Abstract
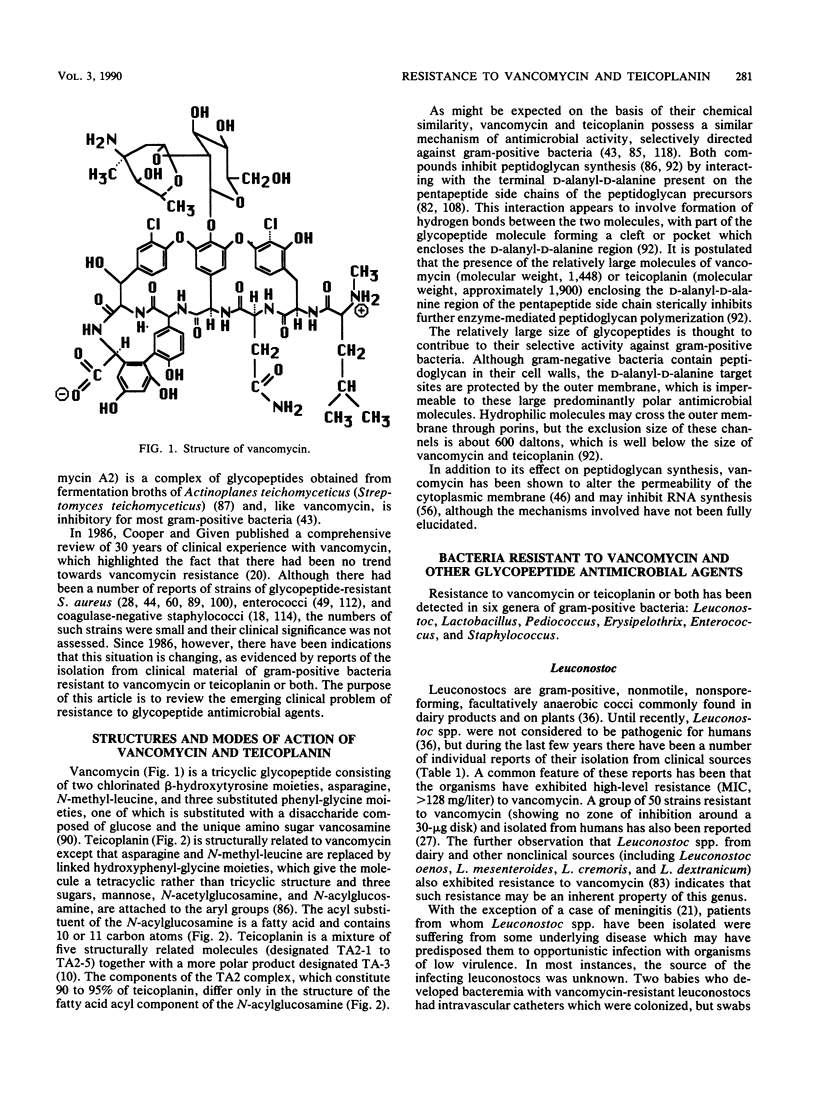
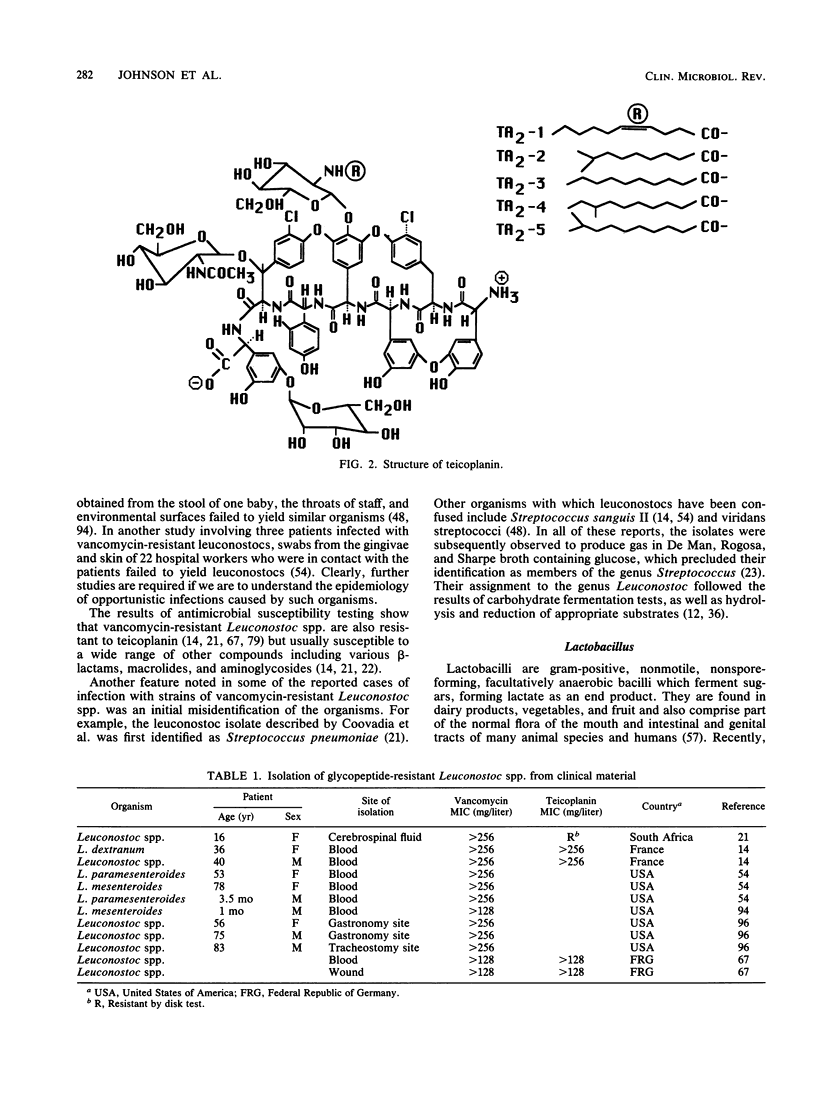
Vancomycin and teicoplanin are glycopeptides active against a wide range of gram-positive bacteria. For 30 years following the discovery of vancomycin in 1956, vancomycin resistance was not detected among normally susceptible bacteria recovered from human specimens. Since 1986, however, bacteria resistant to vancomycin or teicoplanin or both have been described. Strains of the genera Leuconostoc, Lactobacillus, Pediococcus, and Erysipelothrix seem inherently resistant to glycopeptides. Species and strains of enterococci and coagulase-negative staphylococci appear to have acquired or developed resistance. There are at least two categories of glycopeptide resistance among enterococci, characterized by either high-level resistance to vancomycin (MIC, greater than or equal to 64 mg/liter) and teicoplanin (MIC, greater than or equal to 8 mg/liter) or lower-level vancomycin resistance (MIC, 32 to 64 mg/liter) and teicoplanin susceptibility (MIC, less than or equal to 1 mg/liter). The two categories appear to have similar resistance mechanisms, although genetic and biochemical studies indicate that they have arisen independently. Among coagulase-negative staphylococci, strains for which vancomycin MICs are up to 20 mg/liter or teicoplanin MICs are 16 to 32 mg/liter have been reported, but cross-resistance between these glycopeptides varies. The selective advantage accorded to glycopeptide-resistant bacteria and the observation that high-level resistance in enterococci is transferable suggest that such resistance may be expected to increase in incidence. Clinicians and microbiologists need to be aware of this emerging problem.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel G. B., Neu H. C. The nephrotoxicity of antimicrobial agents (second of three parts). N Engl J Med. 1977 Mar 31;296(13):722–728. doi: 10.1056/NEJM197703312961305. [DOI] [PubMed] [Google Scholar]
- BLOWERS R., MASON G. A., WALLACE K. R., WALTON M. Control of wound infection in a thoracic surgery unit. Lancet. 1955 Oct 15;269(6894):786–794. doi: 10.1016/s0140-6736(55)92385-0. [DOI] [PubMed] [Google Scholar]
- Baker C. N., Thornsberry C. Antimicrobial susceptibility of Streptococcus mutans isolated from patients with endocarditis. Antimicrob Agents Chemother. 1974 Mar;5(3):268–271. doi: 10.1128/aac.5.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Jones R. N. Evaluation of teicoplanin and vancomycin disk susceptibility tests. J Clin Microbiol. 1986 Jan;23(1):100–103. doi: 10.1128/jcm.23.1.100-103.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E., Lambert-Zechovsky N., Mariani-Kurkdjian P., Cezard J. P., Navarro J. Bacteremia caused by a vancomycin-resistant Enterococcus. Pediatr Infect Dis J. 1989 Jul;8(7):475–476. doi: 10.1097/00006454-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Borghi A., Coronelli C., Faniuolo L., Allievi G., Pallanza R., Gallo G. G. Teichomycins, new antibiotics from Actinoplanes teichomyceticus nov. sp. IV. Separation and characterization of the components of teichomycin (teicoplanin). J Antibiot (Tokyo) 1984 Jun;37(6):615–620. doi: 10.7164/antibiotics.37.615. [DOI] [PubMed] [Google Scholar]
- Bourgault A. M., Wilson W. R., Washington J. A., 2nd Antimicrobial susceptibilities of species of viridans streptococci. J Infect Dis. 1979 Sep;140(3):316–321. doi: 10.1093/infdis/140.3.316. [DOI] [PubMed] [Google Scholar]
- Bridge P. D., Sneath P. H. Numerical taxonomy of Streptococcus. J Gen Microbiol. 1983 Mar;129(3):565–597. doi: 10.1099/00221287-129-3-565. [DOI] [PubMed] [Google Scholar]
- Burdon D. W. Treatment of pseudomembranous colitis and antibiotic-associated diarrhoea. J Antimicrob Chemother. 1984 Dec;14 (Suppl 500):103–109. doi: 10.1093/jac/14.suppl_d.103. [DOI] [PubMed] [Google Scholar]
- Buu-Hoï A., Branger C., Acar J. F. Vancomycin-resistant streptococci or Leuconostoc sp. Antimicrob Agents Chemother. 1985 Sep;28(3):458–460. doi: 10.1128/aac.28.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calain P., Krause K. H., Vaudaux P., Auckenthaler R., Lew D., Waldvogel F., Hirschel B. Early termination of a prospective, randomized trial comparing teicoplanin and flucloxacillin for treating severe staphylococcal infections. J Infect Dis. 1987 Feb;155(2):187–191. doi: 10.1093/infdis/155.2.187. [DOI] [PubMed] [Google Scholar]
- Calderwood S. A., Wennersten C., Moellering R. C., Jr, Kunz L. J., Krogstad D. J. Resistance to six aminoglycosidic aminocyclitol antibiotics among enterococci: prevalence, evolution, and relationship to synergism with penicillin. Antimicrob Agents Chemother. 1977 Sep;12(3):401–405. doi: 10.1128/aac.12.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. Y., Williams J. D. Transferable resistance and aminoglycoside-modifying enzymes in enterococci. J Med Microbiol. 1985 Oct;20(2):187–196. doi: 10.1099/00222615-20-2-187. [DOI] [PubMed] [Google Scholar]
- Cherubin C. E., Corrado M. L., Sierra M. F., Gombert M. E., Shulman M. Susceptibility of gram-positive cocci to various antibiotics, including cefotaxime, moxalactam, and N-formimidoyl thienamycin. Antimicrob Agents Chemother. 1981 Oct;20(4):553–555. doi: 10.1128/aac.20.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986;40:635–659. doi: 10.1146/annurev.mi.40.100186.003223. [DOI] [PubMed] [Google Scholar]
- Coovadia Y. M., Solwa Z., van den Ende J. Meningitis caused by vancomycin-resistant Leuconostoc sp. J Clin Microbiol. 1987 Sep;25(9):1784–1785. doi: 10.1128/jcm.25.9.1784-1785.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Wennersten C., Zighelboim-Daum S., Reiszner E., Goldmann D., Moellering R. C., Jr High-level resistance to gentamicin in clinical isolates of Streptococcus (Enterococcus) faecium. Antimicrob Agents Chemother. 1988 Oct;32(10):1528–1532. doi: 10.1128/aac.32.10.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Willey S., Reiszner E., Spitzer P. G., Caputo G., Moellering R. C., Jr In vitro and in vivo activity of LY 146032, a new cyclic lipopeptide antibiotic. Antimicrob Agents Chemother. 1986 Oct;30(4):532–535. doi: 10.1128/aac.30.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eykyn S., Phillips I., Evans J. Vancomycin for staphylococcal shunt site infections in patients on regular haemodialysis. Br Med J. 1970 Jul 11;3(5714):80–82. doi: 10.1136/bmj.3.5714.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAIRBROTHER R. W., WILLIAMS B. L. Two new antibiotics; antibacterial activity of novobiocin and vancomycin. Lancet. 1956 Dec 8;271(6954):1177–1178. doi: 10.1016/s0140-6736(56)90052-6. [DOI] [PubMed] [Google Scholar]
- FINLAND M. Emergence of antibiotic-resistant bacteria. N Engl J Med. 1955 Nov 24;253(21):909–contd. doi: 10.1056/NEJM195511242532105. [DOI] [PubMed] [Google Scholar]
- FINLAND M. Emergence of antibiotic-resistant bacteria. N Engl J Med. 1955 Dec 1;253(22):969–contd. doi: 10.1056/NEJM195512012532206. [DOI] [PubMed] [Google Scholar]
- FINLAND M. Emergence of antibiotic-resistant bacteria. N Engl J Med. 1955 Dec 8;253(23):1019–concl. doi: 10.1056/NEJM195512082532306. [DOI] [PubMed] [Google Scholar]
- FINLAND M., HAIGHT T. H. Antibiotic resistance of pathogenic staphylococci; study of five hundred strains isolated at Boston City Hospital from October, 1951, to February, 1952. AMA Arch Intern Med. 1953 Feb;91(2):143–158. doi: 10.1001/archinte.1953.00240140003001. [DOI] [PubMed] [Google Scholar]
- Facklam R., Hollis D., Collins M. D. Identification of gram-positive coccal and coccobacillary vancomycin-resistant bacteria. J Clin Microbiol. 1989 Apr;27(4):724–730. doi: 10.1128/jcm.27.4.724-730.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekety R., Silva J., Buggy B., Deery H. G. Treatment of antibiotic-associated colitis with vancomycin. J Antimicrob Chemother. 1984 Dec;14 (Suppl 500):97–102. doi: 10.1093/jac/14.suppl_d.97. [DOI] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARROD L. P., WATERWORTH P. M. Behaviour in vitro of some new antistaphylococcal antibiotics. Br Med J. 1956 Jul 14;2(4984):61–65. doi: 10.1136/bmj.2.4984.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R. C., Uttley A. H. Susceptibility of enterococci and epidemiology of enterococcal infection in the 1980s. Epidemiol Infect. 1989 Dec;103(3):403–413. doi: 10.1017/s0950268800030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraci J. E., Hermans P. E. Vancomycin. Mayo Clin Proc. 1983 Feb;58(2):88–91. [PubMed] [Google Scholar]
- Gorby G. L., Peacock J. E., Jr Erysipelothrix rhusiopathiae endocarditis: microbiologic, epidemiologic, and clinical features of an occupational disease. Rev Infect Dis. 1988 Mar-Apr;10(2):317–325. doi: 10.1093/clinids/10.2.317. [DOI] [PubMed] [Google Scholar]
- Gorzynski E. A., Amsterdam D., Beam T. R., Jr, Rotstein C. Comparative in vitro activities of teicoplanin, vancomycin, oxacillin, and other antimicrobial agents against bacteremic isolates of gram-positive cocci. Antimicrob Agents Chemother. 1989 Nov;33(11):2019–2022. doi: 10.1128/aac.33.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A. C., Lacey R. W., Brownjohn A. M., Turney J. H. Teicoplanin-resistant coagulase-negative staphylococcus. Lancet. 1986 Nov 15;2(8516):1166–1167. doi: 10.1016/s0140-6736(86)90580-5. [DOI] [PubMed] [Google Scholar]
- Greenwood D. Microbiological properties of teicoplanin. J Antimicrob Chemother. 1988 Jan;21 (Suppl A):1–13. doi: 10.1093/jac/21.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- HINTON N. A., ORR J. H. Studies on the incidence and distribution of antibiotic-resistant Staphylococci. J Lab Clin Med. 1957 Apr;49(4):566–572. [PubMed] [Google Scholar]
- HOWE C. W. Postoperative wound infections due to Staphylococcus aureus. N Engl J Med. 1954 Sep 9;251(11):411–417. doi: 10.1056/NEJM195409092511101. [DOI] [PubMed] [Google Scholar]
- Hancock R., Fitz-James P. C. Some differences in the action of penicillin, bacitracin, and vancomycin on Bacillus megaterium. J Bacteriol. 1964 May;87(5):1044–1050. doi: 10.1128/jb.87.5.1044-1050.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S., Ruoff K. L., Catlin E. A., Ignacio Santos J. Catheter-associated infection with a vancomycin-resistant gram-positive coccus of the Leuconostoc sp. Pediatr Infect Dis J. 1988 Jul;7(7):519–520. doi: 10.1097/00006454-198807000-00018. [DOI] [PubMed] [Google Scholar]
- Harwick H. J., Kalmanson G. M., Guze L. B. In vitro activity of ampicillin or vancomycin combined with gentamicin or streptomycin against enterococci. Antimicrob Agents Chemother. 1973 Oct;4(4):383–387. doi: 10.1128/aac.4.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy D. P., Polk R. E., Garson M. L., Rock D. T., Comstock T. J. Comparison of steady-state pharmacokinetics of two dosage regimens of vancomycin in normal volunteers. Antimicrob Agents Chemother. 1987 Mar;31(3):393–397. doi: 10.1128/aac.31.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliman R. E., Bone G. P. Vancomycin resistance of clinical isolates of lactobacilli. J Infect. 1988 May;16(3):279–283. doi: 10.1016/s0163-4453(88)97676-1. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bieth G., Delbos F. High-level, plasmid-borne resistance to gentamicin in Streptococcus faecalis subsp. zymogenes. Antimicrob Agents Chemother. 1979 Nov;16(5):686–689. doi: 10.1128/aac.16.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz H. W., Handwerger S., van Horn K. G., Wormser G. P. Leuconostoc, an emerging vancomycin-resistant pathogen. Lancet. 1987 Dec 5;2(8571):1329–1330. doi: 10.1016/s0140-6736(87)91217-7. [DOI] [PubMed] [Google Scholar]
- JORDAN D. C., INNISS W. E. Selective inhibition of ribonucleic acid synthesis in Staphylococcus aureus by vancomycin. Nature. 1959 Dec 12;184(Suppl 24):1894–1895. doi: 10.1038/1841894b0. [DOI] [PubMed] [Google Scholar]
- KNIGHT V., HOLZER A. R. Studies on staphylococci from hospital patients. I. Predominance of strains of group III phage patterns which are resistant to multiple antibiotics. J Clin Invest. 1954 Sep;33(9):1190–1198. doi: 10.1172/JCI102992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. H., Gilligan P. H., Facklam R. R. Recovery of resistant enterococci during vancomycin prophylaxis. J Clin Microbiol. 1988 Jun;26(6):1216–1218. doi: 10.1128/jcm.26.6.1216-1218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchmer A. W., Archer G. L., Dismukes W. E. Staphylococcus epidermidis causing prosthetic valve endocarditis: microbiologic and clinical observations as guides to therapy. Ann Intern Med. 1983 Apr;98(4):447–455. doi: 10.7326/0003-4819-98-4-447. [DOI] [PubMed] [Google Scholar]
- Klastersky J., Beumer J., Daneau D. Bacteriophage types and antibiotic susceptibility of Staphylococcus aureus. Appl Microbiol. 1971 Dec;22(6):1000–1007. doi: 10.1128/am.22.6.1000-1007.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWBURY E. J. Clinical problems of drug-resistant pathogens. Br Med Bull. 1960 Jan;16:73–78. doi: 10.1093/oxfordjournals.bmb.a069796. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988 Jul 21;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Weber M., Duval J., Courvalin P. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1989 Jan;33(1):10–15. doi: 10.1128/aac.33.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz L. D., Saunders J., Chalkley L. J., Koornhof H. J. In vitro selection of bacteria resistant to LY146032, a new cyclic lipopeptide. Antimicrob Agents Chemother. 1988 Jan;32(1):24–26. doi: 10.1128/aac.32.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütticken R., Kunstmann G. Vancomycin-resistant Streptococcaceae from clinical material. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Jan;267(3):379–382. doi: 10.1016/s0176-6724(88)80054-3. [DOI] [PubMed] [Google Scholar]
- Maple P. A., Hamilton-Miller J. M., Brumfitt W. World-wide antibiotic resistance in methicillin-resistant Staphylococcus aureus. Lancet. 1989 Mar 11;1(8637):537–540. doi: 10.1016/s0140-6736(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Mastro T. D., Spika J. S., Lozano P., Appel J., Facklam R. R. Vancomycin-resistant Pediococcus acidilactici: nine cases of bacteremia. J Infect Dis. 1990 May;161(5):956–960. doi: 10.1093/infdis/161.5.956. [DOI] [PubMed] [Google Scholar]
- McHenry M. C., Gavan T. L. Vancomycin. Pediatr Clin North Am. 1983 Feb;30(1):31–47. doi: 10.1016/s0031-3955(16)34318-8. [DOI] [PubMed] [Google Scholar]
- Mickelsen P. A., Plorde J. J., Gordon K. P., Hargiss C., McClure J., Schoenknecht F. D., Condie F., Tenover F. C., Tompkins L. S. Instability of antibiotic resistance in a strain of Staphylococcus epidermidis isolated from an outbreak of prosthetic valve endocarditis. J Infect Dis. 1985 Jul;152(1):50–58. doi: 10.1093/infdis/152.1.50. [DOI] [PubMed] [Google Scholar]
- Miniter P. M., Patterson T. F., Johnson M. A., Andriole V. T. Activity of LY146032 in vitro and in experimental enterococcal pyelonephritis. Antimicrob Agents Chemother. 1987 Aug;31(8):1199–1203. doi: 10.1128/aac.31.8.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse G. D., Farolino D. F., Apicella M. A., Walshe J. J. Comparative study of intraperitoneal and intravenous vancomycin pharmacokinetics during continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother. 1987 Feb;31(2):173–177. doi: 10.1128/aac.31.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Tsao J., Panida J. Enterococci from Bangkok, Thailand, with high-level resistance to currently available aminoglycosides. Antimicrob Agents Chemother. 1983 Jun;23(6):799–802. doi: 10.1128/aac.23.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newfield P., Roizen M. F. Hazards of rapid administration of vancomycin. Ann Intern Med. 1979 Oct;91(4):581–581. doi: 10.7326/0003-4819-91-4-581. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Cole C. T., Preston D. A., Schabel A. A., Nagarajan R. Activity of glycopeptides against vancomycin-resistant gram-positive bacteria. Antimicrob Agents Chemother. 1989 Sep;33(9):1477–1481. doi: 10.1128/aac.33.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Wu C. Y., Hobbs J. N., Jr, Preston D. A., Allen N. E. Characterization of vancomycin resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob Agents Chemother. 1989 Jul;33(7):1121–1124. doi: 10.1128/aac.33.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H. E., Sørensen I., Hansen H. E. Peritoneal transport of vancomycin during peritoneal dialysis. Nephron. 1979;24(6):274–277. doi: 10.1159/000181735. [DOI] [PubMed] [Google Scholar]
- Nieto M., Perkins H. R. Modifications of the acyl-D-alanyl-D-alanine terminus affecting complex-formation with vancomycin. Biochem J. 1971 Aug;123(5):789–803. doi: 10.1042/bj1230789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orberg P. K., Sandine W. E. Common occurrence of plasmid DNA and vancomycin resistance in Leuconostoc spp. Appl Environ Microbiol. 1984 Dec;48(6):1129–1133. doi: 10.1128/aem.48.6.1129-1133.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSDORF R. G., CURTIN J. A., BENNETT I. L., Jr The sensitivity of two hundred strains of hemolytic Staphylococcus to a series of antibiotics. AMA Arch Intern Med. 1957 Dec;100(6):927–936. doi: 10.1001/archinte.1957.00260120071008. [DOI] [PubMed] [Google Scholar]
- Pallanza R., Berti M., Goldstein B. P., Mapelli E., Randisi E., Scotti R., Arioli V. Teichomycin: in-vitro and in-vivo evaluation in comparison with other antibiotics. J Antimicrob Chemother. 1983 May;11(5):419–425. doi: 10.1093/jac/11.5.419. [DOI] [PubMed] [Google Scholar]
- Parenti F., Beretta G., Berti M., Arioli V. Teichomycins, new antibiotics from Actinoplanes teichomyceticus Nov. Sp. I. Description of the producer strain, fermentation studies and biological properties. J Antibiot (Tokyo) 1978 Apr;31(4):276–283. doi: 10.7164/antibiotics.31.276. [DOI] [PubMed] [Google Scholar]
- Parenti F. Structure and mechanism of action of teicoplanin. J Hosp Infect. 1986 Mar;7 (Suppl A):79–83. doi: 10.1016/0195-6701(86)90011-3. [DOI] [PubMed] [Google Scholar]
- Patterson J. E., Masecar B. L., Kauffman C. A., Schaberg D. R., Hierholzer W. J., Jr, Zervos M. J. Gentamicin resistance plasmids of enterococci from diverse geographic areas are heterogeneous. J Infect Dis. 1988 Jul;158(1):212–216. doi: 10.1093/infdis/158.1.212. [DOI] [PubMed] [Google Scholar]
- ROGERS D. E. The current problem of Staphylococcal infections. Ann Intern Med. 1956 Nov;45(5):748–781. doi: 10.7326/0003-4819-45-5-748. [DOI] [PubMed] [Google Scholar]
- Reboli A. C., Farrar W. E. Erysipelothrix rhusiopathiae: an occupational pathogen. Clin Microbiol Rev. 1989 Oct;2(4):354–359. doi: 10.1128/cmr.2.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. G., Vellozzi E., Shapiro J., Isenberg H. D. Infection with vancomycin-resistant "streptococci" due to Leuconostoc species. J Infect Dis. 1988 Jan;157(1):216–216. doi: 10.1093/infdis/157.1.216. [DOI] [PubMed] [Google Scholar]
- Ruhen R. W., Darrell J. H. Antibiotic synergism against group D streptococci in the treatment of endocarditis. Med J Aust. 1973 Jul 21;2(3):114–116. doi: 10.5694/j.1326-5377.1973.tb128693.x. [DOI] [PubMed] [Google Scholar]
- Ruoff K. L., Kuritzkes D. R., Wolfson J. S., Ferraro M. J. Vancomycin-resistant gram-positive bacteria isolated from human sources. J Clin Microbiol. 1988 Oct;26(10):2064–2068. doi: 10.1128/jcm.26.10.2064-2068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Kissinger J., Gilmore M. S., Murray P. R., Mulder R., Solliday J., Clarke B. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1989 Sep;33(9):1588–1591. doi: 10.1128/aac.33.9.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Koburov G. T. In vitro activities of quinolones against enterococci resistant to penicillin-aminoglycoside synergy. Antimicrob Agents Chemother. 1989 Jan;33(1):71–77. doi: 10.1128/aac.33.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapico F. L., Canawati H. N., Ginunas V. J., Gilmore D. S., Montgomerie J. Z., Tuddenham W. J., Facklam R. R. Enterococci highly resistant to penicillin and ampicillin: an emerging clinical problem? J Clin Microbiol. 1989 Sep;27(9):2091–2095. doi: 10.1128/jcm.27.9.2091-2095.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalbe R. S., Stapleton J. T., Gilligan P. H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987 Apr 9;316(15):927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Al-Obeid S., Shlaes J. H., Boisivon A., Williamson R. Inducible, transferable resistance to vancomycin in Enterococcus faecium, D399. J Antimicrob Chemother. 1989 Apr;23(4):503–508. doi: 10.1093/jac/23.4.503. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Al-Obeid S., Shlaes J. H., Williamson R. Activity of various glycopeptides against an inducibly vancomycin-resistant strain of Enterococcus faecium (D366). J Infect Dis. 1989 Jun;159(6):1132–1135. doi: 10.1093/infdis/159.6.1132. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Bouvet A., Devine C., Shlaes J. H., al-Obeid S., Williamson R. Inducible, transferable resistance to vancomycin in Enterococcus faecalis A256. Antimicrob Agents Chemother. 1989 Feb;33(2):198–203. doi: 10.1128/aac.33.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Marino J., Jacobs M. R. Infection caused by vancomycin-resistant Streptococcus sanguis II. Antimicrob Agents Chemother. 1984 Apr;25(4):527–528. doi: 10.1128/aac.25.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth E. G., Stevens P. J., Holliman R. E. Prevalence and susceptibility of highly gentamicin resistant Enterococcus faecalis in a south London teaching hospital. J Antimicrob Chemother. 1989 Apr;23(4):633–639. doi: 10.1093/jac/23.4.633. [DOI] [PubMed] [Google Scholar]
- Somma S., Gastaldo L., Corti A. Teicoplanin, a new antibiotic from Actinoplanes teichomyceticus nov. sp. Antimicrob Agents Chemother. 1984 Dec;26(6):917–923. doi: 10.1128/aac.26.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell T. C., Packham D. R., Shanker S., Foldes M., Munro R. Vancomycin therapy for methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):344–350. doi: 10.7326/0003-4819-97-3-344. [DOI] [PubMed] [Google Scholar]
- Standiford H. D., De Maine J. B., Kirby W. M. Antibiotic synergism of enterococci. Relation to inhibitory concentrations. Arch Intern Med. 1970 Aug;126(2):255–259. [PubMed] [Google Scholar]
- Swenson J. M., Hill B. C., Thornsberry C. Problems with the disk diffusion test for detection of vancomycin resistance in enterococci. J Clin Microbiol. 1989 Sep;27(9):2140–2142. doi: 10.1128/jcm.27.9.2140-2142.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toala P., McDonald A., Wilcox C., Finland M. Susceptibility of group D streptococcus (enterococcus) to 21 antibiotics in vitro, with special reference to species differences. Am J Med Sci. 1969 Dec;258(6):416–430. doi: 10.1097/00000441-196912000-00006. [DOI] [PubMed] [Google Scholar]
- Traber P. G., Levine D. P. Vancomycin Ototoxicity in patient with normal renal function. Ann Intern Med. 1981 Oct;95(4):458–460. doi: 10.7326/0003-4819-95-4-458. [DOI] [PubMed] [Google Scholar]
- Tuazon C. U., Miller H. Clinical and microbiologic aspects of serious infections caused by Staphylococcus epidermidis. Scand J Infect Dis. 1983;15(4):347–360. doi: 10.3109/inf.1983.15.issue-4.05. [DOI] [PubMed] [Google Scholar]
- Uttley A. H., George R. C., Naidoo J., Woodford N., Johnson A. P., Collins C. H., Morrison D., Gilfillan A. J., Fitch L. E., Heptonstall J. High-level vancomycin-resistant enterococci causing hospital infections. Epidemiol Infect. 1989 Aug;103(1):173–181. doi: 10.1017/s0950268800030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovo M., Morelli L., Bottazzi V. Drug resistance plasmids in Lactobacillus acidophilus and Lactobacillus reuteri. Appl Environ Microbiol. 1982 Jan;43(1):50–56. doi: 10.1128/aem.43.1.50-56.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS R. E., JEVONS M. P., SHOOTER R. A., HUNTER C. J., GIRLING J. A., GRIFFITHS J. D., TAYLOR G. W. Nasal staphylococci and sepsis in hospital patients. Br Med J. 1959 Oct 10;2(5153):658–662. doi: 10.1136/bmj.2.5153.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanakunakorn C., Bakie C. Synergism of vancomycin-gentamicin and vancomycin-streptomycin against enterococci. Antimicrob Agents Chemother. 1973 Aug;4(2):120–124. doi: 10.1128/aac.4.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanakunakorn C. Mode of action and in-vitro activity of vancomycin. J Antimicrob Chemother. 1984 Dec;14 (Suppl 500):7–18. doi: 10.1093/jac/14.suppl_d.7. [DOI] [PubMed] [Google Scholar]
- Watanakunakorn C. Treatment of infections due to methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):376–378. doi: 10.7326/0003-4819-97-3-376. [DOI] [PubMed] [Google Scholar]
- Westenfelder G. O., Paterson P. Y., Reisberg B. E., Carlson G. M. Vancomycin-streptomycin synergism in enterococcal endocarditis. JAMA. 1973 Jan 1;223(1):37–40. [PubMed] [Google Scholar]
- Williamson R., Al-Obeid S., Shlaes J. H., Goldstein F. W., Shlaes D. M. Inducible resistance to vancomycin in Enterococcus faecium D366. J Infect Dis. 1989 Jun;159(6):1095–1104. doi: 10.1093/infdis/159.6.1095. [DOI] [PubMed] [Google Scholar]
- Wilson A. P., O'Hare M. D., Felmingham D., Grüneberg R. N. Teicoplanin-resistant coagulase-negative staphylococcus. Lancet. 1986 Oct 25;2(8513):973–973. doi: 10.1016/s0140-6736(86)90622-7. [DOI] [PubMed] [Google Scholar]
- Woodford N., Johnson A. P., Morrison D., Chin A. T., Stephenson J. R., George R. C. Two distinct forms of vancomycin resistance amongst enterococci in the UK. Lancet. 1990 Jan 27;335(8683):226–226. doi: 10.1016/0140-6736(90)90317-x. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Dembinski S., Mikesell T., Schaberg D. R. High-level resistance to gentamicin in Streptococcus faecalis: risk factors and evidence for exogenous acquisition of infection. J Infect Dis. 1986 Jun;153(6):1075–1083. doi: 10.1093/infdis/153.6.1075. [DOI] [PubMed] [Google Scholar]
- al-Obeid S., Collatz E., Gutmann L. Mechanism of resistance to vancomycin in Enterococcus faecium D366 and Enterococcus faecalis A256. Antimicrob Agents Chemother. 1990 Feb;34(2):252–256. doi: 10.1128/aac.34.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Maza L., Ruoff K. L., Ferraro M. J. In vitro activities of daptomycin and other antimicrobial agents against vancomycin-resistant gram-positive bacteria. Antimicrob Agents Chemother. 1989 Aug;33(8):1383–1384. doi: 10.1128/aac.33.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]



