Abstract
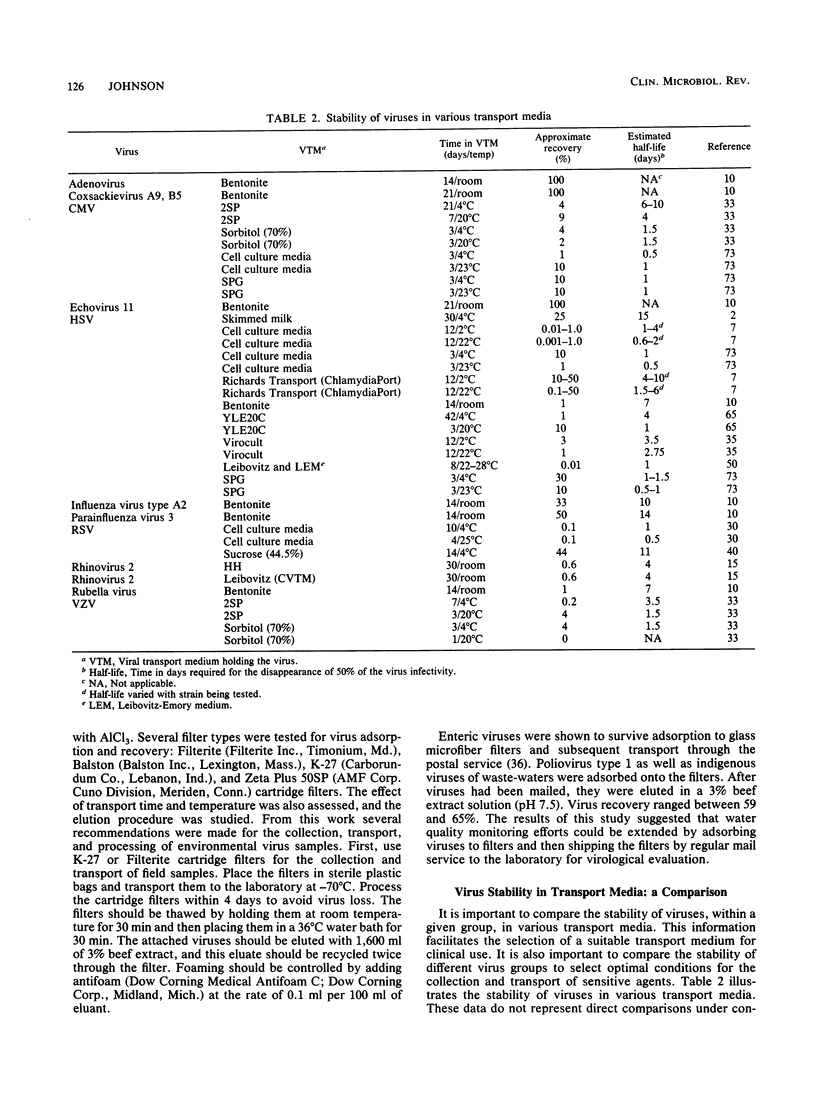
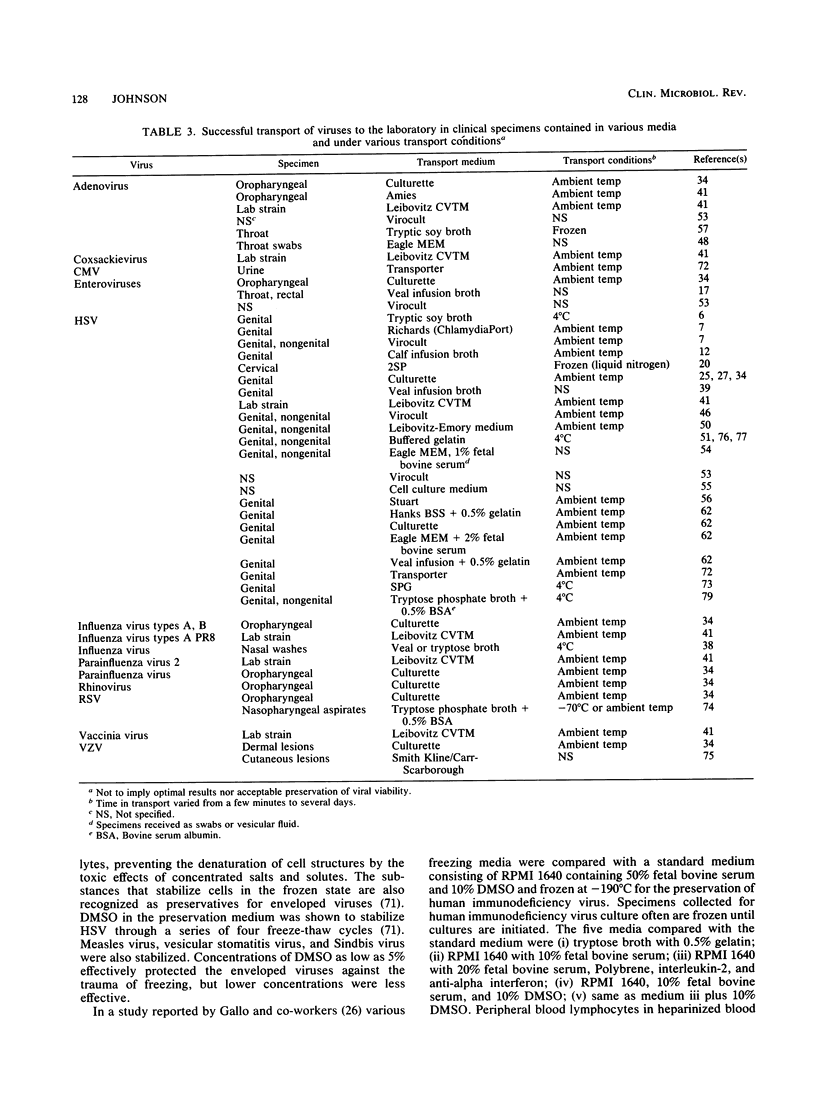
The diagnosis of viral infections by culture relies on the collection of proper specimens, proper care to protect the virus in the specimens from environmental damage, and use of an adequate transport system to maintain virus activity. Collection of specimens with swabs that are toxic to either virus or cell culture should be avoided. A variety of transport media have been formulated, beginning with early bacteriological transport media. Certain swab-tube combinations have proven to be both effective and convenient. Of the liquid transport media, sucrose-based and broth-based media appear to be the most widely accepted and used. Studies on virus stability show that most viruses tested are sufficiently stable in transport media to withstand a transport time of 1 to 3 days. Some viruses may withstand longer transport times. In many cases, it is not necessary to store virus specimens in a refrigerator or send them to the laboratory on wet ice or frozen on dry ice. However, the specimen should not be exposed to environmental extremes. Modern viral transport media allow for more effective use of viral culture and culture enhancement techniques for the diagnosis of human viral infections.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN E. G., KANEDA B., GIRARDI A. J., SCOTT T. F. M., SIGEL M. M. Preservation of viruses of the psittacosis-lymphogranuloma venerum group and herpes simplex under various conditions of storage. J Bacteriol. 1952 Mar;63(3):369–376. doi: 10.1128/jb.63.3.369-376.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander I., Ashley C. R., Smith K. J., Harbour J., Roome A. P., Darville J. M. Comparison of ELISA with virus isolation for the diagnosis of genital herpes. J Clin Pathol. 1985 May;38(5):554–557. doi: 10.1136/jcp.38.5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amies C. R. A modified formula for the preparation of Stuart's Transport Medium. Can J Public Health. 1967 Jul;58(7):296–300. [PubMed] [Google Scholar]
- Arvin A. M., Hensleigh P. A., Prober C. G., Au D. S., Yasukawa L. L., Wittek A. E., Palumbo P. E., Paryani S. G., Yeager A. S. Failure of antepartum maternal cultures to predict the infant's risk of exposure to herpes simplex virus at delivery. N Engl J Med. 1986 Sep 25;315(13):796–800. doi: 10.1056/NEJM198609253151303. [DOI] [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard D. L., Farnes K., Richards D. F., Croft G. F., Johnson F. B. Suitability of new chlamydia transport medium for transport of herpes simplex virus. J Clin Microbiol. 1986 Nov;24(5):692–695. doi: 10.1128/jcm.24.5.692-695.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettoli E. J., Brewer P. M., Oxtoby M. J., Zaidi A. A., Guinan M. E. The role of temperature and swab materials in the recovery of herpes simplex virus from lesions. J Infect Dis. 1982 Mar;145(3):399–399. doi: 10.1093/infdis/145.3.399. [DOI] [PubMed] [Google Scholar]
- Bishai F. R., Labzoffsky N. A. Stability of different viruses in a newly developed transport medium. Can J Microbiol. 1974 Jan;20(1):75–80. doi: 10.1139/m74-012. [DOI] [PubMed] [Google Scholar]
- Brown Z. A., Vontver L. A., Benedetti J., Critchlow C. W., Sells C. J., Berry S., Corey L. Effects on infants of a first episode of genital herpes during pregnancy. N Engl J Med. 1987 Nov 12;317(20):1246–1251. doi: 10.1056/NEJM198711123172002. [DOI] [PubMed] [Google Scholar]
- Chaniot S. C., Holmes M. J., Stott E. J., Tyrrell D. A. An investigation of media for the long term storage of three respiratory viruses. Arch Gesamte Virusforsch. 1974;44(4):396–400. doi: 10.1007/BF01251022. [DOI] [PubMed] [Google Scholar]
- Chonmaitree T., Ford C., Sanders C., Lucia H. L. Comparison of cell cultures for rapid isolation of enteroviruses. J Clin Microbiol. 1988 Dec;26(12):2576–2580. doi: 10.1128/jcm.26.12.2576-2580.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane L. R., Gutterman P. A., Chapel T., Lerner A. M. Incubation of swab materials with herpes simplex virus. J Infect Dis. 1980 Apr;141(4):531–531. doi: 10.1093/infdis/141.4.531. [DOI] [PubMed] [Google Scholar]
- Dahling D. R., Wright B. A. Processing and transport of environmental virus samples. Appl Environ Microbiol. 1984 Jun;47(6):1272–1276. doi: 10.1128/aem.47.6.1272-1276.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darougar S., Walpita P., Thaker U., Goh B. T., Dunlop E. M. A rapid and sensitive culture test for the laboratory diagnosis of genital herpes in women. Genitourin Med. 1986 Apr;62(2):93–96. doi: 10.1136/sti.62.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung J. C., Shanley J., Tilton R. C. Comparison of the detection of herpes simplex virus in direct clinical specimens with herpes simplex virus-specific DNA probes and monoclonal antibodies. J Clin Microbiol. 1985 Nov;22(5):748–753. doi: 10.1128/jcm.22.5.748-753.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo D., Kimpton J. S., Johnson P. J. Isolation of human immunodeficiency virus from peripheral blood lymphocytes stored in various transport media and frozen at -60 degrees C. J Clin Microbiol. 1989 Jan;27(1):88–90. doi: 10.1128/jcm.27.1.88-90.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleaves C. A., Wilson D. J., Wold A. D., Smith T. F. Detection and serotyping of herpes simplex virus in MRC-5 cells by use of centrifugation and monoclonal antibodies 16 h postinoculation. J Clin Microbiol. 1985 Jan;21(1):29–32. doi: 10.1128/jcm.21.1.29-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMBLING M. H. SURVIVAL OF THE RESPIRATORY SYNCYTIAL VIRUS DURING STORAGE UNDER VARIOUS CONDITIONS. Br J Exp Pathol. 1964 Dec;45:647–655. [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B. E., Jungkind D. L., Haller G. J., Sharrar R., Baker R. A., Weisberg M. Evaluation of two rapid methods for the detection of herpes simplex virus antigen in patient specimens. Ann Clin Lab Sci. 1985 Sep-Oct;15(5):418–427. [PubMed] [Google Scholar]
- Howell C. L., Miller M. J. Effect of sucrose phosphate and sorbitol on infectivity of enveloped viruses during storage. J Clin Microbiol. 1983 Sep;18(3):658–662. doi: 10.1128/jcm.18.3.658-662.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntoon C. J., House R. F., Jr, Smith T. F. Recovery of viruses from three transport media incorporated into culturettes. Arch Pathol Lab Med. 1981 Aug;105(8):436–437. [PubMed] [Google Scholar]
- Johnson F. B., Leavitt R. W., Richards D. F. Evaluation of the virocult transport tube for isolation of herpes simplex virus from clinical specimens. J Clin Microbiol. 1984 Jul;20(1):120–122. doi: 10.1128/jcm.20.1.120-122.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joret J. C., Block J. C. Survie de virus entériques adsorbés sur microfibre de verre aucours d'un transport postal. Can J Microbiol. 1981 Feb;27(2):246–248. [PubMed] [Google Scholar]
- Langenberg A., Zbanyszek R., Dragavon J., Ashley R., Corey L. Comparison of diploid fibroblast and rabbit kidney tissue cultures and a diploid fibroblast microtiter plate system for the isolation of herpes simplex virus. J Clin Microbiol. 1988 Sep;26(9):1772–1774. doi: 10.1128/jcm.26.9.1772-1774.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law T. J., Hull R. N. The stabilizing effect of sucrose upon respiratory syncytial virus infectivity. Proc Soc Exp Biol Med. 1968 Jun;128(2):515–518. doi: 10.3181/00379727-128-33054. [DOI] [PubMed] [Google Scholar]
- Leibovitz A. A transport medium for diagnostic virology. Proc Soc Exp Biol Med. 1969 May;131(1):127–130. doi: 10.3181/00379727-131-33820. [DOI] [PubMed] [Google Scholar]
- Mayo D. R., Brennan T., Egbertson S. H., Moore D. F. Rapid herpes simplex virus detection in clinical samples submitted to a state virology laboratory. J Clin Microbiol. 1985 May;21(5):768–771. doi: 10.1128/jcm.21.5.768-771.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaustland K. A., Bond W. W., Bradley D. W., Ebert J. W., Maynard J. E. Survival of hepatitis A virus in feces after drying and storage for 1 month. J Clin Microbiol. 1982 Nov;16(5):957–958. doi: 10.1128/jcm.16.5.957-958.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias A., Wickliffe C., Pipkin J., Leibocitz A., Hutton R. Transport media for herpes simplex virus types 1 and 2. Appl Microbiol. 1971 Sep;22(3):451–454. doi: 10.1128/am.22.3.451-454.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oefinger P. E., Loo S. H., Gander R. M. Modified spin-amplified adsorption procedure with conventional tissue culture tubes for rapid detection and increased recovery of herpes simplex virus from clinical specimens. J Clin Microbiol. 1988 Oct;26(10):2195–2199. doi: 10.1128/jcm.26.10.2195-2199.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez T. R., Mosman P. L., Juchau S. V. Experience with Virocult as a viral collection and transportation system. Diagn Microbiol Infect Dis. 1984 Jan;2(1):7–9. doi: 10.1016/0732-8893(84)90017-8. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Hughes B. L., Aarnaes S. L., de la Maza L. M. Comparison of primary rabbit kidney and MRC-5 cells and two stain procedures for herpes simplex virus detection by a shell vial centrifugation method. J Clin Microbiol. 1988 Feb;26(2):222–224. doi: 10.1128/jcm.26.2.222-224.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips L. E., Magliolo R. A., Stehlik M. L., Whiteman P. A., Faro S., Rogers T. E. Retrospective evaluation of the isolation and identification of herpes simplex virus with Cultureset and human fibroblasts. J Clin Microbiol. 1985 Aug;22(2):255–258. doi: 10.1128/jcm.22.2.255-258.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodin P., Hare M. J., Barwell C. F., Withers M. J. Transport of herpes simplex virus in Stuart's medium. Br J Vener Dis. 1971 Jun;47(3):198–199. doi: 10.1136/sti.47.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutala W. A., Shelton D. F., Arbiter D. Comparative sensitivities of viruses to cell cultures and transport media. Am J Clin Pathol. 1977 Apr;67(4):397–400. doi: 10.1093/ajcp/67.4.397. [DOI] [PubMed] [Google Scholar]
- STUART R. D. Transport medium for specimens in public health bacteriology. Public Health Rep. 1959 May;74(5):431–438. [PMC free article] [PubMed] [Google Scholar]
- Schoenemann W. Haltbarkeit von Herpesviren in verschiedenen Transportmedien. Z Hautkr. 1980 Jul 15;55(14):938–942. [PubMed] [Google Scholar]
- Smith T. F. Clinical uses of the diagnostic virology laboratory. Med Clin North Am. 1983 Sep;67(5):935–951. doi: 10.1016/s0025-7125(16)31160-9. [DOI] [PubMed] [Google Scholar]
- Smith T. F., Weed L. A., Pettersen G. R., Segura J. W. Recovery of Chlamydia and Genital Mycoplasma transported in sucrose phosphate buffer and urease color test medium. Health Lab Sci. 1977 Jan;14(1):30–34. [PubMed] [Google Scholar]
- Tada A., Sekine N., Toba M., Yoshino K. An analysis of factors influencing the isolation rate of herpes simplex virus. Microbiol Immunol. 1977;21(4):219–229. doi: 10.1111/j.1348-0421.1977.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Tannock G. A., Hierholzer J. C., Bryce D. A., Chee C. F., Paul J. A. Freeze-drying of respiratory syncytial viruses for transportation and storage. J Clin Microbiol. 1987 Sep;25(9):1769–1771. doi: 10.1128/jcm.25.9.1769-1771.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti W. M., Menegus M. A. Nosocomial viral infections: IV. Guidelines for cohort isolation, the communicable disease survey, collection, and transport of specimens for virus isolation, and considerations for the future. Infect Control. 1981 May-Jun;2(3):236–245. doi: 10.1017/s0195941700055132. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Stabilization of enveloped viruses by dimethyl sulfoxide. J Virol. 1968 Sep;2(9):953–954. doi: 10.1128/jvi.2.9.953-954.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warford A. L., Eveland W. G., Strong C. A., Levy R. A., Rekrut K. A. Enhanced virus isolation by use of the transporter for a regional laboratory. J Clin Microbiol. 1984 Apr;19(4):561–562. doi: 10.1128/jcm.19.4.561-562.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warford A. L., Rekrut K. A., Levy R. A., Drill A. E. Sucrose phosphate glutamate for combined transport of chlamydial and viral specimens. Am J Clin Pathol. 1984 Jun;81(6):762–764. doi: 10.1093/ajcp/81.6.762. [DOI] [PubMed] [Google Scholar]
- Waris M., Halonen P., Ziegler T., Nikkari S., Obert G. Time-resolved fluoroimmunoassay compared with virus isolation for rapid detection of respiratory syncytial virus in nasopharyngeal aspirates. J Clin Microbiol. 1988 Dec;26(12):2581–2585. doi: 10.1128/jcm.26.12.2581-2585.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West P. G., Aldrich B., Hartwig R., Haller G. J. Increased detection rate for varicella-zoster virus with combination of two techniques. J Clin Microbiol. 1988 Dec;26(12):2680–2681. doi: 10.1128/jcm.26.12.2680-2681.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods G. L., Mills R. D. Conventional tube cell culture compared with centrifugal inoculation of MRC-5 cells and staining with monoclonal antibodies for detection of herpes simplex virus in clinical specimens. J Clin Microbiol. 1988 Mar;26(3):570–572. doi: 10.1128/jcm.26.3.570-572.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods G. L., Mills R. D. Effect of dexamethasone on detection of herpes simplex virus in clinical specimens by conventional cell culture and rapid 24-well plate centrifugation. J Clin Microbiol. 1988 Jun;26(6):1233–1235. doi: 10.1128/jcm.26.6.1233-1235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager A. S., Morris J. E., Prober C. G. Storage and transport of cultures for Herpes simplex virus, type 2. Am J Clin Pathol. 1979 Dec;72(6):977–979. doi: 10.1093/ajcp/72.6.977. [DOI] [PubMed] [Google Scholar]
- Ziegler T., Waris M., Rautiainen M., Arstila P. Herpes simplex virus detection by macroscopic reading after overnight incubation and immunoperoxidase staining. J Clin Microbiol. 1988 Oct;26(10):2013–2017. doi: 10.1128/jcm.26.10.2013-2017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


