Background: A large number of calmodulin-binding sites have been proposed in TRPV6.
Results: We have identified the site that is responsible for inhibition of TRPV6 by calmodulin in excised inside-out patch clamp experiments.
Conclusion: Calmodulin and PI(4,5)P2 antagonistically regulate TRPV6, but not through direct competition.
Significance: This study provides mechanistic insight into Ca2+-induced inactivation of TRPV6.
Keywords: Calcium, Calcium Channels, Calmodulin, Inositol Phospholipid, Ion Channels, Phosphatidylinositol Signaling, TRP Channels, PIP2
Abstract
The epithelial Ca2+ channel transient receptor potential vanilloid 6 (TRPV6) undergoes Ca2+-induced inactivation that protects the cell from toxic Ca2+ overload and may also limit intestinal Ca2+ transport. To dissect the roles of individual signaling pathways in this phenomenon, we studied the effects of Ca2+, calmodulin (CaM), and phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) in excised inside-out patches. The activity of TRPV6 strictly depended on the presence of PI(4,5)P2, and Ca2+-CaM inhibited the channel at physiologically relevant concentrations. Ca2+ alone also inhibited TRPV6 at high concentrations (IC50 = ∼20 μm). A double mutation in the distal C-terminal CaM-binding site of TRPV6 (W695A/R699E) essentially eliminated inhibition by CaM in excised patches. In whole cell patch clamp experiments, this mutation reduced but did not eliminate Ca2+-induced inactivation. Providing excess PI(4,5)P2 reduced the inhibition by CaM in excised patches and in planar lipid bilayers, but PI(4,5)P2 did not inhibit binding of CaM to the C terminus of the channel. Overall, our data show a complex interplay between CaM and PI(4,5)P2 and show that Ca2+, CaM, and the depletion of PI(4,5)P2 all contribute to inactivation of TRPV6.
Introduction
The epithelial Ca2+ channel TRPV6 is a member of the transient receptor potential (TRP)3 superfamily of ion channels. TRPV6 is a Ca2+-selective inwardly rectifying channel expressed in the apical membrane of duodenal epithelial cells, where it is thought to be responsible for Ca2+ entry from the lumen of the intestine (1). TRPV6 expression in the duodenum is regulated at the transcription level by active vitamin D. Calcium entering through this channel is pumped out on the basolateral side of the cell by the plasma membrane Ca2+-ATPase, leading to vectorial transport of Ca2+ from the lumen of the intestine to the intersitium then to the blood. TRPV6 is also expressed in several other tissues, including the placenta and epididymal epithelium. Consistent with the latter, male TRPV6 mice carrying the D541A mutation that renders the channel nonfunctional show severely reduced fertility (2). TRPV5, a close homologue of TRPV6, is expressed in the kidney, where it plays an important role in Ca2+ reabsorption in the distal convoluted tubule (1). Both these channels are constitutively active and undergo Ca2+-induced inactivation (3), which is thought to protect cells from toxic Ca2+ levels, and it was also proposed to limit intestinal Ca2+ transport (4).
The membrane phospholipid phosphatidylinositol 4,5-bisphophate (PI(4,5)P2) commonly known as PIP2, is a general regulator of many ion channels (5–7), including TRP channels (8, 9). The activity of TRPV5 and TRPV6 depends on the presence of PI(4,5)P2 (10–13). It was proposed that Ca2+-induced inactivation of TRPV6 proceeds through Ca2+ influx activating phospholipase C and depletion of PI(4,5)P2 (4).
The ubiquitous Ca2+ sensor calmodulin (CaM) has also been proposed to play a role in the Ca2+-induced inactivation of TRPV6 (14, 15) and TRPV5 (16). A distal C-terminal binding site was implicated in this phenomenon both for TRPV6 (14, 15) and for TRPV5 (16). Removal of that binding site, however, only resulted in a partial inhibition of Ca2+-induced inactivation in whole cell patch clamp experiments. A large number of additional binding sites in the C and N termini, as well as in the intertransmembrane loops, have also been described (17–19), and it is unclear whether any of those sites contribute to the physiological effects of CaM. All studies so far relied on the whole cell patch clamp technique to study the effects of CaM on TRPV6 or TRPV5, where the concentration of CaM cannot be controlled, nor can the effects of Ca2+ and CaM be differentiated.
A recent study proposed that CaM and phosphoinositides bind to overlapping binding sites in a large number of TRP channels and that these signaling molecules generally affect TRP channels by physically displacing each other from their respective binding sites (20). Again this study was based on whole cell patch clamp experiments, and the direct effects of CaM and PI(4,5)P2 were not tested in excised patches.
Here we have reconstituted and characterized the effect of CaM in excised inside-out patches on TRPV6. This technique allows the dissection of the effects of Ca2+ and CaM and can unequivocally identify the site responsible for the inhibitory effect of CaM. This technique is also largely devoid of secondary effects through various cellular components, which are present in whole cell patch clamp experiments, and thus allows us to examine the effects of phosphoinositides and CaM in isolation.
We found that CaM inhibited TRPV6 in excised patches in a concentration-dependent manner in the presence of 3 μm Ca2+. CaM in the absence of Ca2+ did not inhibit TRPV6, whereas Ca2+ alone at higher concentrations also inhibited TRPV6 activity (IC50 = ∼20 μm). We also found that the full-length protein and the purified C terminus, but not the N terminus, of TRPV6 binds to CaM in the presence of Ca2+. CaM binding to the full-length protein was eliminated by a combined mutation of Trp-695 and Arg-699 residues in the distal C-terminal CaM-binding site of TRPV6. In excised patches, this mutant was essentially not inhibited by CaM; in whole cell patch clamp experiments, Ca2+-induced inactivation was reduced but not eliminated. The inhibitory effect of CaM on wild-type TRPV6 in excised patches and planar lipid bilayers was reduced by higher PI(4,5)P2 concentrations, but we have not observed direct competition between PI(4,5)P2 and CaM in biochemical binding experiments. Our data show complex regulation of TRPV6 activity by the interplay between PI(4,5)P2, Ca2+, and CaM.
EXPERIMENTAL PROCEDURES
Reagents
Natural long acyl chain PI(4,5)P2, purified from porcine brain, mainly containing arachydonyl and stearyl side chains (AASt) (Avanti Polar Lipids), was dissolved in water (1 mm stock), followed by 5 min of sonication; then it was aliquoted and stored at −80 °C. Working solutions were prepared daily by dilution of stock aliquots followed by sonication for 5 min. DiC8 PI(4,5)P2 (Cayman Chemical) was dissolved in water (2.5 mm), aliquoted, and stored at −80 °C. Working solutions were diluted from the stock on the day of the experiments. ATP (as Na2ATP; Sigma-Aldrich) was dissolved in the perfusion solution to the final concentration of 2 mm on the day of experiments. After the addition of 2 mm MgCl2, the pH values of the solutions were adjusted to 7.4 before use. Calmodulin purified from bovine testes, purity of ≥98% (Sigma-Aldrich) was dissolved as a 100 μm stock in Cl−-free bath solution (see below), aliquoted, stored at −80 °C, and diluted in the working solution on the day of the experiments.
Molecular Biology and Expression Vectors
For mammalian expression, the coding region of human TRPV6 (hTRPV6) subcloned into pCMV-tag3A (Stratagene) was used (21). This resulted in a c-Myc epitope tag on the N terminus, and we used this tag for TRPV6 detection. For oocyte expression, the hTRPV6 was subcloned into the pGEMSH vector. The pGEMSH-TRPV6 cDNA was linearized and purified using the QIAquick PCR purification kit (Qiagen). cRNA of hTRPV6 was in vitro transcribed using the mMESSAGE mMACHINE kit (Ambion). For bacterial expression, cDNA fragments encoding hTRPV6 wild-type C terminus (residues 579–725), truncated C terminus (residues 579–694), and the wild-type N terminus (residues 1–326) were amplified by PCR and subcloned into pMAL-c4x (New England Biolabs) using the restriction enzymes XbaI and HindIII. The cloned gene was inserted downstream from the malE gene of Escherichia coli, which encoded the maltose-binding protein (MBP). This resulted in the expression of MBP fusion proteins. All of the constructs were confirmed by DNA sequencing. Mutations were introduced with the QuikChange site-directed mutagenesis kit (Stratagene).
Xenopus Oocyte Electrophysiology
The oocytes were extracted from mature female Xenopus laevis frogs (Xenopus Express) and digested with 0.2 mg/ml collagenase (Sigma) in OR2 solution (82.5 mm NaCl, 2 mm KCl, 1 mm MgCl2, and 5 mm HEPES, pH 7.4) for ∼16 h at 18 °C. Defolliculated oocytes were selected and then maintained in OR2 solution plus 1.8 mm CaCl2 and 1% penicillin/streptomycin (Mediatech) at 18 °C. cRNA (20 ng) was microinjected into each oocyte using a nanoliter injector system (World Precision Instruments). The experiments were performed 72 h after injection.
Excised inside-out macropatch experiments were performed with borosilicate glass pipettes (World Precision Instruments) of 0.8–1.7 megaohm resistance. The electrode pipette solution contained 96 mm LiCl, 1 mm EGTA, and 5 mm HEPES, pH 7.4. For the measurements shown in Fig. 2, the electrode pipette was first filled half with Cl−-free bath solution containing 93 mm potassium gluconate, 5 mm HEDTA, 5 mm HEPES, with the pH adjusted to 7.4; and the other half of the pipette was filled with Cl−-containing pipette solution (22). After establishing giagohm resistance seals on devitellinized Xenopus oocytes, the currents were measured using an Axopath 200B amplifier (Molecular Devices). For the measurement in Fig. 2, we used a ramp protocol from −100 to + 100 mV (0.25 mV/ms), immediately preceded by a 100-ms step to −100 mV. The protocol was applied every second; holding potential was 0 mV. For all other measurements, we used a ramp protocol from −103 to +100 mV, performed once a second, immediately preceded by a 100-ms step to −103 mV. The perfusion solution contained 93 mm potassium gluconate, 5 mm HEDTA, 5 mm HEPES, with the pH adjusted to 7.4. To obtain various free Ca2+ concentrations, we added the appropriate amount of Ca2+ in the gluconate and/or HEDTA solution as calculated by the Maxchelator (WinMaxC32 2.50, Stanford University) program (23). Ca2+ at 1, 3, and 10 μm was buffered with HEDTA, and Ca2+ at 30 and 100 μm was not buffered with HEDTA. It has been previously shown that gluconate is a weak Ca2+ buffer, with a Kd of ∼20 mm (22). Thus in the presence of 5 mm HEDTA in the 93 mm gluconate solution, 1.24 mm Ca2+ was added to obtain 1 μm free Ca2+, 2.5 mm to obtain 3 μm free Ca2+, and 3.9 mm to obtain 10 μm free Ca2+. In the 93 mm gluconate solution without HEDTA, 174 μm Ca2+ was added to obtain 30 μm free Ca2+ and 579 μm to obtain 100 μm free Ca2+. For these measurements, the bath was connected with the ground electrode through an agar bridge.
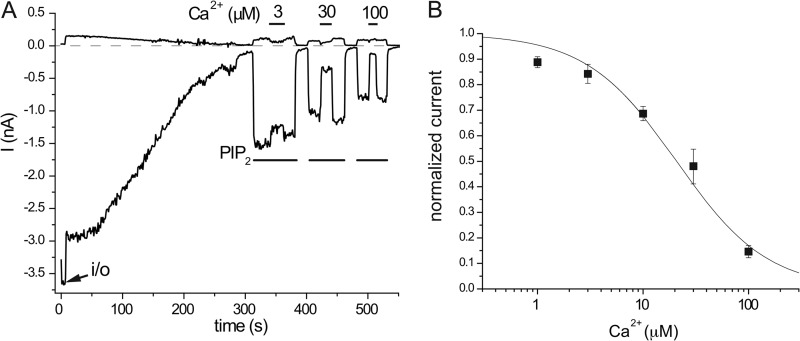
FIGURE 2.
Concentration dependence of the Ca2+ inhibition of TRPV6 activity in excised inside-out macropatches. A, representative trace for the application of 3, 30, and 100 μm free Ca2+ on wild-type TRPV6 activity induced by 25 μm diC8 PI(4,5)P2. This experiment was performed in symmetrical Cl−-free conditions, as described under “Experimental Procedures”; traces at −100 and +100 mV are shown. Note the absence of the Cl− current. B, summary of the data (n = 5–18). The IC50 for Ca2+ inhibition is 20.8 μm, and the Hill coefficient is 1.01.
Mammalian Electrophysiology
Whole cell patch clamp experiments were performed as described earlier (4, 10). Briefly, human embryonic kidney (HEK293) cells were transfected with either the wild-type or mutant TRPV6 and GFP as a transfection marker, using the Effectene transfection reagent. Recordings were performed 36–72 h post-transfection on HEK293 cells in an extracellular solution containing 137 mm NaCl, 5 mm KCl, 10 mm glucose, 10 mm HEPES, with the pH adjusted to 7.4, to which 1 mm MgCl2, 2 or 10 mm CaCl2, or 2 mm EGTA was added, depending on the experimental conditions (see further details in the figure legends). Borosilicate glass pipettes (Sutter Instruments) of 2–4-megaohm resistance were filled with a solution containing 135 mm potassium-gluconate, 5 mm KCl, 5 mm EGTA, 1 mm MgCl2, 2 mm Na2ATP, 10 mm HEPES, with the pH adjusted to 7.2. The cells were kept in extracellular solution containing 1 mm Mg2+ but no Ca2+ for 20 min before measurements. After formation of gigaohm resistance seals, whole cell configuration was established, and currents were measured using an Axopatch 200B amplifier (Molecular Devices). The data were collected and analyzed with the pCLAMP 9.0 software (Molecular Devices). All of the measurements were performed at room temperature (20–25 °C).
Planar Lipid Bilayer Experiments
The TRPV6 protein was purified from HEK293 cells with anti-Myc beads as described earlier (12). Planar lipid bilayers were formed from a solution of synthetic 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine and 1-palmitoyl-2-oleoyl-glycero-3-phosphoethanolamine; both from Avanti Polar Lipids, in a 3:1 ratio in n-decane (Sigma-Aldrich). The solution was used to paint a bilayer in an aperture of ∼150-μm diameter in a Delrin cup (Warner Instruments) between symmetric solutions of 150 mm KCl, 0.02 mm MgCl2, and 20 mm HEPES (pH 7.4) at 22 °C. Bilayer capacitances were in the range of 50–75 picofarad. After the bilayers were formed, 0.2 μl of the TRPV6 micellar solution (0.02 μg/ml) was added to the cis compartment with gentle stirring. Currents were recorded with an Axopatch 200B amplifier. The data were collected and analyzed with the pCLAMP 9.0 software (Molecular Devices).
Expression and Purification of MBP Fusions from Bacteria
A single colony of E. coli BL21 (DE3) transformed with the MBP fusion constructs was inoculated into 30 ml of LB medium containing 150 μg/ml ampicillin and 20 mm glucose and incubated with 250 rpm shaking at 37 °C overnight. The culture was used to inoculate 500 ml of LB containing 150 μg/ml ampicillin and 20 mm glucose. When A600 reached to ∼0.5, isopropyl-β-d-thiogalactopyranoside (Roche Applied Science) at 0.5 mm was added, and the culture was shaken continuously at 37 °C for an additional 3 h, or at 28 °C for 16 h. The cells were harvested by centrifugation at 5000 × g for 20 min and stored at −20 °C before use. A cell pellet from 50 ml of the culture was resuspended in 25 ml of column buffer containing 20 mm Tris, pH 7.4, 500 mm NaCl, 4 mm EDTA, and 15% glycerol with the addition of 1 mm of the protease inhibitor PMSF, 20 mm β-mercaptoethanol, and 2 μg/ml lysozyme. The cells were then lysed by sonication twice, each time for 1 min followed by 1-min incubations on ice. The lysate was centrifuged at 18,000 × g for 45 min. The supernatant was transferred to a fresh 50-ml centrifuge tube with 1 ml of amylase resin, freshly washed with 0.1% SDS, and equilibrated with column buffer. Ethanol (5%) was added to increase the binding efficiency. After 1 h of incubation at 4 °C with gentle shaking, the beads were washed three times with 10 ml of column buffer and one time with 10 ml of column buffer in the presence of 1 μm maltose. MBP fusion proteins were then eluted with column buffer in the presence of 20 μm maltose and stored at 4 °C for use in a week. Protein concentrations were determined using the Bio-Rad protein assay. To detect the protein purity, freshly eluted proteins were electrophoretically separated on 10% SDS-PAGE gel (Bio-Rad) using Tris-glycine SDS buffer (Bio-Rad) at a constant voltage of 194 volts. The protein bands were visualized by staining with Coomassie G-250 (Bio-Rad).
Expression and Preparation of Full-length TRPV6 Protein from HEK293 Cells
HEK293 cells were maintained in minimal essential medium with 10% fetal bovine serum (HyClone) and 1% penicillin/streptomycin (Mediatech) in a humidified, 5% CO2 incubator at 37 °C. Wild-type or mutant hTRPV6 in pCMVtag3A vector was used for full-length TRPV6 protein expression. The cells were transfected using the Effectene transfection reagent (Qiagen) with 1.7 μg of DNA in each 100-mm culture dish. The cells were washed and collected in PBS ∼40 h after transfection and stored at −80 °C before use. A cell pellet collected from three transfected 100-mm dishes was resuspended in 5 ml of NCB buffer containing 500 mm NaCl, 50 mm NaH2PO4, 20 mm HEPES, and 10% glycerol, pH 7.5, with the addition of 1 mm of the protease inhibitor PMSF, 1 mm CaCl2, and 5 mm β-mercaptoethanol. Then the cells were lysed by the freeze-thawing method and centrifuged at 40,000 × g for 2.5 h, separating cytosolic proteins from membrane proteins. The pellet (crude membrane fraction) was then resuspended in 1 ml of NCB buffer containing half tablet of the protease inhibitor mixture (Roche Applied Science), 15 μl of proteCEASETM-50 (G-Biosciences), 1% Nonidet P-40 (Roche Applied Science), and 0.1% Triton X-100 (Sigma) overnight at 4 °C and cleared by centrifugation. The supernatant (solubilized crude membrane fractions) was stored at 4 °C before use.
CaM-Sepharose Pulldown Assay
For the CaM pulldown assay with MBP fusion proteins, 60 μl of CaM-Sepharose 4B (GE Healthcare) slurry was used that was prewashed with CaM binding buffer (50 mm Tris, pH 7.5, 250 mm NaCl, 0.2% Triton X-100) in the presence of 2 mm EGTA and then equilibrated in the CaM binding buffer with various Ca2+ concentrations. 80 ng of freshly purified MBP fusion proteins was incubated with the beads in 300 μl of CaM binding buffer at 4 °C for 1 h. After several washings, the bound fusion proteins were eluted with 80 μl of 2× SDS sample buffer and boiled for 10 min. 20 μl of the bound proteins was fractionated with 10% SDS-PAGE gels and transferred onto PVDF membranes (Bio-Rad) in an IDEA Scientific (Minneapolis, MN) cell filled with blotting buffer (25 mm Tris, 192 mm glycine, and 20% methanol) at 12 volts for 40 min. The membranes were blocked in 5% nonfat dry milk (Bio-Rad) in 1× Tris-buffered saline/Tween 20 and probed with HRP-conjugated anti-MBP monoclonal antibody (1:3500; New England Biolabs) overnight at 4 °C. After thorough washings, protein signals were detected using Supersignal West Pico Chemiluminescent Substrate (Thermo Scientific) with Premium Autoradiography films (Denville Scientific). For studying Ca2+ dependence of hTRPV6 and CaM interaction, 2 mm EGTA or the desired concentrations of CaCl2 were included in the CaM binding buffer. For detecting competition between Ca2+-CaM and PI(4,5)P2, 80 ng of MBP fusion proteins were first incubated with the desired concentrations of PI(4,5)P2 overnight at 4 °C with gentle shaking before loading onto the CaM-Sepharose beads.
For the CaM pulldown assay with the Myc-tagged full-length TRPV6 protein, 100 μl of freshly prepared, solubilized crude membrane fractions were loaded on 100 μl of CaM-Sepharose slurry equilibrated with NCB buffer in the presence of 20 μm Ca2+. After incubation on ice for 2.5 h or at 4 °C for overnight with gentle shaking, the beads were washed four times in 1 ml of NCB buffer containing 1% Nonidet P-40 and 0.1% Triton X-100. The bound proteins were eluted with 60 μl of 2× SDS sample buffer and boiled for 10 min. 30 μl of each sample was fractionated by 10% SDS-PAGE and transferred onto PVDF membranes in an IDEA Scientific cell filled with blotting buffer (25 mm Tris, 192 mm glycine, 0.03% SDS, and 10% methanol) at 22 volts for 1.5 h. Membranes were blocked with 1.2% BSA (Sigma-Aldrich) in 1× Tris-buffered saline/Tween 20 and probed with monoclonal anti-c-Myc antibody (1:3000; Sigma-Aldrich) overnight at 4 °C. After thorough washings, the blots were incubated in HRP-conjugated goat/anti-mouse IgG (1:5000; PerkinElmer Life Sciences), followed by washings. Protein signals were detected using chemiluminescent reagents. On the Western blots, most of the full-length TRPV6 protein was detected at molecular masses higher than the predicted molecular mass of the monomer (83.2 kDa), representing either the tetramers, or other oligomers, consistent with earlier results (24). No signal was observed at any molecular mass using the anti-Myc antibody using HEK cells not transfected with Myc-tagged TRPV6.
Statistics
The mean ± S.E. is shown in the figures. Statistical significance was calculated using t tests.
RESULTS
We have studied the effects of Ca2+ and CaM on the activity of the human TRPV6 in large excised inside-out patches from Xenopus oocytes expressing this channel (Fig. 1). The oocytes have a large endogenous Ca2+-activated Cl− current; thus we used a gluconate-based Cl−-free intracellular solution to avoid contamination of TRPV6 currents with the Cl− current when Ca2+ was applied. TRPV6 currents were measured at −103 mV, and we also monitored the Cl− current at +100 mV in most measurements (10). Fig. 1A shows that in noninjected oocytes, application of 3 μm Ca2+ to the inner surface of the patch membrane induced a large apparent outward current at +100 mV, which corresponds to the inward Cl− current, and essentially no current at −103 mV.
FIGURE 1.
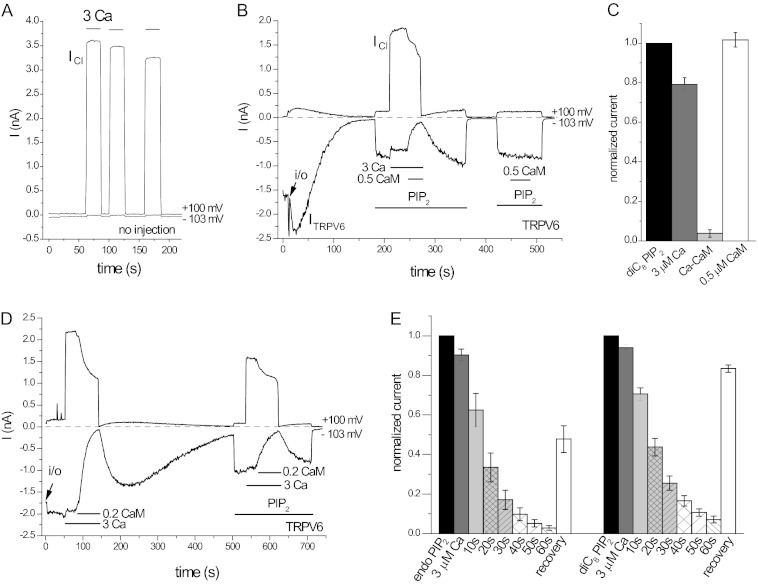
Ca2+-CaM inhibits TRPV6 activity in excised inside-out macropatches. Measurements on TRPV6-expressing and noninjected oocytes were performed as described under “Experimental Procedures.” Currents are shown at −103 mV (lower traces, Li+ current through TRPV6) and at +100 mV (upper traces, Cl− current through the endogenous Ca2+-activated Cl− channels). A, representative current traces in response to 3 μm Ca2+ in a noninjected oocyte. B, representative trace for a TRPV6-expressing oocyte, the applications of 25 μm diC8 PI(4,5)P2, 3 μm Ca2+, 0.5 μm CaM in the presence of 3 μm Ca2+, and 0.5 μm CaM alone are indicated by the horizontal lines. C, summary of the effects of Ca2+, Ca2+-CaM, and CaM in the absence of Ca2+ (n = 6). D, representative traces for the effect Ca2+-CaM in a patch where rundown of TRPV6 currents was very slow. E, summary of the time course of inhibition without application of exogenous PI(4,5)P2 (endo PIP2) and on currents stimulated by diC8 PI(4,5)P2 from five experiments.
In TRPV6-expressing oocytes (Fig. 1, B and D), a large inwardly rectifying TRPV6 current was detected at −103 mV, and only minimal current was detected at +100 mV in the absence of Ca2+, consistent with TRPV6 being an inwardly rectifying channel. Upon excision, TRPV6 activity decreased (rundown) to a variable extent; Fig. 1B shows an example of a typical fast rundown, which is due to the dephosphorylation of PI(4,5)P2 by lipid phosphatases in the patch membrane (12). In some patches rundown was much slower, probably because of the lower phosphatase activity in the patch membrane. Fig. 1D shows an example, where essentially no rundown was observed for ∼100 s; thus the effects of Ca2+ and CaM could be tested without the application of exogenous PI(4,5)P2. When we applied 3 μm Ca2+, a large current appeared at +100 mV, corresponding to the Ca2+ activated Cl− current. At −103 mV, a small inhibition of TRPV6 was observed. When CaM was applied in the presence of 3 μm Ca2+, we observed an almost complete inhibition and partial recovery of current activity upon washout of CaM, followed by rundown of channel activity (Fig. 1D). Fig. 1E summarizes five measurements, where rundown was sufficiently slow to be able to quantify the inhibition by CaM.
Rundown in most patches was fast, however, making it difficult to test the effects of CaM. To induce stable channel activity, we reactivated TRPV6 in most experiments using the water soluble diC8 PI(4,5)P2. When applied in the presence of 25 μm diC8 PI(4,5)P2, 0.5 μm CaM induced an almost complete inhibition of TRPV6 activity in the presence of 3 μm Ca2+ (Fig. 1, B and D). No inhibition was observed by CaM in the absence of Ca2+ (Fig. 1, B and C). It is noteworthy that the Ca2+-induced Cl− current was also partially inhibited by CaM (Fig. 1, B and D).
Ca2+ at 3 μm fully saturates CaM, and this concentration is probably higher than maximal bulk cytoplasmic Ca2+ concentrations even in stimulated cells. Close to the pore of Ca2+-permeable ion channels, however, local Ca2+ concentrations can reach hundreds of micromolars (25). Thus we tested whether Ca2+ alone exerts substantial inhibition on TRPV6 at higher concentrations. Fig. 2 shows the concentration dependence of TRPV6 inhibition by Ca2+ applied to excised patches. TRPV6 was almost fully inhibited by 100 μm Ca2+, and half-maximal inhibition was observed at ∼20 μm. These measurements were performed using symmetrical Cl−-free solutions, to avoid any contamination by the Cl− currents, (see “Experimental Procedures” for further details). Inhibition by Ca2+ alone was almost instantaneous as opposed to the relatively slowly developing inhibition by CaM. Similarly currents recovered immediately upon returning to Ca2+-free perfusion solution, but the effect of CaM washed out much more slowly. Overall, these data show that the effects of Ca2+-CaM and that of Ca2+ alone have different characteristics and can be dissected in excised inside-out patches.
In some patches, such as the measurement shown in Fig. 2, current amplitudes evoked by consecutive applications of diC8 PI(4,5)P2 decreased over time. We have noticed this earlier both with TRPV6 (10) and other PI(4,5)P2-sensitive ion channels such as Kir2.1 (26) and TRPM8 (11). This PI(4,5)P2-independent rundown is quite variable; it is not seen in all patches, its mechanism is unknown, and we have not investigated it further. To avoid overestimating the effect of Ca2+ and other inhibitory compounds caused by this decrease, we always included a control period before testing the next concentration of either Ca2+ or Ca2+-CaM (see below).
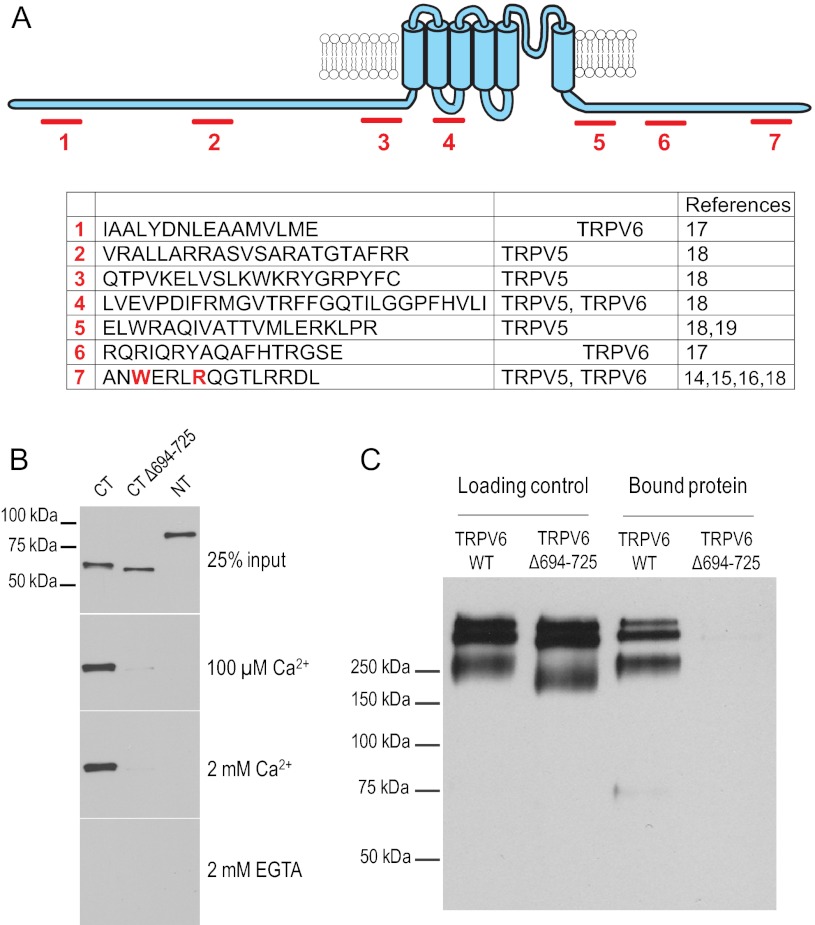
Multiple regions have been suggested in the TRPV6 protein to bind CaM. Fig. 3A shows the seven different putative binding sites identified in various articles (14–19). To evaluate which region is responsible for the inhibition by CaM, we have expressed and purified the C- and N-terminal cytoplasmic regions of TRPV6 in bacteria, using a MBP tag. We measured the binding of the isolated cytoplasmic domains to CaM-sepharose beads and detected the fragments with anti-MBP antibodies. Fig. 3B shows that the cytoplasmic C terminus (residues 579–725) showed a strong binding to CaM beads, whereas the N terminus (residues 1–326) showed no binding. Binding of the C terminus depended on Ca2+. We focused then on the very distal C-terminal region in the human TRPV6 (14) (15), which was also demonstrated in the closely related TRPV5 to bind CaM (16). Removing the distal part of the C terminus (Δ694–725) eliminated CaM binding (Fig. 3B).
FIGURE 3.
CaM binds to TRPV6 via a distal C-terminal binding site. A, proposed CaM-binding sites in TRPV5 and TRPV6. The amino acid sequences correspond to the equivalent fragments in the human TRPV6. B, CaM-Sepharose pulldown assay using MBP fusion proteins of TRPV6 wild-type cytoplasmic C terminus (amino acids 579–725), truncated C terminus without the distal region (Δ694–725), and wild-type cytoplasmic N terminus (amino acids 1–326) in the presence of various Ca2+ concentrations or 2 mm EGTA. Bound proteins were detected by Western blot analysis using anti-MBP antibody. The images are representative of five or six experiments. C, CaM binding assays were performed using the crude membrane fractions from HEK293 cells expressing full-length wild-type Myc-hTRPV6 or mutant Myc-hTRPV6Δ694–725 in the presence of 20 μm Ca2+. Bound proteins eluted from the CaM beads were detected by Western blot analysis using an anti-Myc antibody. The images are representative of eight experiments.
We have also deleted this region from the full-length TRPV6, expressed the mutant and wild-type protein in mammalian cells, and measured binding to CaM. Fig. 3C shows that the full-length wild-type TRPV6 displayed robust binding to CaM, but the truncated (Δ694–725) TRPV6 did not bind CaM at all. The equivalent of this region in TRPV5 was further analyzed in a study by de Groot et al. (16), who identified individual amino acids responsible for CaM binding in TRPV5 with NMR spectroscopy. We have introduced into the human TRPV6 the equivalent mutations to the two residues that had the most dramatic effect in TRPV5 (Fig. 4A). We then tested the effects of this double mutation on CaM binding. Fig. 4B shows that the W695A/R699E double mutant of the C terminus of TRPV6 failed to bind CaM. The same mutations also eliminated binding of the full-length TRPV6 to CaM beads (Fig. 4C). Fig. 4 (D and E) shows that 0.5 μm CaM did not inhibit the W695A/R699E either in the presence or absence of 3 μm Ca2+ in excised patches.
FIGURE 4.
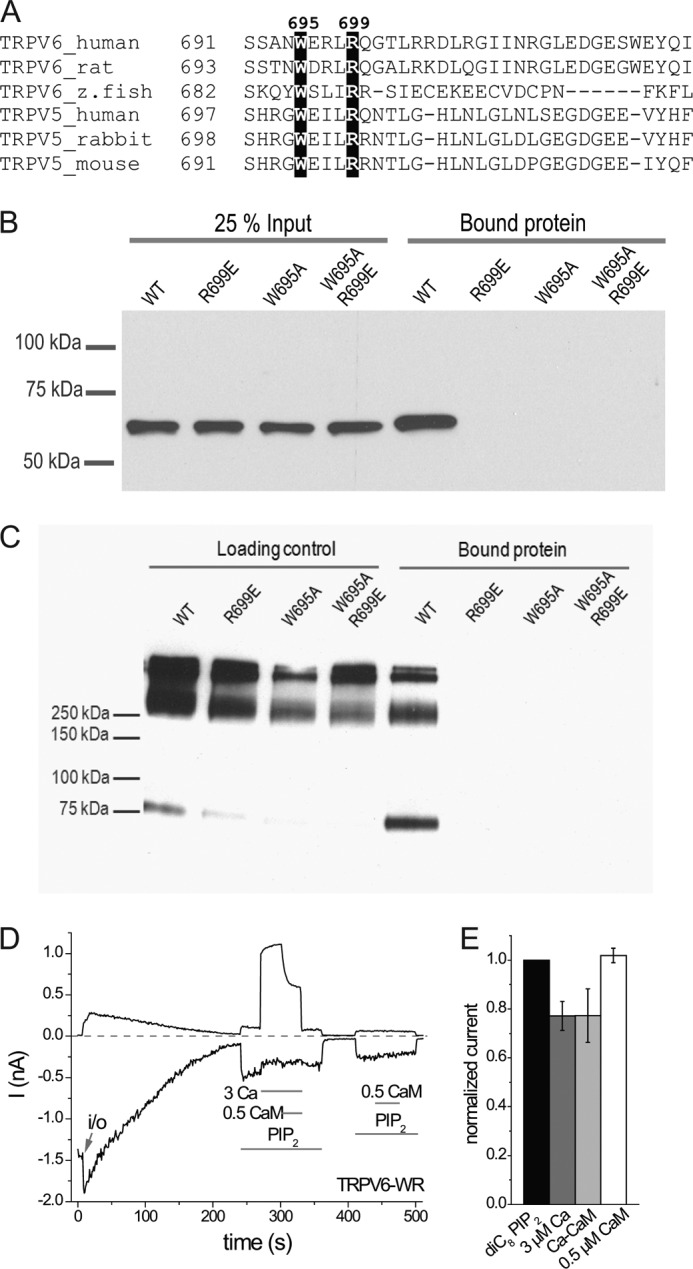
Two highly conserved amino acid residues in the distal C terminus of TRPV6 are responsible for interacting with CaM. A, sequence alignment shows that residues Trp-695 and Arg-699 in hTRPV6 are fully conserved among all studied TRPV5 and TRPV6 species (16). B, CaM-Sepharose pulldown assays were performed in the presence of 100 μm Ca2+ on the isolated C terminus of wild-type and mutant TRPV6. The image is representative of five experiments. C, CaM-Sepharose pulldown assays were performed in the presence of 20 μm Ca2+ for full-length, wild-type, and three mutated TRPV6 proteins transiently expressed in HEK293 cells. Input proteins for loading control and bound proteins eluted from the CaM beads are detected by Western blot using an anti-Myc antibody. The images are representative of three experiments. D, representative trace for the effect of Ca2+, Ca2+-CaM, and CaM on double mutant W695A/R699E (TRPV6-WR) channel activity stimulated by 25 μm diC8 PI(4,5)P2 in excised inside-out macropatches. E, summary data for D normalized to the current evoked by diC8 PI(4,5)P2 (n = 5).
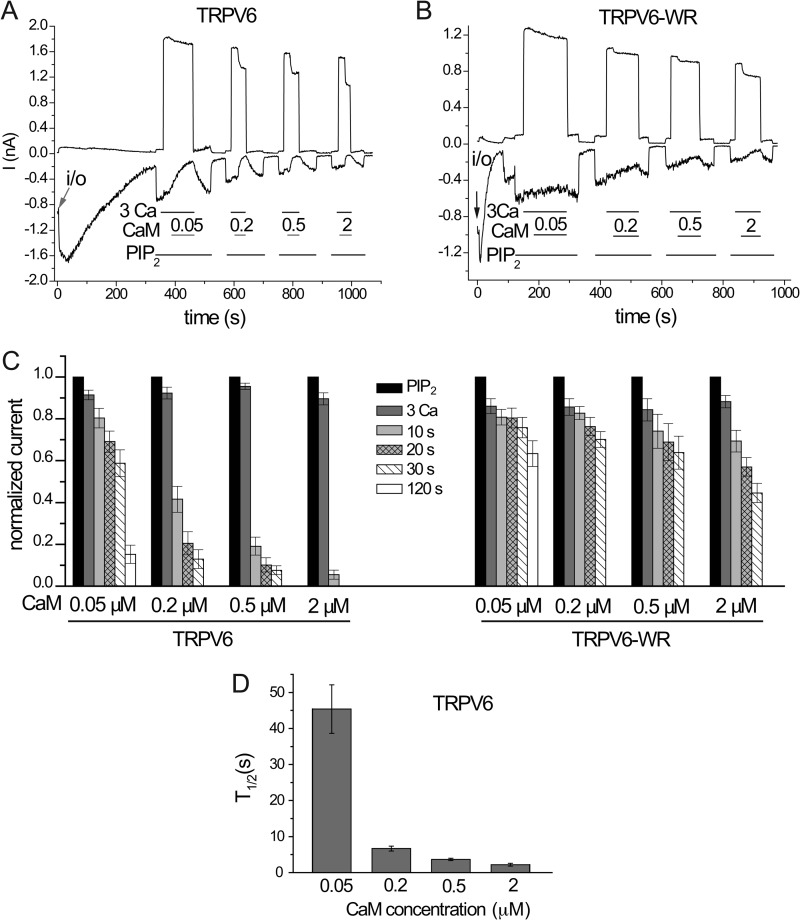
Next we compared the effects of CaM in excised patches on the mutant and wild-type channels in more detail. Fig. 5 shows that the wild-type channel is inhibited in a dose-dependent manner by CaM in the presence of 3 μm Ca2+. In our hands 50 nm CaM still almost completely inhibited TRPV6 activity, even though its effect developed much more slowly than those of higher concentrations (Fig. 5, A, C, and D). It was estimated that free Ca2+-CaM concentration in cells reaches up to 45 nm (27); thus the inhibition we observe in excised patches is likely to be physiologically relevant. When the same concentrations of CaM were tested on the W695A/R699E double mutant, we observed essentially no inhibition at lower CaM concentrations, and much slower and smaller effects than in the wild-type channel at higher CaM concentrations (Fig. 5, B and C). These data indicate that the inhibitory effect of CaM in the physiological range is almost exclusively due to binding to the distal C-terminal region. At supraphysiological CaM concentrations, other binding sites or nonspecific effects by CaM may also contribute to inhibition.
FIGURE 5.
Concentration dependence of the effect of CaM on TRPV6 in excised inside-out macropatches. A and B, representative traces for the effects of different concentrations of CaM in the presence of 3 μm Ca2+ on wild-type TRPV6 (TRPV6) and double mutant W695A/R699E (TRPV6-WR) activated by 25 μm diC8 PI(4,5)P2. C, summary data, normalized to the current evoked by 25 μm diC8 PI(4,5)P2 (n = 5–8). D, T½ values for the different concentrations of CaM.
We also compared the effects of CaM on TRPV6 when channel activity was maintained by MgATP, which serves as a substrate for endogenous lipid kinases in the patch membrane to allow resynthesis of PI(4,5)P2 (12). Fig. 6 shows that when MgATP is applied to excised patches after current rundown, it activated TRPV6 with slower kinetics than diC8 PI(4,5)P2. CaM (0.2 μm) inhibited the activity of the wild-type TRPV6 stimulated by either PI(4,5)P2 or MgATP (Fig. 6, A and C), and the W695A/R699E double mutant was not inhibited or only minimally inhibited in either case (Fig. 6, B and D).
FIGURE 6.
Ca2+-CaM inhibits TRPV6 activity stimulated by MgATP in excised inside-out patches. A and B, representative traces for the application of Ca2+ (3 μm) and Ca2+-CaM (3 μm Ca2+ and 0.2 μm CaM) on wild-type TRPV6 and the W695A/R699E double mutant TRPV6 (TRPV6-WR) activity, respectively. Channel activity was stimulated by 25 μm diC8 PI(4,5)P2 and 2 mm MgATP (2 mm NaATP and 2 mm Mg2+). C and D, summary of the data for six or seven experiments.
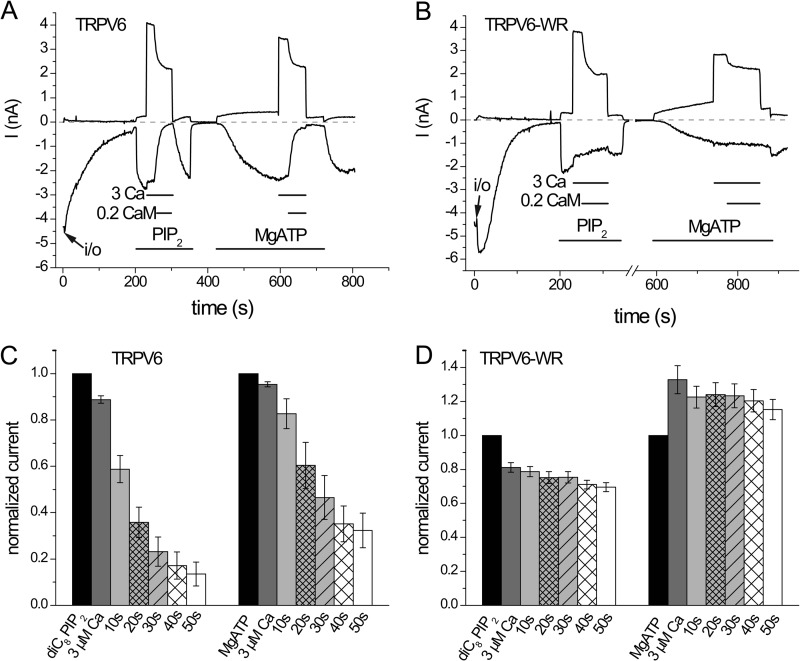
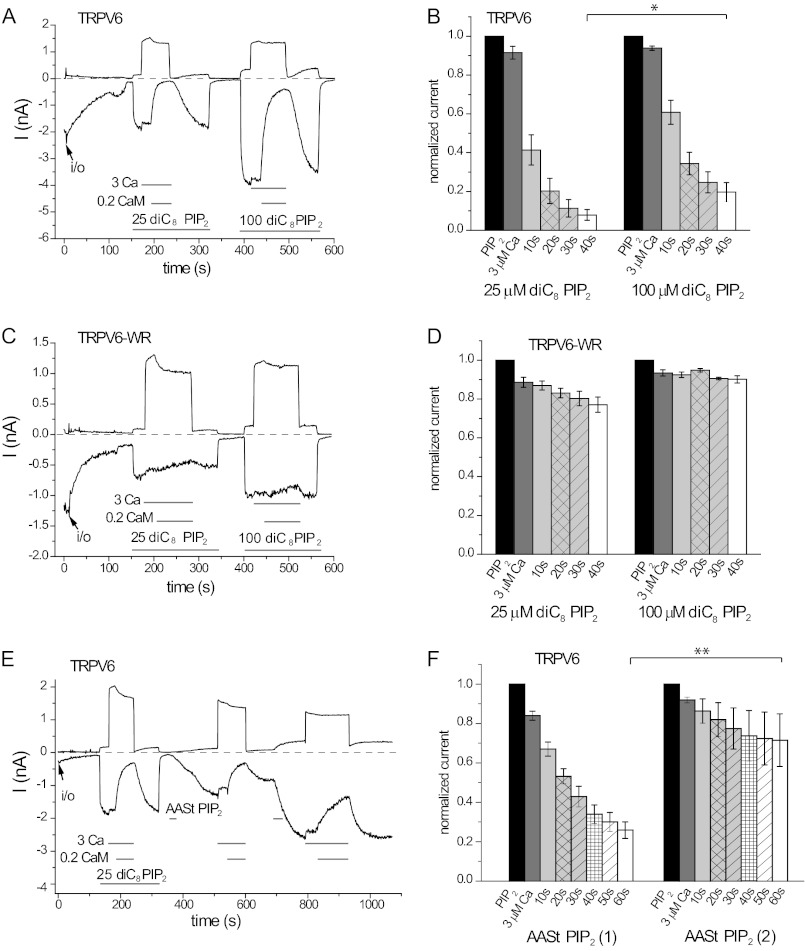
It was proposed for many TRP channels mainly based on biochemical binding experiments that phosphoinositides affect their activity by competing with CaM (20). Because TRPV6 activity depends on PI(4,5)P2, and CaM inhibits it, we tested whether this model can apply to this channel. First, we measured the effects of CaM in the presence of different concentrations and forms of PI(4,5)P2. Fig. 7 (A and B) shows that 0.2 μm CaM inhibited TRPV6 slightly but significantly less in the presence of 100 μm diC8 PI(4,5)P2 than in the presence of 25 μm diC8 PI(4,5)P2. The W695A/R699E double mutant was essentially not inhibited in the presence of either concentration of DiC8 PI(4,5)P2 (Fig. 7, C and D). We also tested the effects of CaM in the presence of the long chain natural AASt PI(4,5)P2 (Fig. 7, E and F). This compound accumulates in the membrane; thus higher effective membrane concentrations can be reached than with the diC8 analogue (28). The effect of AASt PI(4,5)P2 took longer time to develop, and even on washout, channel activity increased further for a while, consistent with our earlier report (12). This is likely due to the slow incorporation of the lipid micelles and lateral diffusion to reach the channel. Because this compound accumulates in the membrane, we used a two-pulse protocol to test whether CaM inhibits the channel less in the presence of higher PI(4,5)P2 concentrations. Fig. 7E shows that current activity was significantly increased upon the second application of AASt PI(4,5)P2 to the same patch, indicating a higher concentration of the lipid in the membrane. CaM inhibited current activity significantly less after the second application of AASt PI(4,5)P2 than after the first. Excess PI(4,5)P2 also reduced the inhibition by CaM in planar lipid bilayers (Fig. 8, A and B).
FIGURE 7.
PI(4,5)P2 competes with Ca2+-CaM on TRPV6 activity in excised inside-out patches. A, representative trace for the effect of 3 μm Ca2+ and Ca2+-CaM (3 μm Ca2+ and 0.2 μm CaM) on wild-type TRPV6, in the presence of 25 and 100 μm diC8 PI(4,5)P2. B, summary data for A, normalized to the current evoked by diC8 PI(4,5)P2 with the corresponding concentration for the wild-type TRPV6 channel. The inhibition by CaM at 40 s is significantly less at 100 μm diC8 PI(4,5)P2 than at 25 μm (n = 9, p = 0.0104). C, representative experiment for the double mutant TRPV6 (TRPV6-WR). D, summary for the effect of CaM on the W695A/R699E mutant of TRPV6 (n = 4). E, representative trace for the effect of 3 μm Ca2+ and 0.2 μm Ca2+-CaM on wild-type TRPV6 activity, in the presence of natural AASt PI(4,5)P2 (10 μm). Because of the micelle form of AASt PI(4,5)P2 in the bath solution, the amount of AASt PI(4,5)P2 incorporated into the patch membrane was associated with the time of application. F, summary data for E, normalized to the current evoked by AASt PI(4,5)P2. The inhibition by CaM was significantly less at the second application of AASt PI(4,5)P2 than at the first one, when measured at 60 s (n = 6, p = 0.0056). Asterisks denote statistical significance.
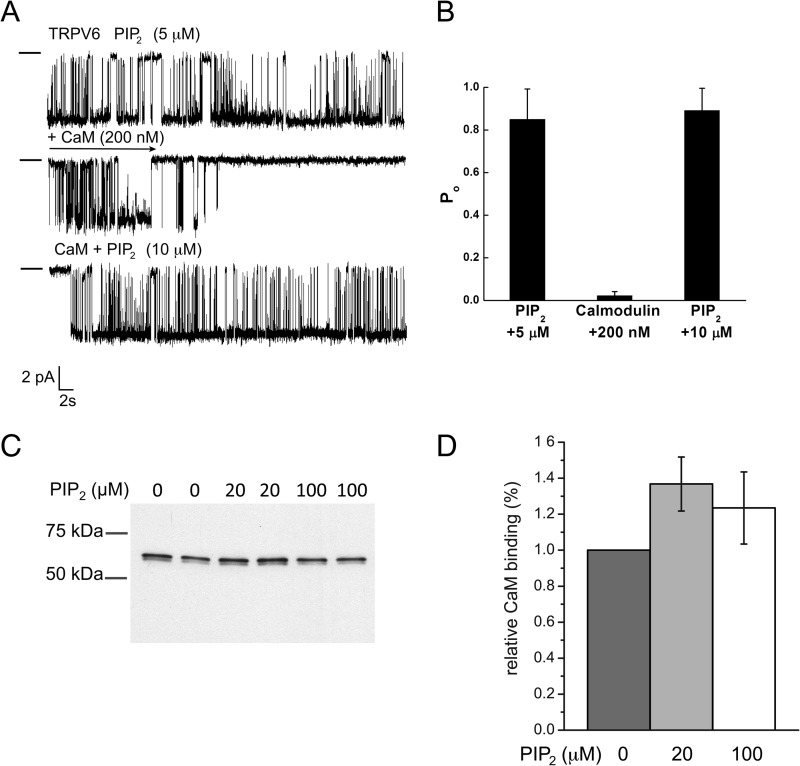
FIGURE 8.
PI(4,5)P2 competes with CaM in planar lipid bilayers, but not in biochemical binding experiments. A, planar lipid bilayer measurements were performed as described under “Experimental Procedures.” TRPV6 activity was stimulated by 5 μm diC8 PI(4,5)P2. Then 200 nm CaM was applied, and PI(4,5)P2 concentration was increased to 10 μm. Clamping potential was −100 mV. The closed state is indicated by a horizontal line on the left side of traces. B, summary of the bilayer experiments (n = 5 for CaM inhibition and n = 3 for PI(4,5)P2 reactivation). C, CaM-Sepharose pulldown assay was performed using MBP fusion proteins of TRPV6 wild-type C terminus in the absence and the presence of 20 or 100 μm diC8 PI(4,5)P2 as described under “Experimental Procedures.” Free Ca2+ concentration was 100 μm. Bound proteins were detected by SDS-PAGE and Western-blotting using anti-MBP antibodies. D, statistical summary based on five measurements.
Our data show so far that PI(4,5)P2 can functionally compete with CaM both in excised patches and in planar lipid bilayers. This can happen either through competition for the same or overlapping binding site or via allosteric effects. To differentiate between these two possibilities, we tested whether PI(4,5)P2 competes with CaM for binding to the C terminus of TRPV6. Fig. 8 (C and D) shows that the C terminus of TRPV6 showed very similar binding to CaM beads in the absence or the presence of 20 and 100 μm diC8 PI(4,5)P2. This measurement indicates that PI(4,5)P2 does not directly compete with CaM for the same binding site.
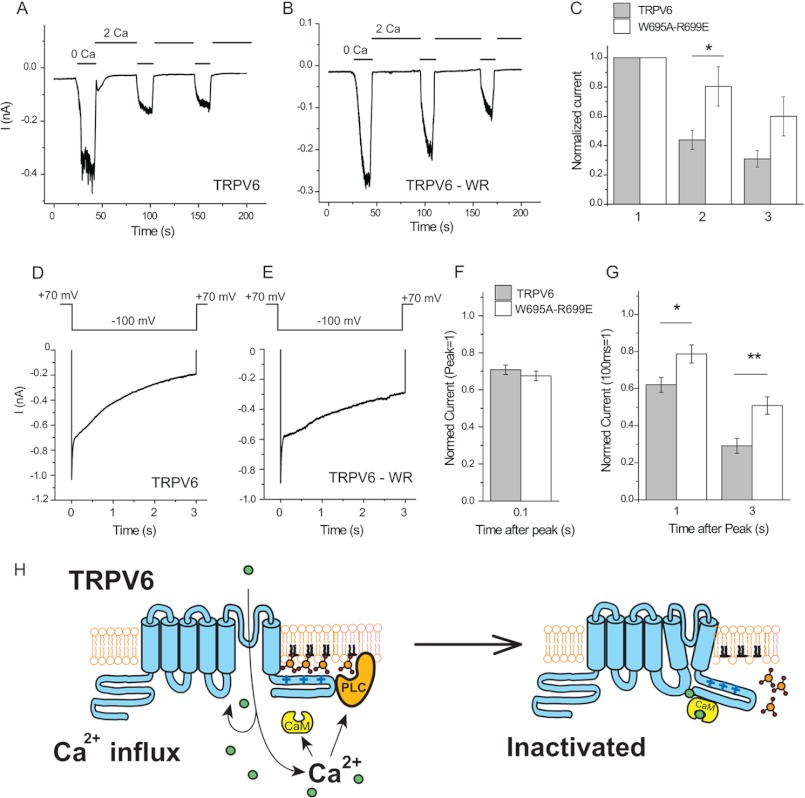
To assess the role of CaM in Ca2+-induced inactivation, we have performed whole cell patch clamp experiments in mammalian cells expressing wild-type and W695A/R699E double mutant TRPV6 channels. Fig. 9 (A–C) shows measurements in which monovalent currents through TRPV6 were measured, interspersed with applications of 2 mm Ca2+. In wild-type channels, monovalent currents decreased, on average, by 60% after the first and ∼70% after second application of 2 mm Ca2+. In the double mutant, only ∼20% inactivation was observed, on average, after the first application of Ca2+, with a ∼40% decrease after the second. These data show that CaM is involved in Ca2+-induced inactivation, but it is probably not the only factor.
FIGURE 9.
Ca2+-induced inactivation is reduced but not eliminated in the W695A/R699E mutant. Whole cell patch clamp experiments in TRPV6-expressing HEK cells were performed as described under “Experimental Procedures.” A and B, representative measurements for wild-type and W695A/R699E (WR) TRPV6 channels at constant −60 mV holding potential. At the beginning of the measurements, the cells were kept in a nominally Ca2+-free solution containing 1 mm Mg2+. Monovalent currents were evoked by the application of 2 mm EGTA in bivalent free extracellular solution (0 Ca); then Ca2+-induced inactivation was induced by the application of a solution containing 2 mm Ca2+ and no Mg2+ (2 Ca). C, summary of current amplitudes in the first, second, and third applications of EGTA. The difference between wild-type and the mutant channel was statistically significant in the second (p = 0.024, n = 11), but not at the third pulse (p = 0.058). D and E, Ca2+-induced inactivation of Ca2+ currents for wild-type and mutant TRPV6 channels. Ca2+ currents were initiated by a 3-s voltage step from +70 to −100 mV in an extracellular solution containing 10 mm Ca2+. The traces shown are averages for six measurements for both groups. F, summary of inactivation kinetics at 100 ms; the data are normalized to the point 3 ms after the voltage step, to avoid capacitative artifacts. G, summary at 1 and 3 s after the −100 mV voltage step; the data are normalized to the current 100 ms after the voltage step, after the initial fast phase inactivation. The difference between wild-type and mutant channel was statistically significant both at 1 s (p = 0.025) and at 3 s (p = 0.0054). Asterisks denote statistical significance. H, model for Ca2+-induced inactivation of TRPV6.
We have also measured Ca2+-induced inactivation in a different protocol often used by several laboratories (3, 14, 15), where Ca2+ influx was initiated with a 3-s voltage step from +70 to −100 mV in the presence of 10 mm Ca2+. Fig. 9 (D–F) shows that in this protocol, a quickly inactivating phase that is complete after ∼50 ms is followed by a slower phase throughout the 3-s voltage pulse. The fast phase was not different between the wild-type and the W695A/R699E mutant; however, inactivation of the mutant channel was significantly smaller in the slow phase (Fig. 9G), in accordance with earlier results using different mutations in this region of TRPV6 (14, 15) or equivalent mutations in TRPV5 (16).
DISCUSSION
TRPV6, similarly to many other Ca2+ permeable channels, undergoes Ca2+-induced inactivation. Given that this channel is constitutively active, this process is especially important for protecting the cell from toxic Ca2+ overload. To understand the roles of individual signaling molecules, we have used excised inside-out patch clamp measurements to study the effects of CaM, PI(4,5)P2 and Ca2+ on TRPV6 channels. Using this technique, we fully control the concentration of CaM and avoid the effects of cellular components present in whole cell patch clamp experiments, and we can dissect the direct effects of Ca2+ from those mediated by CaM.
We found that CaM applied to the intracellular surface of the patch membrane reproducibly inhibited TRPV6 channel activity. In the absence of Ca2+, channel activity was not inhibited by CaM. This is consistent with earlier reports showing that CaM binding to TRPV6 requires Ca2+ (15). For most CaM experiments, we used 3 μm Ca2+, which by itself only caused a small inhibition. CaM is a major intracellular protein, its total cellular concentration is though to be ∼10 μm, but most of it binds to other proteins, and the maximal free concentration of Ca2+-CaM was estimated to be 45 nm at saturating Ca2+ concentrations (3 μm or above) (27). CaM inhibited TRPV6 activity at concentrations as low as 50 nm in excised patches, suggesting that the effect of CaM on TRPV6 is physiologically relevant.
Ca2+ itself without the application of CaM also inhibited TRPV6 activity in excised patches, but substantial inhibition required very high concentrations (EC50 = ∼20 μm). CaM associates with many different ion channels in the absence of Ca2+ (29). Can the effect of Ca2+ be due to CaM preassociated to TRPV6? We think this is unlikely for the following reasons: 1) Our data and earlier reports (15) show that CaM association with the channel requires Ca2+. 2) The CaM1234 mutant that cannot bind Ca2+ is often used to study the effects of preassociated CaM. It was shown that this dominant negative CaM did not bind TRPV6 and had no effect on its Ca2+-induced inactivation (15). 3) Binding of Ca2+ to CaM is though to saturate ∼3 μm, and in our experiments, we needed Ca2+ concentrations as high as 100 μm for full inhibition.
Although global intracellular Ca2+ hardly reaches concentrations over 1 μm, in the subplasmalemmal regions near the mouth of an open Ca2+ channel, local Ca2+ concentrations may reach hundreds of micromolars (25). Despite the requirement for very high Ca2+ concentrations, it is possible that direct inhibition by Ca2+ contributes to Ca2+-induced inactivation. The lack of effect of the CaM-binding site mutation on the first very fast phase of TRPV6 inactivation (Fig. 9, D and E), also described by others (14), is consistent with the idea of local high concentrations of Ca2+ directly inhibiting the channel.
At the physiologically relevant CaM concentrations, the inhibitory effect of CaM developed quite slowly and was partial, suggesting that other factors also contribute to Ca2+-induced inactivation. To be able to address the role of CaM and other signaling pathways, we used the excised inside-out patch technique to unequivocally identify the site responsible for CaM-mediated inhibition. CaM binds to linear α-helical protein segments ∼20 amino acids long (30). Various CaM-binding sites have been proposed in TRPV6 in the N- and C-terminal cytoplasmic domains and in the cytoplasmic loop between the second and third transmembrane domains (Fig. 3A). Here we focused on the distal C-terminal CaM-binding region, residues 694–716 in the human TRPV6 (14, 15). A recent article found that CaM also binds to TRPV5, a close relative of TRPV6, via the equivalent distal C-terminal site (16). That study also identified individual amino acids that are important in this region for CaM binding. Our data show that mutation of two conserved residues (W695A/R699E) in the distal C-terminal region of TRPV6 eliminated CaM binding to the isolated C terminus of TRPV6 and to the full-length channel protein. Furthermore, this double mutant showed dramatically reduced inhibition by Ca2+-CaM in excised patches. Some residual inhibition remained at supraphysiological CaM concentrations, which may be due to nonspecific interactions of CaM with the channel, or interactions with some of the additional CaM-binding sites. Overall, these data show that the inhibitory effect of CaM at physiological CaM levels is mediated by the distal CaM-binding site.
An earlier study showed using FRET in live cells that the cyan fluorescent protein-tagged CaM interacts with the full-length YFP-tagged TRPV6 in a Ca2+-dependent manner (15). Consistent with our results, they also found that the Ca2+-dependent FRET between the full-length TRPV6 and CaM was eliminated by the removal of the distal C-terminal CaM-binding site (15).
The activity of TRPV6 requires the presence of PI(4,5)P2 (10, 12). This appears to be a full dependence, because channel activity invariably showed a complete loss over time in excised patches when MgATP or PI(4,5)P2 was not supplied and sufficient time has passed in the inside-out configuration. We also could not detect any TRPV6 activity in planar lipid bilayers without supplying PI(4,5)P2 (12). Without the presence of some PI(4,5)P2, the effects of CaM could not have been studied. We have used four different ways to supply PI(4,5)P2 to support channel activity: 1) performing the experiment immediately after excision, relying on the endogenous PI(4,5)P2 in the patch membrane; 2) applying short acyl chain diC8 PI(4,5)P2; 3) applying natural long acyl chain PI(4,5)P2; and 4) applying MgATP to allow the lipid kinases in the patch to resynthesize PI(4,5)P2. In all of these cases, CaM inhibited TRPV6 activity.
Both CaM (31) and PI(4,5)P2 (32) regulate a large number of TRP channels. Are there any general principles for the interaction of these two regulating molecules? It was proposed for TRPC6 that phosphoinositides, especially PI(3,4,5)P3, regulate channel activity by disrupting binding to CaM (20). In the same article, CaM and phosphoinositides were shown to bind to overlapping binding sites to isolated C-terminal fragments of several other TRP channels (not including TRPV6), and this direct competition was proposed to be a general mechanism for regulation of TRP channels by these two intracellular signaling molecules. TRPV6 activity fully depends on the presence of PI(4,5)P2. Does CaM inhibit this channel by displacing PI(4,5)P2 from its binding site? This is quite an attractive hypothesis, because the distal C-terminal CaM-binding site has a number of positively charged residues, which are invariably involved in interactions of phosphoinositides with proteins, including ion channels (33). We have found that higher concentrations of PI(4,5)P2 could reduce inhibition by CaM in excised patches and planar lipid bilayers, which is compatible with this model. This finding, however, can also be explained by allosteric effects, for example if PI(4,5)P2 stabilizes the open states, whereas CaM stabilizes the closed state.
To explore this idea further, we have tested whether PI(4,5)P2 can interfere with binding of the isolated C terminus to CaM. We have used the isolated C terminus because it is far less likely than the full-length channel to undergo significant conformational change upon binding of either molecules, which could reduce the binding of the other, without any actual overlap between their respective binding sites (34). We found no competition by PI(4,5)P2 in these experiments, which argues against a simple model in which CaM displaces PI(4,5)P2 from its binding site through direct competition.
As mentioned earlier, CaM invariably binds to short α-helical polypeptide segments. Phosphoinositides may also bind to short highly positively charged peptides (35), in most cases, however, well defined three-dimensional structures such as the pleckstrin homology domain are responsible for binding, where the actual binding residues come together from various parts of the linear sequence. Ion channels generally do not have well defined phosphoinositide-binding domains with homology to other lipid-binding domains. Among ion channels, phosphoinositide interactions are best characterized in inwardly rectifying K+ (Kir) channels. It is thought that the PI(4,5)P2-binding pocket is formed by a three-dimensional structure where residues contributing to lipid binding come from various parts of the C and N terminus (33, 36). This picture was confirmed by the recent co-crystal structure of Kir2.2 and PI(4,5)P2 (37, 38). Most residues playing significant roles in PI(4,5)P2 interactions are located in proximal regions, relatively close to the transmembrane domains. If PI(4,5)P2 interactions in TRP channels are similar to those in Kir channels, it is quite unlikely that the very distal C terminus by itself could serve as the sole PI(4,5)P2-binding domain. We found that when the whole distal CaM binding segment is removed, the channel is still fully functional (data not shown), consistent with earlier findings (14, 15). Because our data show full dependence of channel activity on PI(4,5)P2, it is unlikely that this short segment contributes significantly to PI(4,5)P2 binding. This, again, also argues against CaM inhibiting the channel by displacing PI(4,5)P2 from its binding site.
What is the contribution of CaM inhibition to the overall Ca2+-induced inactivation of TRPV6 in a cellular context? To address this, we have tested the effect of the double mutation in the CaM-binding region on the Ca2+-induced inactivation of TRPV6 in whole cell patch clamp measurements. We have measured the decrease of monovalent currents through TRPV6, interspersed with applications of 2 mm Ca2+, as we described earlier (4, 10). Here we have found that the double mutant TRPV6, lacking CaM binding and displaying no inhibition by CaM in excised patches, showed significantly reduced Ca2+-induced inactivation compared with wild-type TRPV6. Some inactivation did occur, however, especially after the second application of Ca2+, where the difference between wild-type and mutant channels was not statistically significant. In our earlier work, we have shown that inclusion of PI(4,5)P2 in the patch pipette completely eliminated Ca2+-induced inactivation of TRPV6 in this protocol (10). These data suggest that both CaM and PI(4,5)P2 depletion is needed for maximal inactivation.
We have also measured Ca2+-induced inactivation in a different protocol, where Ca2+ influx is initiated by a voltage step to −100 mV, from a positive voltage that does not allow Ca2+ influx. This protocol allows detection of very fast events at the onset of Ca2+ influx. We have found that the first fast phase of inactivation, which was complete at ∼50 ms, was not affected by the CaM-binding site mutation. Because the effects of Ca2+ developed very fast in excised patches, it is quite likely that this fast phase is caused by the direct effect of Ca2+ on the channel. Similar to the protocol measuring monovalent currents, Ca2+-induced inactivation in the second, slower phase was reduced but not eliminated in the CaM-binding site mutant, pointing to the involvement of other factors in Ca2+-induced inactivation.
Our excised patch measurements show that excess PI(4,5)P2 can reduce the effect of CaM on the channel, and the effect of PI(4,5)P2 is less in the presence of CaM; in other words there is functional competition between these two signaling molecules. Overall, these data suggest a model in which Ca2+ plays a dual role in inactivation: by binding to CaM it reduces the effectiveness of PI(4,5)P2, and by reducing PI(4,5)P2 levels, it enhances the effect of CaM. Overall, it is quite likely that neither CaM, nor PI(4,5)P2 depletion alone is sufficient to induce full inactivation of the channel, but the two act together to reduce further Ca2+ influx.
Our data show that although CaM did inhibit TRPV6 at 50 nm, that effect developed much more slowly than at higher concentrations. Thus physiological CaM levels, even when saturated with Ca2+, are suboptimal for inhibiting TRPV6. Channel activity fully depends on the presence of PI(4,5)P2; thus taking this lipid away, should fully inhibit the channel by itself. Indeed when we artificially depleted PI(4,5)P2 with a rapamycin-inducible 5′ phosphatase, we saw a ∼75% inhibition (10) of TRPV6 activity. Ca2+ influx through TRPV6, however, results in only a moderate reduction in PI(4,5)P2 levels (10) when compared with that induced by capsaicin in TRPV1-expressing cells (39). Thus upon Ca2+ influx through TRPV6, two suboptimal inhibitory signals converge and act together to reduce channel activity.
In conclusion, we found that Ca2+-CaM inhibits TRPV6 activity via direct binding to the distal C-terminal region. Increased PI(4,5)P2 levels may override this inhibition, but the effect of CaM is not due to the displacement of PI(4,5)P2 via direct competition. During Ca2+-induced inactivation, Ca2+, CaM, and PI(4,5)P2 contribute to reduced channel activity. Fig. 9H shows our model for Ca2+-induced inactivation of TRPV6.
Acknowledgment
The clone for the human Myc-tagged TRPV6 was generously provided by Dr. T. V. McDonald (Albert Einstein College of Medicine, New York, NY).
This work was supported, in whole or in part, by National Institutes of Health Grants NS055159 and GM093290 (to T. R.) and GM098052 (to E. Z.). This work was also supported by a grant from the University of Medicine and Dentistry of New Jersey Foundation (to T. R.).
- TRP
- transient receptor potential
- TRPV6
- TRP vanilloid 6
- HEDTA
- N-(2Hydroxyethyl)ethylenediamine-N,N′,N′-triacetic acid
- hTRPV6
- human TRP vanilloid 6
- PI(4,5)P2
- phosphatidylinositol 4,5-bisphophate
- AASt
- arachydonyl and stearyl side chains
- MBP
- maltose-binding protein.
REFERENCES
- 1. Hoenderop J. G., Nilius B., Bindels R. J. (2005) Calcium absorption across epithelia. Physiol. Rev. 85, 373–422 [DOI] [PubMed] [Google Scholar]
- 2. Weissgerber P., Kriebs U., Tsvilovskyy V., Olausson J., Kretz O., Stoerger C., Vennekens R., Wissenbach U., Middendorff R., Flockerzi V., Freichel M. (2011) Male fertility depends on Ca2+ absorption by TRPV6 in epididymal epithelia. Sci. Signal. 4, ra27. [DOI] [PubMed] [Google Scholar]
- 3. Nilius B., Prenen J., Hoenderop J. G., Vennekens R., Hoefs S., Weidema A. F., Droogmans G., Bindels R. J. (2002) Fast and slow inactivation kinetics of the Ca2+ channels ECaC1 and ECaC2 (TRPV5 and TRPV6). Role of the intracellular loop located between transmembrane segments 2 and 3. J. Biol. Chem. 277, 30852–30858 [DOI] [PubMed] [Google Scholar]
- 4. Thyagarajan B., Benn B. S., Christakos S., Rohacs T. (2009) Phospholipase C-mediated regulation of transient receptor potential vanilloid 6 channels. Implications in active intestinal Ca2+ transport. Mol. Pharmacol. 75, 608–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suh B. C., Hille B. (2008) PIP2 is a necessary cofactor for ion channel function. How and why? Annu. Rev. Biophys. 37, 175–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hilgemann D. W., Feng S., Nasuhoglu C. (2001) The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE. 2001, re19. [DOI] [PubMed] [Google Scholar]
- 7. Logothetis D. E., Nilius B. (2007) Dynamic changes in phosphoinositide levels control ion channel activity. Pflugers Arch. 455, 1–3 [DOI] [PubMed] [Google Scholar]
- 8. Rohacs T. (2009) Phosphoinositide regulation of non-canonical transient receptor potential channels. Cell Calcium 45, 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nilius B., Owsianik G., Voets T. (2008) Transient receptor potential channels meet phosphoinositides. EMBO J. 27, 2809–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thyagarajan B., Lukacs V., Rohacs T. (2008) Hydrolysis of phosphatidylinositol 4,5-bisphosphate mediates calcium-induced inactivation of TRPV6 channels. J. Biol. Chem. 283, 14980–14987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rohács T., Lopes C. M., Michailidis I., Logothetis D. E. (2005) PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat. Neurosci. 8, 626–634 [DOI] [PubMed] [Google Scholar]
- 12. Zakharian E., Cao C., Rohacs T. (2011) Intracellular ATP supports TRPV6 activity via lipid kinases and the generation of PtdIns(4,5)P2. FASEB J. 25, 3915–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee J., Cha S. K., Sun T. J., Huang C. L. (2005) PIP2 activates TRPV5 and releases its inhibition by intracellular Mg2+. J. Gen. Physiol. 126, 439–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Niemeyer B. A., Bergs C., Wissenbach U., Flockerzi V., Trost C. (2001) Competitive regulation of CaT-like-mediated Ca2+ entry by protein kinase C and calmodulin. Proc. Natl. Acad. Sci. U.S.A. 98, 3600–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Derler I., Hofbauer M., Kahr H., Fritsch R., Muik M., Kepplinger K., Hack M. E., Moritz S., Schindl R., Groschner K., Romanin C. (2006) Dynamic but not constitutive association of calmodulin with rat TRPV6 channels enables fine tuning of Ca2+-dependent inactivation. J. Physiol. 577, 31–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Groot T., Kovalevskaya N. V., Verkaart S., Schilderink N., Felici M., van der Hagen E. A., Bindels R. J., Vuister G. W., Hoenderop J. G. (2011) Molecular mechanisms of calmodulin action on TRPV5 and modulation by parathyroid hormone. Mol. Cell. Biol. 31, 2845–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lambers T. T., Weidema A. F., Nilius B., Hoenderop J. G., Bindels R. J. (2004) Regulation of the mouse epithelial Ca2+ channel TRPV6 by the Ca2+-sensor calmodulin. J. Biol. Chem. 279, 28855–28861 [DOI] [PubMed] [Google Scholar]
- 18. Kovalevskaya N. V., Bokhovchuk F. M., Vuister G. W. (2012) The TRPV5/6 calcium channels contain multiple calmodulin binding sites with differential binding properties. J. Struct. Funct. Genomics 13, 91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holakovska B., Grycova L., Bily J., Teisinger J. (2011) Characterization of calmodulin binding domains in TRPV2 and TRPV5 C-tails. Amino Acids 40, 741–748 [DOI] [PubMed] [Google Scholar]
- 20. Kwon Y., Hofmann T., Montell C. (2007) Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol. Cell. 25, 491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cui J., Bian J. S., Kagan A., McDonald T. V. (2002) CaT1 contributes to the stores-operated calcium current in Jurkat T-lymphocytes. J. Biol. Chem. 277, 47175–47183 [DOI] [PubMed] [Google Scholar]
- 22. Csanády L., Törocsik B. (2009) Four Ca2+ ions activate TRPM2 channels by binding in deep crevices near the pore but intracellularly of the gate. J. Gen. Physiol. 133, 189–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bers D. M., Patton C. W., Nuccitelli R. (2010) A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 99, 1–26 [DOI] [PubMed] [Google Scholar]
- 24. Park E. J., Kim B. J., Kim S. H., Kim S. Y., Sung T. S., Chae H. G., Kim S. J., Kim J., Park H. H., So I., Jeon J. H. (2009) Altered biochemical properties of transient receptor potential vanilloid 6 calcium channel by peptide tags. Biol. Pharm. Bull. 32, 1790–1794 [DOI] [PubMed] [Google Scholar]
- 25. Parekh A. B. (2008) Ca2+ microdomains near plasma membrane Ca2+ channels. Impact on cell function. J. Physiol. 586, 3043–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rohács T., Lopes C. M., Jin T., Ramdya P. P., Molnár Z., Logothetis D. E. (2003) Specificity of activation by phosphoinositides determines lipid regulation of Kir channels. Proc. Natl. Acad. Sci. U.S.A. 100, 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Persechini A., Cronk B. (1999) The relationship between the free concentrations of Ca2+ and Ca2+-calmodulin in intact cells. J. Biol. Chem. 274, 6827–6830 [DOI] [PubMed] [Google Scholar]
- 28. Rohács T., Lopes C., Mirshahi T., Jin T., Zhang H., Logothetis D. E. (2002) Assaying phosphatidylinositol bisphosphate regulation of potassium channels. Methods Enzymol. 345, 71–92 [DOI] [PubMed] [Google Scholar]
- 29. Saimi Y., Kung C. (2002) Calmodulin as an ion channel subunit. Annu. Rev. Physiol. 64, 289–311 [DOI] [PubMed] [Google Scholar]
- 30. Crivici A., Ikura M. (1995) Molecular and structural basis of target recognition by calmodulin. Annu. Rev. Biophys. Biomol. Struct. 24, 85–116 [DOI] [PubMed] [Google Scholar]
- 31. Zhu M. X. (2005) Multiple roles of calmodulin and other Ca2+-binding proteins in the functional regulation of TRP channels. Pflugers Arch. 451, 105–115 [DOI] [PubMed] [Google Scholar]
- 32. Rohacs T., Nilius B. (2007) Regulation of transient receptor potential (TRP) channels by phosphoinositides. Pflugers Arch. 455, 157–168 [DOI] [PubMed] [Google Scholar]
- 33. Rosenhouse-Dantsker A., Logothetis D. E. (2007) Molecular characteristics of phosphoinositide binding. Pflugers Arch. 455, 45–53 [DOI] [PubMed] [Google Scholar]
- 34. Colquhoun D. (1998) Binding, gating, affinity and efficacy. The interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br. J. Pharmacol. 125, 924–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McLaughlin S., Murray D. (2005) Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438, 605–611 [DOI] [PubMed] [Google Scholar]
- 36. Lopes C. M., Zhang H., Rohacs T., Jin T., Yang J., Logothetis D. E. (2002) Alterations in conserved Kir channel-PIP2 interactions underlie channelopathies. Neuron 34, 933–944 [DOI] [PubMed] [Google Scholar]
- 37. Hansen S. B., Tao X., MacKinnon R. (2011) Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477, 495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whorton M. R., MacKinnon R. (2011) Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell 147, 199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lukacs V., Thyagarajan B., Varnai P., Balla A., Balla T., Rohacs T. (2007) Dual regulation of TRPV1 by phosphoinositides. J. Neurosci. 27, 7070–7080 [DOI] [PMC free article] [PubMed] [Google Scholar]