Abstract
Background
Experiences of early life stress, increased psychological arousal and the body’s physiologic stress response seem to play an important role in the pathogenesis and maintenance of borderline personality disorder (BPD). In the present study, we investigated alterations in grey matter of central stress-regulating structures in female patients with BPD.
Methods
We examined T1-weighted structural magnetic resonance imaging scans of unmedicated, right-handed female patients with BPD (according to DSM-IV criteria) and healthy controls matched for age, intelligence and education using fully automated DARTEL voxel-based morphometry. Our regions of interest analyses included the hippocampus, amygdala, anterior cingulate cortex (ACC) and hypothalamus.
Results
We enrolled 30 patients and 33 controls in our study. The grey matter of patients with BPD was reduced in the hippocampus, but increased in the hypothalamus compared with healthy participants. Hypothalamic volume correlated positively with the history of traumatization in patients with BPD. No significant alterations were found in the amygdala and ACC.
Limitations
This study is limited by the lack of measures of corticotropin-releasing hormone, adrenocorticotropic hormone and cortisol levels. Furthermore, moderate sample size and comorbid disorders need to be considered.
Conclusion
Our findings provide new evidence for grey matter alterations in the hypothalamus and replicate previously reported decrements in hippocampal volume in patients with BPD. Understanding the role of the hypothalamus and other central stress-regulating structures could help us to further understand the neurobiological underpinnings of this complex disorder.
Introduction
Psychosocial stress plays an important role in the development and manifestation of borderline personality disorder (BPD). Early life stress and traumatization are risk factors for BPD, and reports of traumatic childhood abuse are frequent in patients with BPD.1 Furthermore, patients with BPD experience psychosocial daily hassles more intensely than healthy individuals, indicating a reactivity and vulnerability to stress.2 Kuo and Linehan3 measured higher skin conductance response in patients with BPD, which is positively correlated with stress. Moreover, the acute symptoms of patients with BPD are precipitated by acute stress. They show higher dissociative symptom scores than subjective stress ratings in comparison to healthy individuals and patients with major depressive and panic disorders.4 Furthermore, high dissociation scores in patients with BPD correlate with greater childhood trauma and greater hypothalamus–pituitary–adrenal (HPA) axis reactivity to stress,5 indicating a link between traumatic childhood experiences, endocrine stress response and acute symptoms. Under subjective stress, patients with BPD display a higher pain threshold,6 and self-injurious behaviour is used as a means of stress reduction.7 It seems plausible that these stress-related symptoms may be linked to dysregulations in the functioning of the HPA axis, which is crucially involved in the body’s response to stress. Correspondingly, patients with BPD show signs of abnormal cortisol reactivity and feedback regulation, indicating disturbed HPA axis functioning, although findings on baseline cortisol, HPA feedback sensitivity and cortisol responses to psychosocial stress in studies of BPD are rather mixed (for example, see the review by Zimmerman and Choi-Kain8). Comorbid disorders, such as posttraumatic stress disorder (PTSD) and major depressive disorder (MDD), which are frequent among patients with BPD and affect HPA axis functioning, as well as other confounding variables, such as sex, menstrual cycle and age, may at least partly explain these inconsistencies.8 The cause of HPA axis dysregulations is thought to result from adverse experiences in early life, with traumatic childhood experiences and sexual abuse in childhood impairing neurobiological systems involved in the stress response.9,10
In addition to disturbances in HPA axis functioning, brain imaging studies have revealed volumetric alterations in structures crucially involved in the regulation of stress responses in patients with BPD. Across studies, patients with BPD have shown reduced hippocampal and amygdalar volumes (although results are less consistent for the latter; for example, see the meta-analysis by Nunes and colleagues11). The hippocampus and amygdala have inverse regulatory effects on the hypothalamic stress response. Whereas the hippocampus inhibits hypothalamic and HPA axis activity,12 the amygdala exhibits activating effects on the HPA axis and stimulates glucocorticoid release.13 Amygdalar hyperactivity and reduced prefrontal amygdalar inhibition to (negative) emotional stimuli is also one of the most consistent findings of functional brain imaging studies of BPD14 (for example, see the review by Mauchnik and Schmahl15). In addition, some studies found reduced volumes in prefrontal brain areas involved in stress regulation,13 in particular the anterior cingulate cortex (ACC) and orbitofrontal cortex.16,17
Thus, there is evidence for increased stress vulnerability, disturbed HPA axis functioning and alterations in the size and activation of structures involved in central stress regulation in patients with BPD. However, so far nothing is known about alterations in hypothalamic volume of patients with BPD. This is astonishing, as the hypothalamus is the main structure connecting the central nervous system to the endocrine system13 and represents a link between endocrine and behavioural processes.18
The aim of the present structural magnetic resonance imaging (MRI) study was to investigate grey matter alterations of central stress-regulating structures in women with BPD by applying a DARTEL voxel-based morphometry (VBM) algorithm. Voxel-based morphometry is an objective, automated method, secure of bias and interrater inaccuracy. It involves a voxel-wise comparison of local grey matter concentration between 2 groups.19 We first performed an exploratory analysis on the whole brain volume. Owing to their influence on stress response, the hippocampus, amygdala and ACC represent the main target points for structural neuroimaging studies investigating central stress regulation.20 Based on prior findings, we hypothesized that patients with BPD would have smaller volumes in the hippocampus, and most probably in the amygdala and the ACC, than healthy women. We also expected that patients with BPD would differ with respect to hypothalamic volume in comparison to healthy women. To test these hypotheses, we determined distinct a priori regions of interest (ROIs). We then explored whether grey matter volume of these 4 structures is associated with childhood trauma. To our knowledge, this is the first study analyzing hypothalamic grey matter alterations using DARTEL VBM in patients with BPD.
Methods
Participants
We recruited right-handed, unmedicated female patients with BPD between 18 and 35 years of age and healthy controls matched for age, IQ and education. Patients were recruited at the Department of General Psychiatry at the University of Heidelberg and through advertisement and were included in the study after confirmation of diagnosis. Healthy participants were also recruited through advertisement. We excluded individuals with a lifetime diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder or substantial neurologic disease; current alcohol/drug dependence; an IQ of 85 or lower; or hormonal or neurologic disorders as well as those who were pregnant. All patients with BPD had a DSM-IV-defined diagnosis of BPD, and none of the healthy participants had a current psychiatric disorder, had ever received a psychiatric diagnosis, or had ever undergone any psychological or psychiatric treatment. The study was approved by the Ethics Committee of the Faculty of Medicine, University of Heidelberg. All participants gave written informed consent after the study procedures were fully explained to them and before participation. This study was part of a larger study investigating neuropeptides in patients with BPD.
Measures
We assessed the diagnosis of BPD as well as comorbidity or absence of Axis II disorders using the German version of the International Disorder Examination for DSM-IV.27 Comorbidity or absence of Axis I disorders and the Global Assessment of Functioning (GAF) score were assessed with the German version of the Structured Clinical Interview for DSM-IV (SCID-I).26 Qualified diagnosticians (K.B. and I.S.) conducted the interviews. Four subtests of the German version of the Hamburg–Wechsler Intelligence Test29 were used to provide an estimate for intelligence (IQ). We also used the Borderline Symptom List–23 (BSL-23)23 to assess borderline symptom severity, the Beck Depression Inventory (BDI)21 to measure depressive psychopathology, the Barratt Impulsiveness Scale (BIS-11)22 to assess impulsive personality traits, the trait scale of the State-Trait Anger Expression Inventory (STAXI)28 to measure trait levels of anger, and the Questionnaire for the Assessment of Factors of Aggression (FAF)25 and the Childhood Trauma Questionnaire (CTQ)24 to assess traumatization history. Healthy controls underwent the same diagnostic assessments as the patients.
Magnetic resonance imaging procedures
For the volumetric assessment, a 3-dimensional (3-D) sagittal isotropic magnetization prepared rapid acquisition gradient echo sequence was obtained using a 3 T scanner (Tim Trio; Siemens) and a 32-channel standard head coil (flip angle 9°, repetition time [TR] 2300 ms, echo time [TE] 2.96 ms, field of view 256 mm, matrix size 256 × 256 pixels, slice thickness 1 mm); 280 slices with an isotropic voxel size of 1 × 1 × 1 mm were acquired. In addition, an axial T2-weighted fluid-attenuated inversion recovery sequence (TR 10000 ms, TE 96 ms) was performed. Experienced neuroradiologists reviewed both sequences to exclude clinically important abnormalities.
Data analysis
Volumetric data were analyzed with SPM8 (Statistical Parametric Mapping, Wellcome Trust Center for Neuroimaging, www.fil.ion.ucl.ac.uk/spm) using the diffeomorphic registration algorithm implemented in the DARTEL toolbox for SPM8,30 which has been found to improve the sensitivity and accuracy of such analysis in comparison to standard SPM.31,32 After the manual setting of the origin (anterior commissure) using the display tool, images were segmented into grey matter, white matter and cerebrospinal fluid using the unified segmentation model included in SPM8.33 The resulting grey and white matter files were imported into the DARTEL procedure. The outputs were rigidly aligned tissue class images, resliced to 1.5 × 1.5 × 1.5 mm3 voxel size. Next, we created a study-specific brain template from the individual tissue class images of all participants. A modulation was installed to correct for changes in volume induced by nonlinear normalization. The DARTEL processed images were spatially normalized to Montreal Neurological Institute (MNI) space. The images were smoothed using a 4 mm full-width at half-maximum Gaussian kernel, which gives consideration to the small size of the hypothalamus and amygdala. We then used these smoothed, modulated and normalized images for statistical analyses.
Statistical analysis
We compared demographic and psychometric characteristics of patients and controls using χ2 tests for categorical variables and 2-sample t tests for continuous variables. Statistical analyses were performed using SPSS software version 17.
For volumetric data, we calculated 2-sample t tests to compare patients with BPD and controls. Images were thresholded at an absolute level of 0.1, and therefore only voxels that exceeded the threshold were included. To account for volumetric variability associated with individual head size, total intracranial volume was entered into the design matrix as a covariate. Total intracranial volume was obtained by summing the volumes of grey matter, white matter and cerebrospinal fluid using the function “get_totals” (www.cs.ucl.ac.uk/staff/G.Ridgway/vbm/). To correct for multiple comparisons in whole brain analyses, results were considered to be significant at p < 0.05, family-wise error (FWE)–corrected. Additionally, a threshold for voxel cluster size of at least k = 10 voxels was chosen. In a second step, we performed an ROI analysis of the hippocampus, amygdala, ACC and hypothalamus. Provided that an an priori hypothesis is made, ROI analyses are more sensitive than whole brain analysis. Masks for these analyses were derived from Wake Forest University WFU Pickatlas (www.ansir.wfubmc.edu/). We considered results to be significant at p < 0.05, FWE-corrected. In an additional analysis, the BPD group was divided into patients with and without comorbid PTSD to test influences of this comorbidity on our ROIs. In a third step, we calculated a regression analysis with the individual results of the CTQ for the BPD group. The total scores of the CTQ were inserted in the design matrix as a covariate to explore whether hypothalamic volume is associated with the extent of traumatic childhood experience. Additional regression analyses with individual IQ, BDI, BIS, BSL, FAF and STAXI scores and the volumes in our ROIs (amygdala, hippocampus, ACC and hypothalamus) were performed to check for influences of these psychometric variables in the BPD group.
Results
Participant demographic and psychometric data
We enrolled 30 women with BPD and 33 controls in our study. Nine patients came from the Department of General Psychiatry at the University of Heidelberg (5 inpatients, 4 outpatients) and 21 were recruited through advertisements. Patients and controls did not differ significantly for age, IQ and education (all t61 ≤ 1.18, p ≥ 0.49; Table 1). As expected, patients with BPD scored higher than controls in all psychometric ratings (except for STAXI and anger control; Table 1). Comorbid psychiatric diagnoses of the BPD group included depression (n = 20 [9 acute]); PTSD (n = 9); remitted substance abuse (n = 8); anxiety disorders (n = 10); eating disorders (n = 9); adjustment disorder (n = 1); and paranoid (n = 4), antisocial (n = 2), histrionic (n = 2), narcissistic (n = 2), compulsive (n = 2), dependent (n = 2) and avoidant (n = 2) personality disorders.
Table 1.
Demographic characteristics of patients with borderline personality disorder and healthy controls
| Group; mean (SD) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | BPD | Control | t61 | p value* |
| Age, yr | 23.7 (4.6) | 24.4 (4.1) | −0.69 | 0.49 |
| Education, school yr | 11.8 (1.6) | 12.0 (1.4) | −1.18 | 0.66 |
| IQ† | 112.4 (12.2) | 112.7 (10.9) | −0.11 | 0.91 |
| GAF score | 69.3 (8.57) | 100 (0.0) | −5.83 | < 0.001 |
| Intracranial volume, mL | 1338.0 (121.1) | 1378.2 (116.7) | −0.73 | 0.19 |
| BPD criteria, IPDE score | 6.9 (1.4) | 0.0 (0.0) | 8.84 | < 0.001 |
| BDI score | 24.3 (12.9) | 5.7 (8.7) | 6.75 | < 0.001 |
| BIS score | 62.2 (13.9) | 60.8 (4.7) | 0.71 | 0.48 |
| BSL score | 1.6 (0.9) | 0.1 (0.2) | 9.27 | < 0.001 |
| CTQ score | 56.2 (17.5) | 30.6 (7.5) | 7.65 | < 0.001 |
| FAF score | ||||
| Spontaneous aggression | 3.9 (2.9) | 1.4 (2.1) | 4.12 | < 0.001 |
| Reactive aggression | 3.9 (2.2) | 1.4 (1.6) | 5.21 | < 0.001 |
| Irritability | 7.7 (3.4) | 3.6 (2.7) | 5.46 | < 0.001 |
| Self-aggression | 7.7 (2.8) | 1.9 (2.6) | 8.71 | < 0.001 |
| Inhibition | 5.5 (2.0) | 5.5 (1.9) | 0.16 | 0.87 |
| Openness | 6.8 (1.7) | 4.3 (2.2) | 5.04 | < 0.001 |
| Aggression sum | 15.6 (7.2) | 6.3 (5.497) | 5.79 | < 0.001 |
| STAXI score | ||||
| Anger temperament | 11.8 (4.3) | 7.9 (3.0) | 4.29 | < 0.001 |
| Anger reaction | 12.3 (4.2) | 8.2 (2.8) | 4.65 | < 0.001 |
| Anger, in | 20.7 (6.5) | 13.7 (4.6) | 5.05 | < 0.001 |
| Anger, out | 15.1 (5.3) | 10.5 (3.0) | 4.29 | < 0.001 |
| Anger, control | 19.9 (5.3) | 24.0 (3.8) | −3.56 | < 0.001 |
BDI = Beck Depression Inventory;21 BIS = Barratt Impulsiveness Scale;22 BPD = borderline personality disorder; BSL = Borderline Symptom List;23 CTQ = Childhood Trauma Questionnaire;24 FAF = Questionnaire for the Assessment of Factors of Aggression;25 GAF = Global Assessment Functioning;26 IPDE = International Disorder Examination for DSM–IV;27 SD = standard deviation; STAXI = State–Trait Anger Inventory.28
Uncorrected for multiple comparisons.
Hamburg–Wechsler Intelligence Test.29
Imaging findings
The results of the whole brain analysis are listed in Table 2. Only results surviving a significance level of p< 0.05, FWE-corrected, were considered to be relevant. Patients with BPD showed significantly reduced grey matter volume in the left hippocampus, vermis 10, parietal inferior left gyrus and temporal mid left/occipital mid left gyrus. In addition, patients with BPD showed significant increases in grey matter volume in the putamen bilaterally and in the Heschl right/temporal superior right gyrus.
Table 2.
Voxel-based morphometric differences between patients with borderline personality disorder and healthy controls in whole brain analysis
| MNI peak voxel | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Contrast; location of peak voxels* | x | y | z | t | p value† | z | Cluster size, k |
| Controls > BPD | |||||||
| Left hippocampus | −26 | −30 | −4 | 6.67 | 0.001 | 5.75 | 59 |
| Vermis 10 | 2 | −42 | −40 | 6.34 | 0.002 | 5.52 | 25 |
| Left inferior parietal gyrus | −30 | −82 | 44 | 6.1 | 0.005 | 5.36 | 24 |
| Left middle temporal gyrus | −58 | −72 | 0 | 5.5 | 0.036 | 4.93 | 32 |
| Right hippocampus | 28 | −30 | −2 | 4.93 | 0.20 | 4.5 | 17 |
| Left superior parietal gyrus | −26 | −72 | 54 | 4.91 | 0.21 | 4.48 | 19 |
| Left middle temporal gyrus | −70 | −38 | −2 | 4.91 | 0.21 | 4.48 | 46 |
| Left precentral gyrus | −36 | −20 | 60 | 4.84 | 0.26 | 4.43 | 20 |
| Left paracentral lobe | −22 | −26 | 74 | 4.75 | 0.32 | 4.36 | 58 |
| Right superior frontal gyrus | 28 | −10 | 70 | 4.74 | 0.33 | 4.35 | 10 |
| Left middle occipital gyrus | −22 | −98 | 10 | 4.74 | 0.33 | 4.35 | 42 |
| Inferior triangular frontal part | 48 | 26 | 6 | 4.64 | 0.42 | 4.27 | 12 |
| — | −2 | −6 | 18 | 4.62 | 0.44 | 4.25 | 25 |
| Left medial superior frontal gyrus | −8 | 70 | 16 | 4.28 | 0.78 | 3.98 | 18 |
| Right postcentral gyrus | 26 | −34 | 66 | 4.17 | 0.87 | 3.89 | 22 |
| Right middle temporal gyrus | 46 | −50 | 12 | 4.11 | 0.91 | 3.85 | 12 |
| Right paracentral lobe | 10 | −40 | 64 | 4.10 | 0.92 | 3.83 | 43 |
| Right inferior occipital gyrus | 34 | −96 | −2 | 4.06 | 0.94 | 3.8 | 23 |
| Right caudate | 6 | 18 | 6 | 4.02 | 0.96 | 3.76 | 10 |
| Left inferior temporal gyrus | −64 | −56 | −14 | 3.97 | 0.97 | 3.73 | 11 |
| Left middle temporal gyrus | −60 | −44 | −12 | 3.95 | 0.98 | 3.71 | 32 |
| Left middle temporal pole | −22 | 12 | −38 | 3.89 | 0.99 | 3.66 | 18 |
| Left postcentral lobe | −58 | −8 | 44 | 3.87 | 0.99 | 3.64 | 16 |
| Right medial orbitofrontal gyrus | 14 | 46 | −4 | 3.82 | > 0.99 | 3.6 | 10 |
| — | −50 | −80 | −12 | 3.73 | > 0.99 | 3.52 | 10 |
| BPD > controls | |||||||
| Left putamen | −32 | −22 | 0 | 6.21 | 0.003 | 5.44 | 86 |
| Right Heschl | 42 | −26 | 4 | 5.87 | 0.011 | 5.2 | 27 |
| Right putamen right | 32 | −12 | 2 | 5.69 | 0.019 | 5.07 | 111 |
| Left supplementary motor area | 2 | −6 | 50 | 5.30 | 0.07 | 4.78 | 21 |
| Left cerebellum 7 | −20 | −70 | −42 | 5.13 | 0.11 | 4.65 | 15 |
| Right middle frontal gyrus | 34 | 24 | 46 | 5.09 | 0.13 | 4.62 | 12 |
| Left superior temporal gyrus | −40 | −28 | 2 | 5.08 | 0.13 | 4.61 | 11 |
| Right middle cingulum | 8 | 6 | 34 | 4.71 | 0.36 | 4.32 | 14 |
| Left superior occipital gyrus | −22 | −80 | 32 | 4.18 | 0.87 | 3.9 | 41 |
| Left cerebellum 6 | −22 | −50 | −32 | 4.15 | 0.89 | 3.87 | 20 |
| — | 16 | −50 | −30 | 3.88 | 0.99 | 3.65 | 10 |
| — | −6 | −50 | −24 | 3.86 | > 0.99 | 3.64 | 10 |
| — | 4 | −36 | −42 | 3.85 | > 0.99 | 3.62 | 70 |
| Left cerebellum | −30 | −66 | −20 | 3.80 | > 0.99 | 3.58 | 38 |
| Vermis 6/Vermis 4/5 | 4 | −66 | −10 | 3.66 | > 0.99 | 3.47 | 18 |
BPD = borderline personality disorder; MNI = Montreal Neurological Institute.
According to Automated Anatomic Labelling atlas.
p < 0.05, family-wise error–corrected in bold; all others p < 0.05, uncorrected.
Region of interest analyses were conducted for the hippocampus, amygdala, ACC and hypothalamus (Table 3). Patients with BPD had significantly decreased grey matter concentration in the right and left hippocampus. The spatial location of the between-group difference was similar in both the left and right hippocampus. However, no significant difference was detected in the amygdala and the ACC. In addition, patients with BPD had significantly increased grey matter volume in the left hypothalamus (Fig. 1). Comparisons between subgroups of patients with BPD with and without PTSD did not reveal any significant differences in brain morphology.
Table 3.
Voxel-based morphometric differences between patients with borderline personality disorder and healthy controls in regions of interest analysis
| Contrast; location of peak voxels* | MNI peak voxel | t | p value† | z | Cluster size, k | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| Controls > BPD | |||||||
| Right hippocampus | 28 | −30 | −4 | 6.67 | < 0.001 | 5.75 | 111 |
| Left hippocampus | −26 | −30 | −4 | 4.87 | 0.003 | 4.45 | 53 |
| BPD > controls | |||||||
| Left hypothalamus | −6 | −4 | −4 | 3.57 | 0.023 | 3.39 | 10 |
| −8 | −2 | −8 | 3.42 | 0.033 | 3.25 | ||
| BPD with CTQ covaried | |||||||
| Right hypothalamus | 4 | −6 | −12 | 3.69 | 0.07 | 3.29 | 68 |
| −4 | −6 | −12 | 3.51 | 0.07 | 3.51 | ||
BPD = borderline personality disorder; CTQ = Childhood Trauma Questionnaire;24 MNI = Montreal Neurological Institute.
According to Automated Anatomic Labelling atlas.
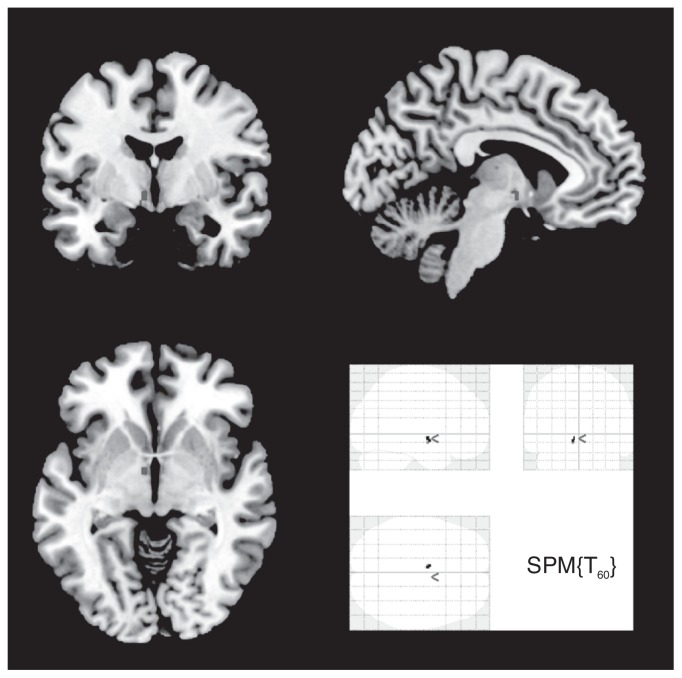
Fig. 1.
Increased grey matter volume in the left hypothalamus of patients with borderline personality disorder compared with healthy controls (region of Interest analysis p≤ 0.05, family-wise error–corrected).
The scores in the CTQ were positively correlated with hypothalamic grey matter volume (r = 0.58, p = 0.001). The spatial location of the correlating areas was similar in the right and left hypothalamus. No correlation was found between CTQ scores and grey matter volumes in the hippocampus, amygdala and ACC. Additional regression analyses revealed no further significant correlations between clinical variables (i.e., IQ, GAF, BDI, BIS, BSL, FAF and STAXI scores) and grey matter volumes in our predefined ROIs in the BPD group.
Discussion
Applying DARTEL VBM, we were able to demonstrate new evidence for increased grey matter volume in the hypothalamus and decreased hippocampal volume in women with BPD compared with healthy women. Covarying for the extent of traumatic childhood experience (CTQ) delivered a positive correlation of hypothalamic volume and CTQ scores in patients with BPD.
Reduction of hippocampal volume has consistently been reported in patients with BPD. Two studies found that hippocampal volume loss was more pronounced in patients with BPD who experienced childhood trauma than in healthy controls34 or patients with BPD who did not experience childhood trauma.35 Hippocampal volume loss has been associated with stress and glucocorticoid admission, which can be explained by a high expression of glucocorticoid receptors in this structure.12,36 The fact that we did not find any correlation between hippocampal grey matter volume and CTQ scores in our patients indicates that the extent of childhood trauma had no direct influence on hippocampal grey matter volume in patients with BPD in our study. This is different from data reported by Dannlowsky and colleagues37 in healthy adults; they found a negative correlation between CTQ scores and hippocampal volume.
In the present study, CTQ scores were positively correlated with grey matter volume in the hypothalamus of patients with BPD, indicating increased hypothalamic volumes in patients with a history of traumatization. According to the atlas by Baroncini and colleagues,38 the alterations found by our group are, in fact, localized in the hypothalamus. To our knowledge, ours is the first study reporting alterations in hypothalamic grey matter volume in patients with BPD. This is astonishing, as the hypothalamus is not only regarded as one of the key structures of central stress regulation, but also represents an important link between the endocrine system and behavioural reactions, such as fight and flight.18
These findings might come about as a consequence of early life stress and may be the result of an interplay of different central nervous structures. In animal studies, early life stress has been reported to lead to increased corticotropin-releasing hormone (CRH) mRNA expression9 and exaggerated glucocorticoid response to subsequent stressors.39 Depressed patients also show heightened CRH mRNA levels,40 and an increased total number of CRH neurons has been reported in patients with mood disorders.41 Similar mechanisms might be involved in traumatized individuals and may account for the hypothalamic grey matter alterations found in our BPD sample. Dysfunctions of the amygdala may be an additional explanation for increased hypothalamic CRH expression. The amygdala evaluates the environment for potential dangers and threats,42 and amygdala activation to threat-related facial expressions correlates with CTQ scores.37 This may suggest that traumatic experiences in childhood lead to a hyper-reagibility of the amygdala to threatening stimuli in adulthood, which has been reported in patients with BPD.43 As the amygdala sustains connections to the hypothalamus, its increased activity could generate an exaggerated stimulation of the HPA axis, increasing glucocorticoid secretion.13 The hippocampus, on the other hand, is a structure particularly damaged by glucocorticoid neurotoxicity due to multiple glucocorticoid receptors.36 Hippocampal damage might lead to the inability of the individual to terminate HPA axis response to stress,13 which is supported by the finding that cortisol response to a stress task correlates with hippocampal volume.44 Taken together, amygdalar hyperactivity and hippocampal damage may lead to increased hypothalamic and HPA axis activity. Increased hypothalamic activity could result in increased grey matter volume and explain HPA axis alterations. The positive correlation between the extent of traumatic childhood experience and hypothalamic grey matter volume supports this hypothesis. Corticotropin-releasing hormone binding sites in the pituitary gland may be reduced to protect the organism from the toxic effects of glucocorti-coids,45 which could explain hypocortisolism in children exposed to traumatic experiences46 and the blunted response of adrenocorticotropic hormone to CRH in traumatized women.9 In fact, pituitary volume seems to be associated with childhood trauma as well as parasuicidal behaviour in teenagers with BPD, which could be the consequence of severe stress during childhood and reflect a hyperactive HPA axis in individuals with this disorder.47,48 Contrary to this explanation and our findings, rodents have shown reduced numbers of cells in the parvocellular part of the hypothalamic paraventricular nucleus after 10 days of neonatal handling.49 Despite this reduction in cell number, the volume of the nuclei remained unchanged, indicating that cell loss does not directly lead to volume loss. It also has to be considered that the rodent brain at birth is far less developed than the human brain, which complicates the translation of animal to human studies.10 Moreover, childhood trauma is more complex than animal models of early stress, and more factors need to be taken into account.50 We did not find a difference in hypothalamic volume between patients with and without PTSD. As only 9 of our 30 patients had a diagnosis of PTSD, the statistical power of this comparison remains weak and deters us from strong conclusions.
Contrary to our expectations, we did not find significant grey matter alterations in the amygdala and ACC. The literature, however, provides heterogeneous results on both structures. Concerning the amygdala, some studies reported a decreased volume,35 some did not find any significant difference51–53 and others revealed increased amygdalar volume in patients with BPD.16 This inconsistency may at least partly be due to small sample sizes, psychotropic medications and comorbidities.11 Zetzsche and colleagues,52 for instance, found increased amygdalar volumes in patients with BPD who had comorbid MDD compared with those without MDD. The heterogeneity of results is even greater for volumetric alterations in the ACC. Recent VBM studies have provided evidence for reduced volumes in a sample of male and female patients with BPD,16,54,55 reduced volumes in male but not in female patients with BPD,17 and no volumetric alterations at all.56 The high morphometric variability of the ACC makes spatial normalization in VBM more difficult and susceptible to errors.57,58 If regional morphological differences exist, these are minimized by the VBM registration algorithm,59 which may explain heterogeneous results for ACC volume in patients with BPD. In addition, age effects resulting from differential developmental effects on brain areas may contribute to heterogeneity in morphometric data in patients with BPD since reduced ACC volume, but no abnormalities of the hippocampus and amygdala, have been reported in adolescent patients with a first presentation of BPD.57,60
The increase in grey matter volume in the putamen of our patients with BPD is consistent with the results of Brambilla and colleagues.34 In this study, alterations in the putamen/basal ganglia were particularly pronounced in patients with BPD and current substance abuse. Current substance abuse was an exclusion criterion in the present study, and comparing participants with remitted substance abuse to those without revealed no significant differences. The putamen and the basal ganglia are involved not only in planning and execution of movement, but also in higher cognitive functions, such as attention and decision-making,61 which at least in some studies have been reported to be impaired in patients with BPD.62
We also found reduced grey matter in the cerebellar vermis. Recent findings suggest a major involvement of cerebellar structures in emotional behaviour,63 which is mediated by reciprocal connections to structures of the limbic system.64 Reduced vermal volume has already been reported in patients with bipolar disorder and PTSD,65,66 suggesting an involvement in the pathology of psychiatric disorders. Schraa-Tam and colleagues67 found prominent activation of the vermis while healthy participants viewed negative facial expressions. The vermis might thus be involved in an individual’s reactions to another person’s negative facial expressions. As patients with BPD are hypervigilant to negative stimuli,68 vermal alterations could represent a morphological correlate of the reactions of patients with BPD to negative facial expressions.
The present study also revealed volumetric alterations in the left inferior parietal gyrus, the left middle temporal gyrus and the right Heschl gyrus. Reductions in the inferior parietal lobe are in line with the findings of Brunner and colleagues69 in adolescent patients with BPD. In a recently published study, patients with BPD showed decreased connectivity of the left inferior parietal lobe and right middle temporal regions compared with healthy controls,70 which was interpreted as a biological substrate of a decreased attentional capacity for relevant somatosensory stimuli. Changes in grey matter volume in the parietal lobe could explain attentional capacity deficits described in patients with BPD.62 The Heschl gyrus is located in the primary auditory cortex and is involved in the processing of auditory information.71 Studies addressing the involvement of the auditory system in patients with BPD have been scarce. However, there is evidence for auditory processing abnormalities in children with BPD.72 Comparable with our findings, Goldstein and colleagues73 reported increased grey matter volume in the Heschl gyrus of patients with BPD, which might reflect compensatory mechanisms for dysfunctions in the processing of auditory information. The middle temporal gyrus is another structure involved in auditory processing,71 in which volumetric reductions have previously been reported in patients with BPD.17 Taken together, these findings suggest alterations in structures of the central auditory system in patients with BPD that need to be addressed in further studies.
Our study has several strengths, as it expands on other studies in the field by investigating volumetric alterations in central stress-regulating structures of female patients with BPD. We used DARTEL VBM, a fully automated and sensitive technique, which is independent of rater inaccuracy and biases, to assess morphometric alterations between well-matched and relatively large, unmedicated samples of women with and without BPD.
Limitations
Some limitations have to be addressed. First, even though our study has a reasonable sample size, negative results could be a consequence of small statistical power. Second, our findings are limited by the lack of measures of CRH, adrenocorticotropic hormone and cortisol levels. Further studies are thus needed to replicate our findings and to address the association between hypothalamic volume and HPA axis activity and reactivity to stress. Third, psychiatric comorbidities were prevalent in our sample and might have confounded the results. The high frequency and number of comorbidities in patients with BPD, however, belongs to the clinical picture of this disorder, and excluding patients with comorbid disorders would not be representative of the clinical population. Future studies should therefore include clinical control groups and compare grey matter volumes of patients with BPD and patients with MDD or PTSD to examine the specificity of volumetric alterations in central stress-regulating structures, such as the hypothalamus and hippocampus. The hypothalamus is a structure that contains many specialized cell groups,38 and future studies should investigate alterations of these different subgroups in particular.
Conclusion
Taken together, our results provide new evidence for grey matter alterations in the hypothalamus and replicate previously reported decrements in hippocampal grey matter in patients with BPD that may be induced by experiences of early life stress. As there have been no other studies on hypothalamic volume and activity in patients with BPD, more research is needed to investigate the nature of this finding. Understanding the role of the hypothalamus and other structures of the stress-regulating system could help us to further understand the neurobiological underpinnings of this complex disorder.
Acknowledgements
The authors are grateful to the Department of Neuroradiology, in particular to Dr. Sabine Heiland, Dr. Martin Bendszus, Dipl.-Ing. Thorsten Kästel and Dipl.-Ing. Marcel Prager for supporting the MRI measurements. This study was supported by the grant 01GW0784 of the German Ministry of Research awarded to S.C. Herpertz.
Footnotes
A. Kuhlmann and K. Bertsch share first authorship.
Competing interests: I. Schmidinger declares having received grant support through her institution from the German Federal Ministry of Education and Research (BMBF). As above for S.C. Herpertz. None declared for A. Kuhlmann, K. Bertsch and P.A. Thomann.
Contributors: K Bertsch and S.C. Herpertz designed the study. A. Kuhlmann, K. Bertsch and I. Schmidinger acquired the data, which were analyzed by A. Kuhlmann, K. Bertsch, P.A. Thomann and S.C. Herpertz. A. Kuhlmann and K. Bertsch wrote the article, which all authors reviewed and approved for publication.
References
- 1.Zanarini MC, Williams AA, Lewis RE, et al. Reported pathological childhood experiences associated with the development of borderline personality disorder. Am J Psychiatry. 1997;154:1101–6. doi: 10.1176/ajp.154.8.1101. [DOI] [PubMed] [Google Scholar]
- 2.Jovev M, Jackson HJ. The relationship of borderline personality disorder, life events and functioning in an Australian psychiatric sample. J Pers Disord. 2006;20:205–17. doi: 10.1521/pedi.2006.20.3.205. [DOI] [PubMed] [Google Scholar]
- 3.Kuo JR, Linehan MM. Disentangling emotion processes in borderline personality disorder: physiological and self-reported assessment of biological vulnerability, baseline intensity, and reactivity to emotionally evocative stimuli. J Abnorm Psychol. 2009;118:531–44. doi: 10.1037/a0016392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stiglmayr CE, Ebner-Priemer UW, Bretz J, et al. Dissociative symptoms are positively related to stress in borderline personality disorder. Acta Psychiatr Scand. 2008;117:139–47. doi: 10.1111/j.1600-0447.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 5.Simeon D, Knutelska M, Smith L, et al. A preliminary study of cortisol and norepinephrine reactivity to psychosocial stress in borderline personality disorder with high and low dissociation. Psychiatry Res. 2007;149:177–84. doi: 10.1016/j.psychres.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Ludascher Ludäscher P, Bohus M, Lieb K, et al. Elevated pain thresholds correlate with dissociation and aversive arousal in patients with borderline personality disorder. Psychiatry Res. 2007;149:291–6. doi: 10.1016/j.psychres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Kleindienst N, Bohus M, Ludascher P, et al. Motives for nonsuicidal self-injury among women with borderline personality disorder. J Nerv Ment Dis. 2008;196:230–6. doi: 10.1097/NMD.0b013e3181663026. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman DJ, Choi-Kain LW. The hypothalamic-pituitary-adrenal axis in borderline personality disorder: a review. Harv Rev Psychiatry. 2009;17:167–83. doi: 10.1080/10673220902996734. [DOI] [PubMed] [Google Scholar]
- 9.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 10.Lupien SJ, McEwen BS, Gunnar MR, et al. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 11.Nunes PM, Wenzel A, Borges KT, et al. Volumes of the hippocampus and amygdala in patients with borderline personality disorder: a meta-analysis. J Pers Disord. 2009;23:333–45. doi: 10.1521/pedi.2009.23.4.333. [DOI] [PubMed] [Google Scholar]
- 12.Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci U S A. 1984;81:6174–7. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herman JP, Figueiredo H, Mueller NK, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo–pituitary–adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Prehn K, Schulze L, Roßmann S, et al. Effects of emotional stimuli on working memory processes in male criminal offenders with borderline and antisocial personality disorder. World J Psychiatr. 2012 Mar 1; doi: 10.3109/15622975.2011.584906. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Mauchnik J, Schmahl C. The latest neuroimaging findings in borderline personality disorder. Curr Psychiatry Rep. 2010;12:46–55. doi: 10.1007/s11920-009-0089-7. [DOI] [PubMed] [Google Scholar]
- 16.Minzenberg MJ, Fan J, New AS, et al. Frontolimbic structural changes in borderline personality disorder. J Psychiatr Res. 2008;42:727–33. doi: 10.1016/j.jpsychires.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soloff P, Nutche J, Goradia D, et al. Structural brain abnormalities in borderline personality disorder: a voxel-based morphometry study. Psychiatry Res. 2008;164:223–636. doi: 10.1016/j.pscychresns.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruk MR, Westphal K, Van Erp A, et al. The hypothalamus: crossroads of endocrine and behavioural regulation in grooming and aggression. Neurosci Biobehav Rev. 1998;23:163–77. doi: 10.1016/s0149-7634(98)00018-9. [DOI] [PubMed] [Google Scholar]
- 19.Ashburner J, Friston KJ. Voxel-based morphometry — the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 20.Pruessner JC, Dedovic K, Pruessner M, et al. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations — 2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35:179–91. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA. Beck-Depressions-Inventar (BDI): Testhandbuch. Bern, Switzerland: Huber; 1994. [Google Scholar]
- 22.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Bohus M, Limberger M, Frank U, et al. Psychometric properties of the Borderline Symptom List (BSL) Psychopathology. 2007;40:126–32. doi: 10.1159/000098493. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein DP, Fink L. Childhood Trauma Questionnaire A retrospective self-report manual. San Antonio (TX): The Psychological Corporation; 1998. [Google Scholar]
- 25.Hampel R, Selg H. Fragebogen zur Erfassung von Aggressivitätsfaktoren. [Questionnaire for the Assessment of Factors of Aggression]. Göttingen: Hogrefe; 1975. [Google Scholar]
- 26.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV (SCID-I) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 27.Loranger E. International Personality Disorder Examination (IPDE): DSM-IV and ICD-10 modules. Odessa (FL): Psychological Assessment Resources; 1999. [Google Scholar]
- 28.Spielberger CD, Johnson EH, Russell SF, et al. The experience and expression of anger; construction and validation of an Anger Expression Scale. In: Chesney MA, Rosenman RH, editors. Anger and hostility in cardiovascular and behavioral disorders. Washington: Hemisphere; 1985. pp. 5–30. [Google Scholar]
- 29.Tewes U. Hamburg-Wechsler-Intelligenztest für Erwachsene (HAWIE-R) Bern, Switzerland: Huber; 1991. [Google Scholar]
- 30.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Bergouignan L, Chupin M, Czechowska Y, et al. Can voxel based morphometry, manual segmentation and automated segmentation equally detect hippocampal volume differences in acute depression? Neuroimage. 2009;45:29–37. doi: 10.1016/j.neuroimage.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Pereira JM, Xiong L, Acosta-Cabronero J, et al. Registration accuracy for VBM studies varies according to region and degenerative disease grouping. Neuroimage. 2010;49:2205–15. doi: 10.1016/j.neuroimage.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 33.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Brambilla P, Soloff PH, Sala M, et al. Anatomical MRI study of borderline personality disorder patients. Psychiatry Res. 2004;131:125–33. doi: 10.1016/j.pscychresns.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Driessen M, Herrmann J, Stahl K, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000;57:1115–22. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 36.Uno H, Tarara R, Else JG, et al. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–11. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–93. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 38.Baroncini M, Jissendi P, Balland E, et al. MRI atlas of the human hypothalamus. Neuroimage. 2012;59:168–80. doi: 10.1016/j.neuroimage.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Bremner JD, Randall P, Verrnetten E, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse — a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, et al. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. Am J Psychiatry. 1995;152:1372–6. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- 41.Bao AM, Hestiantoro A, Van Someren EJ, et al. Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain. 2005;128(Pt 6):1301–13. doi: 10.1093/brain/awh448. [DOI] [PubMed] [Google Scholar]
- 42.Amaral DG. The amygdala, social behavior, and danger detection. Ann N Y Acad Sci. 2006;1000:337–47. doi: 10.1196/annals.1280.015. 2003. [DOI] [PubMed] [Google Scholar]
- 43.Herpertz SC, Dietrich TM, Wenning B, et al. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol Psychiatry. 2001;50:292–8. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- 44.Pruessner JC, Baldwin MW, Dedovic K, et al. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. Neuroimage. 2005;28:815–26. doi: 10.1016/j.neuroimage.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Anisman H, Zaharia MD, Meaney M, et al. Do early life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998;16:149–64. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 46.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 47.Garner B, Chanen AM, Phillips L, et al. Pituitary volume in teenagers with first-presentation borderline personality disorder. Psychiatry Res. 2007;156:257–61. doi: 10.1016/j.pscychresns.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Jovev M, Garner B, Phillips L, et al. An MRI study of pituitary volume and parasuicidal behavior in teenagers with first-presentation borderline personality disorder. Psychiatry Res. 2008;162:273–7. doi: 10.1016/j.pscychresns.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Winkelmann-Duarte EC, Todeschin AS, Fernandes MC, et al. Plastic changes induced by neonatal handling in the hypothalamus of female rats. Brain Res. 2007;1170:20–30. doi: 10.1016/j.brainres.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 50.Bremner JD, Vermetten E. Stress and development: behavioral behavioral and biological consequences. Dev Psychopathol. 2001;13:473–89. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- 51.New AS, Hazlett EA, Buchsbaum MS, et al. Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology. 2007;32:1629–40. doi: 10.1038/sj.npp.1301283. [DOI] [PubMed] [Google Scholar]
- 52.Zetzsche T, Frodl T, Preuss UW, et al. Amygdala volume and depressive symptoms in patients with borderline personality disorder. Biol Psychiatry. 2006;60:302–10. doi: 10.1016/j.biopsych.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 53.Chanen AM, Velakoulis D, Carison K, et al. Orbitofrontal, amygdala and hippocampal volumes in teenagers with first-presentation borderline personality disorder. Psychiatry Res. 2008;163:116–25. doi: 10.1016/j.pscychresns.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Hazlett EA, New AS, Newmark R, et al. Reduced anterior and posterior cingulate gray matter in borderline personality disorder. Biol Psychiatry. 2005;58:614–23. doi: 10.1016/j.biopsych.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 55.Soloff PH, Pruitt P, Sharma M, et al. Structural brain abnormalities and suicidal behavior in borderline personality disorder. J Psychiatr Res. 2012;46:516–25. doi: 10.1016/j.jpsychires.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rüsch N, van Elst LT, Ludaescher P, et al. A voxel-based morphometric MRI study in female patients with borderline personality disorder. Neuroimage. 2003;20:385–92. doi: 10.1016/s1053-8119(03)00297-0. [DOI] [PubMed] [Google Scholar]
- 57.Whittle S, Chanen AM, Fornito A, et al. Anterior cingulate volume in adolescents with first-presentation borderline personality disorder. Psychiatry Res. 2009;172:155–60. doi: 10.1016/j.pscychresns.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Yücel M, Stuart GW, Maruff P, et al. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: an MRI morphometric study. Cereb Cortex. 2001;11:17–25. doi: 10.1093/cercor/11.1.17. [DOI] [PubMed] [Google Scholar]
- 59.Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images”. Neuroimage. 2001;14:1454–62. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- 60.Goodman M, Hazlett EA, Avedon JB, et al. Anterior cingulate volume reduction in adolescents with borderline personality disorder and co-morbid major depression. J Psychiatr Res. 2011;45:803–7. doi: 10.1016/j.jpsychires.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 61.Stocco A, Lebiere C, Anderson JR. Conditional routing of information to the cortex: a model of the basal ganglia’s role in cognitive coordination. Psychol Rev. 2010;117:541–74. doi: 10.1037/a0019077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dell’Osso B, Berlin HA, Serati M, et al. Neuropsychobiological aspects, comorbidity patterns and dimensional models in borderline personality disorder. Neuropsychobiology. 2010;61:169–79. doi: 10.1159/000297734. [DOI] [PubMed] [Google Scholar]
- 63.Sacchetti B, Scelfo B, Strata P. Cerebellum and emotional behavior. Neuroscience. 2009;162:756–62. doi: 10.1016/j.neuroscience.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 64.Heath RG, Harper JW. Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: evoked potential and histological studies. Exp Neurol. 1974;45:268–87. doi: 10.1016/0014-4886(74)90118-6. [DOI] [PubMed] [Google Scholar]
- 65.Baldaçara L, Nery-Fernandes F, Rocha M, et al. Is cerebellar volume related to bipolar disorder? J Affect Disord. 2011;135:305–9. doi: 10.1016/j.jad.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 66.Baldaçcara L, Jackowski AP, Schoedl A, et al. Reduced cerebellar left hemisphere and vermal volume in adults with PTSD from a community sample. J Psychiatr Res. 2011;45:1627–33. doi: 10.1016/j.jpsychires.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 67.Schraa-Tam CK, Rietdijk WJ, Verbeke WJ, et al. fMRI activities in the emotional cerebellum: a preference for negative stimuli and goal-directed behavior. Cerebellum. 2012;11:233–45. doi: 10.1007/s12311-011-0301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herpertz SC, Gretzer A, Steinmeyer EM, et al. Affective instability and impulsivity in personality disorder. Results of an experimental study. J Affect Disord. 1997;44:31–7. doi: 10.1016/s0165-0327(97)01444-4. [DOI] [PubMed] [Google Scholar]
- 69.Brunner R, Henze R, Parzer P, et al. Reduced prefrontal and orbitofrontal gray matter in female adolescents with borderline personality disorder: Is it disorder specific? Neuroimage. 2010;49:114–20. doi: 10.1016/j.neuroimage.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 70.Wolf RC, Sambataro F, Vasic N, et al. Aberrant connectivity of resting-state networks in borderline personality disorder. J Psychiatry Neurosci. 2011;36:402–11. doi: 10.1503/jpn.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brancucci A, Franciotti R, D’Anselmo A, et al. The sound of consciousness: neural underpinnings of auditory perception. J Neurosci. 2011;31:16611–8. doi: 10.1523/JNEUROSCI.3949-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lincoln AJ, Bloom D, Katz M, et al. Neuropsychological and neurophysiological indices of auditory processing impairment in children with multiple complex developmental disorder. J Am Acad Child Adolesc Psychiatry. 1998;37:100–12. doi: 10.1097/00004583-199801000-00023. [DOI] [PubMed] [Google Scholar]
- 73.Goldstein KE, Hazlett EA, New AS, et al. Smaller superior temporal gyrus volume specificity in schizotypal personality disorder. Schizophr Res. 2009;112:14–23. doi: 10.1016/j.schres.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]