Abstract
Enterococci are important human pathogens that are increasingly resistant to antimicrobial agents. These organisms were previously considered part of the genus Streptococcus but have recently been reclassified into their own genus, called Enterococcus. To date, 12 species pathogenic for humans have been described, including the most common human isolates, Enterococcus faecalis and E. faecium. Enterococci cause between 5 and 15% of cases of endocarditis, which is best treated by the combination of a cell wall-active agent (such as penicillin or vancomycin, neither of which alone is usually bactericidal) and an aminoglycoside to which the organism is not highly resistant; this characteristically results in a synergistic bactericidal effect. High-level resistance (MIC, greater than or equal to 2,000 micrograms/ml) to the aminoglycoside eliminates the expected bactericidal effect, and such resistance has now been described for all aminoglycosides. Enterococci can also cause urinary tract infections; intraabdominal, pelvic, and wound infections; superinfections (particularly in patients receiving expanded-spectrum cephalosporins); and bacteremias (often together with other organisms). They are now the third most common organism seen in nosocomial infections. For most of these infections, single-drug therapy, most often with penicillin, ampicillin, or vancomycin, is adequate. Enterococci have a large number of both inherent and acquired resistance traits, including resistance to cephalosporins, clindamycin, tetracycline, and penicillinase-resistant penicillins such as oxacillin, among others. The most recent resistance traits reported are penicillinase resistance (apparently acquired from staphylococci) and vancomycin resistance, both of which can be transferred to other enterococci. It appears likely that we will soon be faced with increasing numbers of enterococci for which there is no adequate therapy.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acar J. F., Buu-Hoi A. Y. Resistance patterns of important gram-positive pathogens. J Antimicrob Chemother. 1988 Apr;21 (Suppl 100):41–47. doi: 10.1093/jac/21.suppl_c.41. [DOI] [PubMed] [Google Scholar]
- Appelbaum P. C., Chaurushiya P. S., Jacobs M. R., Duffett A. Evaluation of the rapid strep system for species identification of streptococci. J Clin Microbiol. 1984 May;19(5):588–591. doi: 10.1128/jcm.19.5.588-591.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum P. C., Jacobs M. R., Heald J. I., Palko W. M., Duffett A., Crist R., Naugle P. A. Comparative evaluation of the API 20S system and the automicrobic system gram-positive identification card for species identification of streptococci. J Clin Microbiol. 1984 Feb;19(2):164–168. doi: 10.1128/jcm.19.2.164-168.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum P. C., Jacobs M. R., Palko W. M., Frauenhoffer E. E., Duffett A. Accuracy and reproducibility of the IDS rapID STR system for species identification of streptococci. J Clin Microbiol. 1986 May;23(5):843–846. doi: 10.1128/jcm.23.5.843-846.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson B. A., Lorian V. Antimicrobial agent susceptibility patterns of bacteria in hospitals from 1971 to 1982. J Clin Microbiol. 1984 Oct;20(4):791–796. doi: 10.1128/jcm.20.4.791-796.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auckenthaler R., Wilson W. R., Wright A. J., Washington J. A., 2nd, Durack D. T., Geraci J. E. Lack of in vivo and in vitro bactericidal activity of N-formimidoyl thienamycin against enterococci. Antimicrob Agents Chemother. 1982 Sep;22(3):448–452. doi: 10.1128/aac.22.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNES E. M. Tetrazolium reduction as a means of differentiating Streptococcus faecalis from Streptococcus faecium. J Gen Microbiol. 1956 Feb;14(1):57–68. doi: 10.1099/00221287-14-1-57. [DOI] [PubMed] [Google Scholar]
- Barrall D. T., Kenney P. R., Slotman G. J., Burchard K. W. Enterococcal bacteremia in surgical patients. Arch Surg. 1985 Jan;120(1):57–63. doi: 10.1001/archsurg.1985.01390250049008. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Onderdonk A. B., Louie T., Kasper D. L., Gorbach S. L. A review. Lessons from an animal model of intra-abdominal sepsis. Arch Surg. 1978 Jul;113(7):853–857. doi: 10.1001/archsurg.1978.01370190075013. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Seidel J. S., Yoshikawa T. T., Anthony B. F., Guze L. B. Group D enterococcal meningitis. Clinical and therapeutic considerations with report of three cases and review of the literature. Arch Intern Med. 1976 Aug;136(8):883–886. doi: 10.1001/archinte.136.8.883. [DOI] [PubMed] [Google Scholar]
- Beargie R., Lynd P., Tucker E., Duhring J. Perinatal infection and vaginal flora. Am J Obstet Gynecol. 1975 May 1;122(1):31–33. doi: 10.1016/0002-9378(75)90611-0. [DOI] [PubMed] [Google Scholar]
- Beaty H. N., Turck M., Petersdorf R. G. Ampicillin in the treatment of enterococcal endocarditis. Ann Intern Med. 1966 Oct;65(4):701–707. doi: 10.7326/0003-4819-65-4-701. [DOI] [PubMed] [Google Scholar]
- Benno Y., Suzuki K., Suzuki K., Narisawa K., Bruce W. R., Mitsuoka T. Comparison of the fecal microflora in rural Japanese and urban Canadians. Microbiol Immunol. 1986;30(6):521–532. doi: 10.1111/j.1348-0421.1986.tb02978.x. [DOI] [PubMed] [Google Scholar]
- Bergan T., Delin C., Johansen S., Kolstad I. M., Nord C. E., Thorsteinsson S. B. Pharmacokinetics of ciprofloxacin and effect of repeated dosage on salivary and fecal microflora. Antimicrob Agents Chemother. 1986 Feb;29(2):298–302. doi: 10.1128/aac.29.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk S. L., Verghese A., Holtsclaw S. A., Smith J. K. Enterococcal pneumonia. Occurrence in patients receiving broad-spectrum antibiotic regimens and enteral feeding. Am J Med. 1983 Jan;74(1):153–154. doi: 10.1016/0002-9343(83)91132-4. [DOI] [PubMed] [Google Scholar]
- Bosley G. S., Facklam R. R., Grossman D. Rapid identification of enterococci. J Clin Microbiol. 1983 Nov;18(5):1275–1277. doi: 10.1128/jcm.18.5.1275-1277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braude A. I. Current concepts of pyelonephritis. Medicine (Baltimore) 1973 Jul;52(4):257–264. doi: 10.1097/00005792-197307000-00004. [DOI] [PubMed] [Google Scholar]
- Brisson-Noël A., Arthur M., Courvalin P. Evidence for natural gene transfer from gram-positive cocci to Escherichia coli. J Bacteriol. 1988 Apr;170(4):1739–1745. doi: 10.1128/jb.170.4.1739-1745.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchino J. J., Ciambarella E., Light I. Systemic group D streptococcal infection in newborn infants. Am J Dis Child. 1979 Mar;133(3):270–273. doi: 10.1001/archpedi.1979.02130030046007. [DOI] [PubMed] [Google Scholar]
- Burdett V., Inamine J., Rajagopalan S. Heterogeneity of tetracycline resistance determinants in Streptococcus. J Bacteriol. 1982 Mar;149(3):995–1004. doi: 10.1128/jb.149.3.995-1004.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V. Streptococcal tetracycline resistance mediated at the level of protein synthesis. J Bacteriol. 1986 Feb;165(2):564–569. doi: 10.1128/jb.165.2.564-569.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush L. M., Boscia J. A., Kaye D. Daptomycin (LY146032) treatment of experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1988 Jun;32(6):877–881. doi: 10.1128/aac.32.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush L. M., Calmon J., Cherney C. L., Wendeler M., Pitsakis P., Poupard J., Levison M. E., Johnson C. C. High-level penicillin resistance among isolates of enterococci. Implications for treatment of enterococcal infections. Ann Intern Med. 1989 Apr 1;110(7):515–520. doi: 10.7326/0003-4819-110-7-515. [DOI] [PubMed] [Google Scholar]
- Calderwood S. A., Wennersten C., Moellering R. C., Jr, Kunz L. J., Krogstad D. J. Resistance to six aminoglycosidic aminocyclitol antibiotics among enterococci: prevalence, evolution, and relationship to synergism with penicillin. Antimicrob Agents Chemother. 1977 Sep;12(3):401–405. doi: 10.1128/aac.12.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. B., Wennersten C., Moellering R. C., Jr Resistance to antibiotic synergism in Streptococcus faecalis: further studies with amikacin and with a new amikacin derivative, 4'-deoxy, 6'-N-methylamikacin. Antimicrob Agents Chemother. 1981 Apr;19(4):549–555. doi: 10.1128/aac.19.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J., McGowan D. A., MacFarlane T. W. The prevalence of enterococci in the dental plaque of chronic hospital patients. Br J Oral Surg. 1983 Sep;21(3):171–174. doi: 10.1016/0007-117x(83)90038-0. [DOI] [PubMed] [Google Scholar]
- Caprioli T., Zaccour F., Kasatiya S. S. Phage typing scheme for group D streptococci isolated from human urogenital tract. J Clin Microbiol. 1975 Oct;2(4):311–317. doi: 10.1128/jcm.2.4.311-317.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizosa J., Kaye D. Antibiotic synergism in enterococcal endocarditis. J Lab Clin Med. 1976 Jul;88(1):132–141. [PubMed] [Google Scholar]
- Carrizosa J., Levison M. E. Minimal concentrations of aminoglycoside that can synergize with penicillin in enterococcal endocarditis. Antimicrob Agents Chemother. 1981 Sep;20(3):405–409. doi: 10.1128/aac.20.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar P. H., Smith B. R., LeFrock J. L., Carr B. Enterococcal superinfection and colonization with aztreonam therapy. Antimicrob Agents Chemother. 1984 Aug;26(2):280–282. doi: 10.1128/aac.26.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. Y., Williams J. D. Transferable resistance and aminoglycoside-modifying enzymes in enterococci. J Med Microbiol. 1985 Oct;20(2):187–196. doi: 10.1099/00222615-20-2-187. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986;40:635–659. doi: 10.1146/annurev.mi.40.100186.003223. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol Rev. 1981 Sep;45(3):409–436. doi: 10.1128/mr.45.3.409-436.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes T., Carlier C., Courvalin P. Aminoglycoside-modifying enzyme content of a multiply resistant strain of Streptococcus faecalis. J Antimicrob Chemother. 1983 Jan;11(1):41–47. doi: 10.1093/jac/11.1.41. [DOI] [PubMed] [Google Scholar]
- Conn J. H., Hardy J. D., Chavez C. M., Fain W. R. Infected arterial grafts: experince in 22 cases with empsis on unusual bactia and technics. Ann Surg. 1970 May;171(5):704–714. doi: 10.1097/00000658-197005000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudron P. E., Mayhall C. G., Facklam R. R., Spadora A. C., Lamb V. A., Lybrand M. R., Dalton H. P. Streptococcus faecium outbreak in a neonatal intensive care unit. J Clin Microbiol. 1984 Dec;20(6):1044–1048. doi: 10.1128/jcm.20.6.1044-1048.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider S. R., Colby S. D. Susceptibility of enterococci to trimethoprim and trimethoprim-sulfamethoxazole. Antimicrob Agents Chemother. 1985 Jan;27(1):71–75. doi: 10.1128/aac.27.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry N., McCallum R. W., Guth P. H. Spontaneous peritonitis in cirrhotic ascites. A decade of experience. Am J Dig Dis. 1974 Aug;19(8):685–692. doi: 10.1007/BF01844937. [DOI] [PubMed] [Google Scholar]
- DEIBEL R. H. THE GROUP D STREPTOCOCCI. Bacteriol Rev. 1964 Sep;28:330–366. doi: 10.1128/br.28.3.330-366.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty S. H., Flohr A. B., Simmons R. L. 'Breakthrough' enterococcal septicemia in surgical patients. 19 cases and a review of the literature. Arch Surg. 1983 Feb;118(2):232–238. doi: 10.1001/archsurg.1983.01390020076013. [DOI] [PubMed] [Google Scholar]
- Dougherty S. H. Role of enterococcus in intraabdominal sepsis. Am J Surg. 1984 Sep;148(3):308–312. doi: 10.1016/0002-9610(84)90460-4. [DOI] [PubMed] [Google Scholar]
- EIGLER J. O., WELLMAN W. E., ROOKE E. D., KEITH H. M., SVIEN H. J. Bacterial meningits. I. General review (294 cases). Proc Staff Meet Mayo Clin. 1961 Jul 19;36:357–365. [PubMed] [Google Scholar]
- Edelstein H., McCabe R. E. Perinephric abscess. Modern diagnosis and treatment in 47 cases. Medicine (Baltimore) 1988 Mar;67(2):118–131. [PubMed] [Google Scholar]
- Edlund C., Lidbeck A., Kager L., Nord C. E. Comparative effects of enoxacin and norfloxacin on human colonic microflora. Antimicrob Agents Chemother. 1987 Nov;31(11):1846–1848. doi: 10.1128/aac.31.11.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Farber B. F., Murray B. E., Wennersten C., Moellering R. C., Jr Ribosomal resistance of clinical enterococcal to streptomycin isolates. Antimicrob Agents Chemother. 1984 Mar;25(3):398–399. doi: 10.1128/aac.25.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Wennersten C., Zighelboim-Daum S., Reiszner E., Goldmann D., Moellering R. C., Jr High-level resistance to gentamicin in clinical isolates of Streptococcus (Enterococcus) faecium. Antimicrob Agents Chemother. 1988 Oct;32(10):1528–1532. doi: 10.1128/aac.32.10.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzensberger R., Shah P. M., Knothe H. Impact of oral ciprofloxacin on the faecal flora of healthy volunteers. Infection. 1985 Nov-Dec;13(6):273–275. doi: 10.1007/BF01645437. [DOI] [PubMed] [Google Scholar]
- Etheridge M. E., Yolken R. H., Vonderfecht S. L. Enterococcus hirae implicated as a cause of diarrhea in suckling rats. J Clin Microbiol. 1988 Sep;26(9):1741–1744. doi: 10.1128/jcm.26.9.1741-1744.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A. C., Chinn A. L. The Enterococci: With Special Reference to Their Association with Human Disease. J Bacteriol. 1947 Oct;54(4):495–512. doi: 10.1128/jb.54.4.495-512.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R., Collins M. D. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989 Apr;27(4):731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R. Recognition of group D streptococcal species of human origin by biochemical and physiological tests. Appl Microbiol. 1972 Jun;23(6):1131–1139. doi: 10.1128/am.23.6.1131-1139.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R., Rhoden D. L., Smith P. B. Evaluation of the Rapid Strep system for the identification of clinical isolates of Streptococcus species. J Clin Microbiol. 1984 Nov;20(5):894–898. doi: 10.1128/jcm.20.5.894-898.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R., Bosley G. S., Rhoden D., Franklin A. R., Weaver N., Schulman R. Comparative evaluation of the API 20S and AutoMicrobic gram-positive identification systems for non-beta-hemolytic streptococci and aerococci. J Clin Microbiol. 1985 Apr;21(4):535–541. doi: 10.1128/jcm.21.4.535-541.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R., Hollis D., Collins M. D. Identification of gram-positive coccal and coccobacillary vancomycin-resistant bacteria. J Clin Microbiol. 1989 Apr;27(4):724–730. doi: 10.1128/jcm.27.4.724-730.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow J. A., Jones D., Phillips B. A., Collins M. D. Taxonomic studies on some group D streptococci. J Gen Microbiol. 1983 May;129(5):1423–1432. doi: 10.1099/00221287-129-5-1423. [DOI] [PubMed] [Google Scholar]
- Fass R. J., Wright C. A. Comparative efficacies of mezlocillin and ampicillin alone or in combination with gentamicin in the treatment of Streptococcus faecalis endocarditis in rabbits. Antimicrob Agents Chemother. 1984 Apr;25(4):408–410. doi: 10.1128/aac.25.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
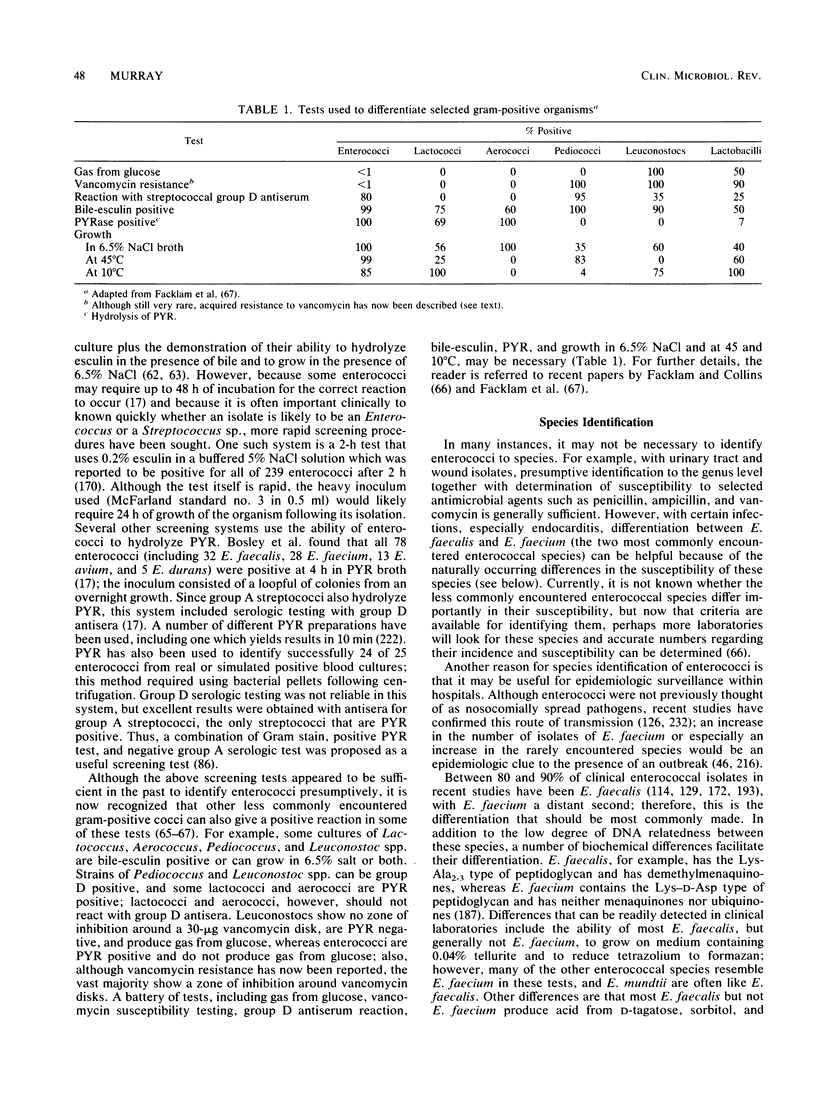
- Feeley T. W., Du Moulin G. C., Hedley-Whyte J., Bushnell L. S., Gilbert J. P., Feingold D. S. Aerosol polymyxin and pneumonia in seriously ill patients. N Engl J Med. 1975 Sep 4;293(10):471–475. doi: 10.1056/NEJM197509042931003. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guerrero M., Rouse M. S., Henry N. K., Geraci J. E., Wilson W. R. In vitro and in vivo activity of ciprofloxacin against enterococci isolated from patients with infective endocarditis. Antimicrob Agents Chemother. 1987 Mar;31(3):430–433. doi: 10.1128/aac.31.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Guerrero M. L., Barros C., Rodriguez Tudela J. L., Fernández Roblas R., Soriano F. Aortic endocarditis caused by gentamicin-resistant Enterococcus faecalis. Eur J Clin Microbiol Infect Dis. 1988 Aug;7(4):525–527. doi: 10.1007/BF01962605. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertally S. S., Facklam R. Comparison of physiologic tests used to identify non-beta-hemolytic aerococci, enterococci, and streptococci. J Clin Microbiol. 1987 Oct;25(10):1845–1850. doi: 10.1128/jcm.25.10.1845-1850.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg C. K., Rosen K. M., Bienstock P. A. Vancomycin therapy for enterococcal and Streptococcus viridans endocarditis. Successful treatment of six patients. Arch Intern Med. 1968 Aug;122(2):134–140. [PubMed] [Google Scholar]
- GERACI J. E., MARTIN W. J. Antibiotic therapy of bacterial endocarditis. VI. Subacute enterococcal endocarditis; clinical, pathologic and therapeutic consideration of 33 cases. Circulation. 1954 Aug;10(2):173–194. doi: 10.1161/01.cir.10.2.173. [DOI] [PubMed] [Google Scholar]
- GRAUDAL H. The classification of motile Streptococci within the Enterococcus group. Acta Pathol Microbiol Scand. 1957;41(5):403–410. doi: 10.1111/j.1699-0463.1957.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Garrison R. N., Fry D. E., Berberich S., Polk H. C., Jr Enterococcal bacteremia: clinical implications and determinants of death. Ann Surg. 1982 Jul;196(1):43–47. doi: 10.1097/00000658-198207000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey G. J., Neu H. C. Infective endocarditis--an evolving disease. A review of endocarditis at the Columbia-Presbyterian Medical Center, 1968-1973. Medicine (Baltimore) 1978 Mar;57(2):105–127. [PubMed] [Google Scholar]
- Gibbs R. S., Listwa H. M., Dreskin R. B. A pure enterococcal abscess after cesarean section. J Reprod Med. 1977 Jul;19(1):17–20. [PubMed] [Google Scholar]
- Glew R. H., Moellering R. C., Jr Effect of protein binding on the activity of penicillins in combination with gentamicin against enterococci. Antimicrob Agents Chemother. 1979 Jan;15(1):87–92. doi: 10.1128/aac.15.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodhart G. L. In vivo v in vitro susceptibility of enterococcus to trimethoprim-sulfamethoxazole. A pitfall. JAMA. 1984 Nov 16;252(19):2748–2749. [PubMed] [Google Scholar]
- Gordon L. P., Damm M. A., Anderson J. D. Rapid presumptive identification of streptococci directly from blood cultures by serologic tests and the L-pyrrolidonyl-beta-naphthylamide reaction. J Clin Microbiol. 1987 Feb;25(2):238–241. doi: 10.1128/jcm.25.2.238-241.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorensek M. J., Lebel M. H., Nelson J. D. Peritonitis in children with nephrotic syndrome. Pediatrics. 1988 Jun;81(6):849–856. [PubMed] [Google Scholar]
- Gross P. A., Harkavy L. M., Barden G. E., Flower M. F. The epidemiology of nosocomial enterococcal urinary tract infection. Am J Med Sci. 1976 Jul-Aug;272(1):75–81. doi: 10.1097/00000441-197607000-00009. [DOI] [PubMed] [Google Scholar]
- Gullberg R. M., Homann S. R., Phair J. P. Enterococcal bacteremia: analysis of 75 episodes. Rev Infect Dis. 1989 Jan-Feb;11(1):74–85. doi: 10.1093/clinids/11.1.74. [DOI] [PubMed] [Google Scholar]
- Gutschik E. Experimental endocarditis in rabbits. 6. Results of long-term combined therapy of Streptococcus faecalis endocarditis with penicillin and streptomycin. Acta Pathol Microbiol Immunol Scand B. 1982 Feb;90(1):37–47. [PubMed] [Google Scholar]
- HUGH R. Motile streptococci isolated from the oropharyngeal region. Can J Microbiol. 1959 Aug;5:351–354. doi: 10.1139/m59-043. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Reversal of activity of trimethoprim against gram-positive cocci by thymidine, thymine and 'folates'. J Antimicrob Chemother. 1988 Jul;22(1):35–39. doi: 10.1093/jac/22.1.35. [DOI] [PubMed] [Google Scholar]
- Harding G. K., Ronald A. R. A controlled study of antimicrobial prophylaxis of recurrent urinary infection in women. N Engl J Med. 1974 Sep 19;291(12):597–601. doi: 10.1056/NEJM197409192911203. [DOI] [PubMed] [Google Scholar]
- Hemsell D. L., Wendel G. D., Gall S. A., Newton E. R., Gibbs R. S., Knuppel R. A., Lane T. W., Sweet R. L. Multicenter comparison of cefotetan and cefoxitin in the treatment of acute obstetric and gynecologic infections. Am J Obstet Gynecol. 1988 Mar;158(3 Pt 2):722–727. doi: 10.1016/s0002-9378(16)44535-7. [DOI] [PubMed] [Google Scholar]
- Henry N. K., Wilson W. R., Geraci J. E. Treatment of streptomycin-susceptible enterococcal experimental endocarditis with combinations of penicillin and low- or high-dose streptomycin. Antimicrob Agents Chemother. 1986 Nov;30(5):725–728. doi: 10.1128/aac.30.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzstein J., Ryan J. L., Mangi R. J., Greco T. P., Andriole V. T. Optimal therapy for enterococcal endocarditis. Am J Med. 1984 Feb;76(2):186–191. doi: 10.1016/0002-9343(84)90772-1. [DOI] [PubMed] [Google Scholar]
- Hindes R. G., Willey S. H., Eliopoulos G. M., Rice L. B., Eliopoulos C. T., Murray B. E., Moellering R. C., Jr Treatment of experimental endocarditis caused by a beta-lactamase-producing strain of Enterococcus faecalis with high-level resistance to gentamicin. Antimicrob Agents Chemother. 1989 Jul;33(7):1019–1022. doi: 10.1128/aac.33.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S. A., Moellering R. C., Jr The enterococcus: "putting the bug in our ears". Ann Intern Med. 1987 May;106(5):757–761. doi: 10.7326/0003-4819-106-5-757. [DOI] [PubMed] [Google Scholar]
- Hook E. W., 3rd, Roberts R. B., Sande M. A. Antimicrobial therapy of experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1975 Nov;8(5):564–570. doi: 10.1128/aac.8.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bieth G., Delbos F. High-level, plasmid-borne resistance to gentamicin in Streptococcus faecalis subsp. zymogenes. Antimicrob Agents Chemother. 1979 Nov;16(5):686–689. doi: 10.1128/aac.16.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Hashimoto H., Clewell D. B. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J Clin Microbiol. 1987 Aug;25(8):1524–1528. doi: 10.1128/jcm.25.8.1524-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda D. P., Barry A. L., Andersen S. G. Emergence of Streptococcus faecalis isolates with high-level resistance to multiple aminocyclitol aminoglycosides. Diagn Microbiol Infect Dis. 1984 Jun;2(3):171–177. doi: 10.1016/0732-8893(84)90027-0. [DOI] [PubMed] [Google Scholar]
- Ingerman M., Pitsakis P. G., Rosenberg A., Hessen M. T., Abrutyn E., Murray B. E., Levison M. E. beta-Lactamase production in experimental endocarditis due to aminoglycoside-resistant Streptococcus faecalis. J Infect Dis. 1987 Jun;155(6):1226–1232. doi: 10.1093/infdis/155.6.1226. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. H., Crawford S. A., Alexander G. A. Rapid identification of group D streptococci with the API 20S system. J Clin Microbiol. 1983 Jun;17(6):1096–1098. doi: 10.1128/jcm.17.6.1096-1098.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOENIG M. G., KAYE D. Enterococcal endocarditis. Report of nineteen cases with long-term follow-up data. N Engl J Med. 1961 Feb 9;264:257–264. doi: 10.1056/NEJM196102092640601. [DOI] [PubMed] [Google Scholar]
- Kaplan A. H., Gilligan P. H., Facklam R. R. Recovery of resistant enterococci during vancomycin prophylaxis. J Clin Microbiol. 1988 Jun;26(6):1216–1218. doi: 10.1128/jcm.26.6.1216-1218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchmer A. W., Moellering R. C., Jr, Watson B. K. Susceptibility of various serogroups of streptococci to clindamycin and lincomycin. Antimicrob Agents Chemother. 1975 Feb;7(2):164–167. doi: 10.1128/aac.7.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Korfhagen T. R., Moellering R. C., Jr, Wennersten C., Swartz M. N. Aminoglycoside-inactivating enzymes in clinical isolates of Streptococcus faecalis. An explanation for resistance to antibiotic synergism. J Clin Invest. 1978 Aug;62(2):480–486. doi: 10.1172/JCI109149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Pargwette A. R. Defective killing of enterococci: a common property of antimicrobial agents acting on the cell wall. Antimicrob Agents Chemother. 1980 Jun;17(6):965–968. doi: 10.1128/aac.17.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrle E., Bhaduri S., Krieger D., Gaus W., Heimpel H., Pflieger H., Arnold R., Vanek E. Risk factors for infections of the oropharynx and the respiratory tract in patients with acute leukemia. J Infect Dis. 1981 Aug;144(2):128–136. doi: 10.1093/infdis/144.2.128. [DOI] [PubMed] [Google Scholar]
- Kékessy D. A., Piguet J. D. Bactériocinogénie et typisation de Streptococcus faecalis. Pathol Microbiol (Basel) 1971;37(2):113–121. [PubMed] [Google Scholar]
- Kühnen E., Richter F., Richter K., Andries L. Establishment of a typing system for group D streptococci. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Jan;267(3):322–330. doi: 10.1016/s0176-6724(88)80048-8. [DOI] [PubMed] [Google Scholar]
- Kühnen E., Rommelsheim K., Andries L. Combined use of phage typing, enterococcinotyping and species differentiation of group D streptococci as an effective epidemiological tool. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Oct;266(3-4):586–595. doi: 10.1016/s0176-6724(87)80242-0. [DOI] [PubMed] [Google Scholar]
- LANGSTON C. W., GUTIERREZ J., BOUMA C. Motile enterococci (Streptococcus faecium var. mobilis var. n.) isolated from grass silage. J Bacteriol. 1960 Nov;80:714–718. doi: 10.1128/jb.80.5.714-718.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEWE L., CANDEL S., EIBER H. B. Therapy of subacute enterococcus (Streptococcus fecalis) endocarditis. Ann Intern Med. 1951 Mar;34(3):717–736. doi: 10.7326/0003-4819-34-3-717. [DOI] [PubMed] [Google Scholar]
- LeBlanc D. J., Inamine J. M., Lee L. N. Broad geographical distribution of homologous erythromycin, kanamycin, and streptomycin resistance determinants among group D streptococci of human and animal origin. Antimicrob Agents Chemother. 1986 Apr;29(4):549–555. doi: 10.1128/aac.29.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Lee L. N., Titmas B. M., Smith C. J., Tenover F. C. Nucleotide sequence analysis of tetracycline resistance gene tetO from Streptococcus mutans DL5. J Bacteriol. 1988 Aug;170(8):3618–3626. doi: 10.1128/jb.170.8.3618-3626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988 Jul 21;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Weber M., Duval J., Courvalin P. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1989 Jan;33(1):10–15. doi: 10.1128/aac.33.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledger W. J., Norman M., Gee C., Lewis W. Bacteremia on an obstetric-gynecologic service. Am J Obstet Gynecol. 1975 Jan 15;121(2):205–212. doi: 10.1016/0002-9378(75)90641-9. [DOI] [PubMed] [Google Scholar]
- Leigh D. A. Peritoneal infections in patients on long-term peritoneal dialysis before and after human cadaveric renal transplantation. J Clin Pathol. 1969 Sep;22(5):539–544. doi: 10.1136/jcp.22.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine L., Hunter P. R. Enterococcal urinary tract infections in a teaching hospital. Eur J Clin Microbiol. 1987 Oct;6(5):574–575. doi: 10.1007/BF02014250. [DOI] [PubMed] [Google Scholar]
- Lleó M. M., Canepari P., Cornaglia G., Fontana R., Satta G. Bacteriostatic and bactericidal activities of beta-lactams against Streptococcus (Enterococcus) faecium are associated with saturation of different penicillin-binding proteins. Antimicrob Agents Chemother. 1987 Oct;31(10):1618–1626. doi: 10.1128/aac.31.10.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luginbuhl L. M., Rotbart H. A., Facklam R. R., Roe M. H., Elliot J. A. Neonatal enterococcal sepsis: case-control study and description of an outbreak. Pediatr Infect Dis J. 1987 Nov;6(11):1022–1026. [PubMed] [Google Scholar]
- Lütticken R., Kunstmann G. Vancomycin-resistant Streptococcaceae from clinical material. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Jan;267(3):379–382. doi: 10.1016/s0176-6724(88)80054-3. [DOI] [PubMed] [Google Scholar]
- MACAULAY D. Acute endocarditis in infancy and early childhood. AMA Am J Dis Child. 1954 Dec;88(6):715–731. doi: 10.1001/archpedi.1954.02050100717003. [DOI] [PubMed] [Google Scholar]
- Maki D. G., Agger W. A. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine (Baltimore) 1988 Jul;67(4):248–269. [PubMed] [Google Scholar]
- Malone D. A., Wagner R. A., Myers J. P., Watanakunakorn C. Enterococcal bacteremia in two large community teaching hospitals. Am J Med. 1986 Oct;81(4):601–606. doi: 10.1016/0002-9343(86)90544-9. [DOI] [PubMed] [Google Scholar]
- Mandell G. L., Kaye D., Levison M. E., Hook E. W. Enterococcal endocarditis. An analysis of 38 patients observed at the New York Hospital-Cornell Medical Center. Arch Intern Med. 1970 Feb;125(2):258–264. doi: 10.1001/archinte.125.2.258. [DOI] [PubMed] [Google Scholar]
- Matsumoto J. Y., Wilson W. R., Wright A. J., Geraci J. E., Washington J. A., 2nd Synergy of penicillin and decreasing concentration of aminoglycosides against enterococci from patients with infective endocarditis. Antimicrob Agents Chemother. 1980 Dec;18(6):944–947. doi: 10.1128/aac.18.6.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L. M., Park B. H., Burdett V., Levy S. B. Energy-dependent efflux mediated by class L (tetL) tetracycline resistance determinant from streptococci. Antimicrob Agents Chemother. 1987 Oct;31(10):1648–1650. doi: 10.1128/aac.31.10.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead G. C. Streptococci in the intestinal flora of man and other non-ruminant animals. Soc Appl Bacteriol Symp Ser. 1978;7:245–261. [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Korzeniowski O. M., Sande M. A., Wennersten C. B. Species-specific resistance to antimocrobial synergism in Streptococcus faecium and Streptococcus faecalis. J Infect Dis. 1979 Aug;140(2):203–208. doi: 10.1093/infdis/140.2.203. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Murray B. E., Schoenbaum S. C., Adler J., Wennersten C. B. A novel mechanism of resistance to penicillin-gentamicin synergism in Streptococcus faecalis. J Infect Dis. 1980 Jan;141(1):81–86. doi: 10.1093/infdis/141.1.81. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Watson B. K., Kunz L. J. Endocarditis due to group D streptococci. Comparison of disease caused by streptococcus bovis with that produced by the enterococci. Am J Med. 1974 Aug;57(2):239–250. doi: 10.1016/0002-9343(74)90448-3. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Weinberg A. N. Studies on antibiotic syngerism against enterococci. II. Effect of various antibiotics on the uptake of 14 C-labeled streptomycin by enterococci. J Clin Invest. 1971 Dec;50(12):2580–2584. doi: 10.1172/JCI106758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Wennersten C., Weinstein A. J. Penicillin-tobramycin synergism against enterococci: a comparison with penicillin and gentamicin. Antimicrob Agents Chemother. 1973 Apr;3(4):526–529. doi: 10.1128/aac.3.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A. J., Jr, Wenzel R. P. Nosocomial urinary tract infections due to enterococcus. Ten years' experience at a university hospital. Arch Intern Med. 1986 Aug;146(8):1549–1551. [PubMed] [Google Scholar]
- Mundt J. O., Graham W. F. Streptococcus faecium var. casselifavus, nov. var. J Bacteriol. 1968 Jun;95(6):2005–2009. doi: 10.1128/jb.95.6.2005-2009.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., An F. Y., Clewell D. B. Plasmids and pheromone response of the beta-lactamase producer Streptococcus (Enterococcus) faecalis HH22. Antimicrob Agents Chemother. 1988 Apr;32(4):547–551. doi: 10.1128/aac.32.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Church D. A., Wanger A., Zscheck K., Levison M. E., Ingerman M. J., Abrutyn E., Mederski-Samoraj B. Comparison of two beta-lactamase-producing strains of Streptococcus faecalis. Antimicrob Agents Chemother. 1986 Dec;30(6):861–864. doi: 10.1128/aac.30.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Mederski-Samaroj B. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J Clin Invest. 1983 Sep;72(3):1168–1171. doi: 10.1172/JCI111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Mederski-Samoraj B., Foster S. K., Brunton J. L., Harford P. In vitro studies of plasmid-mediated penicillinase from Streptococcus faecalis suggest a staphylococcal origin. J Clin Invest. 1986 Jan;77(1):289–293. doi: 10.1172/JCI112289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Tsao J., Panida J. Enterococci from Bangkok, Thailand, with high-level resistance to currently available aminoglycosides. Antimicrob Agents Chemother. 1983 Jun;23(6):799–802. doi: 10.1128/aac.23.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachamkin I., Axelrod P., Talbot G. H., Fischer S. H., Wennersten C. B., Moellering R. C., Jr, MacGregor R. R. Multiply high-level-aminoglycoside-resistant enterococci isolated from patients in a university hospital. J Clin Microbiol. 1988 Jul;26(7):1287–1291. doi: 10.1128/jcm.26.7.1287-1291.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar A., Murray B. E. Failure to demonstrate a consistent in vitro bactericidal effect of trimethoprim-sulfamethoxazole against enterococci. Antimicrob Agents Chemother. 1987 May;31(5):808–810. doi: 10.1128/aac.31.5.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Wu C. Y., Hobbs J. N., Jr, Preston D. A., Allen N. E. Characterization of vancomycin resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob Agents Chemother. 1989 Jul;33(7):1121–1124. doi: 10.1128/aac.33.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble C. J. Carriage of group D streptococci in the human bowel. J Clin Pathol. 1978 Dec;31(12):1182–1186. doi: 10.1136/jcp.31.12.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowlan S. S., Deibel R. H. Group Q streptococci. I. Ecology, serology, physiology, and relationship to established enterococci. J Bacteriol. 1967 Aug;94(2):291–296. doi: 10.1128/jb.94.2.291-296.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odendaal H., de Kock M. Acute salpingitis in pregnancy. S Afr Med J. 1973 Jan 6;47(1):21–22. [PubMed] [Google Scholar]
- Patterson J. E., Colodny S. M., Zervos M. J. Serious infection due to beta-lactamase-producing Streptococcus faecalis with high-level resistance to gentamicin. J Infect Dis. 1988 Nov;158(5):1144–1145. doi: 10.1093/infdis/158.5.1144. [DOI] [PubMed] [Google Scholar]
- Patterson J. E., Masecar B. L., Zervos M. J. Characterization and comparison of two penicillinase-producing strains of Streptococcus (Enterococcus) faecalis. Antimicrob Agents Chemother. 1988 Jan;32(1):122–124. doi: 10.1128/aac.32.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleceas P., Bogdan C., Vereanu A. Enterocine-typing of group D streptococci. Zentralbl Bakteriol Orig A. 1972 Jul;221(2):173–181. [PubMed] [Google Scholar]
- Poindexter A. N., 3rd, Sweet R., Ritter M. Cefotetan in the treatment of obstetric and gynecologic infections. Am J Obstet Gynecol. 1986 Apr;154(4):946–950. doi: 10.1016/0002-9378(86)90495-3. [DOI] [PubMed] [Google Scholar]
- Pérez J. L., Riera L., Valls F., Berrocal C. I., Berrocal L. A comparison of the in-vitro activity of seventeen antibiotics against Streptococcus faecalis. J Antimicrob Chemother. 1987 Sep;20(3):357–362. doi: 10.1093/jac/20.3.357. [DOI] [PubMed] [Google Scholar]
- Qadri S. M., Flournoy D. J., Qadri S. G. Sodium chloride-esculin hydrolysis test for rapid identification of enterococci. J Clin Microbiol. 1987 Jun;25(6):1107–1108. doi: 10.1128/jcm.25.6.1107-1108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAYCROFT R. E., ZIMMERMAN L. N. NEW MODE OF GENETIC TRANSFER IN STREPTOCOCCUS FAECALIS VAR. LIQUEFACIENS. J Bacteriol. 1964 Apr;87:799–801. doi: 10.1128/jb.87.4.799-801.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS W. C., TOMPSETT R. Treatment of enterococcal endocarditis and bacteremia; results of combined therapy with penicillin and streptomycin. Am J Med. 1951 Mar;10(3):278–299. doi: 10.1016/0002-9343(51)90273-2. [DOI] [PubMed] [Google Scholar]
- Rao D. S., Parfitt A. M., Villanueva A. R., Dorman P. J., Kleerekoper M. Hypophosphatemic osteomalacia and adult Fanconi syndrome due to light-chain nephropathy. Another form of oncogenous osteomalacia. Am J Med. 1987 Feb;82(2):333–338. doi: 10.1016/0002-9343(87)90081-7. [DOI] [PubMed] [Google Scholar]
- Reiner N. E., Gopalakrishna K. V., Lerner P. I. Enterococcal endocarditis in heroin addicts. JAMA. 1976 Apr 26;235(17):1861–1863. [PubMed] [Google Scholar]
- Rice L. B., Eliopoulos G. M., Moellering R. C., Jr In vitro synergism between daptomycin and fosfomycin against Enterococcus faecalis isolates with high-level gentamicin resistance. Antimicrob Agents Chemother. 1989 Apr;33(4):470–473. doi: 10.1128/aac.33.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigilano J., Mahapatra R., Barnhill J., Gutierrez J. Enterococcal endocarditis following sigmoidoscopy and mitral valve prolapse. Arch Intern Med. 1984 Apr;144(4):850–851. [PubMed] [Google Scholar]
- Romero-Vivas J., Rodríguez-Créixems M., Bouza E., Hellín T., Guerrero A., Martínez-Beltrán J., García de la Torre M. Evaluation of aztreonam in the treatment of severe bacterial infections. Antimicrob Agents Chemother. 1985 Aug;28(2):222–226. doi: 10.1128/aac.28.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
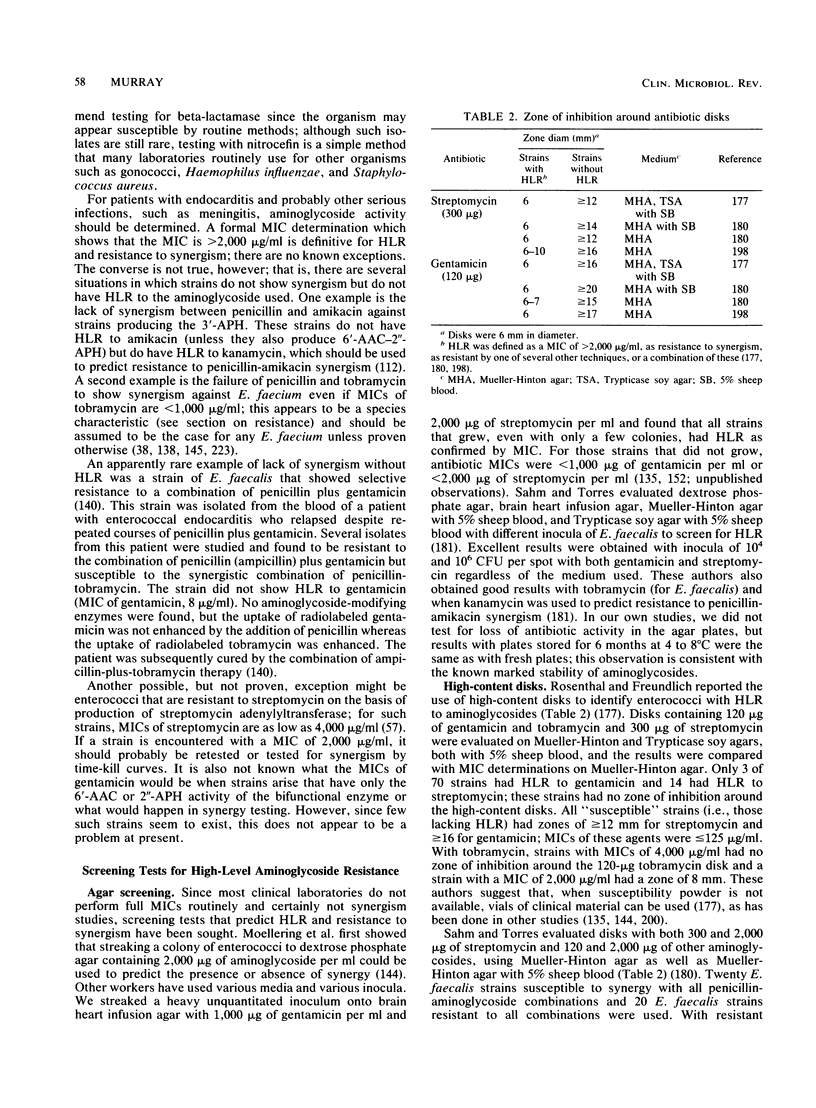
- Rosenthal S. L., Freundlich L. F. An aminoglycoside disk sensitivity test for use with enterococci. J Antimicrob Chemother. 1982 Nov;10(5):459–462. doi: 10.1093/jac/10.5.459. [DOI] [PubMed] [Google Scholar]
- Ryan J. L., Pachner A., Andriole V. T., Root R. K. Enterococcal meningitis: combined vancomycin and rifampin therapy. Am J Med. 1980 Mar;68(3):449–451. doi: 10.1016/0002-9343(80)90118-7. [DOI] [PubMed] [Google Scholar]
- Sahm D. F., Baker C. N., Jones R. N., Thornsberry C. Influence of growth medium on the in vitro activities of second- and third-generation cephalosporins against Streptococcus faecalis. J Clin Microbiol. 1984 Sep;20(3):561–567. doi: 10.1128/jcm.20.3.561-567.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Kissinger J., Gilmore M. S., Murray P. R., Mulder R., Solliday J., Clarke B. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1989 Sep;33(9):1588–1591. doi: 10.1128/aac.33.9.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Torres C. Effects of medium and inoculum variations on screening for high-level aminoglycoside resistance in Enterococcus faecalis. J Clin Microbiol. 1988 Feb;26(2):250–256. doi: 10.1128/jcm.26.2.250-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Torres C. High-content aminoglycoside disks for determining aminoglycoside-penicillin synergy against Enterococcus faecalis. J Clin Microbiol. 1988 Feb;26(2):257–260. doi: 10.1128/jcm.26.2.257-260.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sande M. A., Scheld W. M. Combination antibiotic therapy of bacterial endocarditis. Ann Intern Med. 1980 Mar;92(3):390–395. doi: 10.7326/0003-4819-92-3-390. [DOI] [PubMed] [Google Scholar]
- Schaberg D. R., Zervos M. J. Intergeneric and interspecies gene exchange in gram-positive cocci. Antimicrob Agents Chemother. 1986 Dec;30(6):817–822. doi: 10.1128/aac.30.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Keeley J. M. Imipenem therapy of experimental Staphylococcus aureus and Streptococcus faecalis endocarditis. J Antimicrob Chemother. 1983 Dec;12 (Suppl 500):65–78. doi: 10.1093/jac/12.suppl_d.65. [DOI] [PubMed] [Google Scholar]
- Scheld W. M., Mandell G. L. Enigmatic enterococcal endocarditis. Ann Intern Med. 1984 Jun;100(6):904–905. doi: 10.7326/0003-4819-100-6-904. [DOI] [PubMed] [Google Scholar]
- Schoenbaum S. C., Gardner P., Shillito J. Infections of cerebrospinal fluid shunts: epidemiology, clinical manifestations, and therapy. J Infect Dis. 1975 May;131(5):543–552. doi: 10.1093/infdis/131.5.543. [DOI] [PubMed] [Google Scholar]
- Senghas E., Jones J. M., Yamamoto M., Gawron-Burke C., Clewell D. B. Genetic organization of the bacterial conjugative transposon Tn916. J Bacteriol. 1988 Jan;170(1):245–249. doi: 10.1128/jb.170.1.245-249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman J. M. THE STREPTOCOCCI. Bacteriol Rev. 1937 Dec;1(1):3–97. doi: 10.1128/br.1.1.3-97.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Bouvet A., Devine C., Shlaes J. H., al-Obeid S., Williamson R. Inducible, transferable resistance to vancomycin in Enterococcus faecalis A256. Antimicrob Agents Chemother. 1989 Feb;33(2):198–203. doi: 10.1128/aac.33.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Levy J., Wolinsky E. Enterococcal bacteremia without endocarditis. Arch Intern Med. 1981 Apr;141(5):578–581. [PubMed] [Google Scholar]
- Siegel J. D., McCracken G. H., Jr Group D streptococcal infections. J Pediatr. 1978 Sep;93(3):542–543. doi: 10.1016/s0022-3476(78)81207-4. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H. Interaction of ciprofloxacin with ampicillin and vancomycin for Streptococcus faecalis. Diagn Microbiol Infect Dis. 1988 Apr;9(4):239–243. doi: 10.1016/0732-8893(88)90115-0. [DOI] [PubMed] [Google Scholar]
- Smyth C. J., Halpenny M. K., Ballagh S. J. Carriage rates of enterococci in the dental plaque of haemodialysis patients in Dublin. Br J Oral Maxillofac Surg. 1987 Feb;25(1):21–33. doi: 10.1016/0266-4356(87)90153-7. [DOI] [PubMed] [Google Scholar]
- Spiegel C. A. Laboratory detection of high-level aminoglycoside-aminocyclitol resistance in Enterococcus spp. J Clin Microbiol. 1988 Nov;26(11):2270–2274. doi: 10.1128/jcm.26.11.2270-2274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford H. D., De Maine J. B., Kirby W. M. Antibiotic synergism of enterococci. Relation to inhibitory concentrations. Arch Intern Med. 1970 Aug;126(2):255–259. [PubMed] [Google Scholar]
- Sullam P. M., Täuber M. G., Hackbarth C. J., Sande M. A. Antimicrobial activity of gentamicin in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1985 Feb;27(2):224–226. doi: 10.1128/aac.27.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Hill B. C., Thornsberry C. Problems with the disk diffusion test for detection of vancomycin resistance in enterococci. J Clin Microbiol. 1989 Sep;27(9):2140–2142. doi: 10.1128/jcm.27.9.2140-2142.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira O. H., Carpenter B., Vlad P. Enterococcal endocarditis in early infancy. Can Med Assoc J. 1982 Oct 1;127(7):612–613. [PMC free article] [PubMed] [Google Scholar]
- Thadepalli H., Mandal A. K., Rambhatla K., Bach V. T. Is penicillin alone effective in enterococcal endocarditis? An experimental study in rabbits. Chemotherapy. 1981;27(5):340–349. doi: 10.1159/000238002. [DOI] [PubMed] [Google Scholar]
- Thauvin C., Eliopoulos G. M., Willey S., Wennersten C., Moellering R. C., Jr Continuous-infusion ampicillin therapy of enterococcal endocarditis in rats. Antimicrob Agents Chemother. 1987 Feb;31(2):139–143. doi: 10.1128/aac.31.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. T., Berk S. L., Thomas E. Enterococcal liver abscess associated with moxalactam therapy. Review of literature on enterococcal superinfections in association with moxalactam therapy. Arch Intern Med. 1983 Sep;143(9):1780–1781. [PubMed] [Google Scholar]
- Tight R. R. Ampicillin therapy of experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1980 Aug;18(2):307–310. doi: 10.1128/aac.18.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toala P., McDonald A., Wilcox C., Finland M. Susceptibility of group D streptococcus (enterococcus) to 21 antibiotics in vitro, with special reference to species differences. Am J Med Sci. 1969 Dec;258(6):416–430. doi: 10.1097/00000441-196912000-00006. [DOI] [PubMed] [Google Scholar]
- Tofte R. W., Solliday J. A., Crossley K. B. Susceptibilities of enterococci to twelve antibiotics. Antimicrob Agents Chemother. 1984 Apr;25(4):532–533. doi: 10.1128/aac.25.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Clewell D. B. A transposon (Tn917) in Streptococcus faecalis that exhibits enhanced transposition during induction of drug resistance. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1217–1221. doi: 10.1101/sqb.1979.043.01.138. [DOI] [PubMed] [Google Scholar]
- Tzannetis S., Leonardopoulos J., Papavassiliou J. Enterocinogeny and lysogeny in enterococci. J Appl Bacteriol. 1970 Jun;33(2):358–362. doi: 10.1111/j.1365-2672.1970.tb02207.x. [DOI] [PubMed] [Google Scholar]
- Uttley A. H., Collins C. H., Naidoo J., George R. C. Vancomycin-resistant enterococci. Lancet. 1988 Jan 2;1(8575-6):57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- Warren J. W., Tenney J. H., Hoopes J. M., Muncie H. L., Anthony W. C. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982 Dec;146(6):719–723. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]
- Warren R. E. Difficult streptococci. J Hosp Infect. 1988 Feb;11 (Suppl A):352–357. doi: 10.1016/0195-6701(88)90210-1. [DOI] [PubMed] [Google Scholar]
- Watanakunakorn C., Bakie C. Synergism of vancomycin-gentamicin and vancomycin-streptomycin against enterococci. Antimicrob Agents Chemother. 1973 Aug;4(2):120–124. doi: 10.1128/aac.4.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein A. J., Moellering R. C., Jr Studies of cephalothin: aminoglycoside synergism against enterococci. Antimicrob Agents Chemother. 1975 May;7(5):522–529. doi: 10.1128/aac.7.5.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M. P., Iannini P. B., Stratton C. W., Eickhoff T. C. Spontaneous bacterial peritonitis. A review of 28 cases with emphasis on improved survival and factors influencing prognosis. Am J Med. 1978 Apr;64(4):592–598. doi: 10.1016/0002-9343(78)90578-8. [DOI] [PubMed] [Google Scholar]
- Weinstein W. M., Onderdonk A. B., Bartlett J. G., Louie T. J., Gorbach S. L. Antimicrobial therapy of experimental intraabdominal sepsis. J Infect Dis. 1975 Sep;132(3):282–286. doi: 10.1093/infdis/132.3.282. [DOI] [PubMed] [Google Scholar]
- Wells L. D., von Graevenitz A. Clinical significance of enterococci in blood cultures from adult patients. Infection. 1980;8(4):147–151. doi: 10.1007/BF01639121. [DOI] [PubMed] [Google Scholar]
- Wellstood S. A. Rapid, cost-effective identification of group A streptococci and enterococci by pyrrolidonyl-beta-naphthylamide hydrolysis. J Clin Microbiol. 1987 Sep;25(9):1805–1806. doi: 10.1128/jcm.25.9.1805-1806.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey S. H., Hindes R. G., Eliopoulos G. M., Moellering R. C., Jr Effects of clindamycin and gentamicin and other antimicrobial combinations against enterococci in an experimental model of intra-abdominal abscess. Surg Gynecol Obstet. 1989 Sep;169(3):199–202. [PubMed] [Google Scholar]
- Williamson R., Al-Obeid S., Shlaes J. H., Goldstein F. W., Shlaes D. M. Inducible resistance to vancomycin in Enterococcus faecium D366. J Infect Dis. 1989 Jun;159(6):1095–1104. doi: 10.1093/infdis/159.6.1095. [DOI] [PubMed] [Google Scholar]
- Williamson R., le Bouguénec C., Gutmann L., Horaud T. One or two low affinity penicillin-binding proteins may be responsible for the range of susceptibility of Enterococcus faecium to benzylpenicillin. J Gen Microbiol. 1985 Aug;131(8):1933–1940. doi: 10.1099/00221287-131-8-1933. [DOI] [PubMed] [Google Scholar]
- Wilson S. E., Boswick J. A., Jr, Duma R. J., Echols R. M., Jemsek J. G., Lerner R., Lewis R. T., Najem A. Z., Press R. A., Rittenbury M. S. Cephalosporin therapy in intraabdominal infections. A multicenter randomized, comparative study of cefotetan, moxalactam, and cefoxitin. Am J Surg. 1988 May 31;155(5A):61–66. doi: 10.1016/s0002-9610(88)80215-0. [DOI] [PubMed] [Google Scholar]
- Wilson W. R., Wilkowske C. J., Wright A. J., Sande M. A., Geraci J. E. Treatment of streptomycin-susceptible and streptomycin-resistant enterococcal endocarditis. Ann Intern Med. 1984 Jun;100(6):816–823. doi: 10.7326/0003-4819-100-6-816. [DOI] [PubMed] [Google Scholar]
- Wright A. J., Wilson W. R., Matsumoto J. Y., Washington J. A., 2nd, Geraci J. E. Influence of gentamicin dose size on the efficacies of combinations of gentamicin and penicillin in experimental streptomycin-resistant enterococcal endocarditis. Antimicrob Agents Chemother. 1982 Dec;22(6):972–975. doi: 10.1128/aac.22.6.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y., Clewell D. B. Plasmid-determined tetracycline resistance in Streptococcus faecalis: tandemly repeated resistance determinants in amplified forms of pAMalpha1 DNA. J Mol Biol. 1976 Apr 15;102(3):583–600. doi: 10.1016/0022-2836(76)90336-3. [DOI] [PubMed] [Google Scholar]
- You M. S., Facklam R. R. New test system for identification of Aerococcus, Enterococcus, and Streptococcus species. J Clin Microbiol. 1986 Oct;24(4):607–611. doi: 10.1128/jcm.24.4.607-611.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. L. Enterococcal superinfection and colonization after therapy with moxalactam, a new broad-spectrum antibiotic. Ann Intern Med. 1981 Jun;94(6):784–785. doi: 10.7326/0003-4819-94-6-784. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Bacon A. E., 3rd, Patterson J. E., Schaberg D. R., Kauffman C. A. Enterococcal superinfection in patients treated with ciprofloxacin. J Antimicrob Chemother. 1988 Jan;21(1):113–115. doi: 10.1093/jac/21.1.113. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Kauffman C. A., Therasse P. M., Bergman A. G., Mikesell T. S., Schaberg D. R. Nosocomial infection by gentamicin-resistant Streptococcus faecalis. An epidemiologic study. Ann Intern Med. 1987 May;106(5):687–691. doi: 10.7326/0003-4819-106-5-687. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Mikesell T. S., Schaberg D. R. Heterogeneity of plasmids determining high-level resistance to gentamicin in clinical isolates of Streptococcus faecalis. Antimicrob Agents Chemother. 1986 Jul;30(1):78–81. doi: 10.1128/aac.30.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervos M. J., Patterson J. E., Edberg S., Pierson C., Kauffman C. A., Mikesell T. S., Schaberg D. R. Single-concentration broth microdilution test for detection of high-level aminoglycoside resistance in enterococci. J Clin Microbiol. 1987 Dec;25(12):2443–2444. doi: 10.1128/jcm.25.12.2443-2444.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervos M. J., Schaberg D. R. Reversal of the in vitro susceptibility of enterococci to trimethoprim-sulfamethoxazole by folinic acid. Antimicrob Agents Chemother. 1985 Sep;28(3):446–448. doi: 10.1128/aac.28.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R. A., Moellering R. C., Jr, Weinberg A. N. Mechanism of resistance to antibiotic synergism in enterococci. J Bacteriol. 1971 Mar;105(3):873–879. doi: 10.1128/jb.105.3.873-879.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zscheck K. K., Hull R., Murray B. E. Restriction mapping and hybridization studies of a beta-lactamase-encoding fragment from Streptococcus (Enterococcus) faecalis. Antimicrob Agents Chemother. 1988 May;32(5):768–769. doi: 10.1128/aac.32.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Graevenitz A. Pathogenicity of enterococci outside of urinary tract and blood stream. Klin Wochenschr. 1982 Jul 15;60(14):696–698. doi: 10.1007/BF01716556. [DOI] [PubMed] [Google Scholar]



