Abstract
Association between H. pylori infection, iron deficiency and iron deficiency anaemia has been described, but the mechanisms involved have not been established. We hypothesized that in H. pylori infected children increased gastric concentrations of IL-1β and/or TNF-α, both potent inhibitors of gastric acid secretion that is essential for iron absorption, are predictors for low blood concentrations of ferritin and haemoglobin, markers of early depletion of iron stores and anaemia, respectively. We evaluated 125 children undergoing endoscopy to clarify the origin of gastrointestinal symptoms. Gastric specimens were obtained for H. pylori status and cytokine evaluation and blood samples for determination of iron deficiency/iron deficiency anaemia parameters and IL1 cluster and TNFA polymorphisms that are associated with increased cytokine secretions. Higher IL-1β and TNF-α gastric concentrations were observed in H. pylori-positive (n = 47) than in -negative (n = 78) children. Multiple linear regression models revealed gastric IL-1β, but not TNF-α, as a significant predictor of low ferritin and haemoglobin concentrations; results were reproduced in young children in whom IL1RN polymorphic genotypes associated with higher gastric IL-1β expression and lower blood ferritin and haemoglobin concentrations. In conclusion, high gastric levels of IL-1β can be the link between H. pylori infection and iron deficiency/iron deficiency anaemia in childhood.
Introduction
Anaemia is a major public health problem in developing countries and approximately half of all cases are due to iron deficiency (ID) [1], [2]. Iron deficiency anaemia (IDA) is the final stage in the spectrum of a persistent negative iron balance, being preceded by an iron-restricted erythropoiesis characterized by low iron stores. The greater demand for iron due to growth, expansion of red cell mass and menstrual blood loss in female adolescents favors the development of ID/IDA in childhood/adolescence [2]. Factors which contribute to the high frequency of ID/IDA in developing countries include poor iron intake, low dietary iron bioavailability and blood loss due to gastrointestinal parasitic infections [2], [3].
Helicobacter pylori colonizes the stomach of more than a half of the world’s population. Concomitant high prevalence of H. pylori infection and ID/IDA in some areas, particularly in developing countries, has led to the hypothesis that H. pylori may contribute to ID/IDA. Potential mechanisms proposed include increased blood loss due to H. pylori-induced gastric lesions [4], iron uptake by H. pylori [5], deficient iron absorption due to decreased gastric acidity [6], [7], that isessential for the reduction and solubilization of non-heme iron, and reduced gastric juice ascorbic acid concentrations [6].
The acute phase of H. pylori infection is accompanied by transient hypochlorydria of variable duration [8]–[10]. Similar perturbations in gastric acid secretion occur in animals following infection with gastric Helicobacter species [11] or H. pylori [12]. In the latter study, reversal of the hypochlorhydria induced by H. pylori infection in gerbils by treatment with recombinant IL-1 receptor antagonist implicated the IL-1B gene cluster in hypochlorhydric response to H. pylori. As H. pylori infection is mainly acquired in childhood [13], it is biologically plausible that infected children are at increased risk of developing ID/IDA as a consequence of hypochlorhydria.
Concentrations of IL-1β and TNF-α, potent inhibitors of gastric acid secretion [14], are increased in the gastric mucosa of H. pylori infected adults and children [15]–[17]. As both cytokines are capable of directly inhibiting gastric acid secretion by parietal cells, they might be one of the links between ID and H. pylori infection in childhood [13]. Furthermore, H. pylori infected adults with functional polymorphisms in the IL1 gene cluster associated with over expression of IL-1β have increased hypochlorydria [17] that could, theoretically, interfere with intestinal iron absorption leading to ID.
We hypothesized that in H. pylori infected children without the known common causes of ID, increased gastric concentrations of IL-1β and/or TNF-α could be predictors for low blood concentrations of ferritin and haemoglobin, markers of early depletion of iron stores and anaemia, respectively.
Patients and Methods
This study was approved by the Ethics Committee of the Universidade Federal de Minas Gerais, Belo Horizonte, Brazil and the National Ethics Committee on Research from the Health Ministry of Brazil. Signed informed consent to participate was obtained from the children (whenever possible) and adolescents and their parents.
Patients
Between June 2007 and July 2010 125 children and adolescents (74 girls and 51 boys, mean age 11.1±2.9 years, range 4–16 years) undergoing gastrointestinal endoscopy to clarify the origin of symptoms related to the upper gastrointestinal tract were prospectively studied. All the patients were from Minas Gerais state, localized in the Southeast Brazil. To avoid confounding factors which can modify the gastritis classification and the diagnosis of H. pylori infection, or which independently can modify iron stores, rigorous exclusion criteria were used. Exclusions included children who had received antimicrobial drugs, anti-cholinergic and steroidal and non-steroidal anti-inflammatory agents for at least 30 days, or proton pump inhibitors for at least 15 days before endoscopy; children with peptic ulcer disease, coeliac disease and intestinal parasitic infections; children with gastrooesophageal varices, coagulation disorders, acquired or congenital immunosuppression, inflammatory diseases, renal failure, hematological disorders and neoplasias. Following endoscopy, additional exclusion criteria were previously undiagnosed coeliac disease or any histological non-specific duodenal inflammation in the absence of duodenal gastric metaplasia. From each female adolescent data was obtained on age of menarche, interval between the menses, duration and amount of monthly flow. Those with heavy menstrual blood loss were not included in the study. The menstrual cycle was considered normal when the interval between flows was 25–31 days and duration between 3 and 5 days [18].
Biopsy specimens were obtained from the antral and corpus gastric mucosa for evaluation of H. pylori status, histological parameters and cytokine concentrations. Duodenal biopsies were taken for histological analysis and the exclusion of coeliac disease and tropical enteropathy. In addition, stool samples were obtained for parasitology and H. pylori monoclonal stool antigen assay (HpSA Plus; Meridian Bioscience, Cincinnati, OH).
Blood samples were collected to determine the haemoglobin concentration, haematocrit value, IL1B, IL1RN and TNFA polymorphisms and serum ferritin concentration.
H. pylori Status
H. pylori status was evaluated by culture, preformed urease test, carbolfuchsin-stained histological section, polymerase chain reaction (PCR) for ureA, monoclonal HpSA and 13C-urea breath test as previously described [19], [20]. Patients were considered H. pylori-positive when culture was positive or at least two of the other tests were positive and H. pylori-negative when the results of all tests were negative.
Histology
Endoscopic biopsy specimens were fixed in 10% formalin and embedded in paraffin wax, and 4-µm-thick histological sections were stained with haematoxylin and eosin for histological analysis according to the revised Sydney System [21] and with carbolfuchsin for detection of spiraled organisms. Mononuclear (MN) and polymorphonuclear (PMN) cell infiltrations as well as intestinal metaplasia and atrophy were graded as absent (0), mild (1), moderate (2), or marked (3).
Blood Haemoglobin, Haematocrit and Ferritin Values
Blood haemoglobin concentration and haematocrit values were determined by using an automated electronic counter, Sysmex XT 1800i (Sysmex Corporation, Kobe, Japan). The serum ferritin concentration was determined by a chemiluminescence method employing the ADVIA Centaur® Immunoassay CP System (Siemens Healthcare, Erlangen, Germany).
Gastric Cytokine Levels
IL-1β and TNF-α gastric concentrations were evaluated separately in biopsies from the lesser curvature of the antrum and from the greater curvature of the corpus by ELISA and were expressed as picogram of cytokine per milligram of protein (pg/mg protein) as previously described [14].
IL1B-31T/C, IL1RN VNTR and TNFA-307G/A Genotyping
As IL1B/IL1RN and TNFA polymorphisms might affect transcript levels of IL1B and TNFA, respectively, they were investigated in blood DNA extracted by the QIAamp DNA mini kit (QIAGEN, GmbH, Hilden, Germany). IL1B-31T/C genotypes were determined with Taqman double-stranded SNP ID: rs4986790 (Applied Biosystems, Foster City, CA). IL1RN penta-allelic variable number tandem repeats (VNTR) was genotyped according to Mansfield et al [22]. The alleles 1, 3, 4 and 5 were classified into long and the allele 2 into short categories [22]. The TNFA-307G/A genotypes were investigated by PCR-restriction length polymorphism (RFLP) [22].
Statistical Analysis
Data were analysed with SPSS statistical software package version 17.0 (SPSS Inc., Chicago, IL). Hardy-Weinberg equilibrium of alleles at individual loci was tested by χ2-test with Yate’s correction or Fisher’s exact text. The Kolmogorov-Smirnov goodness-of-fit was used to assess the normality of the data. When significant departures from normality were detected, the data were log-transformed. The histopathological scores in binary variables were transformed by combining absent/mild and moderate/severe scores to compare histopathological data with IL-1β and TNF-α gastric concentrations. The degree of gastric chronic (mononuclear) and active (polymorphonuclear) in H. pylori-positive and -negative children was compared by the two-tailed Mann Whitney U test. Correlations were evaluated by the Pearsońs correlation (continuous normally distributed data) or Spearmańs correlation (scores). The comparisons between antral and corpus cytokine concentrations were undertaken by two-tailed paired Student’s t test or Wilcoxon test. The level of significance was set at p≤0.05. Multiple linear regression analyses (“enter option”) were used in order to quantify the simultaneous and mutually independent contribution, of selected relevant predictor candidates, e.g. IL-1β and TNF-α gastric concentrations, for low ferritin and haemoglobin blood concentration (dependent variables) while controlling for confounders such as gender and age. Variables with p values ≤0.20 in the univariate analyses were selected for the multivariate analyses. The optimum sample size, based on a significant level of 0.05 and a statistical power of 0.80 (type II error 0.02) is 125 cases, even when only a small effect size (f = 0.10) is expected.
Results
Among the 125 children, 47 (37.6%) were H. pylori-positive (mean age 11.7±2.7 years; 29 girls) and 78 (62.4%) were H. pylori-negative (mean age 10.7±2.9 years, 45 girls).
Gastric Cytokine Concentrations
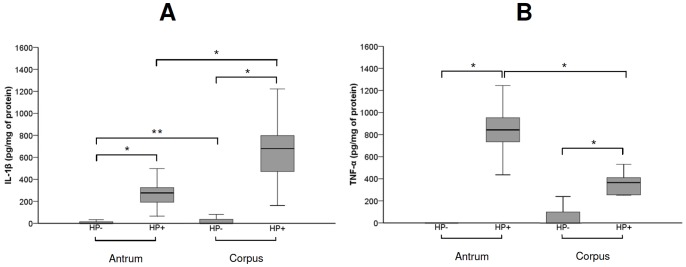
A 17.2-fold increase in corpus and antral gastric concentration of IL-1β as well as a 7.0-fold increase in corpus and 57.7-fold increase in antral gastric TNF-α concentrations were observed in infected children when compared with non-infected children (p<0.001). Comparisons between H. pylori-negative and -positive children are shown in Figure 1A and 1B. The concentrations of IL-1β were higher in the corpus than in the antral gastric mucosa (2.5-fold increase) in both H. pylori-positive (p<0.001) and H. pylori-negative (p = 0.02) children. Conversely, the TNF-α concentration was higher in the antral than in the corpus gastric mucosa in H. pylori-positive children (2.5-fold increase, p<0.001) (Figure 1A and 1B).
Figure 1. Box plots representing the comparison of gastric IL-1β (A) and TNF-α (B) concentrations (pg/mg of protein) between H. pylori-positive (HP+, n = 47) and -negative (HP-, n = 78) children, and between antral and corpus concentration in H. pylori-positive and -negative groups.
The upper and lower limits of the boxes represent the 75th and 25th percentiles, respectively. The horizontal bar across the box indicates the median and the capped bars indicate the minimum and maximum data values. Statistical analysis by Student’s t test after log transformation in the case of IL-1β; *p<0.001 and **p = 0.02.
Blood IDA Parameters and Gastric Cytokine Concentrations
The multiple regression analyses revealed the gastric concentration of IL-1β, but not TNF-α, as a significant independent predictor for low serum ferritin and haemoglobin concentrations (Table 1). In addition, the male gender was an independent predictor of ferritin increase and the age was an independent predictor of haemoglobin increase. As H. pylori infection is mainly acquired by young children and we have previously observed differences in the inflammatory and immune response between children younger and older than 10–12 years [23], we stratified children by age: 12 years old or younger, and those older than 12 years of age. IL-1β remained a predictor of low ferritin concentration and was a stronger predictor of low haemoglobin concentration in the younger age group. The male gender was an independent predictor of ferritin increasing (Table 1).
Table 1. Multiple linear regression model including ferritin or haemoglobin as dependent variables and IL-1β and TNF-α gastric corpus concentrations, gender and age as independent variables.
| Univariate analysis | Multivariate analysis | |||
| Betacoefficient | P value | Betacoefficient | P value | |
| FERRITIN | ||||
| Children of all ages (n = 125) | ||||
| age | 0.119 | 0.19 | 0.126 | 0.15 |
| male gender | 0.280 | 0.002 | 0.279 | 0.02 |
| IL-1β | −0.200 | 0.006 | −0.205 | 0.04 |
| TNF-α | −0.075 | 0.41 | – | – |
| Children ≤12 years of age (n = 84) | ||||
| age | −0.040 | 0.32 | – | – |
| male gender | 0.208 | 0.20 | 0.229 | 0.02 |
| IL-1β | −0.202 | 0.03 | −0.219 | 0.02 |
| TNF-α | −0.106 | 0.35 | – | – |
| HAEMOGLOBIN | ||||
| Children of all ages (n = 125) | ||||
| age | 0.285 | 0.001 | 0.301 | 0.001 |
| male gender | 0.151 | 0.09 | 0.158 | 0.07 |
| IL-1β | −0.220 | 0.02 | −0.244 | 0.03 |
| TNF-α | −0.135 | 0.14 | −0.001 | 0.99 |
| Children ≤12 years of age (n = 84) | ||||
| age | 0.145 | 0.19 | 0.112 | 0.28 |
| male gender | −0.170 | 0.12 | −0.094 | 0.37 |
| IL-1β | −0.399 | <0.001 | −0.439 | <0.001 |
| TNF-α | −0.167 | 0.13 | 0.092 | 0.49 |
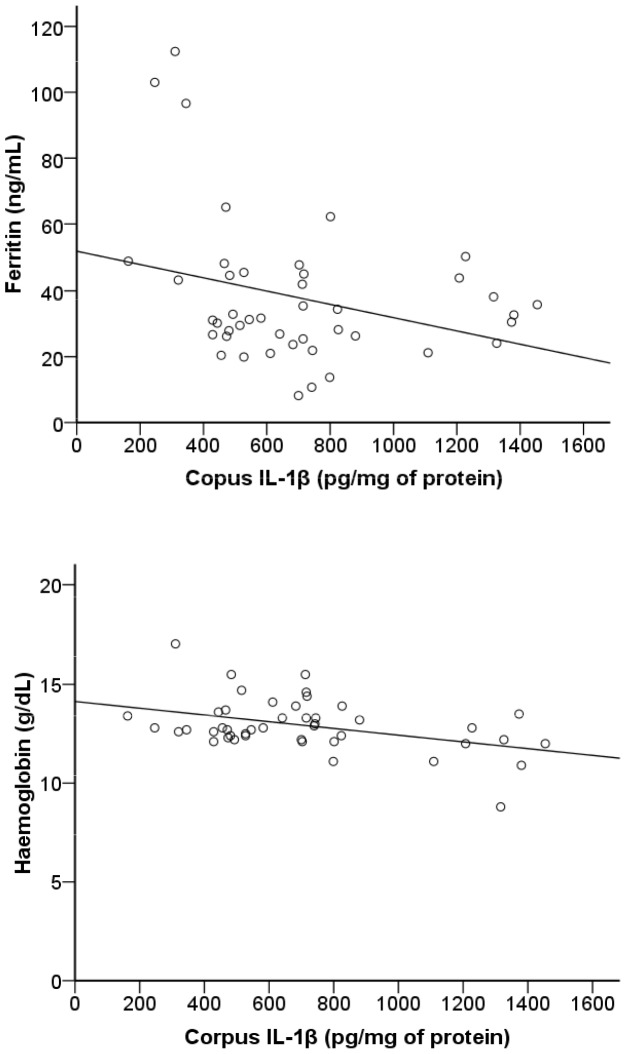
When H. pylori-positive and -negative children were separately analyzed, significant correlations were observed only in the H. pylori-positive group [ferritin (r = −0.42, p = 0.004) and haemoglobin (r = −0.35, p = 0.02)] (Figure 2). The values of haematocrit were also negatively correlated with gastric IL-1β concentrations (r = −0.40, p = 0.007]. In contrast, corpus and antral TNF-α concentrations neither correlated with serum ferritin (p≥0.48), nor with haemoglobin (p≥0.74) or haematocrit (p≥0.40) values.
Figure 2. Correlations between corpus IL-1β concentrations and concentrations of ferritin and haemoglobin in H. pylori-positive children (n = 47).
Statistical analysis by Pearson’s correlation after log transformation in the case of IL-1β and ferritin.
Gastric Inflammation and Cytokine Concentrations
The degrees of antral and corpus chronic and active inflammation were significantly higher (p<0.001 for all) in H. pylori-positive than in -negative children (Table 2). In H. pylori-positive children, the corpus concentration of IL-1β was significantly higher in children with a mild/moderate degree of corpus chronic inflammation than in those without corpus inflammation (672.48±300.37 pg/mg vs. 504.39±28.81 pg/mg, respectively, p = 0.002). Furthermore, the corpus concentration of TNF-α was higher (p<0.001) in infected children with a mild/moderate degree of corpus active inflammation than in those without corpus PMN cell infiltration (369.2±75.3 pg/mg vs. 277.8±75.3 pg/mg).
Table 2. Histological comparison of antral and corpus gastric mucosa of H. pylori (HP)-positive (n = 47) and -negative (n = 78) childrena.
| Inflammation | Absentn (%) | Mildn (%) | Moderaten (%) | Markedn (%) | P value |
| Antrum | |||||
| Chronic inflammation | |||||
| HP-positive | 01 (2.2) | 09 (19.5) | 35 (76.1) | 01 (2.2) | |
| HP-negative | 34 (47.9) | 37 (52.1) | 0 | 0 | <0.001 |
| Active inflammation | |||||
| HP-positive | 04 (8.7) | 26 (56.5) | 16 (34.8) | 0 | |
| HP-negative | 66 (93.0) | 5 (7.0) | 0 | 0 | <0.001 |
| Corpus | |||||
| Chronic inflammation | |||||
| HP-positive | 03 (6.8) | 38 (86.4) | 03 (6.8) | 0 | |
| HP-negative | 42 (54.5) | 35 (45.5) | 0 | 0 | <0.001 |
| Active inflammation | |||||
| HP-positive | 15 (34.1) | 29 (65.9) | 0 | 0 | |
| HP-negative | 74 (96.1) | 03 (3.9) | 0 | 0 | <0.001 |
One antral and 3 corpus gastric biopsy specimens from HP-positive and 7 antral and 1 corpus biopsy specimens from HP-negative children were deemed to be inadequate for histology assessment; n, number.
Neither atrophy, nor intestinal metaplasia was observed.
IL1B, IL1RN and TNFA Polymorphisms
IL1B (p = 0.45), IL1RN (p = 0.36) and TNFA (p = 0.78) polymorphic genotypes did not associate with H. pylori status (Table 3). No association between IL1B and TNFA polymorphic alleles and blood ID/IDA parameters (p≥0.17) was observed.
Table 3. IL1B-31, IL1RN and TNFA-307 genotypic frequencies in H. pylori-positive (n = 47) and –negative children (n = 78).
| All childrenn (%) | H. pylori-positiven (%) | H. pylori-negativen (%) | |
| IL1B-31a | |||
| T/T | 37 (29.8) | 16 (34.8) | 21 (26.9) |
| T/C | 63 (50.8) | 20 (43.5) | 42 (55.2) |
| C/C | 24 (19.4) | 10 (21.7) | 14 (17.9) |
| IL1RN VNTRb | |||
| 1/1 | 82 (66.2) | 30 (65.2) | 52 (66.7) |
| 1/2 | 35 (28.2) | 15 (32.6) | 20 (25.6) |
| 2/2 | 07 (5.6) | 01 (2.2) | 06 (7.7) |
| TNFA-307 | |||
| G/G | 87 (69.6) | 32 (68.1) | 55 (70.5) |
| G/A | 38 (30.4) | 15 (31.9) | 23 (29.5) |
It was not possible to genotype 1 H. pylori-positive children for IL1B-31 and 1 for IL1RN.
1 indicates all the long alleles and 2 the short allele. The loci did not deviate significantly from the expected Hardy-Weinberg distribution (P = 0.90 for IL1B-31, P = 0.26 for IL1RN and P = 0.08 for TNFA-307) and all segregated independently.
In the H. pylori-positive group, carriers of the polymorphic IL1RN allele 2 had increased corpus inflammatory scores (p = 0.02) and significantly higher (p = 0.01 for both) IL-1β concentrations than the non-carriers (antrum 354.4±189.3 pg/mg vs. 253.5±74.3 pg/mg and corpus 873.4±466.6 pg/mg vs. 624.7±183.1 pg/mg). In the H. pylori-positive group of children of 12 years of age or younger (16 non-carriers vs. 9 carriers), a significant negative association between IL1RN polymorphic genotype and haemoglobin (13.0±0.9 g/dL vs. 11.9±1.3 g/dL, p = 0.03) or haematocrit (39.5±2.2% vs. 36.0±4.2%, p = 0.01) was observed.
The mean gastric concentration of antral (p>0.12) and corpus IL-1β (p>0.15) and antral (p>0.32) and corpus TNF-α (p>0.28) did not differ between carriers and non-carriers of IL1B and TNFA polymorphic genotypes, independently of the H. pylori-status.
Discussion
ID is a very common micronutrient deficiency that affects individuals globally, but is a particular problem in developing countries. Children are considered to be at high-risk of ID that associates with deficits of immune, cognitive and motor functions [24], [25], in addition to the possible development of anaemia.
Accumulating evidence from epidemiological studies and clinical trials implicates H. pylori infection in the aetiology of ID/IDA [22], [26], [27]. Notably, ID in H. pylori infected subjects is resistant to iron supplementation, but this resistance is reversible by H. pylori eradication [28], [29]. However, the mechanism, or mechanisms, by which the infection might cause ID/IDA are still not determined. In the present study, we demonstrate that in H. pylori-infected children without common known causes of ID/IDA increased gastric IL-1β concentration is an independent predictor for low blood concentration of ferritin and haemoglobin.
Although it would be expected that in H. pylori infected children gastric IL-1β concentrations would be higher in the antrum, which is more inflamed than the corpus, a 2.5-fold increase in corpus IL-1β was observed. This could be explained either by increased corpus IL-1β production, or by a higher number of IL-1 receptors in the gastric corpus. In the former, as there was no corresponding increase in inflammation, IL-1β sources other than inflammatory cells have to be considered. However, taking in account that IL-1β inhibits gastric acid secretion by acting directly on parietal cells [14], [17], [30], the latter hypothesis seems more likely. Importantly, both murine gastric mucous and parietal cell lineages express the IL-1R1 receptor [31]. In contrast to IL-1β, the concentration of TNF-α was higher in the antrum than in the corpus, but TNF-α was not associated with blood iron parameters. However TNF-α concentrations were correlated with the scores of PMN cells in the corpus mucosa and inflammation has been considered a putative mechanism of disruption of the acid homeostasis.
The negative correlation between gastric IL-1β and ID/IDA blood parameters observed in this study could be due to the powerful capability of IL-1β in inhibiting gastric acid secretion [14]. Gastric acid is essential for iron absorption by reducing the ferric iron to a more soluble and absorbable ferrous iron form [2], [6]. The results of this study would support the argument that in the early phase of H. pylori infection, high gastric secretion of IL-1β that inhibits acid secretion impairs the absorption of iron. IL1β would also participate in the impairment of iron absorption by up-regulating hepcidin as demonstrated in vivo [31], [32]. However, in a recent study, Schwarz et al [33] did not observe associations between the serum concentrations of hepcidin and H. pylori infection.
In chronic H. pylori infection in adulthood, gastric atrophy leading to hypochlorhydria is considered a mechanism of iron deficiency. Gastric corpus atrophic changes are more frequently observed in adult carriers of the IL1 gene cluster polymorphisms [17]. In the present study, IL1RN, but not IL1B polymorphism, was associated with increased gastric IL-1β concentration in concordance with studies in our adult population [34]. The latter study demonstrated that polymorphism of IL1RN, but not IL1B, is associated with increased risk of atrophic gastric changes and gastric carcinoma in this region of Brazil. In the current study we demonstrate that in the group of the H. pylori-positive youngest children, the haemoglobin and haematocrit values are lower in carriers of IL1RN polymorphic alleles than in children with the wild genotype. The high production of IL-1β in the former group might account for a more severe hypochlorhydria in the acute phase of H. pylori infection that is mainly acquired in early childhood.
In conclusion, we provide additional and convincing evidence of a role of gastric IL-1β induced by H. pylori infection in decreasing iron absorption in children. Thus, H. pylori infected children with ID/IDA may benefit from H. pylori eradication therapy.
Funding Statement
The study was supported by the Sixth Framework Programme of the European Union, Project CONTENT (INCO-DEV-3-032136); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil) and Instituto de Biomedicina do Semi-Árido (INCT-IBISAB/Brazil). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO/UNICEF/UNU (2001) Iron deficiency anemia assessment, prevention, and control. Geneva: World Health Organization. Available: http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf. Accessed 24 November 2012.
- 2. Zimmermann MB, Hurrell RF (2007) Nutritional iron deficiency. Lancet 370: 511–520. [DOI] [PubMed] [Google Scholar]
- 3. Crompton DWT, Nesheim MC (2002) Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr 22: 35–59. [DOI] [PubMed] [Google Scholar]
- 4. Yip R, Limburg PJ, Ahlquist DA, Carpenter HA, ÓNeill A, et al. (1997) Pervasive occult gastrointestinal bleeding in an Alaska native population with prevalent iron deficiency. JAMA 277: 1135–1139. [DOI] [PubMed] [Google Scholar]
- 5. Yokota S, Kono M, Mino E, Sato K, Takahashi M, et al. (2008) Enhanced Fe ion-uptake activity in Helicobacter pylori strains isolated from patients with iron-deficiency anemia. Clin Inf Dis 46: e31–33. [DOI] [PubMed] [Google Scholar]
- 6. Annibale B, Capurso G, Lahner E, Passi S, Ricci R, et al. (2003) Concomitant alterations in intragastric pH and ascorbic acid concentrations in patients with Helicobacter pylori gastritis and associated iron deficiency anaemia. Gut 52: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Windle HJ, Kelleher D, Crabtree JE (2007) Childhood Helicobacter pylori infection and growth impairment in developing countries: a vicious cycle? Pediatrics 119: E754–759. [DOI] [PubMed] [Google Scholar]
- 8. Sobala GM, Crabtree JE, Dixon MF, Schorah CJ, Taylor JD, et al. (1991) Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut 32: 1415–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harford WV, Barnett C, Lee E, Perez-Perez G, Blaser MJ, et al. (2000) Acute gastritis with hypochlorhydria: report of 35 cases with long term follow-up. Gut 47: 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson KT, Crabtree JE (2007) Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology 133: 288–308. [DOI] [PubMed] [Google Scholar]
- 11. Fox JG, Otto G, Taylor NS, Rosenblad W, Murphy JC (1991) Helicobacter mustelae-induced gastritis and elevated gastric pH in the ferret (Mustela putorius furo). Infect Immun 59: 1875–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takashima M, Furuta T, Hanai H, Sugimura H, Kaneko E (2001) Effects of Helicobacter pylori infection on gastric acid secretion and serum gastrin levels in Mongolian gerbils. Gut 48: 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Correa P, Piazuelo MB (2008) Natural history of Helicobacter pylori infection. Dig Liver Dis 40: 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beales IL, Calam J (1998) Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut 42: 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI (1991) Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori-associated gastritis. Gut 32: 1473–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Melo FF, Rocha AMC, Rocha GA, Pedroso SHSP, Batista SA (2012) A regulatory instead of an IL-17 T response predominates in Helicobacter pylori-associated gastritis in children. Microbes and Infection 14: 341–347. [DOI] [PubMed] [Google Scholar]
- 17. El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, et al. (2000) Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404: 398–402. [DOI] [PubMed] [Google Scholar]
- 18. Vannella L, Aloe Spirit MA, Gozza G, Tardellas L, Monarca B, et al. (2008) Benefit of concomitant gastrointestinal and gynaecological evaluation in premenopausal women with iron deficiency anaemia. Aliment Pharmacol Ther 28: 422–430. [DOI] [PubMed] [Google Scholar]
- 19. Cardinali LC, Rocha GA, Rocha AM, Moura SB, Soares TF, et al. (2003) Evaluation of [13C]urea-breath test and Helicobacter pylori stool antigen test for diagnosis of H. pylori infection in children from a developing country. J Clin Microbiol 41: 3334–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Queiroz DMM, Guerra JB, Rocha GA, Rocha AMC, Santos A (2004) IL1B and IL1RN polymorphic genes and Helicobacter pylori cagA strains decrease the risk of reflux esophagitis. Gastroenterology 127: 73–79. [DOI] [PubMed] [Google Scholar]
- 21. Dixon MF, Genta RM, Yardley JH, Correa P (1996) Classification and grading of gastritis. The updated Sydney System. Am J Surg Pathol 20: 1161–1181. [DOI] [PubMed] [Google Scholar]
- 22. Mansfield JC, Holden H, Tarlow JK, Di Giovine FS, McDowell TL, et al. (1994) Novel genetic association between ulcerative colitis and the anti-inflammatory cytokine interleukin-1 receptor antagonist. Gastroenterology 106: 637–642. [DOI] [PubMed] [Google Scholar]
- 23. Soares TF, Rocha GA, Rocha AM, Corrêa-Oliveira R, Martins-Filho AO, et al. (2005) Phenotypic study of peripheral blood lymphocytes and humoral immune response in Helicobacter pylori infection according to age. Scand J Immunol 62: 63–70. [DOI] [PubMed] [Google Scholar]
- 24. Idiradinata P, Pollitt E (1993) Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet 341: 1–4. [DOI] [PubMed] [Google Scholar]
- 25. Hurtado EK, Claussen AH, Scott KG (1999) Early childhood anemia and mild or moderate mental retardation. Am J Clin Nutr 69: 115–119. [DOI] [PubMed] [Google Scholar]
- 26. Kurekci AE, Atay AA, Sarici SU, Yesilkaya E, Senses Z, et al. (2005) Is there a relationship between childhood Helicobacter pylori infection and iron deficiency anemia? J Trop Ped 51: 166–169. [DOI] [PubMed] [Google Scholar]
- 27. Baggett H, Parkinson AJ, Muth PT, Gold BD, Gessner B (2006) Endemic iron deficiency associated with Helicobacter pylori infection among school-aged children in Alaska. Pediatrics 117: e396–404. [DOI] [PubMed] [Google Scholar]
- 28. Choe YH, Lee JE, Kim SK (2000) Effect of Helicobacter pylori eradication on sideropenic refractory anaemia in adolescent girls with Helicobacter pylori infection. Acta Paediatr 89: 154–157. [DOI] [PubMed] [Google Scholar]
- 29. Marignani M, Angeletti S, Bordi C, Malagnino F, Mancino C, et al. (1997) Reversal of long standing iron deficiency anaemia alter eradication of Helicobacter pylori infection. Scan J Gastroenterol 32: 617–622. [DOI] [PubMed] [Google Scholar]
- 30. Waghray M, Zavros Y, Saqui-Salces M, El-Zaatari M, Alamelumangapuram CB (2010) Interleukin-1 promotes gastric atrophy through suppression of sonic hedgehog. Gastroenterology 138: 562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Inamura J, Ikuta K, Jimbo J, Shindo M, Sato K, et al. (2005) Upregulation of hepcidin by interleukin-1β in human hepatoma cell lines. Hepatol Res 33: 198–205. [DOI] [PubMed] [Google Scholar]
- 32. Lee P, Peng H, Gelbart T, Wang L, Beutler E (2005) Regulation of hepcidin transcription by interleukin-1 and interleukin 6. PNAS 102: 1906–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwarz P, Kübler JAM, Strnad P, Katrin M, Barth TFE, et al. (2012) Hepcidin is localised in gastric parietal cells, regulates acid secretion and is induced by Helicobacter pylori infection. Gut 61: 193–201. [DOI] [PubMed] [Google Scholar]
- 34. Rocha GA, Guerra JB, Rocha AM, Saraiva IE, Silva DA, et al. (2005) IL1RN polymorphic gene and cagA-positive status independently increase the risk of noncardia gastric carcinoma. Int J Cancer 115: 678–683. [DOI] [PubMed] [Google Scholar]