI00092575) were purchased from Qiagen. IL-33 was purchased from PeproTech.
Cell Culture
HEK-293 cells stably expressing human ST2L (HEK-293-hST2L) and HEK-293T cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) FCS and 1% (v/v) penicillin/streptomycin. HEK-293-hST2L cells were also cultured with 1 μg/ml puromycin. The human mast cell line HMC-1.1 was cultured in Iscove's modified Dulbecco's medium with 10% (v/v) FCS, 1% (v/v) penicillin/streptomycin, and 1.2 mm α-thioglycerol. Human umbilical vein endothelial cells (HUVECs) were obtained from Millipore and cultured using the EndoGRO-LS complete media kit (Millipore) with 10% (v/v) FCS and 1% penicillin/streptomycin.
Co-immunoprecipitation Assay
HEK-293-hST2L and HEK-293T cells were seeded at 2 × 105 cells/ml in 10-cm dishes. 24 h later, cells were transfected with a total of 8 μg of the indicated plasmids using GeneJuice® and cultured for an additional 48 h before harvesting. 5 × 106 HMC-1.1 cells per point were used for each co-immunoprecipitation. Co-immunoprecipitations performed with GFP-Trap beads (ChromoTek) were carried out according to the manufacturer's instructions. Cell lysates were incubated with the beads at 4 °C for 1–2 h. For co-immunoprecipitations performed with Protein A/G Plus-agarose beads, cells were lysed in 10% (v/v) glycerol, 50 mm NaF, 20 mm Tris-Cl (pH 8.0), 2 mm EDTA, 137 mm NaCl, 1% (v/v) Nonidet P-40, 100 μg/ml PMSF, 1 mm sodium orthovanadate, 1 μg/ml leupeptin, and 6 μg/ml aprotinin for 15 min at 4 °C. Lysates were then centrifuged at maximum speed for 10 min at 4 °C, and 50 μl of the samples was removed as the whole cell lysate. Prior to incubation with the remainder of the sample, 1 μg of the relevant antibodies was incubated with 25 μl of Protein A/G Plus-agarose beads overnight at 4 °C and washed three times with lysis buffer. The remainder of the lysates were then incubated with antibody-coupled beads for 2 h at 4 °C with rotation. The lysates and beads were centrifuged at 2200 × g for 3 min at 4 °C, the supernatant was removed, and the beads were washed three times with 1 ml of lysis buffer. The immune complexes were eluted by the addition of 50 μl of SDS/Laemmli buffer and analyzed by SDS-PAGE and Western blotting.
Confocal Microscopy
HEK-293T cells were set up at 0.5 × 105 cells/ml on poly-l-lysine-coated coverslips. 24 h later, they were cotransfected with plasmids encoding TMED1-GFP and ST2L-HA or TMED1-GFP and ER-cyan fluorescent protein (CFP) or Golgi-CFP. 48 h post-transfection, cells were washed twice with PBS and fixed by incubation with 2% formaldehyde for 15 min at room temperature. If required, samples were washed three times with PBS and then permeabilized with 0.2% (v/v) Triton X-100 (Sigma) in PBS on ice for 10 min. Cells were washed again and blocked with 2% (w/v) BSA in PBS for 1 h. Primary anti-HA and secondary Alexa Fluor 647-labeled anti-mouse antibodies were diluted in 2% BSA, and each one was incubated with cells at room temperature for 1 h. Coverslips were mounted on glass slides with Aqua-Poly/Mount (PolySciences, Inc.), sealed, and viewed with and Olympus FluoView FV1000 imaging system.
Site-directed Mutagenesis of TMED1 and ST2L
The QuikChange site-directed mutagenesis kit (Stratagene) was used to mutate or delete certain bases in the TMED1 and ST2L genes and to add a HA tag to the C terminus of ST2L. The manufacturer's instructions were followed using primers incorporating the desired mutations, deletions, or insertions.
Biochemical Protease Protection Assay
HEK-293T cells were set up at 2 × 105 cells/ml. 24 h later, cells were trypsinized and washed twice with Dulbecco's modified Eagle's medium and three times with Krebs-Henseleit buffer (110 mm potassium acetate, 20 mm HEPES, and 2 mm MgCl2). Whole cell extracts were prepared from one-quarter of the cells, and the remaining three-quarters were transferred into three reaction tubes. Cells in the three tubes were permeabilized on ice for 10 min in Krebs-Henseleit buffer containing 20 μm digitonin. After permeabilization, one tube was spun down at 100,000 ×g for 30 min at 4 °C. The supernatant (cytosolic fraction) and the pellet (membrane fraction) were separated and boiled in SDS/Laemmli buffer. The two other tubes were incubated with 1 mm trypsin (Sigma) in the absence or presence of 1% (v/v) Triton X-100 on ice for 30 min and then ultracentrifuged. The pellets were boiled in SDS/Laemmli buffer and analyzed by SDS-PAGE and Western blotting.
Transfection of HEK-293-hST2L Cells and HUVECs with siRNA
HEK-293-hST2L cells were seeded at 1 × 105 cells/ml, and HUVECs were seeded at 7 × 104 cells/ml. 24 h later, HEK-293-hST2L cells or HUVECs were transfected with a final concentration of 5 nm or 10 mm siRNA oligonucleotides, respectively, using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After 48 h, cells were stimulated with IL-33 for the times indicated. Supernatants were then collected for analysis of cytokine levels.
Western Blotting
Protein samples were resolved on SDS-polyacrylamide gels and transferred onto PVDF membranes using a semidry transfer system (Cleaver Scientific). Membranes were blocked in 5% (w/v) dried milk in TBS-T (50 mm Tris-HCl (pH 7.6), 150 mm NaCl, and 0.1% (v/v) Tween 20) for 1 h at room temperature. The membranes were incubated with primary antibody diluted 1:1000 in 5% (w/v) dried milk in TBS-T at 4 °C overnight or at room temperature for at least 2 h. The membranes were then incubated with the appropriate horseradish peroxidase-conjugated secondary antibody diluted 1:2000 in 5% (w/v) dried milk in TBS-T for 1 h before being developed by ECL (Cell Signaling Technology, Inc.) according to the manufacturer's instructions. Some membranes were stripped using RestoreTM PLUS Western blot stripping buffer (Thermo Scientific) according to the manufacturer's instructions before being reprobed.
RT-PCR
Total RNA from cells seeded in 24-well plates was extracted using the RNeasy kit (Qiagen) and was reverse-transcribed by the SuperScript II reverse transcription kit (Invitrogen) according to the manufacturers' instructions. The cDNA generated served as a template for the amplification of TMED1 or GAPDH by real-time PCR with Platinum SYBR Green qPCR SuperMix (Invitrogen) to determine the relative amounts of TMED1 mRNA. The 7900HT fast real-time PCR system (Applied Biosystems) was used for real-time PCR, and the cycling threshold method (2−ΔΔCt) was used for relative quantification by the comparative method after normalization to GAPDH expression. The TMED1 primers used were 5′-TCCGAGAAGCTGGTGTTCTT-3′ (forward) and 5′-CAGTAGCGTGAGCATCTGGA-3′ (reverse).
Enzyme-linked Immunosorbent Assay
For cytokine measurements, HEK-293-hST2L cells or HUVECs were transfected with siRNA in triplicate. 48 h post-transfection, they were stimulated in triplicate with IL-33 at 20 or 40 ng/ml for the required time. Supernatants were then removed and analyzed for IL-8 or IL-6 using ELISA kits according to the manufacturer's instructions.
RESULTS
TMED1 Interacts with ST2L
A previous study carried out to identify ST2L-binding proteins led to the identification of a novel protein, now known as TMED1. The putative ST2L-binding protein was identified by screening cell lines for their ability to bind a ST2L-Fc fusion protein, and cDNA clones of the putative binding protein were then isolated using an expression cloning strategy (22). Although this method resulted in the identification of TMED1, the purported interaction was not confirmed by any other means, and the functional consequence of this interaction remained unknown.
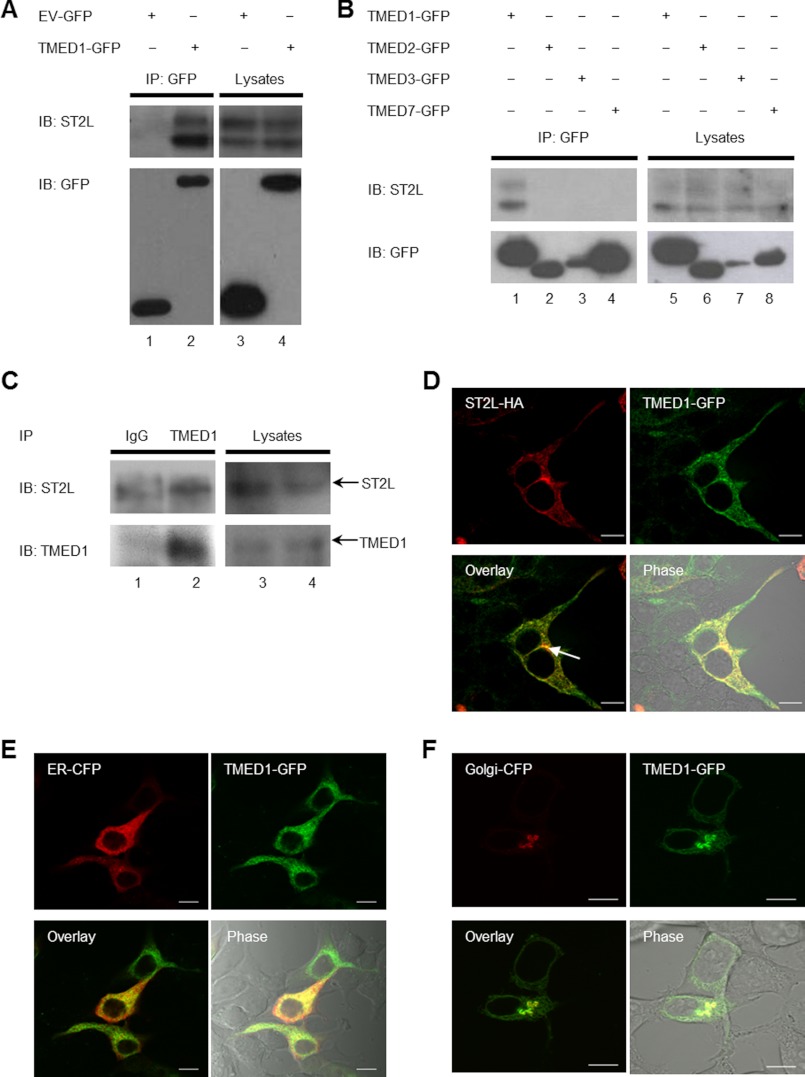
We sought to confirm the interaction using alternative approaches. Co-immunoprecipitation assays were performed in HEK-293-hST2L cells. As shown in Fig. 1A, TMED1-GFP immunoprecipitated together with ST2L in HEK-293-hST2L cells, whereas empty vector-GFP did not (compare lanes 1 and 2). ST2L appears as a doublet on the Western blot, and this is more than likely due to glycosylation of ST2L. To determine the specificity of this interaction, we utilized the related TMED proteins TMED2-GFP, TMED3-GFP, and TMED7-GFP and found that none of these could interact with ST2L (Fig. 1B, compare lane 1 with lanes 2–4). The association between TMED1 and ST2L was also confirmed with endogenous proteins in the human mast cell line HMC-1.1. Fig. 1C shows that endogenous TMED1 co-immunoprecipitated with endogenous ST2L in human mast cells (compare lanes 1 and 2).
FIGURE 1.
TMED1 and ST2L interaction. A and B, HEK-293-hST2L cells were transfected with plasmids encoding empty vector-GFP, TMED1-GFP, TMED2-GFP, TMED3-GFP, or TMED7-GFP as indicated. 48 h post-transfection, cells were lysed, 50 μl was kept as the whole cell lysate, and the remainder was immunoprecipitated (IP) with GFP-Trap beads for 1–2 h at 4 °C. Whole cell lysates and immunoprecipitated samples were analyzed by Western blotting using anti-GFP and anti-ST2L antibodies. Results presented are representative of five (A) and two (B) independent experiments. IB, immunoblot. C, 2 × 107 HMC-1.1 cells were lysed, and 50 μl was kept as the whole cell lysate. The remainder was immunoprecipitated with anti-rabbit IgG, anti-TMED1, or anti-ST2L antibody-coupled Protein A/G Plus-agarose beads for 2 h at 4 °C. Whole cell lysates and immunoprecipitated samples were analyzed by Western blotting using anti-TMED1 and anti-ST2L antibodies. Results presented are representative of four independent experiments. D, HEK-293T cells were transiently transfected with plasmids encoding TMED1-GFP and ST2L-HA. 48 h later, cells were fixed, stained with anti-HA antibodies, and visualized by confocal microscopy. Data shown are representative of a total of 70 cells analyzed in three independent experiments. The GFP tag is represented in green, and ST2L-HA is represented in red. HEK-293T cells were transiently transfected with plasmids encoding TMED1-GFP and ER-CFP (E) or TMED1-GFP and Golgi-CFP (F). 48 h later, cells were fixed and visualized by confocal microscopy. Data shown are representative of 82 (E) and 10 (F) cells from three experiments in which a total of 82 cells were analyzed. The GFP tag is represented in green, and the CFP tag is represented in red.
We also examined the co-localization of TMED1 and ST2L in HEK-293T cells using confocal microscopy. As shown in Fig. 1D (lower left panel), transiently overexpressed TMED1-GFP and ST2L-HA co-localized. TMED1-GFP primarily formed a reticular pattern throughout the cell. This is a characteristic ER pattern, and TMED1 localization in the ER was confirmed with the use of ER-CFP (Fig. 1E, lower left panel). TMED1 was also capable of Golgi localization, which was confirmed using confocal microscopy, although this was less frequent than its localization at the ER (Fig. 1F, lower left panel). ST2L also had an ER-like pattern with a more prominent concentration in a perinuclear Golgi region (Fig. 1D).
Defining the Domains Responsible for the Interaction
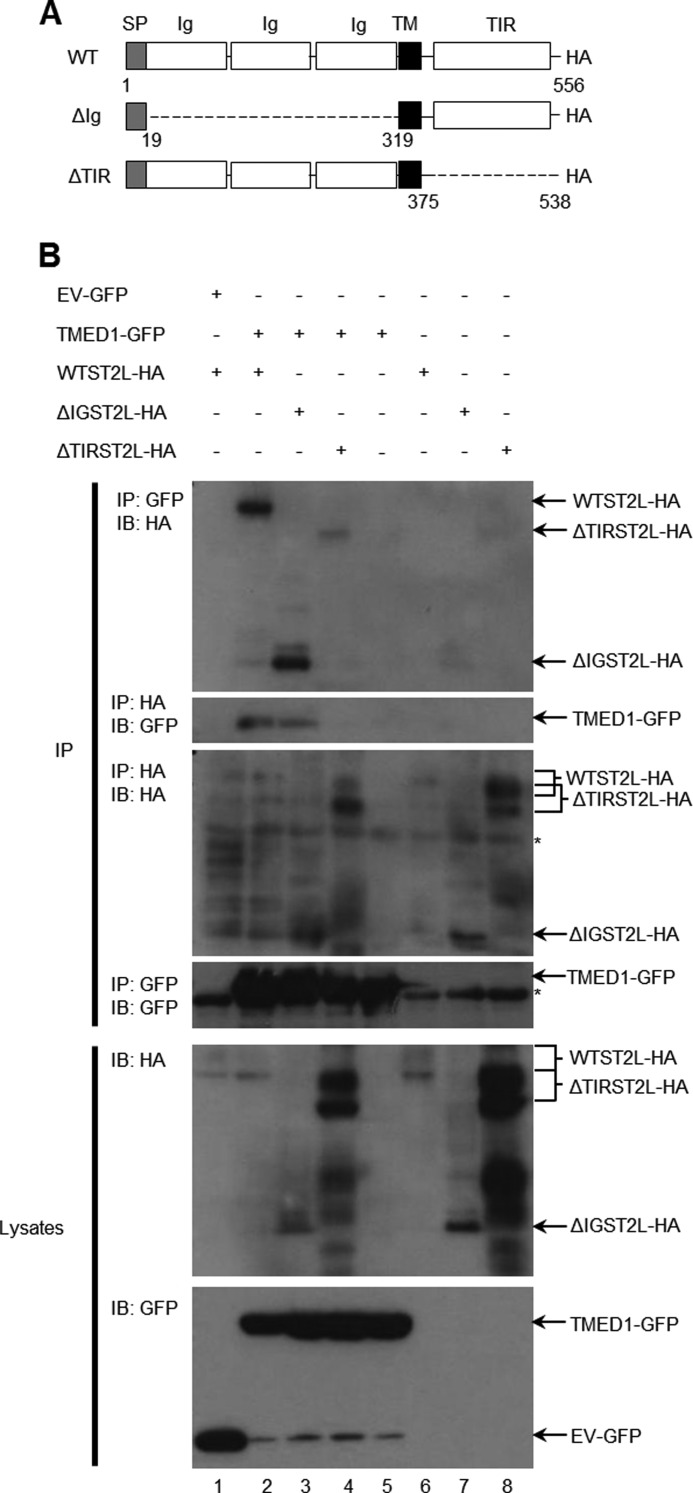
Having confirmed an interaction between TMED1 and ST2L, we next investigated which domains of both proteins mediate the interaction. Two additional ST2L constructs were generated and are depicted Fig. 2A. As shown in Fig. 2A, the three Ig domains of ST2L were removed, resulting in the construct termed ΔIGST2L, whereas the TIR domain was removed in ΔTIRST2L. Fig. 2B illustrates the results from the interaction analysis with these constructs. Lane 2 in the first and second panels of Fig. 2B shows the interaction between ST2L and TMED1 as seen before. The Ig domains of ST2L were not required for the interaction (Fig. 2B, first panel, lane 3). However, although ΔTIRST2L was expressed better in lysates (Fig. 2B, fifth panel, lanes 4 and 8) and pulled itself down to a greater degree than WT ST2L (third panel, lanes 4 and 8), its ability to interact with TMED1 was greatly impaired (first panel, lane 4). These results were confirmed by immunoprecipitating HA-tagged ST2L and blotting for TMED1-GFP (Fig. 2B, second panel). The interaction was observed again (Fig. 2B, lane 2) and was also evident with the Ig domains deleted from ST2L (lane 3). However, removal of the TIR domain obliterated the interaction (Fig. 2B, lane 4).
FIGURE 2.
The TIR domain of ST2L is required for the TMED1/ST2L interaction. A, schematic representation of full-length and truncated ST2L-HA constructs tested for their ability to bind TMED1-GFP. SP, signal peptide; Ig, immunoglobulin domain; TM, transmembrane domain. B, HEK-293T cells were transfected with plasmids encoding empty vector-GFP or TMED1-GFP along with wild-type ST2L-HA or truncated ST2L constructs (ΔIGST2L or ΔTIRST2L). 48 h following transfection, cells were lysed, 50 μl was kept as the whole cell lysate, and the remainder was immunoprecipitated (IP) with anti-GFP or anti-HA antibody-coupled Protein A/G Plus-agarose beads for 3 h at 4 °C. Whole cell lysates and immunoprecipitated samples were analyzed by Western blotting using anti-GFP and anti-ST2L antibodies. Asterisks indicate antibody heavy chain bands. Results presented are representative of three independent experiments. IB, immunoblot.
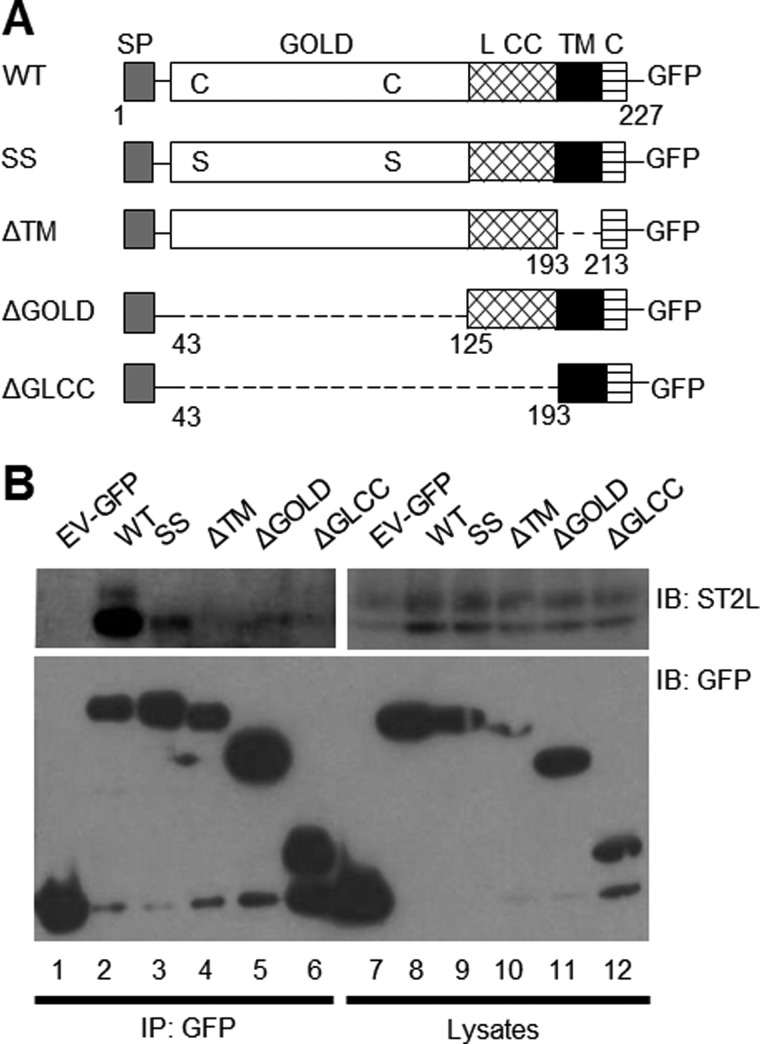
To determine which domains in TMED1 are responsible for the interaction, four additional TMED1 constructs were generated shown in Fig. 3A. The GOLD domain of TMED1 contains two cysteine residues at positions 45 and 106, which are well conserved throughout the TMED family and are thought to form a disulfide bond within this domain (18). In the construct termed SSTMED1, both of these cysteine residues were converted to serine residues, whereas domains were removed in each of the other TMED1 constructs as shown in Fig. 3A. TMED1 co-immunoprecipitated with ST2L (Fig. 3B, upper panel, lane 2); however, each of the changes made to TMED1 greatly reduced its ability to interact with ST2L. Mutation of the cysteine residues and deletion of the GOLD domain both significantly disrupted the interaction to a similar degree (Fig. 3B, upper panel, lanes 3 and 5, respectively). This demonstrates that correct folding of the GOLD domain is of critical importance. However, removal of the linker and coiled-coiled regions (Fig. 3B, upper panel, lane 6) further disrupted the interaction. Deletion of the transmembrane domain also had a negative impact on the interaction (Fig. 3B, upper panel, lane 4).
FIGURE 3.
The GOLD domain of TMED1 is required for the TMED1/ST2L interaction. A, schematic representation of full-length, mutated, and truncated TMED1-GFP constructs tested for their ability to bind ST2L. SP, signal peptide; L CC, linker and coiled-coiled domain; TM, transmembrane domain; C, C-terminal tail. B, HEK-293-hST2L cells were transfected with plasmids encoding empty vector-GFP (EV-GFP), wild-type TMED1-GFP (WT), TMED1-GFP mutant (SS), or truncated constructs (ΔTM, ΔGOLD, or ΔGLCC) as shown. 48 h post-transfection, cells were lysed, 50 μl was kept as the whole cell lysate, and the remainder was immunoprecipitated (IP) with GFP-Trap beads for 1–2 h at 4 °C. Whole cell lysates and immunoprecipitated samples were analyzed by Western blotting using anti-GFP and anti-ST2L antibodies. Results presented are representative of three independent experiments. IB, immunoblot.
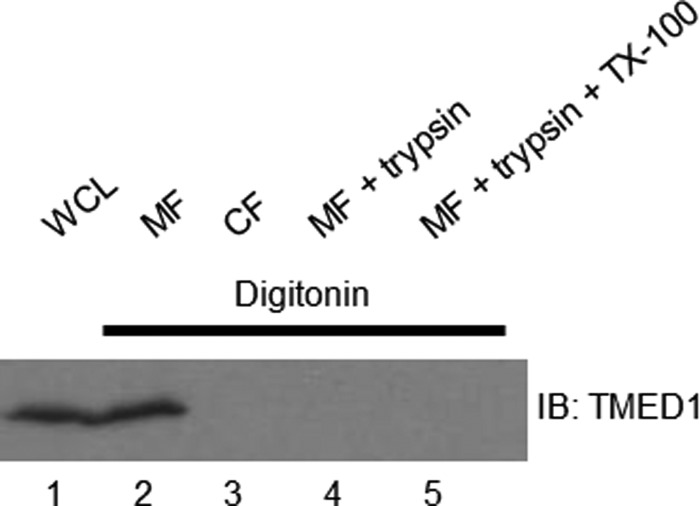
These experiments mapping the domains of TMED1 and ST2L responsible for the interaction suggest that this is mediated primarily by the GOLD domain of TMED1 and the TIR domain of ST2L. The TIR domain of IL-1R/TIR family members is known to be a cytosolic domain, required for intracellular signal transduction (23, 24). Therefore, whether ST2L is positioned in a compartment of the early secretory pathway (such as the ER or Golgi), in an endocytic compartment, or at the plasma membrane, the TIR domain will be in the cytosol. To mediate the interaction, the GOLD domain needs to be in the same cellular compartment. We have demonstrated that TMED1 localizes to the ER and Golgi and wished to investigate if the GOLD domain of TMED1 resides in the lumen of the ER and Golgi or if it projects into the cytosol. We therefore carried out a biochemical protease protection assay to determine this (25). Western blots were immunoblotted using anti-TMED1 antibody generated using the immunogen sequence from positions 26 to 91 of TMED1 and therefore directed against the first half of the GOLD domain. In Fig. 4, lane 1 shows TMED1 expression in whole cell lysates, and lane 2 reveals TMED1 in the membrane fraction. No TMED1 was detected in the cytosolic fraction (Fig. 4, lane 3). Permeabilization of the plasma membrane with digitonin allowed trypsin to digest the GOLD domain of TMED1, as revealed by the disappearance of immunoreactivity to TMED1 using the antibody to its GOLD domain (Fig. 4, lane 4). Permeabilization of all membranes with Triton X-100 also allowed TMED1 to be digested (Fig. 4, lane 5).
FIGURE 4.
The GOLD domain of TMED1 projects into the cytosol. Shown are the results from a biochemical protease protection assay of HEK-293T cells. Lane 1, whole cell lysate (WCL); lane 2, membrane fraction (MF); lane 3, cytosolic fraction (CF); lane 4, membrane fraction plus trypsin; lane 5, membrane fraction plus trypsin plus Triton X-100 (TX-100). Samples were analyzed by Western blotting and probed with anti-TMED1 antibody. Results are representative of three independent experiments. IB, immunoblot.
This assay revealed that in digitonin-permeabilized cells, the GOLD domain of endogenous TMED1 is sensitive to trypsin digestion, suggesting that the GOLD domain extends into the cytosol. This demonstrated, as expected, that TMED1 is membrane-associated, but also showed that the GOLD domain of TMED1 actually projects into the cytosol, thus allowing it to associate with the TIR domain of ST2L.
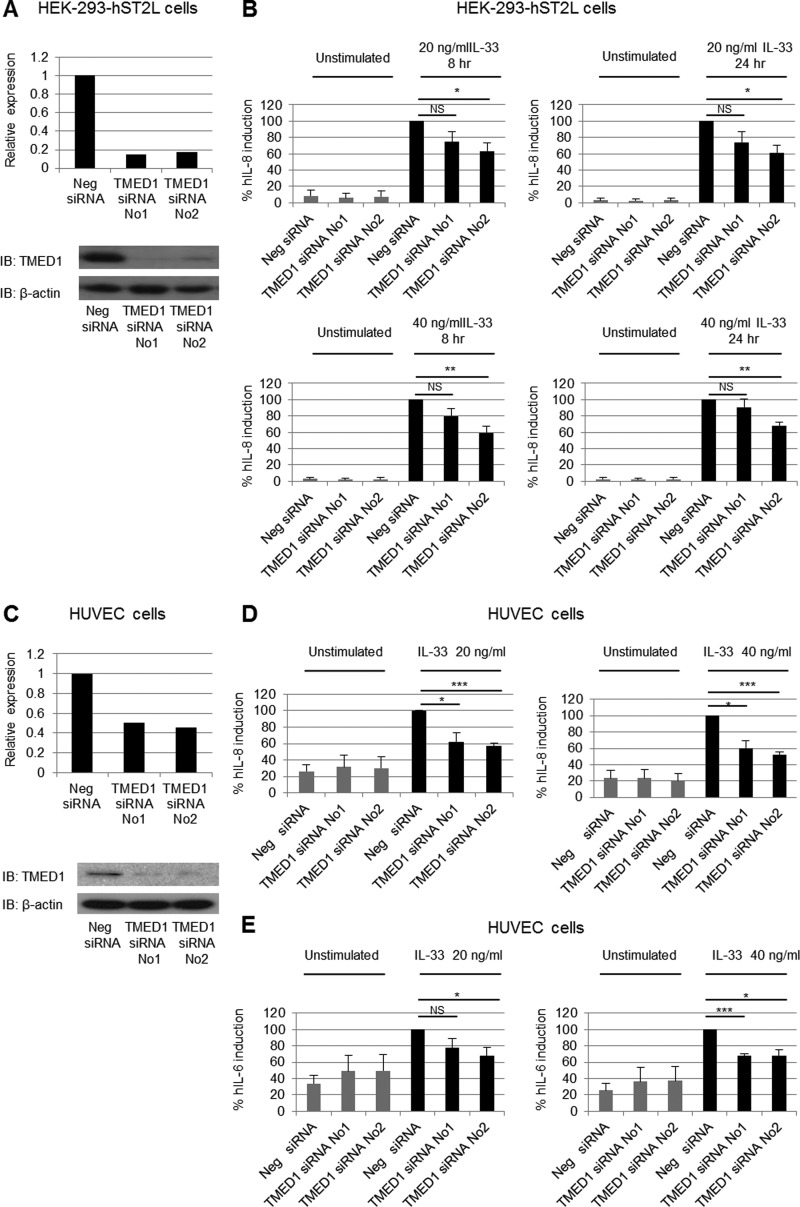
TMED1 Is Involved in IL-33-mediated IL-8 and IL-6 Production
As TMED1 interacts with ST2L, we next investigated if TMED1 plays a role in IL-33 signaling. Using two different siRNA oligonucleotides, we knocked down endogenous TMED1 expression in HEK-293-hST2L cells. Fig. 5A shows that TMED1-targeted siRNA reduced TMED1 mRNA and protein levels in HEK-293-hST2L cells. Following siRNA transfection, HEK-293-hST2L cells were stimulated with either 20 or 40 ng/ml IL-33 for 8 or 24 h. TMED1 knockdown with both siRNAs resulted in reduced IL-33-induced IL-8 production using both concentrations of IL-33 and following stimulation for either 8 or 24 h (Fig. 5B). Impaired IL-8 production was statistically significant in all cases when TMED1 was knocked down using siRNA-2. Although the effect did not reach statistical significance when TMED1 was knocked down using siRNA-1, the same trend of reduced IL-8 production in the absence of TMED1 was also observed in each case, again at both concentrations of IL-33 and with stimulation for either 8 or 24 h.
FIGURE 5.
TMED1 is involved in IL-33 signaling. HEK-293-hST2L cells (A and B) or HUVECs (C–E) were transfected with control siRNA, TMED1 siRNA-1, or TMED1 siRNA-2. RNA or protein from HEK-293-hST2L cells (A) or HUVECs (C) transfected with siRNA was analyzed by real-time PCR and Western blotting to determine the levels of TMED1 knockdown. mRNA expression was normalized to GAPDH and is presented relative to negative siRNA-transfected cells, which was set to 1. Data in A and C are representative of three independent experiments. IB, immunoblot. 48 h following transfection, HEK-293-hST2L cells (B) or HUVECs (D and E) were stimulated with 20 or 40 ng/ml IL-33 for the times indicated, and supernatants were analyzed by ELISA. Results are expressed as percent human (h) IL-8 or IL-6 induction and represent the mean ± S.E. of results for three independent experiments, each carried out in triplicate. The asterisks indicate statistical significance (unpaired two-tailed Student's t test): *, p < 0.05; **, p < 0.01; ***, p < 0.001. NS, not significant.
To further examine the involvement of TMED1 in IL-33-induced signal transduction, we evaluated the levels of IL-33-induced IL-8 and IL-6 production in primary HUVECs following TMED1 knockdown. Analysis of mRNA and protein levels revealed that TMED1-targeted siRNAs were also successful in reducing TMED1 mRNA and protein levels in primary HUVECs (Fig. 5C). Knockdown of TMED1 in HUVECs clearly impaired the IL-33-induced response, resulting in a significant reduction in IL-8 production (Fig. 5D). Furthermore, TMED1 knockdown also resulted in reduced IL-33-induced IL-6 production (Fig. 5E). These results demonstrate that results obtained with HEK-293-hST2L cells are extendable to primary cells and further strengthen the evidence of a role for TMED1 in the IL-33 signaling pathway.
DISCUSSION
This study indicates a role for TMED1 in the ST2L signaling pathway by demonstrating an interaction between TMED1 and ST2L and the impairment of IL-33-induced IL-8 and IL-6 production when TMED1 is knocked down. The IL-1R/TLR superfamily member ST2L was classified as an orphan receptor for many years until the identification of its ligand, IL-33, in 2005 (3). One study to identify the ST2L ligand identified the putative ST2L-binding protein TMED1 (22), a 227-amino acid type I transmembrane protein that contains a GOLD domain, a domain found mainly in proteins involved in vesicular transport. There is mounting evidence of a role for GOLD domain-containing proteins in the innate immune system. A TMED homolog in Drosophila known as LOGJAM has been implicated in the negative regulation of a large number of immune-related genes, including targets of the Toll and Imd signaling pathways (19). In mammals, TMED10 can bind to the MHC class I heavy chain and may protect free MHC class I heavy chains from destruction by ER-associated degradation (26). TAG and TMED7, which contain GOLD domains, have been demonstrated to negatively regulate MyD88-independent TLR4 signaling by displacing the adaptor TRIF from TRAM in the TLR4 signaling complex (20, 21). Given that TMED7 regulates TLR4 signaling and that TMED1 has been shown to potentially interact with ST2L, we sought to determine whether TMED1 is involved in the ST2L signaling pathway.
Since the discovery of TMED1, no further progress has been made with regard to the functional consequence of its purported association with ST2L. As the putative interaction was revealed by a screening method to identify novel ST2L-binding proteins and was not confirmed using other means, we first sought to verify the interaction. Based on co-immunoprecipitation and co-localization data, we can confirm an association between TMED1 and ST2L by overexpression as well as between the endogenous proteins.
Confocal microscopy revealed that TMED1 has a distinct reticular pattern and localizes primarily to the ER but is also capable of localizing to the Golgi. TMED proteins are membrane-spanning proteins that were thought to have their N terminus and GOLD domain located in the ER/Golgi lumen and their C terminus on the cytosolic side of the membrane. However, recent data regarding TMED7 have demonstrated that it possesses an N-terminal transmembrane domain that is responsible for its ability to traverse the membrane and that its GOLD domain is actually located in the cytosolic compartment (20). Transmembrane prediction programs used to analyze the TMED1 sequence suggest that it does not possess a second transmembrane domain located at the N terminus, which corresponds to the region in which the transmembrane domain in TMED7 is found. However, protease protection experiments have demonstrated that, like TMED7, the GOLD domain of TMED1 projects into the cytosol. This topology provides a rationale for how the TIR domain of ST2L, which is known to be cytosolic (23, 24), and the GOLD domain of TMED1 can associate, as it implies that both domains are in the cytosol.
As TMED7 has been implicated in the regulation of TLR4 signaling, we next sought to determine whether TMED1 is involved in ST2L signaling. siRNA-mediated knockdown of TMED1 did affect IL-33-induced signal activation, resulting in reduced IL-8 and IL-6 production. Although TMED7 has been implicated in the negative regulation of TLR4 signaling, TMED1 has been found to positively modulate the ST2L signaling pathway.
Receptor trafficking is now known to be an important player in signal transduction, whether it is to aid receptor trafficking to the correct location to allow signal transduction or to negatively regulate signaling (27). The exact mechanism whereby TMED1 is required for ST2L function still remains unclear. One possibility is that TMED1 is needed to aid ST2L trafficking to the cell surface by modulating the movement of ST2L through the early secretory pathway. Currently, there is limited information available on TMED1. It is known to localize to the ER, ER/Golgi intermediate compartment, and Golgi, and in the Golgi, it can co-localize with TMED10 (16, 28). TMED1 may also localize to endocytic compartments, such as early endosomes, as the related family member TMED7 has been shown to do so (20). Although the internalization of ST2L ultimately results in the degradation of the receptor, acting as a mechanism to limit signal activation (14), it is possible that signal activation can occur from intracellular endocytic compartments as well as from the plasma membrane while ST2L is being internalized. The localization of TMED1 at endocytic compartments may allow TMED1 to associate with ST2L following the internalization of the receptor complex (14) after IL-33 stimulation, thereby allowing TMED1 to carry out its role in the IL-33/ST2L signaling pathway.
In conclusion, we have confirmed the binding of the trafficking protein TMED1 to the IL-33 receptor ST2L and have demonstrated that TMED1 is required for optimal IL-33 responses. Further investigation into the role of TMED1 in the IL-33/ST2L pathway will aim to decipher the mechanism by which this occurs.
Acknowledgments
We thank Dr. K. Bulek (Lerner Research Institute, Cleveland Clinic Foundation, Cleveland, OH) and Professor J. Butterfield (Mayo Clinic, Rochester, MN) for the kind gifts of HEK-293-hST2L and HMC-1.1 cells, respectively. TMED2-GFP and TMED3-GFP were kindly provided by Robert Blum (Institute for Clinical Neurobiology, University Hospital Würzburg, Würzburg, Germany), and the plasmid encoding ST2L was kindly provided by Seamus Martin (Institute of Genetics, Trinity College Dublin).
This work was supported by Science Foundation Ireland.
- IL-1R
- IL-1 receptor
- TLR
- Toll-like receptor
- ER
- endoplasmic reticulum
- HUVEC
- human umbilical vein endothelial cell
- CFP
- cyan fluorescent protein.
REFERENCES
- 1. Tago K., Noda T., Hayakawa M., Iwahana H., Yanagisawa K., Yashiro T., Tominaga S. (2001) Tissue distribution and subcellular localization of a variant form of the human ST2 gene product, ST2V. Biochem. Biophys. Res. Commun. 285, 1377–1383 [DOI] [PubMed] [Google Scholar]
- 2. Li H., Tago K., Io K., Kuroiwa K., Arai T., Iwahana H., Tominaga S., Yanagisawa K. (2000) The cloning and nucleotide sequence of human ST2L cDNA. Genomics 67, 284–290 [DOI] [PubMed] [Google Scholar]
- 3. Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T. K., Zurawski G., Moshrefi M., Qin J., Li X., Gorman D. M., Bazan J. F., Kastelein R. A. (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 [DOI] [PubMed] [Google Scholar]
- 4. Mirchandani A. S., Salmond R. J., Liew F. Y. (2012) Interleukin-33 and the function of innate lymphoid cells. Trends Immunol. 33, 389–396 [DOI] [PubMed] [Google Scholar]
- 5. Palmer G., Lipsky B. P., Smithgall M. D., Meininger D., Siu S., Talabot-Ayer D., Gabay C., Smith D. E. (2008) The IL-1 receptor accessory protein (AcP) is required for IL-33 signaling, and soluble AcP enhances the ability of soluble ST2 to inhibit IL-33. Cytokine 42, 358–364 [DOI] [PubMed] [Google Scholar]
- 6. Chackerian A. A., Oldham E. R., Murphy E. E., Schmitz J., Pflanz S., Kastelein R. A. (2007) IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J. Immunol. 179, 2551–2555 [DOI] [PubMed] [Google Scholar]
- 7. Funakoshi-Tago M., Tago K., Hayakawa M., Tominaga S., Ohshio T., Sonoda Y., Kasahara T. (2008) TRAF6 is a critical signal transducer in IL-33 signaling pathway. Cell. Signal. 20, 1679–1686 [DOI] [PubMed] [Google Scholar]
- 8. Hayakawa H., Hayakawa M., Kume A., Tominaga S. (2007) Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J. Biol. Chem. 282, 26369–26380 [DOI] [PubMed] [Google Scholar]
- 9. Lüthi A. U., Cullen S. P., McNeela E. A., Duriez P. J., Afonina I. S., Sheridan C., Brumatti G., Taylor R. C., Kersse K., Vandenabeele P., Lavelle E. C., Martin S. J. (2009) Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31, 84–98 [DOI] [PubMed] [Google Scholar]
- 10. Talabot-Ayer D., Lamacchia C., Gabay C., Palmer G. (2009) Interleukin-33 is biologically active independently of caspase-1 cleavage. J. Biol. Chem. 284, 19420–19426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liew F. Y., Pitman N. I., McInnes I. B. (2010) Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat. Rev. Immunol 10, 103–110 [DOI] [PubMed] [Google Scholar]
- 12. Oboki K., Ohno T., Kajiwara N., Saito H., Nakae S. (2010) IL-33 and IL-33 receptors in host defense and diseases. Allergol. Int. 59, 143–160 [DOI] [PubMed] [Google Scholar]
- 13. Bulek K., Swaidani S., Qin J., Lu Y., Gulen M. F., Herjan T., Min B., Kastelein R. A., Aronica M., Kosz-Vnenchak M., Li X. (2009) The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of Th2 immune response. J. Immunol. 182, 2601–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao J., Wei J., Mialki R. K., Mallampalli D. F., Chen B. B., Coon T., Zou C., Mallampalli R. K., Zhao Y. (2012) F-box protein FBXL19-mediated ubiquitination and degradation of the receptor for IL-33 limits pulmonary inflammation. Nat. Immunol. 13, 651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Strating J. R., Martens G. J. (2009) The p24 family and selective transport processes at the ER-Golgi interface. Biol. Cell 101, 495–509 [DOI] [PubMed] [Google Scholar]
- 16. Jenne N., Frey K., Brugger B., Wieland F. T. (2002) Oligomeric state and stoichiometry of p24 proteins in the early secretory pathway. J. Biol. Chem. 277, 46504–46511 [DOI] [PubMed] [Google Scholar]
- 17. Carney G. E., Bowen N. J. (2004) p24 proteins, intracellular trafficking, and behavior: Drosophila melanogaster provides insights and opportunities. Biol. Cell 96, 271–278 [DOI] [PubMed] [Google Scholar]
- 18. Anantharaman V., Aravind L. (2002) The GOLD domain, a novel protein module involved in Golgi function and secretion. Genome Biol. 3, research0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boltz K. A., Carney G. E. (2008) Loss of p24 function in Drosophila melanogaster causes a stress response and increased levels of NF-κB-regulated gene products. BMC Genomics 9, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doyle S. L., Husebye H., Connolly D. J., Espevik T., O'Neill L. A., McGettrick A. F. (2012) The GOLD domain-containing protein TMED7 inhibits TLR4 signalling from the endosome upon LPS stimulation. Nat. Commun. 3, 707. [DOI] [PubMed] [Google Scholar]
- 21. Palsson-McDermott E. M., Doyle S. L., McGettrick A. F., Hardy M., Husebye H., Banahan K., Gong M., Golenbock D., Espevik T., O'Neill L. A. (2009) TAG, a splice variant of the adaptor TRAM, negatively regulates the adaptor MyD88-independent TLR4 pathway. Nat. Immunol. 10, 579–586 [DOI] [PubMed] [Google Scholar]
- 22. Gayle M. A., Slack J. L., Bonnert T. P., Renshaw B. R., Sonoda G., Taguchi T., Testa J. R., Dower S. K., Sims J. E. (1996) Cloning of a putative ligand for the T1/ST2 receptor. J. Biol. Chem. 271, 5784–5789 [DOI] [PubMed] [Google Scholar]
- 23. Brint E. K., Xu D., Liu H., Dunne A., McKenzie A. N., O'Neill L. A., Liew F. Y. (2004) ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat. Immunol. 5, 373–379 [DOI] [PubMed] [Google Scholar]
- 24. O'Neill L. A. (2008) The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol. Rev. 226, 10–18 [DOI] [PubMed] [Google Scholar]
- 25. Lorenz H., Hailey D. W., Lippincott-Schwartz J. (2006) Fluorescence protease protection of GFP chimeras to reveal protein topology and subcellular localization. Nat. Methods 3, 205–210 [DOI] [PubMed] [Google Scholar]
- 26. Jun Y., Ahn K. (2011) Tmp21, a novel MHC-I interacting protein, preferentially binds to Β2-microglobulin-free MHC-I heavy chains. BMB Rep. 44, 369–374 [DOI] [PubMed] [Google Scholar]
- 27. McGettrick A. F., O'Neill L. A. (2010) Localisation and trafficking of Toll-like receptors: an important mode of regulation. Curr. Opin. Immunol. 22, 20–27 [DOI] [PubMed] [Google Scholar]
- 28. Emery G., Rojo M., Gruenberg J. (2000) Coupled transport of p24 family members. J. Cell Sci. 113, 2507–2516 [DOI] [PubMed] [Google Scholar]