Abstract
Interactions of selectins with cell surface glycoconjugates mediate the first step of the adhesion and signaling cascade that recruits circulating leukocytes to sites of infection or injury. P-selectin dimerizes on the surface of endothelial cells and forms dimeric bonds with P-selectin glycoprotein ligand-1 (PSGL-1), a homodimeric sialomucin on leukocytes. It is not known whether leukocyte L-selectin or endothelial cell E-selectin are monomeric or oligomeric. Here we used the micropipette technique to analyze two-dimensional binding of monomeric or dimeric L- and E-selectin with monomeric or dimeric PSGL-1. Adhesion frequency analysis demonstrated that E-selectin on human aortic endothelial cells supported dimeric interactions with dimeric PSGL-1 and monomeric interactions with monomeric PSGL-1. In contrast, L-selectin on human neutrophils supported monomeric interactions with dimeric or monomeric PSGL-1. Our work provides a new method to analyze oligomeric cross-junctional molecular binding at the interface of two interacting cells.
Introduction
During acute inflammation, leukocytes are recruited from the circulation to sites of infection and injury. This multistep adhesion and signaling cascade is initiated by interactions between selectins and their glycoconjugates that mediate leukocyte tethering to and rolling on the surface of endothelial cells [1]. The selectin family includes three members. L-selectin is expressed on leukocytes. P- and E-selectin are expressed on activated platelets and/or activated endothelial cells. Their common structure includes a ligand-binding lectin domain, an epidermal growth factor-like module, multiple copies of consensus repeats characteristic of complement-binding proteins, a transmembrane segment, and a short cytoplasmic domain [1]–[3]. The best characterized selectin ligand is P-selectin glycoprotein ligand-1 (PSGL-1), a high-affinity ligand for P-selectin that also binds to L- and E-selectin [1]–[3].
P-selectin [4], [5] and PSGL-1 [6]–[8] form dimers on the respective surfaces of endothelial cells and leukocytes. Dimerization of P-selectin and PSGL-1 stabilizes cell rolling and enhances tether strength in shear flow [9] and prolongs bond lifetime [10]. Induced cell-surface dimerization of L-selectin by cross-linking its cytoplasmic or extracellular domains increases binding to soluble ligand mimic and adhesion to immobilized ligand or to vascular endothelium under shear [11], [12]. However, biochemical analyses of the L-selectin transmembrane and cytoplasmic domains suggest that they are monomeric in bacterial or synthetic membranes [13]. PSGL-1 does not form dimeric bonds with E-selectin purified from lysates of CHO cell transfectants after it is reconstituted into glass-supported lipid bilayers [14]. However, it is not known whether E-selectin forms monomers or oligomers on the surfaces of endothelial cells.
Like other cell adhesion receptors, selectins and their ligands also transduce signals [1], [15]. Antibody-mediated crosslinking of L-selectin or PSGL-1 on leukocytes or of E- or P-selectin on endothelial cells triggers intracellular calcium fluxes and protein tyrosine phosphorylation. Receptor tyrosine kinases [16] and multichain immune recognition receptors [17] must form dimers to transduce signals. On the other hand, both dimeric and monomeric forms of PSGL-1 transduce signals after engaging P-selectin [18]. Whether other selectin ligands or selectins themselves must dimerize to signal under physiological conditions is not known.
The goal of the present work was to determine whether L- and E-selectin form oligomers or monomers on cell surfaces. We used the micropipette adhesion frequency assay to analyze two-dimensional (2D) binding between selectins and ligands [19]–[22]. Our strategy was to use purified molecular systems with structurally well-defined multimericities to define the requirements and properties of monomeric and dimeric interactions between the same receptor-ligand pair. By comparing binding to the same densities of native dimeric PSGL-1 and recombinant monomeric soluble (s) PSGL-1 [10], we showed that endothelial cell E-selectin supported dimeric interactions whereas neutrophil L-selectin supported monomeric interactions.
Materials and Methods
Cells and Proteins
Chinese hamster ovary (CHO) cells transfected to stably express human E-selectin were previously described [23]. Human aortic endothelial cells (HAECs, Clonetics, Walkersville, MD) were generous gifts from Dr. Julia Babensee (Georgia Institute of Technology) and were maintained as previously described [24]. CHO cells were cultured in RPMI 1640 plus 5% fetal bovine serum, 5% calf serum, 2 mM glutamine, 1 mM sodium pyruvate, and 1% penicillin/streptomycin. To induce expression of E-selectin, HAECs were stimulated with 100 U/ml interleukin-1β (IL-1β) for 6 h.
Neutrophils were isolated from a drop of whole blood via a finger prick and RBCs were isolated from peripheral blood drawn from healthy volunteers according to protocols approved by Georgia Institute of Technology’s Institutional Review Board [25]. Written informed consent were obtained for all volunteers. Neutrophils were prepared on the day of experiment. After RBCs were lysed by a brief hypotonic shock, neutrophils were spun down and resuspended in Hanks’ balanced salt solution (Sigma-Aldrich, St. Louis, MO) with 1% human serum albumin (ZLB Plasma, Boca Raton, FL), and used immediately. RBCs were prepared and stored for experiments in several weeks. Whole blood was layered over Histopaque 1119 (Sigma) and centrifuged by 2000 g for 30 min at room temperature. The pelleted RBCs were washed, coated with capturing antibodies, and stored in experimental additive solution 45 (EAS 45) (Dumaswala et al., 1996) at 4°C.
Human L-selectin-Ig was expressed and purified as previously described [26]. The same methods were used to construct, express, and purify human E-selectin-Ig, comprising the lectin and epidermal growth factor domains and the first two consensus repeats of human E-selectin fused to the Fc portion of human IgG1. Recombinant human sE-selectin [27], sPSGL-1 [28], membrane PSGL-1 [8], anti-E-selectin capturing and blocking monoclonal antibodies (mAbs) 1D6 [29] and ES1 [23], anti-L-selectin mAb DREG56 [30], and anti-PSGL-1 capturing mAb PL2 [31] have been described previously. Goat anti-human IgG antibody (for capturing selectin-Ig) and FITC-labeled goat anti-mouse IgG mAb A85-1 (used as secondary antibody) or FITC-labeled human IgG antibody (for site density measurement) was from Sigma. PE-labeled anti-PSGL-1 mAb PL1 for site density measurement was from Calbiochem (St. Louis, MO).
Coupling Ligands onto RBC Surface
A previously described modified chromium chloride (CrCl3) method [32] was used to couple capturing antibodies (1D6 for sE-selectin, goat anti-human IgG-Fc for L- and E-selectin-Ig, and PL2 for (s)PSGL-1) on RBC. Briefly, a 1% CrCl3 solution was prepared, aged at pH 5, and diluted in 20 mM acetate-buffered saline, pH 5.5, at ratios ranging from 1∶100 to 1∶2400. RBCs were washed five times in saline and resuspended in 1% hematocrit. Antibodies were added to each 250 µl sample and mixed. An equal volume of diluted CrCl3 solution was added dropwise with constant agitation. After 5 min the reaction was stopped by addition of 0.5 ml phosphate buffered saline plus 1% IgG-free bovine serum albumin (BSA). Aliquots from each sample were examined under light microscopy for aggregation. Cells were subsequently washed and stored in EAS45. Immediately prior to the micropipette experiment, RBCs precoated with 1D6, anti-Fc or PL2 were respectively incubated with sE-selectin (0.5 µg/ml), L- or E-selectin-Ig (0.5 µg/ml), or (s)PSGL-1 (0.2 µg/ml) at room temperature for 30 min.
Micropipette Adhesion Frequency Assay
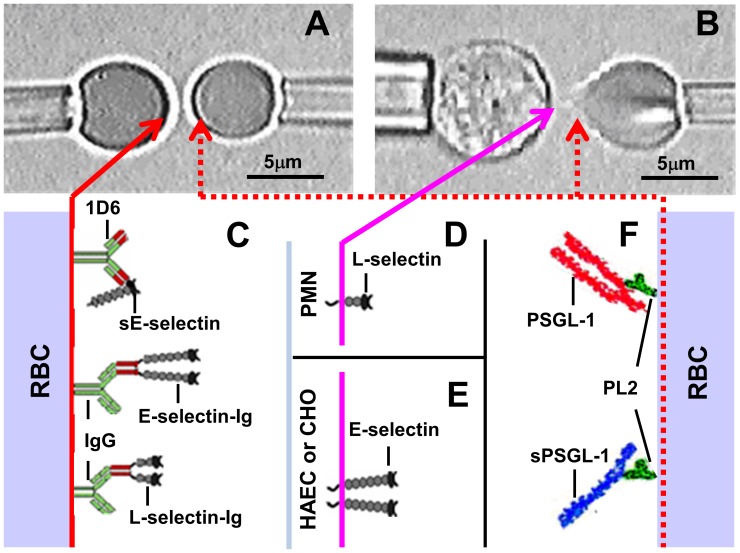
A previously described micropipette adhesion frequency assay [19]–[22] was used to analyze the two-dimensional (2D) binding between various forms of L- or E-selectin and (s)PSGL-1. Briefly, a cell chamber filled with 3 ml of L-15 medium supplemented with 1% BSA was mounted on the stage of an inverted microscope (Zeiss Axiovert 100, Oberkochan, Germany). Cells coated with selectins or ligands were added to different locations separated by sufficient distance to avoid mixing. A selectin-coated cell was picked up by one micropipette and a ligand-coated cells was picked up by an apposing micropipette. The cells were then aligned via micromanipulation. Selectins were either captured on RBCs by precoated antibodies (Fig. 1C), expressed on human neutrophils (for L-selectin, Fig. 1D), or expressed on CHO cell transfectants or induced on HAECs (for E-selectin, Fig. 1E). (s)PSGL-1 was captured on RBCs by precoated mAb (Fig. 1F). One pipette was driven by a computer-controlled piezo translator to move the cell to contact the other cell held stationary for a pre-determined area and duration. Following pipette retraction, the cells were either immediately separated (no adhesion, scored 0) or remained bound with the flexible RBC(s) being stretched (adhesion, scored 1) for a short time until detachment. The likelihood of adhesion was estimated from the frequency of adhesions (P a) observed in 50∼100 repeated contact cycles using a single pair of cells. Three to five cell pairs were tested to obtain a mean P a ± S.E.M. for that contact duration (t). Measurements were made in five contact durations to obtain a P a vs. t binding curve for each receptor-ligand pair respectively expressed on the corresponding cells at given site densities measured separately by flow cytometry.
Figure 1. Micropipette adhesion frequency assay. A and B.
. Photomicrographs of a pair of RBCs (A) or a nucleated cell (left) and a RBC (right) (B) respectively held by two apposing pipettes. C-F. Composite of interacting molecules on respective cell surfaces. The left RBC in A was precoated with anti-E-selectin (1D6) that captured monomeric sE-selectin or precoated with goat anti-human Ig that captured dimeric E-selectin-Ig or L-selectin-Ig (C). The left nucleated cell in B was a PMN that expressed L-selectin (D) but could also be a HAEC or CHO cell that expressed E-selectin (E). Based on the data of this work, L-selectin on PMN is depicted as a monomer whereas E-selectin on HAEC and CHO cell is depicted as a dimer. The right RBCs in both A and B were precoated with anti-PSGL-1 mAb PL2 that captured dimeric membrane PSGL-1 or monomeric recombinant sPSGL-1 (F).
Site Density Determination
Cell samples were incubated with saturating concentrations of fluorescence-conjugated primary antibodies (10 µg/ml or according to manufacturer’s instruction) in FACS solution (RPMI with 1% BSA and 0.05% sodium azide) for 30 min followed by three washes, and analyzed immediately. If non-conjugated primary antibody was used, cells were next incubated with FITC-conjugated secondary antibody (10 µg/ml or per manufacturer’s instruction) and then washed and analyzed immediately. Cells and calibration beads from quantum FITC MESF kits (Bangs Laboratories, Fishers, IN) were analyzed on a BD LSR II flow cytometer (Becton-Dickinson Immunocytometry Systems, San Jose, CA). The mean fluorescent intensities (MFIs) were recorded for samples as well as for each peak of the calibration beads. A standard curve was plotted using the MFIs of the calibration beads, which was used to calculate molecules of equivalent soluble fluorophore (MESF) from the MFIs measured from the cells. Antibodies bound per cell were then calculated by dividing MESF by fluorophore per antibody (supplied by manufacturers), which were then converted to site densities by dividing by the cell surface area [33], [34].
The capacity of capturing E- or L-selectin-Ig by goat anti-human IgG antibody precoated on RBCs was measured using a FITC-conjugated human IgG. At saturating concentration, each binding site for Fc should capture one dimeric E- or L-selectin-Ig. Direct measurement using anti-E-selectin (ES1) and anti-L-selectin (DREG56) mAbs obtained similar results. The site density of sE-selectin was controlled by the site density of its capture antibody (1D6) precoated on RBCs, which was measured using FITC-labeled goat anti-mouse IgG mAb A85-1. The site density of sPSGL-1 was measured using PE-labeled anti-PSGL-1 mAb PL1. Since the same batch of RBCs pre-coated with the same density of PL2 were incubated with saturating concentrations of PSGL-1 or sPSGL-1, only one leg of PSGL-1 would be captured by PL2. Thus, the site density for dimeric PSGL-1 was inferred to be the same as that of monomeric sPSGL-1, whereas the density of selectin binding-sites of PSGL-1 should be twice as that of sPSGL-1. It is possible that a fraction of PL2 bound dimerically with the dimeric PSGL-1 even though a saturating concentration was used. Should this be the case, using our indirect inference would overestimate the selectin binding sites on PSGL-1-coated RBCs. This would increase, rather than decrease, the confidence of our interpretation regarding the formation of dimeric bonds from the observation of higher selectin binding to PSGL-1-coated RBCs than to sPSGL-1-coated RBCs. The site densities of E-selectin on CHO cells and HAECs and of L-selectin on neutrophils were measured using anti-E-selectin mAb ES1 and anti-L-selectin mAb DREG5.
Results
To discriminate the multimericities of E- and L-selectin on their respective cell surfaces, we used the micropipette adhesion frequency assay, which measures cross-junctional ligand interaction with a cell surface receptor in its native membrane environment [21], [33], [35]. In this 2D assay, the measured adhesion frequency depends on both densities of the receptor and the ligand. To control for this, we used the same batch of cells bearing the same receptor density to interact with RBCs coated with the same density of PL2 to capture dimeric (PSGL-1) or monomeric (sPSGL-1). This was done for both L-selectin as well as E-selectin. Using this in situ kinetic analysis, we determined the ability for endothelial cell E-selectin and neutrophil L-selectin to support dimeric interactions by comparing their binding properties with PSGL-1 and sPSGL-1.
Interactions of sPSGL-1 and PSGL-1 with sE-selectin were Indistinguishable Whereas those with E-selectin-Ig were Distinct
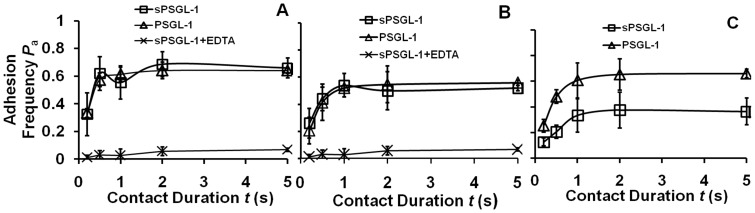
We measured adhesion frequency P a vs. contact duration t curves for RBCs coated with matched densities of either sPSGL-1 or PSGL-1 and RBCs coated with sE-selectin at two site densities (Fig. 2A and B) or with E-selectin-Ig (Fig. 2C). In contact durations from 0.25–5 s, P a increased with t initially and then reached a plateau, as previously observed for E-selectin interaction with carbohydrate ligands on HL-60 cells and Colo-205 cells [36]. Nonspecific adhesion was controlled by adding EDTA to chelate calcium, a divalent cation required for selectin-ligand binding.
Figure 2. Binding curves of two forms of recombinant E-selectin interacting with (s)PSGL-1.
Adhesion frequency vs. contact duration plots of RBCs coated with sE-selectin at site density of 126 µm−2 (A) or 46 µm−2 (B) or E-selectin-Ig at site density of 124 µm−2 (C) interacting with RBCs coated with sPSGL-1 (square) or PSGL-1 (triangle) at matched densities. Control for nonspecific adhesion was measured using 6 mM EDTA to inhibit binding between sE-selectin and sPSGL-1 (A and B, cross). The different adhesion frequency levels of sE-selectin–PSGL-1 (A) and E-selectin-Ig–sPSGL-1 (C) interactions may result from the differences in how the recombinant proteins were made, how sE-selectin and E-selectin-Ig were coated on RBCs, and how their site densities were measured.
It is evident that the two curves of (s)PSGL-1 binding to sE-selectin were indistinguishable regardless of the sE-selectin site densities used (Fig. 2, A and B). This indicates that monomeric sE-selectin formed the same type of monomeric bonds with both monomeric sPSGL-1 and dimeric PSGL-1. It also suggests that each of the two members of dimeric PSGL-1 interacted with sE-selectin with the same binding properties. In sharp contrast, the two curves of (s)PSGL-1 binding to E-selectin-Ig were distinctly different, with higher binding for the dimeric PSGL-1 than for the monomeric sPSGL-1 at all contact durations (Fig. 2C). This indicates that dimeric E-selectin-Ig formed monomeric bonds with monomeric sPSGL-1 but dimeric bonds with dimeric PSGL-1.
Interactions of (s)PSGL-1 with E-selectin on HAECs and CHO Cells Resembled those with E-selectin-Ig on RBCs
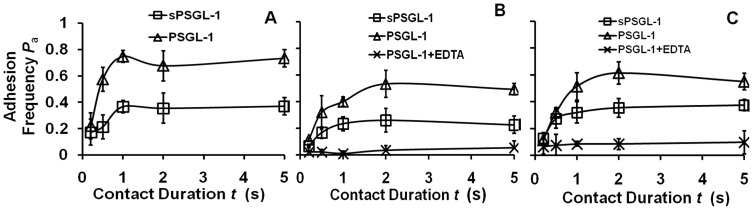
The results of the preceding section indicate that 1) dimeric interactions required both receptor and ligand to be dimeric; 2) dimeric interactions formed more frequently than monomeric interactions; and 3) E-selectin had the same affinity with each leg of dimeric PSGL-1 as with monomeric sPSGL-1. We next compared the 2D binding of sPSGL-1 and PSGL-1 to E-selectin on the surface of two cell types: HAECs stimulated by IL-1β to induce native E-selectin expression at two levels (Fig. 3A and B) and CHO cells transfected to express recombinant E-selectin (Fig. 3C). Nonspecific adhesions were controlled by using an anti-E-selectin mAb ES1 and the calcium chelator EDTA to inhibit specific E-selectin binding. The adhesion frequency vs. contact duration curves of cell surface E-selectin (Fig. 3) showed similar kinetics as purified E-selectin-Ig coated on RBCs (Fig. 2C), imparting confidence to applying the premises established in the preceding section to analyze the data in this section.
Figure 3. Binding curves of cell surface E-selectin interacting with (s)PSGL-1.
Adhesion frequency vs. contact duration plots of HAEC induced to express native E-selectin at 419 µm−2 (A) or 258 µm−2 (B) or CHO cells transfected to express recombinant E-selectin at 410 µm−2 (C) interacting with RBCs coated with matched molecular densities of sPSGL-1 (square) or PSGL-1 (triangle). Control for nonspecific adhesion was measured using 6 mM EDTA to inhibit binding between E-selectin and sPSGL-1 (B and C, cross).
Similar to Fig. 2C, binding of cell surface E-selectin to dimeric PSGL-1 was significantly higher than that to monomeric sPSGL-1 at all contact durations tested regardless of the site densities and cell types (Fig. 3). These data indicate that E-selectin was dimeric on the surface of HAEC and CHO cells because it supported dimeric interactions with PSGL-1 but monomeric interactions with sPSGL-1.
Interactions of (s)PSGL-1 with L-selectin-Ig were Distinctly Different
We next employed the same strategy to analyze L-selectin. In contact durations from 0.125–2 s, RBCs bearing either of two site densities of dimeric L-selectin-Ig adhered to RBCs bearing dimeric PSGL-1 more frequently than to RBCs bearing monomeric sPSGL-1 (Fig. 4). This is similar to the binding curve pattern of E-selectin-Ig observed in Fig. 2C, confirming that dimeric vs. monomeric interactions of (s)PSGL-1 with E-selectin-Ig could be applied to the case of L-selectin-Ig. Thus, dimeric L-selectin-Ig formed dimeric bonds with dimeric PSGL-1 but monomeric bonds with monomeric sPSGL-1.
Figure 4. Binding curves of L-selectin-Ig interacting with (s)PSGL-1.
Adhesion frequency vs. contact duration plots of RBCs coated with L-selectin-Ig at site density of 140 µm−2 (A) or 112 µm−2 (B) interacting with RBCs coated with matched densities of sPSGL-1 (square) or PSGL-1 (triangle).
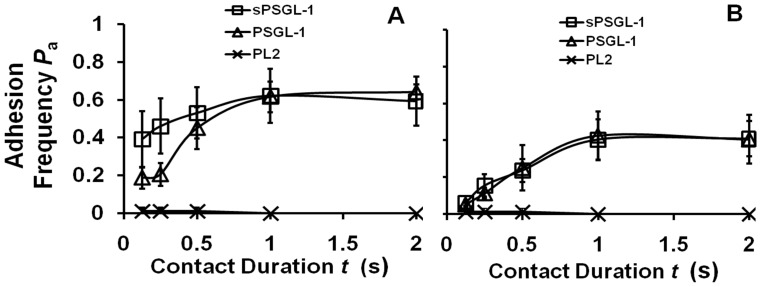
Interactions of sPSGL-1 and PSGL-1 with Neutrophil L-selectin were Indistinguishable
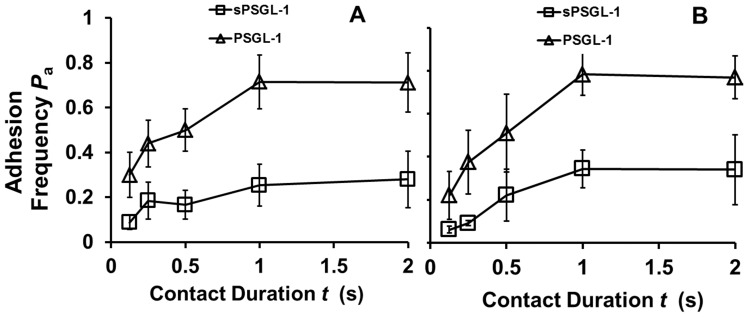
We lastly compared the 2D binding of sPSGL-1 and PSGL-1 at two matched site densities to L-selectin constitutively expressed on human neutrophils. Nonspecific adhesions were controlled by using RBCs precoated with an anti-PSGL-1 capturing mAb PL2 but not further incubated with either form of PSGL-1, which abolished binding (Fig. 5). In contrast to the pattern seen with Fig. 4, the binding curves of dimeric PSGL-1 and monomeric sPSGL-1 were indistinguishable. These results indicate that each of the two members of dimeric PSGL-1 interacted with L-selectin with the same binding affinity, similar to the case of sE-selectin (Fig. 2A and B). Like the sE-selectin data, the data in Fig. 5 also suggest that L-selectin was a monomer on the surface of neutrophils because it supported monomeric interactions with both monomeric sPSGL-1 and dimeric PSGL-1.
Figure 5. Binding curves of neutrophil L-selectin interacting with (s)PSGL-1.
Adhesion frequency vs. contact duration plots of neutrophils constitutively expressing native L-selectin interacting with RBCs coated with matched densities of sPSGL-1 (square) or PSGL-1 (triangle) at 59 µm−2 (A) or 25 µm−2 (B). Control for nonspecific adhesion was measured using RBCs coated with the capture antibody PL2 without incubation with (s)PSGL-1. The differences in the first three data points in (A) between sPSGL-1 and PSGL-1 interacting with L-selectin are not significant as assessed by the Student-t test (p = 0.32, 0.15, and 0.75).
Discussion
Using adhesion frequency measurements, we have demonstrated that E-selectin on endothelial cells or transfected CHO cells supports dimeric interactions with dimeric PSGL-1, whereas L-selectin on neutrophils supports only monomeric interactions with dimeric PSGL-1.
Our observed monomeric interactions of L-selectin with PSGL-1 are consistent with molecular stiffness measurements that L-selectin from human tonsils reconstituted into lipid bilayers forms monomeric bonds with PSGL-1 [14] and with biophysical measurements that L-selectin transmembrane and cytoplasmic domains do not dimerize in bacterial or synthetic cell membranes [13].
Our observed dimeric inteactions of E-selectin with PSGL-1 differ from previous observations that E-selectin from lysates of CHO cell transfectants reconstituted into supported lipid bilayers forms monomeric bonds with PSGL-1, although P-selectin from lysates of human platelets reconstituted in the same lipid bilayers forms dimeric bonds with PSGL-1 [14]. E-selectin oligomerization may be driven by weak noncovalent interactions that are disrupted after detergent solubilization of membranes. P-selectin dimerizes by noncovalent association of transmembrane domains (Ushiyama et al 1993). These associations may require its GxxxG sequence, a known dimerization motif [37], [38]. PSGL-1 dimerizes by noncovalent interactions between both transmembrane and cytoplasmic domains, which are stabilized by a disulfide bond formed immediately above the plasma membrane (Moore et al 1992, Epperson et al 2000, Miner et al 2011). Although the transmembrane domain of E-selectin does not contain a GxxxG sequence, our data suggest that close cis interactions of E-selectin in the membrane enable dimeric bonds with PSGL-1. How an E-selectin dimer is stabilized on a cell is an interesting question for future studies.
Many adhesion molecules are constitutively expressed on the cell surface as homodimers or oligomers. In addition to P-selectin and PSGL-1, the β1 [39], β2 [40], β3 [41]–[43], and αIIb [44] integrin subunits can form clusters. Other examples include intercellular adhesion molecule 1 (ICAM-1) [45], platelet endothelial cell adhesion molecule-1 (PECAM-1) [46], and cadherins [47]. In addition, many cell surface receptors can be induced to form oligomers or increase the size of preformed oligomers as a result of intracellular signaling or ligand engagement [16]. Examples include T cell receptors [48], [49], B cell receptors [50], and Fc receptors [51], [52]. Importantly, in addition to strengthening receptor–ligand interactions, oligomeric binding plays a crucial role in many receptor-mediated signaling processes, making the analysis of oligomeric interaction a highly relevant area of study.
Receptor oligomerization is usually demonstrated by biochemical methods, e.g., chemical crosslinking, as in the case of αIIbβ3 integrin [53]. Because ligand binding to adhesion receptors provides physical linkage to anchor the cell, oligomeric interactions can be analyzed by biomechanical assays. For example, dimeric interactions between P-selectin and PSGL-1 prolong bond lifetimes [10], increase molecular stiffness [14], and stabilize cell rolling in shear flow [9]. Multimeric bonds have also been characterized by increase in bond rupture forces [54]–[57]. The present work has demonstrated a new method of analyzing dimeric receptor-ligand binding by measuring 2D kinetics on the cell membrane. However, these methods also have limitations.
One limitation is the resolution of the our methods. The biomechanical assays based on pico-force techniques usually have sufficiently high resolutions for mechanical variables such as bond lifetime, molecular stiffness, cell rolling velocity, bond rupture force, and adhesion frequency. However, they must be combined with assays to measure site densities of the interacting molecules. Biochemical assays sometimes suffer from inter-experimental variations, as measurements depend on the activities of the reagents that can vary in each preparation. This may explain the apparent discrepancies between Fig. 2A and B, where the measured difference in steady-state adhesion levels is nearly two-fold smaller than that predicted from the 2.7-fold difference in the sE-selectin site densities. For this reason, quantitative comparison should be done in side-by-side experiments using the same preparation of reagents with proper controls to prevent inter-experimental variations from negating key conclusions. This precaution was strictly followed for the comparative data in each panel of Figs. 2–5, ensuring the reliability of our conclusions.
To support dimeric interactions with dimeric PSGL-1, endothelial cell E-selectin must form oligomers of at least two proteins. If E-selectin forms oligomers larger than dimers on the endothelial cell surface, our data cannot determine the actual size of the oligomers. This limitation can be overcome by using oligomeric ligands of larger sizes to dissect the size of E-selectin oligomers combined with mathematical modeling to relate the binding curve to 2D kinetics rates. With such extensions, the present method has the potential to analyze oligomeric cross-junctional molecular binding at the interface of two interacting cells and extract intrinsic kinetic coefficients for such multimeric interactions.
In addition to molecular self-association, selectins cluster in specific membrane domains through interactions with other cellular elements. L-selectin clusters in the tips of leukocyte microvilli, which enhances tethering under flow [58]. P- and E-selectin cluster in clathrin-coated pits and E-selectin clusters in lipid rafts of endothelial cells; these clusters promote slower and more stable leukocyte rolling [59]–[61]. Dimerization of P-selectin may cooperate with P-selectin clustering in clathrin-coated pits to optimize leukocyte rolling [59], [62]. For a given dimeric ligand, whether and how effectively it forms a dimeric bond with a dimeric receptor may depend on the on-rate and the separation distance between the two members of the dimeric receptor, as close proximity of the members may compensate for slow on-rate to promote a dimeric interaction. The on-rate (but not off-rate) of 2D receptor–ligand binding kinetics is impacted by the microtopology [63] and stiffness [64] of the cell surface presenting the receptor as well as the membrane anchor [20], length and orientation [65] of the receptor and/or ligand. These provide potential mechanisms for regulation of dimeric/oligomeric interactions. The biological significance of cells expressing E-selectin as dimers/oligomers but L-selectin as monomers is an important topic for future investigation.
Acknowledgments
We thank Julia Babensee for providing HAECs and Renhao Li for helpful discussion.
Funding Statement
This work was supported by National Institutes of Health grant AI077343 and HL034363. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McEver RP, Zhu C (2010) Rolling cell adhesion. Annu Rev Cell Dev Biol 26: 363–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McEver RP (2001) Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost 86: 746–756. [PubMed] [Google Scholar]
- 3. Vestweber D, Blanks JE (1999) Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev 79: 181–213. [DOI] [PubMed] [Google Scholar]
- 4. Barkalow FJ, Barkalow KL, Mayadas TN (2000) Dimerization of P-selectin in platelets and endothelial cells. Blood 96: 3070–3077. [PubMed] [Google Scholar]
- 5. Ushiyama S, Laue TM, Moore KL, Erickson HP, McEver RP (1993) Structural and functional characterization of monomeric soluble P-selectin and comparison with membrane P-selectin. J Biol Chem 268: 15229–15237. [PubMed] [Google Scholar]
- 6. Epperson TK, Patel KD, McEver RP, Cummings RD (2000) Noncovalent association of P-selectin glycoprotein ligand-1 and minimal determinants for binding to P-selectin. J Biol Chem 275: 7839–7853. [DOI] [PubMed] [Google Scholar]
- 7. Li F, Erickson HP, James JA, Moore KL, Cummings RD, et al. (1996) Visualization of P-selectin glycoprotein ligand-1 as a highly extended molecule and mapping of protein epitopes for monoclonal antibodies. J Biol Chem 271: 6342–6348. [DOI] [PubMed] [Google Scholar]
- 8. Moore KL, Stults NL, Diaz S, Smith DF, Cummings RD, et al. (1992) Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol 118: 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramachandran V, Yago T, Epperson TK, Kobzdej MM, Nollert MU, et al. (2001) Dimerization of a selectin and its ligand stabilizes cell rolling and enhances tether strength in shear flow. Proc Natl Acad Sci U S A 98: 10166–10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marshall BT, Long M, Piper JW, Yago T, McEver RP, et al. (2003) Direct observation of catch bonds involving cell-adhesion molecules. Nature 423: 190–193. [DOI] [PubMed] [Google Scholar]
- 11. Dwir O, Steeber DA, Schwarz US, Camphausen RT, Kansas GS, et al. (2002) L-selectin dimerization enhances tether formation to properly spaced ligand. J Biol Chem 277: 21130–21139. [DOI] [PubMed] [Google Scholar]
- 12. Li X, Steeber DA, Tang ML, Farrar MA, Perlmutter RM, et al. (1998) Regulation of L-selectin-mediated rolling through receptor dimerization. J Exp Med 188: 1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Srinivasan S, Deng W, Li R (2011) L-selectin transmembrane and cytoplasmic domains are monomeric in membranes. Biochim Biophys Acta 1808: 1709–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sarangapani KK, Marshall BT, McEver RP, Zhu C (2011) Molecular stiffness of selectins. J Biol Chem 286: 9567–9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEver RP (2005) Molecular and cellular contributions to selectin-dependent leukocyte adhesion under flow. In: Hamann A, Engelhardt B, editors. Leukocyte Trafficking: Molecular Mechanisms, Therapeutic Targets, and Methods. Weinheim: Wiley-VCH. 217–247. [Google Scholar]
- 16. Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141: 1117–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sigalov AB (2008) Signaling chain homooligomerization (SCHOOL) model. Adv Exp Med Biol 640: 121–163. [DOI] [PubMed] [Google Scholar]
- 18.Shao B, Yago T, Coghill PA, Klopocki AG, Mehta-D’souza P, et al.. (2012) Signal-dependent slow leukocyte rolling does not require cytoskeletal anchorage of P-selectin glycoprotein ligand-1 (PSGL-1) or integrin aLb2. J Biol Chem: in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen W, Zarnitsyna VI, Sarangapani KK, Huang J, Zhu C (2008) Measuring Receptor-Ligand Binding Kinetics on Cell Surfaces: From Adhesion Frequency to Thermal Fluctuation Methods. Cellular and molecular bioengineering 1: 276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chesla SE, Li P, Nagarajan S, Selvaraj P, Zhu C (2000) The membrane anchor influences ligand binding two-dimensional kinetic rates and three-dimensional affinity of FcgammaRIII (CD16). J Biol Chem 275: 10235–10246. [DOI] [PubMed] [Google Scholar]
- 21. Huang J, Zarnitsyna VI, Liu B, Edwards LJ, Jiang N, et al. (2010) The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature 464: 932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zarnitsyna VI, Zhu C (2011) Adhesion frequency assay for in situ kinetics analysis of cross-junctional molecular interactions at the cell-cell interface. J Vis Exp: e3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patel KD, Moore KL, Nollert MU, McEver RP (1995) Neutrophils use both shared and distinct mechanisms to adhere to selectins under static and flow conditions. J Clin Invest 96: 1887–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rose SL, Babensee JE (2007) Complimentary endothelial cell/smooth muscle cell co-culture systems with alternate smooth muscle cell phenotypes. Ann Biomed Eng 35: 1382–1390. [DOI] [PubMed] [Google Scholar]
- 25. Chen W, Lou J, Zhu C (2010) Forcing switch from short- to intermediate- and long-lived states of the alphaA domain generates LFA-1/ICAM-1 catch bonds. The Journal of biological chemistry 285: 35967–35978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sarangapani KK, Yago T, Klopocki AG, Lawrence MB, Fieger CB, et al. (2004) Low force decelerates L-selectin dissociation from P-selectin glycoprotein ligand-1 and endoglycan. J Biol Chem 279: 2291–2298. [DOI] [PubMed] [Google Scholar]
- 27. Xia L, McDaniel JM, Yago T, Doeden A, McEver RP (2004) Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood 104: 3091–3096. [DOI] [PubMed] [Google Scholar]
- 28. Yago T, Leppanen A, Qiu H, Marcus WD, Nollert MU, et al. (2002) Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow. J Cell Biol 158: 787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Erbe DV, Wolitzky BA, Presta LG, Norton CR, Ramos RJ, et al. (1992) Identification of an E-selectin region critical for carbohydrate recognition and cell adhesion. J Cell Biol 119: 215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kishimoto TK, Jutila MA, Butcher EC (1990) Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci U S A 87: 2244–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moore KL, Patel KD, Bruehl RE, Li F, Johnson DA, et al. (1995) P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol 128: 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chesla SE, Selvaraj P, Zhu C (1998) Measuring two-dimensional receptor-ligand binding kinetics by micropipette. Biophys J 75: 1553–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang J, Edwards LJ, Evavold BD, Zhu C (2007) Kinetics of MHC-CD8 interaction at the T cell membrane. J Immunol 179: 7653–7662. [DOI] [PubMed] [Google Scholar]
- 34. Zhang F, Marcus WD, Goyal NH, Selvaraj P, Springer TA, et al. (2005) Two-dimensional kinetics regulation of alphaLbeta2-ICAM-1 interaction by conformational changes of the alphaL-inserted domain. J Biol Chem 280: 42207–42218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang N, Huang J, Edwards LJ, Liu B, Zhang Y, et al. (2011) Two-stage cooperative T cell receptor-peptide major histocompatibility complex-CD8 trimolecular interactions amplify antigen discrimination. Immunity 34: 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Long M, Zhao H, Huang KS, Zhu C (2001) Kinetic measurements of cell surface E-selectin/carbohydrate ligand interactions. Ann Biomed Eng 29: 935–946. [DOI] [PubMed] [Google Scholar]
- 37. Lemmon MA, Treutlein HR, Adams PD, Brunger AT, Engelman DM (1994) A dimerization motif for transmembrane alpha-helices. Nat Struct Biol 1: 157–163. [DOI] [PubMed] [Google Scholar]
- 38. Russ WP, Engelman DM (2000) The GxxxG motif: a framework for transmembrane helix-helix association. J Mol Biol 296: 911–919. [DOI] [PubMed] [Google Scholar]
- 39. Laplantine E, Maurer P, Vallar L, Eble J, Paulsson M, et al. (2002) The integrin beta1 subunit cytoplasmic tail forms oligomers: a potential role in beta1 integrin clustering. Biol Cell 94: 375–387. [DOI] [PubMed] [Google Scholar]
- 40. Myou S, Zhu X, Boetticher E, Qin Y, Myo S, et al. (2002) Regulation of adhesion of AML14.3D10 cells by surface clustering of beta2-integrin caused by ERK-independent activation of cPLA2. Immunology 107: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li R, Babu CR, Lear JD, Wand AJ, Bennett JS, et al. (2001) Oligomerization of the integrin alphaIIbbeta3: roles of the transmembrane and cytoplasmic domains. Proc Natl Acad Sci U S A 98: 12462–12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li R, Mitra N, Gratkowski H, Vilaire G, Litvinov R, et al. (2003) Activation of integrin alphaIIbbeta3 by modulation of transmembrane helix associations. Science 300: 795–798. [DOI] [PubMed] [Google Scholar]
- 43. Hantgan RR, Lyles DS, Mallett TC, Rocco M, Nagaswami C, et al. (2003) Ligand binding promotes the entropy-driven oligomerization of integrin alpha IIb beta 3. J Biol Chem 278: 3417–3426. [DOI] [PubMed] [Google Scholar]
- 44. Li R, Gorelik R, Nanda V, Law PB, Lear JD, et al. (2004) Dimerization of the transmembrane domain of Integrin alphaIIb subunit in cell membranes. J Biol Chem 279: 26666–26673. [DOI] [PubMed] [Google Scholar]
- 45. Chen X, Kim TD, Carman CV, Mi LZ, Song G, et al. (2007) Structural plasticity in Ig superfamily domain 4 of ICAM-1 mediates cell surface dimerization. Proc Natl Acad Sci U S A 104: 15358–15363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Newton JP, Hunter AP, Simmons DL, Buckley CD, Harvey DJ (1999) CD31 (PECAM-1) exists as a dimer and is heavily N-glycosylated. Biochem Biophys Res Commun 261: 283–291. [DOI] [PubMed] [Google Scholar]
- 47. Troyanovsky RB, Laur O, Troyanovsky SM (2007) Stable and unstable cadherin dimers: mechanisms of formation and roles in cell adhesion. Mol Biol Cell 18: 4343–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schamel WW, Arechaga I, Risueno RM, van Santen HM, Cabezas P, et al. (2005) Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J Exp Med 202: 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, et al. (2010) TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol 11: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang J, Reth M (2010) Oligomeric organization of the B-cell antigen receptor on resting cells. Nature 467: 465–469. [DOI] [PubMed] [Google Scholar]
- 51. Powell MS, Barnes NC, Bradford TM, Musgrave IF, Wines BD, et al. (2006) Alteration of the Fc gamma RIIa dimer interface affects receptor signaling but not ligand binding. J Immunol 176: 7489–7494. [DOI] [PubMed] [Google Scholar]
- 52. Dierks SE, Bartlett WC, Edmeades RL, Gould HJ, Rao M, et al. (1993) The oligomeric nature of the murine Fc epsilon RII/CD23. Implications for function. J Immunol 150: 2372–2382. [PubMed] [Google Scholar]
- 53. Hato T, Pampori N, Shattil SJ (1998) Complementary roles for receptor clustering and conformational change in the adhesive and signaling functions of integrin alphaIIb beta3. J Cell Biol 141: 1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sulchek T, Friddle RW, Noy A (2006) Strength of multiple parallel biological bonds. Biophys J 90: 4686–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sulchek TA, Friddle RW, Langry K, Lau EY, Albrecht H, et al. (2005) Dynamic force spectroscopy of parallel individual Mucin1-antibody bonds. Proc Natl Acad Sci U S A 102: 16638–16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kinoshita K, Leung A, Simon S, Evans E (2010) Long-lived, high-strength states of ICAM-1 bonds to beta2 integrin, II: lifetimes of LFA-1 bonds under force in leukocyte signaling. Biophys J 98: 1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Evans E, Leung A, Hammer D, Simon S (2001) Chemically distinct transition states govern rapid dissociation of single L-selectin bonds under force. Proc Natl Acad Sci U S A 98: 3784–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. von Andrian UH, Hasslen SR, Nelson RD, Erlandsen SL, Butcher EC (1995) A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell 82: 989–999. [DOI] [PubMed] [Google Scholar]
- 59. Setiadi H, McEver RP (2003) Signal-dependent distribution of cell surface P-selectin in clathrin-coated pits affects leukocyte rolling under flow. J Cell Biol 163: 1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Setiadi H, McEver RP (2008) Clustering endothelial E-selectin in clathrin-coated pits and lipid rafts enhances leukocyte adhesion under flow. Blood 111: 1989–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Setiadi H, Sedgewick G, Erlandsen SL, McEver RP (1998) Interactions of the cytoplasmic domain of P-selectin with clathrin-coated pits enhance leukocyte adhesion under flow. J Cell Biol 142: 859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ramachandran V, Nollert MU, Qiu H, Liu WJ, Cummings RD, et al. (1999) Tyrosine replacement in P-selectin glycoprotein ligand-1 affects distinct kinetic and mechanical properties of bonds with P- and L-selectin. Proc Natl Acad Sci U S A 96: 13771–13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Williams TE, Nagarajan S, Selvaraj P, Zhu C (2001) Quantifying the impact of membrane microtopology on effective two-dimensional affinity. J Biol Chem 276: 13283–13288. [DOI] [PubMed] [Google Scholar]
- 64. Wu L, Xiao B, Jia X, Zhang Y, Lu S, et al. (2007) Impact of carrier stiffness and microtopology on two-dimensional kinetics of P-selectin and P-selectin glycoprotein ligand-1 (PSGL-1) interactions. J Biol Chem 282: 9846–9854. [DOI] [PubMed] [Google Scholar]
- 65. Huang J, Chen J, Chesla SE, Yago T, Mehta P, et al. (2004) Quantifying the effects of molecular orientation and length on two-dimensional receptor-ligand binding kinetics. J Biol Chem 279: 44915–44923. [DOI] [PubMed] [Google Scholar]